Ammonia Stress Induces Transcriptional Expression Changes in the Mature Eggs of the Acipenser baerii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Siberian Sturgeon
2.3. Treatment of Ammonia Stress
2.4. Mature Egg Collection
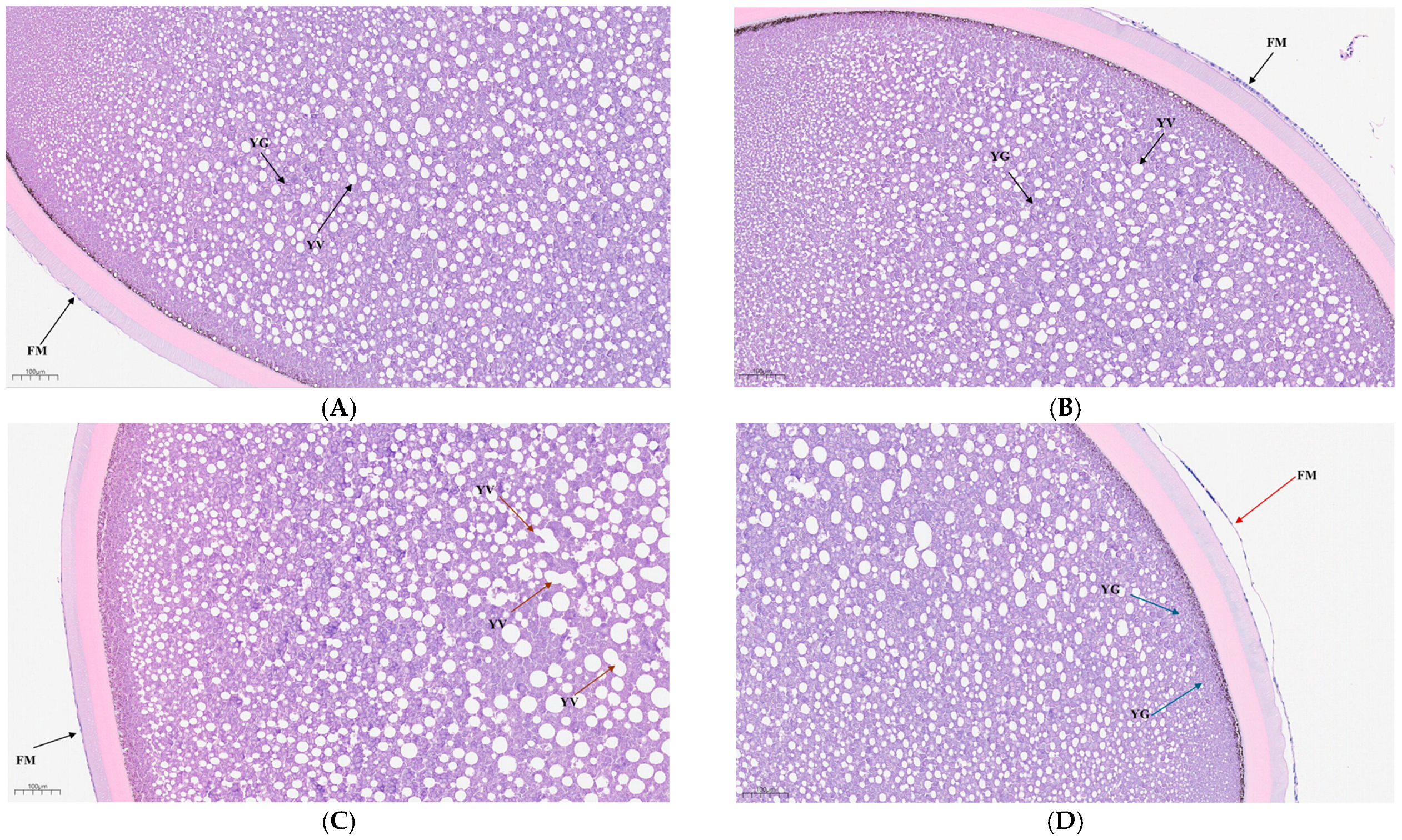
2.5. Analysis of Mature Egg Tissue Structure
2.6. RNA Extraction and Quality Testing
2.7. RNA Library Construction, Sequencing, and Quality Control
2.8. Validation of Data via RT-qPCR
2.9. De Novo Assembly and Functional Annotation
2.10. Data Processing
2.11. WGCNA
3. Results
3.1. Effects of Ammonia Stress on the Microstructure of Mature Eggs of Siberian Sturgeons
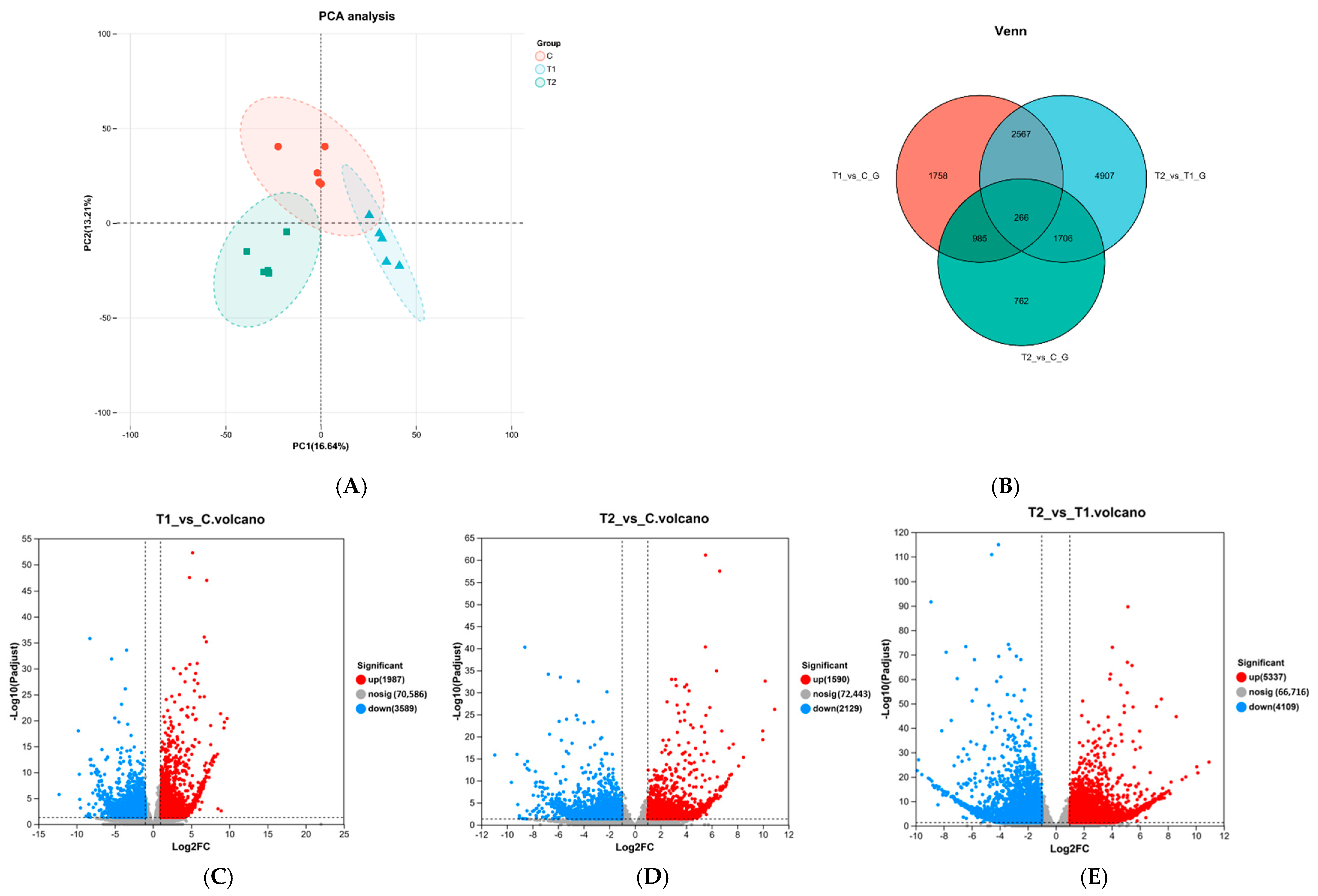
3.2. Transcriptome Sequencing Result Analysis
3.3. Unigenes Function Annotations
3.4. Differential Expression Analysis
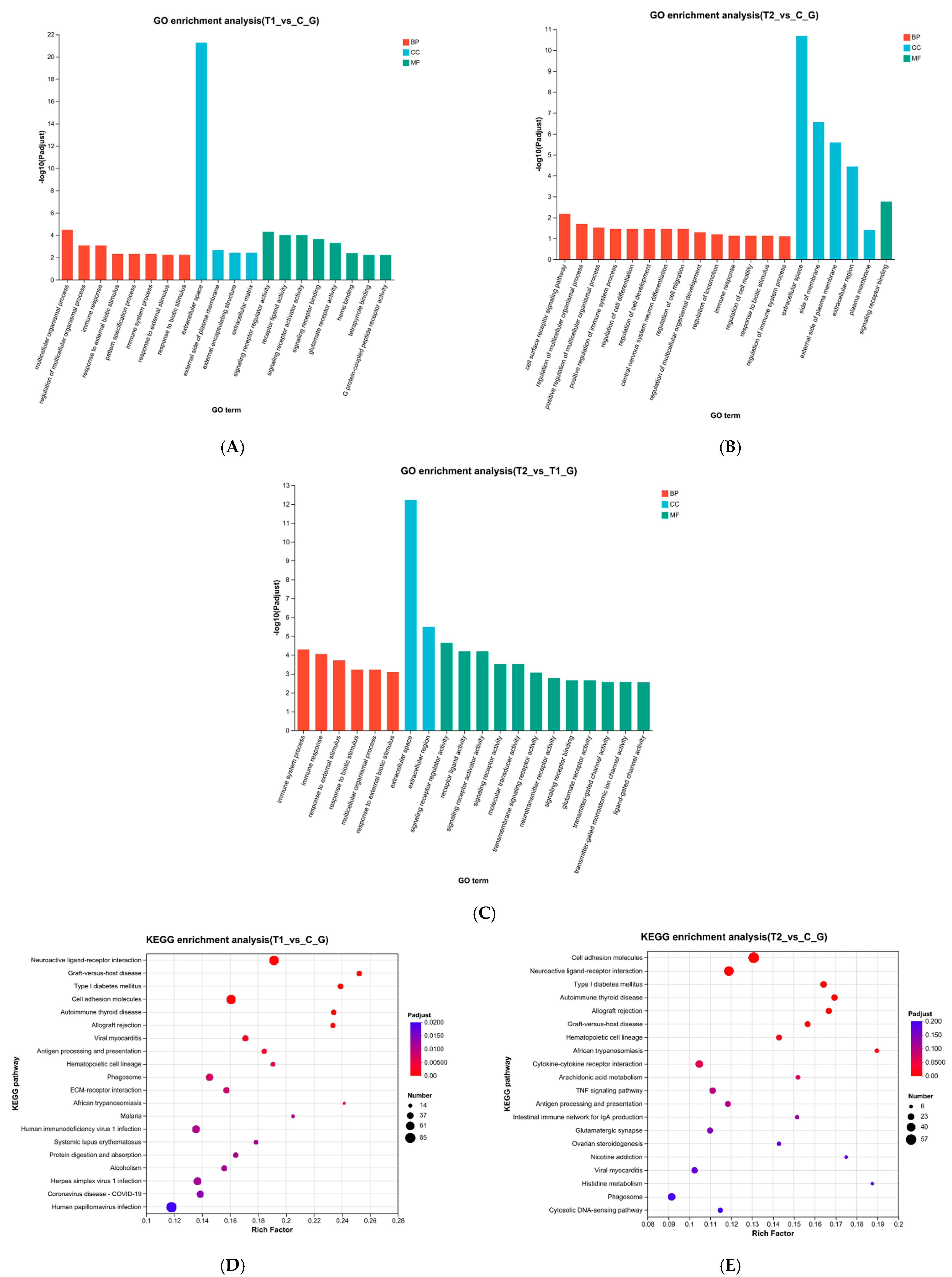
3.5. Enrichment Analysis of DEGs
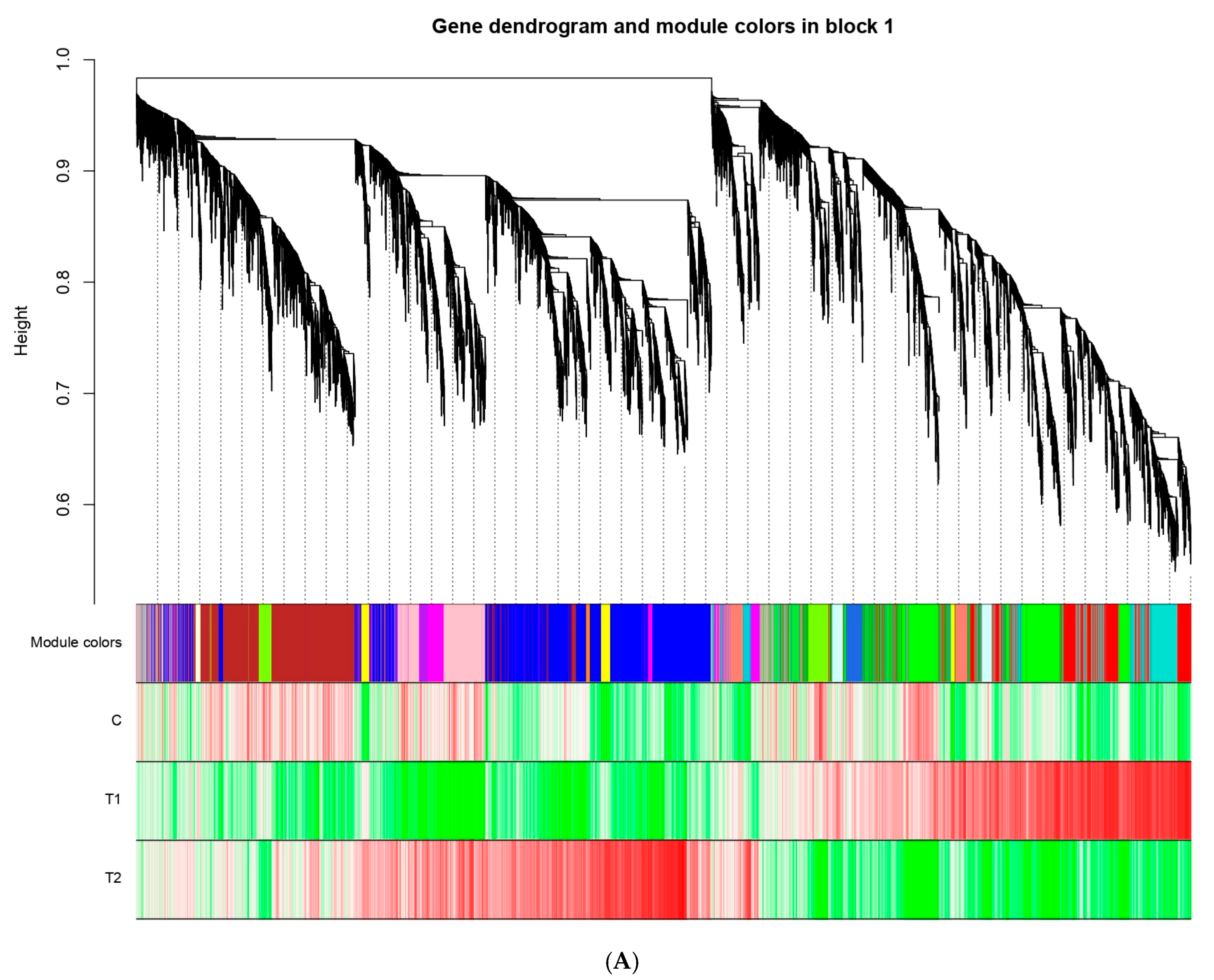
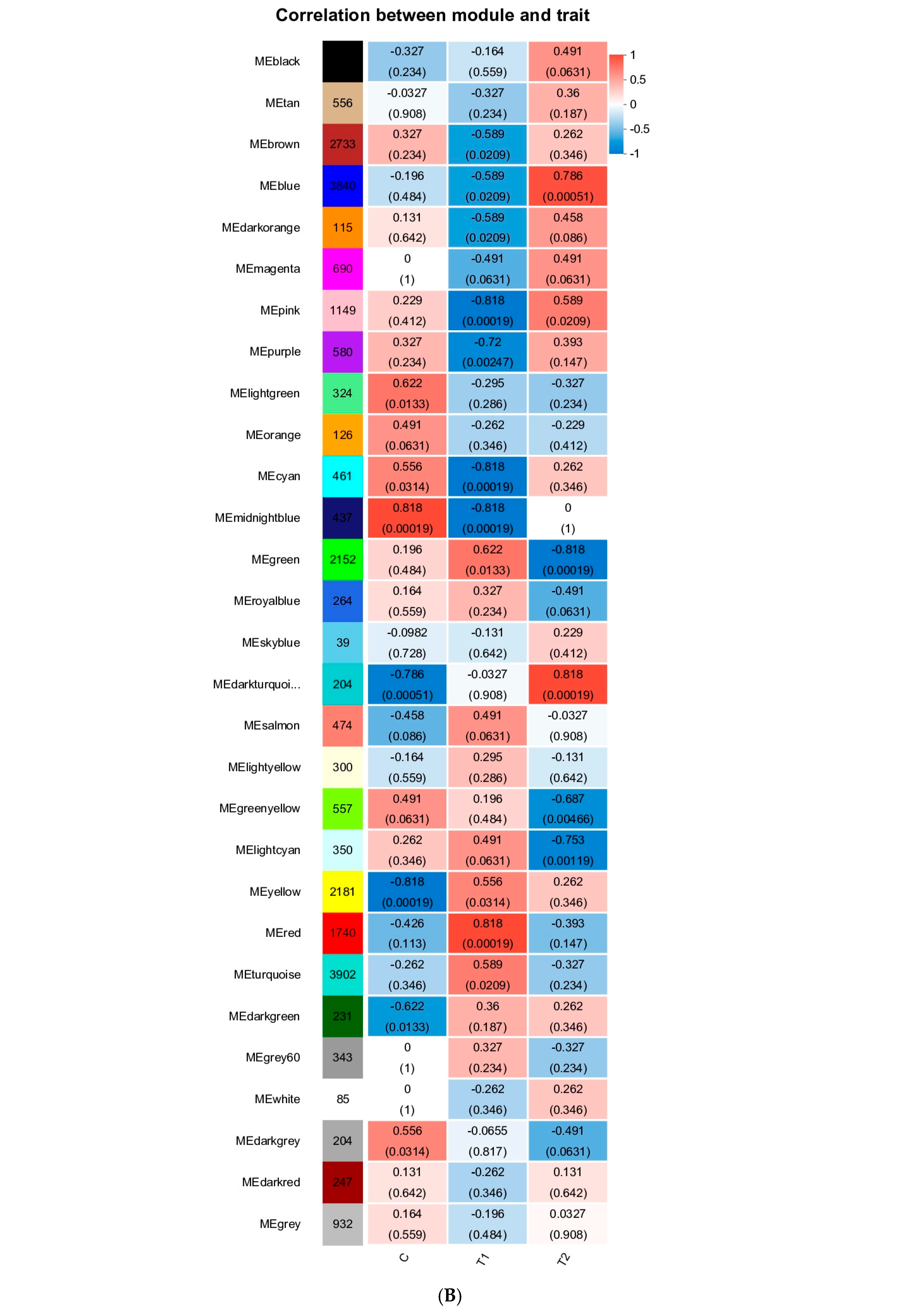
3.6. WCGNA Analysis
3.7. RNA-Seq Expression Analysis
4. Discussion
4.1. Different Performance of the Mature Eggs Under the Ammonia Stress
4.2. Ammonia Stress Increased Leads to a Larger Number of DEGs
4.3. Significant GO and KEGG Pathways in Ammonia Stress Response
4.4. Hunt for Key Modules Through WGCNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagaraju, T.V.; Sunil, B.M.; Chaudhary, B.; Prasad, C.D.; Gobinath, R. Prediction of ammonia contaminants in the aquaculture ponds using soft computing coupled with wavelet analysis. Environ. Pollut. 