Abstract
The Badenian/Sarmatian boundary in the Central Paratethyan basins is characterised by a change from open marine conditions during the late Badenian to the assumed brackish conditions during the early Sarmatian. The foraminiferal and palynological results of the Badenian/Sarmatian boundary interval in the Babczyn 2 borehole (in SE Poland) showed that the studied interval accumulated under variable, unstable sedimentary conditions. The Badenian/Sarmatian boundary, as correlated with a sudden extinction of stenohaline foraminifera, is interpreted as being due to the shallowing of the basin. The lack of foraminifera and marine palynomorphs just above the Badenian/Sarmatian boundary can reflect short-term anoxia. The composition of the euryhaline assemblages, characteristic for the lower Sarmatian part of the studied succession, indicates from marine to hypersaline conditions.
Keywords:
foraminifera; palynofacies; dinocysts; pollen; spores; Miocene; Central Paratethys; Carpathian Foredeep; Poland 1. Introduction
The largest faunal turnover event in the Paratethys—a large epicontinental sea extending from Central Europe to inner Asia since the Oligocene [1]—occurred in the Middle Miocene at the Badenian/Sarmatian boundary, when 94% of Badenian species became extinct (Badenian–Sarmatian Extinction Event—BSEE; [2]). It was proposed that the cause of this event was a drastic basin-wide change in water chemistry from open marine conditions during the late Badenian to brackish conditions in the Central Paratethyan basins during the early Sarmatian. The details of this transformation are still under discussion, though [3,4] concluded that there is no evidence of shallowing associated with the BSEE. Ref. [5] suggested that because of a complex tectonic evolution, the closure of the Paratethyan basins at the end of Badenian was most probably diachronous, and, thus, slightly different ages for the Badenian/Sarmatian boundary across the Paratethys should be expected.
This study presents foraminiferal and palynological results of the upper Badenian–Sarmatian strata in one borehole section in Poland, namely, Babczyn 2, located in the northeastern part of the Carpathian Foredeep, which is the largest Central Paratethyan Basin (Figure 1). The aim of this study was to identify environmental changes around the inferred Badenian/Sarmatian boundary. The studied borehole is a key section of the northern marginal part of the Carpathian Foredeep [3,6,7,8]. A recent biostratigraphic study of two boreholes, including Babczyn 2 [8], confirmed, in general, zonation based on foraminifera that was previously established within the Badenian and Sarmatian strata of the northern margin of the Carpathian Foredeep [9,10,11,12,13] (cf. [14]; Figure 1). In the upper Badenian, the lower Neobulimina longa Zone and the upper Cibicides crassiseptatus (Hanzawaia crassiseptata) Zone occur (Figure 1); the latter is considered to be synchronous with the Velapertina indigena (Łuczkowska) planktonic foraminiferal zone [15].

Figure 1.
Central Paratethys realm in Middle Miocene (modified after [16]) and the location and stratigraphic position of the Babczyn 2 section (modified after [8]); BT—Babczyn Tuff (see [3]); L.—Langhian; T.—Tortonian; foraminiferal and calcareous nannoplankton zones after [8] except of Porosononion subgranozum zone recorded by [17].
Several foraminiferal zones have been identified in the Sarmatian strata (Figure 1). The lowest foraminiferal zone in the Babczyn 2 borehole was the Elphidium angulatum Partial Range Zone occurring below the Anomalinoides dividens Interval Zone [8] that is commonly regarded as the lowest Sarmatian (eco)zone (e.g., [11,13,18,19]).
2. Geological Setting
The Carpathian Foredeep basin developed in front of the West Carpathians in the Early Miocene and is subdivided into inner and outer parts [20]. The fill is mostly composed of Middle Miocene (Badenian and Sarmatian) deposits. The Badenian and Sarmatian strata in this northern marginal part are several hundred metres thick, and towards the axial part of the Carpathian Foredeep, their thickness increases to more than 4 km.
The Langhian red-algal limestones and quartz arenites of the Pińczów Formation (Figure 1) originated from shelf and near-shore environments, and mudstones and clays originated from more basinal locations (e.g., [21]). Their thickness in the Babczyn 2 borehole is approximately 12 m. These are overlain by the Serravallian gypsum of the Krzyżanowice Formation [22] deposited during the Badenian Salinity Crisis ([23], with references therein). The gypsum sequence is 32 m thick [6]. In the uppermost part, an intercalation (2.3 m thick) of marly clays with marine palynomorphs (dinoflagellate cysts) and foraminiferal assemblages occurs [7]. It originated from a short-lived marine transgression to the Badenian evaporite basin prior to the transgression re-installing normal marine conditions in the Carpathian Foredeep Basin, which resulted from the reconnection of the basin with the Mediterranean and Eastern Paratethys primarily by tectonic modification of the interconnecting gateways [24].
Consequently, the Serravallian gypsum is overlain by the sandy–silty series of the Machów Formation (Figure 1). Its upper Badenian part is referred to as the Pecten Beds [25]. They are, in turn, overlain by Syndesmya Beds, which were considered to be Sarmatian by [25]. Ref. [3] confirmed the location of the Badenian–Sarmatian boundary between the Pecten and Syndesmya Beds at the highest occurrence of well-preserved and identifiable pectinid shells. The location was 6.0 m above the Babczyn Tuff found in the middle portion of the Pecten Beds and 3.4 m above the gypsum, which constrained its depositional age to 13.06 ± 0.11 Ma [3]. This age was calibrated against the same astronomically tuned FC standard (e.g., [24]), giving an age of 13.41 ± 0.10 Ma, which was comparable to the age of 13.32 ± 0.07 Ma recorded for a volcanic ash layer located several metres above the Badenian evaporites in the Romanian East Carpathians [24]. This recalibrated age was used as shown in Figure 1.
The Syndesmya Beds form the lowermost part of the Krakowiec Clays ([26,27], with references therein; Figure 1). Ref. [8] proved that the Badenian–Sarmatian boundary lies within the Syndesmya Beds (Figure 1) and not at their base as previously was assumed. The thickness of the Machów Formation in the Babczyn 2 borehole is almost 400 m; however, only the lowermost part of the formation was cored, and its coring started at a depth of 350 m [25].
3. Materials and Methods
A total of 51 samples from the 40.7 m thick interval (368.4–409.1 m deep) in the Babczyn 2 section were studied for foraminifera (samples 8–59); the same sample set was previously [8] used for a biostratigraphic study [8]. The sampling distance was ~1 m in the Pecten beds (samples 8–21), 0.5 m in the lowermost part of the Krakowiec clays (samples 22–29), and ~1 m in the higher part of the studied interval of the Krakowiec clays (samples 32–59). The most common and characteristic species are illustrated in Figure 2 and Figure 3, respectively. The specimens in the figures were deposited at the Institute of Paleobiology, Polish Academy of Sciences in Warszawa, Poland (ZPAL F. 75).
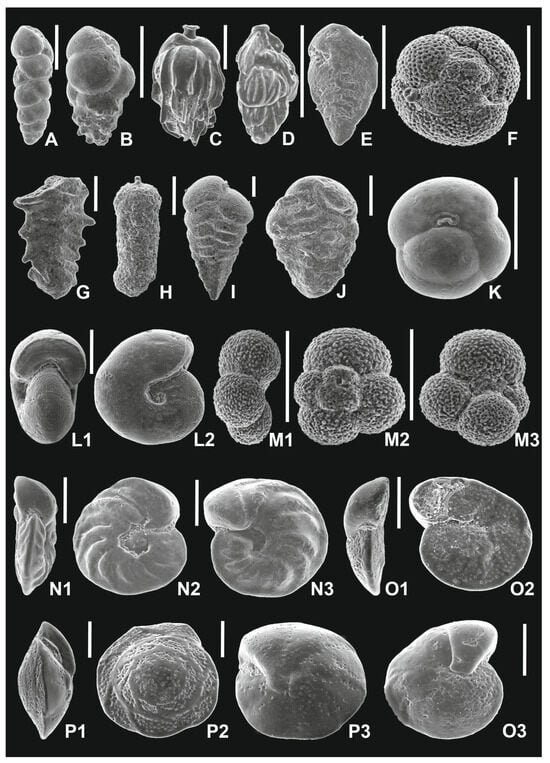
Figure 2.
Foraminifera from the Babczyn 2 borehole. (A)—Neobulimina longa (Venglinskyi), sample 17; (B)—Bulimina subulata (Cushman and Parker), sample 17; (C)—Uvigerina bellicostata (Łuczkowska), sample 19; (D)—Angulogerina angulosa (Williamson), sample 20; (E)—Bolivina dilatata (Reuss), sample 17; (F)—Velapertina indigena (Łuczkowska), sample 28; (G)—Spirorutilus carinatus (d’Orbigny), sample 31; (H)—Martinottiella communis (d’Orbigny), sample 28; (I)—Pseudogaudryina karreriana (Cushman), sample 25; (J)—Siphotextularia inopinata (Łuczkowska), sample 28; (K)—Sphaeroidina bulloides (d’Orbigny), sample 11; (L)—Melonis pompilioides (Fichtel and Moll) [L1: apertural view; L2:lateral view], sample 22; (M)—Globigerina bulloides (d’Orbigny) [M1: edge view; M2: dorsal view; M3: ventral view], sample 28; (N)—Hanzawaia crassiseptata (Łuczkowska) [N1: edge view; N2: dorsal view; N3: ventral view], sample 28; (O)—Lobatula lobatula (Walker and Jacob) [O1: edge view; O2: dorsal view; O3: ventral view], sample 28; (P)—Heterolepa dutemplei (d’Orbigny) [P1: edge view; P2: dorsal view; P3: ventral view],, sample 28; scale bars—200 μm.

Figure 3.
Foraminifera from the Babczyn 2 borehole. (A)—Trilobatus sp. [A1; edge view; A2: dorsal view; A3: ventral view],, sample 28; (B)—Globigerina tarchanensis (Subbotina and Chutzieva) [B1: edge view; B2: dorsal view; B3: ventral view], sample 28; (C)—Elphidium advenum subsp. limbatum (Chapman) [C1: apertural view; C2: lateral view], sample 33; (D)—Elphidium angulatum (Egger) [D1: apertural view; D2: lateral view], sample 29; (E)—Elphidiella serena (Venglinski) [E1: apertural view; E2: lateral view], sample 29; (F)—Quinqueloculina akneriana (d’Orbigny), sample 32; (G)—Porosononion martkobi (Bogdanovich) [G1: apertural view; G2: lateral view], sample 31; (H)—Elphidium angulatum (Egger) [H1: apertural view; H2: lateral view], sample 29; (I,K,L)—Varidentella reussi (Bogdanovich), sample 32; (J)—Anomalinoides dividens (Łuczkowska) [J1: edge view; J2: dorsal view; J3: ventral view], sample 35; scale bars—200 μm.
Depending on the foraminiferal abundances in different samples, all the foraminifera (up to 200 specimens) were picked. The relative abundances of planktonic and benthic foraminifera within the foraminiferal assemblages (P/B ratio), the Shannon–Wiener diversity indices H(S), the relative abundances of the most common genera, and the relative abundances of the infaunal and epifaunal morphogroups within the benthic foraminiferal assemblages were calculated [28,29] (Figure 4). H(S) values > 2.1 indicate normal marine environments [30]. The palaeoenvironmental interpretation based on the foraminifera involves the requirements of the present-day representatives of the recorded taxa [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
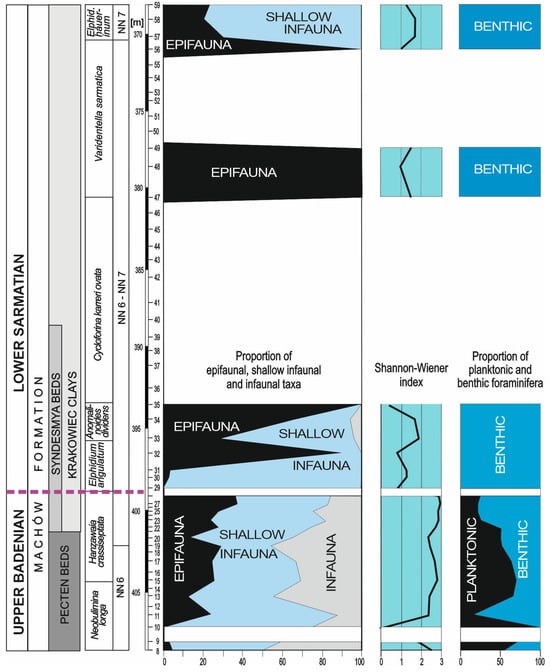
Figure 4.
The relative abundances (in percentages) of epifaunal, shallow infaunal, and infaunal taxa, the Shannon–Wiener indices H(S) and percentages of planktonic foraminifera in foraminiferal assemblages (P/B ratios); purple dashed line indicates the Badenian/Sarmatian boundary.
The following taxa were included in the oxic group: Anomalinoides spp., Cibicidoides spp., Hanzawaia spp., Heterolepa dutemplei, Lobatula lobatula, Valvulineria complanata, Globocassidulina subglobosa, Spirorutilus carinatus, keeled elphidiids, and miliolids [39,43]. Oxic indices represent epifaunal species. Taxa tolerant of suboxic environments included Astrononion perfossum, Hansenisca soldanii, Elphidiella serena, Melonis pompilioides, Pullenia bulloides, Valvulineria complanata, Angulogerina angulosa, Uvigerina spp., and Sphaeroidina bulloides [39,43]; taxa tolerant of dysoxic environments included Bolivina spp. and Bulimina spp. Foraminifera tolerant of suboxic environments represent mostly shallow infaunal species, whereas foraminifera tolerant of dysoxic environments represent mostly deep infauna and species with opportunistic behaviours. These are commonly used as stress markers (e.g., [49,50,51,52]).
For palaeoecological analysis, species or groups of species that exceeded 5% in at least one sample were taken into account. There is a direct relationship between the abundance of species within a community and the environment. Abundance fluctuations of benthic foraminifera are sensitive palaeoceanographic indicators responding to changing palaeotemperature, salinity, nutrient supply, and oxygen conditions.
