Bradoriids (Arthropoda) and the Cambrian Diversification
Abstract
1. Introduction
2. Bradoriid Diversification
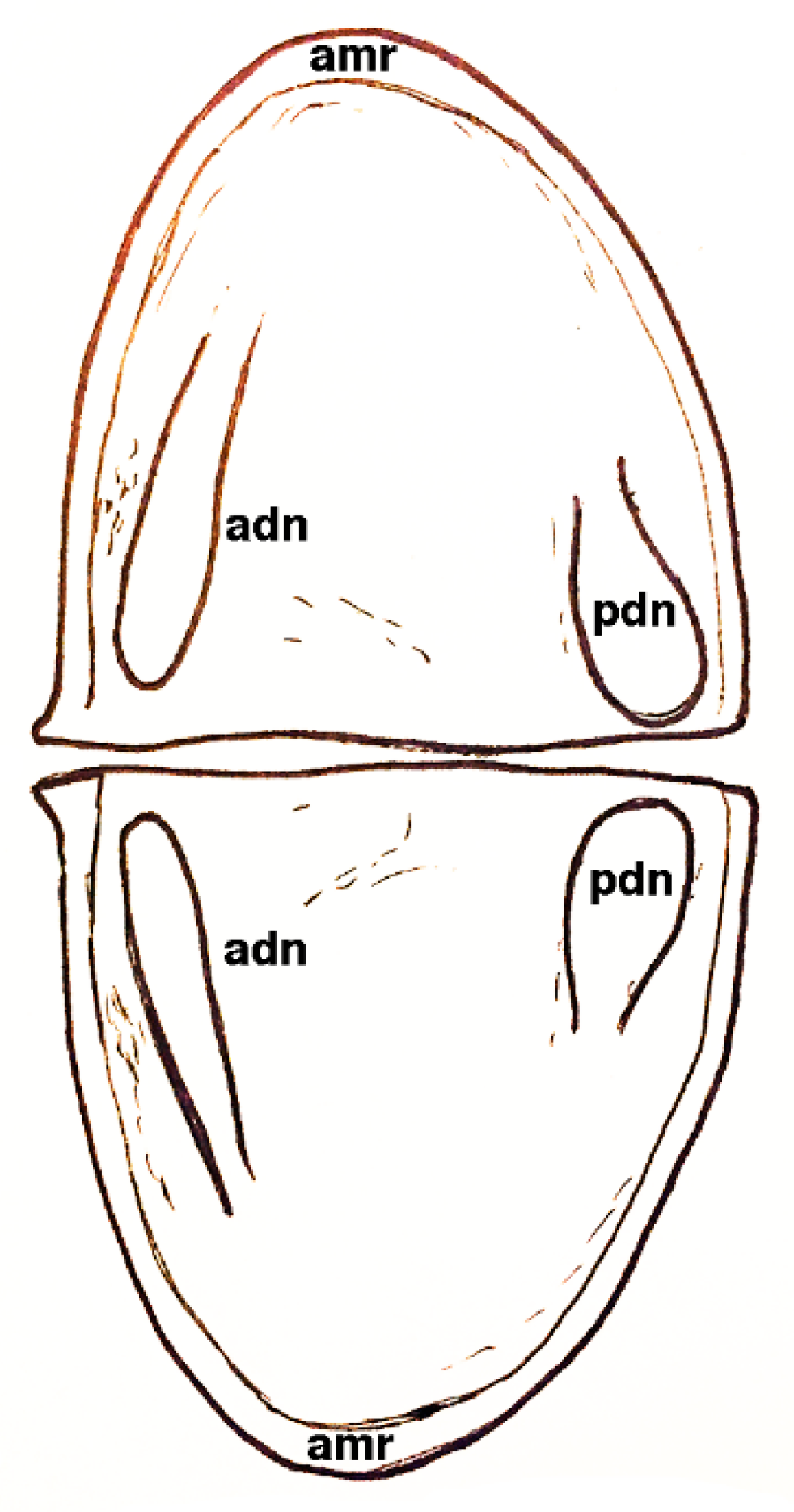
3. Cephalic Appendages in Bradoriids
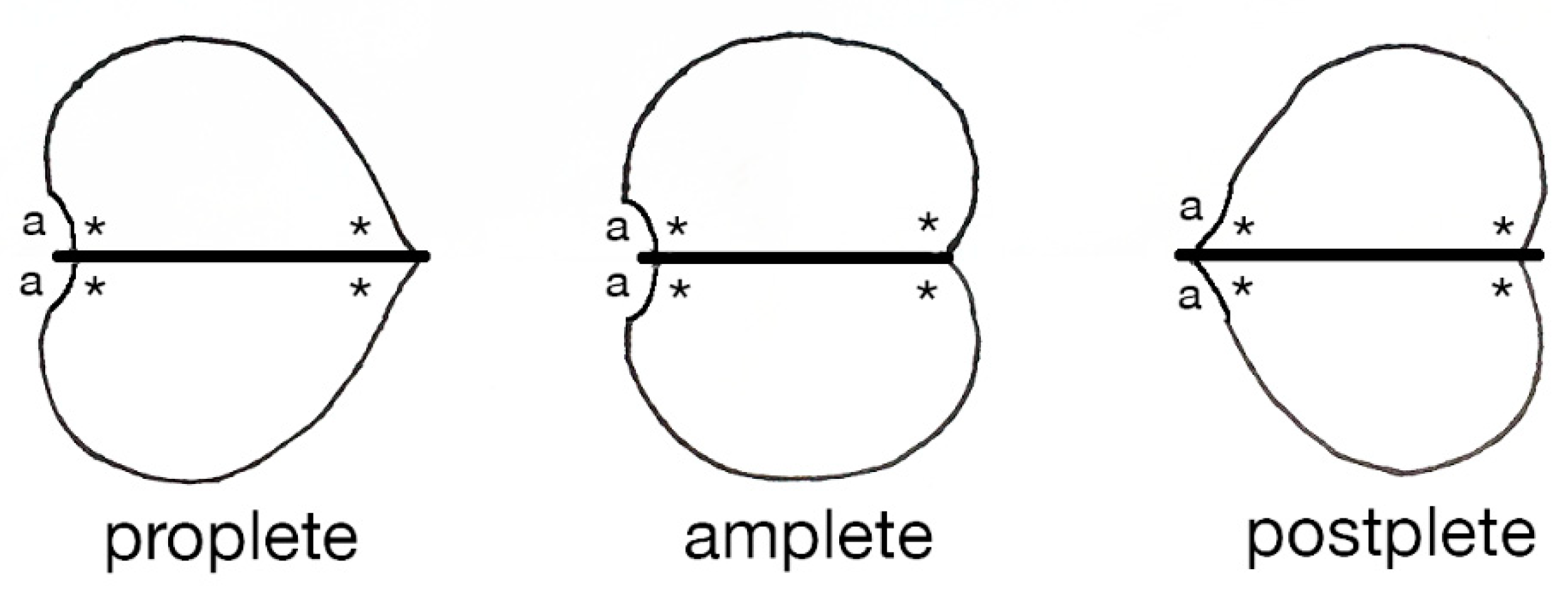
4. Bradoriid Locomotion
5. Bradoriid Developmental Biology
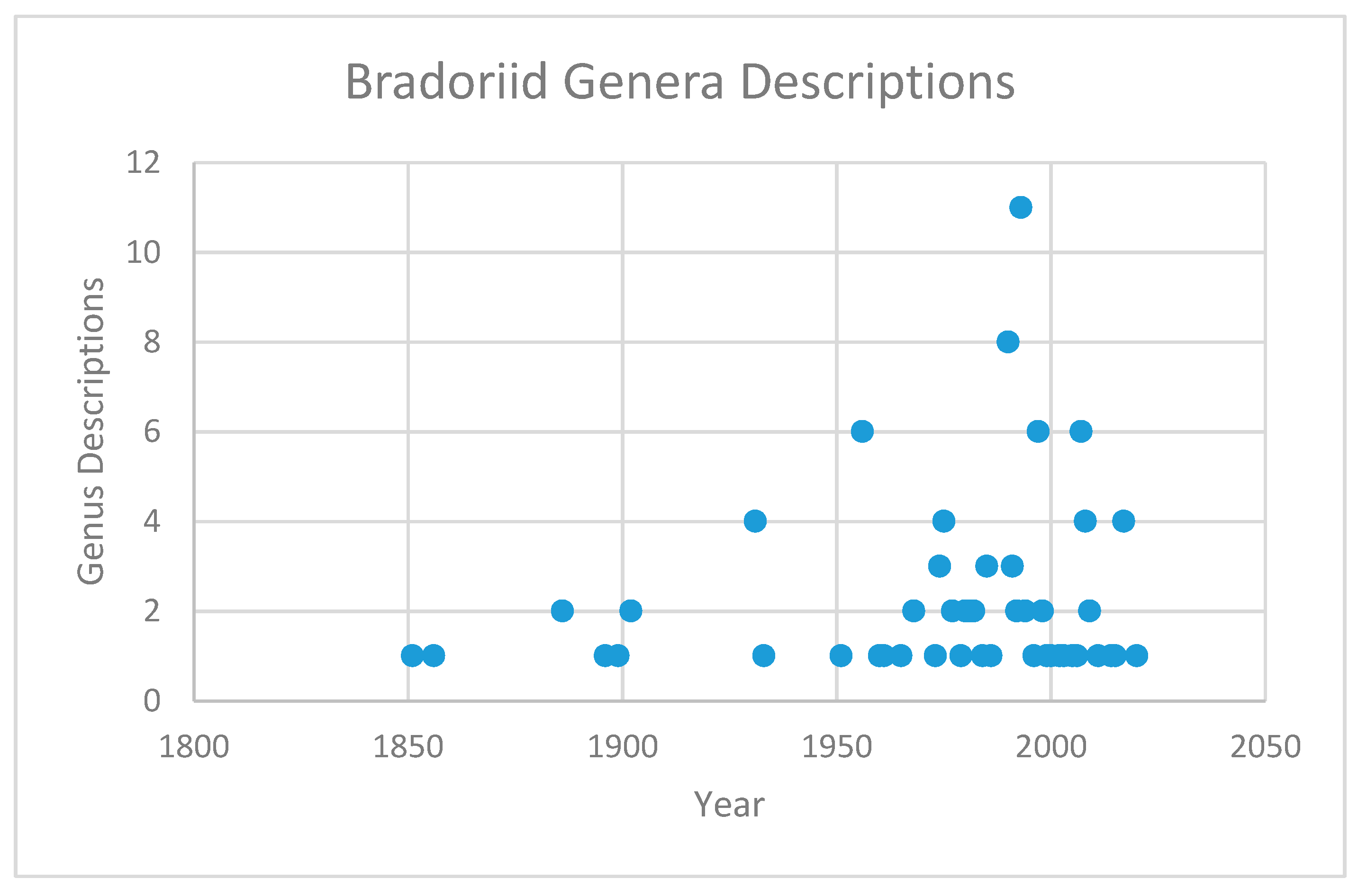
6. Bradoriid Genera
7. Bradoriid Paleobiogeography
8. Systematic Paleontology
| Phylum Euarthropoda Lankester, 1904 [117] |
| Class unknown |
| Order Bradoriida Raymond, 1935 [1] |
| Family Hipponicharionidae Sylvester-Bradley, 1961 [7] |
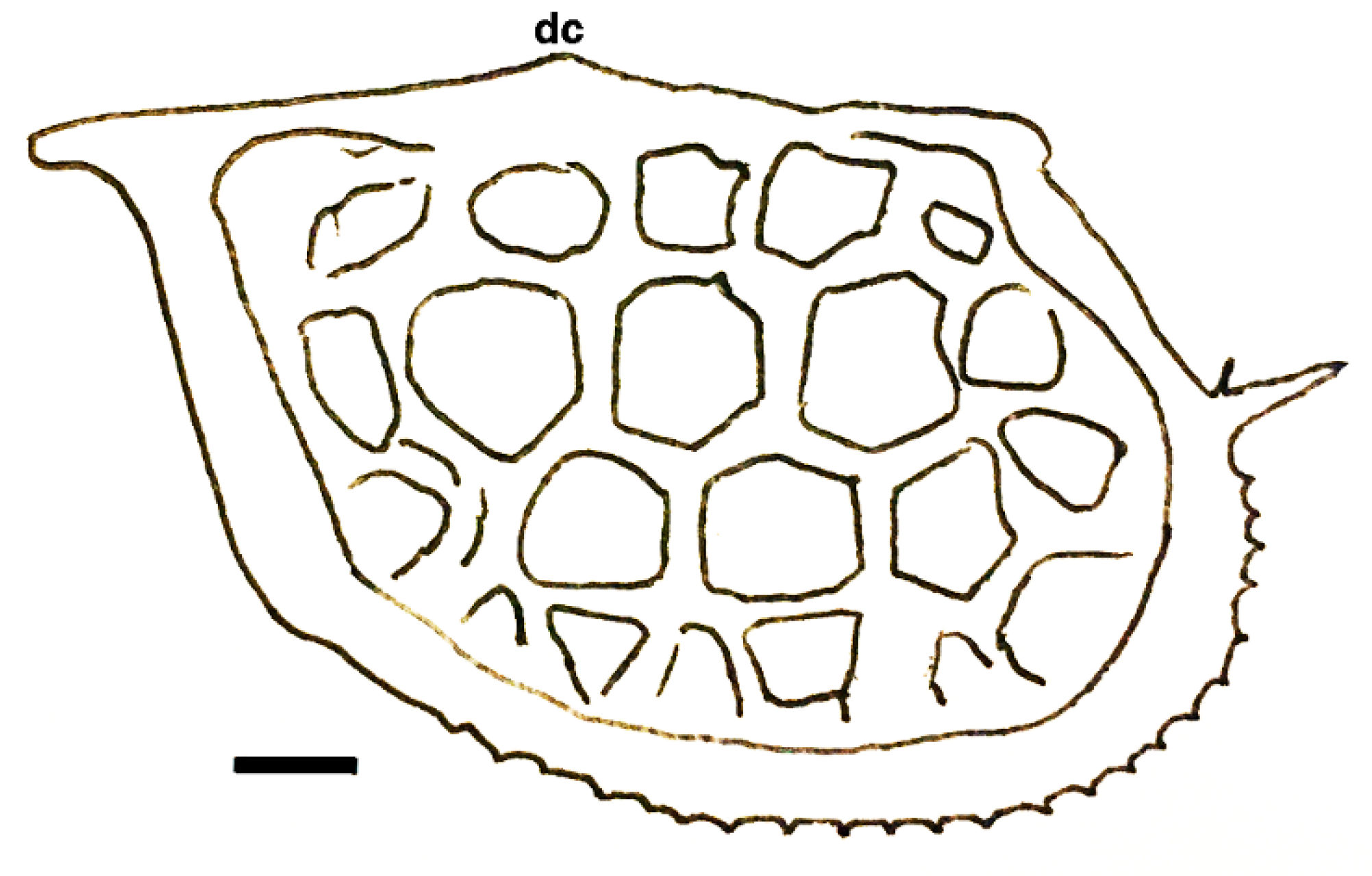
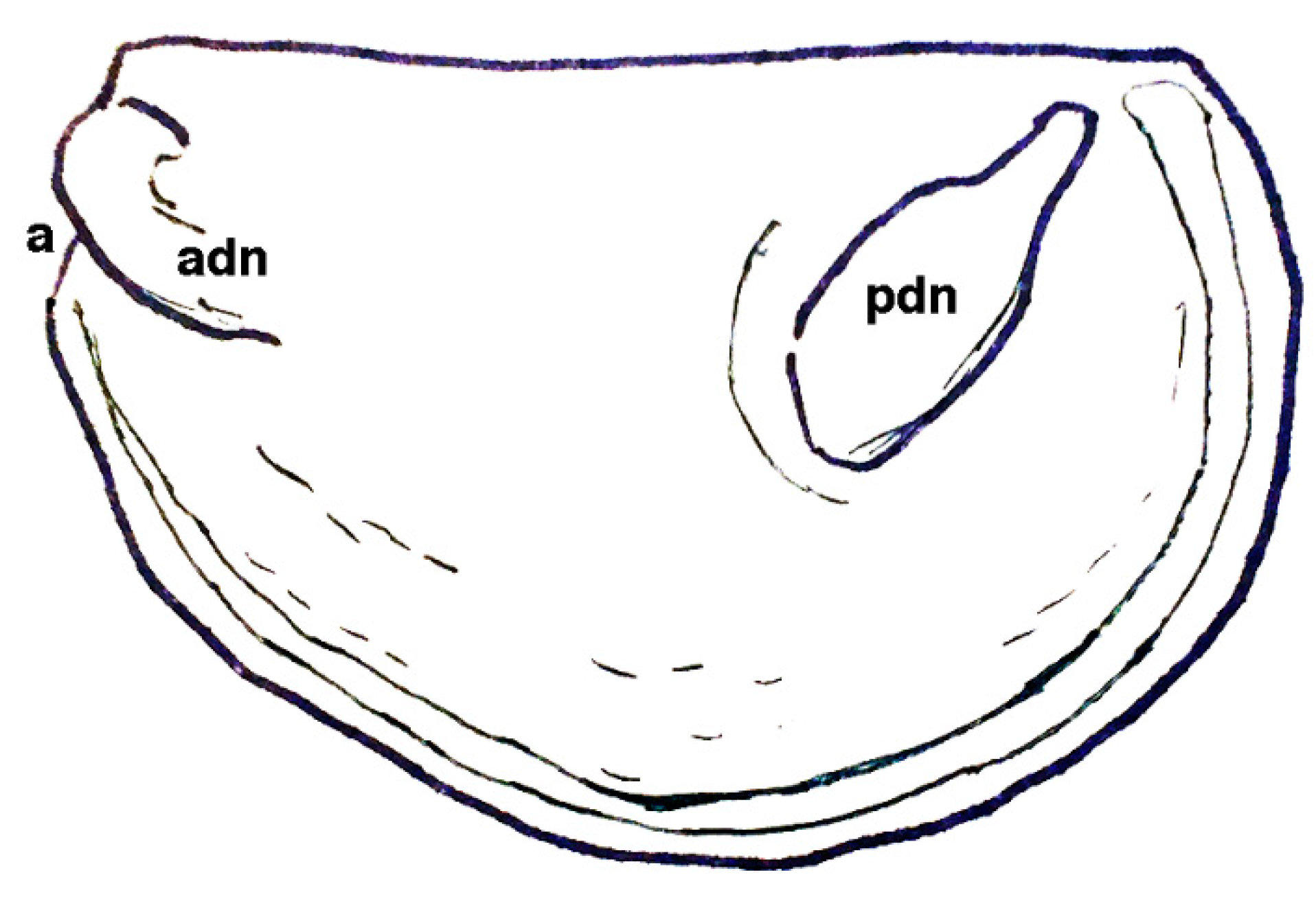
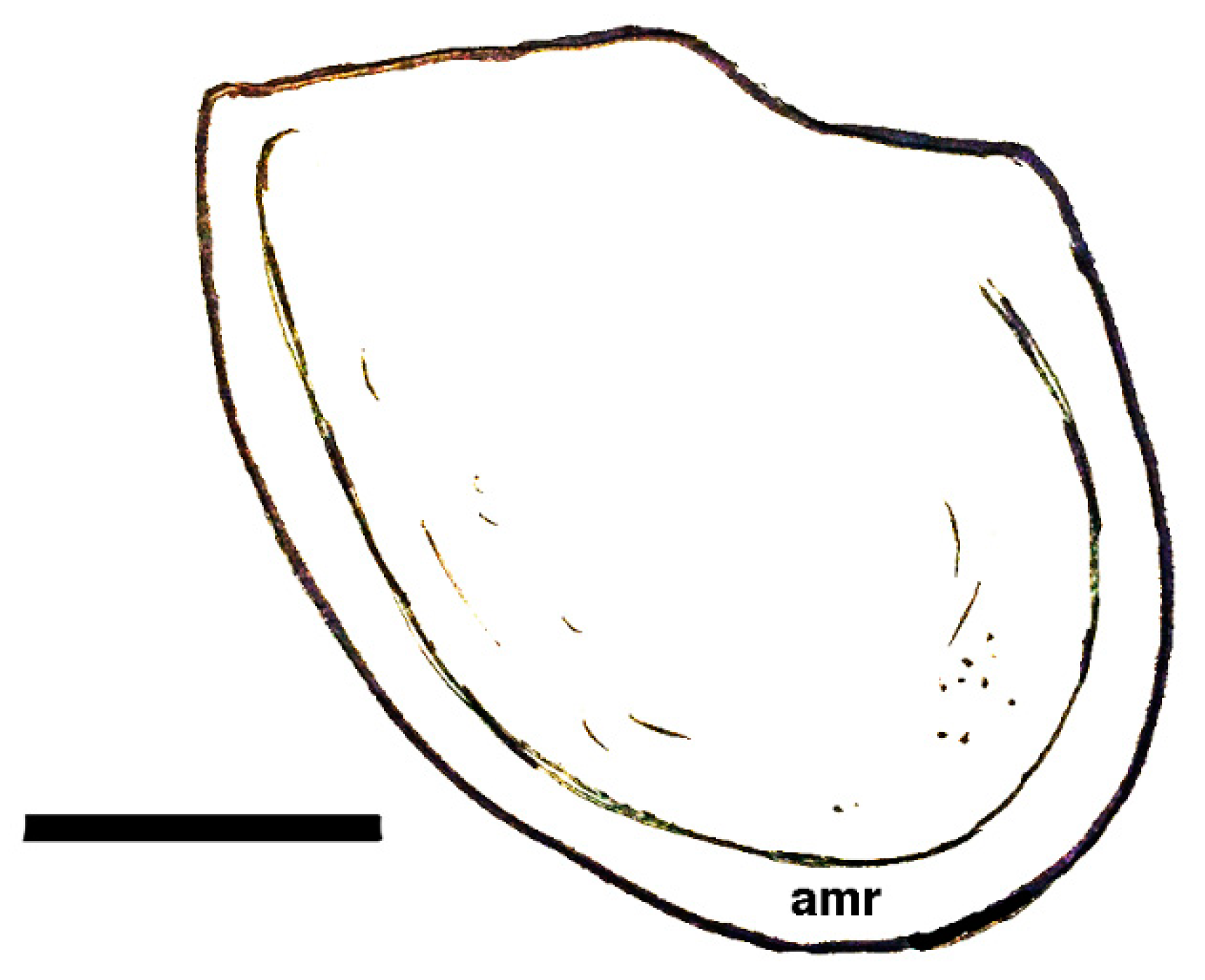
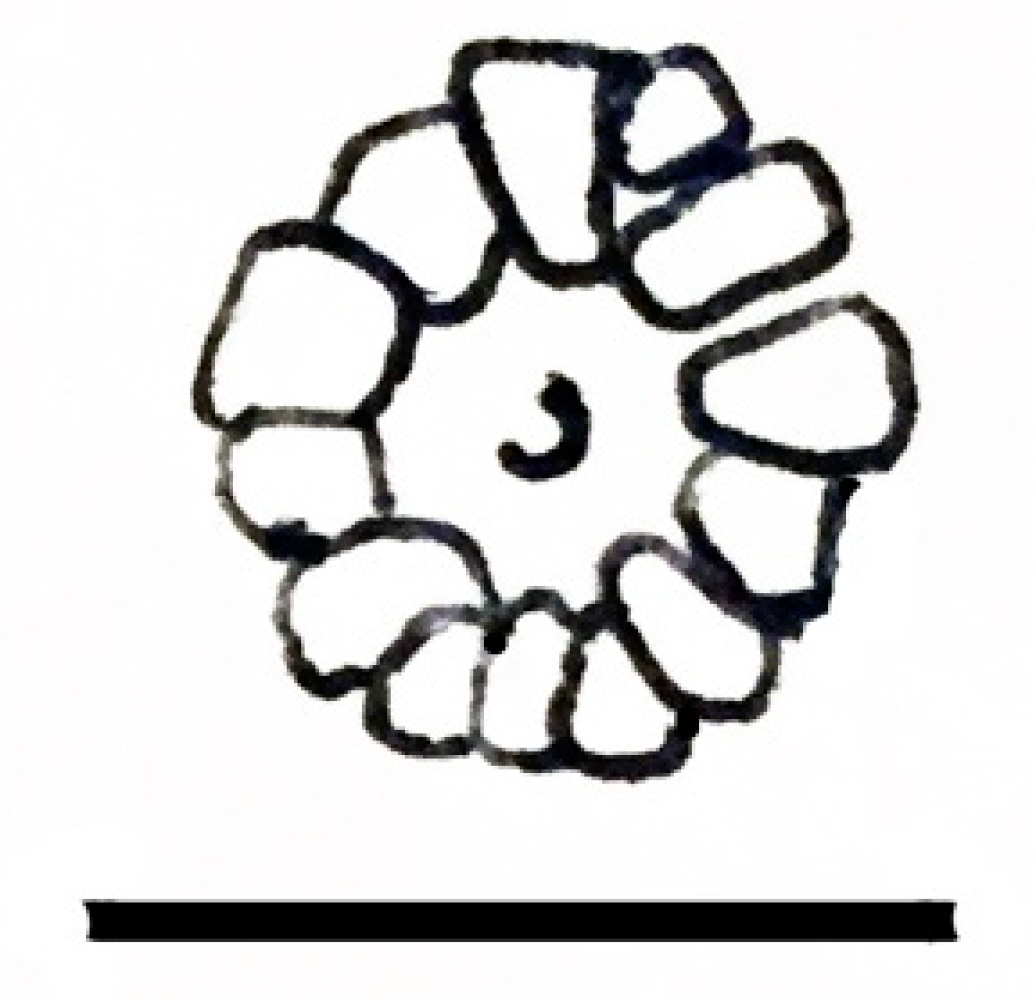
| Genus Cambroarchilocus nov. gen. |
| Ἀσπίδι μὲν Σαΐων τις ἀγάλλεται, ἥν παρὰ θάμνῳ ἔντος ἀμώμητον κάλλιπον οὐκ ἐθέλων· αὐτὸν δ’ ἔκ μ’ ἐσάωσα· τί μοι μέλει ἀσπὶς ἐκείνη; Ἐρρέτω· ἐξαῦτις κτήσομαι οὐ κακίω. One of the Thracians now delights in the shield I discarded Unwillingly near a bush, for it was perfectly fine, But at least I safely escaped. Why should I pine for that shield? Let it go. I’ll later find another one as good. | [118] |
| ostracod cf. Bradoria | McMenamin and McMenamin 1990 [119] |
| phosphatic ostracod | McMenamin et al. 1994 [120] |
| hipponicharionid | Siveter and Williams 1997 [20] |
| bradoriid ostracod | McMenamin 2001 [121] |
| bradoriid | Zhang 2007 [106] |
| ?Albrunnicola | Streng et al. 2008 [69] |
| ?Albrunnicola | Peel 2017 [31] |
| Family unknown |
| Genus Dielymella Ulrich and Bassler, 1931 [66] |
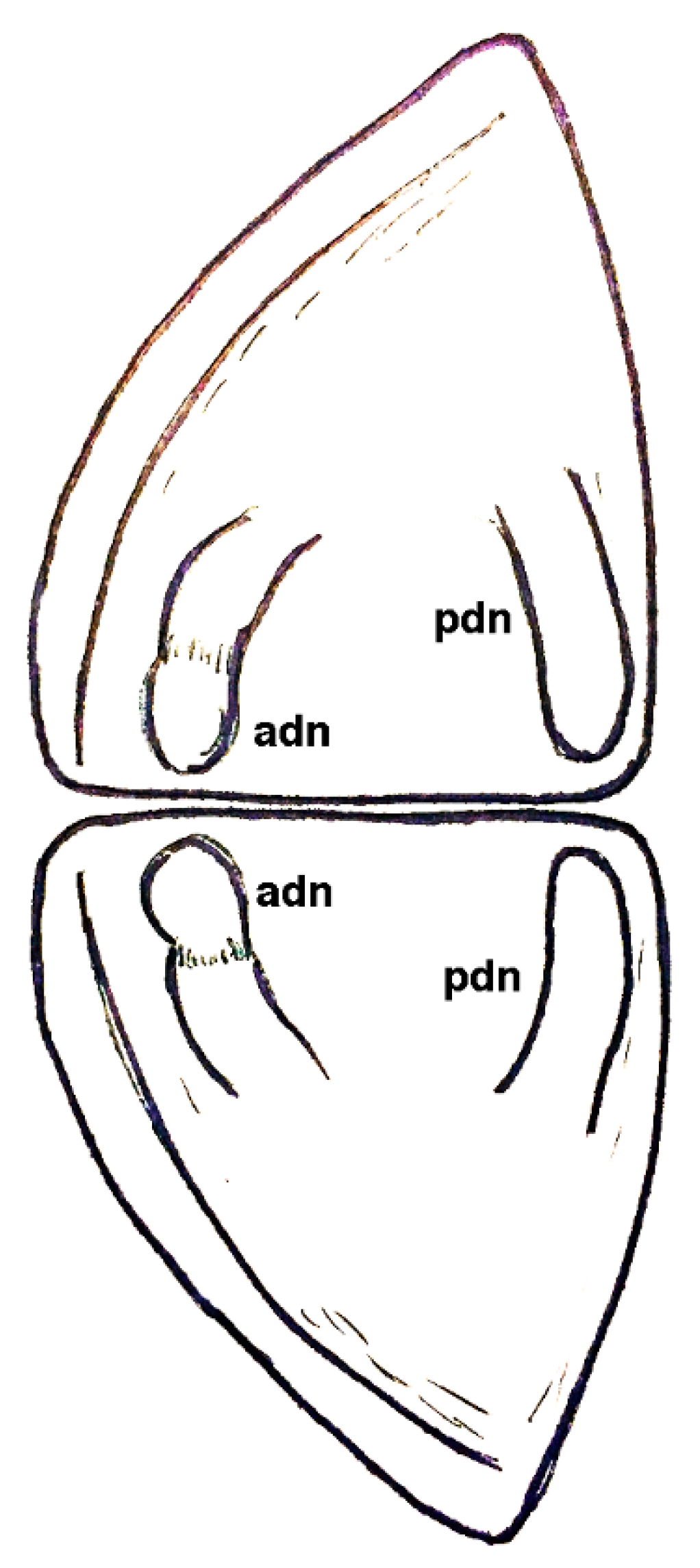
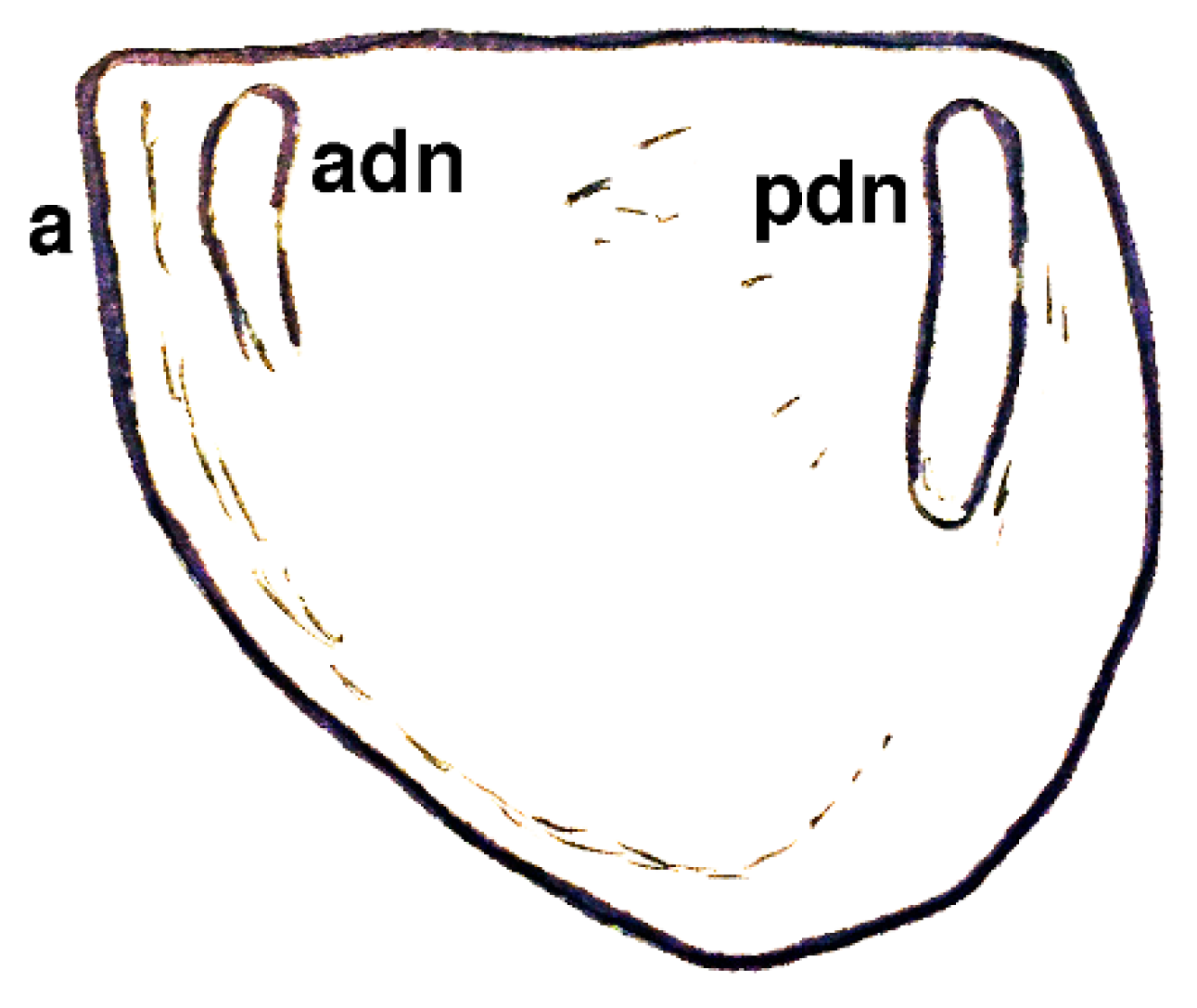
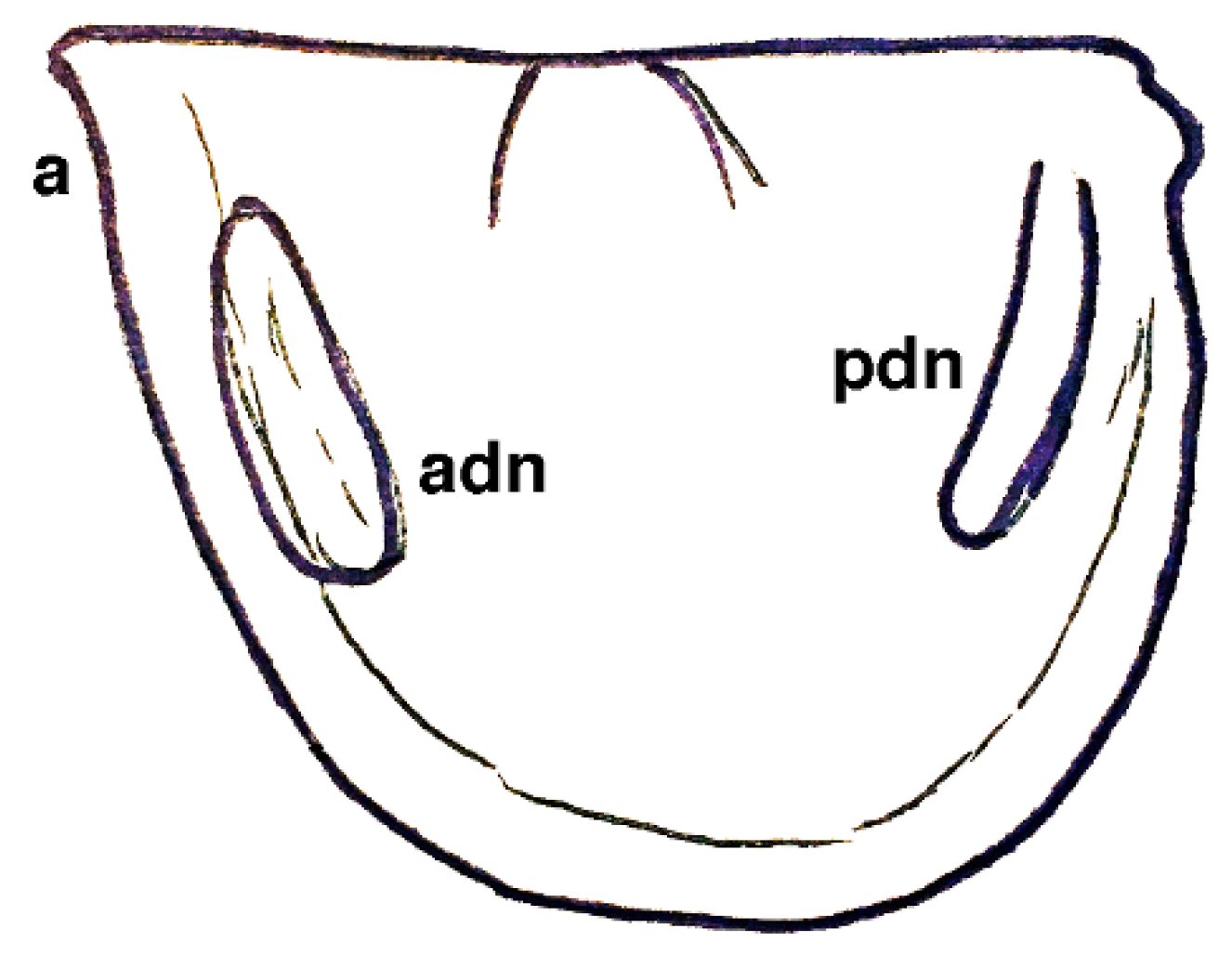
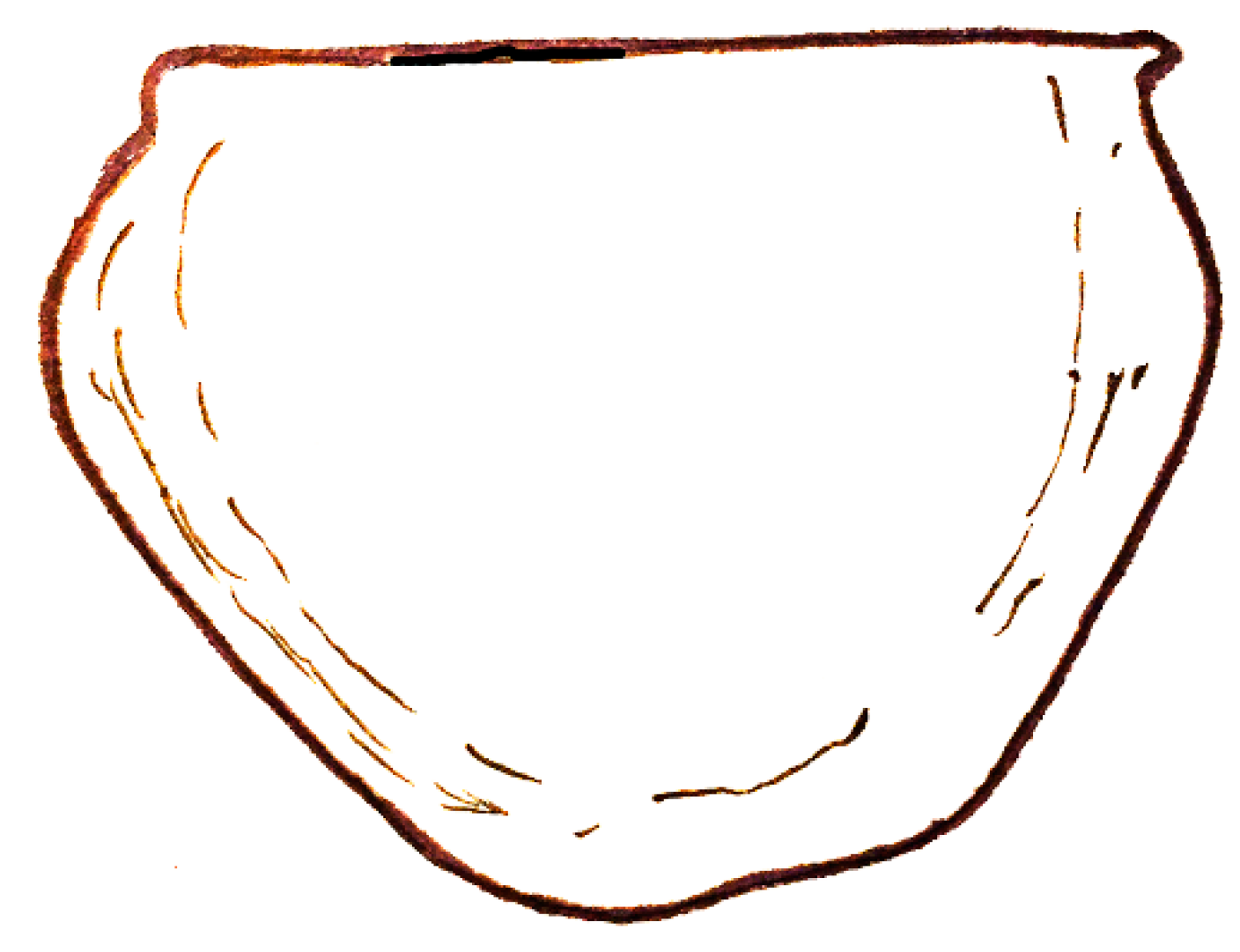
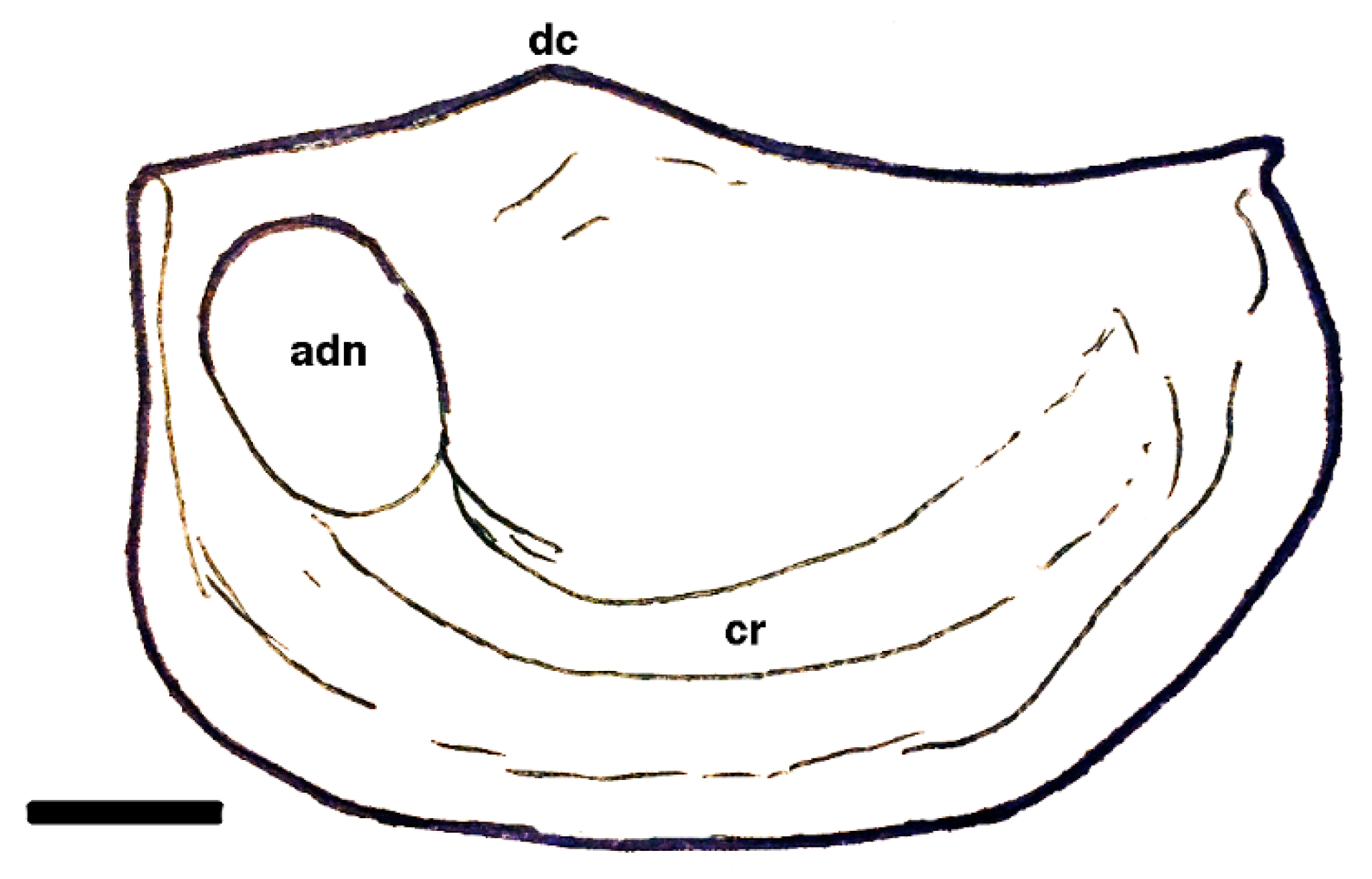
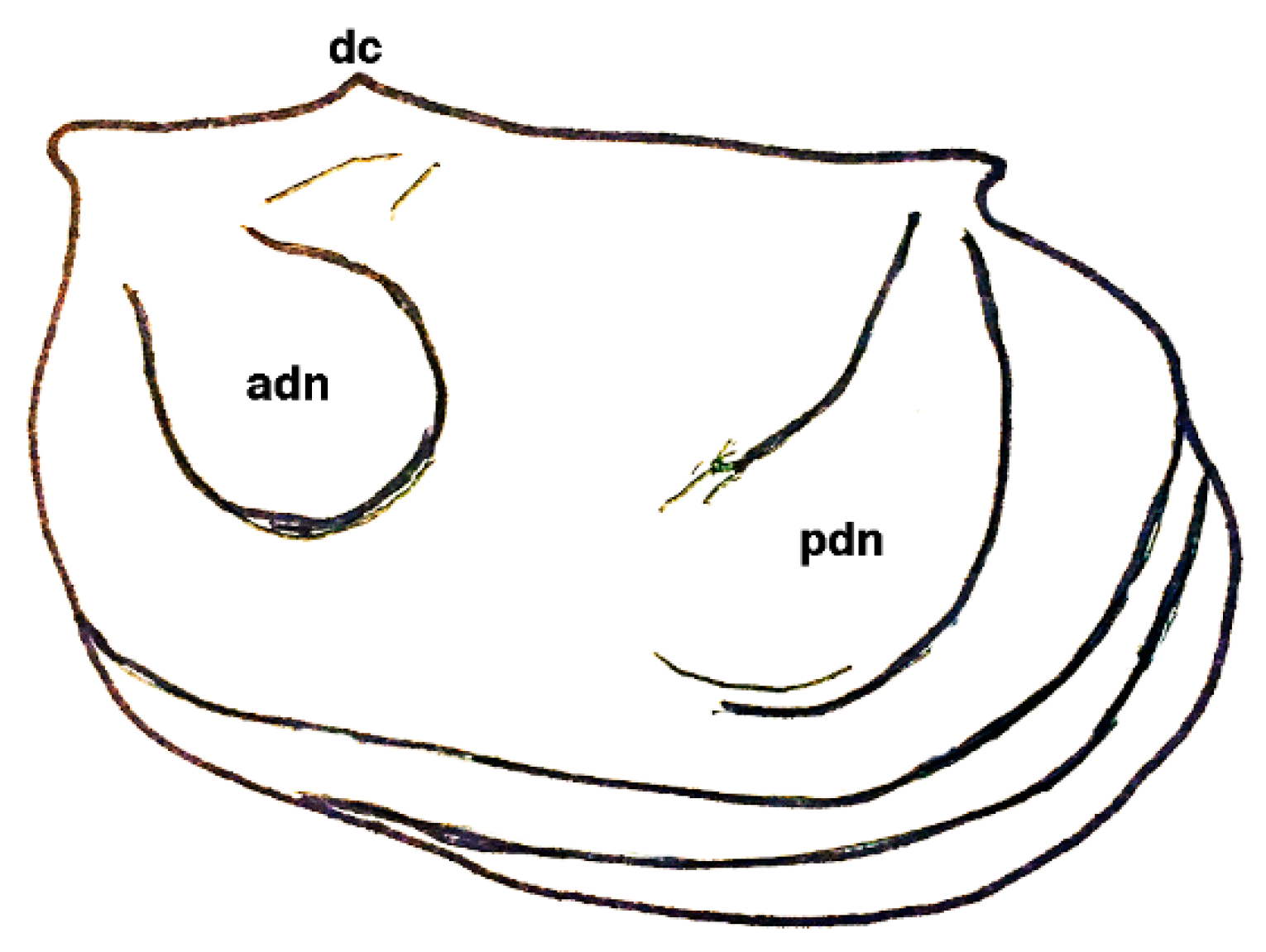
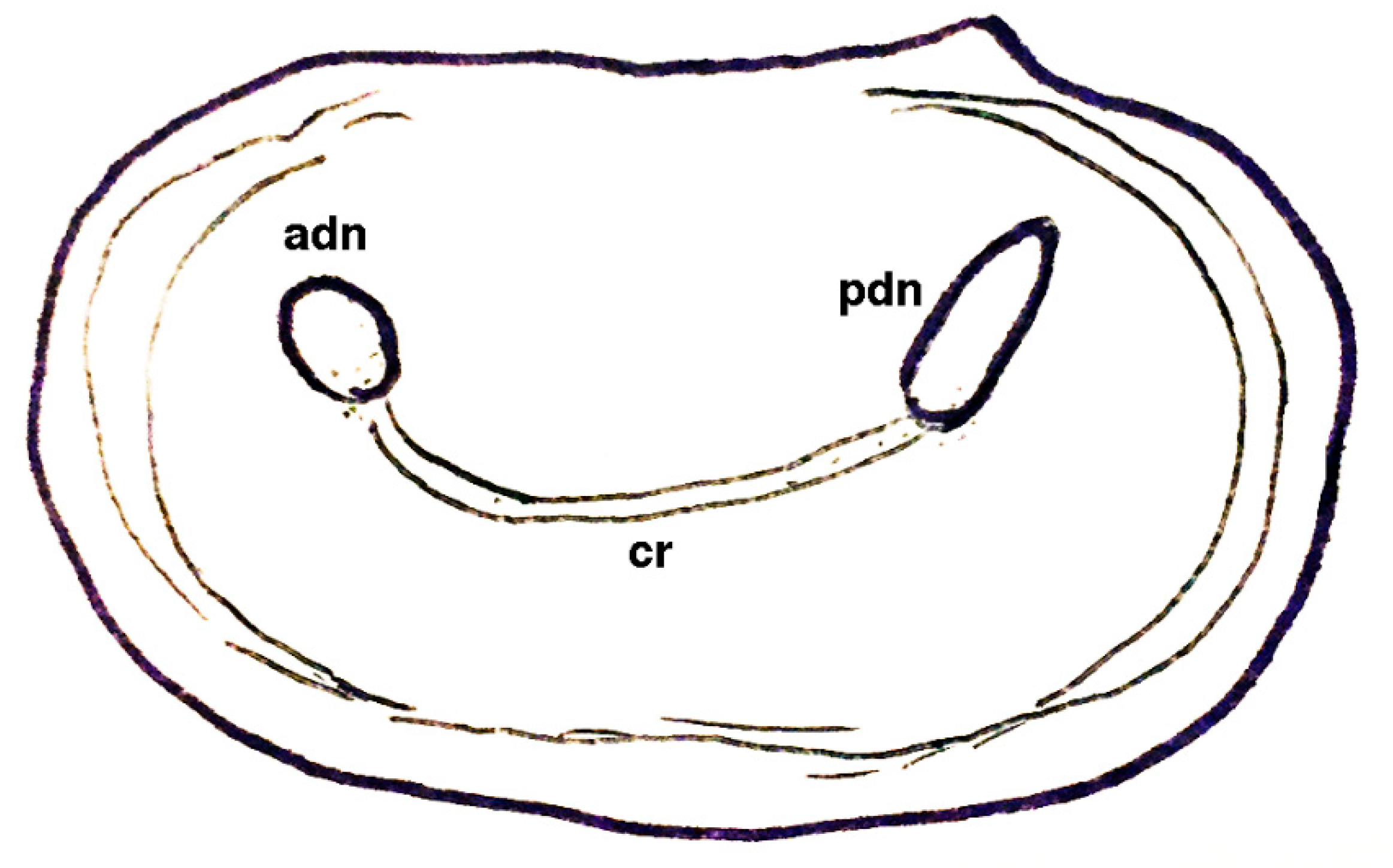
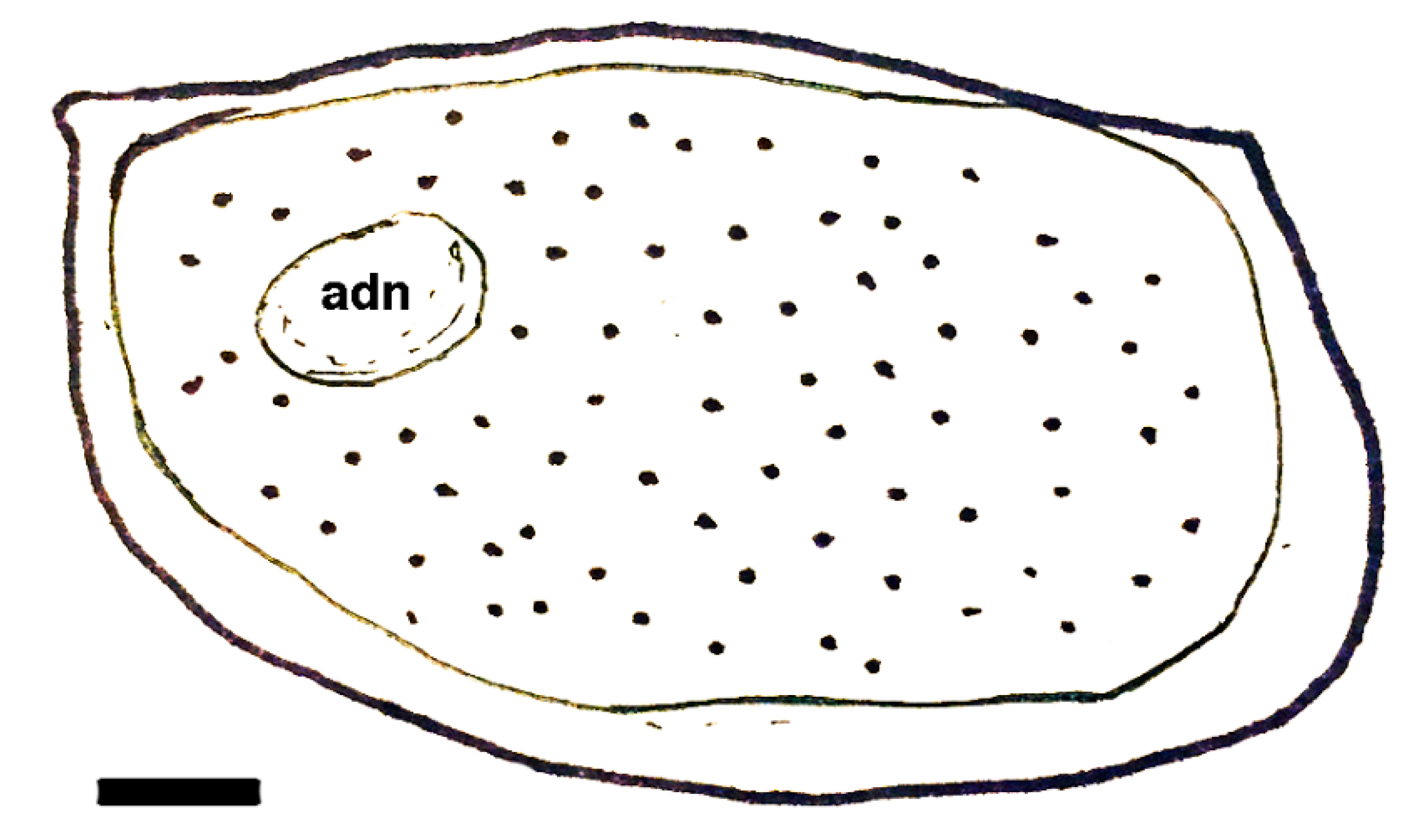
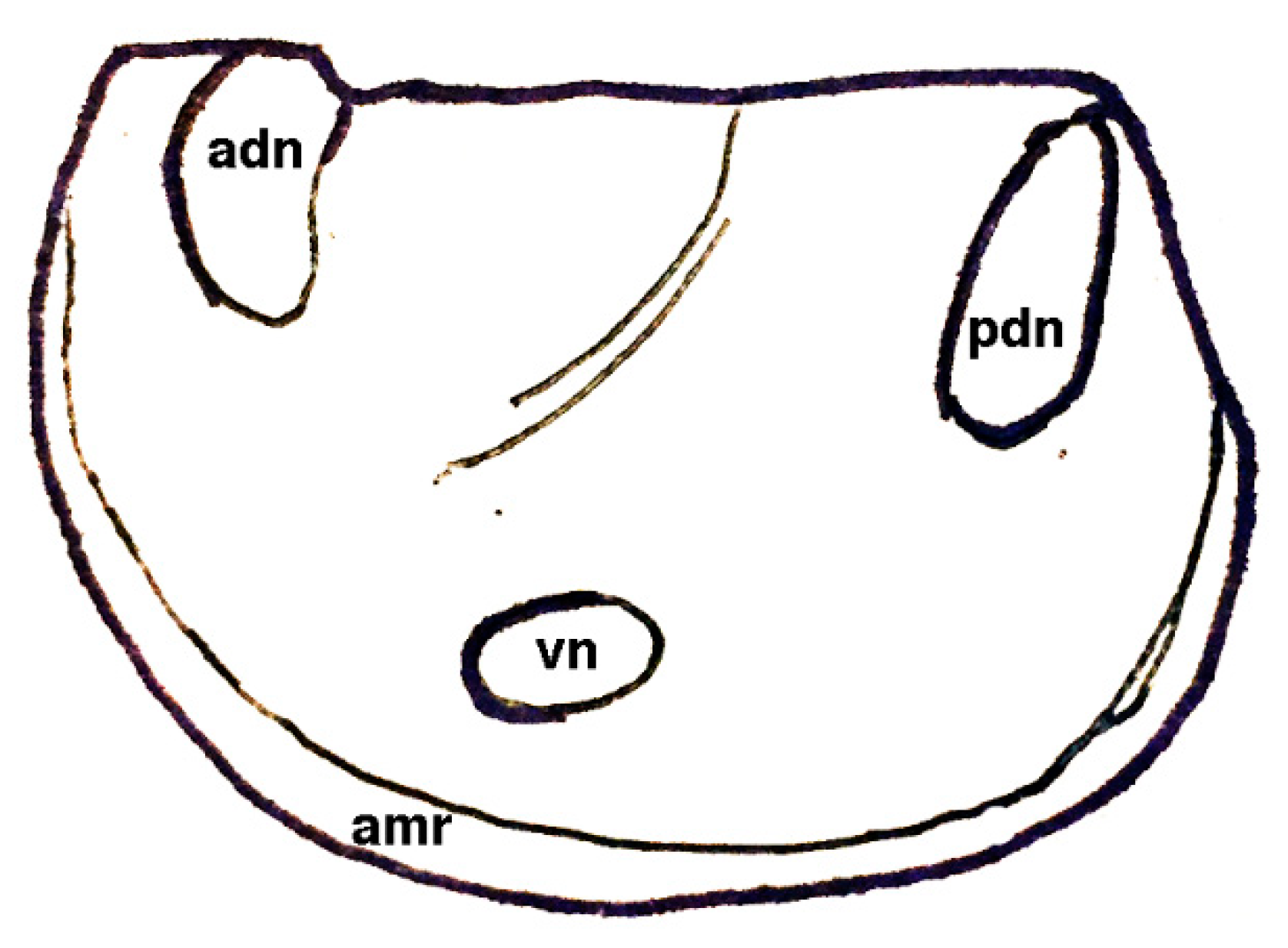
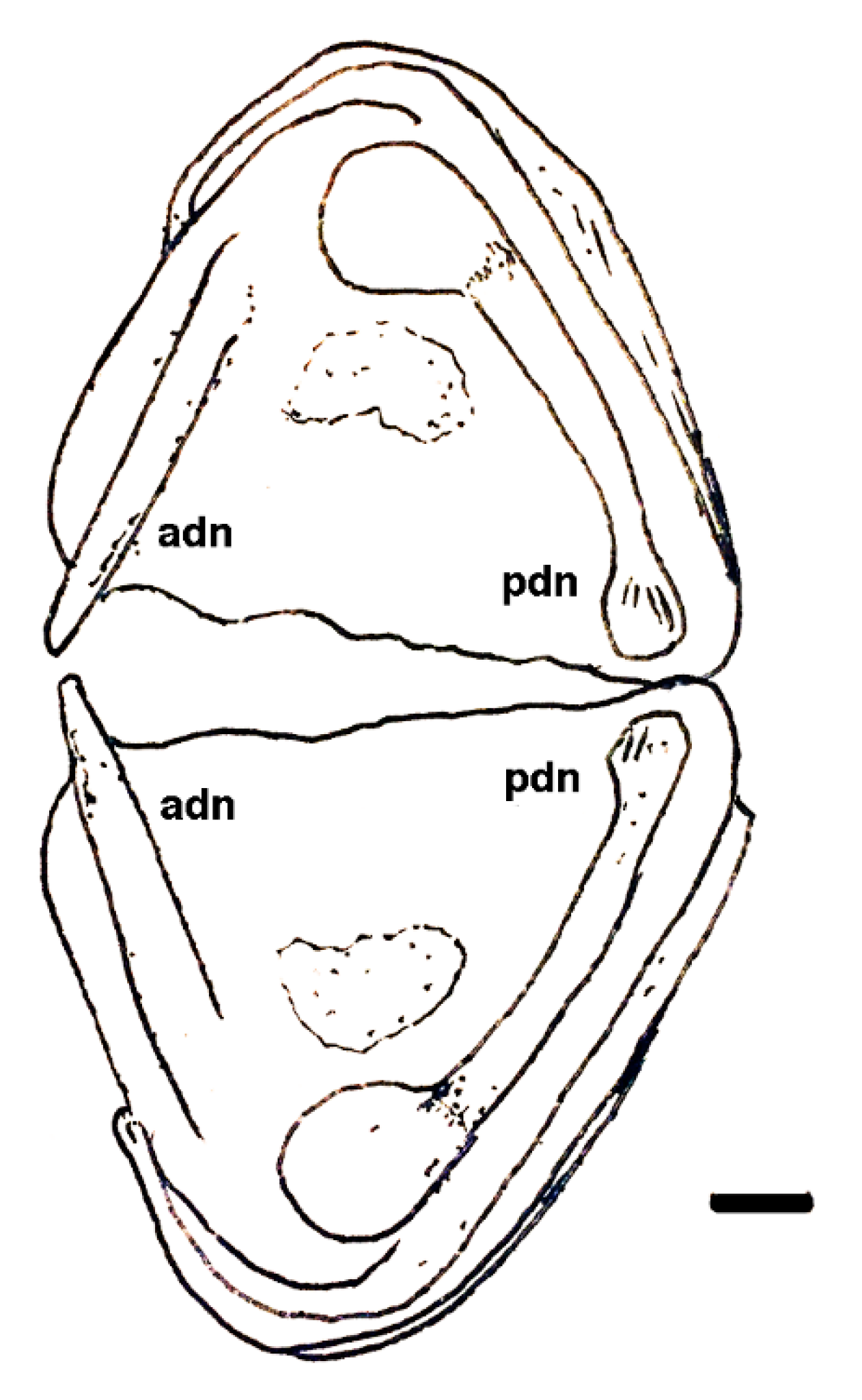
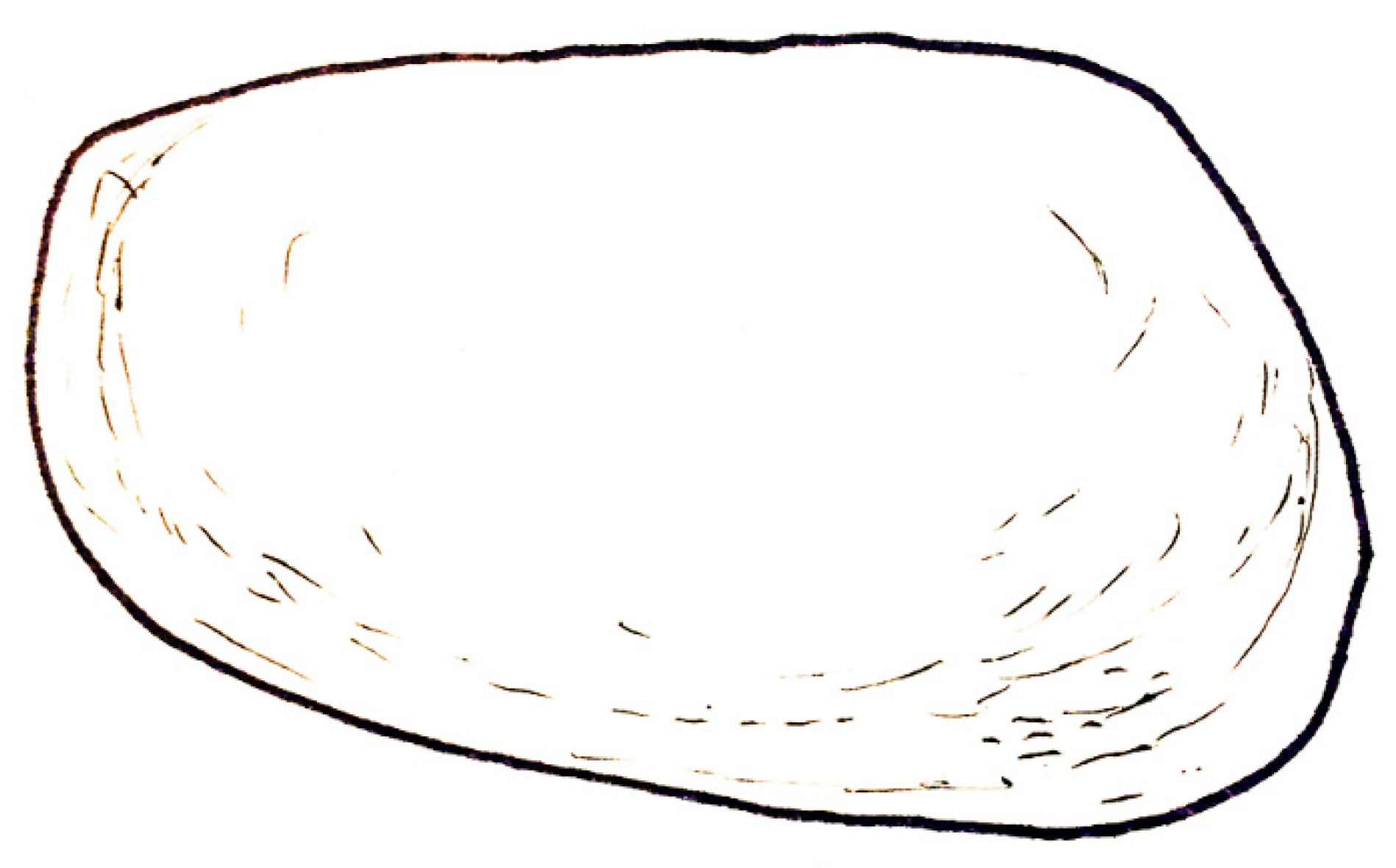
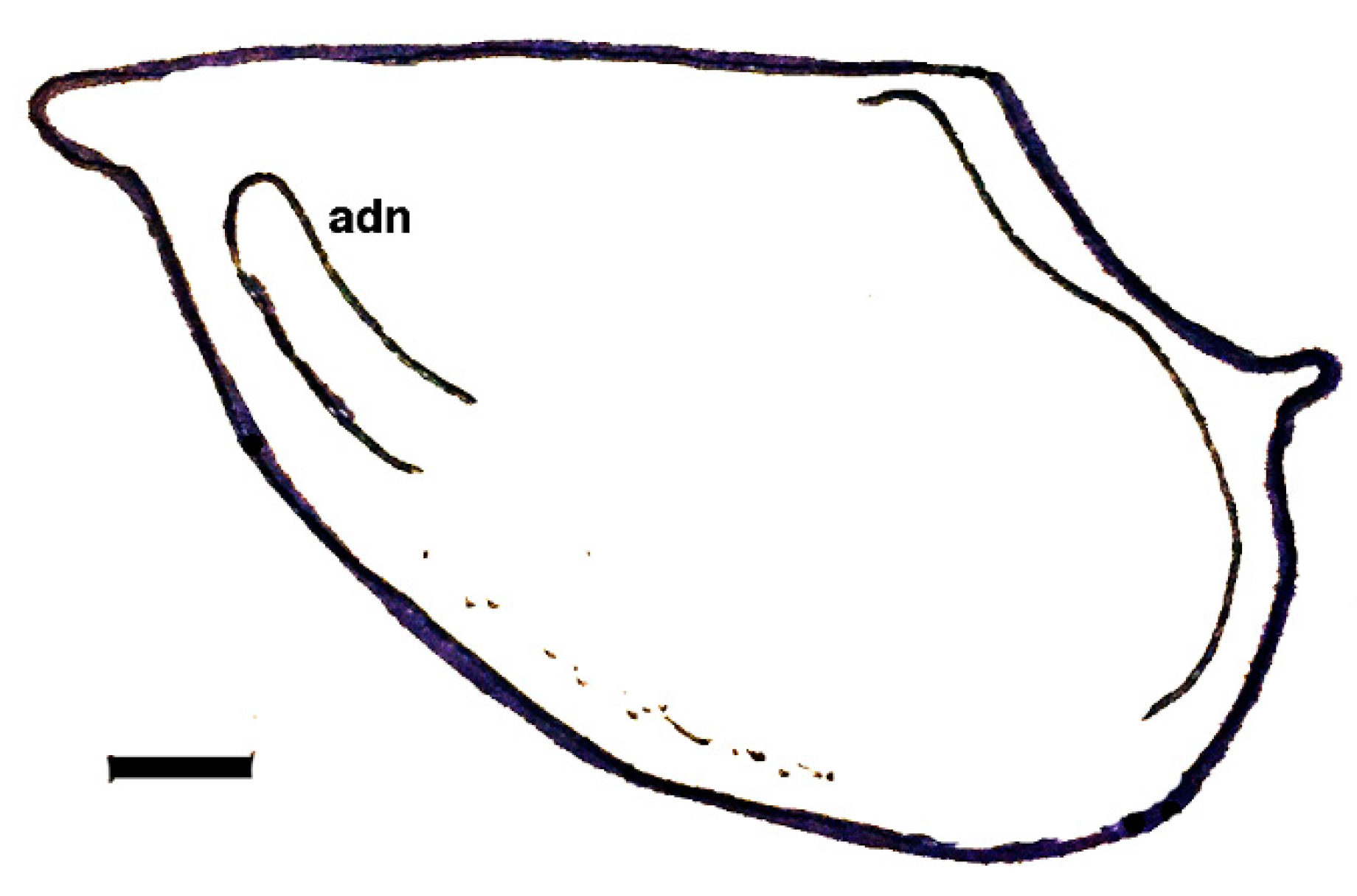
| Dielymella? sp.: Figure 38 |
Competing Interests
Acknowledgments
Conflicts of Interest
References
- Raymond, P.E. Leanchoilia and other mid-Cambrian Arthropoda. Bull. Mus. Compa. Zool. 1935, 76, 205–230. [Google Scholar]
- Williams, M.; Vannier, J.; Corbari, L.; Massabuau, J.-C. Oxygen as a driver of early arthropod micro-benthos evolution. PLoS ONE 2011, 6, e28183. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Siveter, D.J.; Williams, M.; Waloszek, D.; Bergström, J. Appendages of the arthropod Kunmingella from the early Cambrian of China: Its bearing on the systematic position of the bradoriids and the fossil record of the Ostracoda. Philos. Trans. R. Soc. Lond. 1996, 351, 1131–1145. [Google Scholar]
- Hou, X.; Siveter, D.J.; Williams, M.; Feng, X. A monograph of the bradoriid arthropods from the lower Cambrian of SW China. Trans. R. Soc. Edinb. Earth Sci. 2002, 92, 347–409. [Google Scholar]
- Williams, M.; Siveter, D.J.; Popov, L.E.; Vannier, J.M.C. Biogeography and affinities of the bradoriid arthropods: Cosmopolitan microbenthos of the Cambrian seas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 248, 202–232. [Google Scholar] [CrossRef]
- Skovsted, C.B.; Brock, G.A.; Paterson, J.R. Bivalved arthropods from the Lower Cambrian Mernmerna Formation, Arrowie Basin, South Australia and their implications for identification of Cambrian ‘small shelly fossils’. Mem. Assoc. Australas. Palaeontol. 2006, 32, 7–41. [Google Scholar]
