Conservation in the Andean Highlands of South America: A Habitat Enhancement Plan for Tematobius philippii, a Critically Endangered Species in the Ascotán Salt Flat in Chile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Implementation of Refuges
2.3. Refuge Monitoring
2.4. Demographic Analysis
2.5. Biometric Measurements
3. Results
3.1. Refuge Occupancy
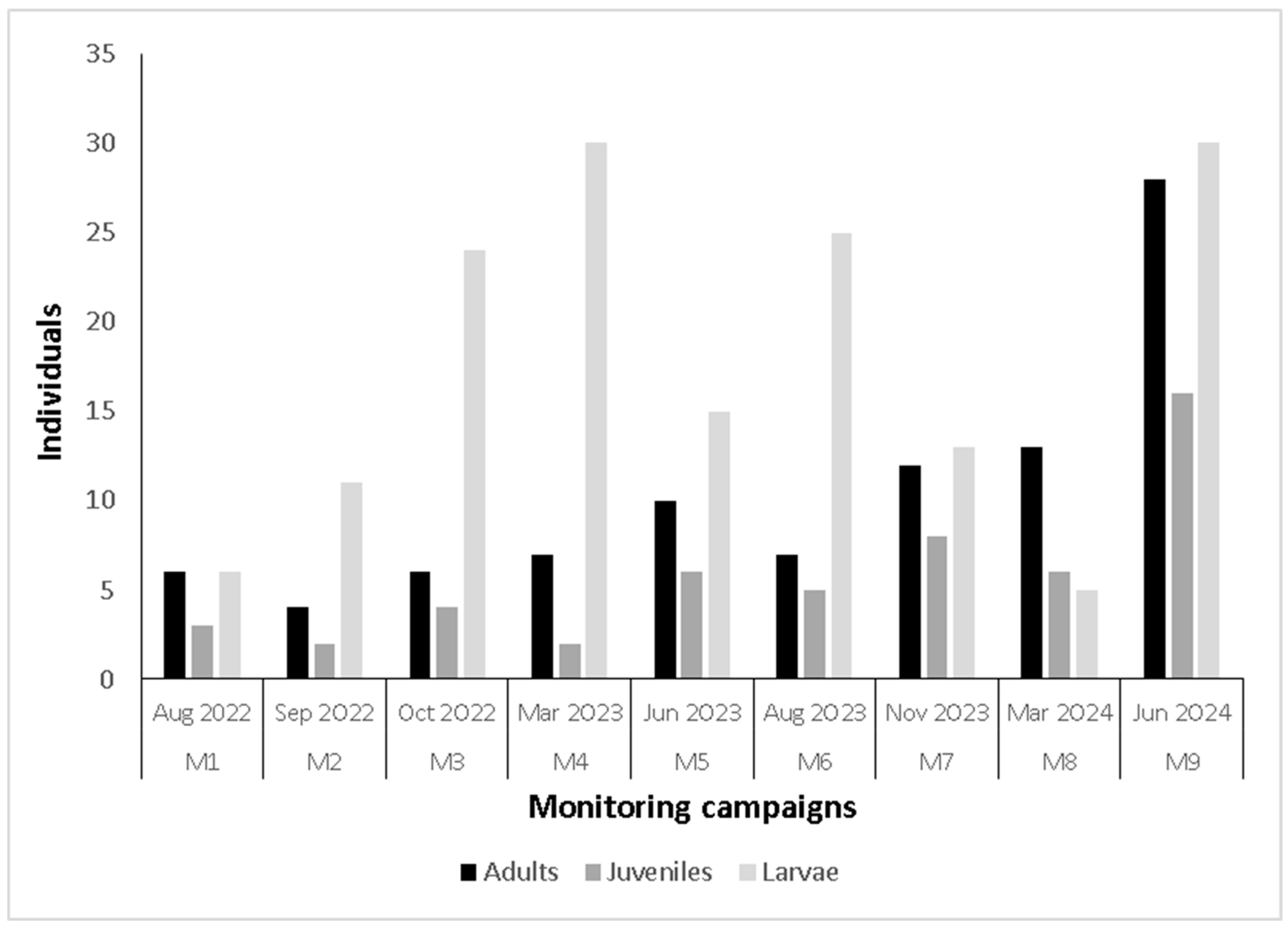
3.2. Population Aspects of Larvae
3.3. Population Aspects of Adults and Juveniles
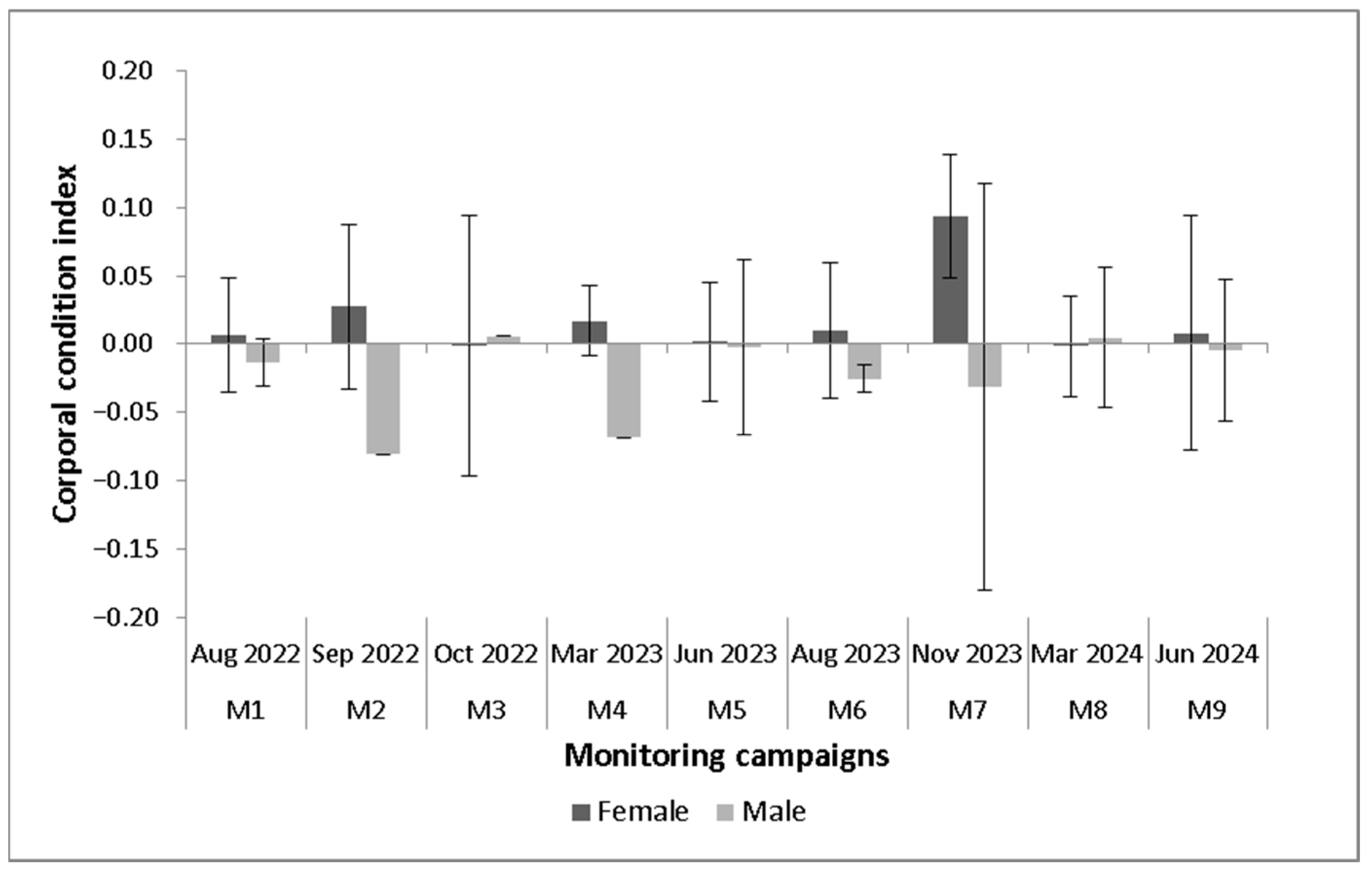
3.4. Biometric Aspects
4. Discussion
5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Catenazzi, A. State of the world’s amphibians. Annu. Rev. Environ. Resour. 2015, 40, 91–119. [Google Scholar] [CrossRef]
- Singh, R. Global Amphibian Decline: Diversity, Threats and Management Strategies. J. Sci. Res. Rep. 2024, 30, 543–562. [Google Scholar] [CrossRef]
- Freda, J. The effects of aluminum and other metals on amphibians. Environ. Pollut. 1991, 71, 305–328. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Romansic, J.M.; Kiesecker, J.M.; Hatch, A.C. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib. 2003, 9, 123–140. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Bickford, D.; Diesmos, A.C.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Sekercioglu, C.H.; Bradshaw, C.J. Measuring the meltdown: Drivers of global amphibian extinction and decline. PLoS ONE 2008, 3, e1636. [Google Scholar] [CrossRef]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Schmidt, T.; Pieper, S.; Alscher, A. Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline? Sci. Rep. 2013, 3, 1135. [Google Scholar] [CrossRef]
- Lips, K.R. Overview of chytrid emergence and impacts on amphibians. Phil. Trans. R. Soc. B 2016, 371, 20150465. [Google Scholar] [CrossRef]