For palynology, 26 samples were studied (i.e., the odd sample numbers from 9 to 55 and the even numbers 8 and 28). The applied palynological procedure included 38% hydrochloric acid (HCl) treatment, 40% hydrofluoric acid (HF) treatment, heavy-liquid (ZnCl2 + HCl; density 2.0 g·cm−3) separation, ultrasonication for 10–15 s, and sieving at 10 µm on nylon mesh. No nitric acid (HNO3) treatment was applied. Each processed rock sample weighed 10 g. Two slides from each sample were prepared using glycerine jelly as a mounting medium. The same set of slides was used for the analyses of dinoflagellate cysts, palynofacies (P.G.), and sporomorphs (E.W. and G.W.). Unprocessed rock samples, palynological residues, and slides were stored in the collection of the Institute of Geological Sciences at the Polish Academy of Sciences Research Centre in Kraków, Poland. The most common and characteristic species are illustrated in Figure 5 and Figure 6, respectively.

Figure 5.
Dinoflagellate cysts from the Babczyn 2 borehole. (A)—Batiacasphaera sphaerica, sample 8; (B)—Pyxidinopsis psilata, sample 8; (C)—Labyrinthodinium truncatum, sample 9; (D)—Reticulatosphaera actinocoronata, sample 9; (E)—Melitasphaeridium pseudorecurvatum, sample 11; (F)—Nematosphaeropsis labyrinthus, sample 11; (G)—Cordosphaeridium minimum, sample 15; (H)—Impagidinium sp., sample 19; (I)—Impagidinium sp., sample 23; (J)—Spiniferites ramosus s.l., sample 23; (K)—Hystrichosphaeropsis obscura, sample 11; (L)—Hystrichosphaeropsis obscura, sample 11; (M)—Operculodinium centrocarpum, sample 11; (N)—Spiniferites pseudofurcatus, sample 15; (O)—Hystrichokolpoma rigaudiae, sample 15; (P)—Pentadinium laticinctum, sample 11; (Q)—Spiniferites ramosus s.l., sample 21; (R,S)—Polysphaeridium subtile, same specimen, various foci, sample 27; (T)—Lingulodinium machaerophorum, sample 23; (U)—Systematophora placacantha, sample 27; (V)—Systematophora ?ancyrea, sample 41; (W)—Selenopemphix nephroides, sample 41; (X)—Selenopemphix nephroides, sample 41. Scale bar in (A) refers to all the photomicrographs.
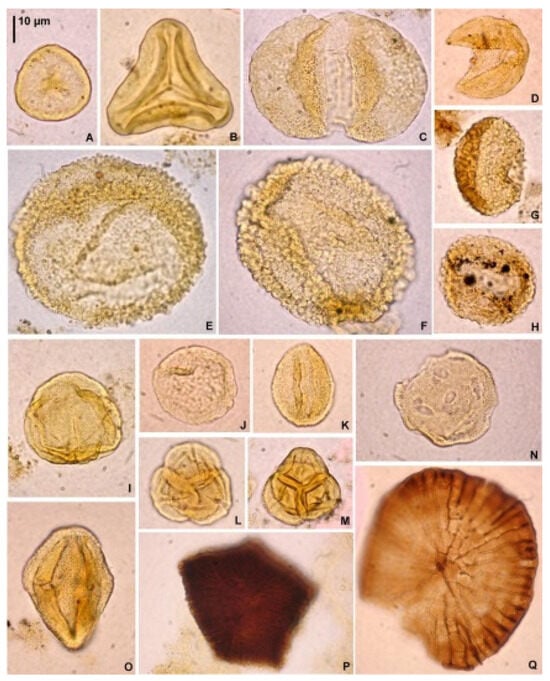
Figure 6.
Terrestrial palynomorphs from the Babczyn 2 borehole. Spores of plants: (A)—Stereisporites sp., sample 55; (B)—Neogenisporis sp., sample 55. Pollen grains: (C)—Cathayapollis sp., sample 47; (D)—Inaperturopollenites cf. verrupapillatus Trevisan, sample 47; (E)—Zonalapollenites gracilis Krutzsch, sample 47; (F)—Zonalapollenites verrucatus Krutzsch, sample 55; (G)—Sciadopityspollenites verticillatiformis (Zauer) Krutzsch, sample 15; (H)—Sciadopityspollenites verticillatiformis (Zauer) Krutzsch, sample 33; (I)—Faguspollenites sp., sample 43; (J)—Ulmipollenites undulosus Wolff, sample 47; (K)—Quercopollenites sp., sample 55; (L)—Ericipites baculatus Nagy, sample 33; (M)—Ericipites sp., sample 55; (N)—Periporopollenites orientaliformis (Nagy) Kohlman-Adamska and Ziembińska-Tworzydło, sample 17; (O)—Edmundipollis sp., sample 9. Fungi: (P)—Cephalothecoidomyces cf. neogenicus G. Worobiec, Neumann and E. Worobiec, sample 47; (Q)—Phragmothyrites sp., sample 15. Scale bar in (A) refers to all the photographs.
A Zeiss Axiolab microscope equipped with 20× lens and 100× oil lens was used for the analyses of the palynofacies and dinoflagellate cysts. The quantitative dinoflagellate cyst analysis was conducted by counting about 300 specimens; in lower-frequency cases, all the specimens from both prepared slides were counted. On this basis, the Shannon–Wiener diversity index (H′) was calculated as follows: H′ = −∑pi ln(pi), where pi is the relative abundance of each taxon and is expressed as eH′. The most frequent and important taxa for palaeoenvironmental reconstructions were grouped into eight dinoflagellate cyst morphogroups as follows:
- Spiniferites morphogroup, including all the Spiniferites and Achomosphaera specimens;
- Batiacasphaera morphogroup, including B. sphaerica, Batiacasphaera sp. A, B. micropapillata, and B. hirsuta;
- Operculodinium morphogroup, including all the Operculodinium specimens;
- Systematophora morphogroup, including S. placacantha and S. ?ancyrea specimens. The latter differed from S. placacantha because of incomplete or absent proximal ridges;
- Lingulodinium morphogroup, including Lingulodinium machaerophorum and a single specimen of Lingulodinium sp. A;
- Nematosphaeropsis morphogroup, including N. labyrinthus;
- Impagidinium morphogroup, including Impagidinium sp. and I. strialatum specimens;
- Polysphaeridium morphogroup, including P. subtile and P. zoharyi.
The palynofacies analysis was performed by counting 500 particles. The palynofacies elements in the present study were assigned to nine groups as follows:
- Black, opaque phytoclasts representing the most resistant coalified land plant remains;
- Dark-brown phytoclasts, which are land plant remains, usually equidimensional, translucent (or with translucent edges), remaining in structureless wood or cortical tissues;
- Cuticle group, including structured land plant tissues and remains with usually preserved cell structures. These are commonly the remains of the leaf epidermis;
- Pollen grains of angiosperms and gymnosperms. The most common are bisaccate pollen grains of gymnosperms;
- Pteridophyte spores;
- Dinoflagellate cysts;
- Algae and the acritarch group, including algae other than dinoflagellate cysts, such as prasinophyte (e.g., Tasmanites, Cymatiosphaera, and Leiosphaera), and incertae sedis microfossils (i.e., acritarchs);
- Foraminifera organic linings (zooclasts), which are the organic cells of foraminifera;
- Amorphous organic matter (AOM), which represents structureless particles of uncertain origins (most likely marine origin in the case of the present study materials), representing a stage in the bacterial decay of organic particles in oxygen-depleted environments.
The identified sporomorph taxa were classified based on the Atlas of Pollen and Spores of the Polish Neogene [53,54,55,56]. In the studied material, the following palaeofloristical elements were distinguished: “palaeotropical” (P), including “tropical” (P1) and “subtropical” (P2); “arctotertiary” (A), including “warm-temperate” (A1) and “temperate” (A2); and cosmopolitan (P/A). The data from the palynological spectra were used to construct a palynological diagram. In the diagram, the percentage shares of the pollen and spore taxa were calculated from the total sum of the pollen grains and spores, and the proportion of non-pollen palynomorphs was computed separately in relation to the total sum using POLPAL software [57].
Microphotographs of the selected sporomorphs and fungal palynomorphs (Figure 6) were obtained using a Nikon Eclipse E400 microscope equipped with a Canon A640 digital camera.
4. Results
4.1. Foraminifera
The Babczyn 2 succession contains well-preserved planktonic, calcareous, and agglutinated benthic foraminifera (Appendix 1 in [8]). Altogether, 60 species, representing the following genera were observed: ArtiSculina, Cycloforina, Pseudotriloculina, Quinqueloculina, Sigmoilinita, Triloculina, Varidentella, Elphidium, Haynesina, Porosononion, Anomalinoides, Heterolepa, Hanzawaia, Lobatula, Cibicidoides, Elphidiella, Melonis, Nonion, Pullenia, Bolivina, Bulimina, Uvigerina, Angulogerina, Sphaeroidina, Favulina, Globigerina, Velapertina, Trilobatus, Ammobaculites, Ammodiscus, Martinottiella, Siphotextularia, Textularia, Spirorutilus, Pavonitina, Pseudogaudryina, Rhabdammina, and Nothia.
In the Badenian part of the studied succession comprising the Pecten Beds and lowermost part of the Syndesmya Beds, both planktonic and benthic foraminifera occur. However, in sample 9, the tests of the foraminifera are damaged in most cases, indicating their transportation and reworking. Sample 10 possesses almost entirely planktonic foraminifera; rare specimens of benthic foraminifera were recorded. Samples from 11 to 28 yielded abundant and well-preserved foraminiferal assemblages, and sample 29 is almost devoid of foraminifera. Upsection, the samples from 30 to 35 from the lowermost Sarmatian yielded only benthic foraminifera, and in the upper part of the section (samples from 35 to 55), foraminifera are rare, and their assemblage is composed of miliolids and reworked forms from the Badenian. Only in a short interval (samples from 47 to 49), the abundant assemblage of miliolids occurs, and in samples 50 and 51, Bolivina sarmatica with miliolids is recorded. Owing to small numbers of specimens, quantitative analysis was not performed for the upper part of the studied section.
The following genera exceed 5% in at least one sample: Bulimina, Bolivina, Uvigerina, Angulogerina, Cibicidoides, Melonis, Sphaeroidina, Hanzawaia, Spirorutilus, Heterolepa, Elphidium, and Porosononion (Figure 7). Quantitative analysis enabled the grouping of samples with homogeneous foraminiferal assemblages. Nine foraminiferal assemblages were recognised in the studied interval (Figure 7).
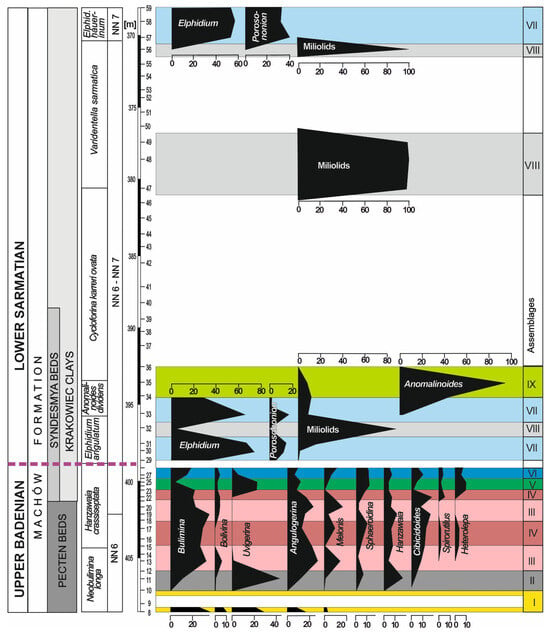
Figure 7.
Relative abundances of the most common benthic foraminiferal genera (in percentages) of the Babczyn 2 borehole; purple dashed line indicates the Badenian/Sarmatian boundary.
Assemblage I occurs at the base of the section (sample 8); sample 9, because of large numbers of resedimented specimens, has not been taken into account (Figure 7). This assemblage is characterised by the dominance of Bulimina (30%) and Uvigerina (30%). Other important components are Angulogerina, which exceeds 17%, and Bolivina (10%). Subsidiary species include Melonis pompilioides, Globocassidulina subglobosa, Astrononion perfossum, Asterigerinata planorbis, and Lobatula lobatula. The P/B ratio exceeds 60%; the H(S) diversity index is 2.5. Infaunal taxa comprise 95% of the assemblage.
Assemblage II is recorded in samples 10 and 11 (Figure 7). It is dominated by Uvigerina, which reaches 40% in the benthic foraminiferal assemblage, and contains Bulimina and Angulogerina, which exceed 10% (18 and 12%, respectively). Hanzawaia (16%) and Cibicidoides (11%) are other common components of this assemblage. The P/B ratio is 17%; the H(S) diversity index ranges from 2.3 to 2.5.
Assemblage III occurs in samples 13, 19, and 20 (Figure 7). It is dominated by Bulimina (from 30 to 40%), Angulogerina (from 16 to 28%), and Melonis (from 14 to 17%). Minor components of the assemblage are Bolivina, Cibicidoides, Sphaeroidina, and Hanzawaia. P/B varies from 70 to 80%; H(S) rangers between 2.3 and 2.7; infaunal species comprise from 75 to 85% of the assemblage (Figure 4).
Assemblage IV is recorded in samples 15, 17, and 22 (Figure 7). The dominant and common species are Bulimina (from 20 to 34%), Angulogerina (from 12 to 17%), Sphaeroidina (from 7 to 10%), Cibicidoides (13%), and Hanzawaia (8%). In this assemblage, for the first time in the studied interval, the following agglutinated foraminifera appear: Spirorutilus, Siphotextularia, and Ammodiscus. P/B is high and reaches 65%; H(S) is from 2.6 to 2.7; infaunal species comprise from 70 to 75% of the assemblage (Figure 4).