2023, 331, 121924. [Google Scholar] [CrossRef]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish welfare in aquaponic systems: Its relation to water quality with an emphasis on feed and faeces—A review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Lin, K.; Zhu, Y.; Zhang, Y.; Lin, H. Determination of ammonia nitrogen in natural waters: Recent advances and applications. Trends Environ. Anal. Chem. 2019, 24, e00073. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and tissue structure in fish exposed to ammonia nitrogen: A review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef] [PubMed]
- Mangang, Y.A.; Pandey, P.K. Hemato-biochemical responses and histopathological alterations in the gill and kidney tissues of Osteobrama belangeri (Valenciennes, 1844) exposed to different sub-lethal unionized ammonia. Aquaculture 2021, 542, 736887. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Ling, R.Z.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71.1. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, D.; Huang, R.; Xiao, Y.; Xu, W.; Liu, X.; Gui, Y.; Li, S.; Xu, J.; Tang, J.; et al. The influence of acute ammonia stress on intestinal oxidative stress, histology, digestive enzymatic activities and PepT1 activity of grass carp (Ctenopharyngodon idella). Aquac. Rep. 2021, 20. [Google Scholar] [CrossRef]
- Liu, M.J.; Guo, H.Y.; Liu, B.; Zhu, K.C.; Guo, L.; Liu, B.S.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.C. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, Z.; Wang, G.; You, K.; Mi, D. High concentrations of environmental ammonia induced changes in large--scale loach (Paramisgurnus dabryanus) immunity. Ecol. Evol. 2021, 11, 8614–8622. [Google Scholar] [CrossRef]
