Advances in the Diagnosis of Reproductive Disorders in Male Camelids
Simple Summary
Abstract
1. Introduction
2. Breeding Soundness Examination Results
3. Congenital Abnormalities
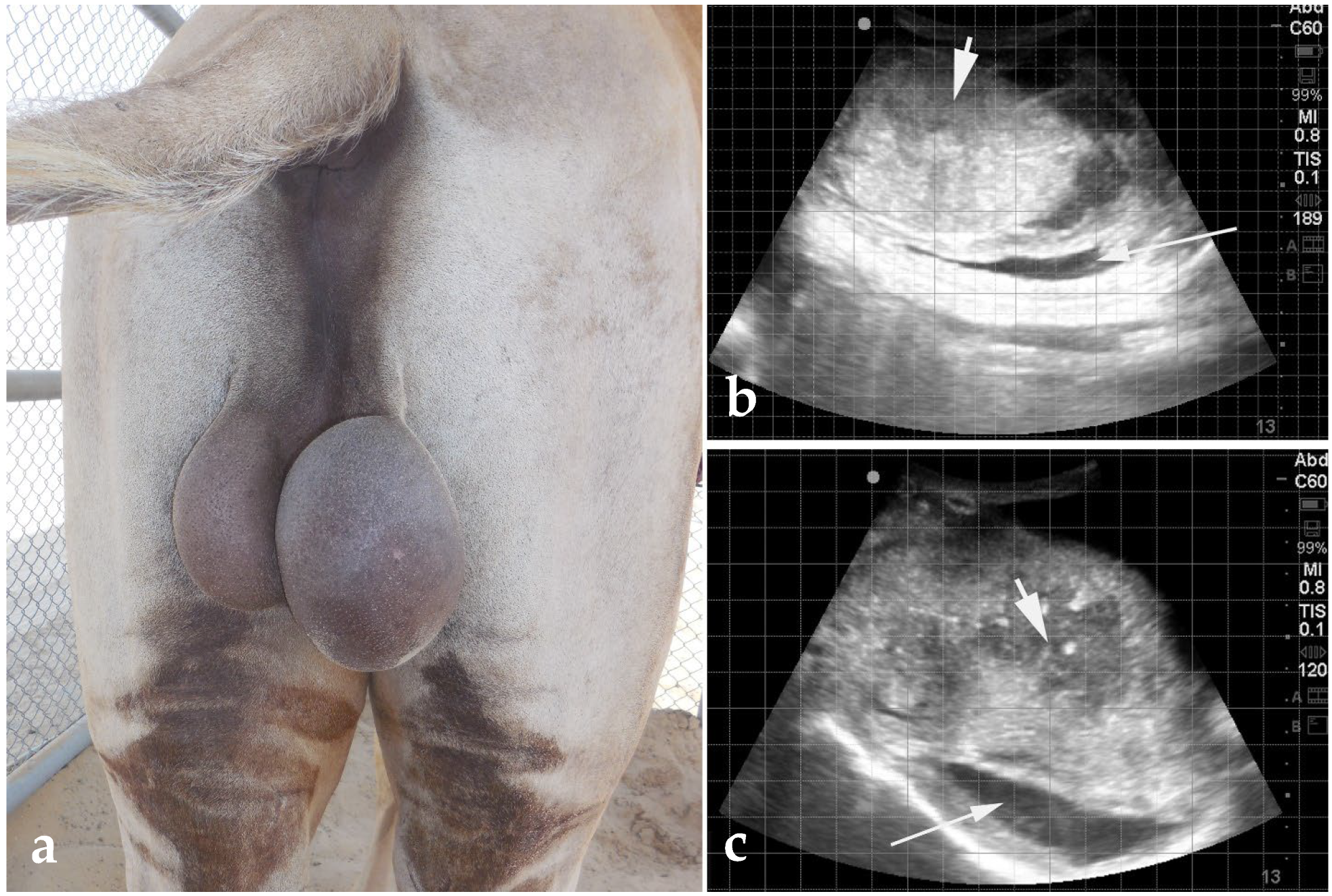
3.1. Cryptorchidism
3.2. Ectopic Testis
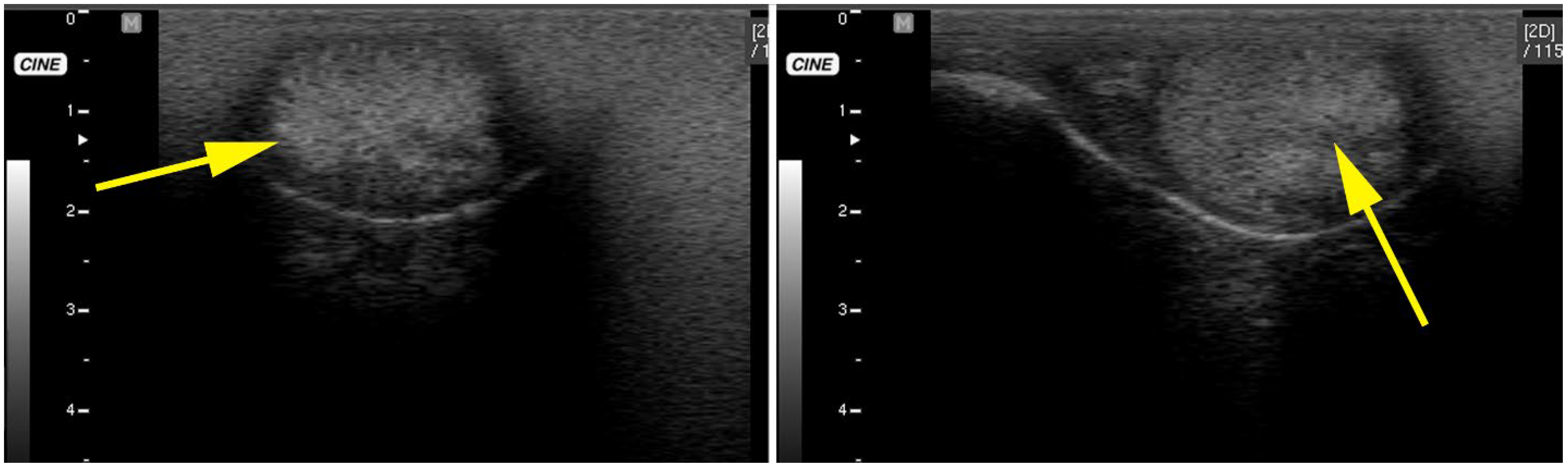
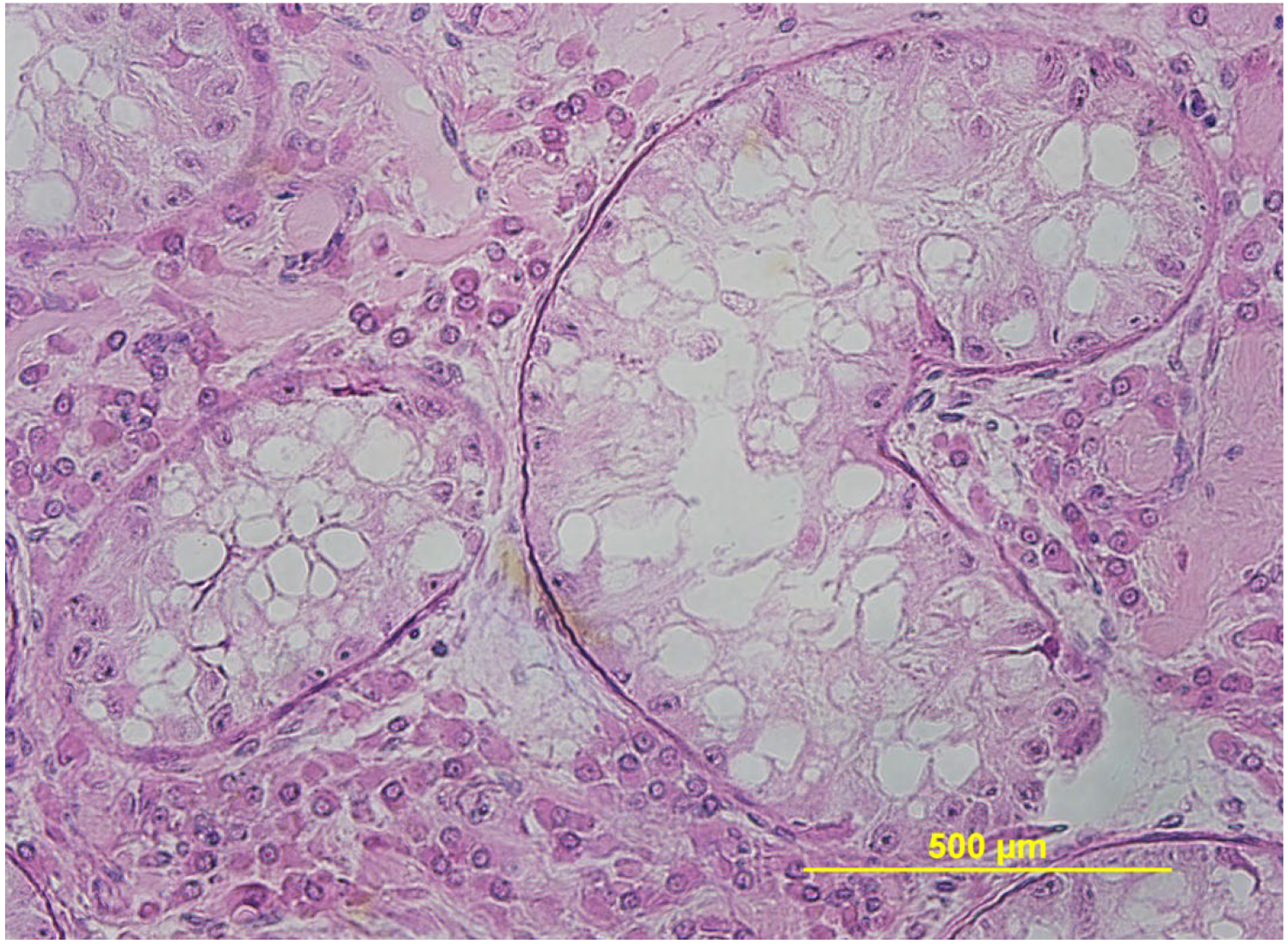
3.3. Testicular and Epididymal Cysts
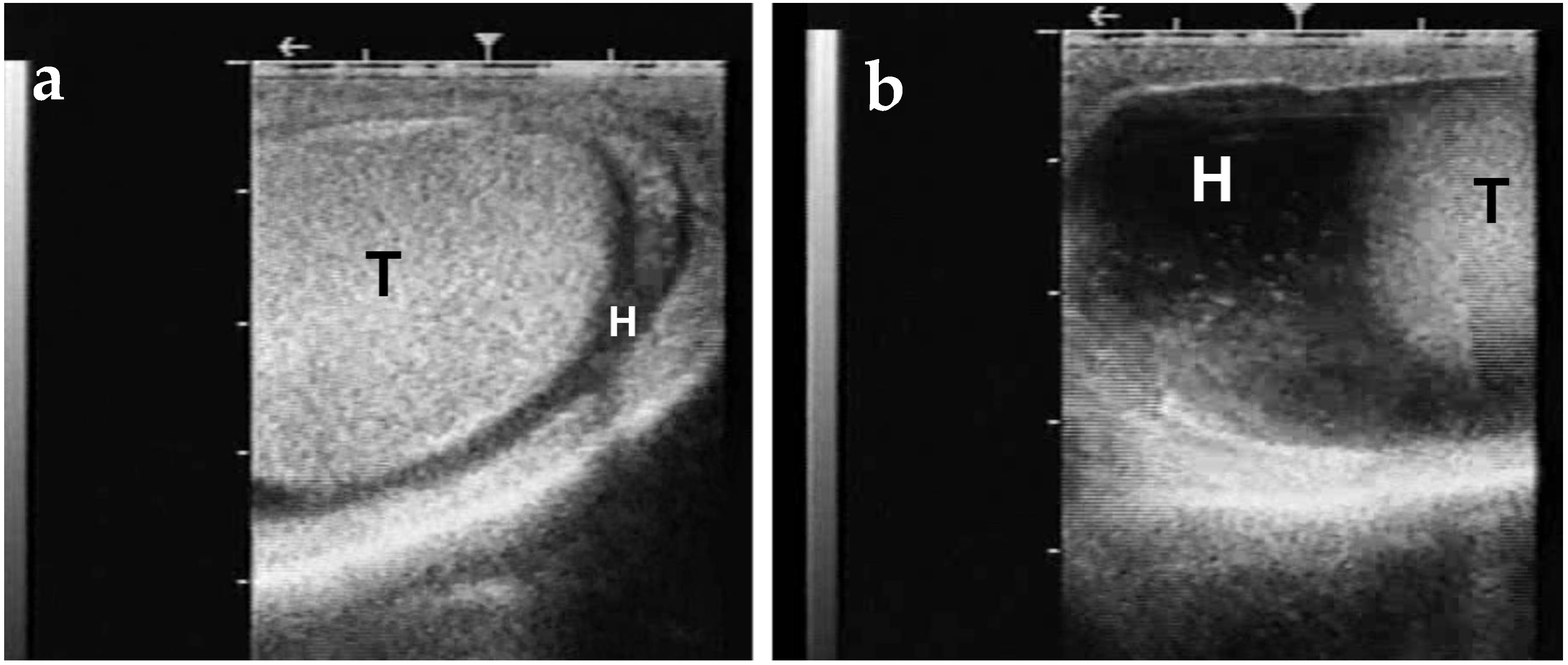
3.4. Testicular Hypoplasia
3.5. Preputial and Penile Developmental Abnormalities
4. Causes of Impotentia Couendi
5. Impotentia Generandi
6. Male Reproductive Emergencies
6.1. Scrotal Enlargement
6.2. Preputial/Penile Injuries
6.3. Preputial Prolapse
6.4. Urolithiasis
6.5. Prolapse of the Soft Palate in the Dromedary
7. Rectal Prolapse
8. Other Causes of Male Infertility
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daborn, T.; Pajor, E.A.; Pearson, J.M. Breeding Bulls in Alberta: A Cross-Sectional Descriptive Survey of Breeding Bull Herds and Current Management Strategies. Transl. Anim. Sci. 2025, 9, 82. [Google Scholar] [CrossRef]
- Koziol, J.H.; Armstrong, C. Society for Theriogenology Manual for Breeding Soundness Examination of Bulls, 2nd ed.; Society for Theriogenology: Pike Road, AL, USA, 2018. [Google Scholar]
- Carretero, M.I.; Giuliano, S.M.; Miragaya, M.H. Male Reproductive Biotechnologies in South American Camelids Part I: Semen Collection, Evaluation and Handling. Anim. Reprod. Sci. 2025, 272, 107634. [Google Scholar] [CrossRef]
- Carretero, M.I.; Neild, D.M.; Bertuzzi, M.L. Male Reproductive Biotechnologies in South American Camelids Part II: Semen Dilution, Cryopreservation and Artificial Insemination. Anim. Reprod. Sci. 2025, 272, 107646. [Google Scholar] [CrossRef] [PubMed]
- Anouassi, A.; Tibary, A. Evaluation of Two Methods of Semen Collection for the Commercial Application of Artificial Insemination in Dromedary Camels. Cabi Agric. Biosci. 2025, 6, 41–50. [Google Scholar] [CrossRef]
- Skidmore, J.A. The Use of Some Assisted Reproductive Technologies in Old World Camelids. Anim. Reprod. Sci. 2019, 207, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Sghiri, A. Evaluation Des Performances de Reproduction d’un Troupeau Camelin à Laayoune (Camelus dromedarius); Institut Agronomique et Veterinaire Hassan II: Rabat, Morocco, 1988. [Google Scholar]
- Ali, A.; Derar, D.; Alsharari, A. Factors Affecting Reproductive Performance in Dromedary Camel Herds in Saudi Arabia. Trop. Anim. Health Prod. 2018, 50, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.A. Reproductive Performance of Camels (Camelus dromedarius) under Pastoral Management and Its Influence on Herd Development. Livest. Prod. Sci. 2005, 92, 17–29. [Google Scholar] [CrossRef]
- Díaz, A.; Huaman, H.; Camacho, J. Serum Oestradiol Level during Mating and Its Effect on Conception Rate in the Alpaca. Rev. Investig. Vet. Perú 2011, 22, 312–317. [Google Scholar]
- Davis, G.H.; Wuliji, T.; Moore, G.H. Growth, Reproduction and Fibre Production of Alpacas Imported from Chile. Proc. N. Z. Soc. Anim. Prod. 1991, 51, 255–258. [Google Scholar]
- Abdel-Raouf, M. Studies on Reproduction in Camels (Camelus dromedarius). III. Testicular Hypoplasia. In Proceedings of the 6th Annual Veterinary Congress, Cairo, Egypt; 1965; pp. 125–130. [Google Scholar]
- Hemeida, N.A.; Ismail, S.T.; El-Wishy, A.B. Studies on Testicular Degeneration in the One-Humped Camel. In Proceedings of the 1st International Congress in Applied Sciences; Zigazig University: Zagazig, Egypt, 1985; Volume 3, pp. 450–458. [Google Scholar]
