Fecal Cortisol Metabolites Indicate Increased Stress Levels in Horses During Breaking-In: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Sample Collection and Processing
2.3. Statistical Analysis
3. Results
3.1. FCM Concentrations Are Higher in Horses in Training
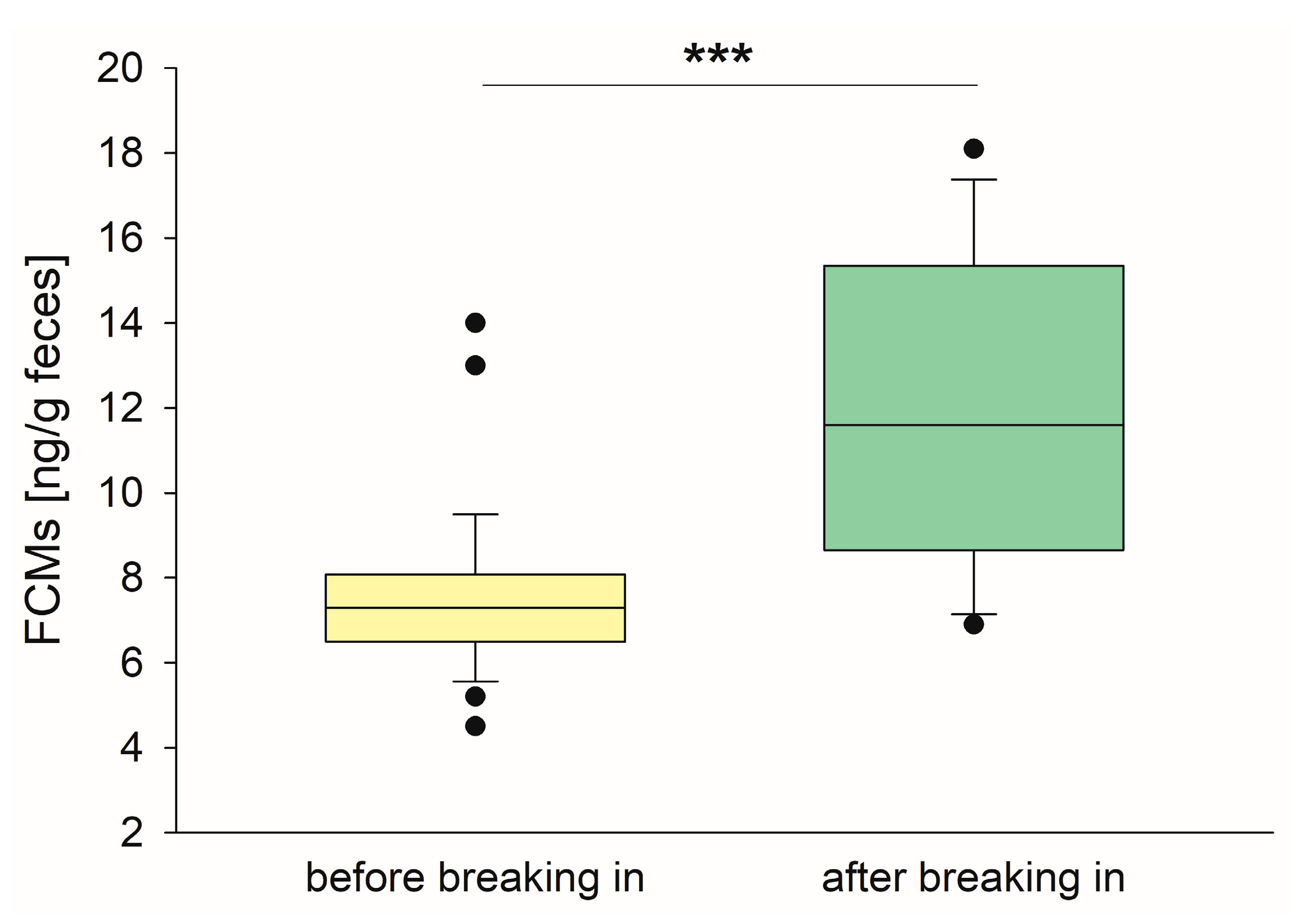
3.2. FCM Concentrations Are Higher Directly After Breaking-In
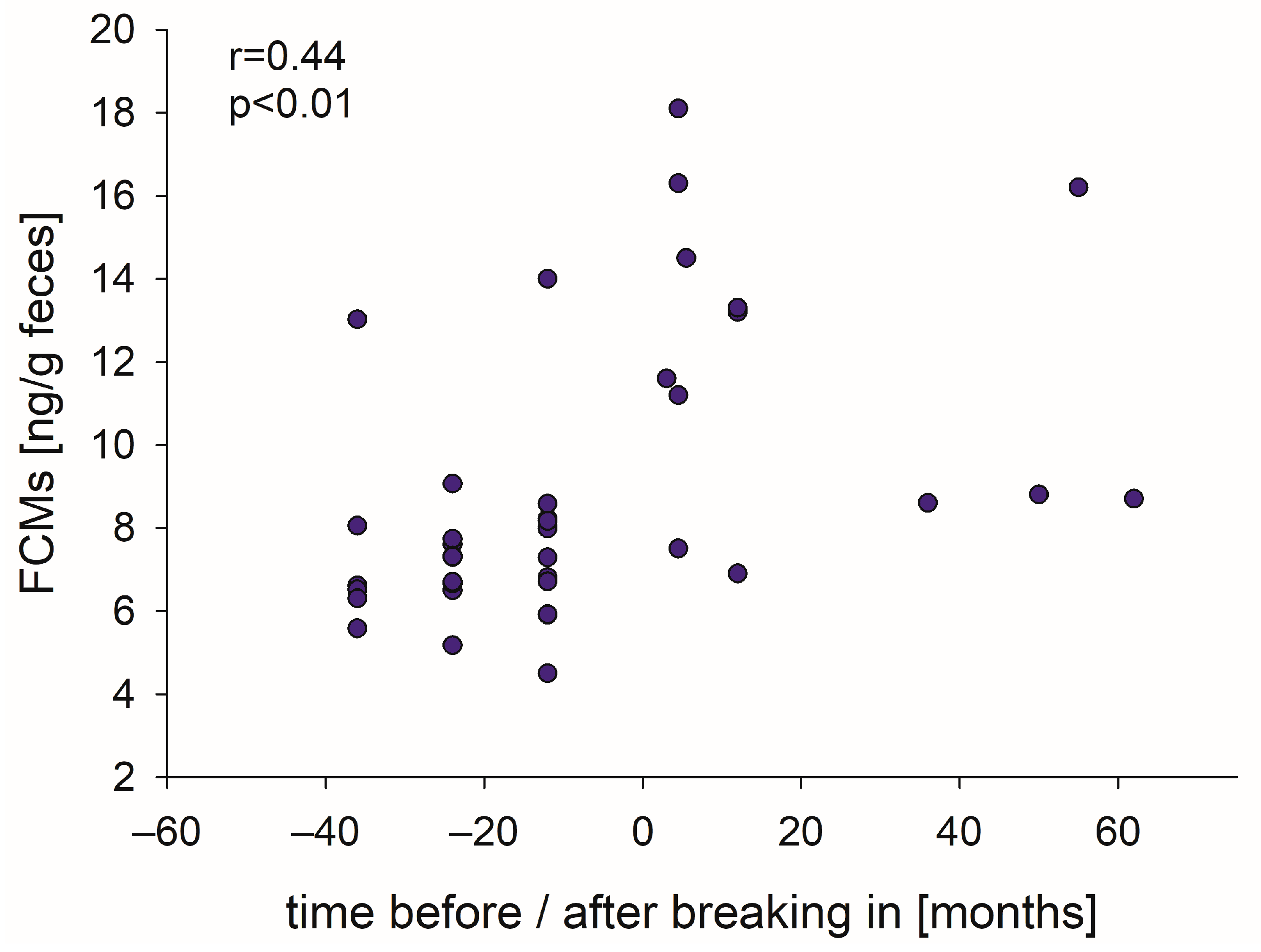
3.3. FCM Concentrations Show a Weak Correlation to Training Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marc, M.; Parvizi, N.; Ellendorff, F.; Kallweit, E.; Elsaesser, F. Plasma cortisol and ACTH concentrations in the warmblood horse in response to a standardized treadmill exercise test as physiological markers for evaluation of training status. J. Anim. Sci. 2000, 78, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Cayado, P.; Muñoz-Escassi, B.; Domínguez, C.; Manley, W.; Olabarri, B.; Sánchez de la Muela, M.; Castejon, F.; Maranon, G.; Vara, E. Hormone response to training and competition in athletic horses. Equine Vet. J. Suppl. 2006, 36, 274–278. [Google Scholar] [CrossRef]
- Schmidt, A.; Möstl, E.; Wehnert, C.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release and heart rate variability in horses during road transport. Horm. Behav. 2010, 57, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Aurich, J.; Möstl, E.; Müller, J.; Aurich, C. Changes in cortisol release and heart rate and heart rate variability during the initial training of 3-year-old sport horses. Horm. Behav. 2010, 58, 628–636. [Google Scholar] [CrossRef]
- Foreman, J.H.; Ferlazzo, A. Physiological responses to stress in the horse. PHK 1996, 12, 401–404. [Google Scholar] [CrossRef]
- Ramos, A.; Mormède, P. Stress and emotionality: A multidimensional and genetic approach. Neurosci. Biobehav. Rev. 1998, 22, 33–57. [Google Scholar] [CrossRef]
- Fraser, D.; Ritchie, J.S.; Fraser, A.F. The term “stress” in a veterinary context. Br. Vet. J. 1975, 131, 653–662. [Google Scholar] [CrossRef]
