Simple Summary
In the late stages of pregnancy and during lactation, sows experience a marked increase in metabolic activity to support fetal growth and milk production. This heightened metabolic demand raises energy requirements and promotes catabolic processes, often leading to oxidative stress, weakened immune function, and negative impacts on reproductive performance as well as piglet growth. To address these challenges, this study evaluated the use of Scutellaria baicalensis and Lonicera japonica (SL) extracts as dietary supplements for sows. The results showed that chlorogenic acid and baicalin, which are key bioactive components in SL, effectively reduced endotoxin levels, lowered inflammatory markers, enhanced antioxidant capacity, and ultimately improved sow reproductive performance and offspring growth.
Abstract
Harnessing the powerful antioxidant and anti-inflammatory properties of Scutellaria baicalensis and Lonicera japonica (SL), SL extract emerges as a natural and effective dietary strategy to enhance sow reproductive performance and overall health. In this study, 100 multiparous Duroc × Landrace × Yorkshire sows were assigned to either a control diet or a diet supplemented with 0.05% SL extract (n = 100), beginning on day 85 of gestation and continuing until day 21 of lactation, with 50 sows in each group. Duroc boars were the source of semen for artificial insemination. While SL supplementation did not affect litter size, birth weight, or milk composition, it significantly reduced piglet mortality during lactation, from 13.11% to 9.72% (p < 0.05). Compared with the control group, feed intake of sows in the SL group increased from 4.56 kg to 4.70 kg (p < 0.01) during lactation. Furthermore, SL extract enhanced the antioxidant capacity of the sows, reduced malondialdehyde and levels of IL-1β, IL-6, and TNF-α, and increased the plasma soluble cluster of differentiation 14 (sCD14) concentrations (p < 0.05). In vitro, pretreatment of mammary epithelial cells with SL extract (2 μg/mL for 24 h) before lipopolysaccharide stimulation significantly upregulated antioxidant markers, suppressed pro-inflammatory cytokine mRNA expression, and inhibited activation of the NF-κB and MAPK pathways (p < 0.05). These findings highlight the potential of SL extract as a natural feed additive to mitigate oxidative stress and inflammation, ultimately supporting improved reproductive performance and health in sows.
1. Introduction
In late pregnancy and lactation, sows face three main challenges: increased nutrient and energy needs for fetal growth and milk production [1,2], heightened maternal metabolism, which can cause oxidative stress, and metabolic fluctuations. These issues compromise immune function and raise risks such as porcine reproductive and respiratory syndrome virus (PRRSV) and miscarriage [3]. These challenges are exacerbated by climate, season, and poor management conditions. Impaired sow health during lactation can alter milk composition, impacting the piglets’ immunity, health, and growth [4,5].
Chinese herbal medicine, particularly Lonicera japonica from Southern China, has become popular as a feed additive for its minimal side effects and numerous benefits. It contains over 2.0% chlorogenic acid, a strong antioxidant that combats oxygen free radicals and lipid peroxidation [5,6]. Chlorogenic acid also shields cellular integrity from oxidative harm by eliminating hydrogen free radicals [7]. There is empirical proof of Lonicera japonica’s efficacy in livestock: Zhang et al. [8] revealed that its extract could significantly reduce oxidative stress markers in pigs and raise antioxidant enzyme activity after they had contracted PRRSV. Similarly, chlorogenic acid was shown to alleviate oxidative stress and inflammation in lipopolysaccharide (LPS)-stimulated bovine mammary epithelial cells, indicating therapeutic promise for treating mastitis [8]. Beyond merely its antioxidant attributes, Lonicera japonica also hosts antiviral, antibacterial, and anti-inflammatory effects. It suppresses inflammatory cytokines, including Tumor necrosis factor α (TNF-α) and Interleukin 18 (IL-18), by inhibiting the activation of the NF-κB and MAPK pathways so as to upgrade immune regulation [9,10]. Scutellaria baicalensis, another traditional Chinese medicine, contains baicalin (over 9.0%), a flavonoid known for its anti-inflammatory and antioxidant effects [11]. Baicalin enhances antioxidant enzymes like Superoxide Dismutase (SOD) and Catalase (CAT), inhibits oxidases, and neutralizes oxidative substances [12]. In broilers, baicalin could directly block reactive oxygen species (ROS) generation and reduce lipid peroxidation [13]. It also slows pathogenic bacteria and decreases pro-inflammatory cytokines, such as IL-1β, 6, and 8 [14]. The combined extracts may offer synergistic benefits. Zhao et al. [15] found that SL extract increased Total Antioxidant Capacity (T-AOC) levels and reduced malondialdehyde (MDA) content in weaned piglets, indicating a joint enhancing effect. Furthermore, Zhang et al. [16] and Li et al. [17] confirmed the safety and efficacy of these herbs in broilers and weaning piglets. They suggested that the observed improved growth performance may have been due to the regulation of gut microbiota, which improves the digestibility of nutrients.
Several studies have confirmed the safety and efficacy of these herbs in different animals and situations. However, existing studies have not clarified how SL additives enhance piglet performance by improving sow health and milk quality during late pregnancy and lactation. The transmission of immunity, antioxidant, and anti-inflammatory capacities from sow to offspring has recently been a major research focus.
Consequently, this study was conducted from day 85 of gestation in sows through to day 21 of lactation, with the objective of examining the effects of SL additives on sow health, reproductive performance, milk composition, endocrine hormones, and the innate immunity of the offspring. In addition, in vitro experiments were performed on porcine mammary epithelial cells (pMECs). The concentration of SL additives was meticulously maintained at a consistent level across both in vitro and in vivo experiments to facilitate a comprehensive investigation of its mechanisms of action.
2. Materials and Methods
2.1. Materials
The extract powder of SL was combined with 2 major components, with the content of chlorogenic acid effective substances being ≥2.2‰, and the content of baicalin effective substances being ≥2.2%. Cell experiment: chlorogenic acid and baicalin were bought from Sigma (St. Louis and Burlington, MA, USA), 94419 and 572667, and the mixing ratio was 1:10.
Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, Gibco, Grand Island, NY, USA); fetal bovine serum (Gibco, USA); PBS solution (Gibco, USA); penicillin-streptomycin (Sigma, USA); 0.25% trypsin (Sigma, USA); hydrocortisone (Sigma, USA); IGF-1 (Sigma, USA); insulin-transferrin-selenium (ITS, ScienCell, Carlsbad, CA, USA); EGF (PeproTech, Cranbury, NJ, USA); CCK-8 (Nanjing, China, Jiancheng); pure LPS (Escherichia coli 055:B5, Sigma, USA); pure neochlorogenic acid (>98%, Sigma, USA); pure baicalin (>95%, Sigma, USA).
2.2. Methods
2.2.1. Animals and Experimental Design
The feeding experiment took place at the Longkou pig farm, Guangdong Daguang Farming Co., Ltd. (Jiangmen, Guangdong, China), from 1 July to 16 September 2021. Healthy, multiparous sows of the “Duroc × Landrace × Large White” breed with three to six parities were selected at day 85 of pregnancy (n = 100). They were evenly divided into two groups based on delivery date, fatness, parity, and past reproductive performance. Each group had 50 replicates with one sow each. The experiment had a single-factor design: (1) Control group with a basal diet; (2) Experimental group with a basal diet plus 0.05% SL extract (Table 1).