Assemblages III and IV alternate twice in the middle and upper parts of the Pecten Beds. They differ from the under- and overlying assemblages by a complete lack of Uvigerina. Instead, Angulogerina angulosa is one of dominant taxa and forms from 12% to 28% of these assemblages.
Assemblage V occurs in samples 23 and 25 (Figure 7). In these samples, Uvigerina reappears, and it forms >20% of the assemblage. The contents of three other species are around 10%: Heterolepa dutemplei (10%), Angulogerina angulosa (8–10%), and Sphaeroidina bulloides (12%). P/B is about 25%, the H(S) diversity index is 2.8, and infaunal morphogroups form 80% of the assemblage (Figure 4).
Assemblage VI is recorded in samples 27 and 28 (Figure 7). Bulimina forms from 15 to 19%, while Uvigerina drops to 0–6%. Angulogerina angulosa forms from 6 to 9%, Sphaeroidina bulloides forms from 8 to 10%, Heterolepa dutemplei forms from 5 to 7%, and agglutinated taxa (Siphotextularia inopinata, Pseudogaudryina karreriana, Ammodiscus sp., and Spirorutilus carinatus) form 13% of the assemblage. P/B is low, from 22 to 27%; H(S) is from 2.9 to 3.0; infaunal species comprise from 65 to 70% of the assemblage (Figure 4). This abundant, highly diversified foraminiferal assemblage suddenly disappeared at a depth of 399.4 m (sample 28).
Assemblage VII is recorded in samples 29, 30, 31, 33, 57, 58, and 59 (Figure 7). It is dominated by small Elphidium, which form from 60 to almost 80% of the assemblage; Porosononion and miliolids form from 10 to 20%. H(S) is from 1.8 to 1.9; infaunal species account for from 70 to 95% (Figure 4).
4.2. Palynofacies
All the samples yielded palynological organic matter, the elements of which showed various ratios reflecting variable environmental conditions and/or various sedimentological processes (Figure 8). The palynofacies of the studied samples were dominated by terrestrial elements represented by palynodebris (black and dark-brown phytoclasts and cuticles) and pollen grains. The shares of these two groups showed variable proportions: palynodebris (cuticles in particular) dominated in the lower part of the studied interval and decreased moving upwards, being replaced by pollen grains in the middle part and AOM in the upper part (Figure 8).
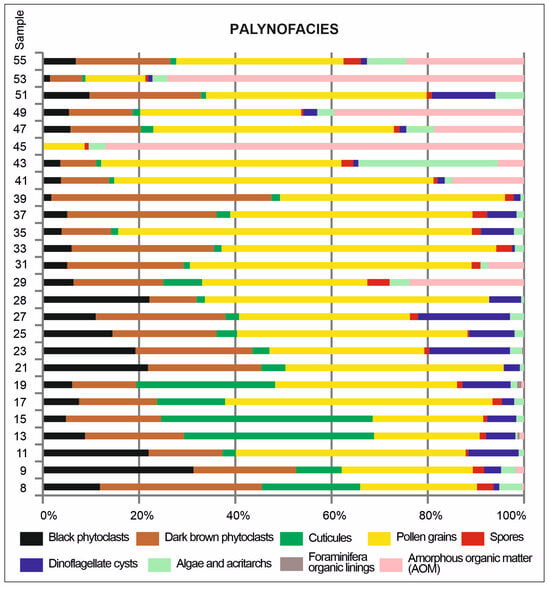
Figure 8.
Palynofacies changes in the Babczyn 2 borehole.
The proportion of marine elements (dinoflagellate cysts—Figure 5, prasinophytes, acritarchs, and rare zooclasts) rarely exceeded 10%. Dinoflagellate cysts were the most common in the lower and middle intervals while becoming rare or absent in the upper part, where prasinophytes and acritarchs predominated among the aquatic palynomorphs (Figure 8).
AOM occurred in two samples from the middle part of the studied interval (samples 29 and 31) and in the upper part, where it dominated in some samples (Figure 8).
4.3. Dinoflagellate Cysts
Virtually all the studied samples (except for samples 29, 31, and 45) involved dinoflagellate cysts. A total of 44–48 taxa assumed to be in situ were distinguished. We note that some taxa, such as Spiniferites ramosus s.l., included several species and/or subspecies, which were not distinguished for the purposes of the present study (Table 1, Table 2, Table 3 and Table 4). Despite this apparent taxonomical richness, the taxonomic diversity of the dinoflagellate cyst assemblages was low, and it oscillated between 2.0 and 6.5 (Figure 9). The majority of the samples yielded assemblages dominated by from three to four species, with the remaining taxa being represented by rare or even single specimens. The dominant taxa included Spiniferites ramosus s.l., Batiacasphaera spp., and Systematophora spp., with the most common ones being Operculodinium spp. and Lingulodinium machaerophorum; their proportions varied from sample to sample and could also be different in neighbouring samples. Some species, like Nematosphaeropsis labyrinthus, Selenopemphix nephroides, Reticulatosphaera actinocoronata, Lingulodinium machaerophorum, and Polysphaeridium subtile, showed increased frequencies only in single samples.

Table 1.
Distribution of aquatic palynomorphs in the upper Badenian of Babczyn 2 borehole (part 1). (*) indicates reworked taxa; their occurrence is not included in the total count.

Table 2.
Distribution of aquatic palynomorphs in the lower Sarmatian of Babczyn 2 borehole (part 1). (*) indicates reworked taxa; their occurrence is not included in the total count.

Table 3.
Distribution of aquatic palynomorphs in the upper Badenian of Babczyn 2 borehole (part 2). (*) indicates reworked taxa; their occurrence is not included in the total count.

Table 4.
Distribution of aquatic palynomorphs in the lower Sarmatian of Babczyn 2 borehole (part 2). (*) indicates reworked taxa; their occurrence is not included in the total count.

Figure 9.
Palaeoenvironmental proxies of aquatic palynomorphs from the Babczyn 2 borehole; KF—Krzyżanowice Formation.
The highest diversity values, between 4.5 and 6.5, were recorded in the lower studied interval, which included the upper Badenian (samples 8–27, except for samples 17 and 25). The diversity decreased in the succession, above the two barren samples (29 and 31), where it oscillated between 2.3 and 4.5 (with a minimal diversity of 0.5 being recorded in sample 51). However, this decreasing upwards diversity trend was not gradual; a characteristic feature was that the diversity in the assemblages from the subsequent samples was alternately higher and lower, thereby forming the zigzag course of the chart (Figure 9). The diversity in dinoflagellate cyst assemblages was generally correlated with the number of taxa. The highest numbers of dinoflagellate cysts (15–22) were recorded in the lower part of the studied interval (samples 9–27). The numbers of taxa from the higher interval were lower, oscillating between 4 and 7, with higher values (i.e., 11, 13, and 16) being present only in three samples (37, 35, and 49, respectively, Figure 9).
Another characteristic feature of the dinoflagellate cyst assemblages from the studied borehole interval was the predominance of gonyaulacoids. Most of the studied samples yielded purely gonyaulacoid assemblages. Peridinioids were represented by Selenopemphix and Lejeunecysta; the latter and S. brevispinosa occurred mainly as single specimens only in a few samples (Table 1, Table 2 and Table 4), whereas S. nephroides formed an acme in sample 41 (up to 25% of the whole assemblage, Figure 9).
The diversity and frequency of the taxa in the studied materials were related to the frequencies of some taxa grouped into eight morphogroups. Representatives of the Spiniferites and Batiacasphaera morphogroups were the most common taxa in the lower part of the studied succession (samples 8–28). Their frequencies showed a negative correlation: samples with common Spiniferites yielded few Batiacasphaera, whereas increased frequencies of Batiacasphaera were associated with decreased Spiniferites (Figure 9). This relationship was further correlated with changes in diversity in this interval; Batiacasphaera-dominated assemblages (samples 17, 21, and 25) showed the lowest diversities. Representatives of the Batiacasphaera morphogroup were virtually absent in the upper interval (Table 1); the only exception was their common occurrence in sample 51, which yielded the most taxonomically impoverished assemblage (Figure 9).
Representatives of the Operculodinium morphogroup occurred in all the positive samples except for sample 33, taken just above the two barren samples; their proportions were not high, i.e., rarely exceeding 20% (Figure 9). They showed a negative correlation with the Batiacasphaera morphogroup, similar to representatives of the Spiniferites morphogroup (Figure 9).
Representatives of the Systematophora morphogroup were rare or absent in the lower and middle parts of the studied borehole interval while becoming more frequent in the higher part, starting from sample 41. Their proportions in this interval oscillated between 30 and a bit over 60% (except for sample 51). The proportions of Systematophora were negatively correlated with those of Batiacasphaera (most visible in sample 51) and Lingulodinium (samples 39 and 51) and showed a positive correlation with Polysphaeridium (Figure 9). The representatives of the latter were very rare or absent except for two samples (33 and 37), which yielded approximately 46 and 18%, respectively. The Lingulodinium share was similar; it was infrequent or absent, being a dominant taxon (almost 80%) in sample 39.
Nematosphaeropsis labyrinthus was a very rare species throughout the studied borehole interval; it occurred infrequently in only four samples from the basal part (Table 1). However, it formed an acme in sample 27, collected below the barren interval (samples 29 and 31), where it reached almost 40% of all the dinoflagellate cysts.
The Impagidinium morphogroup occurred in the basal part of the studied borehole interval (only one specimen was found in the remaining part, sample 49). The distribution of very uncommon specimens of Impagidinium showed a pattern similar to that of Nematosphaeropsis (Figure 9).
4.4. Other Aquatic Palynomorphs
Dinoflagellate cysts were not the only aquatic palynomorphs found in the studied borehole interval. Other aquatic palynomorphs occurred in all the samples while reaching even higher frequencies in some of them. These included acritarchs (e.g., Veryhachium, Nannobarbophora, and unidentified small, sphaeromorphic, spiny, and smooth forms); prasinophytes (e.g., Tasmanitaceae and Leiosphaeridiaceae); and zooclasts (foraminifera organic linings, scolecodonts, and remains of presumably arthropods). The diversity of other aquatic palynomorphs exhibited high variability from sample to sample, thereby resembling the distribution pattern of dinoflagellate cyst assemblages.
The lowermost sample (No. 8), yielded frequent Leiosphaeridiaceae (described below as the genus Leiosphaeridia, although its precise taxonomic position is tentative). Samples 9–13 yielded foraminifera organic linings and Nannobarbophora. Tasmanites co-occurred with Nannobarbophora in sample 15 and Leiosphaeridia in sample 17. Neither sample yielded foraminifera organic linings; these were found in sample 19 (with frequent Nannobarbophora and rare Leiosphaeridia). The latter had an increased frequency in the subsequent sample, sample 21, which yielded no Nannobarbophora and only single foraminifera organic linings. The frequency of Leiosphaearidiaceae in higher samples showed a fluctuating course: it decreased in sample 23, increased in sample 25, and decreased again in sample 27, whereas the occurrences of Tasmanites and Nannobarbophora in these samples showed the opposite trend. Sample 27 also yielded Veryhachium. Rare Tasmanites and Veryhachium were the only prasinophytes and acritarchs, respectively, in sample 28.
The distribution patterns of acritarchs and prasinophytes in the lower part of the studied borehole interval (samples 8–28) showed specific patterns that were further correlated with dinoflagellate cyst assemblages. There was a clear negative correlation between the assemblages dominated by foraminifera organic linings and Nannobarbophora (and Tasmanites to a lesser degree) and those dominated by Leiosphaeridia. Moreover, the samples that yielded the former assemblages yielded relatively diverse dinoflagellate cyst assemblages. These samples, which yielded impoverished assemblages dominated by Batiacasphaera, also exhibited increased frequencies of Leiosphaeridia.
Various spherical forms attributed to Leiosphaeridiaceae became the nearly sole aquatic palynomorphs in samples 29 and 31, which yielded no dinoflagellate cysts; scolecodonts were found in sample 31. Leiosphaeridia were again dominant in the subsequent sample (33), which yielded impoverished dinoflagellate cyst assemblages with relatively frequent Polysphaeridium.
Higher intervals of the studied borehole (samples 35–55) yielded common to very common Leiosphaeridiaceae, represented by morphologically diversified forms, some of which likely represent sphaeromorphic, smooth acritarchs. They were predominant in these samples over other prasinophytes, and acritarchs were commonly the only aquatic palynomorphs (other than dinoflagellate cysts). Rare Nannobarbophora occurred in samples 35, 37, and 49, and a single foraminifera organic lining was found in sample 51.
Reworked dinoflagellate cysts commonly occurred in the lower part of the studied borehole interval (samples 8–28). These were Palaeogene specimens representing mainly Eocene–Oligocene forms, and the Palaeohystrichophora found in sample 27 may have been Cretaceous forms. Higher borehole intervals yielded much less frequently reworked specimens, which were mainly Cretaceous forms (Table 1, Table 2, Table 3 and Table 4; reworked taxa are highlighted with an asterisk).
4.5. Spore–Pollen Analysis
All the samples yielded pollen grains and spores suitable for palynological analysis. In most samples, 300–500 pollen grains and spores (a minimum of 100 in sample 21) were identified. Besides sporomorphs, all the co-occurring non-pollen palynomorphs in the samples were counted. Most sporomorphs were poorly preserved; however, they were still identifiable. A total of 71 fossil-species (including nine species of plant spores, 16 species of gymnosperm pollen, and 46 species of angiosperm pollen) were identified (Table 5). In all the samples, among the pollen grains of the gymnosperms, bisaccate pollen grains (Figure 6C) of the Pinaceae family (mainly Pinus, plus Cathaya, Abies, Picea, Keteleeria/Pseudolarix, and Cedrus); Taxodium/Glyptostrobus (Figure 6D); Tsuga (Figure 6E,F); and Sciadopitys (Figure 6G,H) were the most common. Additionally, several pollen grains of Sequoia/Sequoiadendron/Metasequoia/Cryptomeria were encountered (Figure 10).

