Abstract
Thermal conductivity is a decisive parameter in all geothermal applications. In addition to the influencing factors of density, saturation, porosity, temperature and pressure, it is, above all, the geochemical and mineralogical composition that determines the thermal conductivity in rocks and soils. This study focuses on selected rock samples from Southern and Central Germany regarding major element oxides and minerals as well as distributed thermal conductivity. We examined clastic and chemical sedimentary, as well as igneous and metamorphic rocks, ranging from the Paleozoic to Cenozoic age. Measurements were conducted by X-ray fluorescence analysis (XRF), X-ray diffraction (XRD) and optical scanning with a thermal conductivity scanner (TCS). The results show significant correlations between thermal and geochemical parameters. Chemical composition significantly impacts thermal conductivity. Higher quartz and SiO2 contents generally lead to increased thermal conductivity, while aluminum silicates, common in clay minerals, correlate with lower conductivity. For carbonates, increased density or reduced porosity enhances conductivity. Structural differences and differing mineral concentrations influence the measurement variability along the sampling axis. This is especially visible in clastic sedimentary rock samples, where porosity decreases while cementation of the matrix increases thermal conductivity.
1. Introduction
Knowledge of the thermal conductivity of the subsurface is the key to successful planning and implementation of shallow low enthalpy geothermal systems. Especially in the case of vertical systems, e.g., (double)-U-/coaxial-borehole heat exchangers or energy piles, knowledge of the bedrock is very important. In the case of a simple and homogeneous lithology, the thermal parameters are determined during planning by means of a thermal response test [1,2,3]. An effective thermal conductivity is measured over the entire borehole using a thermal input. In the case of more complex lithologies, it is necessary to examine the subsurface more thoroughly. In practice, depth-resolved tests, e.g., enhanced thermal response tests [4], data logger inspections [5] and core drillings, can be carried out for this purpose. The latter provides a more precise overview of the rock and allows measurements of the thermal properties. These properties are controlled by a variety of petrological, pedological and hydrogeological parameters. Various studies have been made on different materials, including rocks [6,7,8,9,10,11,12], soils [13,14,15] and single minerals [16,17], by using the steady-state or transient method with a variety of measurement instruments, e.g., divided bar [18,19], needle probe [20], thermal cell or guarded parallel/hot plate. In this study, we used an optical high-resolution scanning device, the thermal conductivity scanner (TCS), for measuring sampled rocks occurring in Central and South Germany. The method allows a nondestructive measurement, records the spatial distribution of the thermal conductivity in heterogeneous materials and has no strict requirements. Further studies showed that the TCS method yields consistent results compared to the divided-bar and line-source methods [21]. Subsequently we analyzed the rock samples regarding major element composition and rock-forming minerals and compared this with the thermal conductivity.
The thermal conductivity of rocks is largely determined by the mineral composition, the porosity (ϕ), and, in particular, the total porosity and the degree of saturation (Sr) [22,23]. Depending on the input parameters, different model approaches, either empirical models with input mineralogy [20], porosity [24], p-wave velocities [25] or estimation models based on machine learning [26,27], exist for a variety of rock types. New machine learning models can predict the thermal conductivity of plutonic rocks within a RMSE of 5% using the five main element oxides [26]. In this work, we tried to bring the newly gained data of Germanic rocks into the context of already existing thermal conductivity and geochemical models.
2. Materials and Methods
In order to record different lithological types, a selection of sediments, igneous and metamorphic rocks occurring in Central and Southern Germany (Bavaria, Hesse, Thuringia and Saxony) was chosen for further investigation with regard to their thermal properties, especially thermal conductivity and geochemical composition. For this, three different analytical methods were used, including a thermal conductivity scanner, an X-ray fluorescence spectrometer and an X-ray diffractometer.
2.1. Lithology
The samples of Paleozoic origin are mostly drilled cores from different depth levels up to a depth of almost fifteen meters below ground level (b. g. l.). These samples were taken during preliminary exploration for high-voltage power cable construction. Mesozoic and Cenozoic rocks were sampled on representative outcrops. Lithographic and general information of each sample is listed in Table 1. Figure 1 shows the location of each sample. Samples taken from the boreholes are perpendicular to the bedding. Each drilled core has a diameter of 90 mm.

Table 1.
Main characteristics of each sample used in this study; sorting is in descending order of age. Note: b.g.l.—below ground level.
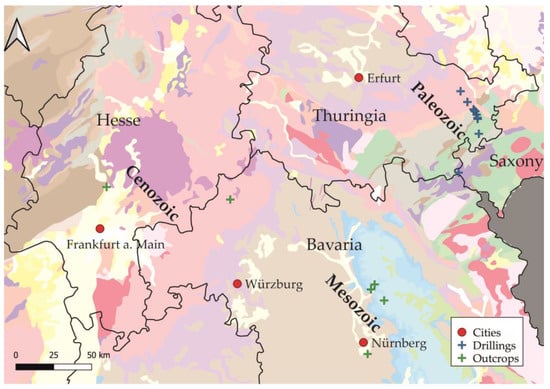
Figure 1.
Locations of sampled rocks in Central and Southern Germany. Blue and green crosses depict sampling areas by drilling and outcrops. Geological eras only indicate age of samples; color scheme of geological units can be found in the base map source: General Geological Map of Germany (GUEK200) [28].
2.2. Thermal Conductivity Measurements
The samples were pre-treated with black paint. The black color applied to the samples is necessary for the absorption of the infrared heat waves emitting from the heat source during the measurement [29]. Drying of the samples was done at 70 °C for at least 48 h. The samples were measured at least three times to test the reproducibility of the measurements. For the calibration of the measurements, three different ranges of thermal conductivity with two standards each were used. The ranges for the standards are as follows: 0.709–1.350 W/(m∙K), 1.350–5.940 W/(m∙K) and 5.940–13.300 W/(m∙K); see also Table 2. The measurements were done using the optical scanning method [21,30] on a thermal conductivity scanner (TCS) by Lippmann and Rauen GbR, Schaufling, Germany at the GeoZentrum Nordbayern Labs at Friedrich-Alexander-Universität Erlangen-Nürnberg, at room temperature (22 ± 2 °C). The key parameters of the TCS are shown in Table 2. The measurement setup is displayed schematically in Figure 2. Measurements were conducted in air-saturated (dry) and water-saturated states. The saturation of samples was done after DIN EN 13755 [31] under atmospheric pressure. The scanning line crosses the most representative areas of each sample and follows the X-axis (longitudinal axis), as indicated in Figure 2. Saturated and dry measurements were conducted along the same lines. Correction of curvature was done automatically by the TCS software v. 5.0.

Table 2.
Key data of the thermal conductivity scanner (TCS) and set of standards at GeoZentrum Nordbayern labs; TC: thermal conductivity, TD: thermal diffusivity.