- Sumar, J. Studies on Reproductive Pathology in Alpacas; Faculty of Veterinary Medicine, University of Agricultural Sciences: Uppsala, Sweden, 1983. [Google Scholar]
- Tibary, A.; Anouassi, A. Male Breeding Soundness Examination. In Theriogenology in Camelidae: Anatomy, Physiology, BSE, Pathology and Artificial Breeding; Tibary, A., Anouassi, A., Eds.; Actes Editions, Institut Agronomique et Veterinaire Hassan II: Abu Dhabi, United Arab Emirates, 1997; pp. 79–111. [Google Scholar]
- Tibary, A.; Anouassi, A.; El Allali, K. Chapter 6. Male Breeding Soundness Examination. In Theriogenology in Camelidae; Tibary, A., Anouassi, A., Eds.; Advanced Scientific Group: Abu Dhabi, United Arab Emirates, 2024. [Google Scholar]
- Waheed, M.M.; Ghoneim, I.M.; Hassieb, M.M. Evaluation of the Breeding Soundness of Male Camels (Camelus dromedarius) via Clinical Examination, Semen Analysis, Ultrasonography and Testicular Biopsy: A Summary of 80 Clinical Cases. Reprod. Domest. Anim. 2014, 49, 790–796. [Google Scholar] [CrossRef]
- Monaco, D. Breeding Soundness Evaluation, a Tool for Improving the Sustainability of Dromedary Camel (Camelus dromedarius) Breeding Systems in Arid and Semi-Arid Lands. Anim. Reprod. Sci. 2025, 277, 107854. [Google Scholar] [CrossRef]
- Pearson, L.K.; Tibary, A. Reproductive Disorders in Male Camelids. Clin. Theriogenol. 2014, 6, 571–577. [Google Scholar]
- Lakritz, J. Congenital/Hereditary Conditions. In Medicine and Surgery of Camelids; John Wiley and Sons: Hoboken, NJ, USA, 2022; pp. 560–589. [Google Scholar]
- Fowler, M.E. Chapter 48—Congenital and Hereditary Conditions of Camelids. In Zoo and Wild Animal Medicine, 6th ed.; Fowler, M.E., Miller, R.E., Eds.; W.B. Saunders: Saint Louis, MI, USA, 2008; pp. 391–393. [Google Scholar]
- El-Shoukary, R.D.; Nasreldin, N.; Osman, A.S. Housing Management of Male Dromedaries during the Rut Season: Effects of Social Contact between Males and Movement Control on Sexual Behavior, Blood Metabolites and Hormonal Balance. Animals 2020, 10, 1621. [Google Scholar] [CrossRef]
- Bott, N.I.; Rodriguez, J.; Sandoval, S. Relationship between Testicular Measurements Using Calipers or Ultrasonography with Testicular Weight in Alpacas (Vicugna pacos). Theriogenology 2008, 70, 576. [Google Scholar] [CrossRef]
- Abraham, M.C.; Puhakka, J.; Ruete, A.; Al-Essawe, E.M.; de Verdier, K.; Morrell, J.M.; Båge, R. Testicular Length as an Indicator of the Onset of Sperm Production in Alpacas under Swedish Conditions. Acta Vet. Scand. 2016, 58, 10. [Google Scholar] [CrossRef] [PubMed]
- Tibary, A.; Vaughan, J. Reproductive Physiology and Infertility in Male South American Camelids: A Review and Clinical Observations. Small Rumin. Res. 2006, 61, 283–298. [Google Scholar] [CrossRef]
- Saleh, B.; Mohammed, A.A.; Mohammad, A.M. Testicular Morphometry, Gonadal and Extra-Gonadal Sperm Reserves of Camel (Camelus dromedarius) during the Hot Dry Season in the Sahelian Region of Nigeria. Niger. J. Anim. Prod. 2021, 48, 53–62. [Google Scholar] [CrossRef]
- Ali, A.; Al-Hassan, M.H.; Jibril, A. Scrotal Circumference and Testicular Morphometric Characteristics of the Camel (Camelus dromedarius) in the Semi-Arid Environment of Northern Nigeria/Circunferencia Escrotal y Características Testiculares Morfométricas Del Camello (Camelus dromedarius) En El Ambiente Semiárido Del Norte de Nigeria. Int. J. Morphol. 2012, 30, 1369–1372. [Google Scholar]