Table 5.
Spores and pollen grains recorded in samples from the Babczyn 2 borehole. Taxonomies and botanical affinities according to [53,54,55,56]. The following palaeofloristical elements have been distinguished: “palaeotropical” (P), including “tropical” (P1) and “subtropical” (P2), and “arctotertiary” (A), including “warm-temperate” (A1) and “temperate” (A2), as well as cosmopolitan (P/A).
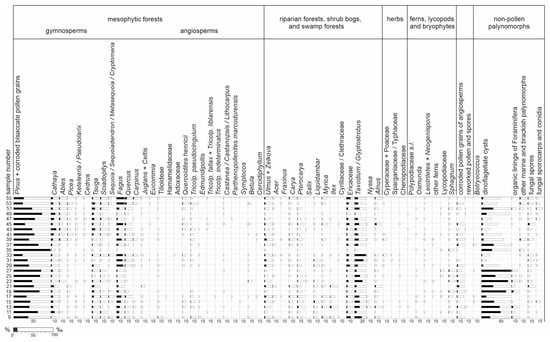
Figure 10.
Palynological diagram for the Babczyn 2 borehole. Black bars show percentages (%); white bars show percentages ×10 (‰).
Among the angiosperm pollen grains, Fagus (Figure 6I), Ericaceae (Figure 6L, M), Quercus (fossil-genus Quercopollenites—Figure 6K—and fossil-species Quercoidites henricii), Ulmus (Figure 6J), Pterocarya, Carya, Zelkova, Liquidambar (Figure 6N), Alnus, and Myrica were the most common. Pollen grains of Acer, Carpinus, fossil-species Tricolporopollenites pseudocingulum, Tilioideae, Betula, Celtis, and Juglans were regularly observed in the samples. Ilex and Nyssa were mainly present in samples from the lower section of the profile. In addition, a few pollen grains of Adoxaceae, Castanea/Castanopsis/Lithocarpus, Cercidiphyllum, Cyrillaceae/Clethraceae, Eucommia, Fraxinus, Salix, and Symplocos, as well as the fossil-species Edmundipollis sp. (Figure 6O), Parthenopollenites marcodurensis, Tricolporopollenites fallax, T. indeterminatus, and T. liblarensis were encountered. Herbs were very rare, represented by members of the Chenopodiaceae, Cyperaceae, Poaceae, and Sparganiaceae/Typhaceae families (Figure 10, Table 5).
Fern spores were also rare, with the Baculatisporites, Laevigatosporites, Leiotriletes, Neogenisporis (Figure 6B), and Verrucatosporites fossil genera being present. In contrast, spores of Sphagnum (Figure 6A) were encountered in all the samples. Several lycopod spores (related to the modern genera Lycopodium and Lycopodiella) were also recorded. Typical freshwater algae were virtually absent, and only scarce Botryococcus colonies were encountered. Microremains of fungi (Figure 6P,Q) were continually found in the palynological profile. Fungal spores were present in all the samples, whereas sporocarps were mainly present in the upper section of the profile (Figure 10).
In all the samples, “arctotertiary” and cosmopolitan taxa prevailed, but “palaeotropical” and “palaeotropical/warm temperate” taxa were also present (Table 5). “Palaeotropical” elements were represented by single specimens of Camarozonosporites sp., Leiotriletes sp., Neogenisporis sp., Cyrillaceaepollenites megaexactus, Ilexpollenites margaritatus, Symplocoipollenites vestibulum, and Tricolporopollenites indeterminatus. The representation of the “palaeotropical/warm temperate” taxa was more significant, and pollen grains of Inaperturopollenites concedipites, I. verrupapillatus, Cupuliferoipollenites oviformis, C. pusillus, Ilexpollenites iliacus, Myricipites sp., Nyssapollenites sp., Parthenopollenites marcodurensis, Quercoidites henricii, and Tricolporopollenites pseudocingulum occurred.
5. Discussion
5.1. Aquatic Palaeoenvironment
The qualitative and quantitative analyses of the foraminifera and aquatic palynomorphs showed that the studied borehole interval accumulated under variable, unstable sedimentary conditions (Figure 11).
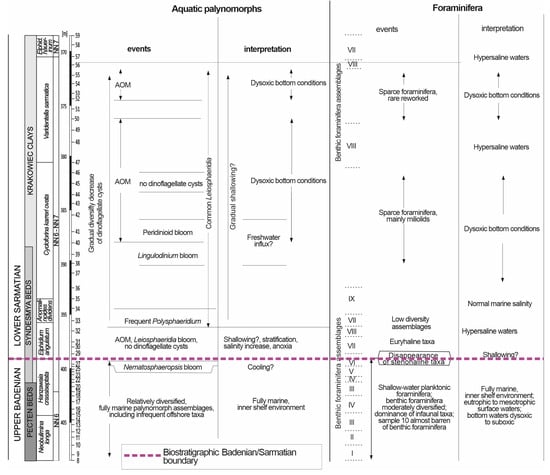
Figure 11.
Palaeoenvironmental changes in the late Badenian and early Sarmatian based on aquatic palynomorph and foraminiferal data from the Babczyn 2 borehole.
5.1.1. Foraminiferal Record
Foraminifera are commonly used as proxies for palaeoenvironmental studies because of the correlation between foraminiferal test shapes and their palaeoenvironmental requirements [32,58]. Planktonic foraminifera can be useful indicators of ancient sea-level changes because of their depth stratification [44,59,60]. The vertical distribution of planktonic foraminifera is directly related to their life cycle. They reproduce at species-specific depths relative to the pycnocline and under distinct temperature and salinity conditions; thus, they require a water column at certain depths for ontogenetic vertical migration and reproduction [60,61]. Simple morphologies (r-strategists), which are representative of the most cosmopolitan and opportunistic taxa, inhabit shallow, more nutrient-rich, eutrophic waters [62,63]. Planktonic foraminifera in the upper Badenian part of the succession included Globigerina (with Globigerina bulloides being dominant within this group) and rarely recorded Trilobatus and Velapertina. Globorotalia, which shows preferences for deeper/intermediate habitats, was not recorded in the studied succession. Globigerina bulloides reproduces primarily within the upper 60 m of the water column and exhibits the maximum abundance at this depth [60,64,65,66]. It is also considered to be an indicator of cooler [67,68] and nutrient-rich eutrophic waters [44,62,69,70]. Velapertina was endemic to the Central Paratethys, where it appeared to have repeated the same sequence of transitional steps as Orbulina [48]. The two genera had broadly overlapping distributions and occupied a niche similar to that of Orbulina in the surface waters [48].
The ratio between the planktonic and benthic foraminifera (P/B) is related to the water depth, and the percentage of planktonic foraminifera generally increases with increasing distance from the shore. However, next to the water depth, the oxygen level of the bottom water has profound effects on the abundance and diversity of benthic foraminifera. This significantly influences the percentage of planktonic foraminifera in assemblages [50]. According to the foraminiferal trophic condition and oxygen concentration (TROX) model [71], the benthic foraminifera distribution is a function of the interplay between the food availability and oxygen concentrations. The dominance of infaunal species is interpreted as an indicator of an increase in the organic matter supply and the dominance of the eutrophic and dysoxic environments. In oligotrophic and well-oxygenated environments, assemblages are dominated by epifaunal species [29,72]. The variations in the proportions of epifaunal and infaunal species indicate distinctive inputs of organic matter (phytodetritus input versus bacterial activity, respectively) [73].
Benthic foraminiferal assemblages from the Badenian part of the studied section (Assemblages from I to VI) are dominated by deep infaunal buliminids (Bulimina) and shallow infaunal uvigerinids (Uvigerina and Angulogerina). Melonis and Sphaeroidina—shallow infaunal species—as well as epifaunal Cibicidoides, Hanzawaia, and Heterolepa are common components of benthic foraminiferal assemblages. The high dominance of infaunal taxa (>95%), mainly buliminids and uvigerinids (Assemblage I) in the lowermost part of the studied interval (samples from 8 to 10), indicates a large supply of organic matter to the sea floor, the dominance of eutrophic conditions, and the oxygen impoverishment of the bottom waters in this area. In the basal part of the studied interval, benthic foraminifera were very rare in sample 10 (Figure 7). Their absence can be explained by a short episode of dysoxic conditions at the bottom of the sea at this site.
The gradual increase in epifauna up the section from 20% in Assemblage II to almost 40% in Assemblage VI indicates a change from eutrophic to mesotrophic conditions, a decrease in the organic matter supply, and an amelioration in oxygen in the bottom waters.
The alternation in the assemblages dominated by infaunal and shallow infaunal taxa, Bulimina, Angulogerina, and, to lesser degrees, Bolivina (Assemblages III, IV, and VI) and Uvigerina (Assemblages II and V), may be the result of slightly different requirements in the types of food and oxygen levels of each taxon. In the uppermost Badenian (samples from 11 to 28), the contribution of the infaunal taxa decreases, and diversity increases, which suggest mesotrophic conditions in the surface waters and increased oxygenation in the bottom waters.
There is also one more factor that could influence the composition of benthic foraminiferal assemblages. Middle Miocene tectonic activity in the Carpathian Foredeep [74] resulted in intensive volcanism with enhanced input of volcanoclastic material [3,75]. Studies and monitoring of the changes in the compositions of benthic foraminiferal assemblages from the South China Sea following the 1991 Mt. Pinatubo eruption have revealed that high tephra settling rates through the water column led to the mass mortality of benthic biota and to significant survival and rapid recovery of the ecosystem in distal parts of the ash fan, where the thickness of the ash layer did not exceed a few millimetres [76,77].
The Badenian/Sarmatian boundary, as correlated with a sudden extinction of stenohaline foraminifera (BSEE), is placed just above sample 28 (within the lowermost part of the Syndesmya Beds; Figure 7). The overlying sample (29) is almost barren of foraminifera from both groups, planktonic and benthic ones. Higher up in the section, assemblage VII is dominated by elphidiids alternating with assemblage VIII, which is dominated by miliolids. These groups of foraminifera are euryhaline and can tolerate a wide range of salinities from brackish to elevated. Keeled species of Elphidium prefer inner-shelf environments characterised by salinities of 30–70‰ and 0–50 m water depths. At this site, they are represented only by very rare specimens of Elphidium fichtelianum and E. macellum. Unkeeled species occur in brackish–hypersaline marshes and lagoons showing salinity ranges of 0–70‰ [29,38]. In the studied section, they are represented by Elphidium excavatum, E. angulatum, E. advenum, and E. hauerinum. Miliolids (Quinqueloculina and Triloculina) occur in hypersaline marine environments (salinity ranging from 32 to 55–65‰), mainly in hypersaline lagoons or on the marine inner shelf [29,78]. The replacement of stenohaline foraminiferal assemblages by euryhaline ones indicates a salinity increase, while the disappearance of planktonic foraminifera suggests a shallowing of the sea.
The next almost monospecific assemblage (assemblage IX), which is dominated by Anomalinoides dividens, probably reflects a salinity lowering to normal marine values. Presently, marine environments are characteristic for Anomalinoides [79], although in the past, its wider environmental range, including brackish salinity conditions, was accepted [46] (with references therein).
5.1.2. Palynomorph Record
The presence of marine palynomorphs suggests that most of the studied section accumulated in marine environments. However, changes in palynofacies and the compositions of aquatic palynomorph assemblages among particular samples suggest that these environmental conditions were unstable while being subjected to numerous changes related to sea-level fluctuations, salinity, and climatic changes. All these factors may have played important roles in the aforementioned changes in aquatic assemblages.
Undoubtedly, the lower part of the studied borehole interval (samples 8–28; the Pecten Beds and the lowermost part of the Syndesmya Beds) accumulated in a marine environment influenced by runoff from the neighbouring land. The upward-decreasing proportions of cuticles suggest that land influences were gradually decreasing; the simultaneous increase in pollen grains (Figure 8) confirms this interpretation, as most of the grains were bisaccate forms, which are airborne, and can be transported for long distances (the so-called ‘Neves effect’; see, e.g., [80,81]). However, there were no other signs of land influences, such as a salinity decrease; there were neither freshwater algae nor dinoflagellate cysts, which prefer low-salinity waters. Despite the relatively intense land influence, this interval was presumably deposited under relatively offshore conditions compared with the interval above sample 28. Only this interval yielded Impagidinium and Nematosphaeropsis, two dinoflagellate cyst genera commonly associated with offshore waters (e.g., [82,83,84]), while the upper interval yielded only a single Impagidinium (sample 49; Table 1).
The interval below the Badenian/Sarmatian boundary (samples 17–28) showed a series of environmental fluctuations manifested by alternating changes in aquatic palynomorph compositions. High-diversity marine palynomorph assemblages dominated by Spiniferites alternated with less-diverse Batiacasphaera-dominated assemblages. Moreover, Nematosphaeropsis labyrinthus occurred during this interval and exhibited a negative correlation with Batiacasphaera (Figure 9).
Spiniferites (mainly S. ramosus and the morphologically similar Achomosphaera) occurs in a broad marine environmental spectrum and is commonly treated as a cosmopolitan taxon (e.g., [82]). But several authors, such as in [85,86], have suggested that its frequency increases offshore. It is also present in the marine Middle Miocene strata of the Carpathian Foredeep and is the most frequent in marine shelf environments (e.g., [87]). This suggests that the samples that yielded diversified assemblages (with frequent Spiniferites) represent strata accumulated in a marine environment, representing an offshore setting, in contrast to those that yielded impoverished assemblages with common Batiacasphaera. Although the latter genus was described by [88] as a Batiacasphaera micropapillata complex, including B. micropapillata and B. minuta, as is typical for outer neritic to oceanic waters with slightly increased salinities, Batiacasphaera (mainly B. sphaerica) tends to exhibit increased frequencies in restricted environments possibly associated with water shallowness and/or increased salinity [87,89]. The negative correlation between Batiacasphaera and Nematosphaeropsis labyrinthus may be an additional clue suggesting sea-level fluctuations during this period. However, Nematosphaeropsis labyrinthus is treated as a cold-water species (e.g., [90]) in contrast to Batiacasphaera, which is believed to be a temperate- to warm-water taxon [88]. This suggests that an acme of N. labyrinthus just below the Badenian/Sarmatian boundary may be the result of a temperature drop in sea-surface waters (a similar inverse correlation between N. labyrinthus and Batiacasphaera micropapillata was noted by [91] and was interpreted as a possible cooling). Notably, high frequencies of N. labyrinthus were found in the Pecten Beds overlying the Badenian evaporites in the Carpathian Foredeep [92]. This might indicate a cooling period (or periods) after the accumulation of the evaporitic series. However, N. labyrinthus is known to be found in warm-water middle Badenian Korytnica Clays and their offshore age equivalents—the Skawina Beds [87,89].