- Wang, T.; Li, W.; Shan, H.; Ma, S. Responses of energy homeostasis and lipid metabolism in Penaeus vannamei exposed to ammonia stress. Aquaculture 2021, 544, 737092. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.-L.; Han, C.; Huang, B.; Lei, J.-L. Effects of ammonia exposure on stress and immune response in juvenile turbot (Scophthalmus maximus). Aquac. Res. 2017, 48, 3149–3162. [Google Scholar] [CrossRef]
- Mutz, K.-O.; Heilkenbrinker, A.; Lönne, M.; Walter, J.-G.; Stahl, F. Transcriptome analysis using next-generation sequencing. Curr. Opin. Biotechnol. 2013, 24, 22–30. [Google Scholar] [CrossRef]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Rong, Z.; Zhao, X.; Gao, X.; Hou, Z.; Zhang, L.; Khor, W.; Xu, Y.; Chen, L.; Wu, C. Transcriptome analysis reveals hypoxic response key genes and modules as well as adaptive mechanism of crucian carp (Carassius auratus) gill under hypoxic stress. Front. Immunol. 2025, 16, 1543605. [Google Scholar] [CrossRef]
- Ruan, L.; Wei, B.; Liu, Y.; Mu, R.; Li, H.; Wei, S. Transcriptome Analysis Revealed the Immune and Metabolic Responses of Grass Carp (Ctenopharyngodon idellus) Under Acute Salinity Stress. Fishes 2025, 10, 380. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Cao, Y.; Gu, L.; Li, T.; Liu, Y.; Song, J.; Wang, W.; Wang, X.; Li, B.; et al. Transcriptome analysis of brain and skin reveals immune responses to acute hypoxia and reoxygenation in Pseudobagrus ussuriensis. Animals 2024, 14, 246. [Google Scholar] [CrossRef]
