Helping Opioid Use Disorder and PTSD with Exposure (HOPE): An Open-Label Pilot Study of a Trauma-Focused, Integrated Therapy for OUD/PTSD
Abstract
1. Introduction
2. Methods
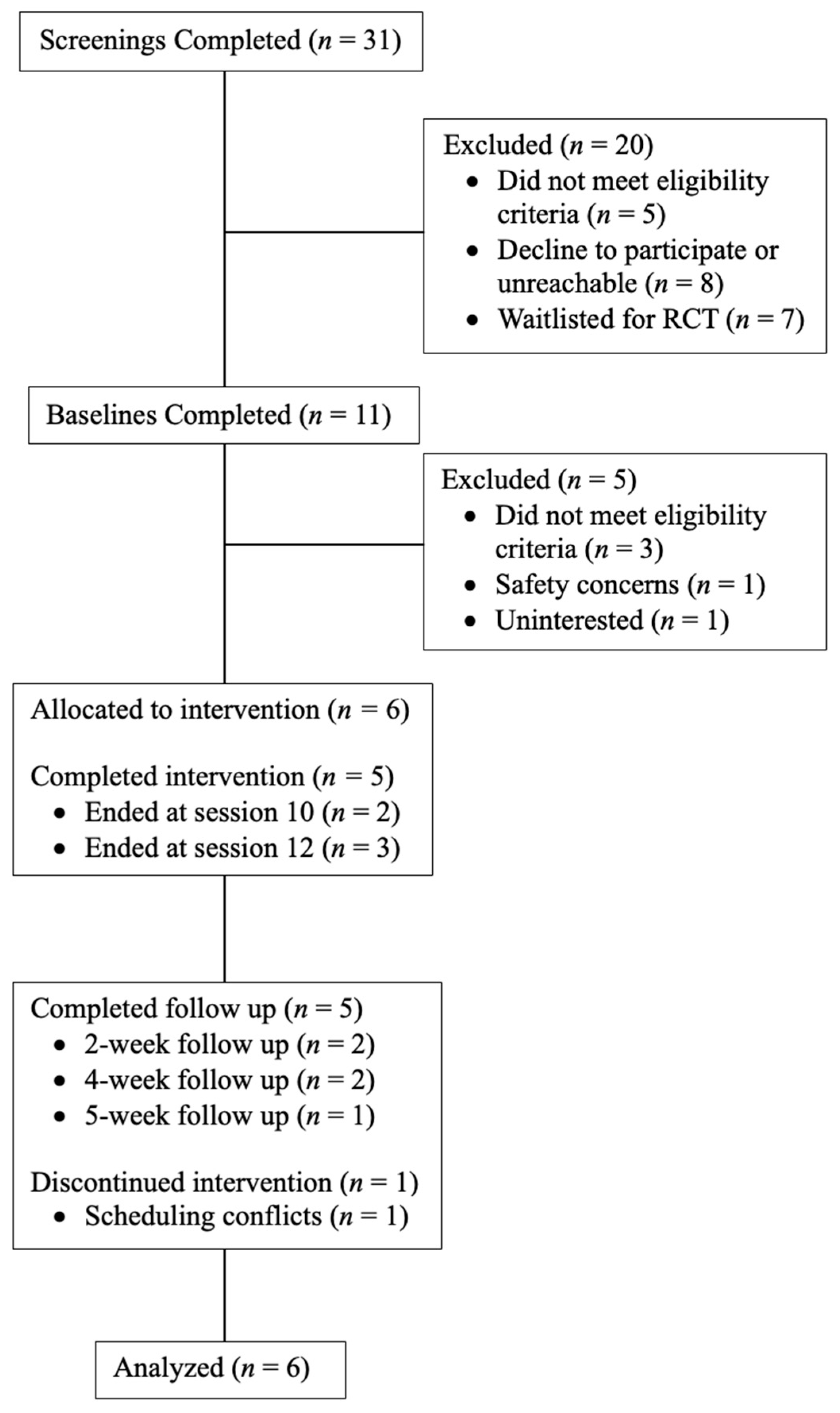
2.1. Participants
2.2. Procedures
2.3. The HOPE Intervention
2.4. Measures
2.5. Data Analysis
3. Results
3.1. Demographics
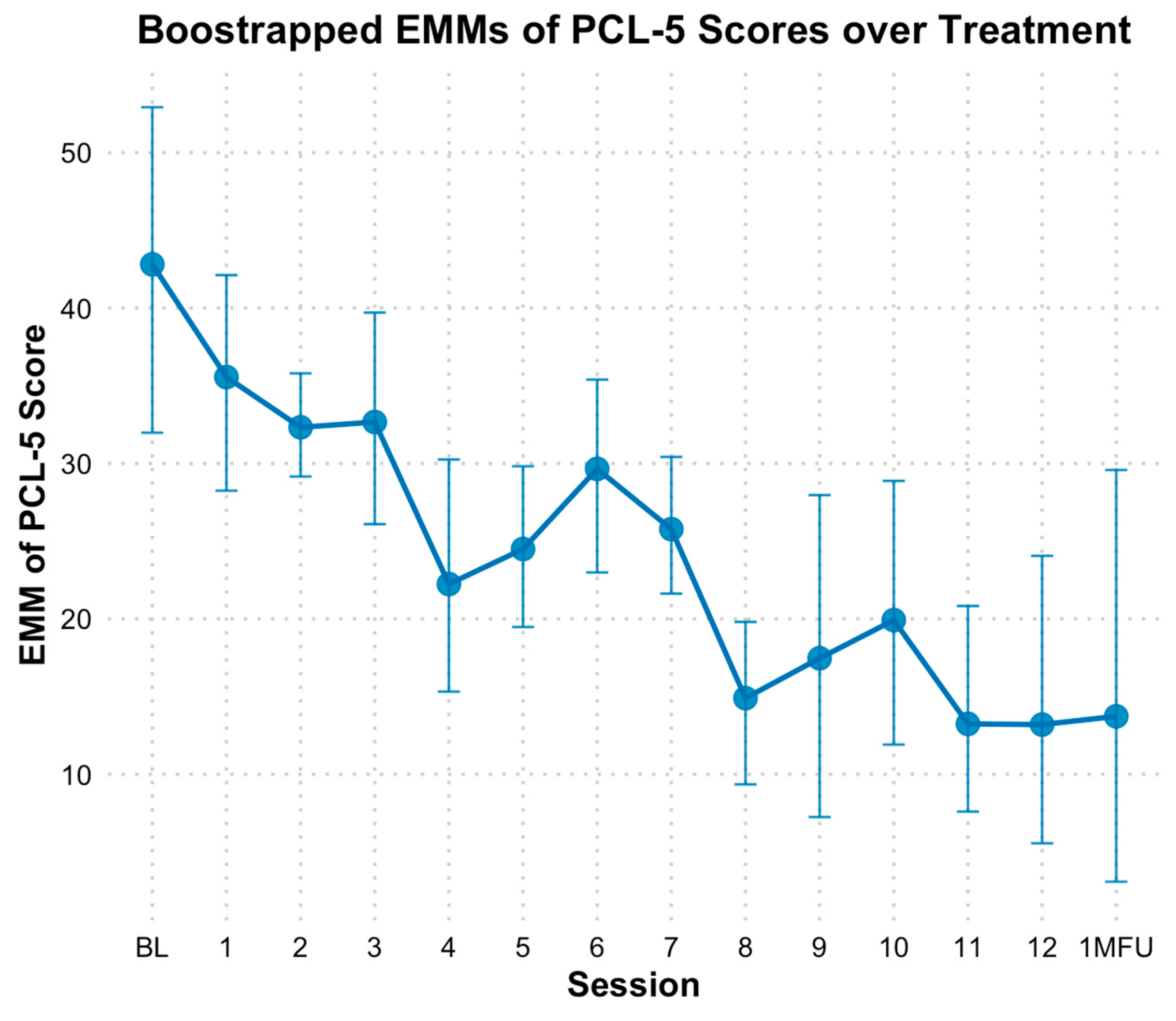
3.2. PTSD Outcomes
3.3. Opioid and Other Substance Use Outcomes
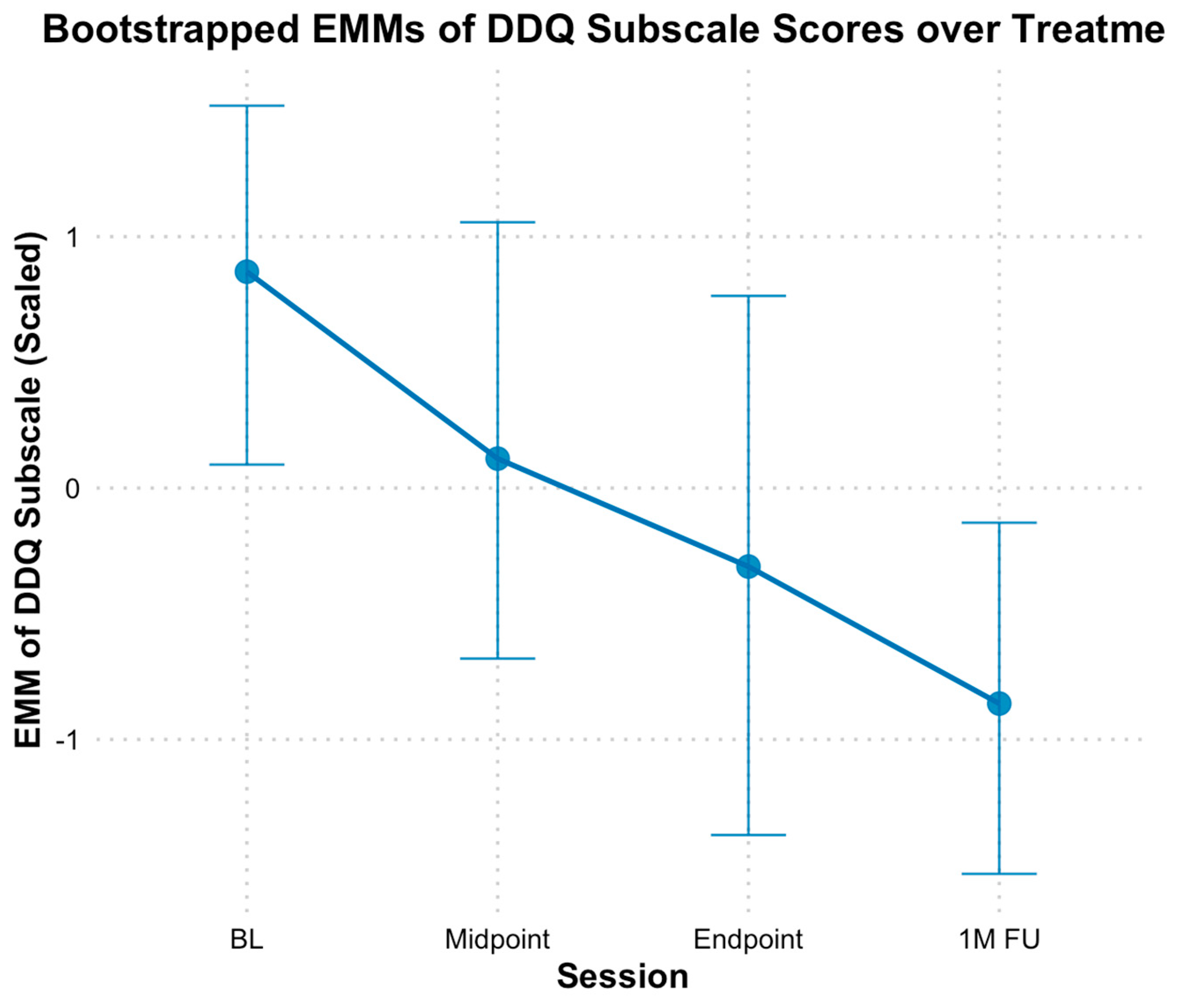
3.4. Opioid Craving and Desire
3.5. Medication for OUD Retention
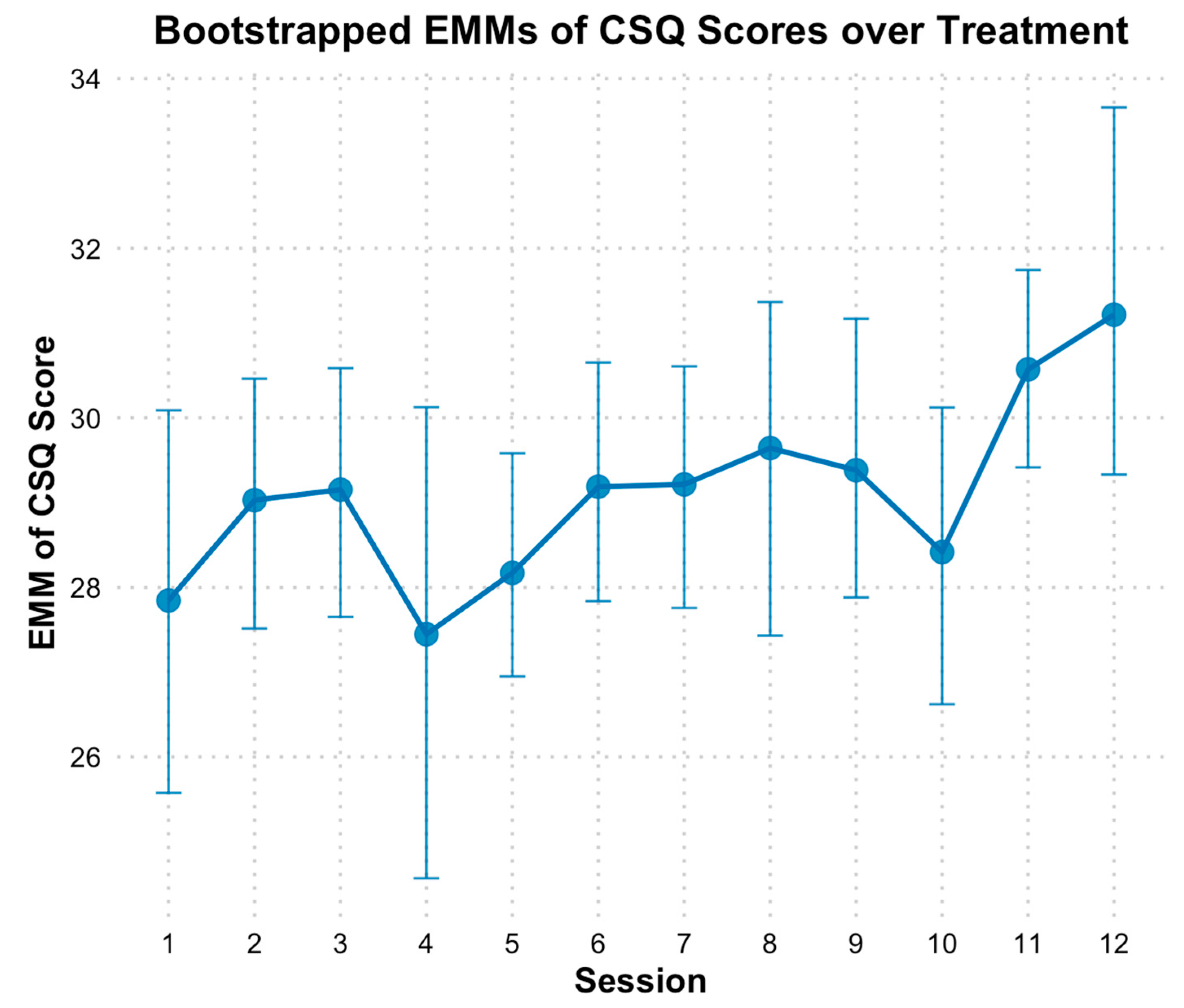
3.6. Client Satisfaction
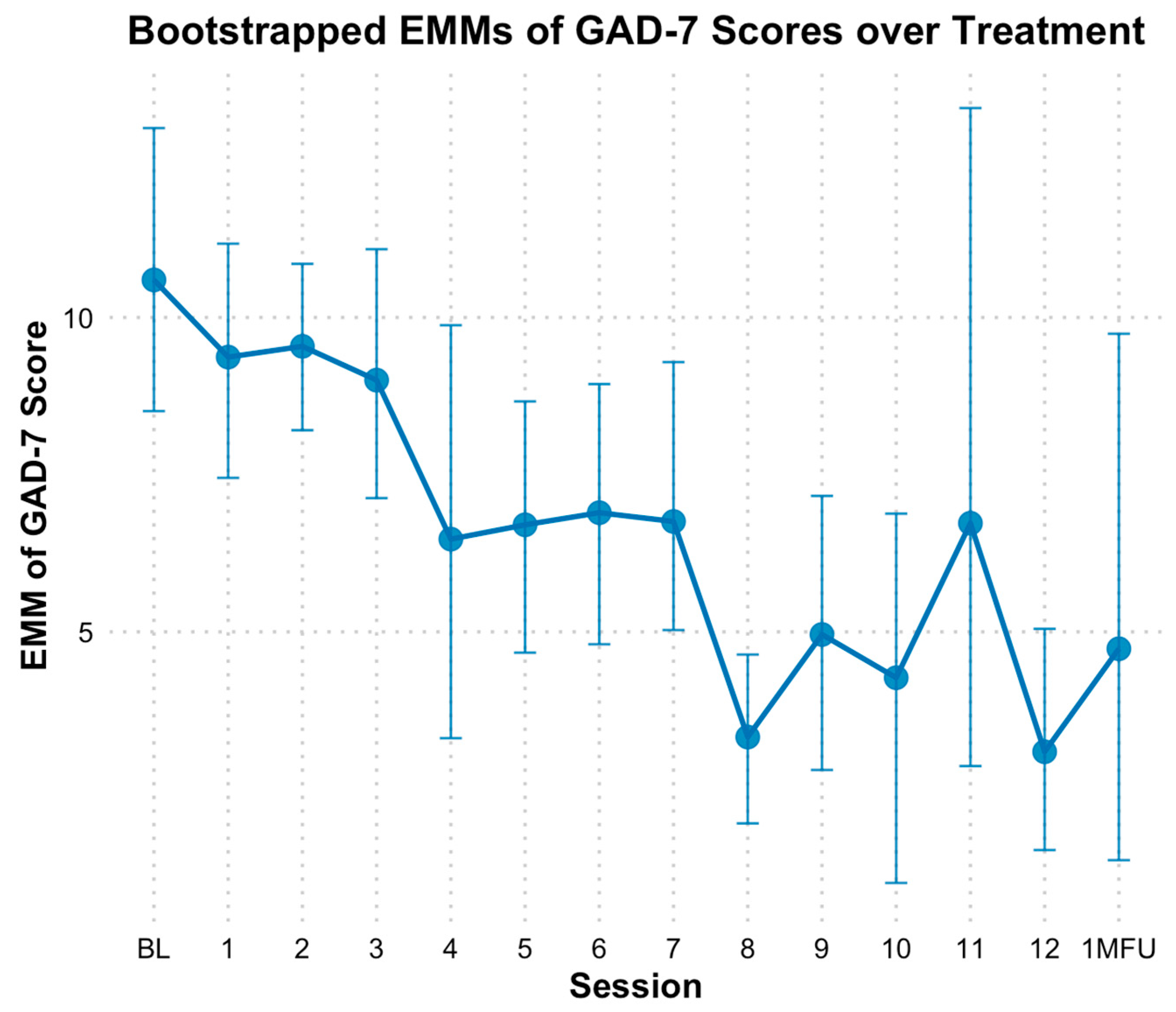
3.7. Depression and Anxiety
3.8. Adverse Events
4. Discussion
4.1. PTSD Outcomes
4.2. Opioid and Substance Use Outcomes
4.3. Client Satisfaction
4.4. Secondary Outcomes
4.5. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aderka, I. M., Gillihan, S. J., McLean, C. P., & Foa, E. B. (2013). The relationship between posttraumatic and depressive symptoms during prolonged exposure with and without cognitive restructuring for the treatment of posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 81(3), 375–382. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing, Inc. [Google Scholar] [CrossRef]
- Attkisson, C. C., & Zwick, R. (1982). The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation, Programming, and Planning, 5(3), 233–237. [Google Scholar] [CrossRef] [PubMed]
- Back, S. E., Foa, E. B., Killeen, T., Mills, K., Teesson, M., Dansky Cotton, B., Carroll, K. M., & Brady, K. T. (2014). Concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE): Therapist Manual. Oxford University Press. [Google Scholar] [CrossRef]
