Abstract
Antiretrovirals (ARVs) present variable toxicity to aquatic organisms. This study reviewed the literature from the Web of Science, Scopus, and PubMed to evaluate ARV ecotoxicity, focusing on aquatic models. Inclusion criteria were studies using ARVs as test substances with toxicity data on aquatic organisms. Quality assessment followed the CRED method, and environmental risk was evaluated via risk quotients (RQs) using the ERA tool. Efavirenz emerged as the most toxic ARV, with EC50 values of 0.011 mg/L (Chlorococcum infusionum) to 0.034 mg/L (Raphidocelis subcapitata), causing growth inhibition, photosynthesis reduction, and oxidative stress. Tenofovir showed lower toxicity, with EC50 values above 300.0 mg/L (Biomphalaria glabrata) and 111.82 mg/L (Artemia salina). Other ARVs, including Lamivudine and Zidovudine, displayed moderate toxicity (EC50 3.013–5.442 mg/L in microalgae). Main effects observed included oxidative damage, altered enzyme activity (catalase and NADH-cytochrome C oxidoreductase), reduced growth and photosynthesis, and bioaccumulation in aquatic plants like Lemna minor. Efavirenz also showed synergistic toxicity when combined with other ARVs. These findings indicate that ARVs, particularly highly toxic compounds such as Efavirenz, pose significant ecological risks, emphasizing the importance of proper disposal and remediation strategies to protect aquatic ecosystems.
1. Introduction
Antiretrovirals (ARVs) are pharmaceutical products used to treat the human immunodeficiency virus (HIV), which causes acquired immunodeficiency syndrome (AIDS). Although they are effective in reducing mortality and improving the quality of life of people living with HIV, they can also affect the aquatic environment when found in water bodies [1,2].
ARVs can enter aquatic ecosystems through different routes, such as improper pharmaceutical waste disposal and disposal of expired or unused medicines in sinks or toilets. However, the predominant pathway is their excretion by treated humans through urine and feces, which leads to their presence in sewage treatment plants and, subsequently, in receiving water bodies [3,4].
Several adverse effects can occur once ARVs are released into the aquatic environment. Scientific studies show that antiretrovirals, such as Lamivudine (3TC), Dolutegravir (DTG), and Tenofovir (TNF), can adversely affect aquatic organisms [5], such as fish [6,7,8], invertebrates [9,10], and algae [11,12,13]. They can interfere with these organisms’ reproduction, growth, and development and affect their physiology and behavior [14,15].
Some ARVs, such as protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs), are known to be highly persistent in the aquatic environment and have a high capacity for bioaccumulation in aquatic organisms. Thus, these substances can remain for long periods, accumulating in sediments and aquatic biota, which can lead to accruement and potentially harmful effects on organisms and aquatic ecosystems [16,17].
In addition to directly impacting aquatic organisms, ARVs can also indirectly affect the structure and stability of aquatic food webs. When a pharmaceutical compromises the survival, growth, or reproduction of primary producers such as cyanobacteria, it reduces the availability of energy and biomass for primary consumers (e.g., zooplankton). This disruption can cascade through the food chain, ultimately affecting higher trophic levels, including fish and aquatic birds, and may result in ecological imbalances and biodiversity loss within aquatic ecosystems [16].
We should stress that ARVs’ impacts on the aquatic environment may vary according to factors such as drug concentration, duration of exposure, and the specific characteristics of the organisms and ecosystem in question. Therefore, more in-depth studies should be conducted to better understand these effects and adopt appropriate prevention and mitigation measures.
Investing in advanced technologies, such as efficient wastewater treatment systems, innovative filtration methods, high-precision environmental monitoring technologies, and advanced degradation processes, is crucial in minimizing ARVs’ impacts on the aquatic environment. Society must also be sensitized to the correct disposal of medicines, encouraging the return of expired or unused medicines to specific collection points [4].
One way to determine and predict the damage caused by these residues in the aquatic environment is through ecotoxicological tests using different aquatic organisms, such as cyanobacteria, microcrustaceans, invertebrates, and fish. These tests are vital in assessing ARVs’ effect on the aquatic environment, and they are designed to measure the impact of chemical compounds on aquatic organisms, allowing a more accurate understanding of the risks and potential effects of these substances [3].
Considering the growing number of ecotoxicity studies and the possible impact of ARV residues on human, animal, and environmental health, this work aimed to conduct a review of ecotoxicological studies of ARVs on aquatic organisms.
2. Materials and Methods
2.1. Search for Articles
We performed a review in the Web of Science, Scopus, and PubMed databases. The search was conducted in the database aggregator using the following keywords and synonyms: “antiretroviral” (study test substance), “environment” (study area), and “toxicity” (biomarker), and the Boolean descriptors that composed the following descriptor: (“antiviral” OR “antiretroviral”) AND “environment” AND “toxicity”. The articles obtained in the search were then transferred to the Rayyan platform to be selected or excluded from the review [18]. The search was carried out until March 2025, which refers to the endpoint of the screening and data collection process. The Google Scholar database aggregator was used to maximize the search strategy using the descriptor “antiretroviral” AND “aquatic organisms” AND “toxicity” AND “environment”.
2.2. Inclusion and Exclusion Criteria
The following inclusion criteria were adopted: (i) ARVs as the test substance; (ii) toxicity data (LC50, EC50, IC50, and similar); (iii) aquatic biological model; (iv) test substance concentration; (v) biological model species; and (vi) test substance description (molecular structure, molecular weight, solubility, and identification).
The exclusion criteria adopted were as follows: (i) estimated toxicity data using software (Toxicity Estimation Software Tool; Ecological Structure-Activity Relationships OR QSAR Toolbox); (ii) unspecified name of the biological model species; (iii) test substance other than ARVs; (iv) duplicates; (v) articles in a language other than English; (vi) reviews; (vii) theses or dissertations; (viii) terrestrial biological models; and (ix) test substance in mixtures with other medicines that are not ARVs.
2.3. Study Screening
Duplicates were initially excluded to screen the studies obtained from the search for articles. Then, the first screening stage was conducted by reading the works’ titles and abstracts. The second stage of the screening process consisted of reading the full articles selected in the first stage.
In both stages, articles were blind-screened by two independent authors (GSS and VAB). After completion, the results were compared, and those with a consensus of both authors were included or excluded from the review. A third author was called in to resolve any disagreement.
2.4. Data Retrieval
After the entire article screening process, we tabulated the selected articles in Excel to obtain detailed information on each article regarding its methodology and main results. Additional procedures were extracting information such as the biological model used, the sample analyzed from the biological model, the type of analysis performed (toxicity, cytotoxicity, and embryotoxicity), the target biomarker (enzymes, proteins, phagocytic activity, movement, heartbeat, and DNA damage), the antiretroviral used as the test substance, the exposure medium used, the test substance solubilization method (using solvent or not), the sample size (n), the number of replicates, the number of experiments, the concentrations evaluated in the study, the exposure time and classification (acute or chronic), and the effective, inhibitory, lethal, or growth concentration value.
2.5. Study Quality Assessment
We adopted the evaluation criteria proposed by Moermond et al. (2016) [19] through the Criteria for Reporting and Evaluating Ecotoxicity Data (CRED) method to assess the quality of the ecotoxicological studies included in this study. This method encompasses strict criteria to assess the reliability and relevance of ecotoxicological studies. The assessment included 20 reliability criteria and 13 relevance criteria (one point per criterion), with detailed guidelines for each, ensuring transparency and consistency.
The reliability criteria evaluated were as follows: (1) Does it include a reference to the methodology used for the tests? (2) Does it include good laboratory practices (standardized method)? (3) Does it include validity control? (4) Does it include solvent, negative, and positive control? (5) Does it include an identification of the substance (name or CAS)? (6) Does it include the substance’s source and purity? (7) Does it include impurities? If so, which ones were included? (8) Does it include a description of the organism (scientific name, weight, age, and size)? (9) Are the organisms kept in culture under controlled conditions (laboratory)? (10) Is the exposure compatible with the test substance? (11) Does it include environmental conditions (pH, temperature, and salinity)? (12) Were the concentrations below the water solubility limit? (13) Does it include correct spacing between concentrations? (14) Does it include the exposure time? (15) Were the initial and final concentrations of the test substance verified? (16) Is the amount of organisms compatible with the environmental amount? (17) Is there a sufficient number of organisms and replicates? (18) Are appropriate statistical methods used? (19) Does it include a dose–response curve? (20) Does it include sufficient data to prove the results? [19].
The relevance criteria evaluated were as follows: (1) Is the species compatible with the substance evaluated? (2) Are the organisms tested relevant to the compound tested? (3) Are the parameters evaluated compatible with the organism? (4) Are the parameters compatible with the test substance’s action? (5) Is the effect relevant at the population level (multi-parameters evaluated)? (6) Are the effect concentrations shown? (7) Are the organism’s life stages appropriate? (8) Are the test conditions relevant to the organism? (9) Is the duration of the test relevant to the organism? (10) If recovery from poisoning is performed, is it relevant? (11) If there is a formulation, is it relevant to the substance tested? (12) Is the exposure scenario tested relevant to the substance? (13) Is the exposure scenario tested relevant to the species? [19].
Based on these criteria, studies were classified as “reliable without restrictions” (30–33 points), “reliable with restrictions” (25–29 points), and “unreliable” (<25 points) based on the total score.
2.6. Environmental Risk Assessment (ERA)
The environmental risk assessment was performed using the Ecological Risk Assessment (ERA) tool [20]. In this study, the ERA was based on the calculation of risk quotients (RQs) (Equation (1)): the ratio between the measured environmental concentration (MEC) and the concentration of ARVs that does not cause an effect (Predicted Non-effect Concentration—PNEC). We adopted the PHARMS-UBA database of The Umweltbundesamt [21] to obtain the environmental concentration data of ARVs.
RQ = MEC/PNEC,
The ecological risk of each target substance was classified into four degrees according to the RQ value, as follows: RQ < 0.01, insignificant risk; 0.01 ≤ RQ < 0.1, low risk; 0.1 ≤ RQ < 1, medium risk; and RQ ≥ 1, high risk. The PNEC was defined as EC50 values (data obtained from the literature) divided by an evaluation factor of 1000 for each organism. This assessment factor was used to ensure a margin of safety when estimating the effects of ARVs on the environment, based on experimental data on unforeseen harmful effects.
3. Results and Discussion
3.1. Article Search
A total of 333 studies were found in the first screening stage (published up to March 2025), and 124 remained after removing duplicates. Next, all titles and abstracts were read to find studies that evaluated the toxicity of ARVs on aquatic organisms. After this second stage, 47 studies remained for full-text reading, and 24 articles published between 2016 and 2025 were considered eligible for the composition of this review (Figure 1) (Table 1).
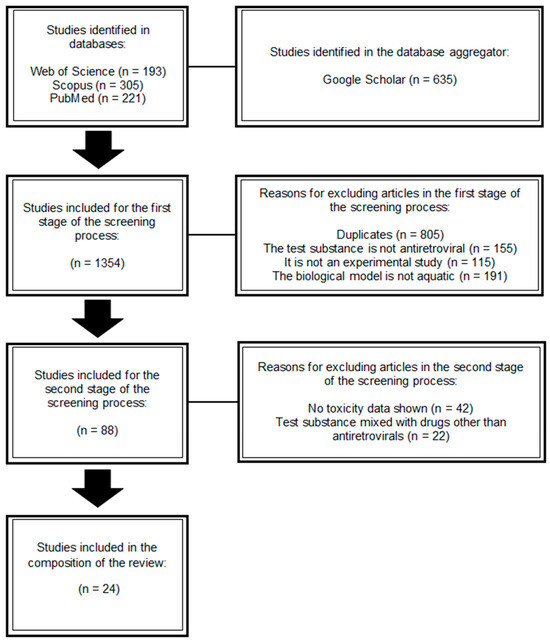
Figure 1.