Table 1.
Division of the experimental group.
2.2.2. Diet and Feeding Management
The basal diet for this trial was a corn–soybean meal formulation based on NRC 2012 [18] nutrient standards (Table 2). Sows were allotted in gestation pens (2.15 × 1.15 m) and fed twice daily at 06:30 and 14:30, with ad libitum water. On day 110 of pregnancy, they were moved to the farrowing house, where limited feeding occurred at 1.0 kg (day of farrowing) plus 0.5 kg/day. Limited feeding continued until day 6 of lactation. From days 6 to 21 of lactation, sows were fed ad libitum, and their feed intake was closely monitored. Sows were cross-fostered within groups 24 h post-farrowing, with 10 to 12 pigs per group.

Table 2.
Diet composition and nutrient level (as fed basis)%.
2.2.3. Data Recording and Sample Collection
When sows farrow, the total piglets born, including stillbirths, mummies, live, healthy, and weak piglets (≤0.8 kg), along with litter and individual birth weights, were recorded. On the 21st day, piglet weaning weights, sow estrus 7 days post-weaning, and piglet average daily weight gain during lactation were noted. Sow feed consumption during the 21-day lactation was tracked to calculate average daily feed intake (ADFI). Backfat thickness was measured at the P2 point (6 cm from the midline of the 10th rib on the left side), using an ultrasonic device (Renco Lean-Meater®, Renco Corporation, Minneapolis, MN, USA), on the 1st and 21st days of farrowing, to determine average backfat loss during lactation.
On gestation day 85, farrowing day, and days 14 and 21 post-farrowing, 10 healthy sows from each group, representing average production performance, were selected. About 10 mL of blood was drawn from their ear vein, placed in EDTA tubes (Becton Dickinson, Franklin Lakes, NJ, USA), and centrifuged at 25 °C at 3500× g for 15 min. The plasma was transferred to sterile cryotubes (Becton Dickinson, Franklin Lakes, NJ, USA), snap frozen in liquid nitrogen, and stored at −80 °C for further research. Similarly, on farrowing day and weaning day 21, 6 average piglets from each group had blood collected from the anterior vena cava, processed, and stored using the same method as the sows.
2.2.4. Measurement of Antioxidant, Inflammatory, and Immunoglobulin Indicators
Concentrations of IL-1β (CSB-E06782p), IL-6 (CSB-E06786p), IL-8 (CSB-E06779p), IL-18 (CSB-E13864p), T-AOC (A015-1), MDA (A003-2), SOD (A001-3), GSH-Px (A031-3), and GSH (A030-1) in plasma were measured with the ELISA kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) following the instructions of the manufacturers. IgA (CSB-E13234p), IgG (CSB-E12734G), and IgM (CSB-E12935f) content was assessed per Wuhan Huamei (Wuhan, China) ELISA kit guidelines. Milk samples were taken within 12 h post-farrowing and on the 14th day of lactation. For the latter, 15 IU oxytocin was injected into the ear vein before collection. About 30 mL of milk was collected each time, transferred to 15 mL sterile tubes, and stored in liquid nitrogen before being moved to a −80 °C freezer. Whey was extracted by centrifuging the milk at 3000× g for 20 min at 4 °C, following Zanello et al.’s [19] method.
2.3. In Vitro Cell Experiments
2.3.1. Cell Recovery and Passage
Remove 1 mL of frozen pMECs from liquid nitrogen using tweezers and thaw them in 37 °C water for 3 min. Transfer the thawed cells to a sterile tube with 9 mL of culture medium. Mix, then centrifuge at 1000 r/min for 3 min. Discard the supernatant, rinse the cells with 9 mL PBS, and centrifuge again. Repeat the rinse and centrifuge process once more. Finally, add 10 mL of culture medium, mix to achieve a uniform cell concentration, and adjust the cell density to 1 × 105/mL. Inoculate into a 25 cm2 sterile culture bottle, shake gently, and place in a 37 °C incubator. Once cell coverage reaches 80%, subculture onto a 12-well plate.
2.3.2. Inflammation Model
We used the Cell Counting Kit-8 (CCK-8, Sigma, USA) method to evaluate how different concentrations and exposure times of bacterial lipopolysaccharide (a) affect pMEC viability. Once pMECs were about 80% confluent, they were digested with 0.25% trypsin, centrifuged, and counted. The cell suspension was adjusted to 2 × 104 cells/mL and inoculated into a 96-well plate with 200 μL per well, then incubated at 37 °C with 5% CO2 for 48 h. After removing the medium, fresh medium with varying LPS concentrations (0, 1, 5, 10, 25, 50, 100, 200 μg/mL) was added, creating 8 groups with 6 replicates each. The plate was incubated again for 12, 24, and 48 h. Absorbance at 450 nm was measured using a CCK-8 kit and a microplate reader (Thermo Scientific, Wilmington, NC, USA) to calculate cell viability. Apoptosis was tested using a flow cytometer (Mindray, Shenzhen, China). Cell viability, apoptosis, and inflammatory factor expression were evaluated to establish the inflammatory model conditions.
2.3.3. Effects of Different Concentrations of SL Extract on pMEC Viability
The CCK-8 method assessed the impact of varying Scutellaria baicalensis extract concentrations on cell viability. When pMEC reached 70–80% confluence, cells were digested with 0.25% trypsin, centrifuged, and counted. The cell suspension was adjusted to 1 × 104 cells/mL, and 200 μL was added per well in 96-well plates and incubated at 37 °C with 5% CO2 for 48 h. Different extract concentrations (0–100 μg/mL) were then added, with six replicates per group, and incubated for another 24 h. Afterward, 20 μL of CCK-8 reagent was added to each well, and incubation continued for 4 h. Absorbance at 450 nm was measured using an automatic microplate reader (Thermo Scientific, USA).
2.4. Protein Blotting
For electrophoresis, use a 10% or 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel (P0012AC, Beyotime, Shanghai, China) to separate proteins based on their molecular weight. Transfer the target proteins to a polyvinylidene difluoride (PVDF) membrane using a tri-glycine system. Rinse the membrane three times with tris-buffered saline with 0.1% Tween® 20 detergent (TBST) buffer, then block with 5–6% skim milk at 26 °C for 2 h. After blocking, rinse again with TBST buffer, apply the primary antibody, and incubate overnight at 4 °C. Rinse the membrane three times, add the secondary antibody, and incubate at 26 °C for 1 h. Finally, use super electrogenerated chemiluminescence (ECL) reagent (P1020 ApplyGen, Beijing, China) to detect chemiluminescent signals and quantify them with ImageJ software (LOCI, University of Wisconsin, WI, USA, version 1.52a).
2.5. Real-Time Fluorescence Quantitative PCR
RNA was extracted from the cell line using the EZB kit (EZbioscience, Roseville, MN, USA). The concentration and quality of the extracted RNA were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, NC, USA). The RNA was then reverse transcribed into cDNA using a reverse transcriptase kit (A0010CGQ, EZBioscience, Roseville, MN, USA). RT-PCR was performed to assess inflammatory factor mRNA expression, using β-actin as a control. The PCR cycle was as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The primer design for this experiment was synthesized by Sangon Biotech Co., Ltd. (Shanghai, China), conducted in accordance with the methodology outlined in a previously published article by Zhang et al. [20] and Tian et al. [21] from our research group. Results were analyzed with the 2−ΔΔCt method [22], and primer details are in Table 3.