- Back, S. E., Killeen, T., Badour, C. L., Flanagan, J. C., Allan, N. P., Ana, E. S., Lozano, B., Korte, K. J., Foa, E. B., & Brady, K. T. (2019). Concurrent treatment of substance use disorders and PTSD using prolonged exposure: A randomized clinical trial in military veterans. Addictive Behaviors, 90, 369–377. [Google Scholar] [CrossRef]
- Ballinger, G. A. (2004). Using generalized estimating equations for longitudinal data analysis. Organizational Research Methods, 7(2), 127–150. [Google Scholar] [CrossRef]
- Banks, D. E., Brown, K., & Saraiya, T. C. (2023). “Culturally responsive” substance use treatment: Contemporary definitions and approaches for minoritized racial/ethnic groups. Current Addiction Reports, 10(3), 422–431. [Google Scholar] [CrossRef]
- Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. [Google Scholar] [CrossRef]
- Becker, W. C., Ganoczy, D., Fiellin, D. A., & Bohnert, A. S. (2015). Buprenorphine/Naloxone dose and pain intensity among individuals initiating treatment for opioid use disorder. Journal of Substance Use Treatment, 48(1), 128–131. [Google Scholar] [CrossRef]
- Belleau, E. L., Chin, E. G., Wanklyn, S. G., Zambrano-Vazquez, L., Schumacher, J. A., & Coffey, S. F. (2017). Pre-treatment predictors of dropout from prolonged exposure therapy in patients with chronic posttraumatic stress disorder and comorbid substance use disorders. Behavior Research Therapy, 91, 43–50. [Google Scholar] [CrossRef]
- Bernstein, D. P., & Fink, L. (1998). Childhood Trauma Questionnaire: A retrospective self-report manual. The Psychological Corporation. [Google Scholar]
- Biondi, B. E., Zheng, X., Frank, C. A., Petrakis, I., & Springer, S. A. (2020). A literature review examining primary outcomes of medication treatment studies for opioid use disorder: What outcome should be used to measure opioid treatment success? American Journal of Addiction, 29(4), 249–267. [Google Scholar] [CrossRef]