- Mear, L.O.; Gnemmi, G.; Echegaray, A. Exploring Novel Approaches for Fertility and Subfertility Prediction in Dromedary Male Camels. Small Rumin. Res. 2024, 240, 107371. [Google Scholar] [CrossRef]
- Kutzler, M.; Tyson, R.; Grimes, M. Determination of Testicular Blood Flow in Camelids Using Vascular Casting and Color Pulsed-Wave Doppler Ultrasonography. Vet. Med. Int. 2011, 1, 638602. [Google Scholar] [CrossRef]
- Samir, H.; ElSayed, M.I.; Radwan, F. An Updated Insight on Testicular Hemodynamics: Environmental, Physiological, and Technical Perspectives in Farm and Companion Animals. Vet. Res. Commun. 2023, 47, 323–345. [Google Scholar] [CrossRef]
- Sary, R.; Khalil, K.; Sindi, R.A. Characteristics of Ultrasound and Magnetic Resonance Imaging of Normal Testes and Epididymis Besides Angiography of Testicular Artery in Dromedary Camel. Front. Vet. Sci. 2022, 9, 899570. [Google Scholar] [CrossRef]
- Pérez-Durand, M.G.; Massa-Guzmán, A.; Luque-Mamani, N. Age-Related Differences in Testosterone Concentration and Its Relation to Testicular Biometrics, Hemodynamics, and Fertility in Alpacas (Vicugna pacos). Vet. Sci. 2023, 10, 429. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Al-Sobayil, F.A.; Al-Halag, M.A. Topographical Anatomy and Desensitization of the Pudendal Nerve in Adult Male Dromedary Camels. Theriogenology 2011, 76, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.K.; Campbell, A.J.; Sandoval, S. Effects of Vasectomy on Seminal Plasma Alkaline Phosphatase in Male Alpacas (Vicugna pacos). Reprod. Domest. Anim. 2013, 48, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Gharu, S.; Diler, S.; Khosa, J.S. Surgical Management of Entrapped Soft Palate in Camels (Camelus dromedarius). Indian Vet. J. 2016, 93, 67–68. [Google Scholar]
- Pearson, L.K.; Rodriguez, J.S.; Tibary, A. Infertility and Subfertility in the Male. In Llama and Alpaca Care; Cebra, C., Anderson, D.E., Tibary, A., Eds.; W.B. Saunders: St. Louis, MI, USA, 2014; pp. 194–216. [Google Scholar]
- Abdel-Ghani, M.A.; Ghoneim, I.M.; Nagano, M.; AlMomen, H.Q.M. Impact of Papain on the Treatment of Raw Diluted Dromedary Semen. Reprod. Domest. Anim. 2024, 59, e14637. [Google Scholar] [CrossRef]
- Kershaw, C.M.; Evans, G.; Rodney, R. Papain and Its Inhibitor E-64 Reduce Camelid Semen Viscosity without Impairing Sperm Function and Improve Post-Thaw Motility Rates. Reprod. Fertil. Dev. 2017, 29, 1107–1114. [Google Scholar] [CrossRef]
- Monaco, D.; Fatnassi, M.; Padalino, B. Effect of α-Amylase, Papain, and Spermfluid® Treatments on Viscosity and Semen Parameters of Dromedary Camel Ejaculates. Res. Vet. Sci. 2016, 105, 5–9. [Google Scholar] [CrossRef]
- Monaco, D.; Lacalandra, G.M.; Ansar, Z. The Effect of Cushioned Centrifugation, with and without Enzymatic Reduction of Viscosity, on the Motility Pattern and Kinematic Parameters of Dromedary Camel Bull Spermatozoa. Animal 2023, 13, 2685. [Google Scholar] [CrossRef]
- Morrell, J.M.; Abraham, M.C. Semen Handling in South American Camelids: State of the Art. Front. Vet. Sci. 2020, 7, 586858. [Google Scholar] [CrossRef]
- Carrasco, R.A.; Bogle, O.A.; Ratto, M.H. Predictive Characteristics of Male Fertility in Alpacas with Special Reference to Seminal NGF. Theriogenology 2024, 216, 177–184. [Google Scholar] [CrossRef]
- Raudsepp, T.; Mendoza, M.; Tibary, A. Clinical and Molecular Cytogenetics of Camelids: A Comprehensive Review. CABI Agric. Biosci. 2025, 6, 38. [Google Scholar] [CrossRef]