- Moore, R.C. Treatise on Invertebrate Paleontology, Part Q, Arthropod 3, Crustacea, Ostracoda; Geological Society of America and University of Kansas Press: Lawrence, KS, USA, 1961. [Google Scholar]
- Ivantsov, A.Y.; Zhuravlev, A.Y.; Leguta, A.V.; Krassilov, V.A.; Melnikova, L.M.; Ushatinskaya, G.T. Palaeoecology of the early Cambrian Sinsk biota from the Siberian Platform. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 220, 69–88. [Google Scholar] [CrossRef]
- Martens, K.; Horne, D.J. Preface: Ostracoda and the four pillars of evolutionary wisdom. Hydrobiologia 2000, 419, 7–11. [Google Scholar] [CrossRef]
- McMenamin, M.A.S. Dynamic Paleontology; Columbia University Press: New York, NY, USA, 2016. [Google Scholar]
- McMenamin, M.A.S. Paleoecological feedback and the Vendian-Cambrian transition. Trends Ecol. Evol. 1988, 3, 205–208. [Google Scholar] [CrossRef]
- Williams, M.; Siveter, D.J. British Cambrian and Tremadoc bradoriid and phosphatocopid arthropods. Monogr. Palaeontol. Soc. Lond. 1998, 153, 1–49. [Google Scholar]
- Hinz, I.; Kanygin, A.V.; Schallreuter, R.E.L. On Chegetella chegitunica Kanygin. Stereo Atlas Ostracod Shells 1990, 17, 85–88. [Google Scholar]
- Von Zittel, K.A. Text-Book of Paleontology, Vol. 1, Invertebrates; Macmillan: London, UK, 1913. [Google Scholar]
- Shu, D.; Vannier, J.; Luo, H.; Chen, L.; Zhang, X.; Hu, S. Anatomy and lifestyle of Kunmingella (Arthropoda, Bradoriida) from the Chengjiang fossil Lagerstätte (lower Cambrian; Southwest China). Lethaia 1999, 32, 279–298. [Google Scholar] [CrossRef]
- Hou, X.; Williams, M.; Siveter, D.J.; Siveter, D.J.; Aldridge, R.J.; Sansom, R.S. Soft-part anatomy of the Early Cambrian bivalved arthropods Kunyangella and Kunmingella: Significance for the phylogenetic relationships of Bradoriida. Proc. R. Soc. B Biol. Sci. 2010, 277, 1835–1841. [Google Scholar] [CrossRef]
- Zhai, D.; Williams, M.; Siveter, D.J.; Harvey, T.H.P.; Sansom, R.S.; Gabbott, S.E.; Siveter, D.J.; Ma, X.; Zhou, R.; Liu, Y.; et al. Variation in appendages in early Cambrian bradoriids reveals a wide range of body plans in stem-euarthropods. Commun. Biol. 2019, 2, 329. [Google Scholar] [CrossRef]
- Edgecombe, G.D.; Legg, D.A. The Arthropod fossil record. In Arthropod Biology and Evolution; Minelli, A., Boxshall, G., Fusco, G., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2013; pp. 393–415. [Google Scholar]
- Streng, M.; Geyer, G. Middle Cambrian Bradoriida (Arthropoda) form the Franconian Forest, Germany, with a review of the bradoriids described from West Gondwana and a revision of material from Baltica. PalZ 2019, 93, 567–591. [Google Scholar] [CrossRef]
- Siveter, D.J.; Williams, M. Cambrian bradoriid and phosphatocopid arthropods of North America. Spec. Pap. Palaeontol. 1997, 57, 1–69. [Google Scholar]
- Peng, J.; Feng, H.; Fu, X.; Zhao, Y.; Yao, L. New bradoriid arthropods from the Early Cambrian Balang Formation of Eastern Guizhou, South China. Acta Geol. Sin. 2010, 84, 56–68. [Google Scholar] [CrossRef]
- Schram, F.R. Arthropods: A convergent phenomenon. Fieldiana Geol. 1978, 39, 61–108. [Google Scholar]
- Jones, P.J.; McKenzie, K.G. Queensland Middle Cambrian Bradoriida (Crustacea): New taxa, palaeobiogeography and biological affinities. Alcheringa 1980, 4, 203–225. [Google Scholar] [CrossRef]