- Soto-Azat, C.; Penafiel-Ricaurte, A.; Price, S.J.; Sallaberry-Pincheira, N.; García, M.P.; Alvarado-Rybak, M.; Cunningham, A.A. Xenopus laevis and emerging amphibian pathogens in Chile. EcoHealth 2016, 13, 775–783. [Google Scholar] [CrossRef]
- Pyron, R.A. Global amphibian declines have winners and losers. Proc. Natl. Acad. Sci. USA 2018, 115, 3739–3741. [Google Scholar] [CrossRef]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. Electronic Database. American Museum of Natural History: New York, NY, USA, 2024. Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 26 October 2025).
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. 2023. Available online: https://www.iucnredlist.org (accessed on 8 May 2023).
- Capurro, L. El género Telmatobius en Chile. Rev. Chil. Hist. Nat. 1954, 3, 31–40. [Google Scholar]
- Hutchison, V.H.; Haines, H.B.; Engbretson, G. Aquatic life at high altitude: Respiratory adaptations in the Lake Titicaca frog, Telmatobius culeus. Respir. Physiol. 1976, 27, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E.; Ostojic, H.; Fago, A.; Dewilde, S.; Van Hauwaert, M.L.; Moens, L.; Monge, C. Novel mechanism for high-altitude adaptation in hemoglobin of the Andean frog Telmatobius peruvianus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 283, R1052–R1060. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, E.O. Lista sistemática y bibliografía comentada sobre el género Telmatobius. Monogr. Herpetol. 2005, 7, 283–349. [Google Scholar]
- Veloso, A. Batracios de las cuencas hidrográficas de Chile: Origen, diversidad y estado de conservación. In Macrófitas y Vertebrados de los Sistemas Límnicos de Chile; Editorial Universitaria: Santiago, Chile, 2006; pp. 103–140. [Google Scholar]