- Tibary, A.; Ruiz, A. Biopsia Testicular En Camélidos: Técnica, Interpretación y Casos Clínicos. In Technicas de Investigation Reproductive en Camelidos; Mellisho, E., Ed.; Biblioteca Nacional del Perú.: Lima, Peru, 2018; pp. 25–32. [Google Scholar]
- Tibary, A.; Campbell, A.; Rodriguez, J.S. Investigation of Male and Female Infertility in Llamas and Alpacas. Reprod. Fertil. Dev. 2021, 33, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Qu, Y.; Li, C. The Histologic and Ultrastructural Characteristics of the Bactrian Camel Testis in Cryptorchidism. Acta Vet. Zootech. Sin. 2016, 47, 993–1000. [Google Scholar]
- Garcia Pereira, F.L.; Allen, A.; Anouassi, A. Parainguinal Cryptorchidectomy under General Anaesthesia in a Bactrian Camel (Camelus bactrianus). J. Camel Pract. Res. 2004, 11, 103–107. [Google Scholar]
- Yuan, L.; Wang, H.; Yang, H. Expression of the NSE, SP, NFH and DβH in Normal and Cryptorchid Testes of Bactrian Camel. Anim. Reprod. 2022, 19, e20210087. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, W. Cryptorchidism in the Vicugna (Lama vicugna). Tierärztliche Prax. 1990, 18, 459–461. [Google Scholar]
- Tibary, A.; Campbell, A.; Rodriguez, J. Urogenital Surgery in Camelids. Clin. Theriogenol. 2020, 12, 271–291. [Google Scholar]
- El-Wishy, A.B. Genital Abnormalities in Camels (Camelus dromedarius). Études Synthèses L’iemvt. 1993, 163–174. [Google Scholar]
- Schwartz, D.; Waqas, M.-S.; Arroyo, E.; Ruiz, A.; Tibary, A. Laparoscopic-Assisted Cryptorchidectomy in South American Camelids and Nigerian Dwarf Goats. Clin. Theriogenol. 2023, 15, 98–102. [Google Scholar] [CrossRef]
- Tibary, A.; Ruiz, A.; Campbell, A. Laparoscopic-Assisted Cryptorchidectomy in Alpacas (Vicugna pacos). In Proceedings of the 5th Conferences od the International Society of Camelid Research and Development, Laayoune, Morocco, 12–15 November 2018; pp. 361–363. [Google Scholar]
- Drew, M.L.; Meyers-Wallen, V.N.; Acland, G.M. Presumptive Sry-Negative XX Sex Reversal in a Llama with Multiple Congenital Anomalies. J. Am. Vet. Med. Assoc. 1999, 215, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.R.; Frazer, G.S.; Hull, B.L. Endocrine Diagnosis of Cryptorchidism in a Llama. Aust. Vet. J. 1996, 74, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Tibary, A.; Campbell, A.J. Effect of Age and Castration on Serum Anti-Mullerian Hormone Concentration in Male Alpacas. Theriogenology 2018, 105, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Kohli, I.S.; Verma, J.K. Note on Dicryptorchidism in a Herd of Bikaneri Camel. Indian J. Anim. Sci. 1981, 51, 684. [Google Scholar]
- El-Hariri, M.N.; Deeb, S. Cryptorchidism with Interstitial Cell Tumour in a Case of Camel (Camelus dromedarius). J. Egypt. Vet. Med. Assoc. 1979, 39, 39–46. [Google Scholar]
- Kutzler, M.A.; Shoemaker, M.; Valentine, B.A. Bilateral Cystic Rete Testis in an Alpaca (Lama pacos). J. Vet. Diagn. Investig. 2006, 18, 303–306. [Google Scholar] [CrossRef]
- Gray, G.A.; Dascanio, J.J.; Kasimanickam, R. Bilateral Epididymal Cysts in an Alpaca Male Used for Breeding. Can. Vet. J. 2007, 48, 741–744. [Google Scholar]
- Tibary, A.; Anouassi, A. Chapter 7. Reproductive Disorders and Infertility in the Male. In Theriogenology in Camelidae; Tibary, A., Anouassi, A., Eds.; Advanced Scientific Group: Abu Dhabi, United Arab Emirates, 2024; pp. 167–206. [Google Scholar]