- Blevins, C. A., Weathers, F. W., Davis, M. T., Witte, T. K., & Domino, J. L. (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. [Google Scholar] [CrossRef]
- Bunting, A. M., Shearer, R., Linden-Carmichael, A. N., Williams, A. R., Comer, S. D., Cerdá, M., & Lorvick, J. (2024). Are you thinking what I’m thinking? Defining what we mean by “polysubstance use.”. American Journal of Drug and Alcohol Abuse, 50(1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. (2018). Annual surveillance report of drug-related risks and outcomes—United States. U.S. Department of Health and Human Services, Atlanta, Georgia.
- Chu, B. C., & Kendall, P. C. (2009). Therapist responsiveness to child engagement: Flexibility within manual-based CBT for anxious youth. Journal of Clinical Psychology, 65(7), 736–754. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A., & Mannarino, A. P. (2015). Trauma-focused cognitive behavior therapy for traumatized children and families. Child and Adolescent Psychiatric Clinic of North America, 24(3), 557–570. [Google Scholar] [CrossRef]
- Cottler, L. B., Compton, W. M., 3rd, Mager, D., Spitznagel, E. L., & Janca, A. (1992). Posttraumatic stress disorder among substance users from the general population. American Journal of Psychiatry, 149(5), 664–670. [Google Scholar] [CrossRef] [PubMed]
- Danovitch, I. (2016). Post-traumatic stress disorder and opioid use disorder: A narrative review of conceptual models. Journal of Addictive Diseases, 35(3), 169–179. [Google Scholar] [CrossRef]
- Ecker, A. H., & Hundt, N. (2018). Posttraumatic stress disorder in opioid agonist therapy: A review. Psychological Trauma: Theory, Research, Practice and Policy, 10(6), 636–642. [Google Scholar] [CrossRef]