- Rode, A.L.; Lieberman, B.S.; Rowell, A.J. A new early Cambrian bradoriid (Arthropoda) from East Antarctica. J. Paleontol. 2003, 77, 691–697. [Google Scholar] [CrossRef]
- Topper, T.P.; Skovsted, C.B.; Brock, G.A.; Paterson, J.R. The oldest bivalved arthropods from the early Cambrian of East Gondwana: Systematics, biostratigraphy and biogeography. Gondwana Res. 2011, 19, 310–326. [Google Scholar] [CrossRef]
- Ferguson, E.; Hutchison, G.E.; Goulden, C.E. Cypria petenensis, a new name for the ostracod Cypria pelagica Brehm 1932. Postilla 1964, 80, 1–4. [Google Scholar]
- Walcott, C.D. Fauna of the “Upper Taconic” of Emmons, in Washington County, N.Y. Am. J. Sci. 1887, 34, 187–199. [Google Scholar] [CrossRef]
- Betts, M.J.; Brock, G.A.; Paterson, J.R. Butterflies of the Cambrian benthos? Shield position in bradoriid arthropods. Lethaia 2016, 49, 478–491. [Google Scholar] [CrossRef]
- Vannier, J.; Williams, M.; Siveter, D.J. The Cambrian origin of the circulatory system of crustaceans. Lethaia 1997, 30, 169–184. [Google Scholar] [CrossRef]
- Koehl, M.A.R. Hairy little legs: Feeding, smelling, and swimming at low Reynolds numbers. Contemp. Math. 1993, 141, 33–64. [Google Scholar]
- Peel, J.S. Systematics and biogeography of some early Cambrian (Series 2) bradoriids (Arthropoda) from Laurentia (Greenland). Can. J. Earth Sci. 2017, 54, 961–972. [Google Scholar] [CrossRef]
- Baldwin, J.; Morris, G.M. Re-examination of the contributions of aerobic and anaerobic energy production during swimming in the bivalve mollusc Limaria fragilis (Family Limidae). Aust. J. Mar. Freshw. Res. 1983, 34, 909–914. [Google Scholar] [CrossRef]
- Vannier, J.; Williams, M.; Álvaro, J.J.; Vaizcaïno, D.; Monceret, S.; Monceret, E. New Early Cambrian bivalved arthropods from southern France. Geol. Mag. 2005, 142, 1–13. [Google Scholar] [CrossRef]
- McMenamin, M.A.S.; McMenamin, S.K. Homeotic genes, the Antennapedia complex in the trilobite genome, and iterative evolution in nevadiid and bristoliid trilobites. In Paleontology Sonora: Lipalian and Cambrian; McMenamin, M.A.S., Ed.; Meanma Press: South Hadley, MA, USA, 2001; pp. 107–113. [Google Scholar]
- Hughes, N.C. Trilobite tagmosis and body patterning from morphological and developmental perspectives. Integ. Comp. Biol. 2003, 43, 185–206. [Google Scholar] [CrossRef]
- Estrada, B.; Sánchez-Herrero, E. The Hox gene Abdominal-B antagonizes appendage development in the genital disc of Drosophila. Development 2001, 128, 331–339. [Google Scholar] [PubMed]
- McMenamin, M.A.S.; Weaver, P.G. Middle Cambrian polymeroid trilobites and correlation of the Carolina and Augusta terranes. Southeast. Geol. 2004, 43, 21–38. [Google Scholar]
- McMenamin, M.A.S. A new nevadiid trilobite recognized from Sonora, Mexico. In Paleontology Sonora: Lipalian and Cambrian; McMenamin, M.A.S., Ed.; Meanma Press: South Hadley, MA, USA, 2001; pp. 103–106. [Google Scholar]
- Webster, M. Ontogeny and evolution of the early Cambrian trilobite genus Nephrolenellus (Olenelloidea). J. Paleontol. 2007, 81, 1168–1193. [Google Scholar] [CrossRef]
- Khadjeh, S.; Turetzek, N.; Pechmann, M.; Schwager, E.E.; Wimmer, E.A.; Damen, W.G.M.; Prpic, N.-M. Divergent role of the Hox gene Antennapedia in spiders is responsible for the convergent evolution of abdominal limb repression. Proc. Natl. Acad. Sci. USA 2012, 109, 4921–4926. [Google Scholar] [CrossRef]