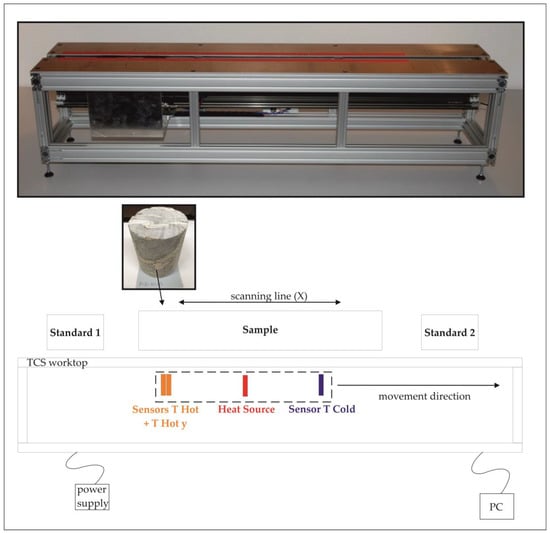
Figure 2.
Picture (from [29]) and schematic illustration of the thermal conductivity scanner (TCS) device. Measurements were conducted at the TCS worktop with two standards for each sample or similar sample type for the calibration range (standard 1: lower than expected thermal conductivity; standard 2: higher than expected thermal conductivity). T Cold and T Hot measure the temperature in line before and after heating; T Hot y measures after heating at a 7 mm distance.
2.3. Geochemical Sample Preparation
The samples were mostly fresh and not altered. To avoid contamination of the chemical data, the rocks were cut into cuboids of approximately 15 cm3 by using different stone-cutting saws at the GeoZentrum Nordbayern labs.
The rectangular cuboids used for geochemical analysis were cleaned in an ultrasonic bath filled with deionized water for 15 min and subsequently dried in a compartment drier for 48 h at 60 °C. Afterward, the samples were crushed by a hydraulic press and ground into a powder with an agate vibratory ring mill, type Retsch RS200. Every crushed sample was ground for 15 min at 700 rpm to a particle size of approx. < 30 μm. Powder aliquots were dried for 24 h at 105 °C for X-ray fluorescence (XRF) and 48 h at 60 °C for X-ray diffraction (XRD) analysis.
The powder of each XRF sample was weighed to 2 × 1.000 g for the magmatic samples and 1 × 0.500 g for the sedimentary and metamorphic samples. One part of the magmatic sample was heated in a muffle furnace to 1050 °C for 12 h to determine the loss-on-ignition (LOI) of each sample. The other part was mixed with 4.83 g of di-lithium tetraborate (Bi4Li2O7) as a disintegration agent and one spatula of di-iodine pentoxide (I2O5) as flux. The sedimentary and metamorphic samples were mixed with 4.00 g of lithium metaborate (LiBO2) and further processed out of the LOI process. The mixture was melted on a five-stage “Oxiflux” burner system to a maximum temperature of 1050 °C. For both procedures, the aim was to generate a homogenous glass bead of each sample.
2.4. Major Element Analytic
X-ray fluorescence spectroscopy (XRF) measurements were carried out on the glass by using a Spectro XEPOS X-ray fluorescence spectrometer at the GeoZentrum Nordbayern. Measurements were conducted in the energy-dispersive mode (EDS). The major elements have been detected in weight percent (wt.%) of their oxides (SiO2, TiO2, Al2O3, Fe2O3, MnO, MgO, CaO, Na2O, K2O and P2O5), while Fe2O3 comprises the total content of Fe2+ and Fe3+. In addition, selected trace elements (Ba, Cr, Ga, Nb, Ni, Pb, Rb, Sr, Th, V, Y, Zn and Zr) have been detected in parts per million (ppm) (see Appendix A Table A1) but will not be used for this subject. To ensure accuracy and precision, three international standards, basalt (BR) and two granites (AC-E, GA), were measured repeatedly. The limits of detection and quantification of the major elements are shown in Table 3, and the values of the trace elements are max. 1.8 ppm for Vanadium.

Table 3.
Limit of detection (LOD) and limit of quantification (LOQ) for major elements from XRF analysis.
2.5. Mineral Phase Analytic
X-ray diffraction (XRD) measurements were conducted using dried powder with <30 μm grain size for the mineral assemblage in powder diagrams. For this purpose, a Siemens D5000 Theta-Theta-diffractometer with Cu K alpha radiation at 35 mA and 40 kV at an angle range of 2–65° 2θ with a stepscan of 0.02° was used. Qualitative and quantitative analyses were carried out using Rietveld analysis (RVA) [32,33].
2.6. Density and Porosity Measurements
The density was calculated by the volume and dry weight of the rectangular prisms. The porosity was measured by two methods using (a) the open porosity calculation by DIN EN 1936 [34] and (b) true density with a Micromeritics AccuPyc II 1345 gas pycnometer as well as (c) the porosity and bulk/envelope density with a Micromeritics GeoPyc 1365 envelope and density analyzer both by Micromeritics Instrument Corporation, Norcross, GA, USA. True density was measured for all samples, while Formula (1) was used for open porosity ϕO to provide a preliminary estimate.
where ms is the saturated weight, md is the dry weight, and mh is the hydrated weight. Specifications of the AccuPyc gas pycnometer are as follows: volume accuracy of 0.02%, volume repeatability of 0.01%, temperature range from 4 °C to 60 °C and a temperature stability of ±0.025 °C. Specifications of the GeoPyc analyzer are reproducibility ±1.1% at a minimum sample size of 25% volume of the sample holder. Each sample was weighed, and the true density was measured via a helium gas pycnometer with 10 measurement cycles. Further processing for porosity measurements was performed by the addition of the Micromeritics “DryFlo” mixture of differing size particles and a small quantity of graphitic lubricant. Each measurement was repeated 10 times with a consolidation force of 145.00 N.
3. Results
We analyzed 17 representative rock samples from Central and South Germany. Investigations of the thermal conductivity and the element and mineral analytics are displayed in two subsections. The petrographic on-site examination and the geochemical composition of the samples are in concordance. The measured thermal conductivity values are within the range specified in VDI 4640 [35].