- Lu, S.; Wei, F.; Li, G. The evolution of the concept of stress and the framework of the stress system. Cell Stress 2021, 5, 76–85. [Google Scholar] [CrossRef]
- Meyer, R.E. Physiologic Measures of Animal Stress during Transitional States of Consciousness. Animals 2015, 5, 702–716. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Snow, D.H.; Rose, R.J. Hormonal changes associated with long distance exercise. Equine Vet. J. 1981, 13, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Baucus, K.L.; Squires, E.L.; Ralston, S.L.; McKinnon, A.O.; Nett, T.M. Effect of transportation on the estrous cycle and concentrations of hormones in mares. J. Anim. Sci. 1990, 68, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.K.; Friend, T.H.; Dellmeier, G. The effect of orientation during trailer transport on heart rate, cortisol and balance in horses. Appl. Anim. Behav. Sci. 1993, 38, 179–189. [Google Scholar] [CrossRef]
- Schmidt, A.; Biau, S.; Möstl, E.; Becker-Birck, M.; Morillon, B.; Aurich, J.; Faure, J.-M.; Aurich, C. Changes in cortisol release and heart rate variability in sport horses during long-distance road transport. Domest. Anim. Endocrinol. 2010, 38, 179–189. [Google Scholar] [CrossRef]
- Dybdal, N.O.; Gribble, D.; Madigan, J.E.; Stabenfelt, G.H. Alterations in plasma corticosteroids, insulin and selected metabolites in horses used in endurance rides. Equine Vet. J. 1980, 12, 137–140. [Google Scholar] [CrossRef]
- Lange, J.; Matheja, S.; Klug, E.; Aurich, C.; Aurich, J.E. Influence of Training and Competition on the Endocrine Regulation of Testicular Function and on Semen Parameters in Stallions. Reprod. Domest. Anim. 1997, 32, 297–302. [Google Scholar] [CrossRef]
- Berghold, P.; Möstl, E.; Aurich, C. Effects of reproductive status and management on cortisol secretion and fertility of oestrous horse mares. Anim. Reprod. Sci. 2007, 102, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, S.; Palme, R.; Vervuert, I.; Glomm, B.; Feige, K.; Macho-Maschler, S.; König von Borstel, U.; Venner, M. Effects of a Gastroscopic Procedure on Salivary Cortisol Release and Fecal Cortisol Metabolites in Young Racehorses. Animals 2024, 14, 3332. [Google Scholar] [CrossRef]