- Betts, M.J.; Paterson, J.R.; Jago, J.B.; Jacquet, S.M.; Skovsted, C.B.; Topper, T.P.; Brock, G.A. Global correlation of the early Cambrian of South Australia: Shelly fauna of the Dailyatia odyssei Zone. Gondwana Res. 2017, 46, 240–279. [Google Scholar] [CrossRef]
- Martinsson, A. Albrunnicola, a new name for the Cambrian ostracode genus Longispina Andres 1969. Lethaia 1979, 12, 27. [Google Scholar] [CrossRef]
- Andres, D. Ostracoden aus dem mittleren Kambrium von Öland. Lethaia 1969, 2, 165–180. [Google Scholar] [CrossRef]
- Wrona, R. Early Cambrian bradoriide and phospatocopide arthropods from King George Island, West Antarctica: Biogeographic implications. Pol. Polar Res. 2009, 30, 347–377. [Google Scholar] [CrossRef]
- Melnikova, L.M. Cambrian Bradoriida (Arthropoda) of the Malyi Karatau (Kyr-Shabakty Section). Paleontol. J. 2003, 37, 394–399. [Google Scholar]
- Melnikova, L.M. The first late Cambrian bradoriids (Ostracoda) from Gorny Altay. Paleontol. Zhurnal 1992, 1992, 80–82. (In Russian) [Google Scholar]
- Matthew, G.F. Faunas of the Paradoxides Beds in eastern North America, No. 1. Trans. N. Y. Acad. Sci. 1896, 15, 192–247. [Google Scholar]
- Kobayashi, T.; Kato, F. On the ontogeny and the ventral morphology of Redlichia chinensis with description of Alutella nakamurain new. gen. and sp. nov. J. Fac. Sci. Univ. Tokyo Sect. 2 1951, 8, 99–143. [Google Scholar]
- Abushik, A.F. First discovery of leperditaceans from the Cambrian of the Siberian Platform. Vestn. Leningr. Univ. Seriya Geol. Geogr. 1960, 6, 93–98. (In Russian) [Google Scholar]
- Hinz-Schallreuter, I.; Gozalo, R.; Liñán, E. New bradorid arthropods from the Lower Cambrian of Spain. Micropaleontology 2008, 53, 497–510. [Google Scholar] [CrossRef][Green Version]
- Collette, J.H.; Hughes, N.C.; Peng, S. The first report of a Himalayan bradoriid arthropod and the paleogeographic significance of this form. J. Paleontol. 2011, 85, 76–82. [Google Scholar] [CrossRef]
- Smith, P.M.; Brock, G.A.; Paterson, J.R.; Topper, T.P. New bradoriid arthropods from the Giles Creek Dolostone (Cambrian Series 3, Stage 5; Templetonian), Amadeus Basin, central Australia. Mem. Assoc. Aust. Palaeontol. 2014, 45, 233–248. [Google Scholar]
- Huo, S.; Shu, D. Cambrian Bradoriida of South China; Northwest University Publishing House: Xi’an, China, 1985. [Google Scholar]
- Öpik, A.A. The geology and palaeontology of the headwaters of the Burke River, Queensland. Bull. Bur. Miner. Resour. Geol. Geophys. 1961, 53, 1–249. [Google Scholar]
- Tan, G. Crustacea. In Stratigraphy, Paleontology and Sedimentary Environment of Sinian in Ganlo Region; Sichuan People’s Press: Chengdu, China, 1980. [Google Scholar]
- Hinz-Schallreuter, I. Ostracodes from the Middle Cambrian of Australia. Neues Jahrb. Geol. Paläontol. Abh. 1993, 188, 305–326. [Google Scholar]
- Matthew, G.F. Illustrations of the fauna of the St. John Group continued. No. 3: Descriptions of new genera and species. Proc. Trans. R. Soc. Can. 1886, 1, 29–84. [Google Scholar]
- Jones, P.J.; Kruse, P.D. New Middle Cambrian bradoriids (Arthropoda) from the Georgina Basin, central Australia. Mem. Assoc. Aust. Palaeontol. 2009, 37, 55–86. [Google Scholar]
- Stigall, A.L. Bicarinellata a new name for Bicarinella Rode, Lieberman and Rowell, 2003 (Arthropoda, Bradoriida) preoccupied by Bicarinella Waterhouse, 1966 (Mollusca, Gastropod) and Bicarinella Akopyan, 1976 (Mollusca, Gastropoda). J. Paleontol. 2008, 82, 1220. [Google Scholar] [CrossRef]
