Effects of Different Forage Sources on Growth Performance, Blood Biochemistry, Hormone Concentrations, and Intestinal Microbiota in Alpacas
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Animals and Experimental Design
2.2. Sample Collections
2.3. Growth Performance and Nutrient Digestibility
2.4. Plasma Biochemical Analysis, Hormones, and Antioxidant Enzymes Activity
2.5. 16S rDNA Gene Sequencing Analysis
2.6. Statistical Analysis
3. Results
3.1. Effects of Different Fiber Sources on Growth Performance and Nutrient Digestibility of Alpacas
3.2. Effects of Different Fiber Sources on Nutrient Digestibility of Alpacas
3.3. Effects of Different Fiber Sources on Blood Biochemical Parameters of Alpacas
3.4. Effects of Different Fiber Sources on Serum Antioxidant Function of Alpacas
3.5. Effects of Different Fiber Sources on Serum Endocrine Hormone Concentrations in Alpacas
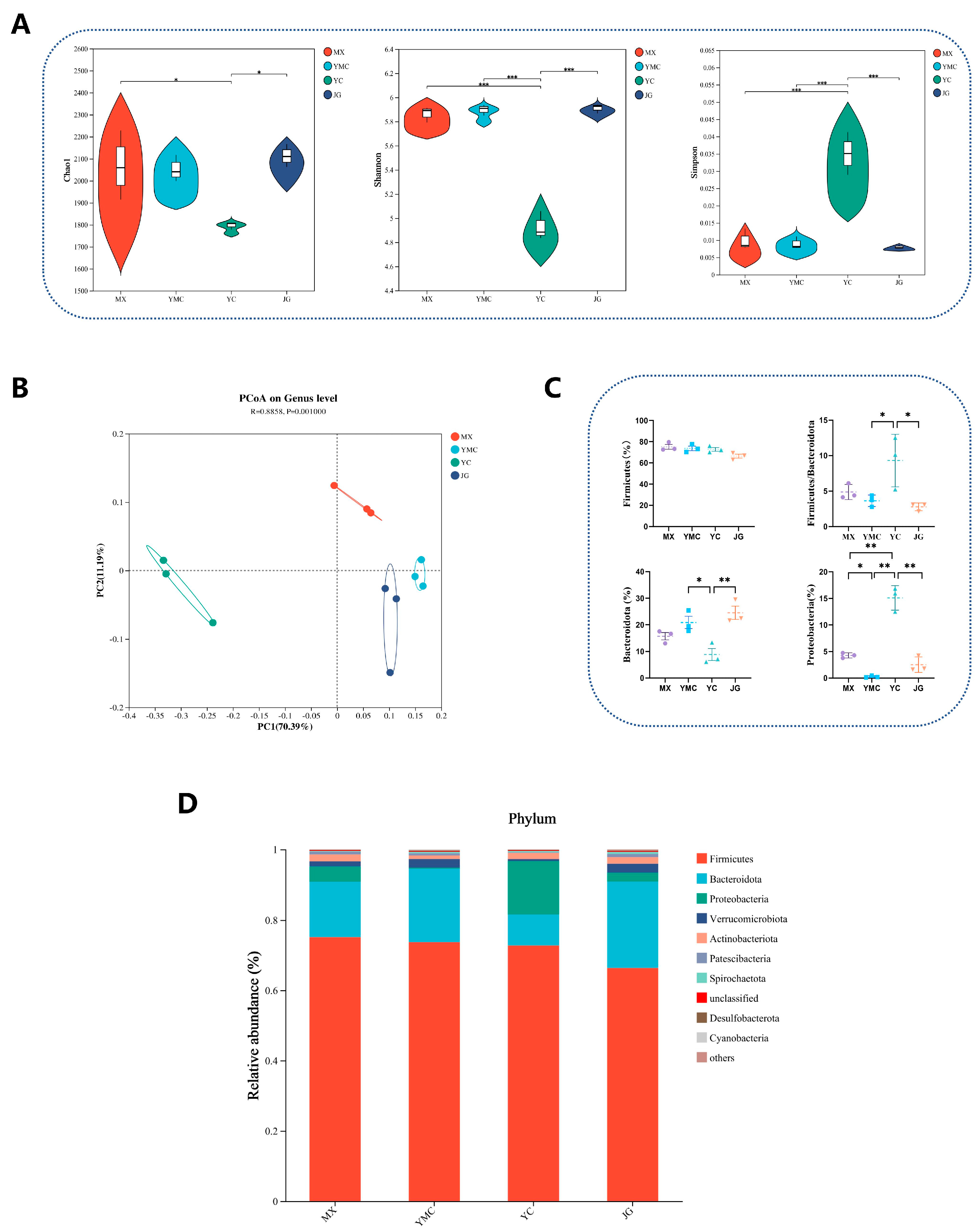
3.6. Effects of Different Fiber Sources on Alpha Fecal Microbial Diversity and Phylum-Level Composition
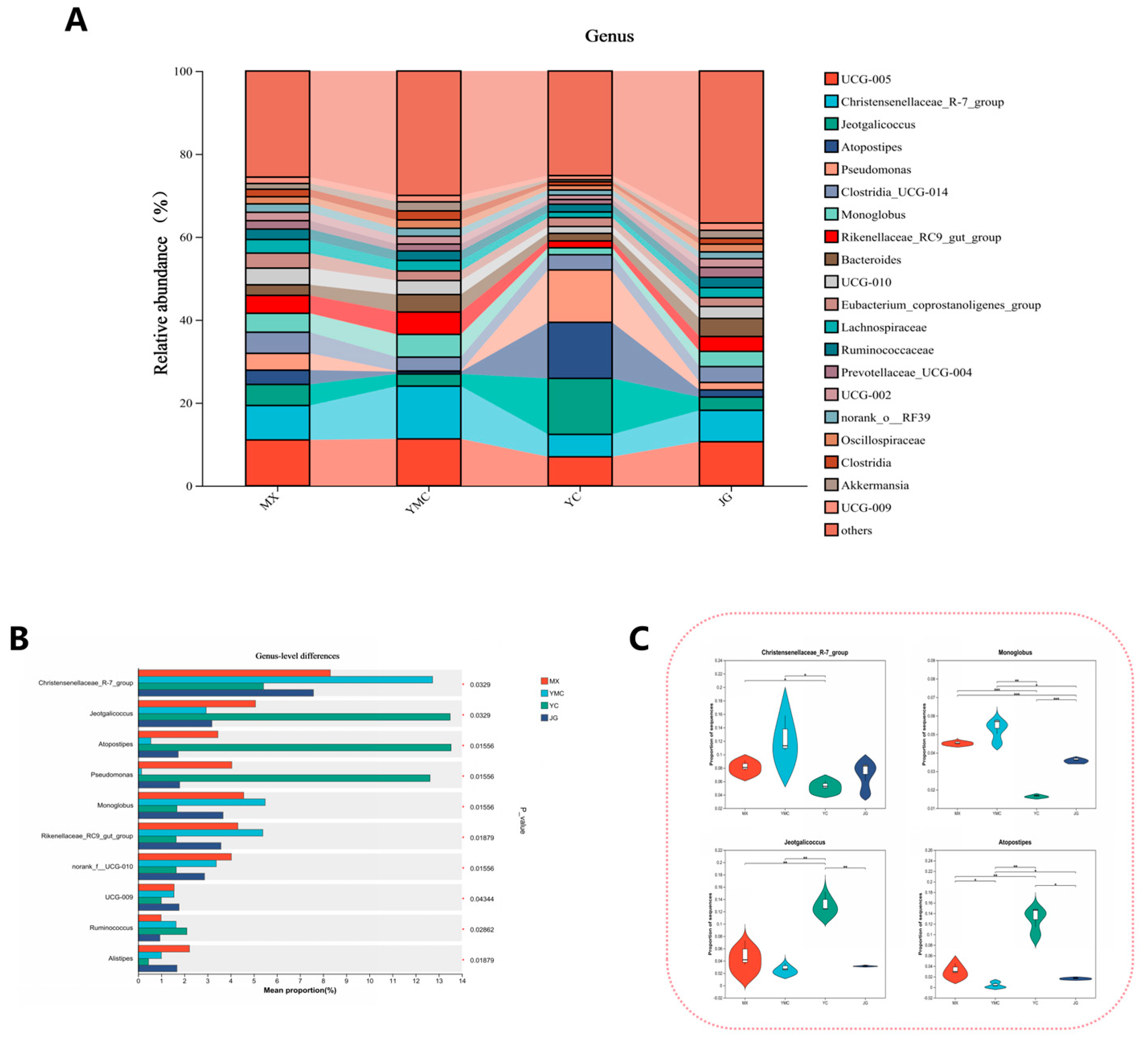
3.7. Effects of Different Fiber Sources on the Alpha Fecal Microbial Genus Level
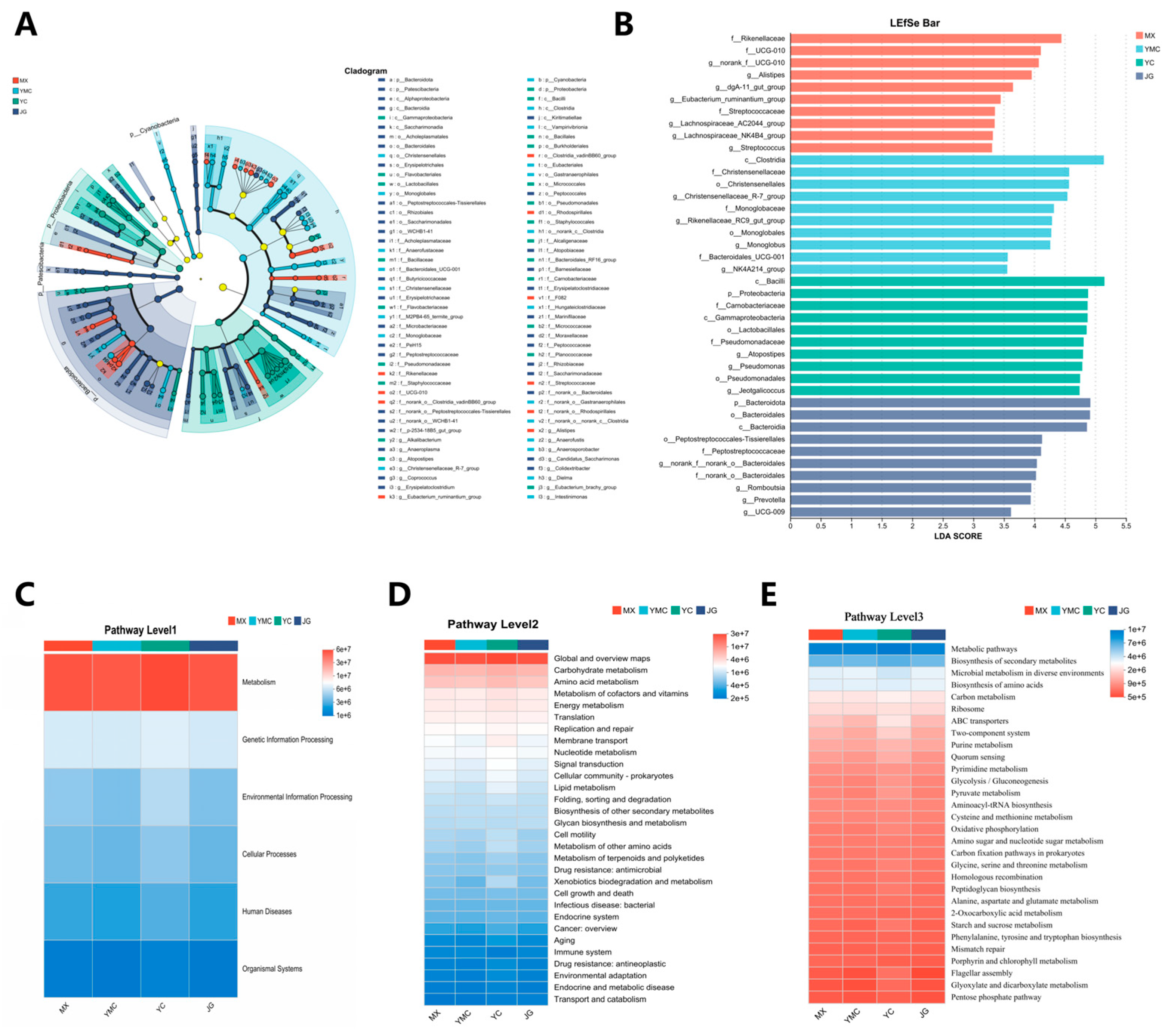
3.8. Effects of Different Fiber Sources on Alpha Fecal Microbial Marker Bacteria and Their Functions
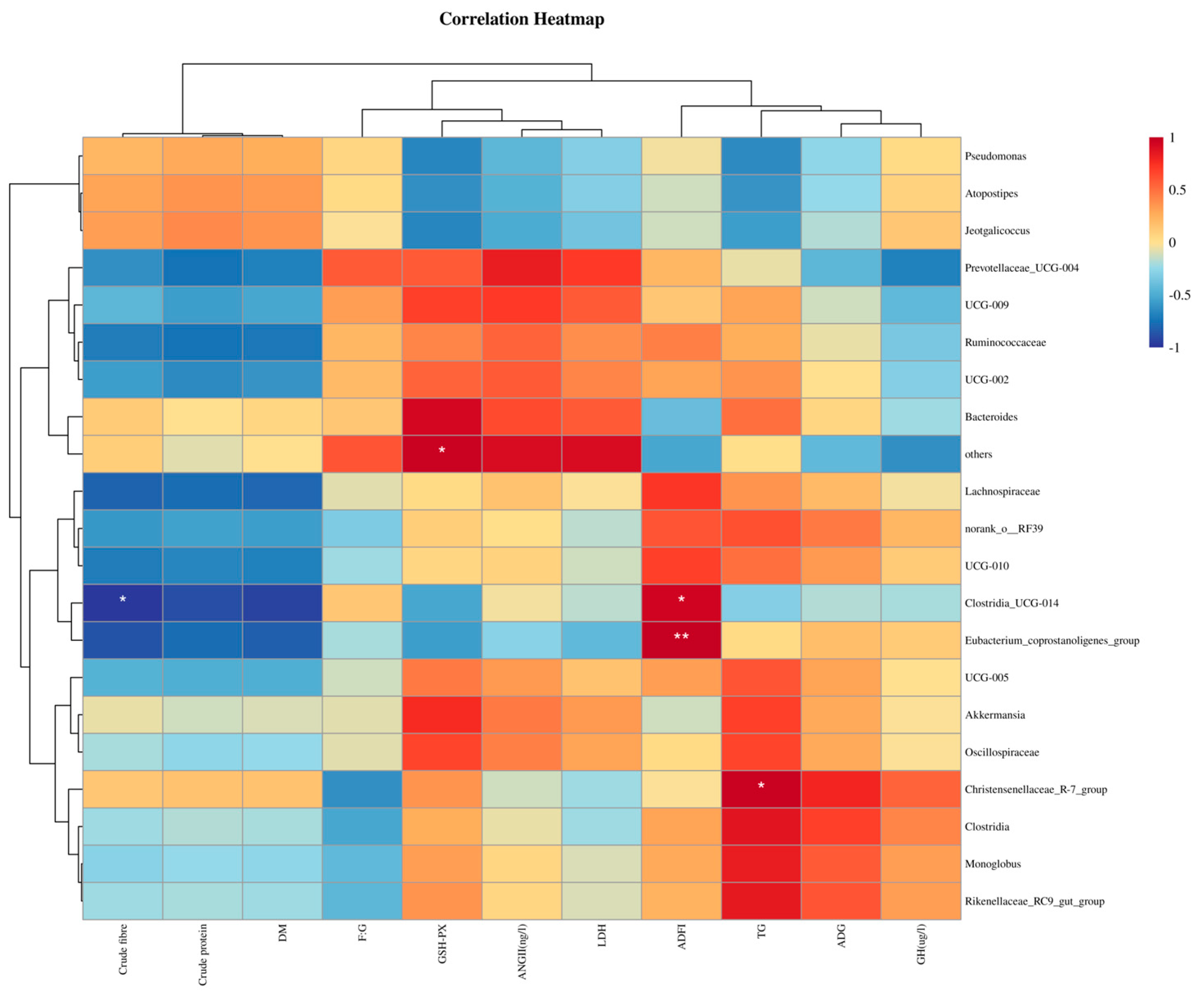
3.9. Effects of Different Fiber Sources on Fecal Microbial Composition in Alpha
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, J.; Ma, Y.T.; Liang, Q.L.; Li, R.L.; Zheng, F.G.; Liu, Q.; Zhu, X.Q.; Gao, W.W. Serological evidence of Toxoplasma gondii and Chlamydia infection in alpacas (Vicugna pacos) in Shanxi Province, northern China. Microb. Pathog. 