Flowchart of the screening process for articles published up to March 2025 to select those included in this review.

Table 1.
Studies on ecotoxicity of antiretrovirals to aquatic organisms covered in this review.
3.2. Study Quality
All articles included in this review obtained scores equal to or higher than 80% regarding reliability, or 16/20 points, with an average of 18 points. Regarding relevance, the scores were equal to or higher than 77%, or 10/13 points, with an average of 12 points. Table 2 shows the score results of the articles included in this review regarding the study’s reliability and relevance.

Table 2.
Reliability and relevance score of articles included in this review using the Criteria for Reporting and Evaluating Ecotoxicity Data (CRED) method.
Based on the assessment of the reliability of the studies included in this review, the criteria with the lowest scores were as follows: (i) including a validity control of the experiments (such as a control chart); (ii) verifying the initial and final concentration of the substance in the test; (iii) showing a positive control for the tests; and (iv) showing the test substance’s dose–response curve.
Regarding the relevance of the studies included in this review, the criteria with the lowest scores were as follows: (i) conducting poisoning recovery tests, (ii) showing effect concentrations (e.g., EC50), and (iii) evaluating multi-parameters in the organisms (such as genotoxicity and cytotoxicity tests).
Therefore, we observed a clear lack of quality control in ecotoxicological studies with ARVs, especially in verifying the initial and final concentrations of the test. Many pharmaceutical residues, especially ARVs, undergo degradation processes, such as hydrolysis and photolysis.
For this reason, evaluating the test’s substance concentration at the beginning and end of the experiment is crucial to ensure that the organism’s exposure to the compound is accurate and consistent throughout the study. Only 8 of the 24 articles included in this review measured the initial and final concentration of the ARVs used in the experiment [6,7,22,23,25,31,32].
3.3. Study Location
Five countries conducted studies evaluating the ecotoxicity of ARVs on different biological models. South Africa (n = 10) stands out with the largest number of studies, followed by Brazil (n = 8), Argentina (n = 2), Italy (n = 3), and France (n = 1).
The high number of studies conducted in South Africa can be explained by factors such as the high HIV infection rate [40,41] and the consumption of ARVs throughout the country [42], which reflects the identification of these residues in different South African water bodies [2,43,44].
3.4. Antiretrovirals
This review evaluated the ARVs Nevirapine (n = 9), Lamivudine (n = 8), Efavirenz (n = 7), Tenofovir (n = 6), Zidovudine (n = 5), Stavudine (n = 3), Abacavir (n = 2), Atazanavir (n = 1), Lopinavir (n = 1), and Ritonavir (n = 1) for ecotoxicological evaluation on aquatic organisms (Table 3).

Table 3.
Physicochemical properties of antiretrovirals relevant for ecotoxicological assessments involving aquatic biological models.
3.5. Aquatic Organisms
The biological models used in the studies to evaluate ARV ecotoxicity comprised different food chains’ trophic levels, such as producers (algae, cyanobacteria, and microalgae), primary consumers (microcrustaceans and mollusks), secondary consumers (fish and amphibians), and decomposers (bacteria). These organisms were also found in both seawater and freshwater, which may influence their sensitivity to xenobiotics.
Figure 2 shows the distribution of EC50 values obtained for the different groups of tested organisms. A high variability in sensitivity was observed, especially among microalgae and cyanobacteria, whose EC50 values ranged across several orders of magnitude. For algae, values varied from extremely low concentrations (0.01 mg/L) to values exceeding 600 mg/L, reflecting possible heterogeneity among species or differential responses to experimental conditions. Similarly, cyanobacteria exhibited a wide data dispersion, revealing both highly sensitive and relatively tolerant organisms. Crustaceans, in turn, showed pronounced sensitivity, with most EC50 values falling within lower concentration ranges, indicative of high toxicity. Mollusks, fish, and echinoderms presented more consistent patterns, with EC50 values mostly within intermediate-to-low ranges. These results highlight the importance of assessing multiple taxonomic groups in the ecotoxicological characterization of substances, considering inherent differences in physiology, metabolism, and modes of action of compounds across different organisms.
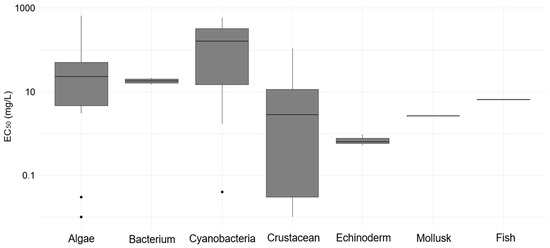
Figure 2.
Distribution of EC50 values (mg/L) for different taxonomic groups of aquatic organisms exposed to the tested compound.
The producer trophic level organisms were the most adopted by the articles (n = 10) among the different trophic levels evaluated in this review. Among these, different species of algae (Lemna minor), cyanobacteria (Microcystis novacekii and Synechococcus elongatus), and microalgae (Raphidocelis subcapitata, Chlorella vulgaris, Chlorococcum infusionum, Coelastrella tenuitheca, Tetradesmus obliquus, and Skeletonema marinoi) were employed to evaluate the toxicity of the ARVs ABC, TNF, EFZ, d4T, NVP, 3TC, RTN, and AZT.
Regarding primary consumer trophic-level organisms, different species of crustaceans (Daphnia magna, Artemia salina, and Thamnocephalus platyurus), microcrustaceans (Ceriodaphnia dubia), snails (Biomphalaria glabrata), sea urchins (Echinometra lucunter), and rotifers (Brachionus calyciflorus) were used in the studies (n = 8) to evaluate the toxicity of the ARVs TNF, EFZ, 3TC, AZT, ATV, RTN, NVP, and d4T.
Next, after the producers, most studies used aquatic organisms at the secondary consumer trophic level (n = 7). Different fish (Danio rerio and Oreochromis mossambicus) and amphibian species (Rhinella arenarum) were used in the studies to evaluate the ecotoxicological effects of the ARVs LPV, 3TC, d4T, AZT, NVP, and EFZ. Among these studies, the most used biological model at the secondary consumer trophic level was Oreochromis mossambicus.
Finally, among the studies, only the bioluminescent bacterial species Aliivibrio fischeri (n = 3), which belongs to the decomposer trophic level and is less commonly used as a biological model, was employed to evaluate the ecotoxicological effects of the ARVs TNF, NVP, dT4, and AZT.
Among the studies examined, EC50 values for ARV compounds showed wide variation across taxonomic groups. Concentrations ranged from 0.011 mg/L for C. infusionum exposed to EFZ, considered extremely low, to values exceeding 600 mg/L for C. infusionum exposed to TDF. Microalgae and cyanobacteria exhibited the greatest range of sensitivity to NNRTIs, with EFZ and NVP demonstrating the highest levels of toxicity. Conversely, NRTIs, such as 3TC, AZT, and TDF, tend to exhibit lower toxicity, with EC50 values generally exceeding 100 mg/L in several aquatic species.
Among consumers, crustaceans such as C. dubia and D. magna showed notable vulnerability, with EC50 values as low as 0.026 mg/L for EFZ and 0.012 mg/L for 3TC, respectively. These data suggest that the toxicity of ARVs depends primarily on the chemical class, environmental persistence, and biological characteristics of the exposed organism. This reinforces the importance of evaluating multiple species to characterize environmental risks.
Overall, the studies analyzed show that aquatic organisms at different trophic levels exhibit varying sensitivity to antiretroviral compounds. Microalgae and cyanobacteria, classified as primary producers, were the most frequently used biological models and, in general, showed greater vulnerability to ARV exposure, especially to substances such as Efavirenz and Nevirapine. Primary and secondary consumers, such as crustaceans, mollusks, and fish, showed intermediate-to-high sensitivity, indicating the possibility of bioaccumulation and trophic transfer in aquatic food chains. The variety of species and responses tested emphasizes the need for a multitrophic approach to accurately assess ecological risks.
3.6. ARV Toxicity in Aquatic Plants
Lemna minor, or duckweed, is an aquatic plant widely used in ecotoxicological studies due to its high sensitivity to contaminants and ability to respond rapidly to environmental stresses [45]. As a fast-growing plant, L. minor allows for efficient assessment of the effects of pollutants, such as heavy metals [46], pesticides [47], and pharmaceuticals [48] in aquatic environments.
L. minor was used to investigate the phytoremediation of the ARVs TNF (412 ng/L), 3TC (5428 ng/L), and EFZ (4000 ng/L) in aquatic environments, where several biomarkers of physiological and biochemical responses were evaluated through the activation of antioxidant defense mechanisms and detoxification. These biomarkers reflect the plant’s ability to cope with oxidative stress and metabolize toxic compounds [23].
EFZ, at a concentration of 4000 ng/L, significantly activated antioxidant responses in L. minor, evidenced by increased activity of cytochrome P450 and the antioxidant enzymes catalase (CAT) and ascorbate peroxidase (APX). These results suggest that the plant activated its endogenous defense mechanisms, specifically the antioxidant pathways, to mitigate the toxic effects induced by the medicine. The activation of these biomarkers indicates the plant’s significant oxidative stress and an attempt at detoxification [23].
On the other hand, TNF administered at a concentration of 412 ng/L showed lower toxicity to L. minor, not resulting in significant changes in growth, photosynthesis, or respiration parameters. Although it induced a mild antioxidant response, the effects were less pronounced when compared to EFZ, suggesting the medicine’s lower aggressiveness against the plant’s physiological health. After seven days of exposure, L. minor accumulated TNF in its tissues and eliminated approximately 70% of the medicine from the aquatic environment, indicating the plant’s ability to adsorb and biotransform this compound, which reinforces its potential as a bioindicator and phytoremediation tool [23].
3TC, administered at a concentration of 5428 ng/L, caused moderate toxicity compared to EFZ. The antioxidant response observed was more discreet, with lower antioxidant defense pathways activation rates, reflecting less intense environmental aggression. Despite this, L. minor tolerated the presence of 3TC well without significant impacts on its basal metabolism. The plant accumulated 3TC in its tissues and removed more than 80% of the medicine from the medium, showing its high efficiency in phytoremediation and its potential to decontaminate aquatic environments contaminated by medicines, such as ARVs [23].
L. minor’s simple morphology and easy handling in the laboratory make it ideal for short/long-term experiments. Parameters such as growth, photosynthesis, and oxidative stress are used as biomarkers to assess contaminants’ toxicity. In addition, L. minor is efficient in absorbing pollutants, making it an excellent candidate for phytoremediation [49].