Table 3.
RT-PCR primers.
2.6. Statistical Analysis
Reproductive performance was analyzed with SPSS 23.0 (IBM, Armonk, New York, NY, USA), presented as mean and standard error. An independent t-test was used to analyze the differences between the two different treatments. The residue normality of the variance of the in vitro cell studies was determined by the Shapiro–Wilk test, then analyzed by one-way ANOVA. Duncan’s method was used to make multiple comparisons. Tendencies were noted at p < 0.10, significant differences at p < 0.05, and highly significant differences at p < 0.01. Graphs were drawn and analyzed using the GraphPad Prism 8.0 software (La Jolla, CA, USA).
3. Results
3.1. Reproductive and Farrowing Performance
In comparison to the control group, the inclusion of SL extract powder in the diet resulted in an 8.70% increase in the litter weight at birth, achieving statistical significance (p < 0.05, Table 4). However, no significant differences were observed in the total number of piglets born, the number of live piglets, the number of healthy piglets, the number of weak piglets, the incidence of stillbirths, the number of mummified fetuses, or the individual weight of the first live piglets (p > 0.05, Table 1).

Table 4.
Effects of dietary SL extract powder on reproductive performance of sows.
3.2. Milk Composition and Lactation Performance
Adding SL extract powder to the diet did not significantly affect colostrum and 14th-day milk composition in lactating sows compared to the control group (p > 0.05, Table 5). However, it significantly improved the survival rate of weaned piglets (p < 0.05, Table 6) and greatly increased sows’ average daily feed intake during lactation (p < 0.01, Table 6). There were no significant changes in litter weight, individual piglet weight, number of weaned piglets, piglet weight gain, sows’ back fat loss during 21 days of lactation, or estrus interval (p > 0.05, Table 7).

Table 5.
Effects of dietary SL extract powder on sow milk composition.

Table 6.
Effects of dietary SL extract on lactation performance of sows.

Table 7.
Effects of dietary SL extract powder on backfat loss and feed intake of lactating sows.
3.3. Plasma Hormone Levels
Compared with the control group, the incorporation of SL extract powder into the sows’ diet significantly elevated the plasma prolactin levels in the sows on the day of farrowing and on the 14th day of lactation (p < 0.10, Table 8), with increases of 8.15% and 5.98%, respectively. However, no significant differences were observed in the levels of corticosterone, insulin, glucagon, T3, T4, and leptin (p > 0.05, Table 8). Furthermore, the addition of SL extract powder was associated with a reduction in plasma endotoxin levels in the sows on the 14th and 21st days of lactation (p < 0.10, Table 9), with decreases of 4.40% and 5.69%, respectively. Additionally, a significant reduction in plasma HSP-70 levels was noted on the 14th day of lactation (p < 0.10, Table 9), with a decrease of 7.57%.

Table 8.
Effects of dietary SL extract powder on plasma-related hormones in sows.

Table 9.
Effects of dietary SL extract powder on endotoxin and HSP-70 in sows.
3.4. Antioxidant Function of Sows and Piglets
According to Table 10, Table 11 and Table 12, compared with the Control group, the inclusion of SL extract powder in the diet of the lactating sows resulted in a statistically significant enhancement of total antioxidant capacity in the plasma, colostrum, milk on day 14, and plasma on day 21 of lactation (p < 0.05), with increases of 13.21%, 9.09%, 5.99%, 12.12%, and 18.52%, respectively. Additionally, there was an observed increase in total antioxidant capacity in the plasma of the sows on the day of farrowing and in the plasma of the newborn piglets (p < 0.10), with increases of 10.91% and 26.0%, respectively. Furthermore, the supplementation significantly reduced malondialdehyde (MDA) content in the plasma, colostrum, and plasma of the newborn piglets on the day of farrowing, as well as on the 14th and 21st days of lactation (p < 0.05), with reductions of 9.97%, 10.03%, 12.38%, 23.01%, and 8.88%, respectively. It also decreased MDA content in the normal milk of the sows on the 14th day and in the plasma of the piglets on the 21st day (p < 0.10), with reductions of 16.02% and 12.76%, respectively.

Table 10.
Effects of dietary SL extract powder on plasma antioxidant indicators in sows.

Table 11.
Effects of dietary SL extract powder on milk antioxidant indicators in sows.

Table 12.
Effects of dietary SL extract powder on plasma antioxidant indicators in piglets.
3.5. Immune Function of Sows
In comparison to the control group, the inclusion of SL extract powder in the diet of the sows resulted in an increase in plasma levels of Ig (immunoglobulin)A, IgG, and IgM by 9.59%, 8.93%, and 9.21%, respectively, on the 14th day of lactation (p < 0.10, Table 13). Additionally, the IgM content in milk on the 14th day showed an increase of 12.07% (p < 0.10, Table 14). However, there was no significant effect on the levels of IgA and IgG in colostrum and milk on the 14th day (p > 0.05).

Table 13.
Effects of a dietary SL extract powder on plasma immunoglobulin levels in serum of sows.