The interval that occurred just above (samples 29 and 31) was characterised by a lack of dinoflagellate cysts and the flowering of Leiosphaeridiaceae. Any of the aforementioned environmental factors—albeit at a larger magnitude—could be the one that led to such a drastic environmental change in the earliest Sarmatian, associated with the collapse of the dinoflagellate floras. The precise reconstruction of environmental changes during the accumulation of these strata is difficult. The absence of dinoflagellate cysts (Figure 8) indicated disastrous conditions for dinoflagellate cysts. However, the most likely reason was that the salinity increased above the tolerable level, even for hypersaline forms (e.g., Polysphaeridium) but was still favourable for Leiosphaeridia. This Prasinophyta genus (e.g., [93]) was described in the Carpathian Foredeep, more specifically, in the middle Badenian evaporitic strata accumulated under increased water salinity conditions (e.g., [89]) and in upper Badenian stress deposits [94]. These possible hypersaline conditions were associated with stagnant, possibly stratified, waters that led to anoxic conditions in the bottom waters, as evidenced by AOM (Figure 8). Hypersaline waters are usually stagnant. If waters are affected by strong circulation, particularly in a deeper basin, then hypersalinar conditions are less likely to appear. The Palynological record shows blooms of Leiosphaera, a prasinophyte alga widespread in the Miocene salt deposits of the Carpathian Foredeep, simultaneously with the appearance of amorphous organic matter (AOM), which is typical for anoxic bottom conditions. We have no indications as to the precise salinity level (certainly above 3.5%).
The cessation of these conditions was caused by a possible sea-level rise and the gradual return of a less-saline water regime. The latter interpretation is supported by the fact that sample 33 yielded a high frequency of Polysphaeridium (a genus known to exist in hypersaline environments, e.g., [95]), which benefited from the transitional salinity levels between the highly saline conditions that were disastrous for the genus (samples 29 and 31) and the conditions that prevailed during the accumulation of the higher interval (samples 35 and above). Polysphaeridium was described at a similar position, i.e., in the strata directly overlying the chemical deposits in other areas of the Miocene of the Carpathian Foredeep (e.g., [94,96,97,98]).
The higher interval (samples 35–55, i.e., the upper part of the Syndesmya Beds and the overlying part of the Krakowiec Clays; Figure 9) was deposited under more restricted and variable environmental conditions (compared to those of the basal interval, namely, samples 8–28).
This interval yielded no offshore species (except for a single Impagidinium specimen in sample 49), and the diversity and number of taxa decreased significantly (Figure 9). However, the factors responsible for these conditions remain unclear. There were no clear indicators of sea-level or salinity changes. An important event was the disappearance of Batiacasphaera, which can be interpreted either as the result of cooling or changes in sea-water chemistry; however, its nature remains uncertain. A drop in the sea-surface water temperature is also suggested by the lack of Nannobarbophora gedlii, which is believed to be a warm-water species [99].
Some recorded events may indicate fluctuations in the salinity level. There are almost no stenohaline foraminiferal organic linings (present in the lower interval, namely, samples 8–28), and some taxa known to benefit from low-salinity, commonly nutrient-rich waters, show temporarily enriched frequencies (e.g., [84]). These include Lingulodinium machaerophorum and heterotrophic Congruentidiaceae (peridinioids are represented in the present material by Selenopemphix and Lejeunecysta). L. machaerophorum dominated sample 39, whereas rare peridinioids occurred in the top interval (samples 39–55), forming an acme of Selenopemphix in sample 41 (Table 4; Figure 9). This interval, in turn, was preceded by samples 33 and 37, which yielded Polysphaeridium—a hypersaline taxon (see above). Another characteristic feature of the strata above the Syndesmya Beds was the common occurrence of Systematophora. This genus was missing or rarely exceeded 10% in the lower interval, whereas it accounted for over 40%, maximally over 60%, in some samples from the upper interval (Figure 9). However, palaeoenvironmental preferences of Systematophora are poorly understood. Ref. [86] included Systematophora placacantha in the Glaphyrocysta eco-group, which included open-marine (i.e., fully marine) and warm-water taxa. Ref. [82] highlighted the widespread occurrence of this genus and linked it to shelf environments in any climatic setting. The analysis of Systematophora (S. placacantha and S. ancyrea) occurrences in the Miocene of the Carpathian Foredeep shows a broad environmental tolerance range of this genus, as it is a member of fully marine (e.g., [87,100]) and impoverished assemblages (e.g., [92]). However, a noticeable feature of the occurrence of this genus is the remarkable frequency increase in Sarmatian deposits (e.g., [92]), which may be linked to the euryhaline nature of this genus. A similar acme of Systematophora (as Clesitosphaeridium) was noted in the uppermost Badenian–lower Sarmatian Paratethyan deposits in Hungary [101].
Another clue suggestive of fluctuating, mainly restricted, environments during the accumulation of the upper part of the Syndesmya Beds and the Krakowiec Clay is the persistent presence of Leiosphaeridia (and presumably some other sphaeromorphic, smooth acritarchs). Their occurrence may reflect not only salinity fluctuations (see above) but also generally cooler surface waters and/or increased nutrient availability (see [91], p. 55 and references therein).
An exception was an assemblage yielded from sample 51, i.e., a low-diversity assemblage dominated by Batiacasphaera, with no peridinioids. Its palynofacies is also exceptional as it includes no AOM in contrast to the remaining samples from this interval. The appearance of Batiacasphaera may be related to the temporal return of fully marine conditions, possibly because of a short-term sea-level rise. The AOM that occurred in most of the samples from this interval indicates stagnant waters with a low oxygen content in the bottom environments. Even a low-magnitude sea-level rise could induce sea-water circulation and better ventilation at the bottom. The appearance of Batiacasphaera in sample 51 may also reflect an increase in surface sea-water temperatures.
5.2. Terrestrial Palaeoenvironment
Although some sporomorphs are corroded, the palynological analysis provided valuable information about the palaeovegetation, palaeogeography, and palaeoclimate. The degree of pollen grain destruction can also be used as a source of information. For example, some bisaccate pollen grains were heavily corroded, particularly in samples with high marine palynomorph contents (at the lower part of the section; Figure 10). This pollen could have easily been transported over long distances, from both the northern seashores and the Carpathians, and their abundance tends to increase offshore (the so-called ‘Neves effect’). Nevertheless, conifers likely played an important role in the coastal forests. For example, the better-preserved non-bisaccate pollen grains of Tsuga and Sciadopitys were the most likely components of mesophytic forests in the vicinity. In the upper section of the profile (above sample 27), some pollen grains were found in clumps (Ericaceae, Fagus, and Salix), which may also indicate the proximity of their habitats to the coast.
The results of the palynological analysis revealed the presence of mesophytic and wetland vegetation along the Paratethys shoreline during sedimentation. Mesophytic forests were composed of Fagus, Quercus (also thermophilous oaks producing pollen of the fossil-species Quercoidites henricii), Tsuga and other conifers, Carpinus, Tilioideae, and others, with a relatively small admixture of Castaneoideae and other thermophilous taxa. Ulmus, Pterocarya, Carya, Zelkova, Liquidambar, Alnus, Acer, Fraxinus, and Salix probably grew in riparian forests in periodically flooded areas, such as river valleys. Taxodium and/or Glyptostrobus, together with Alnus and Nyssa, were likely elements of swamp forests growing in permanently flooded areas in the vicinity. Similarly, Osmunda and other ferns grew in wet places. Various shrubs of the Ericaceae family, as well as Myrica and Ilex, probably were components of shrub communities. The abundance of ericaceous plants may be related to open areas adjacent to the coastline within coastal peat bogs or heathland-type shrub vegetation and partly with forest undergrowth [102,103]. Herbs were represented mainly by the Cyperaceae, Sparganiaceae/Typhaceae, and Poaceae families, and at least some of them could have grown in freshwater margins (e.g., Cyperaceae, Sparganium, and/or Typha). The presence of numerous pollen grains of Chenopodiaceae coincides with the occurrence of high-salinity habitats, such as seashores, because many of the recent species of this family are halophytes that tolerate salty soils.
The changes in the frequency of the spore–pollen taxa were rather small, and no rapid change in vegetation was observed. Nevertheless, the lower section appeared richer in “palaeotropical” taxa (Ilexpollenites margaritatus, Symplocoipollenites vestibulum, and Tricolporopollenites indeterminatus), although it generally had a high content of marine palynomorphs, and the land elements have had little chance of reaching this location. In contrast, the upper section contained more diverse Pinaceae pollen grains, including Abies and Picea, which can be interpreted as a manifestation of the mountain forest development. Such changes are gradual and modified by the changing sea influences; therefore, a boundary between the levels cannot be set.
Diverse fungal microremains (i.a., Asterosporium, Cephalothecoidomyces, Diporotheca, Phragmothyrites, Potamomyces, and cf. Tetraploa) were found, mainly in the upper part of the profile. Their presence indicated a brackish environment and their proximity to the seashore. Cephalothecoidomyces G. Worobiec, Neumann, and E. Worobiec and Potamomyces K.D. Hyde suggest that abundant decaying wood could have accumulated in humid and swampy places [104,105,106]. Contemporary Diporotheca webbiae D. Hawksworth., B. van Geel, and P. Wiltshire, similar to the fossil specimen from Babczyn, is associated with alder carrs [107]. Therefore, it is probable that the coastal areas, at least in some parts, were overgrown by swamp forests, as also indicated by the studied fossil pollen grains. The co-occurrence of the fossil conidia of the Asterosporium asterospermum (Pers.) Hughes fungus with Fagus pollen grains indicates that beech trees grew close to the seashore [108].
The results of the palynological analysis indicated that the climate during the deposition of the sediments was generally warm temperate (warmer than the present-day climate of Poland), mild (without severe winters), and rather humid. Some fungal taxa (notably Potamomyces and probably Tetraploa) indicated a humid and warm, subtropical climate during this period [104,106,109].
Miocene sediments in Poland have a relatively rich palynological documentation, but most studies come from the Polish Lowlands [110,111]. In contrast, palynological (spore–pollen) studies of the Carpathian Foredeep are rare. Moreover, Badenian–Sarmatian marine sediments that were previously analysed were mainly from the western, Silesian region of the Carpathian Foredeep [110,112,113,114,115]. In addition, palynofloras from several boreholes in the Bochnia and Wieliczka regions [116], including borehole Kłaj 1 [117], as well as from the sulphur deposits at Piaseczno near Tarnobrzeg [102], were studied. Although the frequency of sporomorphs in these deposits was usually low, the samples usually had similar compositions, exhibiting abundant conifers with high levels of Pinus as well as Abies, Tsuga, and Picea. Among the deciduous trees, Quercus, Ulmus, Castanea, Engelhardia, and Fagus played the most significant roles, whereas Carya, Pterocarya, and Tricolporopollenites pseudocingulum (in some cases identified as Rhus) were less important. Shrubs and thermophilous ferns were also relatively frequent. Swampy plants, which are characteristic of continental sediments in the Polish Lowlands, were rare (except for Taxodium/Glyptostrobus) and included only taxa, such as Alnus, Liquidambar, Myrica, and Ilex [103].
The Babczyn palynoflora is very similar to the spore–pollen assemblage from the Jamnica S-119 borehole [103] drilled in the upper Badenian and lower Sarmatian marine deposits near Tarnobrzeg in the northeastern part of the Carpathian Foredeep. The Jamnica palynoflora was dominated by coniferous trees, especially Pinus, as well as Taxodium/Glyptostrobus (identified as Taxodiaceae–Cupressaceae), Tsuga, Abies, Picea, Cedrus, and Sciadopitys, whereas the pollen of Sequoia was identified only in some samples. Among deciduous trees and shrubs, Ulmus, Quercus, Alnus, Carya, Fagus, Ericaceae, Engelhardia, Pterocarya, and Quercoidites henricii were the most frequent. Some samples frequently contained small percentages of tree and shrub taxa, such as Betula, Carpinus, Liquidambar, Salix, Acer, Castanea, Fraxinus, Juglans, Nyssa, Parrotia, Myrica, Symplocos, Cyrillaceae/Clethraceae, Ilex, and Tricolporopollenites pseudocingulum. Pollen grains of herbaceous plants were very rare, with sporadic Chenopodiaceae, Cyperaceae, Poaceae, Lamiaceae, and Nuphar. Similarly, the spores of ferns (Polypodiaceae, Osmunda, and Cyatheaceae–Schizaeaceae) and Sphagnum appeared in very small quantities. The composition of plant communities in the entire Jamnica section was quite homogeneous, and no temporal flora changes were observed. The highest content of pollen material was in the middle part of the Jamnica profile, with more abundant pollen from deciduous trees and herbaceous plants, accompanied by a simultaneous decrease in Pinus, which indicated the more landward position of that part of the profile. Differences in the pollen spectra from Jamnica may suggest a shoreline migration at this level rather than changes in palaeovegetation caused by the climate [103].