This organism reacts differently to ARVs, according to experimental results, which also reveal a toxicity gradient among compounds. Increased cytochrome P450, CAT, and APX activity, which indicates the activation of detoxification pathways, were indicative of severe oxidative stress brought on by exposure to EFZ (4000 ng/L). While the plant eliminated roughly 70% of the compound from the medium, TDF (412 ng/L) showed negligible effects, with no discernible changes in growth or photosynthesis. 3TC (5428 ng/L) removed more than 80% of water while causing moderate stress and mild antioxidant activation. It also preserved metabolic stability. While TDF and 3TC demonstrated lower toxicity and higher phytoremediation efficiency, EFZ caused the most significant physiological disruption overall.
L. minor is an effective bioindicator of pharmaceutical contamination in aquatic systems, according to these findings. According to the findings, lipophilic and persistent ARVs, like EFZ, pose a higher ecological risk because of their limited degradation and stronger interaction with cellular membranes. On the other hand, the plant’s potential in phytoremediation programs is highlighted by its ability to absorb and metabolize hydrophilic ARVs, such as 3TC and TDF. Thus, L. minor contributes to ecological restoration and environmental management of contaminated aquatic ecosystems by acting as a model organism for toxicity testing as well as a natural remediation tool that can remove pharmaceutical residues.
3.7. ARV Toxicity in Microalgae
Microalgae are widely used in ecotoxicological tests due to their sensitivity to pollutants such as heavy metals [50], pesticides [51], pharmaceuticals [52], and nanoparticles [53], functioning as bioindicators of environmental quality. Their rapid response to contaminants, ease of cultivation, and role in food chains make them ideal for ecotoxicological studies. With standardized testing methods and the ability to biodegrade pollutants, microalgae can contribute to sustainable water treatment systems [54]. ARVs’ toxicity measured via EC50 values on different microalgae species are shown in the table below (Table 4).

Table 4.
Results of antiretroviral (ARV) toxicity on different microalgae species reported in the evaluated articles.
The ARV TNF’s toxicity was evaluated in different studies on the microalgae Chlorococcum infusionum [12,23] and Raphidocelis subcapitata [11]. TDF at a concentration of 412 ng/L did not produce significant toxic effects when exposed to the microalgae C. infusionum [23]. Even at concentrations of 100 µg/L, TDF did not cause significant toxic effects on cell growth, photosynthesis rate, and oxidative stress markers (H2O2; CAT; MDA) for C. infusionum, with an EC50 value of 671.1 mg/L [12]. However, the microalgae species R. subcapitata showed greater sensitivity to TDF, with an estimated EC50 value of 48.1 mg/L and NOEC of 24.41 µg/L [11].
Another nucleoside analog reverse transcriptase inhibitor (NRTI) evaluated was the ARV 3TC, where the microalgae species Chlorococcum infusionum [12,23] and Raphidocelis subcapitata [13] were used to determine its toxicity on these organisms.
At a concentration of 5428 ng/L of 3TC, we could not observe significant toxic effects on the cell growth of the microalgae C. infusionum, nor on the physiological biomarkers (photosynthesis and NADH-cytochrome) and oxidative stress indicators (H2O2 and MDA) [23].
Although classified as “practically non-toxic” to this organism (GHS, 2017 [55]), 3TC was slightly more toxic to C. infusionum than the ARV TDF, with an EC50 value of 571.1 mg/L [12]. For the microalgae species R. subcapitata, this difference was even greater regarding its sensitivity to 3TC, with an estimated EC50 value of 3.0 mg/L and a risk quotient (RQ) of 1.33, representing a high risk to the environment [13].
Unlike other NRTIs, regarding the ARV AZT, exposure of the microalgae species R. subcapitata to API led to a significant increase in cell growth at concentrations of 1.25 and 2.50 mg/L [13]. Thus, AZT showed a cell-growth-enhancing effect. However, there was no significant inhibition of algal growth at higher concentrations (>10 mg/L). In R. subcapitata, EC50 values were estimated at 5.4 mg/L [13] and 4.5 mg/L [35] at 96 and 72 h, respectively.
Two other NRTIs, ABC [36] and d4T [35], were reported to have toxic effects on the microalgae species R. subcapitata. The ARV ABC had low toxicity in R. subcapitata, with an estimated EC50 value of 57.3 mg/L [36], while the ARV d4T was toxic to the microalgae, with an estimated EC50 of 4.4 mg/L [35].
Regarding the toxicity of the ARV EFZ, different species of microalgae, Chlorococcum infusionum [12,23] and Raphidocelis subcapitata [13], were sensitive to the medicine. Even at a concentration of 4 µg/L, EFZ significantly reduced the C. infusionum photosynthesis rate and enzymatic activity of the enzyme NADH-cytochrome C oxidoreductase. Increasing indicators of oxidative stress were observed, with elevated production of H2O2 and MDA [23] and increased activity of the catalase enzyme. These effects indicate oxidative damage and cellular antioxidant response to stress in C. infusionum cells exposed to EFZ.
This high toxicity was also observed in another microalgae species, R. subcapitata, where exposure to EFZ for 96 h significantly inhibited microalgae growth at 16 to 250 µg/L concentrations. Regarding the risk classification, the ARV EFZ is classified as “very toxic” for the organisms C. infusionum and R. subcapitata, with an estimated EC50 value of 11 µg/L [12] and 34 µg/L [13], respectively.
Toxic effects of the NNRTIs NVP and EFZ have been reported on the microalgae species Chlorella vulgaris [26], Coelastrella tenuitheca, and Tetradesmus obliquus [25]. Cell growth inhibition is dose-dependent for these species, with increasing NVP levels resulting in greater growth inhibition. Exposure of C. tenuitheca to ARVs increased chlorophyll content and efficiency of the photosynthesis system II, which indicates possible protective mechanisms against NVP toxicity. In contrast, in T. obliquus, exposure led to decreasing chlorophyll content, suggesting a stress response to the medicine [25].
Among these organisms, T. obliquus showed greater sensitivity to NVP, with an estimated EC50 value of 18.2 mg/L, followed by C. tenuitheca with an estimated EC50 value of 23.5 mg/L [25] and C. vulgaris with an estimated EC50 of 24.9 mg/L [26]. The commercial medicine based on NVP had an estimated EC50 value of 19.5 mg/L. However, this difference is negligible, which suggests that the NVP formulation’s excipients did not affect the growth inhibition level observed in this species [26].
Finally, the PI RTV showed toxic effects on the microalgae species R. subcapitata, with an estimated EC50 value of 22.9 mg/L, with NOEC and LOEC values of 24.4 and 48.8 µg/L, respectively [11].
The reviewed studies show that microalgae’s sensitivity to ARV exposure varies widely. With EC50 values of 0.011 mg/L for C. infusionum and 0.034 mg/L for R. subcapitata, EFZ was the most toxic compound, resulting in oxidative stress and inhibition of photosynthesis. While nucleoside analogs like 3TC, AZT, and ABC demonstrated moderate-to-low toxicity, with EC50 values ranging from 3.0 to 57.3 mg/L, NVP and RTV displayed intermediate toxicity (18.0 to 22.9 mg/L). The least toxic substance was TDF (EC50 up to 671.0 mg/L). The findings show that, due to variations in solubility, lipophilicity, and cellular uptake mechanisms, non-nucleoside reverse transcriptase inhibitors are more detrimental to microalgae than nucleoside analogs.
All things considered, microalgae were shown to be extremely sensitive bioindicators for assessing the ecological risk of antiretrovirals in aquatic settings. They are perfect for identifying the early, sublethal effects of pharmaceutical residues because of their quick growth and metabolic reactions. Since lipophilic and persistent ARVs can interfere with photosynthesis and oxidative balance even at low concentrations, the persistently high toxicity of EFZ and NVP highlights the environmental concern. On the other hand, TDF and 3TC’s lower toxicity points to slower environmental dissipation and lower cellular uptake. These results highlight the value of microalgae-based bioassays for monitoring new pharmaceutical contaminants and assessing environmental risk.
3.8. ARV Toxicity in Cyanobacteria
Cyanobacteria are widely used in ecotoxicology studies [56,57,58]. These microorganisms are found in several aquatic environments, play an ecological role as primary photosynthetic organisms, and serve as water quality bioindicators, as they respond rapidly to changes in the concentration of pollutants, including heavy metals [59], nanoparticles [60], pesticides [61], and pharmaceutical residues, such as ARVs [12,34]. ARVs’ toxicity measured via EC50 values on two different species of cyanobacteria are shown in the table below (Table 5).

Table 5.
Results of antiretroviral (ARV) toxicity on two different species of cyanobacteria, Synechococcus elongatus and Microcystis novacekii, reported in the evaluated articles.
The ARV TDF is a low-toxicity NRTI in cyanobacteria. In S. elongatus, this ARV alone did not cause significant toxic effects on oxidative stress biomarkers (H2O2 and MDA), antioxidants (CAT) [12], photosynthesis, and enzymatic activity (NADH and Cox) [23]. The EC50 value was estimated at 594.5 mg/L [12]. This low toxicity was also reported in the species M. novacekii [34].
However, although the estimated EC50 value for the API TDF was 161.0 mg/L, a significantly lower estimated EC50 value of 89.00 mg/L was obtained for the TDF-based commercial medicine. These findings indicated that M. novacekii is tolerant to the active API TDF. However, the greater toxicity observed after exposure to commercial medicine suggests that the excipients used in the medicine formulation may contribute to increasing its environmental toxicity [34].
Furthermore, combining different ARVs with TDF potentiated their toxicity on the cyanobacterium M. novacekii. With the mixture of the commercial medicines TDF and 3TC, the EC50 value was estimated at 5.6 ± 0.3 mg/L. However, the mixture of the API TNF with 3TC reduced the toxicity, with an EC50 value higher than 256 mg/L. This same behavior was observed with the mixture of the ARV TNF and DTG, where the EC50 values were estimated at 1.1 ± 0.4 mg/L and 4.79 ± 0.73 mg/L for the mixture of commercial medicines and API, respectively [5].
Similar results were reported for the ARV 3TC, which also belongs to the NRTI class, where low toxicity to cyanobacteria was reported. When tested alone, 3TC did not cause significant effects on oxidative stress biomarkers, such as H2O2 and MDA, nor on antioxidants, such as the CAT enzyme [12]. There was also no relevant impact on photosynthesis and enzyme activity, including NADH and Cox [23]. The estimated EC50 value was 570.3 mg/L [12].
Furthermore, combining different ARVs with 3TC potentiated their toxicity against the cyanobacterium M. novacekii, as observed in the mixtures with TNF. In isolation, the EC50 value of the API 3TC against the cyanobacterium is 184.8 ± 22.1 mg/L, while that of the commercial medicine is 60.3 ± 2.7 mg/L, corresponding to a three-fold increase in toxicity. However, when the API 3TC and DTG were mixed, the potentialized toxicity was even greater, with an EC50 value of 11.4 ± 6.2 mg/L, corresponding to a 16-fold increase in toxicity [5].
The ARV EFZ belongs to the NNRTI class and is highly toxic to the cyanobacterium S. elongatus. At EFZ concentrations starting at 10 µg/L, this ARV significantly increased the H2O2 and MDA levels, indicating oxidative stress and damage to cell membranes and catalase activity. This suggests an attempt by the cyanobacterium to mitigate the effect of oxidative stress [12].
EFZ significantly reduced cell growth (EC50 40.1 µg/L), photosynthesis rate (EC50 19.7 µg/L), and enzymatic activity of NADH-cytochrome C oxidoreductase, leading to an increase in oxidative stress indicators, indicating oxidative damage in S. elongatus cells exposed to ARVs [23].