2020, 149, 104399. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Rashid, M.H.; Zhang, Y.; Vaughan, J.L.; Gasser, R.B.; Jabbar, A. First cross-sectional, molecular epidemiological survey of Cryptosporidium, Giardia and Enterocytozoon in alpaca (Vicugna pacos) in Australia. Parasites Vectors 2018, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shi, Y.; Zhao, M.; Shen, H.; Xu, L.; Luo, Y.; Liu, Y.; Xing, A.; Kang, J.; Jing, H.; et al. Yield and quality properties of alfalfa (Medicago sativa L.) and their influencing factors in China. Eur. J. Agron. 2022, 141, 126637. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, Y. China’s alfalfa market and imports: Development, trends, and potential impacts of the U.S.—China trade dispute and retaliations. J. Integr. Agric. 2020, 19, 1149–1158. [Google Scholar] [CrossRef]
- Dellar, M.; Topp, C.F.E.; Banos, G.; Wall, E. A meta-analysis on the effects of climate change on the yield and quality of European pastures. Agric. Ecosyst. Environ. 2018, 265, 413–420. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, R.; Wang, H.; Liu, B.; Yu, Y.; Jiang, L. Untargeted Metabolome Analyses Revealed Potential Metabolic Mechanisms of Leymus chinensis in Response to Simulated Animal Feeding. Int. J. Mol. Sci. 2024, 25, 6110. [Google Scholar] [CrossRef]
- Liu, T.; Wang, S.; Chen, Y.; Luo, J.; Hao, B.; Zhang, Z.; Yang, B.; Guo, W. Bio-organic fertilizer promoted phytoremediation using native plant Leymus chinensis in heavy Metal(loid)s contaminated saline soil. Environ. Pollut. 2023, 327, 121599. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhao, P.; Song, Z. Effects of High-Voltage Discharge Plasma on Drying Properties, Microstructure, and Nutrients of Oat Grass. Agronomy 2025, 15, 408. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Q.; Cheng, L.; Zhong, R. Effects of mowing regimes on forage yield and crude protein of Leymus chinensis (Trin.) Tzvel in Songnen grassland. Grassl. Sci. 2021, 67, 275–284. [Google Scholar] [CrossRef]
- Hadidi, M.; Rostamabadi, H.; Moreno, A.; Jafari, S.M. Nanoencapsulation of essential oils from industrial hemp (Cannabis sativa L.) by-products into alfalfa protein nanoparticles. Food Chem. 2022, 386, 132765. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana Palacios, J.C.; McClements, D.J.; Mahfouzi, M.; Moreno, A. Alfalfa as a sustainable source of plant-based food proteins. Trends Food Sci. Technol. 2023, 135, 202–214. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Vallenas, A.; Cummings, J.F.; Munnell, J.F. A gross study of the compartmentalized stomach of two new-world camelids, the llama and guanaco. J. Morphol. 1971, 134, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dong, C.S.; Li, H.Q.; Yang, W.Z.; Jiang, J.B.; Gao, W.J.; Pei, C.X.; Liang, Z.Q. Forestomach fermentation characteristics and diet digestibility in alpacas (Lama pacos) and sheep (Ovis aries) fed two forage diets. Anim. Feed Sci. Technol. 2009, 154, 151–159. [Google Scholar] [CrossRef]
- Volkery, J.; Gottschalk, J.; Sobiraj, A.; Wittek, T.; Einspanier, A. Progesterone, pregnanediol-3-glucuronide, relaxin and oestrone sulphate concentrations in saliva, milk and urine of female alpacas (Vicugna pacos) and their application in pregnancy diagnosis. Vet. Rec. 2012, 171, 195. [Google Scholar] [CrossRef]