- Bott, I.; Pearson, L.K.; Rodriguez, J.S. Prevalence and Pathologic Features of Rete Testis Cysts in Alpacas (Vicugna pacos). Clin. Theriogenol. 2010, 2, 395. [Google Scholar]
- Barrios, S.W.; Chavera, C.A.; Huamán, U.H. Testicular Anatomo-Histopathological Alterations in Alpacas (Vicugna pacos) Slaughtered in Nuñoa, Puno. Rev. Investig. Vet. Perú (RIVEP) 2011, 22, 223–232. [Google Scholar]
- Al-Qarawi, A.A. Infertility in the Dromedary Bull: A Review of Causes, Relations and Implications. Anim. Reprod. Sci. 2005, 87, 73–92. [Google Scholar] [CrossRef]
- Ali, A.; Derar, D.R.; Almundarij, T.I. Aetiological Analysis and Diagnosis of Reproductive Disorders in Male Dromedary Camels. Reprod. Domest. Anim. 2021, 56, 1267–1273. [Google Scholar] [CrossRef]
- Lopez, M.J.; Markel, M.D.; Dubielzig, R. Urinary Obstruction in a Hermaphroditic Llama. J. Am. Vet. Med. Assoc. 1998, 212, 710–712. [Google Scholar] [CrossRef]
- Prem, S.; Chawla, S.K.; Chandolia, R.K. Surgical Management of Hypospadias in a New Born Camel Calf. J. Camel Pract. Res. 2010, 17, 209–210. [Google Scholar]
- Monaco, D.; Fatnassi, M.; Padalino, B. Effects of a GnRH Administration on Testosterone Profile, Libido and Semen Parameters of Dromedary Camel Bulls. Res. Vet. Sci. 2015, 102, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.W.; Solis, P.; Ordoñez, C.; Alarcon, V. Fertility of the Male Alpaca: Effect of Daily Consecutive Breeding. Anim. Reprod. Sci. 1997, 46, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Padalino, B.; Monaco, D.; Lacalandra, G.M. Male Camel Behavior and Breeding Management Strategies: How to Handle a Camel Bull during the Breeding Season? Emir. J. Food Agric. 2015, 27, 338–349. [Google Scholar] [CrossRef]
- Lichtenwalner, A.B.; Woods, G.L.; Weber, J.A. Seminal Collection, Seminal Characteristics and Pattern of Ejaculation in Llamas. Theriogenology 1996, 46, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Lichtenwalner, A.B.; Woods, G.L.; Weber, J.A. Ejaculatory Pattern of Llamas during Copulation. Theriogenology 1996, 46, 285–292. [Google Scholar] [CrossRef]
- Ali, A.; Derar, R.; Al-Sobayil, F. Impotentia Generandi in Male Dromedary Camels: Clinical Findings, Semen Characteristics, and Testicular Histopathology. Theriogenology 2014, 82, 890–896. [Google Scholar] [CrossRef]
- Tibary, A.; Campbell, A. Clinical Incidence of Various Reproductive Disorders in Male Alpacas (V. pacos); A Retrospective Study; IVIS: Tours, France, 2016; pp. 106–109. [Google Scholar]
- Al-Qarawi, A.A.; Omar, H.M.; Abdel-Rahman, H.A. Trypanosomiasis-Induced Infertility in Dromedary (Camelus dromedarius) Bulls: Changes in Plasma Steroids Concentration and Semen Characteristics. Anim. Reprod. Sci. 2004, 84, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Derar, D.R.; Alhassun, T.M. Effect of Zinc, Selenium, and Vitamin E Administration on Semen Quality and Fertility of Male Dromedary Camels with Impotentia Generandi. Biol. Trace Elem. Res. 2021, 199, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Tibary, A.; Pearson, L.K. Reproductive Emergencies in Camelids. Clin. Theriogenol. 2014, 6, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.T.; Shigidi, M.T.A. Brucellosis in Camels in Intensive Animal Breeding Areas of Sudan. Implications in Abortion and Early-Life Infections. Rev. D’élevage Médecine Vétérinaire Pays Trop. 2001, 54, 11–15. [Google Scholar]
- Ram, S.M.T.; Satija, K.C.; Chauhan, R.S. Orchitis in a Camel (Camelus dromedarius) Infected with Sarcoptes Cameli. Vet. Parasitol. 1987, 23, 307–309. [Google Scholar] [CrossRef]