- Matthew, G.F. Preliminary notice of the Etcheminian fauna of Cape Breton. Bull. Nat. Hist. Soc. N. B. 1899, 18, 198–208. [Google Scholar]
- Copeland, M.J. Bullaluta kindlei n. gen., n. sp. (Ostracoda, Archaeocopida) from Zone 5 (Late Cambrian, Cedaria-Crepicephalus) of the Cow Head Group, western Newfoundland. Geol. Surv. Can. Pap. Curr. Res. Part B 1986, 86, 339–403. [Google Scholar]
- Neckaja, A.I.; Ivanova, V.A. Pervaya nachodka ostrakod v nizhnem kembrii Vostochnoj Sibiri. Dokl. Akad. Nauk SSSR 1956, 111, 1095–1097. [Google Scholar]
- Hinz, I.C.U. On Capricambria cornucopiae Hinz gen. et sp. nov. Stereo Atlas Ostracod Shells 1991, 18, 65–68. [Google Scholar]
- Kanygin, A.V. Ostrakody ordovika chukotskogo polyostrova. Trudy Inst. Geol. Geofiz. sib. Otd.. 1977, 351, 73–75. [Google Scholar]
- Öpik, A.A. Ordian (Cambrian) Crustacea Bradoriida of Australia. Bull. Bur. Miner. Resour. Geol. Geophys. 1968, 103, 1–44. [Google Scholar]
- Ulrich, E.O.; Bassler, R.S. Cambrian bivalved Crustacea of the order Conchostraca. Proc. USA Natl. Mus. 1931, 78, 1–130. [Google Scholar] [CrossRef]
- Wotte, T.; Sundberg, F.A. Small shelly fossils from the Montezuman—Delamaran of the Great Basin in Nevada and California. J. Paleontol. 2017, 91, 883–901. [Google Scholar] [CrossRef]
- Hinz-Schallreuter, I. Ein ersatzname für Andresia (Bradorida, Kambrium). Geschiebekunde aktuell 2008, 24, 114. [Google Scholar]
- Streng, M.; Ebbestad, J.O.R.; Moczydlowska, M. A Walcottella-like bradoriid (Arthropoda) from the lower Cambrian. GFF 2008, 130, 11–19. [Google Scholar] [CrossRef]
- Shu, D. Cambrian and Lower Ordovician Bradoriida from Zhenjiang, Hunnan and Shaanxi Provinces; Northwest University Press: Xian, China, 1990. [Google Scholar]
- Keij, A.J. Eocene and Oligocene Ostracoda of Belgium. Inst. R. Sci. Nat. Belg, Mém. 1957, 136, 1–210. [Google Scholar]
- Li, Y.-W. On the Cambrian Ostracoda with new materials from Sichuan, Yunnan, and southern Shaanxi, China. Prof. Pap. Stratigr. Paleontol. 1975, 2, 37–72. [Google Scholar]
- Bengtson, S.; Conway Morris, S.; Cooper, B.J.; Jell, P.A.; Runnegar, B.N. Early Cambrian fossils from South Australia. Mem. Assoc. Aust. Palaeontol. 1990, 9, 1–364. [Google Scholar]
- Hinz-Schallreuter, I. Cambrian ostracods mainly from Baltoscandia and Morocco. Arch. Geschiebekd. 1993, 1, 385–448. [Google Scholar]
- Jones, P.J.; McKenzie, K.G. Flemingopsis, a new name for the phosphatocopine ostracode genus Flemingia Jones & McKenzie 1980. Alcheringa 1981, 5, 310. [Google Scholar]
- Peel, J.S.; Streng, M. A new middle Cambrian bradoriid arthropod from Greenland and western Canada. J. Paleontol. 2015, 89, 96–102. [Google Scholar] [CrossRef]
- Hinz-Schallreuter, I.; Jones, P.J. Gladioscutum lauriei Hinz & Jones gen. et sp. nov. (Archaeocopida) from the Middle Cambrian of the Georgina Basin, central Australia. Paläontol. Z. 1994, 68, 361–375. [Google Scholar]
- Huo, S.-C. Brief notes on Lower Cambrian Archaeostraca from Shensi and Yunnan. Acta Palaeontol. Sin. 1956, 4, 425–445. [Google Scholar]
- Matthew, G.F. Ostracoda of the basal Cambrian rocks in Cape Breton. Can. Rec. Sci. 1902, 8, 437–470. [Google Scholar]
- Ulrich, E.O.; Bassler, R.S. Indianites, new name for the Cambrian crustacean Indiana Ulrich and Bassler. J. Wash. Acad. Sci. 1931, 21, 364. [Google Scholar]