3.1. Thermal Conductivity via Thermal Conductivity Scanner (TCS)
The thermal conductivity ranges from average values of 1.19 W/(m∙K) for dry claystone to 6.85 W/(m∙K) for dry quartzite and 2.24 W/(m∙K) for saturated claystone to 8.06 W/(m∙K) for saturated quartzite. Rocks from other sedimentary origin show distinct higher values with λdry = 1.72–3.27 W/(m∙K) and λsat = 2.24–2.96 W/(m∙K) for sandstones and λdry = 2.85–3.91 W/(m∙K) and λsat = 2.35–3.07 W/(m∙K) for limestone and dolomite. Thermal conductivity on igneous rocks was measured between λdry = 1.91 W/(m∙K) and for λsat = 1.57 W/(m∙K) for basalt and λdry = 1.98 W/(m∙K) and λsat = 2.96 W/(m∙K) for granite. Paleozoic metamorphic rocks from Thuringia and Saxony show values of λdry = 1.39–1.79 W/(m∙K) and λsat = 2.44–2.96 W/(m∙K) for clay slates and distinctly higher values for greywackes ranging from λdry = 2.62–3.94 W/(m∙K) and λsat = 3.22–3.75 W/(m∙K). Table 4 shows the thermal conductivity values for each sample as well as the relative standard deviations. Figure 3 depicts the length-resolved measurements of each sample in categories of similar rock types. True density and bulk density were measured via a pycnometer. These ranged from 2.63 to 2.92 g/cm3 (ρTrue) and 1.92 to 2.94 g/cm3 (ρBulk). Total porosity ranged from <0.1 to 28.94% (see Table 4). The standard deviations of the density measurements show low deviations, with the highest value of 0.101 g/cm3 and a mean value of 0.008 g/cm3.

Table 4.
Mean values for thermal conductivity of each sample in dry and saturated conditions with the relative standard deviation (RSD = standard deviation/mean value) of each measurement and measured porosities and densities in dry conditions.
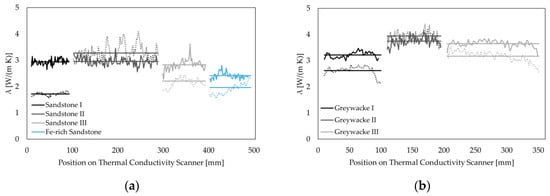
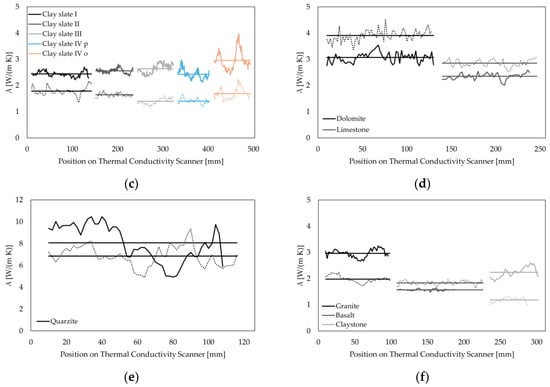
Figure 3.
Thermal conductivity measured with a thermal conductivity scanner (TCS) along each sample. Dashed lines represent thermal conductivity in a dry state; solid lines are thermal conductivity measurements in a saturated state. Solid horizontal bars display the mean values, with the relative standard deviation (RSD) of each measurement displayed in Table 4. (a) Sandstones; (b) greywackes; and (c) clay slates. Note: p: measurement parallel to foliation; o: measurement orthogonal to foliation; (d) carbonates; (e) quartzite; and (f) igneous rocks and claystone.
3.2. Major Element and Mineral Phase Analytic
The major element analytic by XRF provides quantitative results in a weight percent of their oxides. All values can be found in Table 5. The metamorphic rocks’ range shows moderate values of 60.6 to 66.1 wt.% SiO2, high values of 17.8 to 20.9 wt.% Al2O3 and 5.3 to 6.7 wt.% Fe2O3, with the exception of the Quartzite sample. Clastic sediments are represented by high values of 69.7 to 93.2 wt.% SiO2, moderate to low values of 1.35 to 11.8 wt.% Al2O3 and low values of 0.99 to 4.78 wt.% Fe2O3, with the exception of “Eisensandstein Fm” sandstone with 19.05 wt.% Fe2O3. The carbonate sedimentary samples show typical high values of 31.1 wt.% and 53.5 wt.% CaO and high loss-on-ignition (LOI) for dolomite and limestone and additionally a high value of 20.8 wt.% MgO for dolomite. Igneous rocks show characteristic values, as shown in Table 4. Values for MnO and P2O5 are for all samples underneath 0.1 wt.% and 0.7 wt.%, respectively, and are therefore not listed in the table.

Table 5.
Major element oxide results from X-ray fluorescence spectroscopy (XRF) in weight percent (wt.%) and the loss-on-ignition (LOI) for each sample. Trace element values are in Appendix A Table A1.
Measurements of mineral phases via XRD show values for the main rock-forming minerals. An exact quantity for each mineral and the XRD patterns can be found in Table A2 and Figure A1 (Appendix A). Clay slates, greywackes and sandstones have medium to high values of quartz, with 35.9% to 50.2% in clay slates, 54.6% to 56.4% in greywackes and 55.0% to 89.0% in sandstones. Clay slates commonly have high percentages of mica (muscovite) and clay minerals (illite, chlorite). Greywackes have medium values of plagioclase 18.8 ± 1% as well as fluctuating contents of muscovite and chlorite, ranging from 8.3% to 19.1% and 7.8% to 13.2%, respectively. Granite and basalt samples show common values for rock-forming minerals quartz, plagioclase and potassium, as well as pyroxene, plagioclase and olivine (see also Table A2 and Section 4.2).
4. Discussion
4.1. Contributing Factors Not Measurable in Laboratory Scale
As shown in previous studies, the thermal conductivity of the subsoil is subject to many factors, such as grain size, porosity, pressure, temperature, degree of saturation or matrix composition [7,36,37]. This study focuses on rocks in shallow areas. The rock-forming parameters, temperature and pressure were not investigated in this study since they are not applicable in shallow depths. Depending on the location, the geothermal gradient is first visible at depths of approx. 100 m or more [5]. Nevertheless, temperature has a great influence on thermal conductivity and diffusivity [38]. In the applied use of shallow geothermal energy, the hydrogeological surrounding of the system has a big influence on the effectiveness [39,40,41,42,43]. In this case, the thermal properties can be measured more accurately by using depth-resolved solutions, e.g., distributed temperature sensing [5] or the implementation of the moving infinite line source for calculating thermal conductivity during thermal response tests [1,44]. Porosity and density are the main parameters that have a great influence on thermal conductivity [45]. In situ measurements, such as eGRT or TRT [4,5,46], of thermal properties can show depth-resolved values for thermal conductivity and heat capacity but do not consider petrophysical parameters.
4.2. Classification of Samples
The sampled greywackes show common geochemical values. By using the logarithmic relationships between SiO2/Al2O3 and Na2O/K2O [47,48,49], samples greywacke I + II are classified as greywacke while sample greywacke III is, in fact, bordering on being arkose. The density and thermal conductivity of greywackes are similar to samples from other studies, e.g., [50,51]. Sandstone I + II and Fe-rich sandstone are arkoses and a subarkose, respectively, while sandstone III is quartz arenite. Following the classification of log (Fe2O3/K2O), sandstone III and Fe-rich sandstone are iron sandstones. Lithofacies classification of mudstones [52,53] categorizes the claystone sample as clay-rich siliceous mudstone. Following the classification of total alkali vs. silica (TAS) for major element oxides [54], the sampled basalt is on the border between basalt and basanite. This is common for the sampling area Rhön, Northern Bavaria (see Figure 1) [55]. TAS for plutonic rocks [56] classifies the sampled granite as just that. The slate samples are representative of major elements content with a focus on SiO2, Al2O3 and Fe2O3 when compared to other studies [57,58]. The quartzite sample is mainly used as a reference for high SiO2 content at high thermal conductivity.