Table 14.
Effects of dietary SL extract powder on plasma immunoglobulin levels in milk of sows.
3.6. Inflammatory Factor Content in Sows
There was a significant decrease in the levels of TNF-α (L0), IL-1β (L14), IL-6 (L0), and IL-8 (L21) in sow plasma, with reductions of 5.71%, 8.29%, 8.15%, and 6.97%, respectively (p < 0.05, Table 15). Additionally, the incorporation of SL extract powder into the diet of sows significantly reduce the IL-8 concentration in their milk by 9.46% on the 14th day (p < 0.01, Table 16). Furthermore, a downward trend was observed in the concentrations of TNF-α (L14), IL-6 (L14), and IL-18 (L0/L14) in sow plasma, as well as TNF-α and IL-18 in their colostrum, with respective decreases of 8.12%, 6.11%, 8.22%, 6.90%, 6.56%, and 9.11% (p < 0.10, Table 16). The supplementation also significantly increased the soluble cluster of differentiation 14 (sCD14) concentration in sow plasma, by 10.29% on the 14th day (p < 0.05, Table 15), and showed an upward trend in sCD14 levels in sow colostrum, with an increase of 10.24% (p < 0.10, Table 16). Similar tendency was shown in piglets, as the levels of TNF-α (L0) decreased by 10.70% (Table 17).

Table 15.
Effects of dietary SL extract powder on plasma inflammatory cytokines in sows.

Table 16.
Effects of dietary SL extract powder on milk inflammatory cytokines in sows.