- First, M. B., Williams, J. B. W., Karg, R. S., & Spitzer, R. L. (2015). Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). American Psychiatric Association. [Google Scholar]
- Foa, E. B., Hembree, E. A., Rothbaum, B. O., & Rauch, S. A. M. (2019). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences—Therapist guide (2nd ed.). Oxford University. Available online: https://www.oxfordclinicalpsych.com/view/10.1093/med-psych/9780190926939.001.0001/med-9780190926939 (accessed on 1 January 2022).
- Franken, I. H., Hendriksa, V. M., & van den Brink, W. (2002). Initial validation of two opiate craving questionnaires the obsessive compulsive drug use scale and the desires for drug questionnaire. Addictive Behaviors, 27(5), 675–685. [Google Scholar] [CrossRef]
- Galovski, T. E., Nixon, R. D. V., & Kehle-Forbes, S. (2024). Walking the line between fidelity and flexibility: A conceptual review of personalized approaches to manualized treatments for posttraumatic stress disorder. Journal of Traumatic Stress, 37(5), 768–774. [Google Scholar] [CrossRef]
- Gardner, W., Mulvey, E. P., & Shaw, E. C. (1995). Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychological Bulletin, 118(3), 392–404. [Google Scholar] [CrossRef]
- Haller, M., & Chassin, L. (2014). Risk pathways among traumatic stress, posttraumatic stress disorder symptoms, and alcohol and drug problems: A test of four hypotheses. Psychology of Addictive Behaviors, 28(3), 841–851. [Google Scholar] [CrossRef]