- Sáez, P.A.; Fibla, P.; Correa, C.; Sallaberry, M.; Salinas, H.; Veloso, A.; Mella, J.; Iturra, P.; Méndez, M.A. A new endemic lineage of the Andean frog genus Telmatobius (Anura, Telmatobiidae) from the western slopes of the central Andes. Zool. J. Linn. Soc. 2014, 171, 769–782. [Google Scholar] [CrossRef]
- von Tschirnhaus, J.; Correa, C. The definitive rediscovery of Telmatobius halli (Anura, Telmatobiidae) at its historic type locality and its synonymy with T. dankoi and T. vilamensis. ZooKeys 2021, 1079, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Sáez, P.A.; Zúñiga-Reinoso, Á.; Fibla, P.; Cruz-Jofré, F.; Aguilar, C.; Aparicio, J.; Cusi, J.C.; Otálora, K.; Méndez, M.A. Phylogeny of Telmatobius marmoratus complex (Anura, Telmatobiidae) reveals high cryptic diversity in the Andean Altiplano. Mol. Phylogenetics Evol. 2022, 176, 107594. [Google Scholar] [CrossRef]
- Fibla, P.; Sáez, P.A.; Lobos, G.; Rebolledo, N.; Véliz, D.; Pastenes, L.; del Pozo, T.; Méndez, M.A. Delimitation of Endangered Telmatobius Species (Anura: Telmatobiidae) of the Chilean Salt Puna. Animals 2024, 14, 3612. [Google Scholar] [CrossRef]
- Vila, I.; Mendez, M.; Scott, S.; Morales, P.; Poulin, E. Threatened fishes of the world: Orestias ascotanensis Perenti, 1984 (Cyprinodontidae). Environ. Biol. Fishes 2007, 80, 491–492. [Google Scholar] [CrossRef]
- Valladares, M.A.; Méndez, M.A.; Collado, G.L. Influenced but not determined by historical events: Genetic, demographic and morphological differentiation in Heleobia ascotanensis from the Chilean Altiplano. PeerJ Life Environ. 2018, 6, e5802. [Google Scholar] [CrossRef]
- Lobos, G.; Rebolledo, N.; Sandoval, M.; Canales, C.; Perez-Quezada, J.F. Temporal gap between knowledge and conservation needs in high Andean Anurans: The case of the Ascotán salt flat frog in Chile (Anura, Telmatobiidae). S. Am. J. Herpetol. 2018, 13, 33–43. [Google Scholar] [CrossRef]