- Hou, X.-G.; Bergström, J. The arthropods of the Lower Cambrian Chengjiang fauna, with relationships and evolutionary significance. In The Early Evolution of Metazoa and the Significance of Problematic Taxa; Simonetta, A.M., Conway Morris, S., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 179–187. [Google Scholar]
- Betts, M.J.; Paterson, J.R.; Jago, J.B.; Jacquet, S.M.; Skovsted, C.B.; Topper, T.P.; Brock, G.A. A new lower Cambrian shelly fossil biostratigraphy for South Australia. Gondwana Res. 2016, 36, 163–195. [Google Scholar] [CrossRef]
- Reyment, R.A.; Elewa, A.M. Predation by drills on ostracoda. In Predator-Prey Interactions in the Fossil Record; Kelley, P.H., Mowalewski, M., Hansen, T.A., Eds.; Kluwer: New York, NY, USA, 2003; pp. 93–111. [Google Scholar]
- Gridina, N.M. Ostatki konodontov i fosfatnykh problematiki v otlozheniyakh nizhnego kembriya centralnogo Kazakhstana. Paleontol. Zhurnal 1991, 102–108. [Google Scholar]
- Missarzhevsky, V.V.; Mambetov, A.M. Stratigrafiya i fauna progranichnykh sloev kembriya i dokembriya Malogo Karatau. Trudy Geol. Inst. Akad. Nauk SSSR 1981, 362, 1–92. [Google Scholar]
- Šnajdr, M. Konicekion from the middle Cambrian of Bohemia. Věstn. Ústřed. Úst. Geol. 1975, 50, 153–156. [Google Scholar]
- Siveter, D.J.; Briggs, D.E.G.; Siveter, D.J.; Sutton, M.D.; Legg, D.; Joomun, S. A Silurian short-great-appendage arthropod. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132986. [Google Scholar] [CrossRef]
- Huo, S. Additional notes on Lower Cambrian Archaeostraca from Shensi and Yunnan. Acta Palaeontol. Sin. 1965, 13, 291–303. [Google Scholar]
- Jones, T.R. Notes on the Paleozoic bivalved Entomostraca. No. 3, Some species of Leperditia. Ann. Magazine Nat. Hist. Lond. Ser. 2 1856, 17, 81–101. [Google Scholar] [CrossRef][Green Version]
- Rouault, M. Mémoire sur la terrain paléozoïque de environs de Rennes. Soc. Géol. France Bull. Ser. 2 1851, 8, 358–399. [Google Scholar]
- Jeffries, R.P.S.; Lewis, M.; Donovan, S.K. Protocystites menevensis—a stem-group chordate (Cornuta) from the Middle Cambrian of South Wales. Palaeontology 1987, 30, 429–484. [Google Scholar]
- Hirschmann, N. Beitrag zur Kenntnis der Ostracodenfauna des Finnischen Meerbusens. Meddelanden af Societas pro Fauna et Flora Fennica 1909, 35, 282–296. [Google Scholar]
- Luo, H.; Jiang, Z.; Wu, X.; Song, X.; Ouyang, L. The Sinian-Cambrian Boundary in Eastern Yunnan; People’s Publishing House: Yunnan, China, 1982. (In Chinese) [Google Scholar]
- Melnikova, L.; Siveter, D.J.; Williams, M. Cambrian Bradoriida and Phosphatocopida (Arthropoda) of the former Soviet Union. J. Micropaleontol. 1997, 16, 179–191. [Google Scholar] [CrossRef]
- Missarzhevsky, V.V. Konodonty (?) i fosfatnye problematiki kembriya Mongolii i Sibiri. In Bespozvonochnye Paleozoya Mongolii. Trudy Sovmestaya Sovetsko-Mongolskaya Paleontologicheskaya Ekspeditsiya 1977, 5, 10–19. [Google Scholar]
- Melnikova, L.M. A new genus of Bradoriidae (Crustacea) from the Cambrian of northern Eurasia. Paleontol. J. 2000, 34, 180–185. [Google Scholar]
- Skovsted, C.B. A carapace of the bradoriid arthropod Mongolitubulus from the early Cambrian of Greenland. GFF 2005, 127, 217–220. [Google Scholar] [CrossRef]
- Zhang, W.-T. Bradoriida. In Handbook of Stratigraphy and Palaeontology of Southwest China; Nanjing Institute of Geology and Paleontology, Academica Sinica, Science Press: Beijing, China, 1974. [Google Scholar]