All in all, all samples are representative in terms of their geochemical composition. In terms of thermal conductivity, the range of measured values is significantly greater and often highly heterogeneous depending on the microstructural composition. This is particularly the case with sedimentary and metamorphic rocks. The λ-values for the sampled rocks are located in a range of generally applicable literature, such as VDI 4640-1 [35], and can be found in other studies [27,59,60].
4.3. Combination of Geochemical and Geothermal Properties
The chemical composition has a decisive influence on thermal conductivity. Figure 4a,b show the thermal conductivity in relation to the SiO2 and quartz contents. This shows the much-described trend of increasing thermal conductivity with increasing quartz content, as well as SiO2 [7,61,62], with the exception of carbonate rocks. Rocks with a high proportion of aluminum silicates, which are frequently found in clay minerals, have a lower thermal conductivity. The ratio of Al2O3 to SiO2 is the highest in metamorphic slates and claystone, which have the lowest thermal conductivity, also visible in Figure 4c. Bulk density and true density show diverging values when compared with the thermal conductivity; porosity ranges up to approx. 29% without any significant correlations visible (Figure 4f–h). Values of ρtrue provide a better understanding of the mineral and grain densities. The influence of CaO (Figure 4d) is not visible from the data in this study.
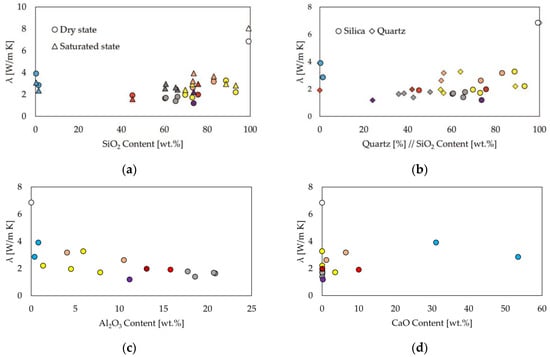
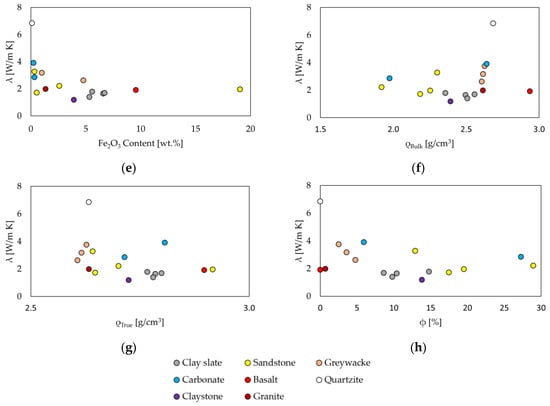
Figure 4.
Relationship between thermal conductivity λ and major element oxide composition, quartz content, density ρ and porosity ϕ. (a) Saturated and dry states λ vs. SiO2; (b) λ vs. SiO2 and quartz contents with same color code; (c) λdry vs. Al2O3; (d) λdry vs. CaO; (e) λdry vs. Fe2O3; (f) λdry vs. ρBulk; (g) λdry vs. ρTrue; and (h) λdry vs. ϕ.
An important factor that must be considered is the measurement concept. The optical scanning method is well-established and delivers reliable data on thermal conductivity [27,30,63]. As this method produces heterogeneous, distributed data along a sample, interpretation must be done carefully. Our results show deviations along the measurement axis, as visible in Figure 2. These deviations are caused, on the one hand, by different minerals and mineral accumulations, e.g., in shales, and on the other hand, by porosities or different measuring distances of the sample, as seen in the results of the quartzite sample (Figure 3e). Previous studies have investigated the significant deviations caused by inhomogeneities in the rocks and developed various approaches [45,64]. These include thermal conductivity and the porosity maps method, which allow a more detailed insight into the thermal conductivity distribution. This comprises porosity, on the one hand, and mineral accumulations from sedimentary or metamorphic arrangement, on the other. The deviation due to mineral arrangement is visible in the clay-slate measurements, especially during orthogonal orientation, but also in certain cases of sedimentary rocks, e.g., sandstone II. In this case, the XRD measurements indicate that the larger fluctuations are caused by matrix-forming potassium feldspar (14%). This can be repeatedly observed in all three greywacke samples. Different orders of magnitude in measurement deviation are potentially driven by the mineralogical content. The basalt and greywacke samples clearly show that the SiO2 content alone is not a direct indication of an increase in thermal conductivity.
4.4. Influence of Porosity and Density
The pore volume or porosity and density of materials have a great influence on thermal properties. Thermal conductivity of bodies with random porosity, as in the case of most rocks, is decreased proportionally to the volume pore fraction [65]. Porosity is related to the internal rock structure and geometry while processes after rock forming can also have a great impact [66]. In the case of the samples in this study, the factor of alteration must be considered since the samples are all from very shallow drillings and outcrops. Furthermore, sedimentary samples and potentially hydrothermal altered samples, such as the metamorphic clay slates, are subject to different forms of intergranular cementation [36,37,66]. Porosity favors possible saturation and can thus contribute to an increase in thermal conductivity. We observed that the Mesozoic sandstones and Paleozoic greywackes show relatively low values of thermal conductivity with increasing porosity (see Figure 5). In the case of these sediments, this correlation appears to be due to the low amount of pore volume or, conversely, the high proportion of cementation or matrix. This can, therefore, also be transferred to the noticeably more compact and finer graywackes. While the true density and bulk density of clay slates are relatively high due to metamorphic overprinting, porosity is more distinctive with the siliciclastic samples.
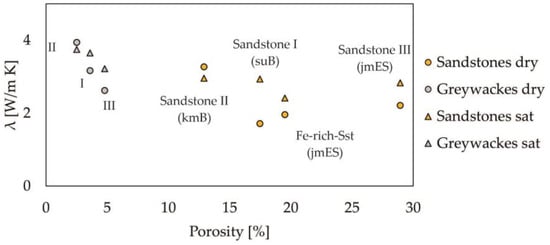
Figure 5.
Relationship of porosity and thermal conductivity of saturated and dry sandstones and greywackes. Note: jmES: Eisensandstein Fm; kmB: Löwenstein-Fm; suB: Bernburg-Fm.