- Zou, J.; Hu, P.; Wang, M.; Chen, Z.; Wang, H.; Guo, X.; Gao, J.; Wang, Q. Liver injury and metabolic dysregulation in largemouth bass (Micropterus salmoides) after ammonia exposure. Metabolites 2023, 13, 274. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.H.; Miao, B.B.; Wu, R.X.; Li, Q.Q.; Tang, B.G.; Liang, Z.B.; Niu, S.F. Liver transcriptome analysis reveal the metabolic and apoptotic responses of Trachinotus ovatus under acute cold stress. Fish Shellfish Immunol. 2024, 148, 109476. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, Y.; Zhong, L.; Liu, H.; Wang, M.; Chen, X. Using comparative transcriptome analysis to identify molecular response mechanisms to salinity stress in channel catfish (Ictalurus punctatus). Environ. Pollut. 2023, 333, 121911. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhang, C.; Xu, R.; Lv, C.; Xue, Y.; Wang, P.; Li, Z.; Chen, M. High Ammonia Nitrogen--Induced Reproductive Toxicity in Goldfish (Carassius auratus) Mature Ovary. Aquac. Res. 2024, 2024, 9577902. [Google Scholar] [CrossRef]
- Callahan, G.T. Investigating Egg Shell Softness in the Sensory Characteristics of Caviar Produced from Farmed White Sturgeon, Acipenser Transmountanus. Ph.D. Thesis, Washington State University, Washington, DC, USA, 2018. [Google Scholar]
- Bobe, J.; Labbé, C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef]
- Pahlevan Kakhki, M. TRIzol-based RNA extraction: A reliable method for gene expression studies. J. Sci. Islam. Repub. IRAN 2014, 25, 13–17. [Google Scholar]