- Hassan, A. N., Le Foll, B., Imtiaz, S., & Rehm, J. (2017). The effect of post-traumatic stress disorder on the risk of developing prescription opioid use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. Drug and Alcohol Dependence, 179, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hien, D. A., Smith, K. Z., Owens, M., Lopez-Castro, T., Ruglass, L. M., & Papini, S. (2018). Lagged effects of substance use on PTSD severity in a randomized controlled trial with modified prolonged exposure and relapse prevention. Journal of Consulting and Clinical Psychology, 86(10), 810–819. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, C., Norberg, M. M., & Tomko, R. L. (2018). Solving the problem of cannabis quantification. The Lancet Psychiatry, 5(4), e8. [Google Scholar] [CrossRef]
- Højsgaard, S., & Lauritzen, S. (2024). On some algorithms for estimation in Gaussian graphical models. Biometrika, 111(4), 1201–1219. [Google Scholar] [CrossRef]
- Hser, Y. I., Saxon, A. J., Huang, D., Hasson, A., Thomas, C., Hillhouse, M., Jacobs, P., Teruya, C., McLaughlin, P., Wiest, K., Cohen, A., & Ling, W. (2014). Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction, 109(1), 79–87. [Google Scholar] [CrossRef]
- Kadden, R., Carroll, K., Donovan, D., Cooney, N., Monti, P., Abrams, D., Litt, M., & Hester, R. (1992). Cognitive-behavioral coping skills therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Khoury, L., Tang, Y. L., Bradley, B., Cubells, J. F., & Ressler, K. J. (2010). Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depressin and Anxiety, 27(12), 1077–1086. [Google Scholar] [CrossRef]
- Kroenke, K., & Spitzer, R. L. (2002). The PHQ-9: A new depression and diagnostic severity measure. Psychiatric Annals, 32, 509–521. [Google Scholar] [CrossRef]
- Lenth, R. (2025). Emmeans: Estimated marginal means, aka least-squares means. (R package version 1.11.1-00001). Available online: https://rvlenth.github.io/emmeans/ (accessed on 1 January 2022).