Table 17.
Effects of dietary SL extract powder on inflammatory cytokines in piglets.
3.7. Establishment of In Vitro Inflammatory Model
According to Figure 1A, the cell viability of pMECs exhibited a general decline with increasing concentrations of LPS and prolonged treatment duration. An optimal LPS concentration of 50 μg/mL and a treatment duration of 24 h were identified for further investigation. Under these conditions, a significant increase in cell apoptosis was observed (p < 0.05, Figure 1B). Concurrently, the expression levels and content of inflammatory factor mRNA were assessed, revealing a significant upregulation at this time point (p < 0.05, Figure 1C,D). Considering the parameters of cell viability, apoptosis, and inflammatory factor expression, the conditions selected for establishing the inflammatory model were an LPS concentration of 50 μg/mL and a treatment duration of 24 h.
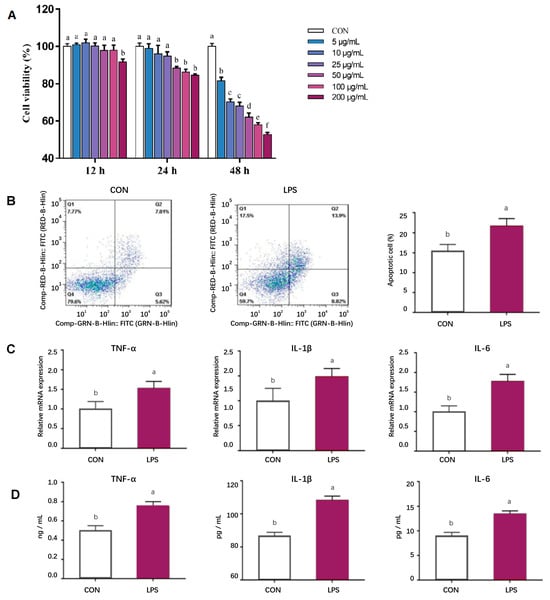
Figure 1.
Establishment of in vitro inflammatory model. (A) Effects of LPS treatment at different levels (0, 5, 10, 25, 50, 100, 200 μg/mL) and times (12, 24, 48 h) on pMEC viability; (B) Effects of adding 50 μg/mL of LPS treatment for 24 h on pMEC apoptosis; (C) Effects of adding 50 μg/mL of LPS treatment for 24 h on the mRNA expression abundance of inflammatory factors in pMECs; (D) Effects of adding 50 μg/mL of LPS treatment for 24 h on the content of inflammatory factors in pMECs-related cells. Data are expressed as mean ± standard error, n = 6; Different superscript letters indicate significant differences (p < 0.05). pMEC: Porcine mammary epithelial cells. CON: Control group. LPS: Challenged by lipopolysaccharide.
3.8. Effect of SL Extract on pMEC Viability
The effects of SL extract on pMEC viability are shown in Figure 2. The viability of pMECs first increased and then decreased in the range of 0–5 μg/mL. Cell viability was highest at 2 μg/mL. Therefore, 2 μg/mL was selected as the optimal pretreatment concentration of SL extract in this experiment. Pretreatment of pMECs with 2 μg/mL SL extract for 24 h significantly alleviated the decrease in cell viability induced by 50 μg/mL of LPS (p < 0.05, Figure 3).
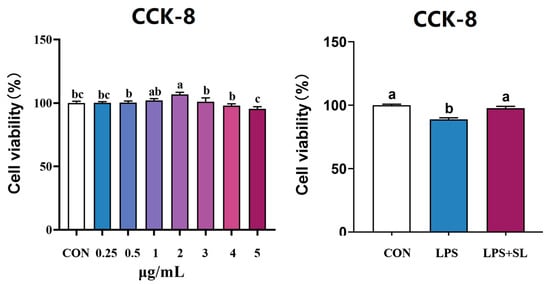
Figure 2.
Effect of SL extract on pMEC viability. Data are expressed as mean ± standard error, n = 6; Different superscript letters indicate significant differences (p < 0.05). CCK-8: Cell Counting Kit-8. CON: Control group. LPS: Challenged by lipopolysaccharide. SL: Scutellaria baicalensis and Lonicera japonica treated.
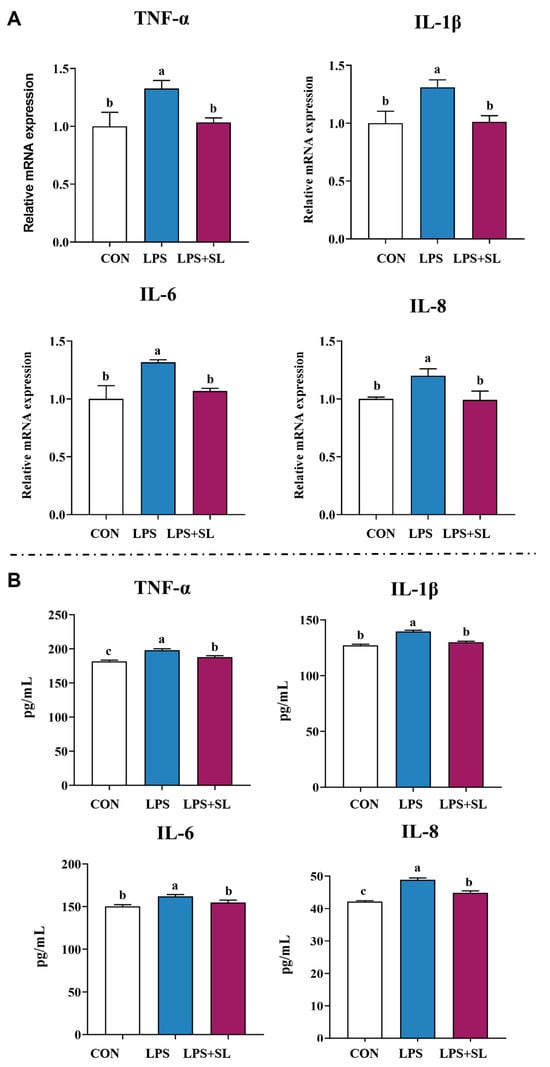
Figure 3.
Effect of SL extract on relative mRNA expression (A) and content (B) of inflammatory cytokines. Data are expressed as mean ± standard error, n = 6; Different superscript letters indicate significant differences (p < 0.05). TNF-α: Tumor necrosis factor α. IL: Interleukin. CON: Control group. LPS: Challenged by lipopolysaccharide.
3.9. Effects of SL Extract on Inflammatory Factors, Antioxidant Status, and Inflammatory Signaling Pathways in pMECs Induced by LPS
A 24 h pretreatment with SL extract significantly inhibited the LPS-induced upregulation of both mRNA expression and the content of inflammatory factors of TNF-α, IL-1β, IL-6, and IL-8, thereby alleviating cellular inflammatory damage (p < 0.05, Figure 3). Moreover, pretreatment with SL extract resulted in a notable increase in total antioxidant capacity (T-AOC) and superoxide dismutase (SOD) levels in cells subjected to inflammatory damage, while simultaneously decreasing intracellular malondialdehyde (MDA) content (p < 0.05, Figure 4).
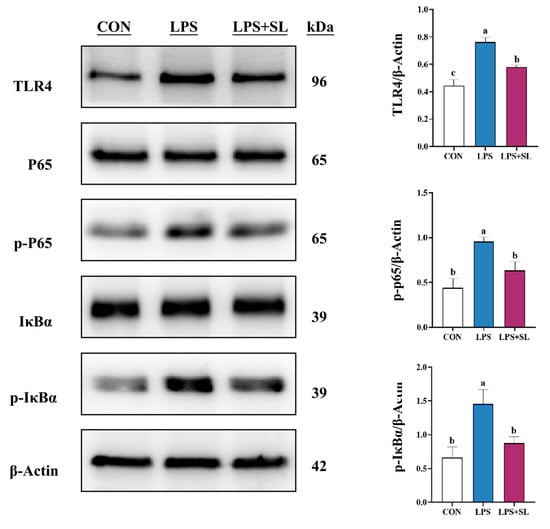
Figure 4.
Effect of SL extract on NF-κB signaling pathway in pMECs. Data are expressed as mean ± standard error, n = 3; Different superscript letters indicate significant differences (p < 0.05). TLR4: Toll-like receptor 4. p65: Transcription factor p65. p-p65: Phosphorylated transcription factor p65. IκBα: Inhibitor kappa B alpha. p-IκBα: Phosphorylated inhibitor kappa B alpha. CON: Control group. LPS: Challenged by lipopolysaccharide. SL: Scutellaria baicalensis and Lonicera japonica treated. kDa: kilodalton.
LPS exposure led to a significant elevation in the phosphorylation levels of proteins involved in the NF-κB and MAPK inflammatory signaling pathways (p < 0.05, Figure 4 and Figure 5). Nonetheless, SL extract pretreatment effectively inhibited the phosphorylation of transcription factor p65 (p65), inhibitor kappa B alpha (IκBα), p38 mitogen-activated protein kinase (p38), extracellular signal-regulated kinase (ERK), and Jun N-terminal kinase (JNK) proteins, thereby substantially mitigating the inflammatory response in mammary epithelial cells (p < 0.05, Figure 4 and Figure 5).
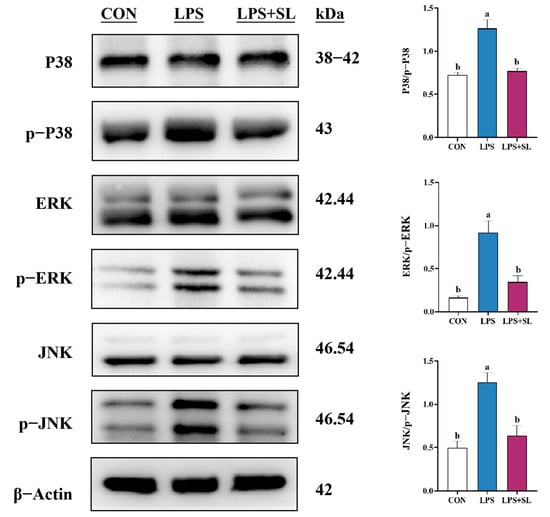
Figure 5.
Effect of SL extract on MAPK signaling pathway in pMECs. Data are expressed as mean ± standard error, n = 3; different superscript letters indicate significant differences (p < 0.05). p38: p38 mitogen-activated protein kinase. p-p38: phosphorylated p38 mitogen-activated protein kinase. ERK: Extracellular signal-regulated kinase. p-ERK: Phosphorylated Extracellular signal-regulated kinase. JNK: Jun N-terminal kinase. p-JNK: phosphorylated Jun N-terminal kinase. CON: Control group. LPS: Challenged by lipopolysaccharide. SL: Scutellaria baicalensis and Lonicera japonica treated. kDa: kilodalton.
4. Discussion
4.1. Challenges for Sows in Late Gestation and Lactation
Animal health relies on sustaining a balanced physiological state. Sows face metabolic changes that can disrupt homeostasis from late pregnancy to lactation. At this time, they are highly vulnerable to oxidative stress, immunosuppression, and inflammation. These issues can be further exacerbated by high temperature and humidity in the summer, intensifying the influence of these negative factors. A key hormone for fetal growth and milk production, prolactin, was first identified in the cow and subsequently in humans. It is produced through the H-P-A axis and regulated by the nervous and hormonal systems [23]. If conditions of oxidative stress persist, the subsequent reduction in prolactin levels usually results in negative outcomes, including smaller litter size, lower survival rate of piglets, and decreased vitality among the piglets [24,25]. In the presence of such virulent diseases as PRRSV and Porcine Epidemic Diarrhea (PED), as the health of sows in late pregnancy is threatened, the number of total-born, live-born, and healthy piglets decreases significantly [26,27].