- Yang, S.; Li, D.; Feng, L.; Zhang, C.; Xi, D.; Liu, H.; Yan, C.; Xu, Z.; Zhang, Y.; Li, Y.; et al. Transcriptome analysis reveals the high temperature induced damage is a significant factor affecting the osmotic function of gill tissue in Siberian sturgeon (Acipenser baerii). BMC Genom. 2023, 24, 2. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, Z.; Qi, M.; Cai, W.; Saraiva, J.L.; Wen, Y.; Liu, G.; Zhu, Z.; Zhu, S.; Zhao, J. Water quality impact on fish behavior: A review from an aquaculture perspective. Rev. Aquac. 2025, 17, e12985. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Wang, A.; Zhu, X.; Chen, C.; Xian, J.; Sun, Z. Isolated and combined exposure to ammonia and nitrite in giant freshwater pawn (Macrobrachium rosenbergii): Effects on the oxidative stress, antioxidant enzymatic activities and apoptosis in haemocytes. Ecotoxicology 2015, 24, 1601–1610. [Google Scholar] [CrossRef]
- Huang, Z.B.; Liang, L.Y.; Li, W.H.; Li, N.; Ding, L.; Hong, M.L. Effects of acute ammonia stress on tissue structure of main organs of Chinese striped-necked turtle mauremys sinensis. Fish. Sci. 2022, 41, 143–149. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; He, M.; Su, J.; Liu, L. Chronic Ammonia Stress in Chinese Perch (Siniperca chuatsi): Oxidative Response, Nitrogen Metabolism, and Multi-Enzyme-Mediated Molecular Detoxification Defense Mechanisms. Antioxidants 2025, 14, 768. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Z.; Zheng, X.; Wang, S.; Fan, J.; Liu, Z. Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea). Fish Shellfish. Immunol. 2020, 99, 514–525. [Google Scholar] [CrossRef]