Although it was not the focus of this review, the possibility of a synergistic effect was verified in the combination of EFZ + 3TC, EFZ + TNF, and EFZ + 3TC + TNF. Tests with the combination of these drugs at environmental concentrations close to 0.3 µg/L demonstrated significant toxicity to S. elongatus. This effect is amplifying the toxic impact on cellular inhibition and photosynthesis, increasing H2O2 and lipid peroxidation levels and further affecting cellular integrity and cyanobacterial metabolism. These results indicate that EFZ, especially in combination with other ARVs, poses a considerable risk to photosynthetic organisms in contaminated aquatic environments [12]. Furthermore, since the drugs are simultaneously present in environmental matrices, the results presented should encourage further studies focusing on the possible interactions of these drugs and their ecotoxicological effects, and on expanding these effects to biodigestibility (ability of organisms metabolize or degrade drugs present in environmental matrices).
Finally, the ARV DTG showed high toxicity in the cyanobacterium M. novacekii, with EC50 values lower than 1 mg/L after chronic exposure (14 days). Furthermore, no stimulatory effects at low doses were observed on this organism after exposure to DTG, either a commercial medicine or an API [5].
Cyanobacterial sensitivity to various ARVs varied significantly, according to the reviewed studies. With EC50 values as low as 0.04 mg/L for S. elongatus, EFZ demonstrated the highest toxicity, resulting in oxidative stress, lipid peroxidation, and inhibition of photosynthesis. While TDF and 3TC showed significantly higher EC50 values (>100.0 mg/L), indicating low toxicity, DTG also caused significant toxicity to M. novacekii (EC50 = 1.7 mg/L). However, synergistic effects significantly increased toxicity when ARVs were tested in mixtures, such as TDF + 3TC or TDF + DTG, bringing EC50 values down to almost 5.0 mg/L. These results show that ARV combinations can intensify negative effects, especially for cyanobacteria, which are extremely vulnerable to photosynthetic disruption and oxidative imbalance.
All things considered, cyanobacteria were found to be extremely sensitive primary producers for evaluating pharmaceutical contamination. They are great markers of environmental stress because of their oxidative metabolism and photosynthetic activity. Given that these substances frequently co-occur in aquatic environments, the synergistic effects among ARV mixtures that have been observed raise serious ecological concerns. Even at concentrations that are relevant to the environment, Efavirenz and Dolutegravir can hinder photosynthesis and energy metabolism, which jeopardizes the growth and stability of cyanobacteria. 3TC and TDF, on the other hand, showed limited toxicity, indicating a weaker membrane interaction and decreased bioavailability. These findings highlight how crucial it is to incorporate cyanobacteria into multitrophic ecotoxicological evaluations in order to more accurately forecast the ecological risks connected to pharmaceutical contamination.
3.9. ARV Toxicity in Crustaceans
Crustaceans are organisms widely used as biological models in ecotoxicological tests because they have ecological, physiological, and behavioral characteristics making them sensitive to environmental changes, such as rapid responses to chemical stress, filter-feeding behavior, and well-characterized life cycles. They are therefore valuable organisms for assessing aquatic pollution [39]. Studies with these organisms are developed to evaluate different pollutants in the aquatic environment, such as heavy metals [62], pesticides [63], medicines [64], nanoparticles [65], and microplastics [66]. Based on EC50 values, ARVs’ toxicity in different microcrustacean species is shown in the table below (Table 6).

Table 6.
Results of antiretroviral (ARV) toxicity on five different species of crustaceans reported in the evaluated articles.
The ARV TNF’s toxicity is explored in different freshwater and saltwater microcrustacean species. TNF’s toxicity was significantly higher in freshwater organisms. Based on acute toxicity tests, TNF had an estimated LC50 value of 29.98 mg/L for C. dubia [11]. For the organisms B. calyciflorus and T. platyurus, even at high concentrations (100 mg/L), mortality after acute exposure was 2.83 and 4.98%, respectively. On the other hand, in chronic tests, TNF toxicity was higher for both C. dubia and B. calyciflorus, where ARVs had EC50 values of 5.74 µg/L and 1.08 µg/L, respectively. No significant subacute effects were observed for the organism T. platyurus, with an effect of 5.60% at concentrations of 100 mg/L of TNF. In genotoxicity and ROS production tests with C. dubia, TNF caused 16.63 ± 2.04% damage at higher concentrations (100 mg/L) [11].
No mortalities were observed in any of the treatment groups during 48 h of exposure to TNF (250 to 1000 µg./L) for the D. magna species. However, for enzymatic biomarkers, such as CAT activity, the activity decreased with increasing concentration, suggesting suppression of the antioxidant system at high concentrations and H2O2 reduction. In contrast, we observed a significant increase in GST activity at 250 and 500 µg/L concentrations. There were no significant changes in MDA levels for TNF [28].
In saltwater organisms, such as A. salina, the organism immobility EC50 value was estimated at 111.82 mg/L for the API and 61.83 mg/L for the medicine, suggesting a possible synergistic effect between the API and the excipients in the commercial medicine [53]. This freshwater organism’s high resistance is also similar to that found for the ARV ABC, where the nauplius larvae had EC50 values higher than 100 mg/L for the species A. salina, reinforcing the resistance of the organism to ARV residues [36].
For the NRTI 3TC, the reported toxicity in aquatic crustaceans was greater than that of the NRTI TNF. The ARV 3TC showed toxicity at environmentally relevant concentrations (10 µg/L), as it caused mortality of 10% and 45% of Daphnia magna individuals in 24 and 48 h of exposure, respectively. Toxicity was greater at higher concentrations (100 µg/L), with mortality of 85 and 100% in 24 and 48 h, respectively. Thus, the 3TC LC50 value was estimated at 34.1 µg/L (24 h) and 12.3 µg/L (48 h) [29,37].
In Ceriodaphnia dubia, the exposure of organisms to higher 3TC concentrations (0.625 to 10 mg/L) did not reduce female fertility. However, it significantly reduced neonates observed at 3TC concentrations of 1.25 to 10 mg/L, with EC50 values estimated at 1.345 mg/L [13]. These results indicate that 3TC has a higher toxic potential for D. magna than C. dubia, especially at high concentrations, suggesting that even small amounts of this medicine can cause lethal impacts for these aquatic organisms [13,29,37].
However, regarding the ARV AZT, the effect was the opposite of TNF’s. In D. magna, concentrations of 4.5 mg/L for AZT were insufficient to cause significant organism immobility, even after treating the sample containing ARVs with high doses of UV254 and UV254/H2O2 [35]. On the other hand, in C. dubia, AZT showed higher toxicity, with an estimated EC50 value of 5.671 mg/L [13].
Besides the ARV AZT, tests with D. magna exposed to 4.35 mg/L of d4T showed that this concentration was insufficient to cause significant organism immobility, even after treating the sample containing ARVs with the application of high doses of UV254 and UV254/H2O2. This suggests that the byproducts formed by degradation are not toxic [35]. In D. magna, exposure of the organism to 100 mg/L of ABC was insufficient to lead to immobility in 50% of the organisms. Therefore, the EC50 value was higher than 100 mg/L, suggesting a possible resistance of this organism to this ARV [36].
Nugnes et al. (2024) [11] evaluated the effects of RTV on the aquatic organisms C. dubia, B. calyciflorus, and T. platyurus. In acute toxicity tests, RTV resulted in the immobility of 38.33% of C. dubia organisms at concentrations of 2 mg/L. Similar results were observed in T. platyurus, where RTV exposure resulted in the immobility of 31.70% of organisms at 0.75 mg/L. Finally, only B. calyciflorus could be estimated to have an EC50 value of 11.35 mg/L.
Furthermore, no significant subacute effects were observed in subacute tests with T. platyurus, with immobility values of organisms exposed to RTV of 7.55% at 0.75 mg/L. On the other hand, RTV showed high toxicity in chronic tests, with EC50 values of 0.14 µg/L and 0.20 µg/L for C. dubia and B. calyciflorus, respectively. In genotoxicity tests, RTV caused 15.38% damage and induced 13.29% ROS production in C. dubia exposed to concentrations of 10 µg/L [11].
Regarding the NNRTI EFZ, the crustacean species Daphnia magna was resistant to acute exposure to concentrations of up to 1000 µg/L of the ARV. Although no mortality was observed in any group during the 48 h experiment, immobilization was concentration-dependent, especially at 250 to 1000 µg/L concentrations. The EC50 was not calculated because immobilization or mortality did not reach 50% even at the maximum concentration of 1000 µg/L [28].
CAT activity was significantly elevated for enzymatic biomarkers for EFZ at all concentrations (62.5 to 1000 µg/L), indicating increased H2O2. Regarding GST, we observed a significant reduction in activity at low concentrations of EFZ and a non-significant increase at intermediate concentrations. A change was observed concerning lipid peroxidation, with increased MDA levels at all concentrations, indicating oxidative stress and damage to the cell membrane [28].
In C. dubia, after exposure, we observed that the tested concentrations of EFZ (0.03 to 0.5 mg/L) reduced female fertility at EFZ concentrations of 0.031 to 0.25 mg/L, showing a high toxicity level, with an EC50 value of 0.026 mg/L [13]. However, although this NNRTI was highly toxic, as observed in other ARVs, saltwater aquatic organisms demonstrated greater resistance to exposure. The NNRTI NVP produced moderate toxicity in A. salina, with EC50 values of 27.77 mg/L [26].
Depending on the compound and length of exposure, crustaceans show a wide range of sensitivity to ARVs, according to toxicity data. With EC50 values ranging from 0.012 to 0.034 mg/L for D. magna and C. dubia, respectively, EFZ and 3TC were among the most toxic. With EC50 values above 100.0 mg/L, TDF and ABC, on the other hand, showed significantly lower toxicity and higher organismal tolerance. With chronic EC50 values close to 0.0002 mg/L, RTV produced severe sublethal effects that suggested oxidative stress and genotoxic reactions. In A. salina, a saltwater species that is more resilient to ARV exposure, NVP showed moderate toxicity (EC50 = 27.0 mg/L). According to these findings, the most sensitive bioindicators of ARV toxicity are freshwater crustaceans, especially Daphnia species.
All things considered, crustaceans make excellent biological models for evaluating the ecotoxicological effects of medications. The ecological hazards that these substances present to aquatic food webs, particularly at the primary consumer level, are highlighted by their sensitivity to low ARV concentrations. The findings show that, due to variations in chemical stability and lipophilicity, non-nucleoside reverse transcriptase inhibitors, like EFZ, exhibit greater toxicity than nucleoside analogs, such as TDF and 3TC. Even sublethal doses can cause oxidative stress and hinder reproduction, according to chronic exposure scenarios. In order to comprehend cumulative ecological damage and to direct environmental risk assessment and pollution management strategies, it is imperative to monitor the effects of ARVs on crustaceans.
3.10. ARV Toxicity in Fish
Fish are organisms widely used as biological models in ecotoxicological tests to assess the impacts of pollutants on the aquatic environment [24]. These organisms are representative of higher trophic levels and key ecological functions within aquatic ecosystems, hold important positions in food chains [67], have species diversity [68], and are environmental bioindicators [69]. ARVs’ main toxicity effects on two different fish species are shown in the table below (Table 7).

Table 7.