- Krupinskaite, A.; Stanislauskiene, R.; Serapinas, P.; Rutkiene, R.; Gasparaviciute, R.; Meskys, R.; Stankeviciute, J. alpha-L-Fucosidases from an Alpaca Faeces Metagenome: Characterisation of Hydrolytic and Transfucosylation Potential. Int. J. Mol. Sci. 2024, 25, 809. [Google Scholar] [CrossRef]
- Boughey, I.; Samsing, F.; Hall, E.; Rodney, R.; Bush, R. Characterisation of the Faecal Microbiome of Alpacas Raised in South Eastern Australia. Animals 2025, 15, 1748. [Google Scholar] [CrossRef]
- Bedenice, D.; Resnick-Sousa, J.; Bookbinder, L.; Trautwein, V.; Creasey, H.N.; Widmer, G. The association between fecal microbiota, age and endoparasitism in adult alpacas. PLoS ONE 2022, 17, e0272556. [Google Scholar] [CrossRef]
- Nair, J.; Xu, S.; Smiley, B.; Yang, H.E.; McAllister, T.A.; Wang, Y. Effects of inoculation of corn silage with Lactobacillus spp. or Saccharomyces cerevisiae alone or in combination on silage fermentation characteristics, nutrient digestibility, and growth performance of growing beef cattle. J. Anim. Sci. 2019, 97, 4974–4986. [Google Scholar] [CrossRef]
- GB/T 6438-2022; Determination of Organic Matter in Feeds. China National Standard. Standards Press of China: Beijing, China, 2022.
- GB/T 6432-2018; Determination of Crude Protein in Feeds. China National Standard. Standards Press of China: Beijing, China, 2018.
- GB/T 6434-2022; Determination of Crude Fiber in Feeds. China National Standard. Standards Press of China: Beijing, China, 2022.
- GB/T 6436-2018; Determination of Calcium in Feeds. China National Standard. Standards Press of China: Beijing, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds. China National Standard. Standards Press of China: Beijing, China, 2018.