- Salica, M.J.; Gastón, M.S.; Akmentins, M.S.; Vaira, M. Threatened aquatic Andean frogs and mining activity in the Lithium Triangle of South America: Can both coexist? Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 34, e4044. [Google Scholar] [CrossRef]
- Hermosilla, V.; Vila, I.; Copaja, S.V. Lithium and boron of spring water and salt crust from Salar de Ascotán, southwestern Altiplano. J. Chil. Chem. Soc. 2019, 64, 4538–4541. [Google Scholar] [CrossRef]
- Hernández-Rojas, M.; Estévez, R.A.; Romero, C.; Perez, S.; Labra, F.A. Integrating Species Richness, Distribution and Human Pressures to Assess Conservation Priorities in High Andean Salares. Sustainability 2025, 17, 8139. [Google Scholar] [CrossRef]
- SCM El Abra. Plan de Manejo Ambiental de la Vertiente 11, Salar de Ascotán; SNIFA: Santiago, Chile, 2009.
- Lobos, G. (Centro de Gestión Ambiental y Biodiversidad, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile, Santiago, Chile). Personal communication, 2025.
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from population of marked animals. Bird Study 1999, 46, S120–S139. [Google Scholar] [CrossRef]
- Gosner, K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Acosta, R.; Vera, R.; Castro, S.; Nuñez, A.; González, N.; Abdenur, F.; Figueroa, R. Aspectos ecológicos de Telmatobius atacamensis (Anura: Telmatobiidae), un microendemismo de la Puna, Salta-Argentina. Rev. Peru. Biol. 2020, 27, 113–120. [Google Scholar] [CrossRef]
- Lajmanovich, R.C. Interpretación ecológica de una comunidad larvaria de anfibios anuros. Interciencia 2000, 25, 71–79. [Google Scholar]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2006; 331p. [Google Scholar]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression and Survival Analysis; Springer: New York, NY, USA, 2001. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org (accessed on 26 October 2025).