4.5. Comparison with Prior Studies
Measurements of thermal conductivity via TCS are a common procedure [21,23,30,64]. This promotes a good comparability of measurements, even though each rock type has a wide range. The saturation method creates deviations, e.g., by use of Ar-gas or oil [12]; therefore, only water-saturated and dry samples are listed in Table 5. As already mentioned, all thermal conductivity values are within the ranges specified in VDI 4640 [35]. The values for the thermal conductivity of sandstone have a great variability in comparison to rock samples [23] from Northern Bavaria, which show significantly higher values. Igneous rocks are within the range of measured samples from other studies [9,10]. The carbonatic samples match similar rock types of fine mudstone. Overall thermal conductivity matches with world-wide published values. An overview of publications with comparable values is shown in Table 6.

Table 6.
Related work with similar lithological investigations. Note: TC: thermal conductivity; suBB: Bernburg-Fm, “Basissandstein”; suBT: Bernburg-Fm, “Tonlagensandststein”.
5. Conclusions
In this study, a set of 17 rock samples from the Paleozoic to Cenozoic age was analyzed regarding their geochemical compositions via XRF and XRD and thermal conductivity via TCS. The samples ranged from clastic and chemical sedimentary rocks, namely sandstones, greywackes, claystone and carbonates, to igneous and metamorphic rocks, and more precisely, basalt, granite, quartzite and clay shales. The results of the investigations are in concordance with previous studies. Our further conclusions are as follows:
- A new dataset of geochemical and thermal properties of rocks from Central Germany was created. The rocks were selected to cover the main areas of central German geology.
- The resulting thermal conductivity values are in a range between 1.19 and 6.85 W/(m∙K) in a dry state and 2.24 and 8.06 W/(m∙K) in a water-saturated state.
- The chemical composition significantly impacts thermal properties, especially a high quartz content rather than SiO2 content, and increases thermal conductivity in SiO2-bearing rocks. Aluminum silicates, occurring in slates and clay stone, correlate with lower thermal conductivity. Rock density correlates partly with thermal conductivity.
- Heterogeneities due to mineral concentrations and structural differences can be well displayed and analyzed with TCS.
In order to analyze the main influencing parameters further, especially upon closer examination of the mineral phases and accumulations and sedimentary cementations, the next step in this research is the use of scanning electron microscopy (SEM). In this way, grain size, grain connection and the porosity effects can be examined in more detail. This can be further supported by 2D mapping of selected heterogeneous rocks regarding thermal conductivity and porosity, e.g., as conducted by [45,64].
Author Contributions
Conceptualization, O.S.; methodology, O.S. and H.H.; software, O.S. and H.H.; validation, O.S.; formal analysis, O.S.; investigation, O.S. and H.H.; resources, D.B.; data curation, O.S. and H.H.; writing—original draft preparation, O.S.; writing—review and editing, O.S. and H.H.; visualization, O.S.; supervision, D.B.; project administration, O.S. and D.B.; funding acquisition, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data, if not included in this study, can be requested from the corresponding author.
Acknowledgments
We want to thank the members of the shallow geothermal working group at Friedrich-Alexander-Universität-Erlangen-Nürnberg (FAU) for their help and valuable discussion. We want to express great gratitude to Melanie Hertel, Stefan Krumm and Eric Salomon from GeoZentrum Nordbayern, FAU, for their help with the sample preparation and geochemical measurements. Last, but not least, we want to thank TCS-Lippmann und Rauen GbR for their custom-made TC scanner.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| Acronyms | |
| b.g.l. | Below ground level |
| RSD | Relative standard deviation |
| TAS | Total alkali vs. silica (content) |
| TCS | Thermal conductivity scanner |
| XRF | X-ray fluorescence |
| XRD | X-ray diffraction |
| Variables | |
| θ | water content [%] |
| λ | thermal conductivity [W/(m∙K)] |
| ρ | density [kg/m3]/[g/cm3] |
Appendix A

Table A1.
Trace element results from X-ray fluorescence spectroscopy (XRF) in parts per million (ppm).
Table A1.
Trace element results from X-ray fluorescence spectroscopy (XRF) in parts per million (ppm).
| Sample Type | Ba | Cr | Ga | Nb | Ni | Pb | Rb |
|---|---|---|---|---|---|---|---|
| Clay slate I | 723 | 74.0 | 22.5 | 11.0 | 46.6 | 2.4 | 148 |
| Clay slate II | 689 | 96.8 | 24.6 | 14.1 | 57.5 | 9.2 | 173 |
| Clay slate III | 566 | 94.7 | 24.4 | 12.1 | 50.1 | 6.2 | 152 |
| Clay slate IV | 660 | 96.2 | 27.3 | 14.7 | 58.5 | 13.6 | 174 |
| Granite | 759 | 11.2 | 22.1 | 37.5 | 6.9 | 5.6 | 112 |
| Greywacke I | 231 | 99.8 | 15.4 | 4.4 | 67.1 | 7.6 | 45.9 |
| Greywacke II | 318 | 96.4 | 15.0 | 5.6 | 48.4 | 7.1 | 58.5 |
| Greywacke III | 287 | 40.5 | 7.8 | 3.0 | 23.6 | 6.0 | 39.0 |
| Claystone | 260 | 97.9 | 13.2 | 5.1 | 61.8 | 3.9 | 57.3 |
| Sandstone I | 842 | 23.6 | 9.5 | 0.9 | 25.6 | 15.6 | 114 |
| Sandstone II | 576 | 19.5 | 8.3 | 2.1 | 11.4 | 14.1 | 86.8 |
| Sandstone III | 106 | 32.1 | 4.0 | 3.4 | 13.5 | 10.3 | 14.1 |
| Fe-rich Sandstone | 102 | 99.3 | 8.8 | 7.6 | 57.4 | 7.1 | 12.7 |
| Dolomite | 20.8 | 22.8 | 2.0 | <0.5 | 2.3 | <1.0 | <0.5 |
| Limestone | 29.3 | 29.2 | 3.9 | 1.0 | <0.5 | 1.7 | <0.5 |
| Basalt | 971 | 136 | 18.1 | 70.9 | 92.3 | 4.1 | 66.6 |
| Quartzite | 54.7 | 16.9 | 2.4 | <0.5 | 13.2 | 19.0 | <0.5 |
| Sample type | Sr | Th | V | Y | Zn | Zr | |
| Clay slate I | 71.9 | 13.5 | 92.7 | 31.5 | 99.3 | 210 | |
| Clay slate II | 109 | 20.3 | 97.0 | 29.8 | 118 | 175 | |
| Clay slate III | 91.0 | 18.4 | 102 | 26.8 | 91.3 | 181 | |
| Clay slate IV | 85.4 | 21.2 | 96.9 | 33.2 | 118 | 184 | |
| Granite | 62.6 | 10.4 | 2.9 | 48.4 | 33.3 | 486 | |
| Greywacke I | 52.5 | 5.5 | 75.5 | 15.8 | 61.2 | 120 | |
| Greywacke II | 63.6 | 5.8 | 92.9 | 17.9 | 42.8 | 139 | |
| Greywacke III | 262 | 4.3 | 31.7 | 6.6 | 29.9 | 65.8 | |
| Claystone | 60.1 | 6.6 | 91.1 | 18.1 | 58.3 | 149 | |
| Sandstone I | 89.5 | 4.2 | 15.4 | 5.9 | 17.6 | 63.6 | |
| Sandstone II | 69.0 | 5.7 | 16.9 | 1.5 | 15.7 | 64.1 | |
| Sandstone III | 23.0 | 5.1 | 32.0 | 7.0 | 98.0 | 60.0 | |
| Fe-rich Sandstone | 536 | 6.0 | 136 | 19.8 | 39.3 | 330 | |
| Dolomite | 47.8 | 1.3 | 16.5 | 2.7 | 18.4 | 5.8 | |
| Limestone | 248 | 2.1 | 20.1 | 5.8 | 34.9 | 10.1 | |
| Basalt | 976 | 6.7 | 222 | 22.7 | 84.2 | 283 | |
| Quartzite | 3.9 | <1.0 | 1.3 | <0.5 | 10.7 | 4.3 |

Table A2.