Results of antiretroviral (ARV) toxicity on two different fish species, Oreochromis mossambicus and Danio rerio.
The ARV NVP’s toxicity was explored in different biomarkers on the fish species O. mossambicus [6,31,32,33]. Exposure of fish (larval stage) to NVP for 60 days at an environmental concentration of 1.48 µg/L showed no changes in survival and development (such as length and mass) of the fish [31]. Also, we observed no changes in mean growth, behavior, and physical deformities in the O. mossambicus larvae [32]. However, at concentrations of 3.74 µg/L of NVP, fish of the species O. mossambicus demonstrated a significant increase in the hepato-somatic and splenic–somatic indices. Histopathology of the fish revealed that exposure to NVP caused hepatocyte necrosis, vacuolization, and moderate fibrosis around blood vessels and bile ducts.
Although hematological parameters such as hemoglobin, hematocrit, and leukocytes were unaffected by ARV exposure, the hepatic index was higher in fish exposed to NVP, indicating greater tissue damage [33]. In addition to these changes, NVP at a concentration of 3.74 µg/L caused significant changes in the ovarian index, an increase in vitellogenic oocyte atresia, and vacuolization of interstitial tissue [6].
The toxic effects of another NNRTI (EFZ) were evaluated on the O. mossambicus fish. Increased liver indices, sinus congestion, and changes in liver color were reported after 96 h of exposure to a concentration of 20.6 ng/L. These observed effects indicate growing liver damage, including steatosis and necrosis, directly reflecting on the fish’s general health [7].
Finally, another fish species, Danio rerio, was used as a biological model to evaluate the ARV LPV’s effects. After exposure, we observed that LPV had an estimated LC50 value of 6.417 mg/L, with dose-dependent toxicity in the first 24 h of exposure [8].
Research evaluating the toxicity of ARVs in fish revealed notable physiological and histopathological changes, especially in O. mossambicus. Hepatocyte necrosis, vacuolization, moderate fibrosis, and elevated hepato-somatic and ovarian indices were all signs of liver and reproductive impairment following exposure to NVP (3.74 µg/L). Even at low concentrations, EFZ (20.6 µg/L) demonstrated hepatotoxic potential by causing sinus congestion, steatosis, and necrosis. LPV exposure caused 50% mortality in D. rerio at 6.42 mg/L, indicating acute toxicity. These findings show that, at environmentally relevant concentrations, non-nucleoside reverse transcriptase inhibitors cause biochemical and histological harm to fish, making them more toxic than protease or nucleoside inhibitors.
Because of their ecological significance and physiological resemblance to higher vertebrates, fish are generally useful biological models for evaluating ARV ecotoxicity. According to the findings, long-term exposure to low ARV concentrations can cause serious tissue damage, particularly to the liver and reproductive organs, endangering the health of the organism and the stability of its population. The persistent and lipophilic ARVs that can bioaccumulate in aquatic organisms pose a threat to the environment, as evidenced by the consistent hepatotoxic effects of EFZ and NVP. Fish-based bioassays are crucial for ecological risk assessment and environmental management initiatives, and these results highlight the necessity of thorough monitoring of ARV residues in aquatic environments.
3.11. ARV Toxicity in Mollusks
Mollusks are widely used as biological models in ecotoxicity studies. Their characteristics facilitate the monitoring of pollutants and make them relatively easy to maintain in the laboratory. These factors make them effective models for assessing the impacts of pollution at the cellular and subcellular levels, contributing to knowledge of the ecological effects of chemical substances in the environment [70]. ARVs’ main toxicity effects on two different mollusk species are shown in the table below (Table 8).

Table 8.
Results of antiretroviral (ARV) toxicity on two different species of mollusk, Biomphalaria glabrata and Echinometra lucunter, reported in the evaluated articles.
Although it did not cause mortality in mollusks of the species B. glabrata, exposure for 21 days to the ARV TNF resulted in behavioral changes such as increased agitation and escape from the water [9]. When evaluating TNF’s cytotoxicity on mollusk hemocytes, exposure to ARVs significantly reduced the total number of circulating hemocytes and cell viability (EC50 of 2.65 mg/L). The ratio between granulocytes and hyalinocytes and metabolic activity (EC50 of 1.62 mg/L) also showed significant differences, indicating a possible toxic effect of TNF on B. glabrata hemocytes [9].
Regarding 3TC and AZT, embryotoxicity and genotoxicity tests of B. glabrata revealed toxic effects of lethality and significant damage to the DNA of hemocytes of mollusks exposed to ARVs, even after the photo-Fenton treatment process (initial concentration 15 mg/L). These results suggest that potentially toxic byproducts may be formed during ARV treatment and potentiate its toxicity [71].
From toxicity tests with E. lucunter exposed to the ARVs ATV, EFZ, and NVP, we observed that EFZ has greater toxicity at the fertilization stage, with a mean inhibitory concentration (EC50) of 11.46 mg/L. At the same time, for the other two ARVs, ATV and NVP, the EC50 value was estimated at 73.04 and 84.61 mg/L, respectively. Regarding the effects observed on larval development, the estimated EC50 values for the ARVs NVP, ATV, and EFZ were 0.52, 0.63, and 0.97 mg/L, respectively [10].
Mollusk toxicity data showed different cytotoxic and physiological reactions to ARV exposure. TDF decreased hemocyte viability and altered behavior in B. glabrata, with cytotoxicity and metabolic activity EC50 values of 2.65 mg/L and 1.62 mg/L, respectively. With EC50 values ranging from 0.52 to 0.63 mg/L during larval development, EFZ and ATV demonstrated developmental and reproductive toxicity to E. lucunter embryos. NVP showed reduced toxicity (0.97–84.6 mg/L), indicating increased tolerance at later stages of life. Furthermore, 3TC and AZT photodegradation byproducts maintained their genotoxic potential and damaged DNA in B. glabrata hemocytes. These results demonstrate how susceptible mollusks are to ARV exposure, especially in the early stages of development.
Mollusks have generally shown themselves to be useful bioindicators for assessing the less serious consequences of pharmaceutical contaminants. Their vulnerability to ARVs, particularly ATV and EFZ, indicates possible ecological hazards related to cellular damage and embryotoxicity. Even low levels of ARVs can affect immunological and metabolic processes, as evidenced by the observed decrease in hemocyte viability and DNA integrity. Additionally, the persistence of toxic byproducts following photodegradation raises the possibility that ecological hazards may not be completely mitigated by traditional wastewater treatments. To more accurately forecast the long-term and multigenerational impacts of antiretroviral residues on aquatic invertebrates and ecosystem health, mollusk-based assays must be included in environmental risk assessments.
3.12. ARV Toxicity in Amphibians
Amphibians are biological models used in ecotoxicity studies and excellent environmental quality bioindicators [72,73]. In direct contact with water and soil at different stages of their life cycle, amphibians are exposed to a variety of pollutants, allowing the assessment of these contaminants’ effects at multiple ecological levels, promoting the preservation of biodiversity and the health of ecosystems [73,74]. Among them, heavy metals [75], pesticides [76], and pharmaceutical residues [77] stand out. The toxicity results of different ARVs are shown in Table 9.

Table 9.
Results of antiretroviral (ARV) toxicity on tadpoles of the species Rhinella arenarum reported in the evaluated articles.
Fernández et al. (2020) [30] observed the ability of the ARVs 3TC, d4T, AZT, and NVP to bioaccumulate in tadpoles of the species R. arenarum, especially NVP. We observed a dose-dependent effect, with higher bioaccumulation with increasing ARV concentration. Another effect caused by these ARVs in tadpoles is the significant increase in glutathione S-transferase (GST) activity, suggesting oxidative stress damage in the organisms, possibly due to increased production of reactive oxygen species (ROS).
Fernández et al. (2022) [27] also evaluated the bioaccumulation of ABC and EFZ in R. arenarum tadpoles. EFZ showed higher bioaccumulation levels at environmentally relevant concentrations than ABC, which correlated with its greater lipophilicity. Although no mortality or behavioral changes were observed, the concentration of ARVs in tadpoles increased with concentration, highlighting the potential for bioaccumulation.
Research involving amphibians has demonstrated significant sublethal and bioaccumulative impacts of ARVs. In R. arenarum, significant bioaccumulation of EFZ and ABC was observed in tissues after 96 h, suggesting limited excretion and a strong affinity for biological membranes. Exposure to 3TC and AZT led to changes in hematological and metabolic parameters, characterized by heightened oxidative stress and diminished enzyme activity. At environmentally relevant concentrations, there was no acute mortality; however, chronic exposure indicated possible developmental and reproductive hazards. The EC50 values for these species ranged from 0.5 to 4.0 mg/L, indicating a moderate level of toxicity.
Because of their dual aquatic–terrestrial life cycle and permeable skin, amphibians are an important bioindicator group for evaluating the ecological risks of pharmaceuticals. ARVs can impair energy metabolism and development, particularly in the embryonic and larval stages, as evidenced by the observed oxidative and bioaccumulative effects. The possibility of biomagnification along food chains poses serious environmental concerns, even though their tolerance seems to be higher than that of microalgae and crustaceans. Because of their propensity for bioaccumulation, persistent substances like ABC and EFZ need special consideration. Thus, understanding ecosystem-level risks and enhancing ecotoxicological management requires ongoing monitoring of ARV residues and their long-term effects on amphibian populations.
3.13. ARV Toxicity in Bacteria
Bacteria are frequently used in ecotoxicological tests to assess contamination of the aquatic environment, as they have high sensitivity, fundamental ecological functions, such as the organic matter’s decomposition, ease of monitoring in laboratory conditions, and speed in conducting tests [38]. Ecotoxicological tests with bacteria are used to evaluate pollutants, such as heavy metals [78], pesticides [79], and medicines [80]. The species Aliivibrio fischeri is a bioluminescent marine bacterium widely used in ecotoxicological tests, especially in the bioluminescence inhibition test, which evaluates the toxicity of a substance based on the impact on the bacteria’s bioluminescence capacity [81]. The main toxicity effects of ARVs on two different fish species are shown in the table below (Table 10).

Table 10.
Results of antiretroviral (ARV) toxicity on the bacterial species Aliivibrio fischeri reported in the evaluated articles.
The toxicity of the ARVs NVP (API and medicine), TNF (API and medicine), AZT, and d4T were evaluated on the bioluminescent bacterium A. fischeri. For NVP, the EC50 value was estimated at 22.18 and 12.40 mg/L for API and medicine, respectively [26]. The toxicity to the ARVs was higher for TNF, with an EC50 value estimated at 14.83 and 8.20 mg/L for API and medicine, respectively [34]. These results show how the excipients in the medicine formulations contribute to the substances’ toxicity. On the other hand, the ARVs AZT and d4T at concentrations of 4.5 and 4.35 mg/L, respectively, were insufficient to cause toxicity effects, even after treating the samples with UV254 or UV254/H2O2, not evidencing acute or chronic effects [35].
Research using bacterial models showed that exposure to ARVs can disrupt vital cellular functions like bioluminescence, membrane integrity, and respiration. With EC50 values ranging from 12.4 to 22.2 mg/L for NVP and 14.8 mg/L for TDF, which indicate moderate acute toxicity, A. fischeri was the most used species. Even though nucleoside analogs like dT4 and AZT showed little toxicity, their breakdown products might still have some bioactivity. On the other hand, even at sublethal concentrations, EFZ and RTV demonstrated greater inhibitory potential on bacterial metabolism. According to these results, ARVs may interfere with bacterial respiration and enzymatic activity, which could have an impact on the equilibrium of the microbial community in aquatic environments.