- Corriero, A.; Zupa, R.; Bello, G.; Mylonas, C.C.; Deflorio, M.; Genovese, S.; Basilone, G.; Buscaino, G.; Buffa, G.; Pousis, C.; et al. Evidence that severe acute stress and starvation induce rapid atresia of ovarian vitellogenic follicles in Atlantic bluefin tuna, Thunnus thynnus (L.) (Osteichthyes: Scombridae). J. Fish Dis. 2011, 34, 853–860. [Google Scholar] [CrossRef]
- Qiang, J.; Duan, X.J.; Zhu, H.J.; He, J.; Tao, Y.F.; Bao, J.W.; Zhu, X.; Xu, P. Some ‘white’oocytes undergo atresia and fail to mature during the reproductive cycle in female genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2021, 534, 736278. [Google Scholar] [CrossRef]
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the tissues: Extracellular matrix and its artificial substitutes: Cell signalling mechanisms. Cells 2022, 11, 914. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, C.; Liang, Y.; Wu, K.; Wen, X. Ammonia nitrogen stress affects immune regulation via TNFα in yellow catfish (Pelteobagrus fulvidraco). Aquaculture 2024, 583, 740593. [Google Scholar] [CrossRef]
- Ijiri, S.; Kazeto, Y.; Lokman, P.M.; Adachi, S.; Yamauchi, K. Characterization of a cDNA encoding P-450 aromatase (CYP19) from Japanese eel ovary and its expression in ovarian follicles during induced ovarian development. Gen. Comp. Endocrinol. 2003, 130, 193–203. [Google Scholar] [CrossRef]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Abdollahzadeh, R.; Osman, M.B.; Sakinah, M.; Nordin, N.; Hamid, H.A. A review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism studies: Candidate susceptibility genes for polycystic ovary syndrome (PCOS) and infertility. Genes 2022, 13, 302. [Google Scholar] [CrossRef]