- Alexander, S.L.; Irvine, C.H. The effect of social stress on adrenal axis activity in horses: The importance of monitoring corticosteroid-binding globulin capacity. J. Endocrinol. 1998, 157, 425–432. [Google Scholar] [CrossRef]
- Sauer, F.J.; Hermann, M.; Ramseyer, A.; Burger, D.; Riemer, S.; Gerber, V. Effects of breed, management and personality on cortisol reactivity in sport horses. PLoS ONE 2019, 14, e0221794. [Google Scholar] [CrossRef]
- Mazzola, S.M.; Colombani, C.; Pizzamiglio, G.; Cannas, S.; Palestrini, C.; Costa, E.D.; Gazzonis, A.L.; Bionda, A.; Crepalid, P. Do You Think I Am Living Well? A Four-Season Hair Cortisol Analysis on Leisure Horses in Different Housing and Management Conditions. Animals 2021, 11, 2141. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Erhard, M.H.; Zeitler-Feicht, M.H. Which animal-to-feeding-place ratio at time-controlled hay racks is animal appropriate? Preliminary analysis of stress responses of horses. Front. Vet. Sci. 2022, 9, 1005102. [Google Scholar] [CrossRef] [PubMed]
- Pilger, F.; Kroschel, L.; Aurich, J.; Nagel, C.; Hoffmann, G.; Hartmann, U.; Aurich, C. Stress response of 18-, 24- and 30-month-old sport horse stallions to a pretraining programme. Animal 2024, 18, 101373. [Google Scholar] [CrossRef]
- Gorgasser, I.; Tichy, A.; Palme, R. Fecal cortisol metabolites in Quarter Horses during initial training under field conditions. Wien. Tierärztl. Monatsschr. 2007, 94, 226–230. [Google Scholar]
- Hovey, M.R.; Davis, A.; Chen, S.; Godwin, P.; Porr, C.A.S. Evaluating Stress in Riding Horses: Part One-Behavior Assessment and Serum Cortisol. J. Equine Vet. Sci. 2021, 96, 103297. [Google Scholar] [CrossRef]