- Bo, P.T.; Dong, Y.; Zhang, R.; Soe Htet, M.N.; Hai, J. Optimization of Alfalfa-Based Mixed Cropping with Winter Wheat and Ryegrass in Terms of Forage Yield and Quality Traits. Plants 2022, 11, 1752. [Google Scholar] [CrossRef]
- Horvat, D.; Viljevac Vuletic, M.; Andric, L.; Balicevic, R.; Kovacevic Babic, M.; Tucak, M. Characterization of Forage Quality, Phenolic Profiles, and Antioxidant Activity in Alfalfa (Medicago sativa L.). Plants 2022, 11, 2735. [Google Scholar] [CrossRef]
- Singh, P.; Verma, A.K.; Sahu, D.S.; Mehra, U.R. Utilization of nutrients as influenced by different restriction levels of feed intake under sub-tropical conditions in crossbred calves. Livest. Sci. 2008, 117, 308–314. [Google Scholar] [CrossRef]
- An, X.; Zhang, L.; Luo, J.; Zhao, S.; Jiao, T. Effects of Oat Hay Content in Diets on Nutrient Metabolism and the Rumen Microflora in Sheep. Animals 2020, 10, 2341. [Google Scholar] [CrossRef] [PubMed]
- Wagener, M.G.; Kornblum, M.; Kiene, F.; Ganter, M.; Teichmann, U. Influence of age on biochemical serum parameters in female alpacas—A retrospective study. Heliyon 2025, 11, e41097. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Taskinen, M.R.; Bjornson, E.; Packard, C.J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, T.; Yu, Q.; Zhang, C.; Zhang, Y.; Xu, L. Mixed oats and alfalfa improved the antioxidant activity of mutton and the performance of goats by affecting intestinal microbiota. Front. Microbiol. 2022, 13, 1056315. [Google Scholar] [CrossRef]
- Geidl-Flueck, B.; Hochuli, M.; Németh, Á.; Eberl, A.; Derron, N.; Köfeler, H.C.; Tappy, L.; Berneis, K.; Spinas, G.A.; Gerber, P.A. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 2021, 75, 46–54. [Google Scholar] [CrossRef]
- Maiboroda, D.; Danchenko, O.; Gryshchenko, V.; Danchenko, M. Enhancing the quality and technological properties of goose meat during low-temperature storage through the action of biologically active substances from oats and alfalfa. East. Eur. J. Enterp. Technol. 2024, 1, 20–28. [Google Scholar] [CrossRef]
- Wunderling, K.; Zurkovic, J.; Zink, F.; Kuerschner, L.; Thiele, C. Triglyceride cycling enables modification of stored fatty acids. Nat. Metab. 2023, 5, 699–709. [Google Scholar] [CrossRef]
- Khajali, F.; Rafiei, F. A review of plant anti-nutritional factors in animal health and production: The classification, biological properties, and the passivation strategy. J. Agric. Food Res. 2024, 18, 101290. [Google Scholar] [CrossRef]
- Husakova, T.; Pavlata, L.; Pechova, A.; Hauptmanova, K.; Tichy, L. Assessment of selenium status in alpaca. Small Rumin. Res. 2014, 117, 176–182. [Google Scholar] [CrossRef]
- Ji, X.; Tong, W.; Sun, X.; Xiao, L.; Wu, M.; Li, P.; Hu, Y.; Liang, Y. Dietary Effects of Different Proportions of Fermented Straw as a Corn Replacement on the Growth Performance and Intestinal Health of Finishing Pigs. Animals 2025, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dun, Y.; Xiang, G.; Wang, S.; Zhang, H.; Zhou, W.; Li, Y.; Liang, Y. The Effects of Pretreated and Fermented Corn Stalks on Growth Performance, Nutrient Digestion, Intestinal Structure and Function, and Immune Function in New Zealand Rabbits. Animals 2025, 15, 1737. [Google Scholar] [CrossRef] [PubMed]