- Craig, Z.R.; Wang, W.; Flaws, J.A. Endocrine-disrupting chemicals in ovarian function: Effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction 2011, 142, 633. [Google Scholar] [CrossRef]
- Tian, H.; Sun, Y.; Wang, H.; Bing, X.; Wang, W.; Ru, S. Monocrotophos pesticide affects synthesis and conv of sex steroids through multiple targets in male goldfish (Carassius auratus). Sci. Rep. 2017, 7, 2306. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.-F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]

| Sample | Raw Reads | Clean Reads | Error Rate | Q20/% | Q30/% | GC/% |
|---|---|---|---|---|---|---|
| C1 | 44,177,344 | 43,759,336 | 0.0117 | 99.25 | 96.56 | 48.77 |
| C2 | 43,025,390 | 42,649,676 | 0.0116 | 99.27 | 96.61 | 48.48 |
| C3 | 43,511,676 | 43,108,596 | 0.0117 | 99.25 | 96.54 | 49.37 |
| C4 | 43,393,402 | 42,969,304 | 0.0117 | 99.25 | 96.53 | 51.12 |
| C5 | 42,964,220 | 42,579,062 | 0.0117 | 99.23 | 96.49 | 49.88 |
| T1_1 | 42,424,566 | 42,089,948 | 0.0116 | 99.3 | 96.73 | 48.85 |
| T1_2 | 43,138,566 | 42,806,416 | 0.0116 | 99.31 | 96.73 | 49.57 |
| T1_3 | 43,132,158 | 42,848,662 | 0.0115 | 99.31 | 96.75 | 49.86 |
| T1_4 | 43,624,112 | 43,297,704 | 0.0115 | 99.33 | 96.81 | 48.48 |
| T1_5 | 43,560,080 | 43,244,660 | 0.0115 | 99.32 | 96.77 | 50.21 |
| T2_1 | 43,536,888 | 43,086,176 | 0.0117 | 99.24 | 96.55 | 49.67 |