All things considered, bacterial bioassays worked well for identifying quick reactions to drug contamination. A sensitive and repeatable endpoint for determining ARV toxicity in aquatic environments is the inhibition of A. fischeri bioluminescence. The findings show that even low levels of some substances, especially RTV and EFZ, can disrupt bacterial metabolism, which may have an impact on the breakdown of organic matter and the cycling of nutrients. ARV contamination presents a possible threat to ecosystem functioning given the ecological function of bacteria as decomposers. To detect early biochemical changes and assess the wider effects of antiretroviral residues on microbial processes, it is crucial to integrate bacterial models into environmental risk assessment frameworks.
3.14. ARV Ecotoxicity
The included ARVs showed a wide variation in concentrations in different aquatic and WWTP environments. The presence of ARVs in the environment has been widely reported across various environmental matrices, reflecting the increasing consumption of these pharmaceuticals and the limited efficiency of conventional wastewater treatment systems in fully removing them. Table 11 presents the ARV concentrations reported in the literature, organized by environmental matrix.

Table 11.
Concentrations of antiretroviral drugs (µg/L) reported in different environmental matrices.
ABC was detected in rivers and streams at concentrations of 0.001 µg/L in Germany [82] and 0.203 µg/L in the USA [83], and in untreated WWTP influents from South Africa at concentrations ranging from 3500 µg/L to 14,000 µg/L [84].
For AZT, concentrations in treated WWTP effluents in South Africa ranged from 0.078 µg/L to 0.740 µg/L; in untreated influents, they reached 1400 µg/L [84]. EFV showed higher concentrations in South African WWTPs, in untreated influents, at concentrations of up to 34,000 µg/L [84], and in treated effluents at concentrations of up to 37,300 µg/L [85]. In Kenya, 3TC was found in extremely high concentrations, with values up to 847,100 µg/L in treated WWTP effluents [86], while in Zambia, the concentrations were up to 118,970 µg/L in untreated WWTP influents [87].
In South Africa, LPV was detected in treated and untreated WWTP effluents at concentrations up to 3800 µg/L and 2500 µg/L, respectively [84]. NVP was found in treated WWTP effluents at concentrations of 9500 µg/L in Kenya [86], 1720 µg/L in Zambia [87], and 1900 µg/L in South Africa [84]. RTV was detected in untreated and treated WWTP effluents from South Africa at concentrations of 3200 µg/L and 1500 µg/L, respectively [84].
d4T has been detected in rivers and streams in South Africa at concentrations ranging from 0.102 µg/L to 0.778 µg/L [2]. TNF has been found at concentrations of up to 0.243 µg/L in rivers and streams in South Africa [2], and TNF has been found at concentrations of up to 0.250 µg/L in untreated WWTP waters in the same country [85]. Finally, AZT has been found at concentrations of up to 66,590 µg/L in untreated WWTP influents in Zambia [87] and 53,000 µg/L in South Africa [84]. Table 12 presents the main results found from this review on the possible impact of ARV on different aquatic organisms based on the EC50 values identified in the studies.

Table 12.
Representative risk of antiretrovirals on different aquatic organisms based on the EC50 values reported in the articles.
We calculated RQ values for the non-target aquatic organisms included in this study to assess the potential ecological risks of ARVs through their environmental concentrations. The maximum ARV concentrations in the final wastewater, surface water, or groundwater effluents were combined using MECs in Equation (1), considering the “worst-case” ecotoxicity scenario. The individual RQ values are provided in Figure 3.
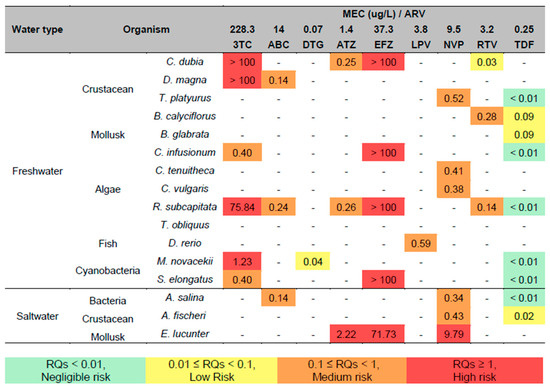
Figure 3.
RQs for antiretrovirals in water samples and receiving water bodies worldwide. The values of the concentration of ARV that do not cause an effect (Predicted Non-effect Concentration—PNEC) on the organisms used to estimate environmental risk are shown in Table 12; MEC = measured environmental concentration.
From the ARVs’ RQ obtained through the EC50 values of the studies included in this study, we can observe that TNF is the ARV with the lowest environmental risk. Although its toxicity to crustaceans and mollusks has been classified as “low” and “moderate”, respectively, due to its low environmental concentration, approximately 250 ng/L in untreated effluents from South Africa, TNF is classified as “negligible risk” and “low risk”. On the other hand, the ARV EFZ demonstrated greater environmental risk. Thus, for both the test organisms and the ecosystem, EFZ was classified as a “high risk” substance for mollusks, cyanobacteria, microalgae, and crustaceans and classified as “high” environmental risk.
3.15. Study Limitations
Reviews are one of the best ways to summarize and synthesize the available evidence on a given topic, in this case, ecotoxicological studies using aquatic organisms to assess the ecotoxicological effects of ARVs. However, some limitations need to be considered, such as:
- (1)
- Language restrictions: This review was limited to articles published in English, which may exclude studies published exclusively in other languages;
- (2)
- Time restrictions: The search was limited to a specific period (until March 2025) and may not include studies published between the screening and publication;
- (3)
- Scope restrictions: The search was restricted to the SciELO, Web of Science, Scopus, and PubMed databases;
- (4)
- Publication bias: There is a greater tendency to publish studies with positive and significant results, which may lead to a tendency towards selective publication and, consequently, to observed toxic effects;
- (5)
- Study heterogeneity: The studies included in this review have different species, interventions, exposure time, and matrix classification, which hampers the correlation of the results;
- (6)
- Data exclusion: Data not published in indexed journals, such as theses, dissertations, and abstracts, and data from the National Medical Information System, were not included in this review, since they did not meet the inclusion criteria for this review.
4. Conclusions
This study highlights growing concern about the environmental impacts of pharmaceutical residues in the environment on aquatic organisms. Although ARVs have been instrumental in preventing and controlling AIDS and improving the quality of life of people living with HIV, our study has shown that many of these medicines can have significant adverse effects on aquatic ecosystems. The evidence gathered in this review indicates that exposure to ARVs can harm aquatic organisms, affecting their behavior, growth, reproduction, and survival.
In some cases, even if there are no changes in these parameters, changes in immunological, biochemical, or histological biomarkers have been observed. Some of these medicines can persist in the environment for long periods, which increases the likelihood of chronic exposure and accumulation of residues in aquatic ecosystems and organisms. These results highlight the importance of more comprehensive environmental risk assessments during the development and use of ARVs, and the implementation of appropriate management strategies to reduce negative impacts by treating these wastes before they reach the different water bodies worldwide.
It is also crucial to foster continued research to better understand the mechanisms of toxicity and long-term effects on aquatic organisms since many regulations assess and standardize only acute exposure tests. Ultimately, this review highlights the need to assess the potential environmental threat and reinforces the importance of taking proactive measures to mitigate negative impacts and preserve the health of aquatic ecosystems, which are essential for life on the planet.
5. Prospects
Aquatic organisms have been used in ARV toxicity testing. However, despite the increasing number of studies, and based on the data from this review, several gaps deserve attention and investigation, such as:
- (1)
- Use of biochemical, histological, and immunological biomarkers to determine ARV toxicity, since some did not cause mortality in the test organisms but rather changes in these biomarkers.
- (2)
- Development of studies aimed at reviewing and standardizing aquatic biological models for toxicity tests of pharmaceutical residues, especially ARVs, since in some cases, acute exposure alone is not enough to determine toxicity, in addition to the recovery capacity of the organisms, which is often not evaluated in the studies.
- (3)
- Expansion of the scope of genotoxicity tests using metabolomic, proteomic, and transcriptomic analyses.
Author Contributions
Conceptualization, G.S.-S., V.A.B. and C.R.d.S.; methodology, G.S.-S., C.R.d.S. and V.A.B.; software, G.S.-S. and M.P.G.M.; validation, G.S.-S., M.M., M.D.A. and C.A.d.J.P.; formal analysis, G.S.-S.; investigation, V.A.B. and M.P.G.M.; data curation, M.D.A. and L.T.R.J.; writing—original draft preparation, G.S.-S., V.A.B. and M.C.V.M.S.; writing—review and editing, M.R.S., M.M., K.P.N. and M.P.G.M.; supervision, G.S.-S. and M.C.V.M.S.; project administration, G.S.-S.; funding acquisition, M.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Council for Scientific and Technological Development (CNPq), grant number 403853/2023-0, and the Minas Gerais State Research Support Foundation (FAPEMIG), grant numbers APQ-03069-23 and BIP-00012-24.