Percentage of mineral phases in each sample measured with XRD.
Table A2.
Percentage of mineral phases in each sample measured with XRD.
| Clay Slate I | Clay Slate II | Clay Slate II | Clay Slate II | ||||
|---|---|---|---|---|---|---|---|
| Quartz | 50.2 | Quartz | 35.9 | Quartz | 42.6 | Quartz | 38.3 |
| Muscovite | 37.1 | Muscovite | 43.1 | Muscovite | 38.2 | Muscovite | 40.7 |
| Chlorite | 11.9 | Chlorite | 14.1 | Chlorite | 11.1 | Chlorite | 15.2 |
| K-Feldspar | 0.7 | Plagioclase | 3.6 | Plagioclase | 6.8 | Plagioclase | 3.7 |
| K-Feldspar | 3.1 | K-Feldspar | 1.1 | K-Feldspar | 1.9 | ||
| Granite | Greywacke I | Greywacke II | Greywacke III | ||||
| Quartz | 42 | Quartz | 55.3 | Quartz | 54.6 | Quartz | 56.4 |
| Muscovite | 7.1 | Muscovite | 8.3 | Muscovite | 16.8 | Muscovite | 19.1 |
| Chlorite | 1.7 | Chlorite | 13.2 | Chlorite | 7.8 | Chlorite | 7.2 |
| Plagioclase | 27.5 | Plagioclase | 19.2 | Plagioclase | 19.9 | Plagioclase | 17.2 |
| K-Feldspar | 21.5 | K-Feldspar | 1 | K-Feldspar | 0.6 | ||
| Dolomite | 2.8 | ||||||
| Sandstone I | Sandstone II | Sandstone III | Fe-rich Sandstone | ||||
| Quartz | 56.3 | Quartz | 74 | Quartz | 89 | Quartz | 55 |
| Muscovite | 3.8 | K-Feldspar | 14 | Goethite | 11 | Goethite | 38 |
| K-Feldspar | 28.5 | Illite | 6 | Hematite | 7 | ||
| Dolomite | 11.3 | Kaolinite | 6 | ||||
| Claystone | Basalt | Quartzite | |||||
| Quartz | 24 | Chlorite | 5 | Quartz | 100 | ||
| Muscovite | 9 | Plagioclase | 36 | Dolomite | |||
| Chlorite | 4 | Diopside | 33 | Dolomite | 100 | ||
| K-Feldspar | 10 | Natrolite | 4 | Limestone | |||
| Hematite | 3 | Analcime | 8 | Quartz | 1 | ||
| Illite | 50 | Leucite | 2 | Calcite | 99 | ||
| Nepheline | 5 | ||||||
| Forsterite | 7 |
Figure A1.
X-ray diffraction pattern for every sample.
References
- Suft, O.; Bertermann, D. One-Year Monitoring of a Ground Heat Exchanger Using the In Situ Thermal Response Test: An Experimental Approach on Climatic Effects. Energies 2022, 15, 9490. [Google Scholar] [CrossRef]
- Sanner, B.; Mands, E.; Sauer, M.K.; Grundmann, E. Thermal Response Test, a routine method to determine thermal ground properties for GSHP design. In Proceedings of the International IEA Heat Pump Conference, Zürich, Switzerland, 20–22 May 2008. [Google Scholar]
- VDI 4640-5; Thermal use of the underground—Part 5: Thermal-Response-Test (TRT). VDI: Düsseldorf, Germany, 2020.
- Dalla Santa, G.; Pasquier, P.; Schenato, L.; Galgaro, A. Repeated ETRTs in a Complex Stratified Geological Setting: High-Resolution Thermal Conductivity Identification by Multiple Linear Regression. J. Geotech. Geoenviron. Eng. 2022, 148, 04022007. [Google Scholar] [CrossRef]
- Bertermann, D.; Suft, O. Determination of the Temperature Development in a Borehole Heat Exchanger Field Using Distributed Temperature Sensing. Energies 2024, 17, 4697. [Google Scholar] [CrossRef]
- Eppelbaum, L.; Kutasov, I.; Pilchin, A. Thermal properties of rocks and density of fluids. In Applied Geothermics; Springer: Berlin/Heidelberg, Germany, 2014; pp. 99–149. [Google Scholar] [CrossRef]
- Clauser, C.; Huenges, E. Thermal Conductivity of Rocks and Minerals; American Geophyscial Union: Washington, DC, USA, 1995. [Google Scholar] [CrossRef]
- Midttømme, K.; Roaldset, E.; Aagaard, P. Thermal conductivity of selected claystones and mudstones from England. Clay Miner. 1998, 33, 131–145. [Google Scholar] [CrossRef]
- Barry-Macaulay, D.; Bouazza, A.; Singh, R.M.; Wang, B.; Ranjith, P.G. Thermal conductivity of soils and rocks from the Melbourne (Australia) region. Eng. Geol. 2013, 164, 131–138. [Google Scholar] [CrossRef]
- Long, M.; Murray, S.; Pasquali, R. Thermal conductivity of Irish rocks. Ir. J. Earth Sci. 2018, 36, 63–80. [Google Scholar] [CrossRef]
- Bloomer, J. Thermal conductivities of mudrocks in the United Kingdoms. Q. J. Eng. Geol. Hydrogeol. 1981, 14, 357–362. [Google Scholar] [CrossRef]
- Alishaev, M.G.; Abdulagatov, I.M.; Abdulagatova, Z.Z. Effective thermal conductivity of fluid-saturated rocks. Eng. Geol. 2012, 135–136, 24–39. [Google Scholar] [CrossRef]