- Marx, B. P., Lee, D. J., Norman, S. B., Bovin, M. J., Sloan, D. M., Weathers, F. W., Keane, T. M., & Schnurr, P. P. (2021). Reliable and clinically significant change in the clinician-administered PTSD Scale for DSM-5 and PTSD Checklist for DSM-5 among male veterans. Psychological Assessment, 34, 197. [Google Scholar] [CrossRef]
- McHugh, R. K., Fitzmaurice, G. M., Carroll, K. M., Griffin, M. L., Hill, K. P., Wasan, A. D., & Weiss, R. D. (2014). Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug and Alcohol Dependence, 145, 121–126. [Google Scholar] [CrossRef]
- McHugh, R. K., Hilton, B. T., Chase, A. M., Griffin, M. L., & Weiss, R. D. (2021). Do people with opioid use disorder and posttraumatic stress disorder benefit from adding individual opioid drug counseling to buprenorphine? Drug and Alcohol Dependence, 113, 106710. [Google Scholar] [CrossRef]
- Meier, A., Lambert-Harris, C., McGovern, M. P., Xie, H., An, M., & McLeman, B. (2014). Co-occurring prescription opioid use problems and posttraumatic stress disorder symptom severity. American Journal of Drug and Alcohol Abuse, 40(4), 304–311. [Google Scholar] [CrossRef]
- Meshberg-Cohen, S., Black, A. C., DeViva, J. C., Petrakis, I. L., & Rosen, M. I. (2019). Trauma treatment for veterans in buprenorphine maintenance treatment for opioid use disorder. Addictive Behaviors, 89, 29–34. [Google Scholar] [CrossRef]
- Mills, K. L., Teesson, M., Ross, J., & Darke, S. (2007). The impact of post-traumatic stress disorder on treatment outcomes for heroin dependence. Addiction, 102(3), 447–454. [Google Scholar] [CrossRef]
- Mills, K. L., Teesson, M., Ross, J., & Peters, L. (2006). Trauma, PTSD, and substance use disorders: Findings from the Australian national survey of mental health and well-Being. American Journal of Psychiatry, 163(4), 652–658. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E. C., Heath, A. C., Lynskey, M. T., Bucholz, K. K., Madden, P. A. F., Statham, D. J., & Martin, N. G. (2006). Childhood sexual abuse and risks for licit and illicit drug-related outcomes: A twin study. Psychological Medicine, 36(10), 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T. D., Attkisson, C. C., & Stegner, B. L. (1983). Assessment of patient satisfaction: Development and refinement of a service evaluation questionnaire. Evaluation, Programming, and Planning, 6(3–4), 299–313. [Google Scholar] [CrossRef] [PubMed]
- Onken, L. S., Carroll, K. M., Shoham, V., Cuthbert, B. N., & Riddle, M. (2014). Reenvisioning clinical science: Unifying the discipline to improve the public health. Clinical Psychological Science, 2(1), 22–34. [Google Scholar] [CrossRef]
- Parida, S., De Aquino, J. P., & Sofuoglu, M. (2019). High potency synthetic opioids: Curbing the third wave of the opioid crisis. Neuroscience and Biobehavioral Reviews, 106, 9–10. [Google Scholar] [CrossRef]
- Peck, K. R., Giannini, J., Badger, G. J., Cole, R., & Sigmon, S. C. (2025). A novel prolonged exposure therapy protocol for improving therapy session attendance and PTSD symptoms among adults receiving buprenorphine or methadone treatment. Drug and Alcohol Dependence, 266, 112507. [Google Scholar] [CrossRef]
- Peck, K. R., Moxley-Kelly, N., Badger, G. J., & Sigmon, S. C. (2021). Posttraumatic stress disorder in individuals seeking treatment for opioid use disorder in vermont. Preventitive Medicine, 152, 106817. [Google Scholar] [CrossRef]
- Peck, K. R., Schumacher, J. A., Stasiewicz, P. R., & Coffey, S. F. (2018). Adults with comorbid posttraumatic stress disorder, alcohol use disorder, and opioid use disorder: The effectiveness of modified Prolonged Exposure. Journal of Traumatic Stress, 31(3), 373–382. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J. M., Brooner, R. K., King, V. L., & Kidorf, M. S. (2016). Effect of traumatic event reexposure and PTSD on substance use disorder treatment response. Drug and Alcohol Dependence, 158, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, N., Miglin, R., Bounoua, N., Beckford, E., Estrada, S., & Baskin-Sommers, A. (2021). Profiles of lifetime substance use are differentiated by substance of choice, affective motivations for use, and childhood maltreatment. Addictive Behaviors, 113, 106710. [Google Scholar] [CrossRef]