4.2. Antioxidant Effects of SL Extract
Chlorogenic acid and baicalin in SL extract possess potent antioxidant activity, reducing deleterious free radicals and protecting the body from oxidative stress [28]. Baicalin, the main active compound of Scutellaria baicalensis, also exhibits powerful antioxidant activity, enhancing antioxidant enzymes and decreasing the activity of oxidase [29]. It has been found that chlorogenic acid (600 mg/kg) can improve T-AOC and T-SOD levels, reduce MDA levels in heat-stressed chicks, and alleviate inflammatory bacteria colonization [30]. In weaned piglets, 10,000 mg per kg chlorogenic acid supplementation increases GSH-Px and CAT, while decreasing MDA. It controls the impairment of the intestinal barrier caused by weaning stress [31].
4.3. Anti-Inflammatory and Immunomodulatory Effects of SL Extract
Other signs of elevated oxidative stress burden include increasing inflammatory cytokines and a decrease in immune function. Levels of immunoglobulins reflect the immune capacity [32]. Studies have shown that SL extract added to the feed of broilers can raise the content of serum IgA and IgG, enhance their immunity, and improve production performance [33]. Dietary inclusion of 0.5 g/kg chlorogenic acid in the feed reduces serum IL-6 and IL-10 levels and improves IgA concentration in broilers, subsequently producing positive effects in their immunity and growth performance [34]. Furthermore, baicalin decreases the level of serum IgE and histamine in mice with nasal sinusitis, thus enhancing their immune response, and has a therapeutic effect [35]. Supplementation with baicalin in the diet reduced the serum concentrations of IgE, IL-1β, IL-4, IL-6, TNF-α, and histamine in rats with allergic rhinitis, which suggests its potential as a therapeutic agent for allergic rhinitis through inhibition of inflammation mediators [36]. Chlorogenic acid attenuates Dextran Sulfate Sodium Salt (DSS)-induced ulcerative colitis in mice by decreasing inflammation and cell apoptosis in the colon through inhibition of the MAPK/ERK/JNK signaling pathway [37]. It reduces levels of TNF-α, IL-1β, and mRNA expressions of these cytokines in hyperuricemic mice [38].
4.4. Intergenerational Transmission of Health Inequalities
If the late pregnancy health of sows is impaired, the proportion of total, live, and healthy litters is decreased compared to that of normal sows [39]. Furthermore, the feed intake and milk composition of sows are altered, which causes negative effects on the weaning weight of piglets when challenged by stress during lactation [40]. Additional experimental data show that weak piglets with lower birth weight probably suffer further developmental retardation and fail to keep up with the growth of their healthier littermates within the same litter [41]. Therefore, we hypothesized that these susceptible piglets nursed by sows subjected to stress that produces low-quality milk will exhibit even greater decreases in growth and total output potential.
In the present study, results indicated that sows and piglets had markedly higher antioxidant levels when the sows’ ration was supplemented with SL extract during late gestation and lactation, and their MDA content was decreased by 33.29% compared to the control group. These findings indicate that SL extract has a potent antioxidant and anti-inflammatory activity. Also, the addition of SL had a minor influence on immunoglobulin levels in sow plasma, milk, and piglet plasma. Additionally, it significantly increased IgG, IgA, and IgM concentrations in sow plasma on day 14 of lactation (p < 0.05), which is consistent with other results. Also, TNF-α, IL-1β, and IL-6 in sow plasma and IL-8 in normal milk and IL-8 and IL-18 in sow plasma, colostrum, and piglet plasma were lower when supplemented by dietary SL extract. The reduction in pro-inflammatory factors and increase in immunoglobulins indicate that SL extract has strong anti-inflammatory properties. This effect supports its potential for clinical use. The enhancement of immune functions is possibly attributed to the antioxidant, anti-inflammatory, and immunoregulatory functions of SL extract. Additionally, the present study revealed that adding 0.5 kg/T of SL extract to the sows’ diet could increase the litter weight of newborn piglets (p < 0.10), significantly enhance the survival rate of weaned piglets (p < 0.05), and dramatically increase the sows’ feed intake during lactation (p < 0.01).
4.5. Antioxidant and Immunoregulatory Effects on Porcine Mammary Epithelial Cells (PMECs)
In order to clarify the impact of sows’ antioxidant capacity and immune function on their milk and offspring, in vitro studies on porcine mammary epithelial cells (PMECs) were conducted.
Previous study shows that chlorogenic acid decreases TNF-α, IL-1β, and IL-6 in LPS-stimulated RAW 264.7 macrophages [42]. SL reduces levels of Nitric Oxide (NO), Prostaglandin E2 (PGE2), IL-12, IL-2, IL-6, IL-7, and TNF-α in LPS-induced Raw 264.7 cells and lowers inflammation and stimulates cell growth [43]. Baicalin attenuates LPS-induced microglial inflammation via suppressing NF-κB and MAPKs pathways’ phosphorylation in BV-2 microglia and plays a role by downregulating inflammation [44]. It decreases apoptosis and downregulates apoptosis-related genes in Glaesserella parasuis (GPS)-stimulated porcine peritoneal mesothelial cells (PMCs), alleviating GPS-induced inflammation by suppressing MAPK-pathway activation [45]. It has been shown that chlorogenic acid could downregulate phosphorylation of proteins of the MAPK and NF-κB pathways in Human gingival fibroblasts 1(HGF-1) cells, ameliorating LPS-induced inflammatory response [46]. Moreover, chlorogenic acid inhibits pro-inflammatory cytokines and mediators such as TNF-α, IL-6, IL-1β, Cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and NO, and inactivates the NF-κB and MAPK pathway, suppressing cellular inflammatory injury [47]. The antioxidant activity of cells can be strengthened by increasing the activity of superoxide dismutase (SOD) and the production of nitric oxide (NO) via the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, thereby reducing the damage of oxidative stress [48]. Chlorogenic acid also inhibits the production of Reactive oxygen species (ROS) in IL-1β-treated human umbilical vein endothelial cells (HUVECs) and upregulates endogenous antioxidant capacity and partially preserves the activity of LPS-reduced autophagy in human chondrocytes by inducing autophagy and reducing ROS-induced cell apoptosis [49]. However, there are limited reports on the impact of SL extract on the anti-oxidative capacity of PMECs.