- Sana, F.; Iahtasham, K.; Amar, N. Serological, Molecular Detection and Potential Risk Factors Associated with Camel Brucellosis in Pakistan. Trop. Anim. Health Prod. 2016, 48, 1711–1718. [Google Scholar]
- Mowlavi, G.; Massoud, J.; Mobedi, I. Hydatidosis and Testicular Filariasis (D. evansi) in Camel (C. dromedarius) in Central Part of Iran. Iran. J. Public Health 1997, 26, 21–28. [Google Scholar]
- Youssef, A.H. Orchidectomy in Camel Filariasis. J. Egypt. Vet. Med. Assoc. 1976, 35, 147–157. [Google Scholar]
- Oryan, A.; Valinezhad, A.; Bahrami, S. Prevalence and Pathology of Camel Filariasis in Iran. Parasitol. Res. 2008, 103, 1125–1131. [Google Scholar] [CrossRef]
- Aubry, P.; Swor, T.M.; Löhr, C.V. Septic Orchitis in an Alpaca. Can. Vet. J. 2000, 41, 704–706. [Google Scholar]
- Nobre, H.; Rush, J.; Jensen, R. Case Report: Bilateral Indirect Inguinal Herniation in a Male Alpaca. Clin. Theriogenol. 2018, 10, 457–462. [Google Scholar] [CrossRef]
- Birincioglu, S.S.; Avcı, H.; Aydogan, A. Seminoma and Cholangiocarcinoma in an 18-Year-Old Male Camel. Turk. J. Vet. Anim. Sci. 2008, 32, 141–144. [Google Scholar]
- Ali, A.; Ahmed, A.F.; Mehana, E.E. Unilateral Seminoma in a Dromedary Camel. Reprod. Domest. Anim. 2013, 48, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Aboellail, T.A.; Waugh, M.; Harvey, A. Neoplasia and Proliferative Lesions of New World Camelids: A Systematic Literature Review and Retrospective Study of Cases Submitted to Colorado State University From 1995 to 2020. Front. Vet. Sci. 2021, 8, 743498. [Google Scholar] [CrossRef]
- Stewart, J.; Brookhart, M.; Clark, S. Theriogenology Question of the Month. J. Am. Vet. Med. Assoc. 2020, 257, 45–47. [Google Scholar] [CrossRef]
- Tibary, A.; Rodriguez, J.; Sandoval, S. Reproductive Emergencies in Camelids. Theriogenology 2008, 70, 515–534. [Google Scholar] [CrossRef]
- Abdeldjalil, D. Acquired Obstructive Urolithiasis in Male Camelus dromedarius from Southeast Algeria: Report of 11 Cases. J. Camelid Sci. 2020, 13, 56–65. [Google Scholar]
- Duesterdieck-Zellmer, K.F.; Van Metre, D.V.; Cardenas, A. Acquired Urethral Obstruction in New World Camelids: 34 Cases (1995–2008). Aust. Vet. J. 2014, 92, 313–319. [Google Scholar] [CrossRef]
- Tharwat, M.; Al-Sobayil, F. Ultrasonographic Findings in Camels (Camelus dromedarius) with Different Urinary Affections. J. Camel Pract. Res. 2016, 23, 301–308. [Google Scholar] [CrossRef]
- Rosser, J.M.; Jacob, S.I.; Brounts, S.H. Use of Tube Cystostomy in the Surgical Management of Obstructive Urolithiasis in a Bactrian Camel. J. Am. Vet. Med. Assoc. 2019, 254, 868–873. [Google Scholar] [CrossRef]
- Van Saun, R.J. Nutritional Diseases of Llamas and Alpacas. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 797–810. [Google Scholar] [CrossRef]
- Tibary, A.; Anouassi, A.; Khan, I.A.; El Allali, A. Chapter 21. Male Urogenital Surgery. In Theriogenology in Camelidae; Tibary, A., Anouassi, A., Eds.; Advanced Scientific Group: Abu Dhabi, United Arab Emirates, 2024; pp. 593–620. [Google Scholar]
- M’Zah, A.; Ben Said, M.S.; Matoussi, A. Ablation of the Soft Palate in a Male Dromedary. Rev. Médicale Vétérinaire 1993, 144, 885–890. [Google Scholar]
- Bolbol, A.E.; Ela Shazly, M.D. Impaction of the Dulaa “Palatine Diverticulum in the Dromedary”. In Proceedings of the 5th Science Congress; Faculty of Veterinary Medicine, Assiut University: Asyut, Egypte, 1992; pp. 193–202. [Google Scholar]