- Santo, T., Campbell, G., Gisev, N., Tran, L. T., Colledge, S., Di Tanna, G. L., & Degenhardt, L. (2021). Prevalence of childhood maltreatment among people with opioid use disorder: A systematic review and meta-analysis. Drug and Alcohol Dependence, 219, 108459. [Google Scholar] [CrossRef]
- Saraiya, T. C., Helpinstill, S., Gray, D., Hien, D. A., Brady, K. T., Hood, C. O., & Back, S. E. (2024). The lived experiences and treatment needs of women with opioid use disorder and PTSD symptoms: A mixed-methods study of women and providers. Journal of Substance Use and Addiction Treatment, 161, 209344. [Google Scholar] [CrossRef] [PubMed]
- Schacht, R. L., Brooner, R. K., King, V. L., Kidorf, M. S., & Peirce, J. M. (2017). Incentivizing attendance to prolonged exposure for PTSD with opioid use disorder patients: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 85(7), 689–701. [Google Scholar] [CrossRef]
- Schiff, M., Levit, S., & Cohen-Moreno, R. (2010). Childhood sexual abuse, post-traumatic stress disorder, and use of heroin among female clients in Israeli methadone maintenance treatment programs (MMTPS). Social Work & Health Care, 49(9), 799–813. [Google Scholar] [CrossRef]
- Schiff, M., Nacasch, N., Levit, S., Katz, N., & Foa, E. B. (2015). Prolonged Exposure for treating PTSD among female methadone patients who were survivors of sexual abuse in Israel. Social Work & Health Care, 54(8), 687–707. [Google Scholar] [CrossRef]
- Sloan, D. M., & Marx, B. P. (2019). Written exposure therapy for PTSD: A brief treatment approach for mental health professionals. American Psychological Association. [Google Scholar] [CrossRef]
- Smith, K. Z., Smith, P. H., Cercone, S. A., McKee, S. A., & Homish, G. G. (2016). Past year non-medical opioid use and abuse and PTSD diagnosis: Interactions with sex and associations with symptom clusters. Addictive Behaviors, 58, 167–174. [Google Scholar] [CrossRef]
- Sobell, L. C., & Sobell, M. B. (2000). Alcohol timeline followback (TFLB). In A. P. Association (Ed.), Handbook of psychiatric measures (pp. 477–479). American Psychiatric Associaiton. [Google Scholar]
- Spitzer, R. L., Kroenke, K., Williams, J. B., & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. [Google Scholar] [CrossRef]
- Szafranski, D. D., Gros, D. F., Acierno, R., Brady, K. T., Killeen, T. K., & Back, S. E. (2019). Heterogeneity of treatment dropout: PTSD, depression, and alcohol use disorder reductions in PTSD and AUD/SUD treatment noncompleters. Clinical Psychology & Psychotherapy, 26(2), 218–226. [Google Scholar] [CrossRef]
- Volkow, N., Benveniste, H., & McLellan, A. T. (2018). Use and misuse of opioids in chronic pain. Annual Review of Medicine, 69, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, S. E., Larochelle, M. R., Ameli, O., Chaisson, C. E., McPheeters, J. T., Crown, W. H., Azocar, F., & Sanghavi, D. M. (2020). Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Network Open, 3(2), e1920622. [Google Scholar] [CrossRef]
- Weathers, F. W., Blake, D. D., Schnurr, P. P., Kaloupek, D. G., Marx, B. P., & Keane, T. M. (2013a). The life events checklist for DSM-5 (LEC-5). National Center for PTSD. [Google Scholar]
- Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan, D. M., Schnurr, P. P., Kaloupek, D. G., Keane, T. M., & Marx, B. P. (2018). The Clinician-Administered PTSD scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30(3), 383–395. [Google Scholar] [CrossRef]
- Weathers, F. W., Litz, B. T., Keane, T. M., Palmieri, P. A., Marx, B. P., & Schnurr, P. P. (2013b). The PTSD Checklist for DSM-5 (PCL-5). (1 August 2020). Available online: www.ptsd.va.gov (accessed on 1 January 2022).
- Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J., Kuhn, M., Pedersen, T. L., Miller, E., Bache, S. M., Müller, K., Ooms, J., Robinson, D., Seidel, D. P., Spinu, V., … Yutani, H. (2019). Welcome to the tidyverse. Journal of Open Source Software, 4(43), 1686. [Google Scholar] [CrossRef]
- Wickham, H., Miller, E., & Smith, D. (2023). Haven: Import and export ‘SPSS’, ‘Stata’ and ‘SAS’ files. (R package version 2.5.3). Available online: https://CRAN.R-project.org/package=haven (accessed on 1 January 2022).