In this study, SL extract significantly raised T-AOC and SOD levels and lowered MDA (p < 0.05), thereby enhancing cellular antioxidant capacity and alleviating inflammation in sow mammary epithelial cells. Also, it had a significantly inhibitory effect on the expression of TLR4, p-P38, p-IκBα, p-p65, p-ERK, p-JNK, MAPK, and NF-κB pathways’ phosphorylation, which led to a dramatic reduction in inflammatory cytokines. These results are not only congruent with previous studies in various cells, but also with the results of our in vivo studies.
5. Conclusions
This experiment examined the effect of dietary SL extract in in vivo and in vitro models. It proved that adding SL extract powder to the diet of sows from late pregnancy to lactation improved their production performance by improving their antioxidant capacity and immunity and reducing the inflammation level of the sows. Part of the positive effect was transmitted to piglets through colostrum and milk, and, furthermore, enhanced the piglets’ performance.
Author Contributions
W.G. administrated the project. H.L. provided resources and labs for in vitro studies. F.C. designed the experiments. W.C. conducted the experiment. H.D., J.Y. and H.J. analyzed the data. N.W. wrote the paper. F.C., W.G. and N.W. conducted the final editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key R&D Program of China (2021YFD1300401-2).
Institutional Review Board Statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the South China Agricultural University Animal Care and Use Committee (Guangzhou, China), with the approval code SCAU#2023018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article. The datasets generated for this study are available on request from the corresponding author.
Conflicts of Interest
Huiyuan Lv is currently employed at Beijing Centre Biology Co., Ltd. However, she provided assistance for this experiment during her doctoral degree from China Agricultural University. The authors declare that the company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Goodband, R.D.; Tokach, M.D.; Goncalves, M.A.D.; Woodworth, J.C.; Dritz, S.S.; DeRouchey, J.M. Nutritional Enhancement during Pregnancy and Its Effects on Reproduction in Swine. Anim. Front. 2013, 3, 68–75. [Google Scholar] [CrossRef]
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient Requirements of the Modern High-Producing Lactating Sow, with an Emphasis on Amino Acid Requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Hörtenhuber, S.J.; Schauberger, G.; Mikovits, C.; Schönhart, M.; Baumgartner, J.; Niebuhr, K.; Piringer, M.; Anders, I.; Andre, K.; Hennig-Pauka, I.; et al. The Effect of Climate Change-Induced Temperature Increase on Performance and Environmental Impact of Intensive Pig Production Systems. Sustainability 2020, 12, 9442. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic Acid: A Dietary Phenolic Acid with Promising PharmacotherapeuticPotential. Curr. Med. Chem. 2023, 30, 3905–3926. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.-C.; Ji, X.-Y.; Che, H.-Y.; Meng, Y.; Wu, H.-Y.; Zhang, J.-B.; Zhang, Y.-H.; Yuan, B. CGA Alleviates LPS-Induced Inflammation and Milk Fat Reduction in BMECs through the NF-κB Signaling Pathway. Heliyon 2024, 10, e25004. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Yu, M.-C.; Chang, Y.-C.; Wu, Y.-H.; Huang, K.-H.; Tsai, M.-M. Lonicera japonica Thunb. Ethanol Extract Exerts a Protective Effect on Normal Human Gastric Epithelial Cells by Modulating the Activity of Tumor-Necrosis-Factor-α-Induced Inflammatory Cyclooxygenase 2/Prostaglandin E2 and Matrix Metalloproteinase 9. Curr. Issues Mol. Biol. 2024, 46, 7303–7323. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological Action and Potential Targets of Chlorogenic Acid. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 87, pp. 71–88. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology and Toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- AlSaad, A.M.; Mohany, M.; Almalki, M.S.; Almutham, I.; Alahmari, A.A.; AlSulaiman, M.; Al-Rejaie, S.S. Baicalein Neutralizes Hypercholesterolemia-Induced Aggravation of Oxidative Injury in Rats. Int. J. Med. Sci. 2020, 17, 1156–1166. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, S.; Zhou, M. Effect of the Flavonoid Baicalein as a Feed Additive on the Growth Performance, Immunity, and Antioxidant Capacity of Broiler Chickens. Poult. Sci. 2019, 98, 2790–2799. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mortazavi, Z.; Mehri, S.; Hosseinzadeh, H. Protective and Therapeutic Effects of Scutellaria baicalensis and Its Main Active Ingredients Baicalin and Baicalein against Natural Toxicities and Physical Hazards: A Review of Mechanisms. DARU J. Pharm. Sci. 2022, 30, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, M.; Xiong, L.; Lin, T.; Zhang, S.; Yue, X.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal Supplementation with Glycerol Monolaurate Improves the Intestinal Health of Suckling Piglets by Inhibiting the NF-κB/MAPK Pathways and Improving Oxidative Stability. Food Funct. 2023, 14, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jin, E.; Liu, X.; Ji, X.; Hu, H. Effect of Dietary Fructus Mume and Scutellaria baicalensis Georgi on the Fecal Microbiota and Its Correlation with Apparent Nutrient Digestibility in Weaned Piglets. Animals 2022, 12, 2418. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Bai, S.; Wang, J.; Zeng, Q.; Peng, H.; Lv, H.; Mu, Y.; Xuan, Y.; Li, S.; et al. Extract of Scutellaria baicalensis and Lonicerae Flos Improves Growth Performance, Antioxidant Capacity, and Intestinal Barrier of Yellow-Feather Broiler Chickens against Clostridium Perfringens. Poult. Sci. 2024, 103, 103718. [Google Scholar] [CrossRef] [PubMed]