- Vasilieva, N.I. Rannekembriiskaya melkaya rakovinnaya fauna iz skvazhin zapadnoi Yakutii. Paleontol. Zhurnal 1994, 1994, 3–9. [Google Scholar]
- Topper, T.P.; Skovsted, C.B.; Brock, G.A.; Paterson, J.R. New bradoriids from the lower Cambrian Mernmerna Formation, South Australia: Systematics, biostratigraphy and biogeography. Mem. Assoc. Aust. Paleontol. 2007, 33, 67–100. [Google Scholar]
- Zhang, X.-G. Moult stages and dimorphism of Early Cambrian bradoriids from Xichuan, Henan, China. Alcheringa 1987, 11, 1–19. [Google Scholar] [CrossRef]
- Hinz, I. On Pejonesia sestina (Fleming). Stereo Atlas Ostracod Shells 1992, 19, 5–8. [Google Scholar]
- Siveter, D.J.; Williams, M.; Peel, J.S.; Siveter, D.J. Bradoriida (Arthropoda) from the early Cambrian of North Greenland. Trans. R. Soc. Edinb. Earth Sci. 1996, 86, 113–121. [Google Scholar] [CrossRef]
- Kornicker, L.S.; Sohn, I.G. Myodocopid Ostracoda from the Late Permian of Greece and a basic classification for Paleozoic and Mesozoic Myodocopida. Smithson. Contrib. Paleobiol. 2000, 91, 1–33. [Google Scholar] [CrossRef]
- Hinz-Schallreuter, I. Systematics and phylogeny of the Oepikatulidae (Cambrian Ostracoda). Greifswald. Geowiss. Beitr. 1999, 6, 23–53. [Google Scholar]
- Zhang, X.-G. Phosphatized bradoriids (Arthropoda) from the Cambrian of China. Palaeontogr. Abt. A 2007, 281, 93–173. [Google Scholar] [CrossRef]
- Voronin, Y.I.; Voronova, L.G.; Grigorieva, N.V.; Drozdova, N.A.; Zhegallo, E.A.; Zhuravlev, A.Y.; Ragozina, A.L.; Rozanov, A.Y.; Sayutina, T.A.; Sysoev, V.A.; et al. Granitsa dokembriya v geosinklinalnykh oblastyakh (opornyj razrez Salany-Gol, MNR). Trudy Sovmestoi Sov. Mong. Paleontol. Ekhspeditsii 1982, 18, 1–150. [Google Scholar]
- Liu, Y.; Zhang, X.-L.; Liu, W.; Zhang, Q. New bradoriids from the Lower Cambrian Yanwangbian formation of southern Shaanxi Province, Central China. Palaeoworld 2008, 17, 102–107. [Google Scholar] [CrossRef]
- Wiman, C. Studien über das Nordbaltische Silurgebiet. I. Olenellussandstein, Obolussandstein und Ceratopygeschiefer. Bull. Geol. Instit. Univ. Upps. 1902, 6, 36–76. [Google Scholar]
- Stubblefield, C.J. On the occurrence of Tremadoc Shales in the Tortworth Inlier (Gloucestershire). Notes on the fossils. Q. J. Geol. Soc. Lond. 1933, 89, 357–375. [Google Scholar]
- Melnikova, L.M. Novyi rod bradoriid (Crustacea) is kembriya Severnoi Evrazii. Paleontol. Zhurnal 1998, 4, 36–40. [Google Scholar]
- Ivantsov, A.Y.; Zhuravlev, A.Y.; Krasilov, V.A.; Leguta, A.V.; Mel’nikova, L.M.; Mel’nikova, L.M.; Urbanek, A.; Ushatinskaya, G.T.; Malakhovskaya, Y.E. Unikalnye Sinskie Mestonakhozhdeniya Rannekembriiskikh Organismov Sibirskaya Platform; Rossiiskaya Akademiya Nauk: Moscow, Russia, 2005. [Google Scholar]
- Koneva, S.P. O pervoi nakhodke ostrakod v nizhnem kembri Kazakhstana. Paleontol. Zhurnal 1978, 1, 150–152. [Google Scholar]
- Khazanovich, K.K.; Popov, L.E.; Melnikova, L.M. Bessamkovije brakhiopody, ostrakody (bradoriidy) I khiolitelmenty i sablinskoi swity leningradskoi oblasti. Paleontologicheskii Zhurnal 1984, 4, 33–47. [Google Scholar]
- Fleming, P.J. Bradoriids from the Xystridura zone of the Georgina Basin, Queensland. Publ. Qld. Geol. Surv. 356 Prof. Pap. 1973, 31, 1–9. [Google Scholar]
- McMenamin, M.A.S. Two new species of the Cambrian genus Mickwitzia. J. Paleontol. 1992, 66, 173–182. [Google Scholar] [CrossRef]