| Variable | N (%) or M (SD) |
|---|---|
| Age | 44.7 (12.9) |
| Sex | |
| Woman | 4 (66.7%) |
| Man | 2 (33.3%) |
| Racial Identity | |
| White | 4 (66.7%) |
| Multiracial (Native American/White) | 1 (16.7%) |
| Pacific Islander | 1 (16.7%) |
| Native American/Alaska Native | 0 (0%) |
| Black/African American | 0 (0%) |
| Asian | 0 (0%) |
| Hispanic/Latino (Central American origin) | 1 (16.7%) |
| Have children (yes/no) | 4 (66.7%) |
| Monthly income | $1650 ($1764) |
| Employment (Part-time or Full-time) | 5 (83.3%) |
| Insurance | |
| Medicaid | 5 (83.3%) |
| Private | 1 (16.7%) |
| Education | |
| Some high school | 1 (16.7%) |
| Completed high school/GED | 4 (66.7%) |
| Some college or Associates | 1 (16.7%) |
| Incarceration History | 4 (66.7%) |
| Religion | |
| Christianity/Catholicism | 3 (50.0%) |
| Islam | 1 (16.7%) |
| Atheist | 2 (33.3%) |
| Relationship status | |
| Single/never married | 3 (50.0%) |
| Living with Partner | 1 (16.7%) |
| Divorced | 1 (16.7%) |
| Children (yes) | 4 (66.7%) |
| Number of children | 1.4 (1.1) |
| Primary caregiver of children (yes) | 1 (16.7%) |
| Variable | N (%) or M (SD) |
|---|---|
| Medication for OUD | |
| Methadone | 1 (16.7%) |
| Buprenorphine/Suboxone | 4 (66.7%) |
| Naltrexone/Vivitrol | 1 (16.7%) |
| Age of first opioid use | 19.7 (7.2) |
| Age of opioid use disorder onset | 21.3 (6.7) |
| Ever overdosed (yes/no) | 1 (16.7%) |
| Current diagnoses | |
| Current OUD | 6 (100.0%) |
| Major depressive episode | 2 (33.3%) |
| Alcohol use disorder | 3 (50.0%) |
| Sedative use disorder | 3 (50.0%) |
| Cannabis use disorder | 2 (33.3%) |
| Stimulant use disorder | 3 (50.0%) |
| Nicotine User | 5 (83.3%) |
| Chronic pain | 2 (33.3%) |
| PHQ-9 at baseline | 12.0 (5.1) |
| GAD-7 at baseline | 10.5 (4.3) |
| PTSD Severity | |
| CAPS-5 at baseline | 32.0 (3.1) |
| PCL-5 at baseline | 42.3 (7.5) |
| Trauma Characteristics | |
| Lifetime trauma exposure | 8.7 (1.5) |
| Index trauma | |
| Sexual assault | 3 (50.0%) |
| Physical assault | 2 (33.3%) |
| Sudden violent death | 1 (16.7%) |
| Natural disaster, fire/explosion, transportation accident, other serious accident, or exposure to toxic substance | 0 (0%) |
| Assault with a weapon | 0 (0%) |
| Other unwanted or uncomfortable sexual experience | 0 (0%) |
| Combat, exposure to a war-zone, or captivity | 0 (0%) |
| Life-threatening illness or injury | 0 (0%) |
| Severe human suffering | 0 (0%) |
| Sudden violent death | 0 (0%) |
| Sudden accidental death | 0 (0%) |
| Serious injury, harm, or death to someone else | 0 (0%) |
| Childhood Trauma Questionnaire | |
| Emotional abuse subscale | 13.3 (2.8) |
| Physical abuse subscale | 11.8 (2.9) |
| Sexual abuse subscale | 13.2 (3.5) |
| Emotional neglect subscale | 14.7 (1.3) |
| Physical neglect subscale | 12.8 (5.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiya, T.C.; Singal, S.; Prakash, K.; Johal, P.; Hameed, S.; Back, S.E.; Mills, K.L.; Hien, D.A. Helping Opioid Use Disorder and PTSD with Exposure (HOPE): An Open-Label Pilot Study of a Trauma-Focused, Integrated Therapy for OUD/PTSD. Behav. Sci. 2025, 15, 874. https://doi.org/10.3390/bs15070874
Saraiya TC, Singal S, Prakash K, Johal P, Hameed S, Back SE, Mills KL, Hien DA. Helping Opioid Use Disorder and PTSD with Exposure (HOPE): An Open-Label Pilot Study of a Trauma-Focused, Integrated Therapy for OUD/PTSD. Behavioral Sciences. 2025; 15(7):874. https://doi.org/10.3390/bs15070874
Chicago/Turabian StyleSaraiya, Tanya C., Sonali Singal, Krithika Prakash, Priya Johal, Sara Hameed, Sudie E. Back, Katherine L. Mills, and Denise A. Hien. 2025. "Helping Opioid Use Disorder and PTSD with Exposure (HOPE): An Open-Label Pilot Study of a Trauma-Focused, Integrated Therapy for OUD/PTSD" Behavioral Sciences 15, no. 7: 874. https://doi.org/10.3390/bs15070874
APA StyleSaraiya, T. C., Singal, S., Prakash, K., Johal, P., Hameed, S., Back, S. E., Mills, K. L., & Hien, D. A. (2025). Helping Opioid Use Disorder and PTSD with Exposure (HOPE): An Open-Label Pilot Study of a Trauma-Focused, Integrated Therapy for OUD/PTSD. Behavioral Sciences, 15(7), 874. https://doi.org/10.3390/bs15070874