- Al-Sobayil, F.A.; Ahmed, A.F. Surgery of the Injured Dulla in Dromedary Camels (Camelus dromedarius). Iran. J. Vet. Surg. 2011, 6, 17–22. [Google Scholar]
- Biswadeep, J.; Abhishek, S. Resection of Entrapped, over-Distended Soft Palate in a Male Dromedary Camel (Camelus dromidarius). Int. J. Livest. Res. 2014, 4, 54–57. [Google Scholar] [CrossRef]
- Reece, J.F.; Chawla, S.K. Prolapse of the Soft Palate in a Male Arabian Camel (Camelus dromidarius). Vet. Rec. 2001, 149, 656–657. [Google Scholar] [CrossRef]
- Sadan, M. Rectal Prolapse in Camels (Camelus dromedarius): Clinical Findings and Treatment Outcomes. J. Camel Pract. Res. 2019, 26, 99–103. [Google Scholar] [CrossRef]
- Sickinger, M.; Brachthäuser, L.; Köhler, K. Rectal Prolapse in a Male Llama: A Case Report. Wien. Tierärztliche Monatsschrift 2012, 99, 47–51. [Google Scholar]
- Zabady, M.K.; Fouda, T.A.; Abdin-Bey, M.R. Rectal Prolapse in Dromedary Camels. J. Camel Pract. Res. 2011, 18, 337–344. [Google Scholar]
- Serin, I.; Ceylan, A.; Kirkan, S. Preputial Bacterial Flora and Antibiotic Susceptibility in Wrestling Dromedary Bulls in Aydin Region of Turkey. J. Anim. Vet. Adv. 2010, 9, 482–485. [Google Scholar] [CrossRef][Green Version]
- Jarvinen, J.A.; Kinyon, J.M. Preputial Microflora of Llamas (Lama glama) and Alpacas (Vicugna pacos). Small Rumin. Res. 2010, 90, 156–160. [Google Scholar] [CrossRef]
- Hassan, N.I.; Ahmed, T.M. Mycoplasma and Ureaplasma of the Genital Tract of Camels in Egypt. Assiut Vet. Med. J. 1997, 38, 104–118. [Google Scholar]
- Derar, D.R.; Ahmed, A.; Osman, S.A. Potential Pathogens in Infertile Male Dromedary Camels and Their Association with the Spermiogram and Clinical Findings. Comp. Clin. Pathol. 2017, 26, 965–970. [Google Scholar] [CrossRef]
- Wernery, U. The Barren Camel with Endometritis—Isolation of Trichomonas Fetus and Different Bacteria. J. Vet. Med. Ser. B 1991, 38, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Pasquel, H.S.; Casas, A.E.; Huanca, L.W. Determination of the Presence of Campylobacter Fetus Subsp. Venerealis in Alpacas and Llamas in the Puno Region. Rev. Investig. Vet. Perú (RIVEP) 2011, 22, 278–282. [Google Scholar]
- Al-Busadah, K.A.; El-Bahr, S.M.; Khalafalla, A.I. Serum Biochemical Profile and Molecular Detection of Pathogens in Semen of Infertile Male Dromedary Camels (Camelus dromedarius). Anim. Reprod. Sci. 2017, 180, 58–65. [Google Scholar] [CrossRef]
- Byers, S.R.; Evermann, J.F.; Holdorson, G. Bovine Viral Diarrhea Virus Detection in Alpacas Following Experimental Infection. In Proceedings of the International Camelid Health Conference; International Camelid Institute: Corvallis, OR, USA, 2007; pp. 27–30. [Google Scholar]
- Byers, S.R.; Evermann, J.F.; Bradway, D.S. The Effects of Exposure of Susceptible Alpacas to Alpacas Persistently Infected with Bovine Viral Diarrhea Virus. Can. Vet. J. 2011, 52, 263–271. [Google Scholar]
- Ali, A.; Derar, D.R.; Abdel-Elmoniem, E.M. Impotentia Generandi in Male Dromedary Camels: Heavy Metal and Trace Element Profiles and Their Relations to Clinical Findings and Semen Quality. Trop. Anim. Health Prod. 2019, 51, 1167–1172. [Google Scholar] [CrossRef]
- Ali, A.; Derar, D.R.; Abdel-Elmoniem, E.M. Cadmium in Seminal Plasma of Fertile and Infertile Male Dromedary Camels. Biol. Trace Elem. Res. 2020, 193, 162–165. [Google Scholar] [CrossRef]