- Kędzierski, W.; Cywińska, A.; Strzelec, K.; Kowalik, S. Changes in salivary and plasma cortisol levels in Purebred Arabian horses during race training session. Anim. Sci. J. 2014, 85, 313–317. [Google Scholar] [CrossRef]
- Soroko, M.; Howell, K.; Dudek, K.; Waliczek, A.; Micek, P.; Flaga, J. Relationship between maximum eye temperature and plasma cortisol concentration in racehorses during intensive training. Pol. J. Vet. Sci. 2021, 24, 393–397. [Google Scholar] [CrossRef]
- Palme, R.; Fischer, P.; Schildorfer, H.; Ismail, M.N. Excretion of infused 14C-steroid hormones via feces and urine in domestic livestock. Anim. Reprod. Sci. 1996, 43, 43–63. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Ann. N. Y. Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef]
- Palme, R.; Möstl, E. Measurement of cortisol metabolites in feces of sheep as a parameter of cortisol concentration in blood. Int. J. Mammal. Biol. 1997, 62, 192–197. [Google Scholar]
- Möstl, E.; Messmann, S.; Bagu, E.; Robia, C.; Palme, R. Measurement of glucocorticoid metabolite concentrations in feces of domestic livestock. Zentralbl. Veterinarmed. A 1999, 46, 621–631. [Google Scholar] [PubMed]
- Palme, R. Measuring fecal steroids: Guidelines for practical application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Merl, S.; Scherzer, S.; Palme, R.; Möstl, E. Pain causes increased concentrations of glucocorticoid metabolites in horse feces. J. Equine Vet. Sci. 2000, 20, 586–590. [Google Scholar] [CrossRef]
- Nowak, A.C.; Macho-Maschler, S.; Biermann, N.M.; Palme, R.; Dengler, F. Investigating the interplay of stressors and health in horses through fecal cortisol metabolite analysis. Front. Vet. Sci. 2025, 12, 1545577. [Google Scholar] [CrossRef]
- Wolframm, I.A.; Micklewright, D. Effects of trait anxiety and direction of pre-competitive arousal on performance in the equestrian disciplines of dressage, showjumping and eventing. Comp. Exerc. Physiol. 2010, 7, 185–191. [Google Scholar] [CrossRef]