| T2_2 | 42,724,446 | 42,363,790 | 0.0117 | 99.25 | 96.53 | 50.09 |
| T2_3 | 40,971,686 | 40,593,188 | 0.0116 | 99.27 | 96.63 | 48.75 |
| T2_4 | 46,351,226 | 45,775,014 | 0.0118 | 99.18 | 96.29 | 49.39 |
| T2_5 | 51,754,234 | 51,172,172 | 0.0118 | 99.17 | 96.26 | 48.66 |
| Length Range/bp | Transcript | Unigene |
|---|---|---|
| 200~500 | 59,670 | 39,270 |
| 501~1000 | 29,440 | 16,100 |
| 1001~2001 | 22,789 | 9621 |
| >2001 | 25,060 | 11,171 |
| Total number | 136,959 | 76,162 |
| N50 length | 2287 | 2153 |
| Mean length | 1195.71 | 1039.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Q.; Zhang, C.; Wu, W.; Zhou, Q.; Lv, C.; Sun, X.; Yang, F. Ammonia Stress Induces Transcriptional Expression Changes in the Mature Eggs of the Acipenser baerii. Animals 2025, 15, 3122. https://doi.org/10.3390/ani15213122
Qi Q, Zhang C, Wu W, Zhou Q, Lv C, Sun X, Yang F. Ammonia Stress Induces Transcriptional Expression Changes in the Mature Eggs of the Acipenser baerii. Animals. 2025; 15(21):3122. https://doi.org/10.3390/ani15213122
Chicago/Turabian StyleQi, Qian, Cheng Zhang, Wenhua Wu, Qi Zhou, Chenran Lv, Xiaohui Sun, and Feng Yang. 2025. "Ammonia Stress Induces Transcriptional Expression Changes in the Mature Eggs of the Acipenser baerii" Animals 15, no. 21: 3122. https://doi.org/10.3390/ani15213122
APA StyleQi, Q., Zhang, C., Wu, W., Zhou, Q., Lv, C., Sun, X., & Yang, F. (2025). Ammonia Stress Induces Transcriptional Expression Changes in the Mature Eggs of the Acipenser baerii. Animals, 15(21), 3122. https://doi.org/10.3390/ani15213122