- Bancila, R.; Hartel, T.; Plaiasu, R.; Smets, J.; Cogalniceanu, D. Comparing three body condition indices in amphibians: A case study of yellow-bellied toad Bombina variegata. Amphib.-Reptil. 2010, 31, 558–562. [Google Scholar] [CrossRef]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; Gonzalez, L.; Tablada, M.; Robledo, C. InfoStat, Versión 2004. Grupo InfoStat, FCA; Universidad Nacional de Córdoba: Córdoba, Argentina, 2004. [Google Scholar]
- Lobos, G.; Vidal, M.; Labra, A.; Correa, C.L.; Rabanal, F.E.; Diaz, H.; Alzamora, A.; Azat, C. Protocolo Para el Control de Enfermedades Infecciosas en Anfibios Durante Estudios de Campo; Red Chilena de Herpetología: Santiago, Chile, 2011. [Google Scholar]
- Cowan, M.A.; Callan, M.N.; Watson, M.J.; Watson, D.M.; Doherty, T.S.; Michael, D.R.; Dunlop, J.A.; Turner, J.M.; Moore, H.A.; Watchorn, D.J.; et al. Artificial refuges for wildlife conservation: What is the state of the science? Biol. Rev. 2021, 96, 2735–2754. [Google Scholar] [CrossRef]
- Vogele, L.E.; Rainwater, W.C. Use of brush shelters as cover by spawning black basses (Micropterus) in Bull Shoals Reservoir. Trans. Am. Fish. Soc. 1975, 104, 264–269. [Google Scholar] [CrossRef]
- Hoff, M.H. Effects of increased nesting cover on nesting and reproduction of smallmouth bass in northern Winsconsin lakes. In First International Smallmouth Bass Symposium; American Fisheries Society: Ocean Springs, MS, USA; Mississippi State University: Starkville, MS, USA, 1991; pp. 39–43. [Google Scholar]
- Hunt, J.; Bacheler, N.; Wilson, D.; Videan, E.; Annett, C.A. Enhancing largemouth bass spawning: Behavioral and habitat considerations. In Black Bass: Ecology, Conservation, and Management; American Fisheries Society Symposium 31; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 277–290. [Google Scholar]
- Bohnsack, J.A.; Sutherland, D.L. Artificial reef research: A review with recommendations for future priorities. Bull. Mar. Sci. 1985, 37, 11–39. [Google Scholar]
- Johnson, D.L.; Beaumier, R.A.; Lynch, W.E., Jr. Selection of habitat structure interstice size by bluegills and largemouth bass in ponds. Trans. Am. Fish. Soc. 1988, 117, 171–179. [Google Scholar] [CrossRef]
- Moring, J.R.; Nicholson, P.H. Evaluation of three types of artificial habitats for fishes in a freshwater pond in Maine, USA. Bull. Mar. Sci. 1994, 55, 1149–1159. [Google Scholar]
- Helfman, G.S. The advantage to fishes of hovering in shade. Copeia 1981, 1981, 392–400. [Google Scholar] [CrossRef]
- Johnson, D.L.; Lynch, W.E., Jr. Panfish use of and angler success at evergreen tree, brush, and stake-bed structures. N. Am. J. Fish. Manag. 1992, 12, 222–229. [Google Scholar] [CrossRef]
- Shoo, L.P.; Olson, D.H.; McMenamin, S.K.; Murray, K.A.; Van Sluys, M.; Donnelly, M.A.; Stratford, D.; Terhivuo, J.; Merino-Viteri, A.; Herbert, S.M.; et al. Engineering a future for amphibians under climate change. J. Appl. Ecol. 2011, 48, 487–492. [Google Scholar] [CrossRef]
- Pérez, M.E. Cría en cautividad y uso sostenible de la rana gigante del lago Titicaca (Telmatobius culeus). Monogr. Herpetol. 2005, 7, 261–271. [Google Scholar]
- Yabiku, R.M.F.; Bermudez, L.; Souza, L.S. Contribuições para conservação da criticamente ameaçada rã do lago titicaca (Telmatobius culeus): Notas sobre a manutenção da espécie e primeiro registro de reprodução ex-situ. In Proceedings of the IX Brazilian Congresso of Herpetology, Sao Paulo, Brazil, 22–26 July 2019. [Google Scholar]
- Knoll, S. Nutrition of the Titicaca Water Frog (Telmatobius culeus). Master’s Dissertation, Faculty of Veterinary Medicine, Ghent University, Gent, Belgium, 2017. Available online: https://libstore.ugent.be/fulltxt/RUG01/002/509/181/RUG01-002509181_2018_0001_AC.pdf (accessed on 26 October 2025).