- Rammler, M.; Schwarz, H.; Wagner, J.; Bertermann, D. Comparison of Measured and Derived Thermal Conductivities in the Unsaturated Soil Zone of a Large-Scale Geothermal Collector System (LSC). Energies 2023, 16, 1195. [Google Scholar] [CrossRef]
- Dissanayaka, S.H.; Hamamoto, S.; Kawamoto, K.; Komatsu, T.; Moldrup, P. Thermal Properties of Peaty Soils: Effects of Liquid-Phase Impedance Factor and Shrinkage. Vadose Zone J. 2012, 11. [Google Scholar] [CrossRef]
- Alrtimi, A.; Rouainia, M.; Haigh, S. Thermal conductivity of a sandy soil. Appl. Therm. Eng. 2016, 106, 551–560. [Google Scholar] [CrossRef]
- Horai, K.-I.; Simmons, G. Thermal conductivity of rock-forming minerals. Earth Planet. Sci. Lett. 1969, 6, 359–368. [Google Scholar] [CrossRef]
- Horai, K.i. Thermal conductivity of rock-forming minerals. J. Geophys. Res. 1971, 76, 1278–1308. [Google Scholar] [CrossRef]
- Chopra, N.; Ray, L.; Dey, S.; Mitra, A. Thermal conductivity, density, petrological and geochemical characteristics of granitoids from Singhbhum Craton, eastern India. Geothermics 2020, 87, 101855. [Google Scholar] [CrossRef]
- Ray, L.; Chopra, N.; Hiloidari, S.; Naidu, N.N.; Kumar, V. Thermal conductivity of granitoids of varying composition up to 300 °C and implications for crustal thermal models. Geophys. J. Int. 2021, 227, 316–332. [Google Scholar] [CrossRef]
- Horai, K.-I.; Baldridge, S. Thermal conductivity of nineteen igneous rocks, II estimation of the thermal conductivity of rock from the mineral and chemical compositions. Phys. Earth Planet. Inter. 1972, 5, 157–166. [Google Scholar] [CrossRef]
- Popov, Y.A.; Pribnow, D.F.C.; Sass, J.H.; Williams, C.F.; Burkhardt, H. Characterization of rock thermal conductivity by high-resolution optical scanning. Geothermics 1999, 28, 253–276. [Google Scholar] [CrossRef]
- Woodside, W.; Messmer, J.H. Thermal Conductivity of Porous Media. I. Unconsolidated Sands. J. Appl. Phys. 1961, 32, 1688–1699. [Google Scholar] [CrossRef]
- Franz, C.; Schulze, M. Bestimmung thermischer Eigenschaften der Gesteine des Unteren und Mittleren Buntsandsteins. Grundwasser 2016, 21, 47–58. [Google Scholar] [CrossRef]
- Maqsood, A.; Kamran, K.; Gul, I.H. Prediction of thermal conductivity of granite rocks from porosity and density data at normal temperature and pressure: In situ thermal conductivity measurements. J. Phys. D Appl. Phys. 2004, 37, 3396. [Google Scholar] [CrossRef]
- Gegenhuber, N.; Schoen, J. New approaches for the relationship between compressional wave velocity and thermal conductivity. J. Appl. Geophys. 2012, 76, 50–55. [Google Scholar] [CrossRef]
- Yu, R.; Jiang, S.; Fuchs, S.; Peng, P.; Li, Y.; Wang, H. Estimating the thermal conductivity of plutonic rocks from major oxide composition using machine learning. Geophys. J. Int. 2023, 234, 2143–2159. [Google Scholar] [CrossRef]
- Jennings, S.; Hasterok, D.; Payne, J. A new compositionally based thermal conductivity model for plutonic rocks. Geophys. J. Int. 2019, 219, 1377–1394. [Google Scholar] [CrossRef]
- BGR. General Geological Map of the Federal Republic of Germany 1:200,000 (GUEK200); BGR: Stuttgart, Germany, 2003. [Google Scholar]
- Popov, Y.; Lippmann, E.; Rauen, A. TCS Manual. Achim, Germany. 2020. Available online: www.tcscan.de (accessed on 2 January 2025).
- Popov, Y.; Tertychnyi, V.; Romushkevich, R.; Korobkov, D.; Pohl, J. Interrelations between thermal conductivity and other physical properties of rocks: Experimental data. In Thermo-Hydro-Mechanical Coupling in Fractured Rock; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1137–1161. [Google Scholar] [CrossRef]
- EN 13755:2008; Natural Stone Test Methods—Determination of Water Absorption at Atmospheric Pressure. DIN: Berlin, Germany, 2008. [CrossRef]
- Bish, D.L.; Howard, S. Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Rietveld, H.M. The rietveld method. Phys. Scr. 2014, 89, 098002. [Google Scholar] [CrossRef]
- EN 1936:2006; Natural Stone Test Method—Determination of Real Density and Apparent Density, and of Total and Open Porosity. Deutsches Institut für Normung e.V.: Berlin, Germany, 2007.
- VDI 4640-1; Thermal Use of the Underground—Part 1: Fundamentals, Approvals, Environmental Aspects. VDI: Düsseldorf, Germany, 2010.