- Pawluski, J.; Jego, P.; Henry, S.; Bruchet, A.; Palme, R.; Coste, C.; Hausberger, M. Low plasma cortisol and fecal cortisol metabolite measures as indicators of compromised welfare in domestic horses (Equus caballus). PLoS ONE 2017, 12, e0182257. [Google Scholar] [CrossRef]
- Saslow, C.A. Understanding the perceptual world of horses. Appl. Anim. Behav. Sci. 2002, 78, 209–224. [Google Scholar] [CrossRef]
- Budzyńska, M. Stress Reactivity and Coping in Horse Adaptation to Environment. J. Equine Vet. Sci. 2014, 34, 935–941. [Google Scholar] [CrossRef]
- Ijichi, C.; Collins, L.M.; Creighton, E.; Elwood, R.W. Harnessing the power of personality assessment: Subjective assessment predicts behaviour in horses. Behav. Process. 2013, 96, 47–52. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Cravana, C.; Fazio, E.; Medica, P. The different hormonal system during exercise stress coping in horses. Vet. World 2020, 13, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Richard-Yris, M.-A.; Hausberger, M. Influence of various early human–foal interferences on subsequent human–foal relationship. Dev. Psychobiol. 2006, 48, 712–718. [Google Scholar] [CrossRef]
- Fureix, C.; Jego, P.; Sankey, C.; Hausberger, M. How horses (Equus caballus) see the world: Humans as significant “objects”. Anim. Cogn. 2009, 12, 643–654. [Google Scholar] [CrossRef]
- Rivera, E.; Benjamin, S.; Nielsen, B.; Shelle, J.; Zanella, A.J. Behavioral and physiological responses of horses to initial training: The comparison between pastured versus stalled horses. Appl. Anim. Behav. Sci. 2002, 78, 235–252. [Google Scholar] [CrossRef]
- Visser, E.K.; Ellis, A.D.; van Reenen, C.G. The effect of two different housing conditions on the welfare of young horses stabled for the first time. Appl. Anim. Behav. Sci. 2008, 114, 521–533. [Google Scholar] [CrossRef]
- Sikorska, U.; Maśko, M.; Ciesielska, A.; Zdrojkowski, Ł.; Domino, M. Role of Cortisol in Horse’s Welfare and Health. Agriculture 2023, 13, 2219. [Google Scholar] [CrossRef]