- Lean, F.Z.X.; Cox, R.; Madslien, K.; Spiro, S.; Nymo, I.H.; Brojer, C.; Neimanis, A.; Lawson, B.; Holmes, P.; Man, C.; et al. Tissue distribution of angiotensin-converting enzyme 2 (ACE2) receptor in wild animals with a focus on artiodactyls, mustelids and phocids. One Health 2023, 16, 100492. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Marroquín, V.M.; Cabezas-Garcia, E.H.; Antezana-Julian, W.; Estellés-Barber, F.; Franco, F.E.; Pinares-Patiño, C.S. Design, operation, and validation of metabolism crates for nutrition studies in alpacas (Vicugna pacos). Small Rumin. Res. 2022, 209, 106660. [Google Scholar] [CrossRef]
- Tong, H.; Capuano, A.W.; Mehta, R.I.; Sood, A.; Bennett, D.A.; Ahima, R.S.; Arnold, S.E.; Arvanitakis, Z. Associations of renin-angiotensin system inhibitor use with brain insulin signaling and neuropathology. Ann. Clin. Transl. Neurol. 2024, 11, 2112–2122. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Abdelgawad, M.A.; Shalaby, K.; Ghoneim, M.M.; AboulMagd, A.M.; Abdelwahab, N.S.; Hassan, H.M.; Othman, A.M. Pioglitazone Synthetic Analogue Ameliorates Streptozotocin-Induced Diabetes Mellitus through Modulation of ACE 2/Angiotensin 1-7 via PI3K/AKT/mTOR Signaling Pathway. Pharmaceuticals 2022, 15, 341. [Google Scholar] [CrossRef]
- Wagener, M.G.; Buchallik-Schregel, J.; Rohrig, P.; Kornblum, M.; Neubert, S.; Altrock, A.V.; Bauer, B.U.; Nicolaisen, T.J.; Ganter, M.; Kiene, F. Hyperfructosaminemia in alpacas (Vicugna pacos) is associated with hyperglycemia, hypofructosaminemia with decreased plasma proteins, and poor nutritional status. Am. J. Vet. Res. 2025, 86, 6. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Granata, R.; Leone, S.; Zhang, X.; Gesmundo, I.; Steenblock, C.; Cai, R.; Sha, W.; Ghigo, E.; Hare, J.M.; Bornstein, S.R.; et al. Growth hormone-releasing hormone and its analogues in health and disease. Nat. Rev. Endocrinol. 2025, 21, 180–195. [Google Scholar] [CrossRef]
- Ma, Y.; Khan, M.Z.; Liu, Y.; Xiao, J.; Chen, X.; Ji, S.; Cao, Z.; Li, S. Analysis of Nutrient Composition, Rumen Degradation Characteristics, and Feeding Value of Chinese Rye Grass, Barley Grass, and Naked Oat Straw. Animals 2021, 11, 2486. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2021, 12, 796025. [Google Scholar] [CrossRef]
- Scepanovic, P.; Hodel, F.; Mondot, S.; Partula, V.; Byrd, A.; Hammer, C.; Alanio, C.; Bergstedt, J.; Patin, E.; Touvier, M.; et al. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zhang, M.; Fu, B.; Wang, X.; Yang, H.; Fang, X.; Li, Z.; Teng, T.; Shi, B. Branched Short-Chain Fatty Acid-Rich Fermented Protein Food Improves the Growth and Intestinal Health by Regulating Gut Microbiota and Metabolites in Young Pigs. J. Agric. Food Chem. 2024, 72, 21594–21609. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.; Sun, G.; Ding, H.; Song, X.; Bai, G.; Shi, B.; Shang, T. Characteristics of glucose and lipid metabolism and the interaction between gut microbiota and colonic mucosal immunity in pigs during cold exposure. J. Anim. Sci. Biotechnol. 2023, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, X.; Liu, Y.; Gao, M.; Wang, M.; Wang, Y.; Wang, X.; Guo, Y. Assessment of the dietary amino acid profiles and the relative biomarkers for amino acid balance in the low-protein diets for broiler chickens. J. Anim. Sci. Biotechnol. 2024, 15, 157. [Google Scholar] [CrossRef]
- Jiao, P.; Wang, Z.; Zhang, X.; Lu, X.; Sun, Q.; Zhao, H.; Xin, H.; Yang, W.; Lv, X.; Xie, X.; et al. Dietary supplementation of Clostridium butyricum and rumen protected fat alters immune responses, rumen fermentation, and bacterial communities of goats. Anim. Feed Sci. Technol. 2024, 314, 116014. [Google Scholar] [CrossRef]