- Meligy, A.M.A.; Waheed, M.M.; El-Bahr, S.M. Effect of Heavy Metals Arsenic, Cadmium, and Lead on the Semen Variables of Dromedary Camels (Camelus dromedarius). Anim. Reprod. Sci. 2019, 208, 106115. [Google Scholar] [CrossRef]
- Waheed, M.M.; Ghoneim, I.M.; Alhaider, A.K. Seminal Plasma and Serum Fertility Biomarkers in Dromedary Camels (Camelus dromedarius). Theriogenology 2015, 83, 650–654. [Google Scholar] [CrossRef]
- Rashad, D.E.M.; Ibrahim, S.; El-Sokary, M.M.M.; Mahmoud, K.G.M.; Kandiel, M.M.M.; Abou El-Roos, M.E.A.; Sosa, G.A.M. Region-specific Gene Expression Profile in the Epididymis of High- and Low-fertile Dromedary Camels. Reprod Domest. Anim. 2024, 59, e14678. [Google Scholar] [CrossRef]
- Rashad, D.E.; Ibrahim, S.; El-Sokary, M.M.; Mahmoud, K.G.M.; Abou El-Roos, M.E.; Sosa, G.A.; Kandiel, M.M. Abundance of Selected Genes Implicated in Testicular Functions in Camelus dromedarius with High and Low Epididymal Semen Quality. Biol. Reprod. 2024, 110, 501–508. [Google Scholar] [CrossRef]
- Malo, C.; Carracedo, S.; Delehedde, M.; Sergeant, N.; Skidmore, J.A. Identification of proAKAP4 Concentration Variations in Dromedary Sperm and Their Correlation with Monthly Semen Parameters. Reprod. Fertil. 2021, 2, 268–279. [Google Scholar] [CrossRef]
- Baily, M.P.; Avila, F.; Das, P.J. An Autosomal Translocation 73,XY,t(12;20)(Q11;Q11) in an Infertile Male Llama (Lama glama) with Teratozoospermia. Front. Genet. 2019, 10, 344. [Google Scholar] [CrossRef] [PubMed]
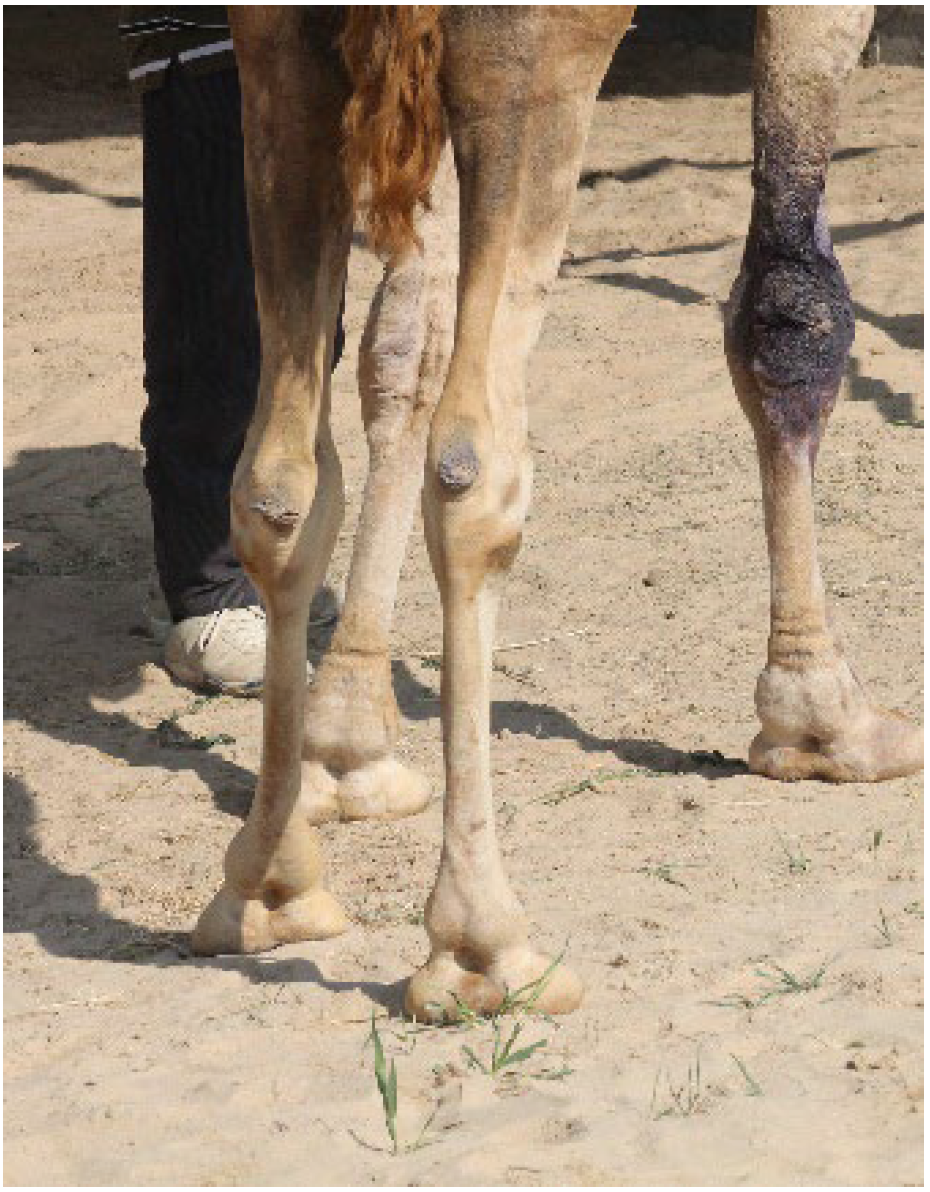

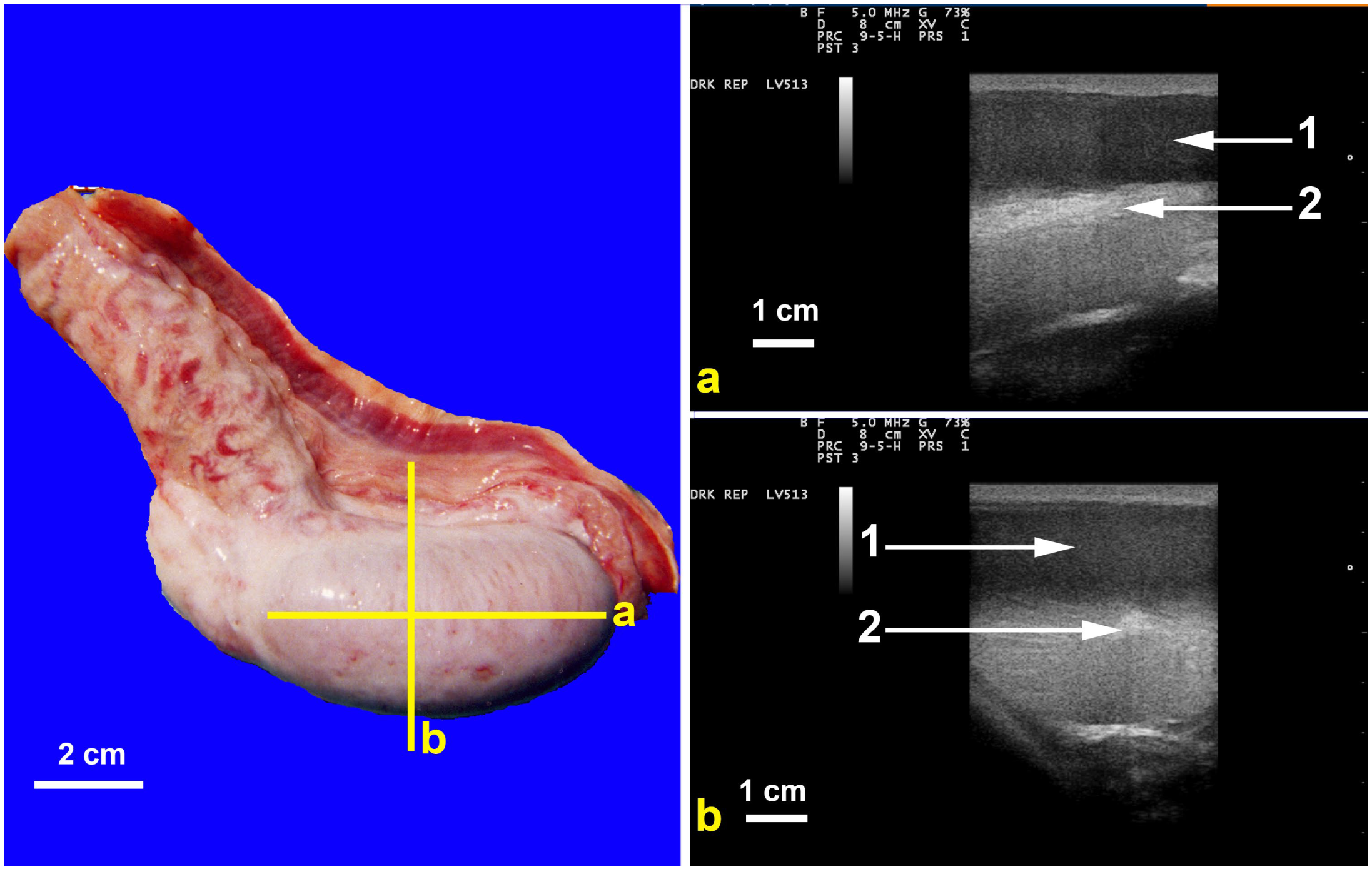
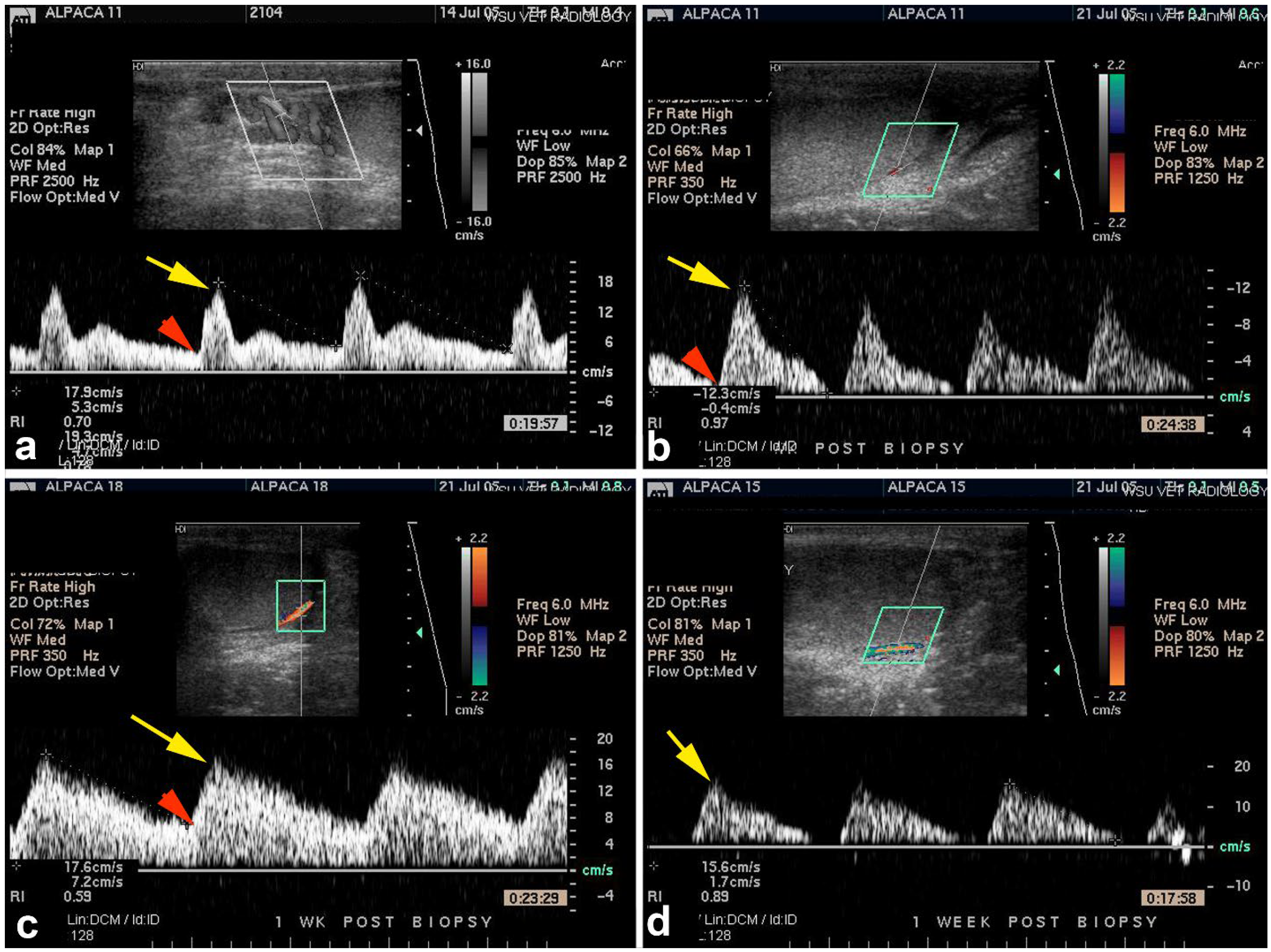


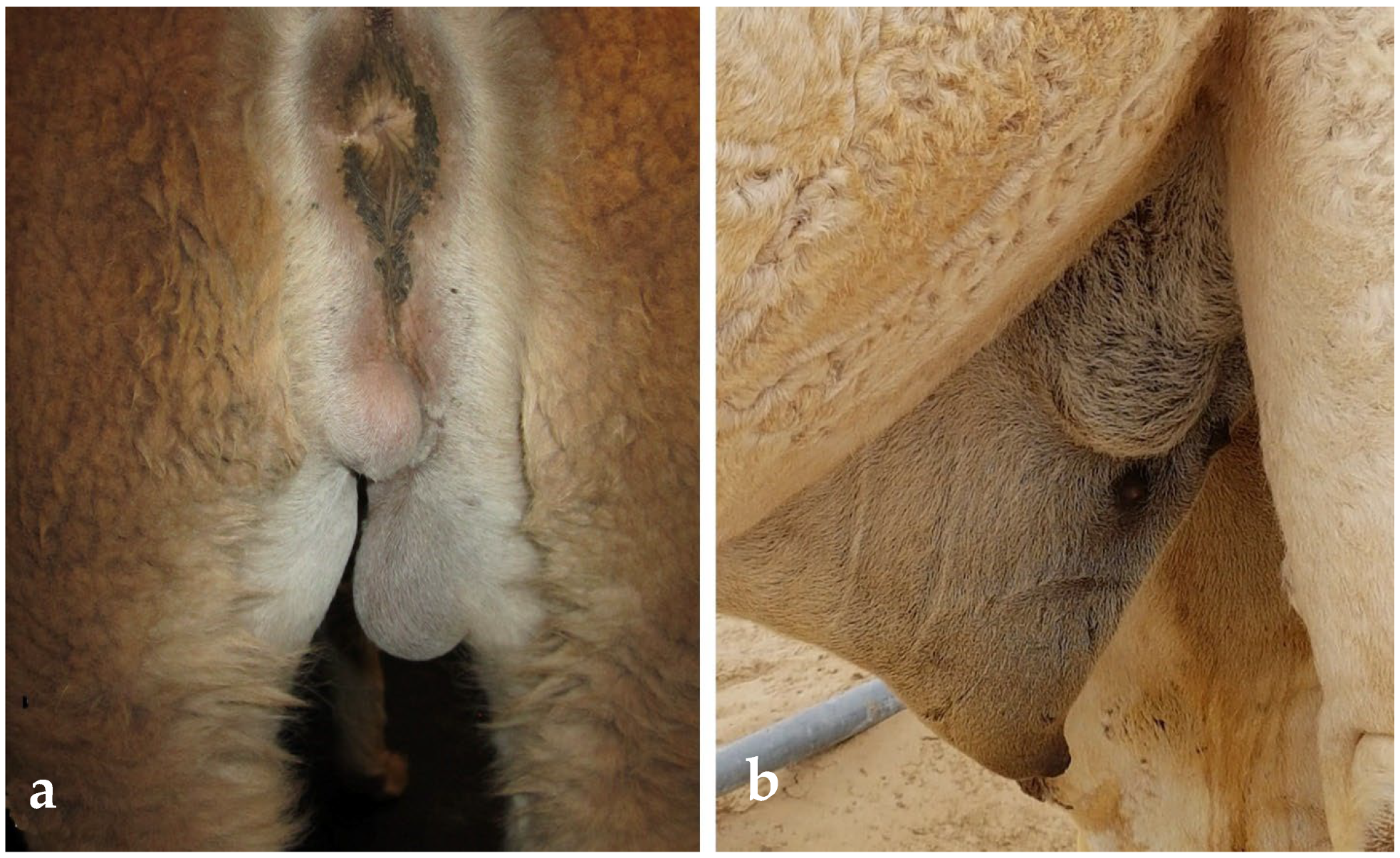





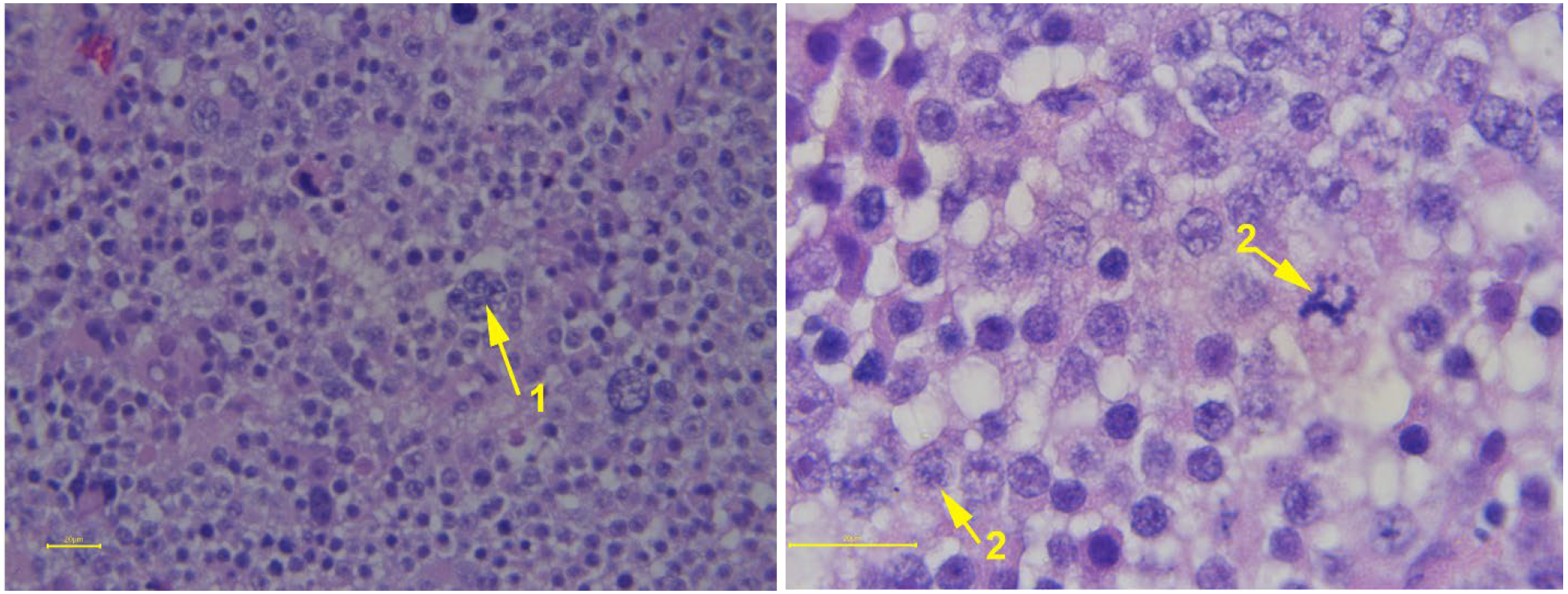
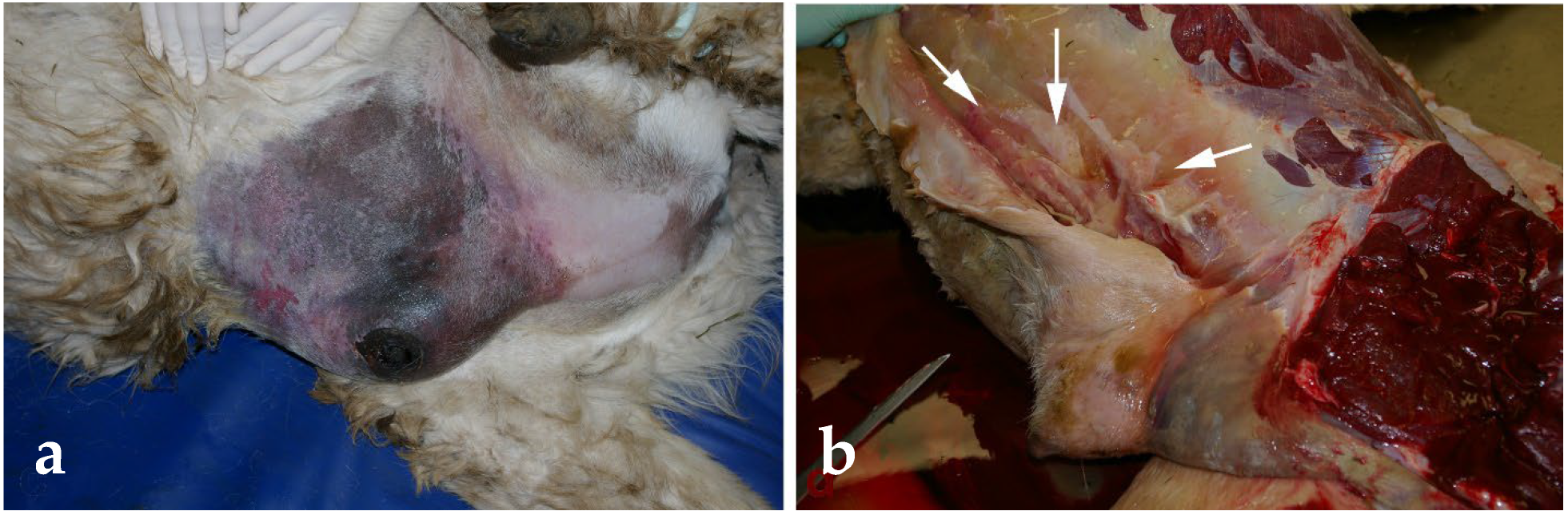
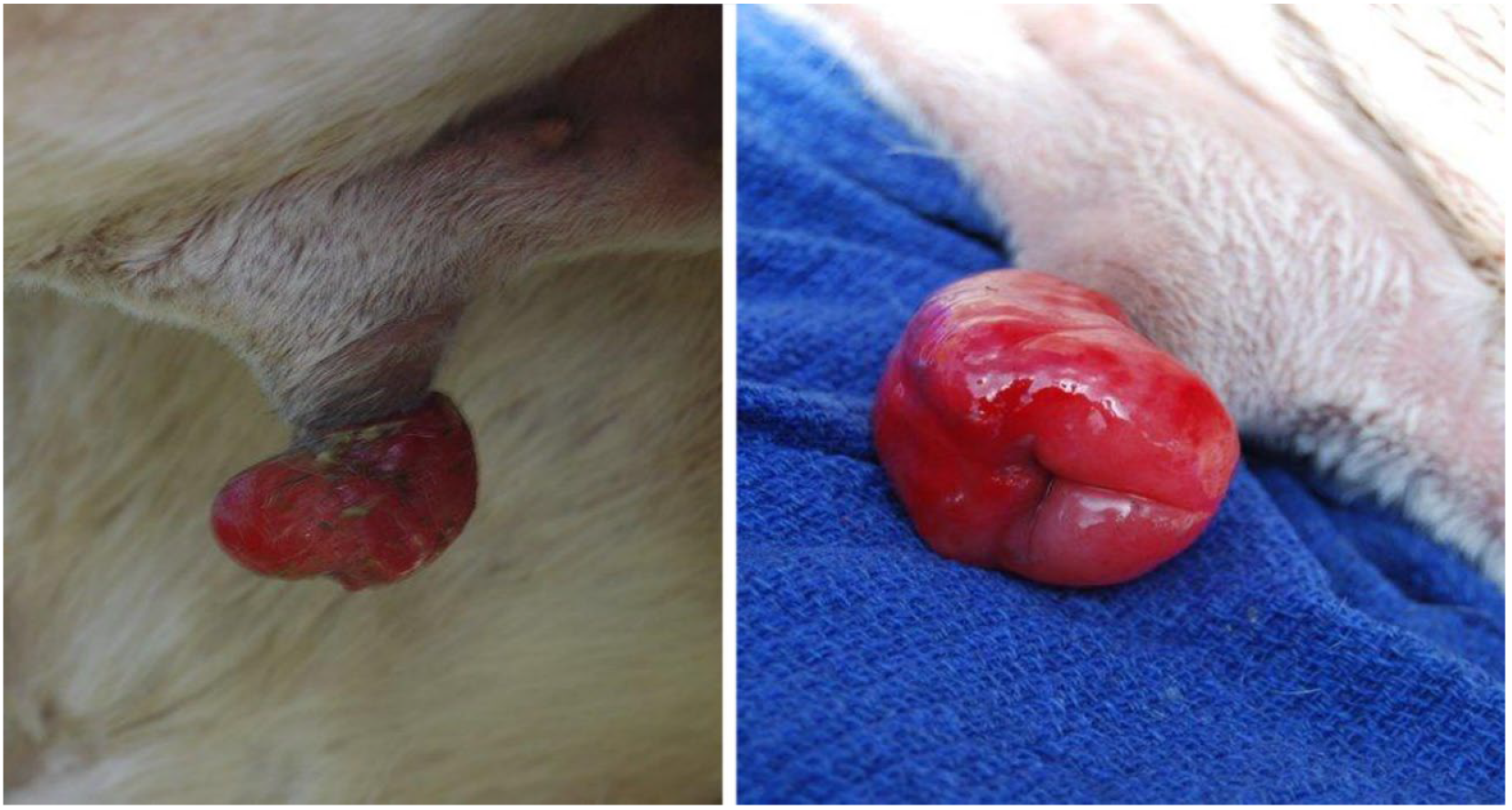




| Condition | Alpacas | Dromedary Camels | |
|---|---|---|---|
| BSE (N = 301) | Infertility (N = 93) | Infertility (N = 48) | |
| N (%) | N (%) | N (%) | |
| Peno-preputial attachment/frenulum | 9 (2.99) | - | 1 (2.08) |
| Phimosis/adhesions | - | 4 (4.30) | 3 (0.63) |
| Cryptorchidism | 4 (1.33) | - | - |
| Ectopic testicle | 3 (0.99) | 1 (1.07) | 1 (2.08) |
| Rete testis cysts (unilateral) | 30 (9.97) | 9 (9.68) | - |
| Rete testis cysts (bilateral) | 48 (15.95) | 16 (17.20) | 1 (2.08) |
| Epididymal cysts | 1 (0.33) | 4 (4.30) | 1 (2.08) |
| Testicular hypoplasia | 24 (7.97) | 2 (4.16) | |
| Testicular degeneration/atrophy | 15 (4.98) | 33 (35.48) | 21 (43.75) |
| Orchitis | 3 (0.99) | 8 (8.60) | 7 (14.58) * |
| Hydrocele | 10 (3.32) | 9 (9.67) | 3 (6.25%) |
| Total abnormalities | 147 (48.84) | 84 (90.32) | (83.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sghiri, A.; Waqas, M.S.; Ciccarelli, M.; Anouassi, A.; Tibary, A. Advances in the Diagnosis of Reproductive Disorders in Male Camelids. Animals 2025, 15, 2931. https://doi.org/10.3390/ani15192931
Sghiri A, Waqas MS, Ciccarelli M, Anouassi A, Tibary A. Advances in the Diagnosis of Reproductive Disorders in Male Camelids. Animals. 2025; 15(19):2931. https://doi.org/10.3390/ani15192931
Chicago/Turabian StyleSghiri, Abdelmalek, Muhammad Salman Waqas, Michela Ciccarelli, Abelhaq Anouassi, and Ahmed Tibary. 2025. "Advances in the Diagnosis of Reproductive Disorders in Male Camelids" Animals 15, no. 19: 2931. https://doi.org/10.3390/ani15192931
APA StyleSghiri, A., Waqas, M. S., Ciccarelli, M., Anouassi, A., & Tibary, A. (2025). Advances in the Diagnosis of Reproductive Disorders in Male Camelids. Animals, 15(19), 2931. https://doi.org/10.3390/ani15192931