- Kalliokoski, O.; Jellestad, F.K.; Murison, R. A systematic review of studies utilizing hair glucocorticoids as a measure of stress suggests the marker is more appropriate for quantifying short-term stressors. Sci. Rep. 2019, 9, 11997. [Google Scholar] [CrossRef]
- Marliani, G.; Vaccari, L.; Cavallini, D.; Montesano, C.S.; Buonaiuto, G.; Accorsi, P.A. Assessing the effectiveness of cannabidiol additive supplementation on canine behavior and cortisol levels. Heliyon 2024, 10, e31345. [Google Scholar] [CrossRef]
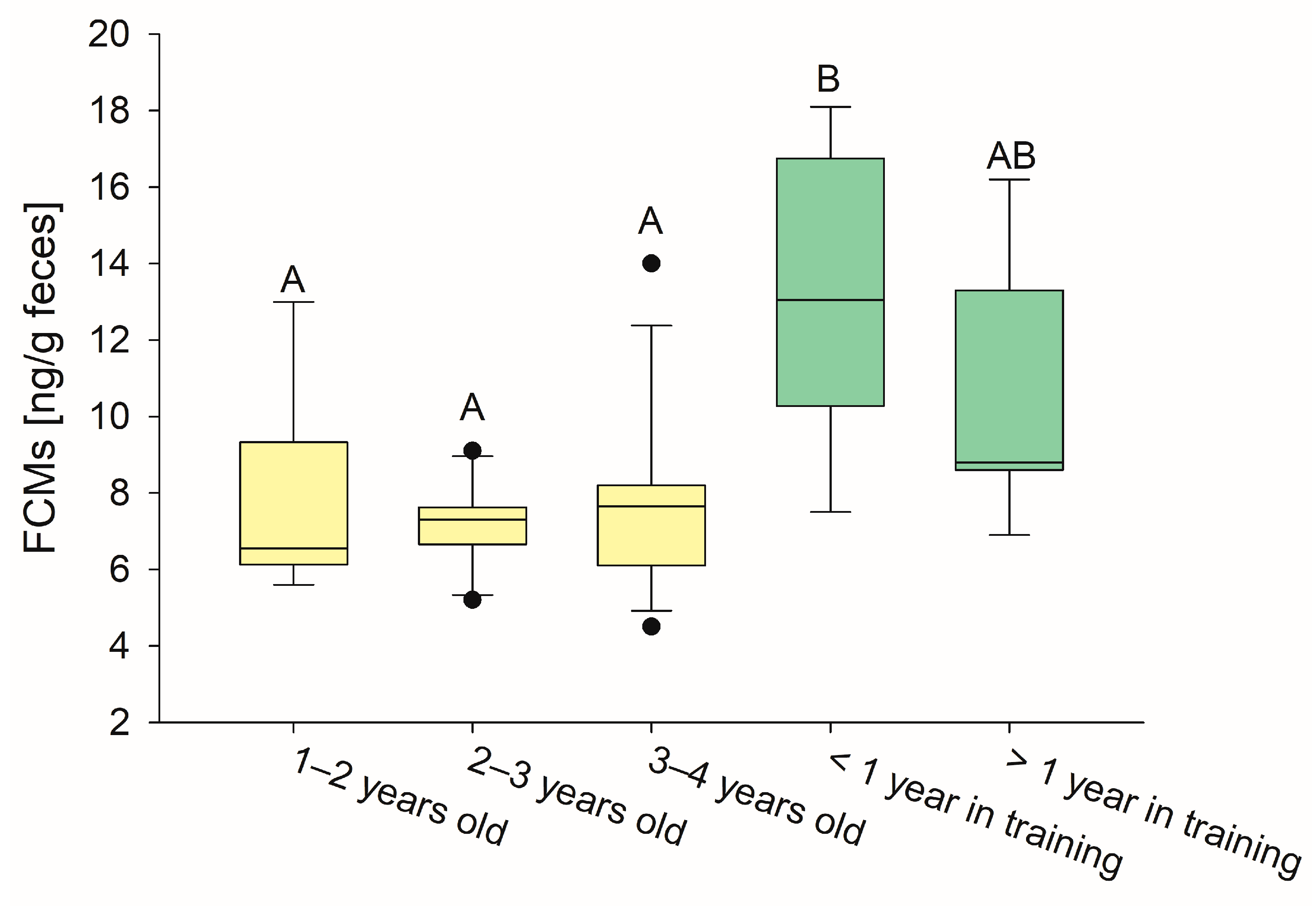
| Group | Median | 75% | 25% |
|---|---|---|---|
| Unridden, 1–2 years old | 6.6 a | 9.3 | 6.1 |
| Unridden, 2–3 years old | 7.3 a | 7.6 | 6.7 |
| Unridden, 3–4 years old | 7.7 a | 8.2 | 6.1 |
| In training for 3–6 months | 13.1 b | 16.8 | 10.3 |
| In training for 1 year | 13.2 ab | 13.3 | 6.9 |
| In training for >3 years | 8.8 ab | 14.4 | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krieber, J.; Nowak, A.C.; Geissberger, J.; Illichmann, O.; Macho-Maschler, S.; Palme, R.; Dengler, F. Fecal Cortisol Metabolites Indicate Increased Stress Levels in Horses During Breaking-In: A Pilot Study. Animals 2025, 15, 1693. https://doi.org/10.3390/ani15121693
Krieber J, Nowak AC, Geissberger J, Illichmann O, Macho-Maschler S, Palme R, Dengler F. Fecal Cortisol Metabolites Indicate Increased Stress Levels in Horses During Breaking-In: A Pilot Study. Animals. 2025; 15(12):1693. https://doi.org/10.3390/ani15121693
Chicago/Turabian StyleKrieber, Julia, Aurelia C. Nowak, Jakob Geissberger, Oliver Illichmann, Sabine Macho-Maschler, Rupert Palme, and Franziska Dengler. 2025. "Fecal Cortisol Metabolites Indicate Increased Stress Levels in Horses During Breaking-In: A Pilot Study" Animals 15, no. 12: 1693. https://doi.org/10.3390/ani15121693
APA StyleKrieber, J., Nowak, A. C., Geissberger, J., Illichmann, O., Macho-Maschler, S., Palme, R., & Dengler, F. (2025). Fecal Cortisol Metabolites Indicate Increased Stress Levels in Horses During Breaking-In: A Pilot Study. Animals, 15(12), 1693. https://doi.org/10.3390/ani15121693