- Mantilla, B. Reproducción de la rana gigante (Telmatobius culeus, Garman 1875) del lago Titicaca en ambientes controlados, Puno. Ph.D Thesis, Universidad Nacional del Altiplano, Puno, Peru, 2018. Available online: http://repositorio.unap.edu.pe/handle/20.500.14082/9193 (accessed on 26 October 2025).
- Cabeza, O.; Peña, L.; Almarza, S.; Caiozzi, A.; Montalba, A. Conservación ex situ de Telmatobius dankoi. In Ecología y Conservación en los Telmatobius Altoandinos de Chile; el caso de la Ranita del Loa; Lobos, G., Rojas, O., Eds.; Museo de Historia Natural y Cultural del Desierto de Atacama: Calama, Chile, 2020; pp. 142–150. [Google Scholar]
- Lobos, G.; Rebolledo, N.; Charrier, A.; Rojas, O. Natural history notes of Telmatobius dankoi (Anura, Telmatobiidae), a critically endangered species from northern Chile. Stud. Neotrop. Fauna Environ. 2016, 51, 152–157. [Google Scholar] [CrossRef]
- Lobos, G.; Rebolledo, N.; Salinas, H.; Fibla, P.; Sáez, P.A.; Méndez, M. Ecological features of Telmatobius chusmisensis (Anura: Telmatobiidae), a poorly known species from northern Chile. S. Am. J. Herpetol. 2021, 20, 1–7. [Google Scholar] [CrossRef]
- Corbalán, V.; Debandi, G.; Martínez, F.; Úbeda, C. Prolonged larval development in the Critically Endangered Pehuenche’s frog Alsodes pehuenche: Implications for conservation. Amphib.-Reptil. 2014, 35, 283–292. [Google Scholar] [CrossRef]



| Models | Abundance | Standard Error | Deviance | d.f. | AIC |
|---|---|---|---|---|---|
| M0 | 100.1 | 11.6 | 111.0 | 509 | 186.9 |
| Mt * | 96.5 | 10.8 | 75.8 | 501 | 167.7 |
| Mh Chao LB | 100.1 | 11.6 | 111.0 | 509 | 186.9 |
| Mh Darroch | 90.5 | 18.1 | 110.7 | 508 | 188.6 |
| Mh Gamma 3.5 | 87.2 | 21.1 | 110.7 | 508 | 188.6 |
| Mth Chao LB * | 96.5 | 10.8 | 75.8 | 501 | 167.7 |
| Mth Darroch | 90 | 17.7 | 75.7 | 500 | 169.5 |
| Mth Gamma 3.5 | 87.6 | 21.4 | 75.7 | 500 | 169.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzamora, A.; Salinas, H.; Trujillo, J.C.; Lobos, G. Conservation in the Andean Highlands of South America: A Habitat Enhancement Plan for Tematobius philippii, a Critically Endangered Species in the Ascotán Salt Flat in Chile. Animals 2025, 15, 3156. https://doi.org/10.3390/ani15213156
Alzamora A, Salinas H, Trujillo JC, Lobos G. Conservation in the Andean Highlands of South America: A Habitat Enhancement Plan for Tematobius philippii, a Critically Endangered Species in the Ascotán Salt Flat in Chile. Animals. 2025; 15(21):3156. https://doi.org/10.3390/ani15213156
Chicago/Turabian StyleAlzamora, Alejandra, Hugo Salinas, Juan Carlos Trujillo, and Gabriel Lobos. 2025. "Conservation in the Andean Highlands of South America: A Habitat Enhancement Plan for Tematobius philippii, a Critically Endangered Species in the Ascotán Salt Flat in Chile" Animals 15, no. 21: 3156. https://doi.org/10.3390/ani15213156
APA StyleAlzamora, A., Salinas, H., Trujillo, J. C., & Lobos, G. (2025). Conservation in the Andean Highlands of South America: A Habitat Enhancement Plan for Tematobius philippii, a Critically Endangered Species in the Ascotán Salt Flat in Chile. Animals, 15(21), 3156. https://doi.org/10.3390/ani15213156