- Wang, S.; Tang, W.; Jiang, T.; Wang, R.; Zhang, R.; Ou, J.; Wang, Q.; Cheng, X.; Ren, C.; Chen, J.; et al. Effect of Dietary Concentrate-to-Forage Ratios During the Cold Season on Slaughter Performance, Meat Quality, Rumen Fermentation and Gut Microbiota of Tibetan Sheep. Animals 2024, 14, 3305. [Google Scholar] [CrossRef]
- Gao, J.; Cheng, B.B.; Liu, Y.F.; Li, M.M.; Zhao, G.Y. Effects of red cabbage extract rich in anthocyanins on rumen fermentation, rumen bacterial community, nutrient digestion, and plasma indices in beef bulls. Animal 2022, 16, 100510. [Google Scholar] [CrossRef]
- Misra, S.; Prasad, P.; Semwal, P.; Mishra, S.K.; Asif, M.H.; Chauhan, P.S. Genomic characterization of the salt-tolerant Bacillus and Jeotgalicoccus strains reveals a diverse metabolism relevant to plant growth promotion and salt stress tolerance. 3 Biotech 2024, 14, 316. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, G.; Lan, J.; Li, X.; Li, X.; Liu, R. Polysaccharide-mediated modulation of gut microbiota in the treatment of liver diseases: Promising approach with significant challenges. Int. J. Biol. Macromol. 2024, 280, 135566. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, Z.; Zhang, J.; Ma, W.; Wang, C. Effects of N-Carbamylglutamate supplementation on cecal morphology, microbiota composition, and short-chain fatty acids contents of broiler breeder roosters. Sci. Rep. 2025, 15, 7489. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xiaojia, L.; Wei, Z.; Jian, Z.; Aiting, W.; Jing, W.; Lin, Y.; Bangwei, C.; Dan, Y. Baicalin circumvents anti-PD-1 resistance by regulating the gut microbiota metabolite short-chain fatty acids. Pharmacol. Res. 2024, 199, 107033. [Google Scholar] [CrossRef]
- Rabee, A.E.; Younan, B.R.; Kewan, K.Z.; Sabra, E.A.; Lamara, M. Modulation of rumen bacterial community and feed utilization in camel and sheep using combined supplementation of live yeast and microalgae. Sci. Rep. 2022, 12, 12990. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zhao, H.; Tu, Y.; Liu, M.; Jiang, L.; Zhao, Y. Characterization of the Dynamic Changes of Ruminal Microbiota Colonizing Citrus Pomace Waste during Rumen Incubation for Volatile Fatty Acid Production. Microbiol. Spectrum. 2023, 11, e03517–e03522. [Google Scholar] [CrossRef]
- Zhu, F.; Shi, Q.; Jiang, Y.H.; Zhang, Y.Q.; Zhao, H. Impaired synaptic function and hyperexcitability of the pyramidal neurons in the prefrontal cortex of autism-associated Shank3 mutant dogs. Mol. Autism 2024, 15, 9. [Google Scholar] [CrossRef]
- Chipa Guillen, P.K.; Antezana Julian, W.O.; Rios Rado, W.M.; Moscoso-Munoz, J.E.; Cabezas-Garcia, E.H. Diet Digestibility and Partitioning of Nutrients in Adult Male Alpacas Fed a Blend of Oat Hay and Alfalfa Pellets at Two Levels of Intake. Animals 2023, 13, 3613. [Google Scholar] [CrossRef]
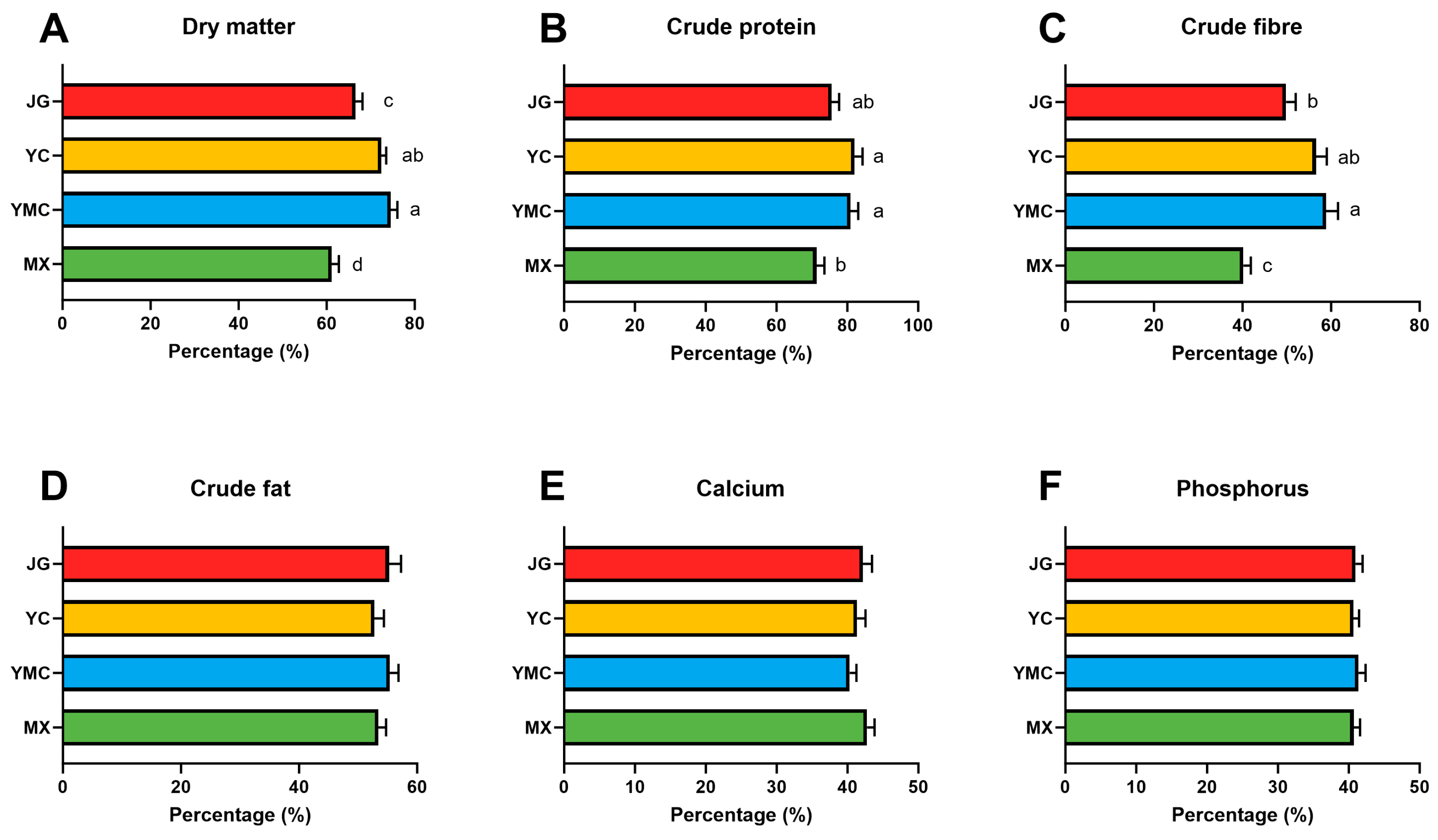
| MX | YMC | YC | JG | |
|---|---|---|---|---|
| Ingredients | ||||
| Alfalfa | 50.0% | 0.0% | 0.0% | 0.0% |
| Oat grass | 0.0% | 50.0% | 0.0% | 0.0% |
| Leymus chinensis | 0.0% | 0.00% | 50.00% | 0.00% |
| Corn Straw | 0.00% | 0.00% | 0.00% | 50.00% |
| Corn | 35.00% | 19.50% | 13.50% | 26.90% |
| Wheat bran | 10.00% | 14.80% | 17.00% | 2.70% |
| Soybean meal | 0.00% | 12.00% | 16.00% | 16.50% |
| Calcarea carbonica | 0.50% | 0.00% | 0.00% | 0.00% |
| Premix a | 0.50% | 0.50% | 0.50% | 0.50% |
| Sodium chloride | 1.00% | 1.00% | 1.00% | 1.00% |
| Calcium bicarbonate | 3.00% | 2.20% | 2.00% | 2.40% |
| Nutrient composition b | ||||
| Digestible energy (MJ) | 10.97 | 10.92 | 10.95 | 10.88 |