Data Availability Statement
The data supporting the findings of this study are available within the article. Additional datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to the National Council for Scientific and Technological Development (CNPq) and the Minas Gerais State Research Support Foundation for their essential funding that made this work possible. We recognize the importance of continued investment in scientific research for environmental preservation and public health, and we are grateful for their trust and encouragement of our work. During the preparation of this manuscript/study, the authors used ChatGPT-5 (OpenAI, San Francisco, CA, USA) as a support tool to increase the manuscript’s readability and linguistic accuracy. The original text was first drafted in Portuguese, and the software was only used for English translation and grammar correction. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- González Peña, O.I.; López Zavala, M.Á.; Cabral Ruelas, H. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef]
- Wood, T.P.; Duvenage, C.S.J.; Rohwer, E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Ecotoxicological assessment of pharmaceuticals and personal care products using predictive toxicology approaches. Green Chem. 2020, 22, 1458–1516. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, G.; Alcantara, M.D.; Souza, C.R.d.; Moreira, C.P.d.S.; Nunes, K.P.; Pereira, C.A.d.J.; Mol, M.P.G.; Silveira, M.R. Toxicity of the Antiretrovirals Tenofovir Disoproxil Fumarate, Lamivudine, and Dolutegravir on Cyanobacterium Microcystis novacekii. Water 2025, 17, 815. [Google Scholar] [CrossRef]
- Nibamureke, U.M.C.; Wagenaar, G.M. Histopathological changes in Oreochromis mossambicus (Peters, 1852) ovaries after a chronic exposure to a mixture of the HIV drug nevirapine and the antibiotics sulfamethoxazole and trimethoprim. Chemosphere 2021, 274, 129900. [Google Scholar] [CrossRef]
- Robson, L.; Barnhoorn, I.E.J.; Wagenaar, G.M. The potential effects of efavirenz on Oreochromis mossambicus after acute exposure. Environ. Toxicol. Pharmacol. 2017, 56, 225–232. [Google Scholar] [CrossRef]
- Aremu, O.S.; Katata-Seru, L.; Mkhize, Z.; Botha, T.L.; Wepener, V. Polyethylene glycol (5,000) succinate conjugate of lopinavir and its associated toxicity using Danio rerio as a model organism. Sci. Rep. 2020, 10, 11789. [Google Scholar] [CrossRef]
- Souza-Silva, G.; Souza, C.R.; Pereira, C.A.d.J.; Lima, W.D.S.; Mol, M.P.G.; Silveira, M.R. Toxicological evaluation of antiretroviral Tenofovir Disoproxil Fumarate on the mollusk Biomphalaria glabrata and its hemocytes. Sci. Total Environ. 2023, 891, 164484. [Google Scholar] [CrossRef]
- Cid, R.S.; Roveri, V.; Vidal, D.G.; Dinis, M.A.P.; Cortez, F.S.; Salgueiro, F.R.; Toma, W.; Cesar, A.; Guimarães, L.L. Toxicity of Antiretrovirals on the Sea Urchin Echinometra lucunter and Its Predicted Environmental Concentration in Seawater from Santos Bay (Brazilian Coastal Zone). Resources 2021, 10, 114. [Google Scholar] [CrossRef]
- Nugnes, R.; Orlo, E.; Russo, C.; Lavorgna, M.; Isidori, M. Comprehensive eco-geno-toxicity and environmental risk of common antiviral drugs in aquatic environments post-pandemic. J. Hazard. Mater. 2024, 480, 135947. [Google Scholar] [CrossRef]
- Gomes, M.P.; Kubis, G.C.; Kitamura, R.S.A.; Figueredo, C.C.; Nogueira, K.d.S.; Vieira, F.; Navarro-Silva, M.A.; Juneau, P. Do anti-HIV drugs pose a threat to photosynthetic microorganisms? Chemosphere 2022, 307, 135796. [Google Scholar] [CrossRef]
- Almeida, L.C.; Mattos, A.C.; Dinamarco, C.P.G.; Figueiredo, N.G.; Bila, D.M. Chronic toxicity and environmental risk assessment of antivirals in Ceriodaphnia dubia and Raphidocelis subcapitata. Water Sci. Technol. 2021, 84, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Escher, M.A.d.S.; Américo-Pinheiro, J.H.P.; Torres, N.H.; Ferreira, L.F.R. A Problemática Ambiental da Contaminação dos Recursos Hídricos Por Fármacos. Rev. Bras. Ciênc. Ambient. 2019, 51, 141–148. [Google Scholar] [CrossRef]
- Mahmood, A.R.; Al-Haideri, H.H.; Hassan, F.M. Detection of Antibiotics in Drinking Water Treatment Plants in Baghdad City, Iraq. Adv. Public Health 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F. What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1453–1496. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral drugs in aquatic environment and wastewater treatment plants: A review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020, 699, 134322. [Google Scholar] [CrossRef]
- Johnson, N.; Phillips, M. Rayyan for systematic reviews. J. Electron. Resour. Librariansh. 2018, 30, 46–48. [Google Scholar] [CrossRef]
- Moermond, C.T.A.; Kase, R.; Korkaric, M.; Ågerstrand, M. CRED: Criteria for reporting and evaluating ecotoxicity data. Environ. Toxicol. Chem. 2015, 35, 1297–1309. [Google Scholar] [CrossRef]
- Riva, F.; Zuccato, E.; Davoli, E.; Fattore, E.; Castiglioni, S. Risk assessment of a mixture of emerging contaminants in surface water in a highly urbanized area in Italy. J. Hazard. Mater. 2019, 361, 103–110. [Google Scholar] [CrossRef]
- UBA. Database—Pharmaceuticals in the Environment. Umwelt Bundesamt. 2025. Available online: https://www.umweltbundesamt.de/en/database-pharmaceuticals-in-the-environment-0#background (accessed on 15 July 2025).
- Silva, F.S.; Vasconcelos Lima, M.d.; Pereira, D.R.; Albuquerque Melo, A.M.M.d.; Cavalcanti, J.V.F.L.; Garcia, R.R.P.; Lucena, A.L.A.d.; Rodríguez-Díaz, J.M.; Honorato, F.A.; Napoleão, D.C. Photocatalytic efficacy of pyrite in the degradation of antiretroviral drugs: Biomphalaria glabrata as a bioindicator of toxic and genotoxic effects. Emerg. Contam. 2025, 11, 100416. [Google Scholar] [CrossRef]
- Kitamura, R.S.A.; Marques, R.Z.; Kubis, G.C.; Kochi, L.Y.; Barbato, M.L.; Maranho, L.T.; Juneau, P.; Gomes, M.P. The phytoremediation capacity of Lemna minor prevents deleterious effects of anti-HIV drugs to nontarget organisms. Environ. Pollut. 2023, 329, 121672. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Philippe, C.; Brendonck, L.; Pinceel, T. Towards improved fish tests in ecotoxicology—Efficient chronic and multi-generational testing with the killifish Nothobranchius furzeri. Chemosphere 2021, 273, 129697. [Google Scholar] [CrossRef]
- Reddy, K.; Renuka, N.; Kumari, S.; Ratha, S.K.; Moodley, B.; Pillay, K.; Bux, F. Assessing the potential for nevirapine removal and its ecotoxicological effects on Coelastrella tenuitheca and Tetradesmus obliquus in aqueous environment. Environ. Pollut. 2023, 317, 120736. [Google Scholar] [CrossRef] [PubMed]
- Diniz, J.S.; Freitas, L.A.d.P.; Vaz, I.C.D.; Barbosa, F.A.R.; Mol, M.P.G.; Magalhães, S.M.S.; Silveira, M.R. Os efeitos tóxicos do antirretroviral nevirapina e um medicamento à base de nevirapina para organismos aquáticos. RSD 2022, 11, e19211225014. [Google Scholar] [CrossRef]
- Fernández, L.P.; Brasca, R.; Repetti, M.R.; Attademo, A.M.; Peltzer, P.M.; Lajmanovich, R.C.; Culzoni, M.J. Bioaccumulation of abacavir and efavirenz in Rhinella arenarum tadpoles after exposure to environmentally relevant concentrations. Chemosphere 2022, 301, 134631. [Google Scholar] [CrossRef]
- Mahaye, N.; Musee, N. Effects of Two Antiretroviral Drugs on the Crustacean Daphnia magna in River Water. Toxics 2022, 10, 423. [Google Scholar] [CrossRef]
- Omotola, E.O.; Genthe, B.; Ndlela, L.; Olatunji, O.S. Environmental Risk Characterization of an Antiretroviral (ARV) Lamivudine in Ecosystems. Int. J. Environ. Res. Public Health 2021, 18, 8358. [Google Scholar] [CrossRef]
- Fernández, L.P.; Brasca, R.; Attademo, A.M.; Peltzer, P.M.; Lajmanovich, R.C.; Culzoni, M.J. Bioaccumulation and glutathione S-transferase activity on Rhinella arenarum tadpoles after short-term exposure to antiretrovirals. Chemosphere 2020, 246, 125830. [Google Scholar] [CrossRef]
- Nibamureke, U.M.C.; Barnhoorn, I.E.J.; Wagenaar, G.M. Assessing the potential effects of nevirapine in South African surface water on fish growth: A chronic exposure of Oreochromis mossambicus. S. Afr. J. Sci. 2019, 115, 1–6. [Google Scholar] [CrossRef]
- Nibamureke, U.M.C.; Barnhoorn, I.E.J.; Wagenaar, G.M. Hatching success and survival of fish early life stages in a chronic exposure to nevirapine: A case study of the Mozambique tilapia. Int. J. Environ. Health Res. 2018, 29, 441–456. [Google Scholar] [CrossRef]
- Nibamureke, U.; Barnhoorn, I.; Wagenaar, G. Nevirapine in African surface waters induces liver histopathology in Oreochromis mossambicus: A laboratory exposure study. Afr. J. Aquat. Sci. 2019, 44, 77–88. [Google Scholar] [CrossRef]
- Silva, S.R.; Barbosa, F.A.R.; Mol, M.P.G.; Magalhães, S.M.S. Toxicity for Aquatic Organisms of Antiretroviral Tenofovir Disoproxil. J. Environ. Prot. 2019, 10, 1565–1577. [Google Scholar] [CrossRef]
- Russo, D.; Siciliano, A.; Guida, M.; Andreozzi, R.; Reis, N.M.; Li Puma, G.; Marotta, R. Removal of antiretroviral drugs stavudine and zidovudine in water under UV254 and UV254/H2O2 processes: Quantum yields, kinetics and ecotoxicology assessment. J. Hazard. Mater. 2018, 349, 195–204. [Google Scholar] [CrossRef]
- Minguez, L.; Pedelucq, J.; Farcy, E.; Ballandonne, C.; Budzinski, H.; Halm-Lemeille, M.-P. Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in northwestern France. Environ. Sci. Pollut. Res. 2014, 23, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Omotola, E.O.; Genthe, B.; Ndlela, L.; Olatunji, O.S. Evaluation of the probable synergistic toxicity of selected potentiated antiretroviral and antibiotics on some aquatic biomarker organisms. Environ. Monit. Assess. 2023, 195, 1489. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, S.; Cavallo, A.; Del Rio, L.; Renzi, M. Impact of global change on environmental hazards of different clays: A case study on Aliivibrio fischeri. J. Hazard. Mater. 2023, 457, 131806. [Google Scholar] [CrossRef]
- Pereira da Silva Alves, D.; Borges Teixeira, F.; Teófilo, M.N.G.; Camilo Cotrim, C.F.; Silva, J.A.d.P.; Madureira Almeida, L.; Luiz Cardoso Bailão, E.F. Use of ecotoxicological bioindicators in effluent monitoring—Legal implications in Brazil. Eng. Sanit. Ambient. 2024, 29, e2023005. [Google Scholar] [CrossRef]
- Gona, P.N.; Gona, C.M.; Ballout, S.; Rao, S.R.; Kimokoti, R.; Mapoma, C.C.; Mokdad, A.H. Burden and changes in HIV/AIDS morbidity and mortality in Southern Africa Development Community Countries, 1990–2017. BMC Public Health 2020, 20, 867. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Cork, M.A.; Sligar, A.; Steuben, K.M.; Wilson, K.F.; Provost, N.R.; Mayala, B.K.; VanderHeide, J.D.; Collison, M.L.; Hall, J.B.; et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019, 570, 189–193. [Google Scholar] [CrossRef]