Ref. [118] made similar assumptions for the entire Carpathian Region. Their palaeofloristic and palaeoclimatic reconstructions showed that the upper Badenian and lower Sarmatian floras were closer to each other than to the flora of the previous and subsequent stages. The evolutionary process during the late Badenian–early Sarmatian continued the main trends of forest floral and vegetational evolutions, stimulated by the gradual cooling of the climate [119]. At this time, the replacement of the subtropical forest communities by warm temperate and temperate forest communities was observed in the Central Paratethys. In the southeastern part of Ukraine (Eastern Paratethys), temperate forests were replaced by herb communities. This process was evident in all the studied sections, albeit involving spatial differences [118,120,121].
5.3. Implications
Although a consensus exists that at the Badenian–Sarmatian boundary, a sudden decrease in biodiversity and the disappearance of marine foraminifer assemblages in the Central Paratethys occurred (e.g., [122]), there are various hypotheses regarding its drivers. Ref. [123] concluded that this change occurred within a time interval of fewer than 10 kyr and that it was related to a change in the configuration of the Central–Eastern Paratethys gateway (Bârlad Strait). It could also have been related to a minor rise in the global sea level that otherwise affected the Sarmatian transgression in many other basins of the Central Paratethys ([124], with references therein). Ref. [125] suggested a continuous sea level rise in the Eastern Paratethys because of freshwater input by rivers, and, thus, the Sarmatian transgression event could have resulted from a full connection between both Paratethyan basins. It was repeatedly suggested that the transgression was accompanied by a drastic change in water chemistry, exemplified by a supposed change from marine to brackish conditions in the Central Paratethys (cf. [16,126]), which otherwise could be expected considering the inflow of Eastern Paratethyan water to the Central Paratethys.
Ref. [16] concluded that tectonic widening and the deepening of the Bârlad Strait generated an effective water exchange between the two domains. However, our data do not support the brackish-water environments at the onset of the Sarmatian, as envisaged by [16,123,127]. Instead, the domination of the foraminiferal assemblages above the Badenian/Sarmatian boundary by miliolids, which is commonly observed in various parts of the Carpathian Foredeep Basin in Poland [8,14], implies from normal to hypersalinity conditions lower than 50 psu [29,128]. As far as the palynological record on the onset of the Sarmatian is concerned, there are no blooms, or increased frequencies, of dinoflagellate cysts that would benefit from such environmental conditions, like euryhaline Lingulodinium machaerophorum or protoperidinioids. The former species is common in low-salinity waters (e.g., [129]) frequently found in recent sediments of the Black and Caspian Seas [130]. There are no freshwater algae, like Botryococcus and Pediastrum. On the contrary, samples from the Elphidium angulatum Zone yielded blooms of Prasinophyta Leiosphaera, which is known to occur in the Middle Badenian strata associated with hypersaline conditions. Moreover, sample 33 yielded the highest proportion of Polysphaeridium (Figure 9), a genus commonly associated with increased salinity [95].
Dinocyst degradation can be excluded, as the same samples yielded other aquatic palynomorphs. The co-occurrence of Elphidium in those samples can be explained by a larger (compared to samples for the palynological study) rock volume taken from the core, which could also include relatively thin, possibly bioturbated layers with benthic communities that appeared during short periods of better sea bottom ventilation.
The authors in [131] concluded that from fully marine to hypersaline conditions existed in the late Sarmatian, and our results suggest that this conclusion is also valid for the early Sarmatian. This conclusion is based on the foraminiferal and aquatic palynomorph records (Figure 11). Warm and humid climatic conditions indicated by pollen obviously are not in favour of hypersalinity. But this may be related to warm/cold current circulations (there is evidence of such changes in intervals where cold-water N. labyrinthus interlayers with warm-water Batiacasphaera). Moreover, hypersaline conditions could appear independent of climatic conditions, for example, in the cases of basin isolation, the temporary cessation of circulation, and the appearance of stratification. Spore–pollen assemblages record changes in vegetation on land and changes in marine salinity, which will not be evident in these assemblages unless they were caused by a very pronounced change in the climate. Ref. [132] recorded only a slight cooling, and a reduction in humidity was marked at 14–13.5 Ma. No clear change was observed there, only fluctuations with a slight trend, despite an important environmental change related to the mid-Badenian Salinity Crisis [23,133]. The pollen record from marine sediments is not as precise as that from terrestrial/freshwater sediments, e.g., from lignite seams, which is something to be aware of. In the case of Babczyn, the spore–pollen assemblages are not very rich but are sufficient to draw some conclusions. Possible climatic fluctuations here could have been masked by changes in pollen transport and preservation conditions (i.e., water chemistry). Significant climate changes are not evident here though.
6. Conclusions
The qualitative and quantitative analyses of the foraminifera and the aquatic palynomorphs in the Badenian–Sarmatian strata of the Babczyn 2 borehole, one of the key sections in SE Poland, showed that the studied interval accumulated under variable, unstable sedimentary conditions in marine environments.
The dominance of infaunal benthic foraminifera species, mainly buliminids and uvigerinids, throughout the upper Badenian part of the studied interval (samples from 8 to 28) indicates mostly dysoxic and suboxic conditions. For the uppermost Badenian, mesotrophic conditions in surface waters and increased oxygenation of the bottom waters were observed. The Badenian/Sarmatian boundary, as correlated with a sudden extinction of stenohaline foraminifera, is placed just above sample 28 (within the lowermost part of the Syndesmya Beds). The overlying sample (29) is almost barren of foraminifera, and the lack of benthic forms may be explained by dysoxic conditions at the bottom of the sea. Euryhaline early Sarmatian benthic foraminiferal assemblages indicate a salinity increase, while the lack of planktonic foraminifera suggests a shallowing of the sea.
An acme of N. labyrinthus just below the Badenian/Sarmatian boundary may be the result of a temperature drop in sea-surface waters. The interval that occurred just above (samples 29 and 31) was characterised by a lack of dinoflagellate cysts and the flowering of Leiosphaeridiaceae, suggesting hypersaline conditions associated with stagnant, possibly stratified waters that led to anoxic conditions in the bottom waters, as evidenced by AOM. The subsequent cessation of these hypersaline conditions was caused by a possible sea-level rise and the gradual return of a less-saline water regime. The higher interval (samples 35–55) was deposited under more restricted and variable environmental conditions, including a brackish environment, as indicated by diverse fungal microremains found in the upper part of the profile.
The results of the spore–pollen analysis revealed the presence of mesophytic and wetland vegetation along shoreline of the Paratethys during sedimentation and indicated that the climate during the deposition of the sediments was generally warm temperate, mild, and rather humid.
Author Contributions
Conceptualisation, D.P. and T.M.P.; methodology, D.P., P.G., E.W. and G.W.; validation, D.P., P.G., E.W. and G.W.; formal analysis, D.P., P.G., E.W., G.W. and T.M.P.; investigation, D.P., P.G., E.W., G.W. and T.M.P.; resources, D.P.; data curation, D.P., P.G., E.W. and G.W.; writing—original draft preparation, D.P., P.G., E.W., G.W. and T.M.P.; writing—review and editing, T.M.P., P.G., D.P., E.W. and G.W.; visualisation, D.P., P.G., E.W., G.W. and T.M.P.; supervision, D.P.; project administration, D.P.; funding acquisition, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland, grant number UMO-2017/27/B/ST10/01129.
Data Availability Statement
The data presented in this study are available on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Laskarev, V. Sur les equivalentes du Sarmatien supérieur en Serbie. In Recueil de Traveaux Ofert a M. Jovan Cvijic par ses amis et Collaborateurs; Vujević, P., Ed.; Državna Štamparija: Beograd, Serbia, 1924; pp. 73–85. [Google Scholar]
- Harzhauser, M.; Piller, W.E. Benchmark data of a changing sea—palaeogeography, palaeobiogeography and events in the Central Paratethys during the Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 253, 8–31. [Google Scholar] [CrossRef]
- Śliwiński, M.; Bąbel, M.; Nejbert, K.; Olszewska-Nejbert, D.; Gąsiewicz, A.; Schreiber, B.C.; Be-Nowitz, J.A.; Layer, P. Badenian–Sarmatian chronostratigraphy in the Polish Carpathian Foredeep. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 326–328, 12–29. [Google Scholar] [CrossRef]
- Palcu, D.V.; Kouwenhoven, T.J.; Krijgsman, W. Reply to “Comment on the Badenian-Sarmatian extinction event in the Carpathian foredeep basin of Romania: Paleogeographic changes in the Paratethys (Palcu et al., 2015)” by Silye and Filipescu (2016). Glob. Planet. Chang. 2016, 145, 141–142. [Google Scholar] [CrossRef]
- Silye, L.; Filipescu, S. Comment on “The Badenian-Sarmatian Extinction Event in the Carpathian foredeep basin of Romania: Paleogeographic changes in the Paratethys domain” (Palcu et al., 2015). Glob. Planet. Chang. 2016, 145, 17–19. [Google Scholar] [CrossRef]
- Peryt, T.M.; Kasprzyk, A. Carbonate-evaporite sedimentary transitions in the Badenian (middle Miocene) basin of southern Poland. Sediment. Geol. 1992, 76, 257–271. [Google Scholar] [CrossRef]
- Peryt, D.; Gedl, P.; Peryt, T.M. Marine transgression(s) to evaporite basin: The case of middle Miocene (Badenian) gypsum in the Central Paratethys, SE Poland. J. Palaeogeogr. 2020, 9, 16. [Google Scholar] [CrossRef]
- Peryt, D.; Garecka, M.; Peryt, T.M. Foraminiferal and calcareous nannoplankton biostratigraphy of the upper Badenian–lower Sarmatian strata in the SE Polish Carpathian Foredeep. Geol. Quart. 2021, 65, 18. [Google Scholar] [CrossRef]
- Łuczkowska, E. Foraminiferal zones in the Miocene, south of the Holy Cross Mts. Bull. Acad. Pol. Sci. Sér. Sci. Géol. Géogr. 1963, 11, 29–34. [Google Scholar]
- Łuczkowska, E. The micropaleontological stratigraphy of the Miocene in the region of Tarnobrzeg-Chmielnik (in Polish with English summary). Pr. Geol. 1964, 20, 1–72. [Google Scholar]
- Łuczkowska, E. A new zone with Praeorbulina indigena (Foraminiferida, Globigerinidae) in the Upper Badenian (Tortonian s.s.) of Central Paratethys. Roczn. Polsk. Tow. Geol. 1971, 40, 445–448. [Google Scholar]
- Odrzywolska-Bieńkowa, E. Micropaleontological stratigraphy of the Miocene of central part of the Carpathian Foredeep (in Polish with English summary). Prz. Geol. 1975, 23, 597–603. [Google Scholar]
- Czepiec, I. Biostratigraphy and paleoenvironment of Sarmatian marginal zone of Poland (in Polish with English summary). Kwart. AGH Geologia 1996, 22, 309–338. [Google Scholar]
- Kirchner, Z. Miocene stratigraphy of the Central Carpathian Foreland based on microfaunal studies (in Polish with English summary). Acta Geol. Pol. 1956, 6, 421–450. [Google Scholar]
- Cicha, I.; Rögl, F.; Rupp, C.; Čtyroká, J. (Eds.) Oligocene-Miocene foraminifera of the Central Paratethys. Abh. Senckenberg. Naturforsch. Ges. 1998, 549, 1–325. [Google Scholar]
- Palcu, D.V.; Golovina, L.A.; Vernyhorova, Y.V.; Popov, S.V.; Krijgsman, W. Middle Miocene paleoenvironmental crises in Central Eurasia caused by changes in marine gateway configuration. Glob. Planet. Chang. 2017, 158, 57–71. [Google Scholar] [CrossRef]
- Odrzywolska-Bieńkowa, E. Porównanie wybranych głębszych profile mikrofaunistycznych rejonu świętokrzyskiego i lubelskiego (In Polish). Sprawozd. Posiedz. Kom. Nauk. PAN Kraków 1972, 16, 493–494. [Google Scholar]
- Filipescu, S. Anomalinoides dividens bioevent at the Badenian/Sarmatian boundary—a response to paleogeographic and paleoenvironmental changes. Studia Univ. Babeş-Bolyai Geologia 2004, 49, 21–26. [Google Scholar] [CrossRef]
- Dumitriu, S.D.; Loghin, S.; Dubicka, Z.; Melinte-Dobrinescu, M.C.; Paruch-Kulczycka, J.; Ionesi, V. Foraminiferal, ostracod, and calcareous nannofossil biostratigraphy of the latest Badenian—Sarmatian interval (Middle Miocene, Paratethys) from Poland, Romania and the Republic of Moldova. Geol. Carpath. 2017, 68, 419–444. [Google Scholar] [CrossRef]