- National Research Council, Division on Earth and Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Zanello, G.; Meurens, F.; Serreau, D.; Chevaleyre, C.; Melo, S.; Berri, M.; D’Inca, R.; Auclair, E.; Salmon, H. Effects of Dietary Yeast Strains on Immunoglobulin in Colostrum and Milk of Sows. Vet. Immunol. Immunopathol. 2013, 152, 20–27. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, L.; Chen, J.; Tian, Z.; Liu, J.; Chen, F.; Ren, M.; Guan, W.; Zhang, S. Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-κB and MAPK Signaling Pathways. Animals 2021, 11, 1528. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, J.; Lin, T.; Zhu, J.; Gan, H.; Chen, F.; Zhang, S.; Guan, W. Dietary Supplementation with Lysozyme–Cinnamaldehyde Conjugates Enhances Feed Conversion Efficiency by Improving Intestinal Health and Modulating the Gut Microbiota in Weaned Piglets Infected with Enterotoxigenic Escherichia Coli. Animals 2023, 13, 3497. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, Function, and Regulation of Secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Loisel, F.; Farmer, C.; Van Hees, H.; Quesnel, H. Relative Prolactin-to-Progesterone Concentrations around Farrowing Influence Colostrum Yield in Primiparous Sows. Domest. Anim. Endocrinol. 2015, 53, 35–41. [Google Scholar] [CrossRef]
- Farmer, C. Altering Prolactin Concentrations in Sows. Domest. Anim. Endocrinol. 2016, 56, S155–S164. [Google Scholar] [CrossRef]
- Rowland, R.R.R. The Interaction between PRRSV and the Late Gestation Pig Fetus. Virus Res. 2010, 154, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Olanratmanee, E.; Kunavongkrit, A.; Tummaruk, P. Impact of Porcine Epidemic Diarrhea Virus Infection at Different Periods of Pregnancy on Subsequent Reproductive Performance in Gilts and Sows. Anim. Reprod. Sci. 2010, 122, 42–51. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The Main Bioactive Compounds of Scutellaria baicalensis Georgi. for Alleviation of Inflammatory Cytokines: A Comprehensive Review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Zhao, N.; Yang, X.; Du, E.; Huang, S.; Guo, W.; Zhang, W.; Wei, J. Effect of Chlorogenic Acid on Intestinal Inflammation, Antioxidant Status, and Microbial Community of Young Hens Challenged with Acute Heat Stress. Anim. Sci. J. 2021, 92, e13619. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic Acid Improves Intestinal Barrier Functions by Suppressing Mucosa Inflammation and Improving Antioxidant Capacity in Weaned Pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, T.; Hammarström, L.; Zhao, Y. The Immunoglobulins: New Insights, Implications, and Applications. Annu. Rev. Anim. Biosci. 2020, 8, 145–169. [Google Scholar] [CrossRef]
- Liu, F.-X.; Sun, S.; Cui, Z.-Z. Analysis of Immunological Enhancement of Immunosuppressed Chickens by Chinese Herbal Extracts. J. Ethnopharmacol. 2010, 127, 251–256. [Google Scholar] [CrossRef]
- Liu, H.; Chen, P.; Lv, X.; Zhou, Y.; Li, X.; Ma, S.; Zhao, J. Effects of Chlorogenic Acid on Performance, Anticoccidial Indicators, Immunity, Antioxidant Status, and Intestinal Barrier Function in Coccidia-Infected Broilers. Animals 2022, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, X.; Fu, F.; Guo, Q.; Zhu, X.; Xu, Y.; Yan, X.; He, X.; Wang, X. Baicalin Ameliorates Inflammatory Response in a Mouse Model of Rhinosinusitis via Regulating the Treg/Th17 Balance. Ear Nose Throat J. 2022, 101 (Suppl. 2), 8S–16S. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, G.; Shu, S.; Xu, Y.; Ma, X. Metabolomics Analysis of Baicalin on Ovalbumin-Sensitized Allergic Rhinitis Rats. R. Soc. Open Sci. 2019, 6, 181081. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, C.; Yu, L.; Sheng, T.; Wu, Z.; Wang, X.; Zhang, D.; Lin, Y.; Gong, Y. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. BioMed Res. Int. 2019, 2019, 6769789. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Wang, J.; Wang, X.; Hu, N.; Wang, S. Chlorogenic Acid Supplementation Ameliorates Hyperuricemia, Relieves Renal Inflammation, and Modulates Intestinal Homeostasis. Food Funct. 2021, 12, 5637–5649. [Google Scholar] [CrossRef]
- Niu, X.; Ding, Y.; Chen, S.; Gooneratne, R.; Ju, X. Effect of Immune Stress on Growth Performance and Immune Functions of Livestock: Mechanisms and Prevention. Animals 2022, 12, 909. [Google Scholar] [CrossRef]
- Su, J.; Kong, X.; Wang, W.; Xie, Q.; Wang, C.; Tan, B.; Wang, J. Weaning Performance Prediction in Lactating Sows Using Machine Learning, for Precision Nutrition and Intelligent Feeding. Anim. Nutr. 2025, 21, 222–233. [Google Scholar] [CrossRef]
- Paredes, S.P.; Jansman, A.J.M.; Verstegen, M.W.A.; Den Hartog, L.A.; Van Hees, H.M.J.; Bolhuis, J.E.; Van Kempen, T.A.T.G.; Gerrits, W.J.J. Identifying the Limitations for Growth in Low Performing Piglets from Birth until 10 Weeks of Age. Animal 2014, 8, 923–930. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, B.; Kang, S.; Jeong, G.; Lee, J.; Yu, Y.-B.; Chun, M. Anti-Inflammatory Effects of Scutellaria baicalensis Extract via Suppression of Immune Modulators and MAP Kinase Signaling Molecules. J. Ethnopharmacol. 2009, 126, 320–331. [Google Scholar] [CrossRef]
- Li, B.; Wang, M.; Chen, S.; Li, M.; Zeng, J.; Wu, S.; Tu, Y.; Li, Y.; Zhang, R.; Huang, F.; et al. Baicalin Mitigates the Neuroinflammation through the TLR4/MyD88/NF-κB and MAPK Pathways in LPS-Stimulated BV-2 Microglia. BioMed Res. Int. 2022, 2022, 3263446. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhou, L.; Wang, Z.; Li, X.; Ding, L.; Qiu, Y.; Guo, P.; Ye, C.; Fu, S.; Wu, Z.; et al. Baicalin Alleviate Apoptosis via PKC-MAPK Pathway in Porcine Peritoneal Mesothelial Cells Induced by Glaesserella parasuis. Molecules 2022, 27, 5083. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Yoon, H.-S. Chlorogenic Acid as a Positive Regulator in LPS-PG-Induced Inflammation via TLR4/MyD88-Mediated NF-κB and PI3K/MAPK Signaling Cascades in Human Gingival Fibroblasts. Mediators Inflamm. 2022, 2022, 2127642. [Google Scholar] [CrossRef] [PubMed]
- Ontawong, A.; Duangjai, A.; Vaddhanaphuti, C.S.; Amornlerdpison, D.; Pengnet, S.; Kamkaew, N. Chlorogenic Acid Rich in Coffee Pulp Extract Suppresses Inflammatory Status by Inhibiting the P38, MAPK, and NF-κB Pathways. Heliyon 2023, 9, e13917. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Jung, H.-J.; Im, S.-S.; Song, D.-K.; Bae, J.-H. Effects of Chlorogenic Acid on Intracellular Calcium Regulation in Lysophosphatidylcholine-Treated Endothelial Cells. BMB Rep. 2017, 50, 323–328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).