- Myers, B.; Parry, C.D.H.; Morojele, N.K.; Nkosi, S.; Shuper, P.A.; Kekwaletswe, C.T.; Sorsdahl, K.R. “Moving Forward with Life”: Acceptability of a Brief Alcohol Reduction Intervention for People Receiving Antiretroviral Therapy in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 5706. [Google Scholar] [CrossRef]
- Adeola, A.O.; Forbes, P.B.C. Antiretroviral Drugs in African Surface Waters: Prevalence, Analysis, and Potential Remediation. Environ. Toxicol. Chem. 2021, 41, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Zitha, A.B.; Ncube, S.; Mketo, N.; Nyoni, H.; Madikizela, L.M. Antiretroviral Drugs in Water: An African Challenge with Kenya and South Africa as Hotspots and Plausible Remediation Strategies. Chem. Afr. 2022, 5, 1237–1253. [Google Scholar] [CrossRef]
- Markovic, M.; Neale, P.A.; Nidumolu, B.; Kumar, A. Combined toxicity of therapeutic pharmaceuticals to duckweed, Lemna minor. Ecotoxicol. Environ. Saf. 2021, 208, 111428. [Google Scholar] [CrossRef]
- Horvat, T.; Vidaković-Cifrek, Ž.; Oreščanin, V.; Tkalec, M.; Pevalek-Kozlina, B. Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci. Total Environ. 2007, 384, 229–238. [Google Scholar] [CrossRef]
- Tagun, R.; Boxall, A.B.A. The Response of Lemna minor to Mixtures of Pesticides That Are Commonly Used in Thailand. Bull. Environ. Contam. Toxicol. 2018, 100, 516–523. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Fajardo-Romero, D.; Rodríguez-Rodríguez, C.E.; Cedergreen, N. Single and mixture toxicity of selected pharmaceuticals to the aquatic macrophyte Lemna minor. Ecotoxicology 2022, 31, 714–724. [Google Scholar] [CrossRef]
- Jmii, S.; Dewez, D. Toxic Responses of Palladium Accumulation in Duckweed (Lemna minor): Determination of Biomarkers. Environ. Toxicol. Chem. 2021, 40, 1630–1638. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghatchouli, N.; Arroussi, H. Overview of the management of heavy metals toxicity by microalgae. J. Appl. Phycol. 2022, 34, 475–488. [Google Scholar] [CrossRef]
- Luz, D.S.; Guimarães, P.S.; Castro, M.S.; Primel, E.G.; Giroldo, D.; Martins, C.d.M.G. Effects of the Pesticide Carbofuran on Two Species of Chlorophyceae (Desmodesmus communis and Pseudopediastrum boryanum) and Their Pesticide Bioremediation Ability. Environ. Toxicol. Chem. 2023, 43, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.-Y.; Lee, Y.-C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201, 110781. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.; An, S.-A.; Khim, J.S. Methodological advances and future directions of microalgal bioassays for evaluation of potential toxicity in environmental samples: A review. Environ. Int. 2023, 173, 107869. [Google Scholar] [CrossRef]
- United-Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 10th ed.; United Nations Publications: New York, NY, USA; Geneva, Switzerland, 2023. [Google Scholar]
- Marić, P.; Ahel, M.; Babić, O.; Simeunović, J.; Smital, T. Ecotoxicological profiling of selected cyanobacterial strains using multi-endpoint effect-directed analysis. Ecotoxicology 2020, 29, 535–550. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Q.; Zhang, Z.; Hu, B.; Chen, J.; Chen, J.; Qian, H. Pollutant toxicology with respect to microalgae and cyanobacteria. J. Environ. Sci. 2021, 99, 175–186. [Google Scholar] [CrossRef]
- Vilar, M.; Ferrão-Filho, A. (Eco)Toxicology of Cyanobacteria and Cyanotoxins: From Environmental Dynamics to Adverse Effects. Toxics 2022, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.P.S.; Dwivedi, V.; Kumar, S.; Kushwaha, A.; Goswami, L.; Reddy, B.S. Cyanobacterial Extracellular Polymeric Substances for Heavy Metal Removal: A Mini Review. J. Compos. Sci. 2020, 5, 1. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Abdi, I.; Alsherif, E.A.; Aloufi, A.S.; Korany, S.M.; Mohamed, M.Y.A.; Aldilami, M.; Selim, S.; Hamed, S.M. Species-specific ecotoxicity of platinum nanoparticles to two cyanobacteria. Mar. Pollut. Bull. 2024, 209, 117054. [Google Scholar] [CrossRef] [PubMed]
- Kapsi, M.; Tsoutsi, C.; Paschalidou, A.; Albanis, T. Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (N.W. Greece). Sci. Total Environ. 2019, 650, 2188–2198. [Google Scholar] [CrossRef]
- Okamoto, A.; Yamamuro, M.; Tatarazako, N. Acute toxicity of 50 metals to Daphnia magna. J. Appl. Toxicol. 2014, 35, 824–830. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Huang, Y.; Zhang, X.; Huang, Y.; Qin, W.C.; Wen, Y.; Zhao, Y.H. Evaluation of modes of action of pesticides to Daphnia magna based on QSAR, excess toxicity and critical body residues. Ecotoxicol. Environ. Saf. 2020, 203, 111046. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna model in the toxicity assessment of pharmaceuticals: A review. Ecotoxicol. Environ. Saf. 2021, 763, 143038. [Google Scholar]
- Liu, Z.; Malinowski, C.R.; Sepúlveda, M.S. Emerging trends in nanoparticle toxicity and the significance of using Daphnia as a model organism. Chemosphere 2022, 291, 132941. [Google Scholar] [CrossRef]
- Yin, J.; Long, Y.; Xiao, W.; Liu, D.; Tian, Q.; Li, Y.; Liu, C.; Chen, L.; Pan, Y. Ecotoxicology of microplastics in Daphnia: A review focusing on microplastic properties and multiscale attributes of Daphnia. Ecotoxicol. Environ. Saf. 2023, 249, 114433. [Google Scholar] [CrossRef]
- Ames, J.; Miragem, A.A.; Cordeiro, M.F.; Cerezer, F.O.; Loro, V.L. Effects of glyphosate on zebrafish: A systematic review and meta-analysis. Ecotoxicology 2022, 31, 1189–1204. [Google Scholar] [CrossRef]
- Badadyan, L. Ecotoxicology and water pollution—Fish disease. BIO Web Conf. 2024, 93, 04008. [Google Scholar] [CrossRef]
- Henke, A.N.; Chilukuri, S.; Langan, L.M.; Brooks, B.W. Reporting and reproducibility: Proteomics of fish models in environmental toxicology and ecotoxicology. Sci. Total Environ. 2024, 912, 168455. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, G.; Souza, C.R.d.; Pereira, C.A.d.J.; Santos Lima, W.d.; Mol, M.P.G.; Silveira, M.R. Using freshwater snail Biomphalaria glabrata (Say, 1818) as a biological model for ecotoxicology studies: A systematic review. Environ. Sci. Pollut. Res. 2023, 30, 28506–28524. [Google Scholar] [CrossRef]
- Amaral, D.F.d.; Guerra, V.; Motta, A.G.C.; Melo e Silva, D.d.; Rocha, T.L. Ecotoxicity of nanomaterials in amphibians: A critical review. Sci. Total Environ. 2019, 686, 332–344. [Google Scholar] [CrossRef]
- Johnson, M.S.; Aubee, C.; Salice, C.J.; Leigh, K.B.; Liu, E.; Pott, U.; Pillard, D. A review of ecological risk assessment methods for amphibians: Comparative assessment of testing methodologies and available data. Integr. Environ. Assess. 2016, 13, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, E.; Rodrigues, C.C.; Jacintho, J.C.; Franco-Belussi, L.; Jones-Costa, M.; Abdalla, F.C.; Rocha, T.L.; Salla, R.F. The amphibian’s spleen as a source of biomarkers for ecotoxicity assessment: Historical review and trends. Sci. Total Environ. 2023, 901, 165915. [Google Scholar] [CrossRef]
- Peluso, J.; Aronzon, C.M.; Martínez Chehda, A.; Cuzziol Boccioni, A.P.; Peltzer, P.M.; De Geronimo, E.; Aparicio, V.; Gonzalez, F.; Valenzuela, L.; Lajmanovich, R.C. Environmental quality and ecotoxicity of sediments from the lower Salado River basin (Santa Fe, Argentina) on amphibian larvae. Aquat. Toxicol. 2022, 253, 106342. [Google Scholar] [CrossRef]
- Jayawardena, U.A.; Wickramasinghe, D.D.; Udagama, P.V. Cytogenotoxicity evaluation of a heavy metal mixture, detected in a polluted urban wetland: Micronucleus and comet induction in the Indian green frog (Euphlyctis hexadactylus) erythrocytes and the Allium cepa bioassay. Chemosphere 2021, 277, 130278. [Google Scholar] [CrossRef]
- Boualit, L.; Cayuela, H.; Cattin, L.; Chèvre, N. The Amphibian Short-Term Assay: Evaluation of a New Ecotoxicological Method for Amphibians Using Two Organophosphate Pesticides Commonly Found in Nature—Assessment of Biochemical, Morphological, and Life-History Traits. Environ. Toxicol. Chem. 2022, 41, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, P.M.; Cuzziol Boccioni, A.P.; Martinuzzi, C.; Bassó, A.; Attademo, A.M.; Culzoni, M.J.; Paradina-Fernandez, L.; Lajmanovich, R.C. Ecotoxicity and Risk Assessment Characterization of Veterinary Pharmaceuticals on Anuran Amphibian Larvae. In Amphibian Species in Environmental Risk Assessment Strategies; Royal Society of Chemistry: London, UK, 2023; Volume 46, pp. 81–101. [Google Scholar]
- Rosado, D.; Usero, J.; Morillo, J. Assessment of heavy metals bioavailability and toxicity toward Vibrio fischeri in sediment of the Huelva estuary. Chemosphere 2016, 153, 10–17. [Google Scholar] [CrossRef]
- Vurm, R.; Tajnaiová, L.; Kofroňová, J. The Influence of Herbicides to Marine Organisms Aliivibrio fischeri and Artemia salina. Toxics 2021, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Mora, J.R.; Méndez-Rivera, M.; Ramírez-Morales, D.; Cambronero-Heinrichs, J.C.; Rodríguez-Rodríguez, C.E. Toxicity of selected pharmaceuticals and their mixtures to the aquatic indicators Daphnia magna and Aliivibrio fischeri. Ecotoxicology 2024, 33, 1047–1061. [Google Scholar] [CrossRef]
- Giner, B.; Lafuente, C.; Lapeña, D.; Errazquin, D.; Lomba, L. QSAR study for predicting the ecotoxicity of NADES towards Aliivibrio fischeri. Exploring the use of mixing rules. Ecotoxicol. Environ. Saf. 2020, 191, 110004. [Google Scholar] [CrossRef] [PubMed]
- Prasse, C.; Schlüsener, M.P.; Schulz, R.; Ternes, T.A. Antiviral Drugs in Wastewater and Surface Waters: A New Pharmaceutical Class of Environmental Relevance? Environ. Sci. Technol. 2010, 44, 1728–1735. [Google Scholar] [CrossRef]
- Carpenter, C.M.G.; Helbling, D.E. Widespread Micropollutant Monitoring in the Hudson River Estuary Reveals Spatiotemporal Micropollutant Clusters and Their Sources. Environ. Sci. Technol. 2018, 52, 6187–6196. [Google Scholar] [CrossRef]
- Abafe, O.A.; Späth, J.; Fick, J.; Jansson, S.; Buckley, C.; Stark, A.; Pietruschka, B.; Martincigh, B.S. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere 2018, 200, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Determination of selected antiretroviral drugs in wastewater, surface water and aquatic plants using hollow fibre liquid phase microextraction and liquid chromatography—Tandem mass spectrometry. J. Hazard. Mater. 2020, 382, 121067. [Google Scholar] [CrossRef]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water 2020, 12, 1376. [Google Scholar] [CrossRef]
- Ngumba, E.; Gachanja, A.; Nyirenda, J.; Maldonado, J.; Tuhkanen, T. Occurrence of antibiotics and antiretroviral drugs in source-separated urine, groundwater, surface water and wastewater in the peri-urban area of Chunga in Lusaka, Zambia. Water 2020, 46, 278–284. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).