- Ney, R.; Burzewski, W.; Bachleda, T.; Górecki, W.; Jakóbczak, K.; Słupczyński, K. Outline of paleogeography and evolution of lithology and facies of Neogene layers on the Carpathian Foredeep (in Polish with English summary). Prace Geol. 1974, 82, 3–65. [Google Scholar]
- Dziadzio, P.; Maksym, A.; Olszewska, B. Miocene deposition in the eastern part of the Carpathian Foredeep in Poland (In Polish with English summary). Prz. Geol. 2006, 54, 413–420. [Google Scholar]
- Alexandrowicz, S.W.; Garlicki, A.; Rutkowski, J. Podstawowe jednostki litostratygraficzne miocenu zapadliska przedkarpackiego (In Polish). Kwart. Geol. 1982, 26, 470–471. [Google Scholar]
- Peryt, T.M. The beginning, development and termination of the Middle Miocene Badenian salinity crisis in Central Paratethys. Sediment. Geol. 2006, 188–189, 379–396. [Google Scholar] [CrossRef]
- de Leeuw, A.; Tulbure, M.; Kuiper, K.F.; Melinte-Dobrinescu, M.C.; Stoica, M.; Krijgsman, W. New 40Ar/39Ar, magnetostratigraphic and biostratigraphic constraints on the termination of the Badenian Salinity Crisis: Indications for tectonic improvement of basin interconnectivity in Southern Europe. Glob. Planet. Chang. 2018, 169, 1–15. [Google Scholar] [CrossRef]
- Pawłowska, K.; Kubica, B. Karta otworu wiertniczego Babczyn-2 (in Polish; unpublished); Archive CUG: Warszawa, Poland, 1960. [Google Scholar]
- Jasionowski, M.; Peryt, T.M. Historia Badań. In Budowa geologiczna Polski, tom I. Stratygrafia, część 3a Kenozoik Paleogen Neogen; Peryt, T.M., Piwocki, M., Eds.; Polish Geological Institute: Warszawa, Poland, 2004; pp. 203–212. [Google Scholar]
- Czapowski, G. Wschodnia Część Zapadliska. In Budowa geologiczna Polski, tom I. Stratygrafia, część 3a Kenozoik Paleogen Neogen; Peryt, T.M., Piwocki, M., Eds.; Polish Geological Institute: Warszawa, Poland, 2004; pp. 239–245. [Google Scholar]
- Buzas, H.A.; Gibson, T.G. Species diversity: Benthonic Foraminifera in Western North Atlantic. Science 1969, 163, 72–75. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; p. 426. [Google Scholar]
- Murray, J.W. Ecology and Palaeoecology of Benthic Foraminifera; Routledge: London, UK, 1991; p. 408. [Google Scholar]
- Van der Zwaan, G.J. Paleoecology of late Miocene Mediterranean Foraminifera. Utrecht Micropaleont. Bull. 1982, 25. [Google Scholar]
- Corliss, B.; Chen, C. Morphotype patterns of Norwegian Sea deep-sea benthic foraminifera and ecological implication. Geology 1988, 16, 716–719. [Google Scholar] [CrossRef]
- Culver, S. New foraminiferal depth zonation of the northwestern Gulf of Mexico. Palaios 1988, 3, 69–85. [Google Scholar] [CrossRef]
- Lutze, G.F.; Thiel, H. Epibenthic foraminifera from elevated microhabitats; Cibicidoides wuellerstorfi and Planulina ariminensis. J. Foram. Res. 1989, 19, 153–158. [Google Scholar] [CrossRef]
- Verhallen, P. Late Pliocene to early Pleistocene Mediterranean mud-dwelling foraminifera; influence of a changing environment on community structure and evolution. Utrecht Micropaleont. Bull. 1991, 40, 1–219. [Google Scholar]
- Sjoerdsma, P.G.; van der Zwaan, G.J. Simulating the effect of changing oceanic flux and oxygen content on the distribution of benthic foraminifers. Marine Micropaleont. 1992, 19, 163–180. [Google Scholar] [CrossRef]
- Langer, M. Epiphytic foraminifera. Mar. Micropaleont. 1993, 20, 235–265. [Google Scholar] [CrossRef]
- Hayward, B.W.; Hollis, C.J.; Grenfell, H.R. Recent Elphidiidae (Foraminiferida) of the South-west Pacific and fossil Elphidiidae of New Zealand. Inst. Geol. Nucl. Sci. Mon. 1997, 16, 170. [Google Scholar]
- Kaiho, K. Effect of organic carbon flux and dissolved oxygen on the benthic foraminiferal oxygen index (BFOI). Mar. Micropaleont. 1999, 37, 67–76. [Google Scholar] [CrossRef]
- Schönfeld, J. A new benthic foraminiferal proxy for near-bottom current velocities in the Gulf of Cadiz, northeastern Atlantic Ocean. Deep-Sea Res. I 2002, 49, 1853–1875. [Google Scholar] [CrossRef]
- Hohenegger, J. Estimation of environmental paleogradient values based on presence/absence data: A case study using benthic foraminifera for paleodepth estimation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 217, 115–130. [Google Scholar] [CrossRef]
- Kouwenhoven, T.J.; van der Zwaan, G.J. A reconstruction of late Miocene Mediterranean circulation patterns using benthic foraminifera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 238, 373–385. [Google Scholar] [CrossRef]
- Kaminski, M.A. Calibration of the Benthic Foraminiferal Oxygen Index in the Marmara Sea. Geol. Quart. 2012, 56, 757–764. [Google Scholar] [CrossRef][Green Version]
- Schiebel, R.; Hemleben, C. Planktic Foraminifers in the Modern Ocean; Springer: Berlin, Germany, 2017; p. 358. [Google Scholar]
- Jorissen, F.; Nardelli, M.P.; Almogi-Labin, A.; Barras, C.; Bergamin, L.; Bicchi, E.; El Kateb, A.; Ferraro, L.; McGann, M.; Morigi, C.; et al. Developing Foram-AMBI for biomonitoring in the Mediterranean: Species assignments to ecological categories. Mar. Micropaleont. 2018, 140, 33–45. [Google Scholar] [CrossRef]
- Dumitriu, S.D.; Dubicka, Z.; Loghin, S.; Melinte-Dobrinescu, M.C.; Paruch-Kulczycka, J. The evolution of the Carpathian Foredeep Basin during the latest Badenian and Sarmatian (Middle Miocene): Inferences from micropalaeontological data. Geol. Quart. 2020, 64, 1004–1022. [Google Scholar] [CrossRef]
- Cavaliere, M.; Scipioni, V.; Francescangeli, F.; Ferraro, L.; Frontalini, F. Paleoenvironmental changes in the Gulf of Gaeta (Central Tyrrhenian Sea, Italy): A perspective from benthic foraminifera after dam construction. Water 2023, 15, 815. [Google Scholar] [CrossRef]
- Kiss, P.; Hudáčková, N.; Titschack, J.; Siccha, M.G.R.; Heřmanová, Z.; Silye, L.; Ruman, A.; Rybár, S.; Kučera, M. Convergent evolution of spherical shells in Miocene planktonic foraminifera documents the parallel emergence of a complex character in response to environmental forcing. Paleobiology 2023, 49, 454–470. [Google Scholar] [CrossRef]
- Van der Zwaan, G.J.; Duijnstee, I.A.P.; den Dulk, M.; Ernst, S.R.; Jannink, N.T.; Kouwenhoven, T.J. Benthic foraminifers: Proxies or problems? A review of paleoecological concepts. Earth-Sci. Rev. 1999, 46, 213–236. [Google Scholar] [CrossRef]
- Van Hinsbergen, D.J.J.; Kouwenhoven, T.J.; van der Zwaan, G.J. Paleobathymetry in the backstripping procedure: Correction for oxygenation effects on depth estimates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 221, 245–265. [Google Scholar] [CrossRef]
- Holcová, K.; Zágoršek, K. Bryozoa, foraminifera and calcareous nannoplankton as environmental proxies of the "bryozoan event" in the Middle Miocene of the Central Paratethys (Czech Republic). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 267, 216–234. [Google Scholar] [CrossRef]
- Hart, M.B.; Fisher, J.K.; Smart, C.W.; Speers, R.; Wall-Palmer, D. Re-colonization of hostile environments by benthic foraminifera: An example from Montserrat, Lesser Antilles Volcanic Arc. Micropaleontology 2022, 69, 1–27. [Google Scholar] [CrossRef]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Ważyńska, H.; Słodkowska, B.; Sadowska, A. Atlas of Pollen and Spores of the Polish Neogene. Vol. 1—Spores; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2001; p. 158. [Google Scholar]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Ważyńska, H.; Sadowska, A. Atlas of Pollen and Spores of the Polish Neogene. Vol. 2—Gymnosperms; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; p. 237. [Google Scholar]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Słodkowska, B.; Ważyńska, H.; Sadowska, A. Atlas of pollen and spores of the Polish Neogene. Vol. 3—Angiosperms (1); W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2009; p. 233. [Google Scholar]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Słodkowska, B.; Worobiec, E.; Durska, E. Atlas of pollen and spores of the Polish Neogene. Vol. 4—Angiosperms (2); W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2014; p. 466. [Google Scholar]
- Nalepka, D.; Walanus, A. Data processing in pollen analysis. Acta Palaeobot. 2003, 43, 125–134. [Google Scholar]
- Corliss, B. Microhabitats of benthic foraminifera within deep-sea sediments. Nature 1985, 314, 435–438. [Google Scholar] [CrossRef]
- Schiebel, R.; Bijma, J.; Hemleben, C. Population dynamics of the planktic foraminifer Globigerina bulloides from the eastern North Atlantic. Deep-Sea Res. I 1997, 44, 1701–1713. [Google Scholar] [CrossRef]
- Schiebel, R.; Hemleben, C. Modern planktonic foraminifera. Paläont. Zt. 2005, 79, 135–148. [Google Scholar] [CrossRef]
- Caron, M. Cretaceous planktic foraminifera. In Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perch-Nielsen, K., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 17–86. [Google Scholar]
- Hemleben, C.; Spindler, M.; Anderson, O.R. Modern Planktonic Foraminifera. Springer: New York, NY, USA, 1989; p. 363. [Google Scholar]
- Hebbeln, D.; Marchant, M.; Wefer, G. Seasonal variations of the particle flux in the Peru-Chile Current at 30°S under normal and El Nino conditions. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 2101–2128. [Google Scholar] [CrossRef]
- Petrizzo, M.R. Palaeoceanographic and palaeoclimatic inferences from Late Cretaceous planktonic foraminiferal assemblages from the Exmouth Plateau (ODP Sites 762 and 763, eastern Indian Ocean). Mar. Micropaleontol. 2002, 45, 117–150. [Google Scholar] [CrossRef]
- Kretschmer, K.; Jonkers, L.; Kucera, M.; Schulz, M. Modeling seasonal and vertical habitats of planktonic foraminifera on a global scale. Biogeosciences 2018, 15, 4405–4429. [Google Scholar] [CrossRef]
- Di Stefano, A.; Verducci, M.; Lirer, F.; Ferraro, L.; Iaccarino, S.M.; Hüsing, S.K.; Hilgen, F.J. Paleoenvironmental conditions preceding the Messinian Salinity Crisis in the Central Mediterranean: Integrated data from the Upper Miocene Trave section (Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 37–53. [Google Scholar] [CrossRef]
- Bé, A.W.H.; Tolderlund, D.S. Distribution and ecology of living planktonic foraminifera in surface waters of the Atlantic and Indian Oceans. In The Micropaleontology of Oceans; Funnell, B.M., Riedel, W.R., Eds.; Cambridge University Press: Cambridge, UK, 1971; pp. 105–149. [Google Scholar]
- Bé, A.W.H. An ecological zoogeographic and taxonomic review of recent planktonic foraminifera. In The Micropaleontology of Oceans; Ramsay, A.T.S., Ed.; Academic Press: London, UK, 1977; pp. 1–100. [Google Scholar]
- Thunell, R.; Reynolds-Sautter, L. Planktonic foraminiferal faunal and stable isotopic indices of upwelling: A sediment trap study in the San Pedro Basin, Southern California Bight. Geol. Soc. Spec. Publ. 1992, 64, 77–91. [Google Scholar] [CrossRef]
- Darling, K.F.; Wade, C.M.; Siccha, M.; Trommer, G.; Schulz, H.; Abdolalipour, S.; Atsushi Kurasawa, A. Genetic diversity and ecology of the planktonic foraminifers Globigerina bulloides, Turborotalita quinqueloba and Neogloboquadrina pachyderma off the Oman margin during the late SW Monsoon. Mar. Micropaleont. 2017, 137, 64–77. [Google Scholar] [CrossRef]
- Jorissen, F.J.; de Stigter, H.C.; Widmark, J.G.V. A conceptual model explaining benthic foraminiferal microhabitats. Mar. Micropaleont. 1995, 26, 3–15. [Google Scholar] [CrossRef]
- Robert, C.M. Global Sedimentology of the Ocean: An Interplay between Geodynamics and Paleoenvironment; Elsevier: Amsterdam, The Netherlands, 2008; p. 496. [Google Scholar]
- Rodrigues, A.R.; Gómez Pivel, M.A.; Schmitt, P.; de Almeida, F.K.; Bonetti, C. Infaunal and epifaunal benthic foraminifera species as proxies of organic matter paleofluxes in the Pelotas Basin, south-western Atlantic Ocean. Mar. Micropaleont. 2018, 144, 38–49. [Google Scholar] [CrossRef]
- Kováč, M.; Márton, E.; Oszczypko, N.; Vojtko, R.; Hók, J.; Králiková, S.; Plašienka, D.; Klučiar, T.; Hudáčková, N.; Oszczypko-Clowes, M. Neogene palaeogeography and basin evolution of the Western Carpathians, Northern Pannonian domain and ad joining areas. Glob. Planet. Chang. 2017, 155, 133–154. [Google Scholar] [CrossRef]
- Bukowski, K.; de Leeuw, A.; Gonera, M.; Kuiper, K.F.; Krzywiec, P.; Peryt, D. Badenian tuffite levels within the Carpathian orogenic front (Gdów–Bochnia area, Southern Poland): Radio-isotopic dating and stratigraphic position. Geol. Quart. 2010, 54, 449–464. [Google Scholar]
- Hess, S.; Kuhnt, W. Deep-sea benthic foraminiferal recolonization of the 1991 Mt Pinatubo ash layer in the South China Sea. Mar. Micropaleont. 1996, 28, 171–197. [Google Scholar] [CrossRef]
- Hess, S.; Kuhnt, W.; Hill, S.; Kaminski, M.A.; Holbourn, A.; de Leon, M. Monitoring the recolonization of the Mt Pinatubo 1991 ash layer by benthic foraminifera. Mar. Micropaleont. 2001, 43, 119–142. [Google Scholar] [CrossRef]
- Debenay, J.-P.; Geslin, E.; Eichler, B.B.; Duleba, W.; Sylvestre, F.; Eichler, P. Foraminiferal assemblages in a hypersaline lagoon, Araruama (R.J.) Brazil. J. Foram. Res. 2001, 31, 133–151. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species, 2024. Available online: https://www.marinespecies.org (accessed on 3 January 2024).