- Lankester, E.R. The structure and classification of Arthropoda. The Quarterly Journal of Microscopical Science, London 1904, 47, 523–582. [Google Scholar]
- Gerber, D.E. Greek Iambic Poetry; Harvard University Press: Cambridge, MA, USA, 1999. [Google Scholar]
- McMenamin, M.A.S.; Schulte McMenamin, D.L.S. The Emergence of Animals—The Cambrian Breakthrough; Columbia University Press: New York, NY, USA, 1990. [Google Scholar]
- McMenamin, M.A.S.; Pittenger, S.L.; Carson, M.R.; Larrabee, E.M. Upper Precambrian-Cambrian faunal sequence, Sonora, Mexico and lower Cambrian fossils from New Jersey, United States. N. Y. State Mus. Bull. 1994, 481, 213–227. [Google Scholar]
- McMenamin, M.A.S. (Ed.) Paleontology Sonora: Lipalian and Cambrian; Meanma Press: South Hadley, MA, USA, 2001. [Google Scholar]
- McMenamin, M.A.S. The Garden of Ediacara: Discovering the First Complex Life; Columbia University Press: New York, NY, USA, 1998. [Google Scholar]
- Aguirre-Velarde, A.; Flye-Sainte-Marie, J.; Mendo, J.; Jean, F. Sclerochronological records and daily microgrowth of the Peruvian scallop (Argopecten purpuratus, Lamarck, 1819) related to environmental conditions in Paracas Bay, Pisco, Peru. J. Sea Res. 2015, 99, 1–8. [Google Scholar] [CrossRef]
- Babcock, L.E. Trilobites in Paleozoic predator-prey systems, and their role in reorganization of early Paleozoic ecosystems. In Predator-Prey Interactions in the Fossil Record; Kelley, P.H., Mowalewski, M., Hansen, T.A., Eds.; Kluwer: New York, NY, USA, 2003; pp. 55–92. [Google Scholar]
- Stewart, J.H.; McMenamin, M.A.S.; Morales-Ramirez, J.M. Upper Proterozoic and Cambrian rocks in the Caborca Region, Sonora, Mexico—Physical stratigraphy, biostratigraphy, paleocurrent studies, and regional relations. USA Geol. Surv. Prof. Pap. 1984, 1309, 1–36. [Google Scholar]
- Slater, B.J.; Willman, S.; Budd, G.E.; Peel, J.S. Widespread preservation of small carbonaceous fossils (SCFs) in the early Cambrian of North Greenland. Geology 2018, 46, 107–110. [Google Scholar] [CrossRef]
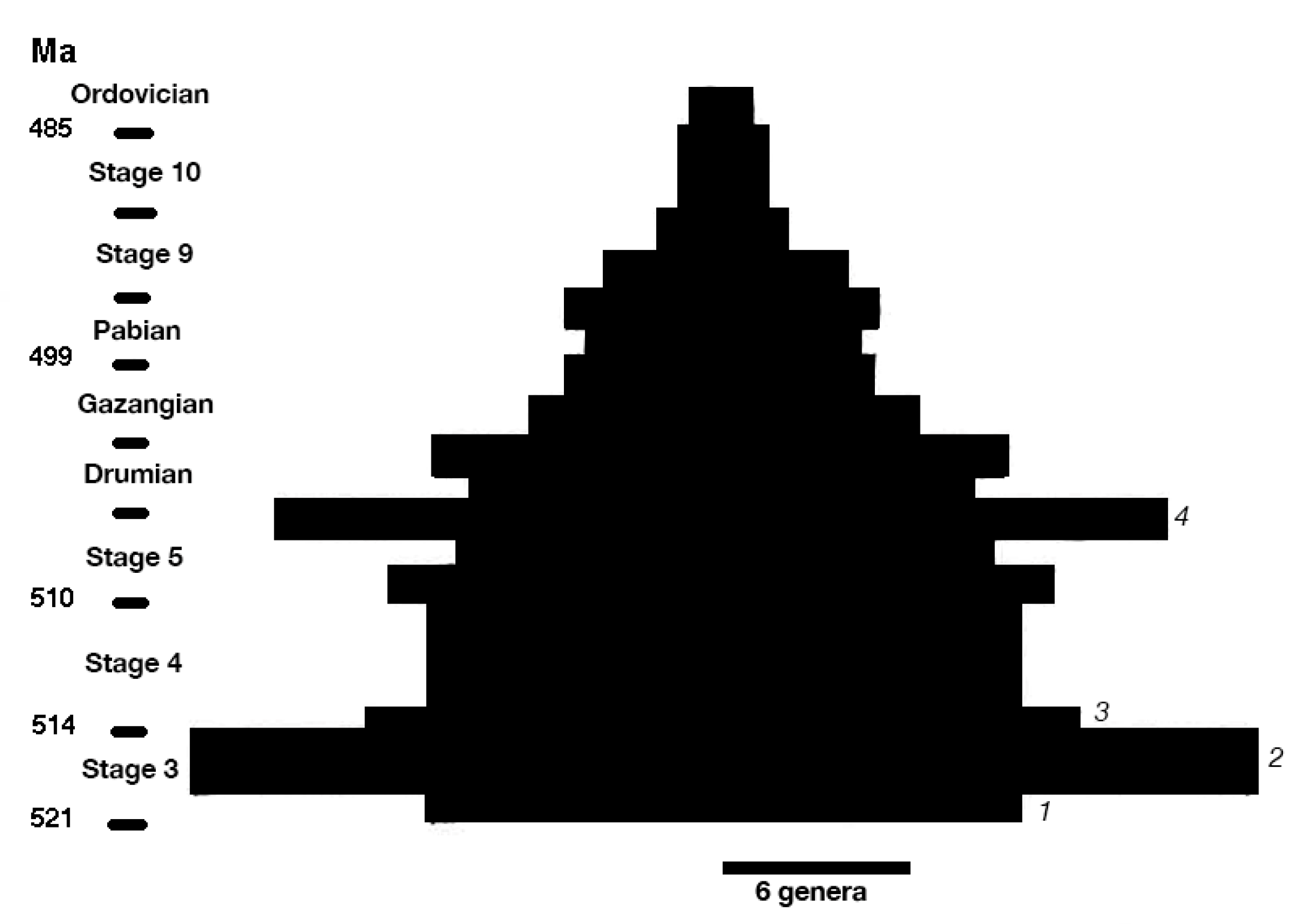


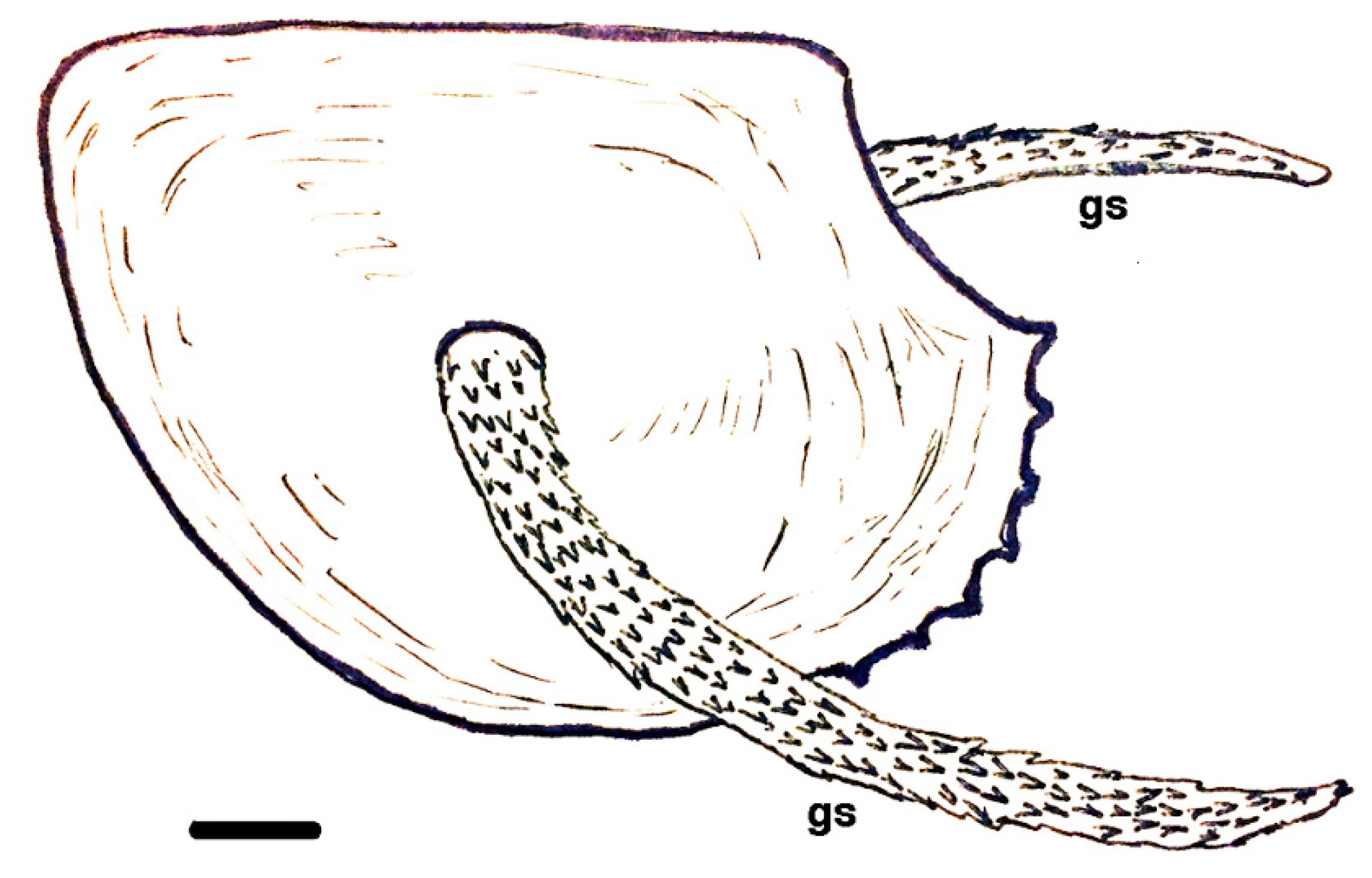
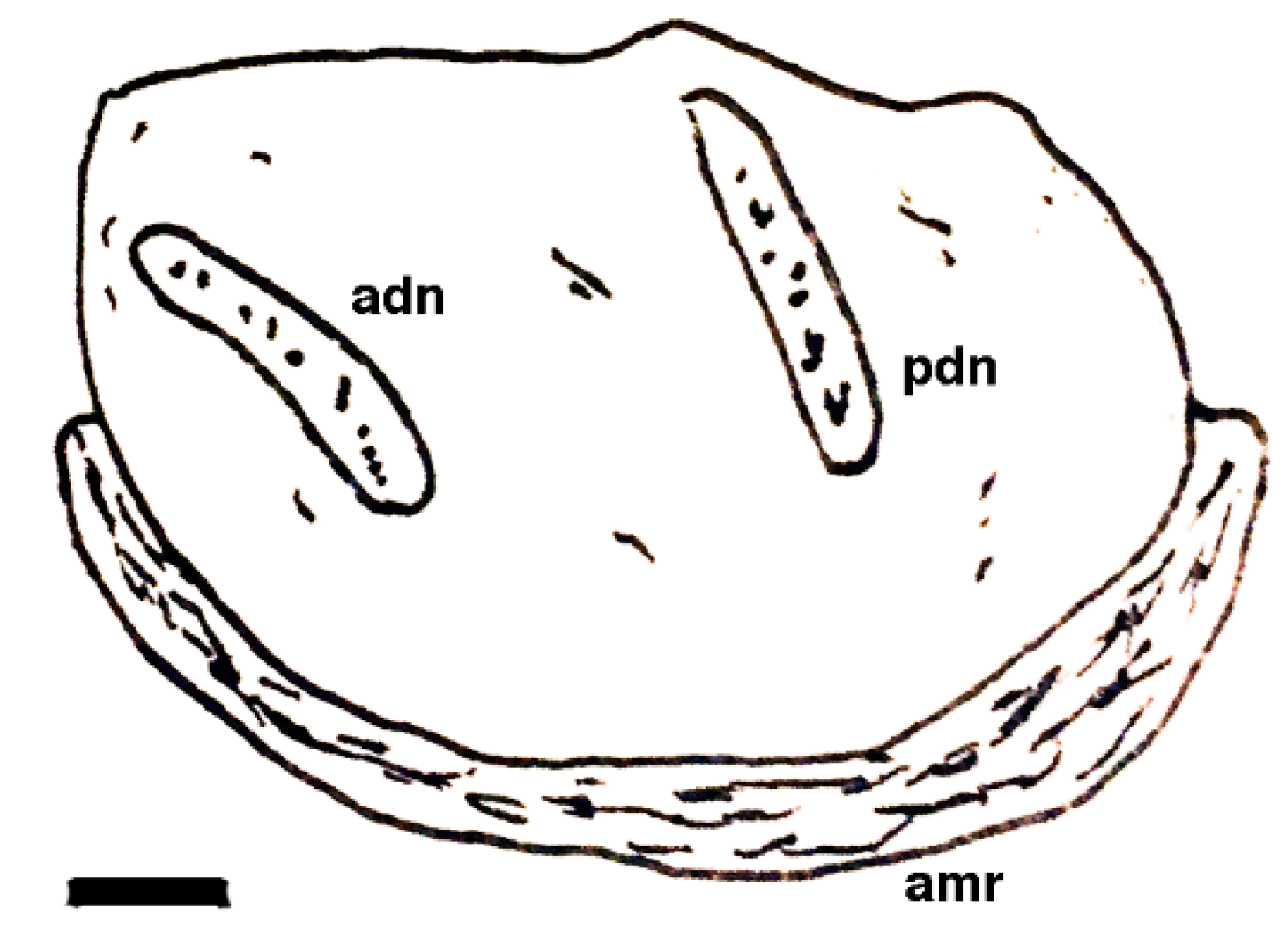
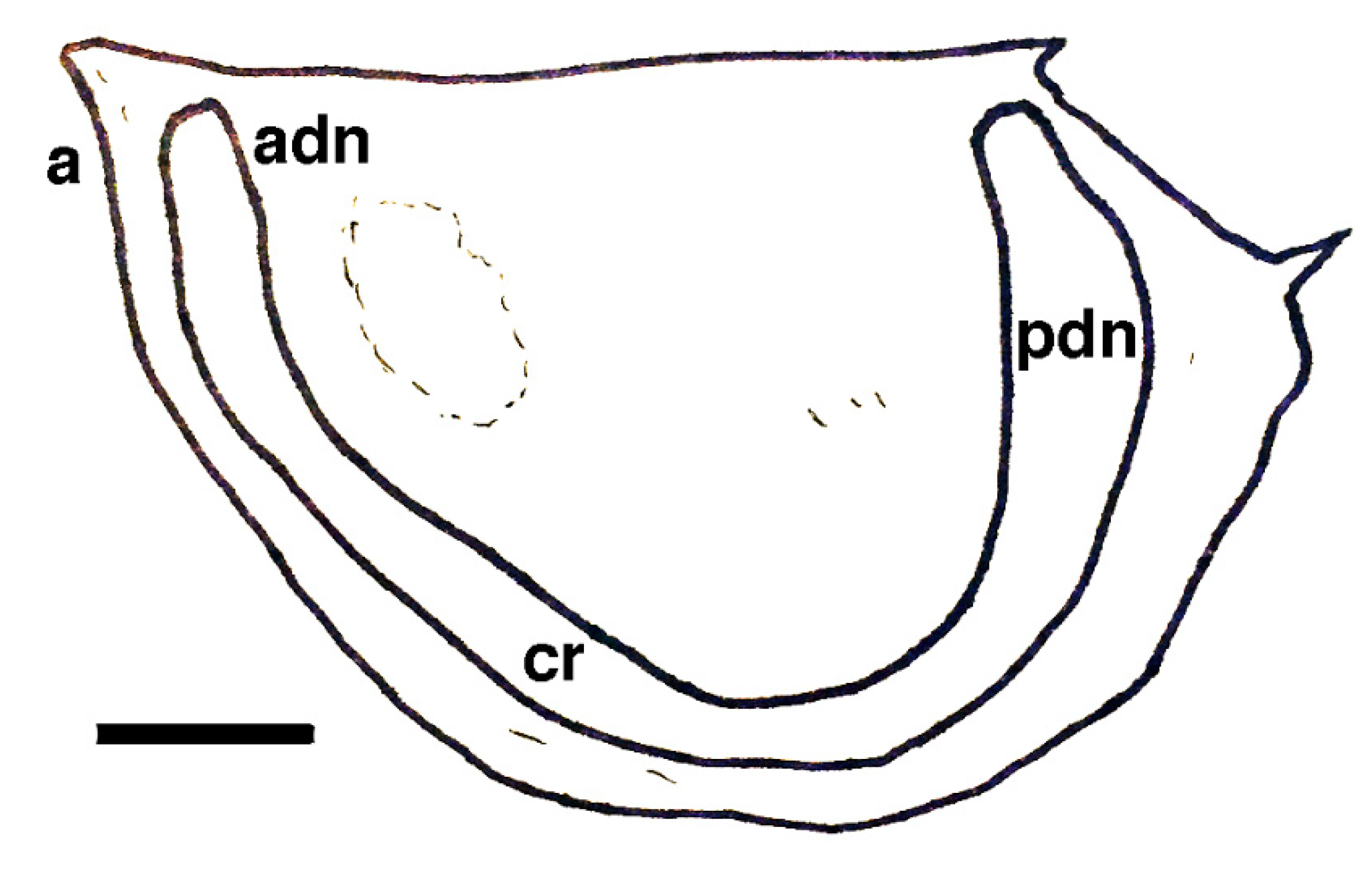
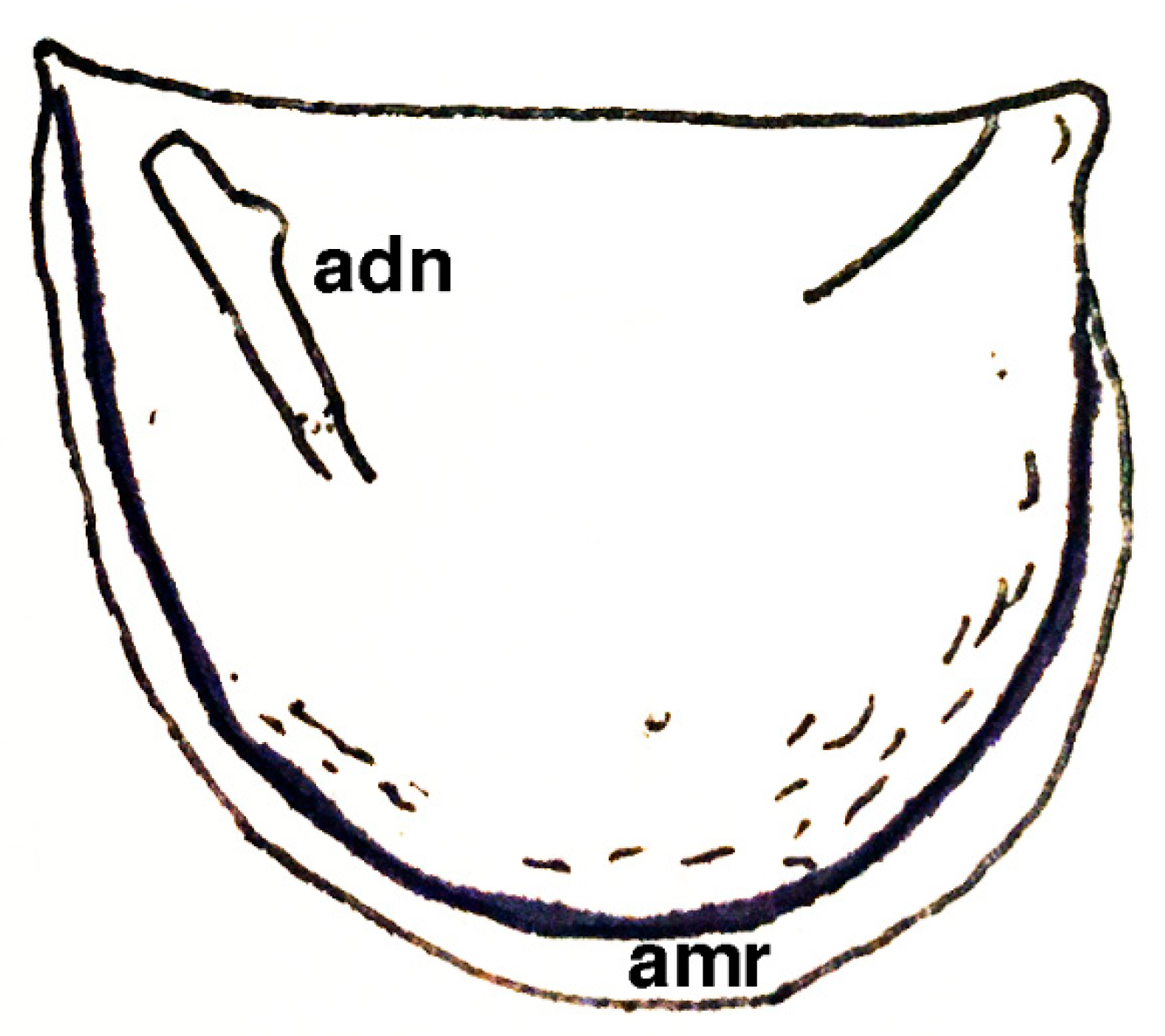
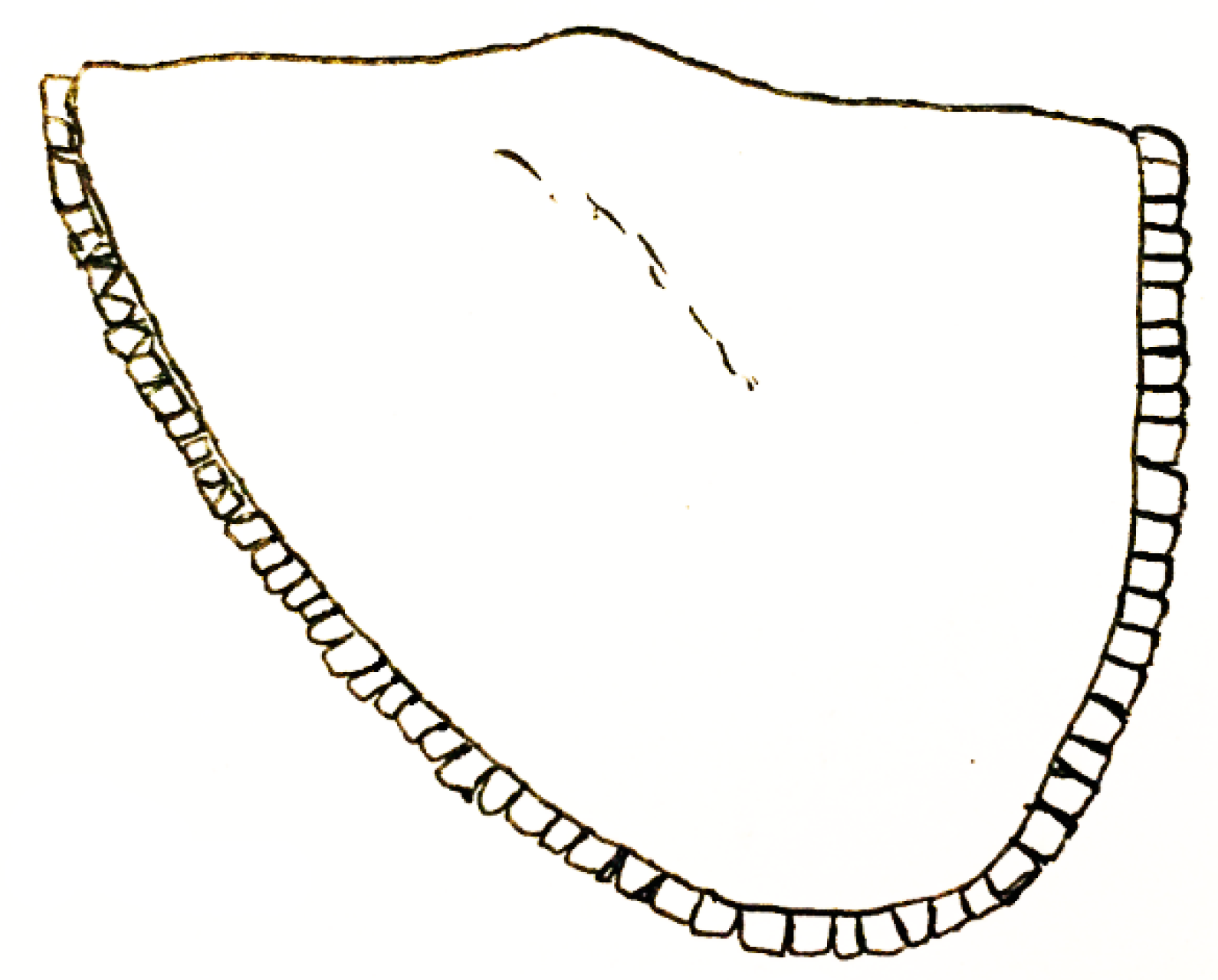
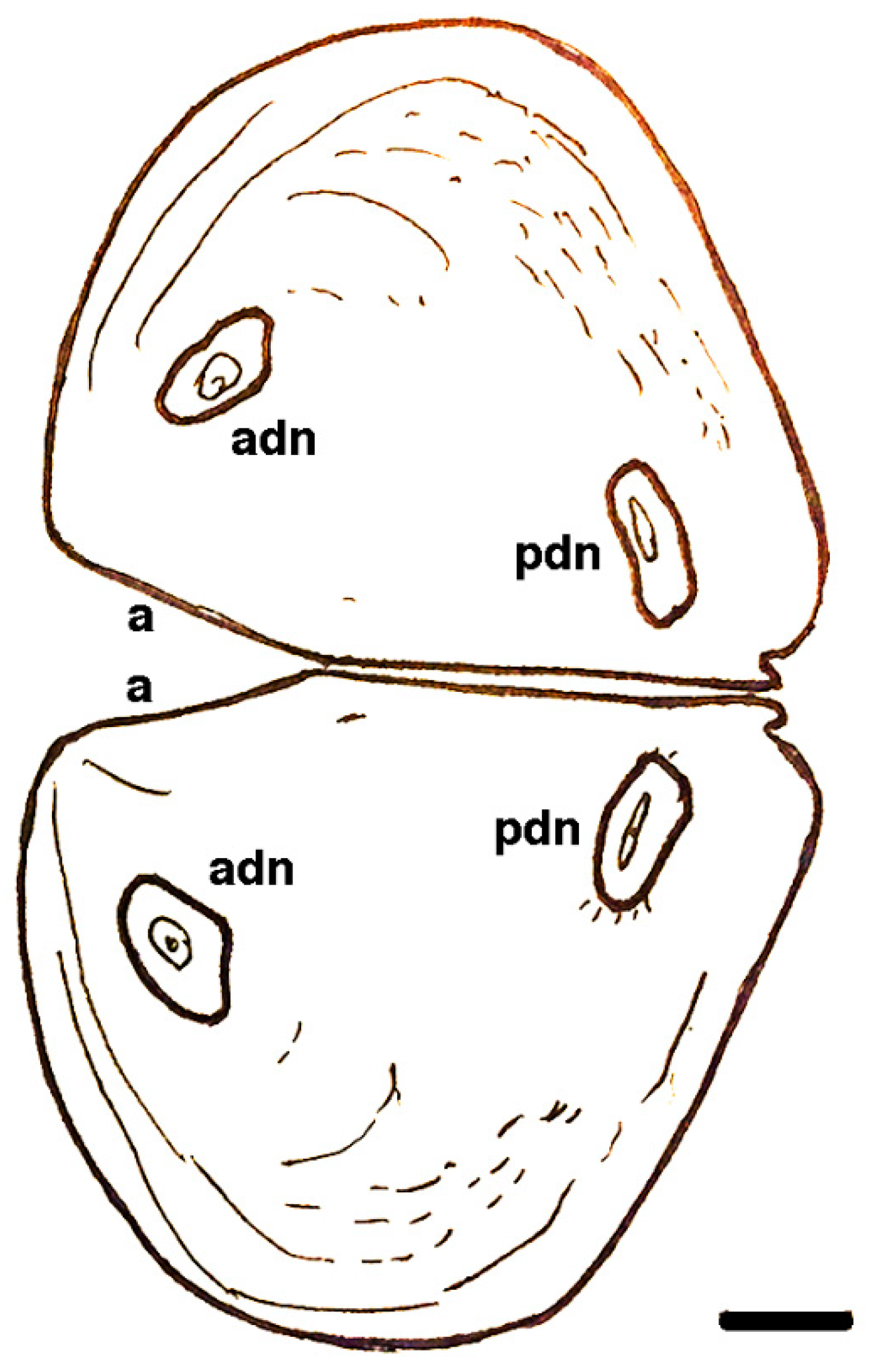
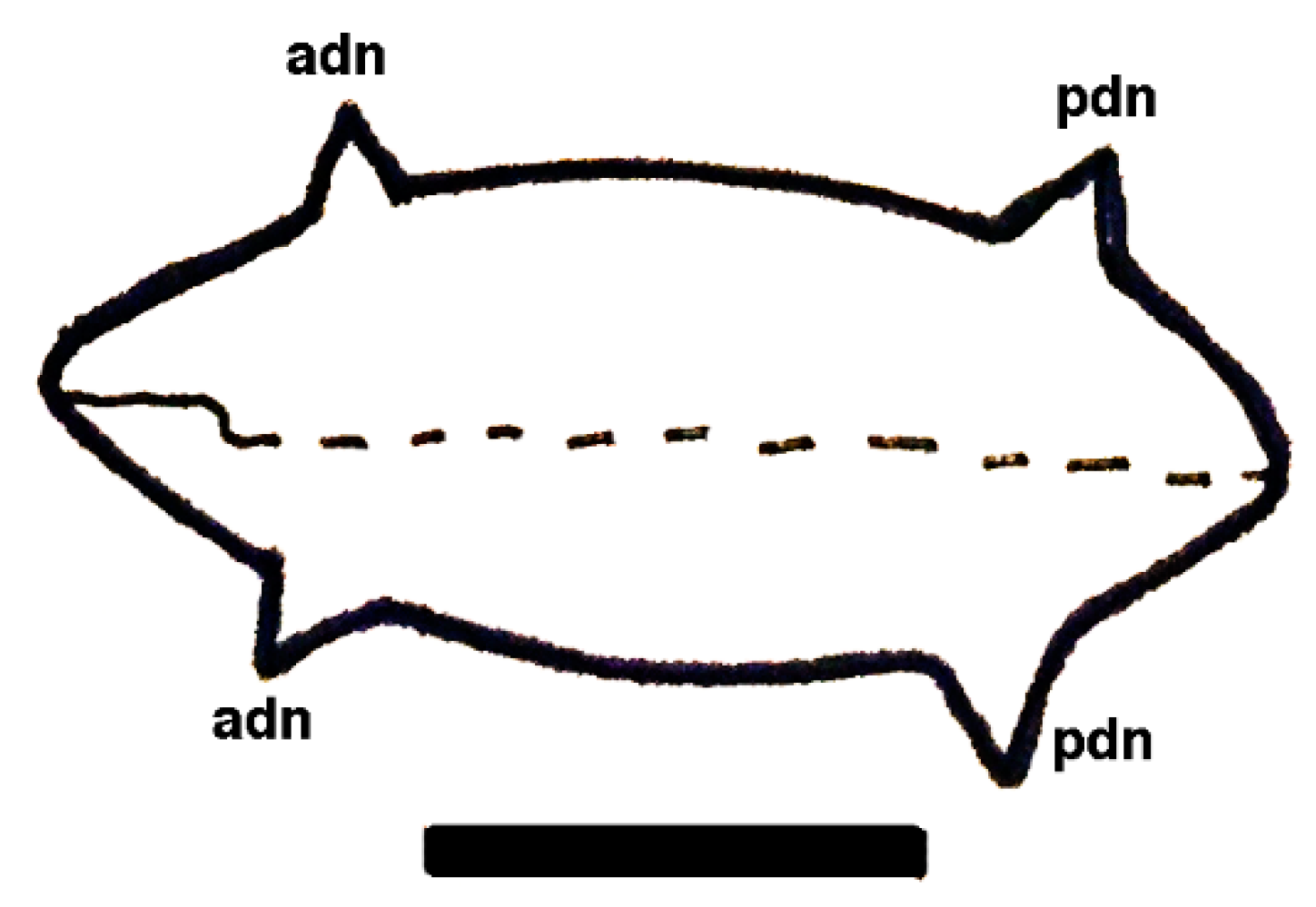
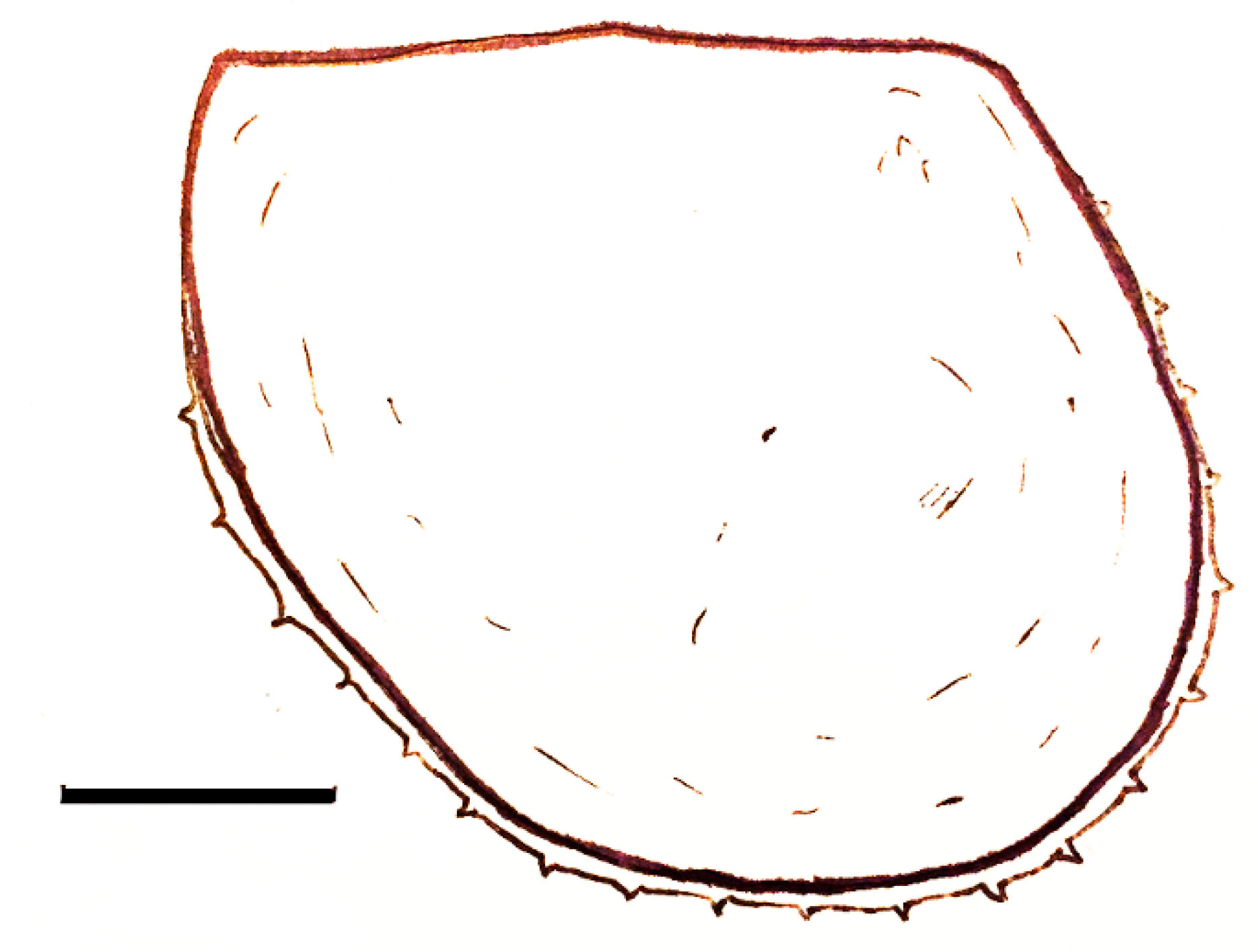







| Short-lived Taxa | Long-lived Taxa | |
|---|---|---|
| Wave 1 (base of Stage 3) | 11 (55%) | 9 (45%) |
| Wave 2 (middle Stage 3) | 16 (76%) | 5 (24%) |
| Wave 3 (late Stage 3) | 11 (73%) | 4 (27%) |
| Wave 4 (late Wuliuan) | 12 (92%) | 1 (8%) |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMenamin, M.A.S. Bradoriids (Arthropoda) and the Cambrian Diversification. Geosciences 2020, 10, 119. https://doi.org/10.3390/geosciences10040119
McMenamin MAS. Bradoriids (Arthropoda) and the Cambrian Diversification. Geosciences. 2020; 10(4):119. https://doi.org/10.3390/geosciences10040119
Chicago/Turabian StyleMcMenamin, Mark A. S. 2020. "Bradoriids (Arthropoda) and the Cambrian Diversification" Geosciences 10, no. 4: 119. https://doi.org/10.3390/geosciences10040119
APA StyleMcMenamin, M. A. S. (2020). Bradoriids (Arthropoda) and the Cambrian Diversification. Geosciences, 10(4), 119. https://doi.org/10.3390/geosciences10040119