| Crude protein | 13.21% | 13.25% | 13.22% | 13.22% |
| Acid detergent fiber | 19.39% | 20.92% | 22.81% | 24.58% |
| Calcium | 0.91% | 0.95% | 0.93% | 0.97% |
| Phosphorus | 0.69% | 0.67% | 0.68% | 0.63% |
| Items | MX | YMC | YC | JG | SEM | p-Value |
|---|---|---|---|---|---|---|
| ADFI (g/d) | 872.87 ± 16.96 a | 878.96 ± 13.8 a | 759.66 ± 12.01 b | 751.77 ± 16.37 b | 19.23 | <0.001 |
| ADG (g/d) | 88.07 ± 4.60 ab | 119.48 ± 2.93 a | 79.26 ± 15.76 b | 55.56 ± 5.56 b | 7.85 | 0.006 |
| F:G | 9.95 ± 0.33 ab | 7.36 ± 0.07 a | 10.21 ± 1.58 ab | 13.76 ± 1.18 b | 0.81 | 0.013 |
| Items | MX | YMC | YC | JG | SEM | p-Value |
|---|---|---|---|---|---|---|
| BUN (mmol/L) | 9.55 ± 0.69 | 9.58 ± 0.79 | 9.54 ± 0.60 | 9.71 ± 0.93 | 0.32 | 0.99 |
| GLU (mmol/L) | 6.13 ± 0.30 | 6.42 ± 0.41 | 6.15 ± 0.36 | 6.23 ± 0.36 | 0.16 | 0.94 |
| TP (g/L) | 53.43 ± 1.73 | 56.4 ± 2.02 | 53.46 ± 2.71 | 52.45 ± 2.21 | 1.04 | 0.63 |
| TG (mmol/L) | 0.24 ± 0.01 a | 0.32 ± 0.02 b | 0.21 ± 0.01 a | 0.22 ± 0.01 a | 0.05 | <0.001 |
| ALT (IU/L) | 18.92 ± 0.65 | 19.32 ± 0.70 | 18.96 ± 0.91 | 21.8 ± 0.92 | 1.72 | 0.099 |
| AST (IU/L) | 189.95 ± 8.74 | 185.09 ± 9.86 | 179.69 ± 8.25 | 217.04 ± 8.84 | 5.77 | 0.072 |
| TBIL (μmol/L) | 0.83 ± 0.05 | 0.84 ± 0.07 | 0.85 ± 0.06 | 0.97 ± 0.06 | 0.11 | 0.398 |
| LDH (IU/L) | 368.63 ± 16.52 a | 371.93 ± 17.45 a | 377.97 ± 17.19 a | 558.03 ± 17.91 b | 25.29 | <0.001 |
| Items | MX | YMC | YC | JG | SEM | p-Value |
|---|---|---|---|---|---|---|
| GSH-Px (U/mL) | 103.7 ± 5.88 a | 137 ± 8.00 ab | 105.88 ± 9.17 a | 155.61 ± 12.52 b | 7.65 | 0.011 |
| T-SOD (U/mL) | 108.86 ± 8.68 | 106.69 ± 11.99 | 133 ± 12.44 | 137.26 ± 8.66 | 6.14 | 0.159 |
| MDA (U/mL) | 2.21 ± 0.13 | 2.13 ± 0.08 | 2.3 ± 0.04 | 2.52 ± 0.17 | 0.07 | 0.182 |
| T-AOC (mmol/L) | 2.61 ± 0.15 | 2.57 ± 0.08 | 2.52 ± 0.21 | 2.51 ± 0.22 | 2.39 | 0.975 |
| Items | MX | YMC | YC | JG | SEM | p-Value |
|---|---|---|---|---|---|---|
| ACTH (ng/L) | 41.96 ± 2.63 | 41.49 ± 1.85 | 40.18 ± 2.05 | 45.61 ± 1.67 | 1.08 | 0.356 |
| ANGII (ng/L) | 24.45 ± 0.32 a | 24.2 ± 0.82 a | 23.74 ± 0.35 a | 27.92 ± 0.89 b | 0.57 | 0.007 |
| NE (ng/L) | 88.5 ± 4.01 | 86.4 ± 6.58 | 88.89 ± 4.29 | 101.84 ± 4.68 | 2.81 | 0.194 |
| GH (μg/L) | 25.61 ± 0.45 a | 29.24 ± 1.07 b | 26.19 ± 0.51 a | 20.7 ± 0.32 c | 0.96 | <0.001 |
| INS (mIU/L) | 42.74 ± 2.31 | 47.92 ± 2.23 | 43.89 ± 2.59 | 40.54 ± 1.78 | 4.34 | 0.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhao, Y.; Xu, L.; Teng, T.; Ma, D. Effects of Different Forage Sources on Growth Performance, Blood Biochemistry, Hormone Concentrations, and Intestinal Microbiota in Alpacas. Animals 2025, 15, 2625. https://doi.org/10.3390/ani15172625
Chen Z, Zhao Y, Xu L, Teng T, Ma D. Effects of Different Forage Sources on Growth Performance, Blood Biochemistry, Hormone Concentrations, and Intestinal Microbiota in Alpacas. Animals. 2025; 15(17):2625. https://doi.org/10.3390/ani15172625
Chicago/Turabian StyleChen, Zhihui, Yang Zhao, Liangmei Xu, Teng Teng, and Deying Ma. 2025. "Effects of Different Forage Sources on Growth Performance, Blood Biochemistry, Hormone Concentrations, and Intestinal Microbiota in Alpacas" Animals 15, no. 17: 2625. https://doi.org/10.3390/ani15172625
APA StyleChen, Z., Zhao, Y., Xu, L., Teng, T., & Ma, D. (2025). Effects of Different Forage Sources on Growth Performance, Blood Biochemistry, Hormone Concentrations, and Intestinal Microbiota in Alpacas. Animals, 15(17), 2625. https://doi.org/10.3390/ani15172625