- Chaloner, W.G.; Muir, M. Spores and floras. In Coal and Coal-Bearing Strata; Murchison, D.G., Westoll, T.S., Eds.; Oliver and Boyd: Edinburgh, UK, 1968; pp. 127–146. [Google Scholar]
- Streel, M.; Richelot, C. Wind and water transport and sedimentation of miospores along two rivers subject to major floods and entering the Mediterranean Sea at Calvi (Corsica, France). In Sedimentation of Organic Particles; Traverse, A., Ed.; Cambridge University Press: Cambridge, UK, 1994; pp. 59–67. [Google Scholar]
- Brinkhuis, H. Late Eocene to Early Oligocene dinoflagellate cysts from the Priabonian type-area (northeast Italy): Biostratigraphy and palaeoenvironmental interpretation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 107, 121–163. [Google Scholar] [CrossRef]
- Stover, L.E.; Brinkhuis, H.; Damassa, S.P.; de Verteuil, L.; Helby, R.J.; Monteil, E.; Partridge, A.D.; Powell, A.J.; Riding, J.B.; Smelror, M.; et al. Mesozoic–Tertiary dinoflagellates, acritarchs and prasinophytes. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Dallas TX, USA, 1996; Volume 2, pp. 641–750. [Google Scholar]
- Sluijs, A.; Pross, J.; Brinkhuis, H. From greenhouse to icehouse; organic-walled dinoflagellate cysts as paleoenvironmental indicators in the Paleogene. Earth-Sci. Rev. 2005, 68, 281–315. [Google Scholar] [CrossRef]
- Williams, G.L. Dinoflagellate cysts, their classification, biostratigraphy and palaeoecology. In Oceanic Micropalaeontology; Ramsay, A.T.S., Ed.; Academic Press: London, UK, 1977; pp. 1231–1325. [Google Scholar]
- Köthe, A. Paleogene dinoflagellates from northwest Germany. Geol. Jb. R. A 1990, 118, 1–111. [Google Scholar]
- Gedl, P. Middle Miocene dinoflagellate cysts from the Korytnica clays (Góry Świętokrzyskie Mountains, Poland). Ann. Soc. Geol. Pol. 1996, 66, 191–218. [Google Scholar]
- Schreck, M.; Matthiessen, J. Batiacasphaera micropapillata: Palaeobiogeographic distribution and palaeoecological implications of a critical Neogene species complex. Micropalaeont. Soc. Spec. Publ. 2013, 5, 301–314. [Google Scholar]
- Gedl, P. Palynofacies of the Miocene deposits in the Gliwice area (Upper Silesia, Poland). Bull. Pol. Acad. Sci. Earth Sci. 1997, 45, 191–201. [Google Scholar]
- Rochon, A.; de Vernal, A.; Turon, J.L.; Matthiessen, J.; Head, M.J. Distribution of recent dinoflagellate cysts in surface sediments from the North Atlantic Ocean and adjacent seas in relation to sea-surface parameters. Am. Assoc. Strat. Palynol. Found. Contrib. Ser. 1999, 35, 146. [Google Scholar]
- Schreck, M.; Meheust, M.; Stein, R.; Matthiessen, J. Response of marine palynomorphs to Neogene climate cooling in the Iceland Sea (ODP Hole 907A). Mar. Micropaleont. 2013, 101, 49–67. [Google Scholar] [CrossRef]
- Gedl, P. Palaeoenvironmental and sedimentological interpretations of the palynofacies analysis of the Miocene deposits from the Jamnica S-119 borehole (Carpathian Foredeep, Poland). Geol. Quart. 1999, 43, 479–492. [Google Scholar]
- Guy-Ohlson, D. Prasinophycean algae. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; Volume 1, pp. 181–189. [Google Scholar]
- Gedl, P. Dinoflagellate cysts and palynofacies from the upper Badenian (Middle Miocene) of the Roztocze area at Józefów and Żelebsko (Carpathian Foredeep Basin, Poland): Palaeoenvironmental implications. Ann. Soc. Geol. Pol. 2016, 86, 273–289. [Google Scholar] [CrossRef][Green Version]
- Bradford, M.R.; Wall, D.A. The distribution of Recent organic-walled dinoflagellate cysts in the Persian Gulf, Gulf of Oman, and northeastern Arabian Sea. Palaeontogr. Abt. B 1984, 192, 16–84. [Google Scholar]
- Gedl, P.; Peryt, D. Dinoflagellate cyst, palynofacies and foraminiferal records of environmental changes related to the Late Badenian (Middle Miocene) transgression at Kudryntsi (western Ukraine). Ann. Soc. Geol. Pol. 2011, 81, 331–349. [Google Scholar]
- Peryt, D.; Gedl, P.; Peryt, T.M. Foraminiferal and palynological records of the Late Badenian (Middle Miocene) transgression in Podolia (Shchyrets near Lviv, western Ukraine). Geol. Quart. 2014, 58, 465–484. [Google Scholar] [CrossRef]
- Gedl, P.; Peryt, D.; Peryt, T.M. Foraminiferal and palynological organic matter records of the Upper Badenian (Middle Miocene) deposits at Anadoly (marginal part of the Ukrainian Carpathian Foredeep Basin). Geol. Quart. 2016, 60, 517–536. [Google Scholar] [CrossRef]
- Head, M.J. Neogene occurrences of the marine acritarch genus Nannobarbophora Habib and Knapp, 1982 emend., and the new species N. gedlii. J. Paleont. 2003, 77, 382–385. [Google Scholar] [CrossRef]
- Gedl, P. In situ and recycled dinoflagellate cysts from Middle Miocene deposits at Bęczyn, Carpathian Foredeep, Poland. Studia Geol. Pol. 2005, 124, 371–394. [Google Scholar]
- Jiménez-Moreno, G.; Head, M.J.; Harzhauser, M. Early and Middle Miocene dinoflagellate cyst stratigraphy of the Central Paratethys, Central Europe. J. Micropaleont. 2006, 25, 113–139. [Google Scholar] [CrossRef]
- Oszast, J. The Miocene vegetation of a sulphur bed at Piaseczno near Tarnobrzeg (Southern Poland) (in Polish with English summary). Acta Palaeobot. 1967, 8, 3–29. [Google Scholar]
- Sadowska, A. Sarmatian palynoflora from Jamnica near Tarnobrzeg (Carpathian Foredeep)—environmental and climatic implications. Geol. Quart. 1999, 43, 493–498. [Google Scholar]
- Schlütz, F.; Shumilovskikh, L.S. On the relation of Potamomyces armatisporus to the fossil form-type Mediaverrunites and its taxonomical and ecological implications. Fung. Ecol. 2013, 6, 309–315. [Google Scholar] [CrossRef]
- Worobiec, G.; Neumann, F.H.; Worobiec, E.; Nitz, V.; Hartkopf-Fröder, C. New fungal cephalothecoid-like fructifications from central European Neogene deposits. Fung. Biol. 2017, 121, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Worobiec, G.; Worobiec, E.; Gedl, P.; Kasiński, J.R.; Peryt, D.; Widera, M. Terrestrial-aquatic wood-inhabiting ascomycete Potamomyces from the Miocene of Poland. Acta Palaeontol. Pol. 2022, 67, 737–744. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; van Geel, B.; Wiltshire, P.E. The enigma of the Diporotheca palynomorph. Rev. Palaeobot. Palynol. 2016, 235, 94–98. [Google Scholar] [CrossRef]
- Worobiec, G.; Worobiec, E.; Gedl, P.; Kowalski, R.; Peryt, D.; Tietz, O. Fossil history of fungus host-specificity: Association of conidia of fossil Asterosporium asterospermum with macro- and microremains of Fagus. Fungal Biol. 2023, 127, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Worobiec, E.; Worobiec, G.; Gedl, P. Occurrence of fossil bamboo pollen and a fungal conidium of Tetraploa cf. aristata in Upper Miocene deposits of Józefina (Poland). Rev. Palaeobot. Palynol. 2009, 157, 211–217. [Google Scholar] [CrossRef]
- Grabowska, I.; Słodkowska, B. Katalog Profili Osadów Trzeciorzędowych Opracowanych Palinologicznie (In Polish); PIG: Warszawa, Poland, 1993; p. 80. [Google Scholar]
- Ważyńska, H. Introduction. Pr. Państw. Inst. Geol. 1998, 160, 5–8. [Google Scholar]
- Oszast, J. Pollen analysis of Tortonian clays from Stare Gliwice in Upper Silesia, Poland (In Polish with English summary). Monogr. Botan. 1960, 9, 1–47. [Google Scholar]
- Dyjor, S.; Sadowska, A. Problem of the Badenian-Sarmatian boundary at Stara Kuźnia region near Kędzierzyn (Silesia) in the light of palinological investigations (In Polish with English summary). Acta Palaeobot. 1984, 24, 27–51. [Google Scholar]
- Sadowska, A. Former palynological studies of the Tertiary in the western part of Carpathian Foredeep in Poland (In Polish with English summary). Prz. Geol. 1996, 44, 1039–1042. [Google Scholar]
- Sadowska, A. Miocene palynology in the Gliwice Region (Upper Silesia), Poland. Bull. Pol. Acad. Sci. Earth Sci. 1997, 45, 203–210. [Google Scholar]
- Durska, E. The Badenian Salinity Crisis in the palynological record: Vegetation during the evaporative event (Carpathian Foredeep, southern Poland). Ann. Soc. Geol. Pol. 2017, 87, 213–228. [Google Scholar] [CrossRef][Green Version]
- Kita, Z. Palynological analysis of Tortonian deposits from the borehole Kłaj 1 (East of Kraków) (In Polish with English summary). Roczn. Polsk. Tow. Geol. 1963, 33, 517–526. [Google Scholar]
- Syabryaj, S.V.; Stuchlik, L. Palaeofloristic and palaeoclimatic reconstruction on the territories of Ukraine and Poland during the Badenian-Sarmatian. Acta Palaeobot. 2004, 44, 55–68. [Google Scholar]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Syabryaj, S.V.; Stuchlik, L. Development of flora and vegetation of the Ukrainian Eastern Carpathians and Polish Western Carpathians in the Neogene. Acta Palaeobot. 1994, 34, 165–194. [Google Scholar]
- Kvaček, Z.; Kováč, M.; Kovar-Eder, J.; Doláková, N.; Jechorek, H.; Parashiv, V.; Kováčová, M.; Sliva, L. Miocene evolution of landscape and vegetation in the Central Paratethys. Geol. Carpath. 2006, 57, 295–310. [Google Scholar]
- Hudáčková, N.; Ruman, A.; Plašienková, I.; Jamrich, M.; Halásová, E.; Kiss, P.; Kováčová, M.; Babejová-Kmecová, J.; Kováč, M. Badenian/Sarmatian foraminifera shift in the Central Paratethys: Two methods comparison—A morphogroup and species approach. In Proceedings of the Geologica Carpathica 70 Conference, Smolenice Castle, Slovakia, 9–11 October 2019; pp. 144–145. [Google Scholar]
- Palcu, D.V.; Tulbure, M.; Bartol, M.; Kouwenhoven, T.J.; Krijgsman, W. The Badenian-Sarmatian Extinction Event in the Carpathian foredeep basin of Romania: Paleogeographic changes in the Paratethys domain. Glob. Planet. Chang. 2015, 133, 346–358. [Google Scholar] [CrossRef]
- Nováková, P.; Rybár, S.; Šarinová, K.; Nagy, A.; Hudáčková, N.; Jamrich, M.; Teodoridis, V.; Kováčová, M.; Šujan, M.; Vlček, T.; et al. The late Badenian-Sarmatian (Serravallian) environmental transition calibrated by sequence stratigraphy (eastern Danube Basin, Central Paratethys). Geol. Carpath. 2020, 71, 291–313. [Google Scholar] [CrossRef]
- Popov, S.V.; Antipov, M.P.; Zastrozhnov, A.S.; Kurina, E.E.; Pinchuk, T.N. Sea-level fluctuations on the northern shelf of the Eastern Paratethys in the Oligocene–Neogene. Stratigr. Geol. Correl. 2010, 18, 200–224. [Google Scholar] [CrossRef]
- Simon, D.; Palcu, D.; Meijer, P.; Krijgsman, W. The sensitivity of middle Miocene paleoenvironments to changing marine gateways in Central Europe. Geology 2019, 47, 35–38. [Google Scholar] [CrossRef]
- Liu, S.; Krijgsman, W.; Dekkers, M.J.; Palcu, D. Early diagenetic greigite as an indicator of paleosalinity changes in the middle Miocene Paratethys Sea of central Europe. Geochem. Geophys. Geosyst. 2017, 18, 2634–2645. [Google Scholar] [CrossRef]
- Brasier, M.D. The ecology and distribution of Recent foraminifera from the reefs and shoals around Barbuda, West Indies. J. Foram. Res. 1975, 5, 193–210. [Google Scholar] [CrossRef]
- Ellegaard, M.; Lewis, J.; Harding, I. Cyst-theca relationship, life cycle, and effects of temperature and salinity on the cyst morphology of Gonyaulax baltica sp. nov. (Dinophyceae) from the Baltic Sea area. J. Phycol. 2002, 38, 775–789. [Google Scholar] [CrossRef]
- Mudie, P.J.; Marret, F.; Mertens, K.; Shumilovskikh, L.; Leroy, S.A.G. Atlas of modern dinoflagellate cyst distributions in the Black Sea Corridor: From Aegean to Aral Seas, including Marmara, Black, Azov and Caspian Seas. Mar. Micropaleont. 2017, 134, 1–152. [Google Scholar] [CrossRef]
- Piller, W.E.; Harzhauser, M. The myth of the brackish Sarmatian Sea. Terra Nova 2005, 17, 450–455. [Google Scholar] [CrossRef]
- Ivanov, D.; Worobiec, E. Middle Miocene (Badenian) vegetation and climate dynamics in Bulgaria and Poland based on pollen data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 467, 83–94. [Google Scholar] [CrossRef]
- de Leeuw, A.; Bukowski, K.; Krijgsman, W.; Kuiper, K.F. Age of the Badenian salinity crisis; impact of Miocene climate variability on the circum-Mediterranean region. Geology 2010, 38, 715–718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).