- Kämmlein, M.; Stollhofen, H. Lithology-specific influence of particle size distribution and mineralogical composition on thermal conductivity measurements of rock fragments. Geothermics 2019, 80, 119–128. [Google Scholar] [CrossRef]
- Kämmlein, M.; Stollhofen, H. Pore-fluid-dependent controls of matrix and bulk thermal conductivity of mineralogically heterogeneous sandstones. Geotherm. Energy 2019, 7, 13. [Google Scholar] [CrossRef]
- Vosteen, H.-D.; Schellschmidt, R. Influence of temperature on thermal conductivity, thermal capacity and thermal diffusivity for different types of rock. Phys. Chem. Earth Parts A/B/C 2003, 28, 499–509. [Google Scholar] [CrossRef]
- Gehlin, S.; Hellström, G. Influence on thermal response test by groundwater flow in vertical fractures in hard rock. Renew. Energy 2003, 28, 2221–2238. [Google Scholar] [CrossRef]
- Magraner, T.; Montero, Á.; Cazorla-Marín, A.; Montagud-Montalvá, C.; Martos, J. Thermal response test analysis for U-pipe vertical borehole heat exchangers under groundwater flow conditions. Renew. Energy 2021, 165, 391–404. [Google Scholar] [CrossRef]
- Diao, N.; Li, Q.; Fang, Z. Heat transfer in ground heat exchangers with groundwater advection. Int. J. Therm. Sci. 2004, 43, 1203–1211. [Google Scholar] [CrossRef]
- Verdoya, M.; Imitazione, G.; Chiozzi, P.; Orsi, M.; Armadillo, E.; Pasqua, C. Interpretation of thermal response tests in borehole heat exchangers affected by advection. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 19–25 April 2015; p. 7. [Google Scholar]
- Luo, J.; Tuo, J.; Huang, W.; Zhu, Y.; Jiao, Y.; Xiang, W.; Rohn, J. Influence of groundwater levels on effective thermal conductivity of the ground and heat transfer rate of borehole heat exchangers. Appl. Therm. Eng. 2018, 128, 508–516. [Google Scholar] [CrossRef]
- Pasquier, P.; Lamarche, L. Analytic expressions for the moving infinite line source model. Geothermics 2022, 103, 102413. [Google Scholar] [CrossRef]
- Haffen, S.; Géraud, Y.; Rosener, M.; Diraison, M. Thermal conductivity and porosity maps for different materials: A combined case study of granite and sandstone. Geothermics 2017, 66, 143–150. [Google Scholar] [CrossRef]
- Luo, J.; Rohn, J.; Xiang, W.; Bayer, M.; Priess, A.; Wilkmann, L.; Steger, H.; Zorn, R. Experimental investigation of a borehole field by enhanced geothermal response test and numerical analysis of performance of the borehole heat exchangers. Energy 2015, 84, 473–484. [Google Scholar] [CrossRef]
- Tao, H.; Sun, S.; Wang, Q.; Yang, X.; Jiang, L. Petrography and geochemistry of lower carboniferous greywacke and mudstones in Northeast Junggar, China: Implications for provenance, source weathering, and tectonic setting. J. Asian Earth Sci. 2014, 87, 11–25. [Google Scholar] [CrossRef]
- Herron, M.M. Geochemical classification of terrigenous sands and shales from core or log data. J. Sediment. Res. 1988, 58, 820–829. [Google Scholar] [CrossRef]
- Pettijohn, F.J.; Potter, P.E.; Siever, R. Sand and Sandstone; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Mielke, P.; Weinert, S.; Bignall, G.; Sass, I. Thermo-physical rock properties of greywacke basement rock and intrusive lavas from the Taupo Volcanic Zone, New Zealand. J. Volcanol. Geotherm. Res. 2016, 324, 179–189. [Google Scholar] [CrossRef]
- Devaraju, T.; Sudhakara, T.; Kaukonen, R.; Viljoen, R.; Alapieti, T.; Ahmed, S.; Sivakumar, S. Petrology and geochemistry of greywackes from Goa-Dharwar sector, western Dharwar Craton: Implications for volcanoclastic origin. J. Geol. Soc. India 2010, 75, 465–487. [Google Scholar] [CrossRef]
- Miao, H.; Guo, J.; Wang, Y.; Jiang, Z.; Zhang, C.; Li, C. Mineralogical and elemental geochemical characteristics of Taodonggou Group mudstone in the Taibei Sag, Turpan–Hami Basin: Implication for its formation mechanism. Solid Earth 2023, 14, 1031–1052. [Google Scholar] [CrossRef]
- Glaser, K.S.; Miller, C.K.; Johnson, G.M.; Toelle, B.; Kleinberg, R.L.; Miller, P.; Pennington, W.D. Seeking the sweet spot: Reservoir and completion quality in organic shales. Oilfield Rev. 2013, 25, 16–29. [Google Scholar]
- Bas, M.L.; Maitre, R.L.; Streckeisen, A.; Zanettin, B.; IUGS Subcommission on the Systematics of Igneous Rocks. A chemical classification of volcanic rocks based on the total alkali-silica diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- Jung, S.; Hoernes, S. The major-and trace-element and isotope (Sr, Nd, O) geochemistry of Cenozoic alkaline rift-type volcanic rocks from the Rhön area (central Germany): Petrology, mantle source characteristics and implications for asthenosphere–lithosphere interactions. J. Volcanol. Geotherm. Res. 2000, 99, 27–53. [Google Scholar] [CrossRef]
- Wilson, M. Igneous Petrogenesis; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Hickman, A.H.; Wright, A.E. Geochemistry and chemostratigraphical correlation of slates, marbles and quartzites of the Appin Group, Argyll, Scotland. Earth Environ. Sci. Trans. R. Soc. Edinb. 1983, 73, 251–278. [Google Scholar] [CrossRef]
- Wagner, W.; Baumann, H.; Negendank, J.; Roschig, F. Geological, petrographic, geochemical and petrophysical investigations on roofing slates. Mainz. Geowiss. Mitt. 1997, 26, 131–184. [Google Scholar]
- Dalla Santa, G.; Galgaro, A.; Sassi, R.; Cultrera, M.; Scotton, P.; Mueller, J.; Bertermann, D.; Mendrinos, D.; Pasquali, R.; Perego, R.; et al. An updated ground thermal properties database for GSHP applications. Geothermics 2020, 85, 101758. [Google Scholar] [CrossRef]
- Balkan, E.; Erkan, K.; Şalk, M. Thermal conductivity of major rock types in western and central Anatolia regions, Turkey. J. Geophys. Eng. 2017, 14, 909–919. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Ren, T.; Horton, R. Quartz contents derived from particle density measurements improve the accuracy of soil thermal conductivity estimates. Geoderma 2023, 436, 116526. [Google Scholar] [CrossRef]
- Tarnawski, V.; Momose, T.; Leong, W. Assessing the impact of quartz content on the prediction of soil thermal conductivity. Géotechnique 2009, 59, 331–338. [Google Scholar] [CrossRef]
- Fuchs, S.; Förster, H.J.; Braune, K.; Förster, A. Calculation of thermal conductivity of low-porous, isotropic plutonic rocks of the crust at ambient conditions from modal mineralogy and porosity: A viable alternative for direct measurement? J. Geophys. Res. Solid Earth 2018, 123, 8602–8614. [Google Scholar] [CrossRef]
- Popov, Y.; Ostrizhniy, D.; Chekhonin, E.; Spasennykh, M.; Romushkevich, R.; Moiseenko, E.; Bogatov, S.; Kirik, S. Investigation of Rock Thermal Properties for Nuclear Waste Disposal Using Advanced Hardware-Methodical Basis. Rock Mech. Rock Eng. 2023, 56, 4583–4612. [Google Scholar] [CrossRef]
- Francl, J.; Kingery, W. Thermal conductivity: IX, experimental investigation of effect of porosity on thermal conductivity. J. Am. Ceram. Soc. 1954, 37, 99–107. [Google Scholar] [CrossRef]
- Surma, F.; Geraud, Y. Porosity and thermal conductivity of the Soultz-sous-Forêts granite. Pure Appl. Geophys. 2003, 160, 1125–1136. [Google Scholar] [CrossRef]
- LGB-RLP. Wärmeleitfähigkeiten von Gesteinen in Rheinland-Pfalz; Landesamt für Geologie und Bergbau Rheinland-Pfalz: Mainz, Germany, 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).