Hypersalinity Drives Dramatic Shifts in the Invertebrate Fauna of Estuaries
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
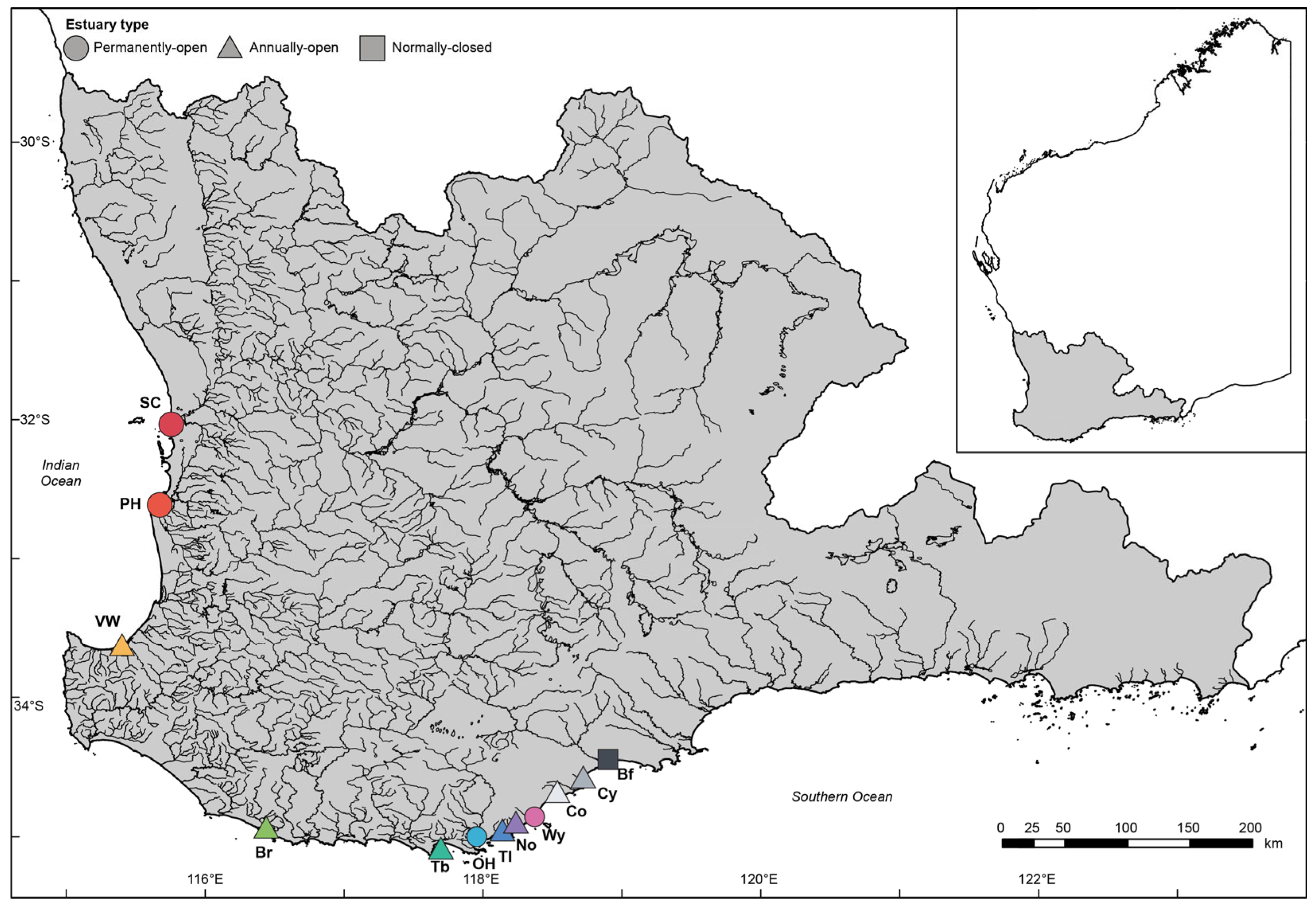
2.1. Site Description and Sampling
2.2. Data Analysis
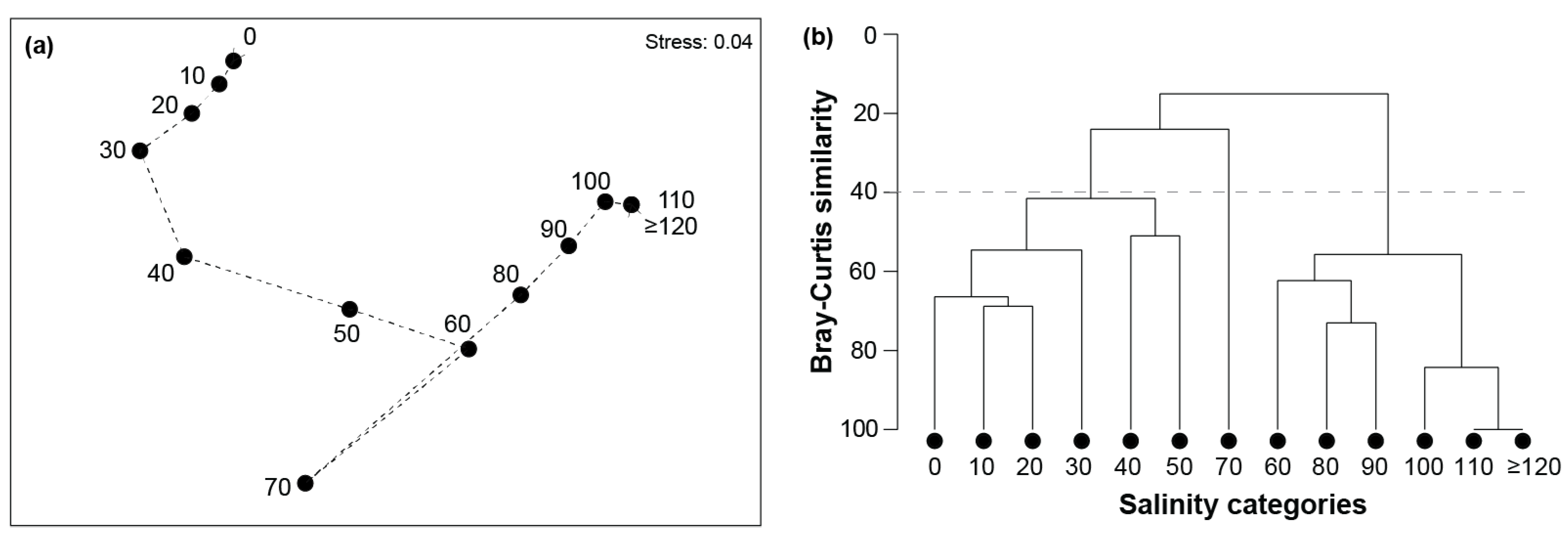
3. Results
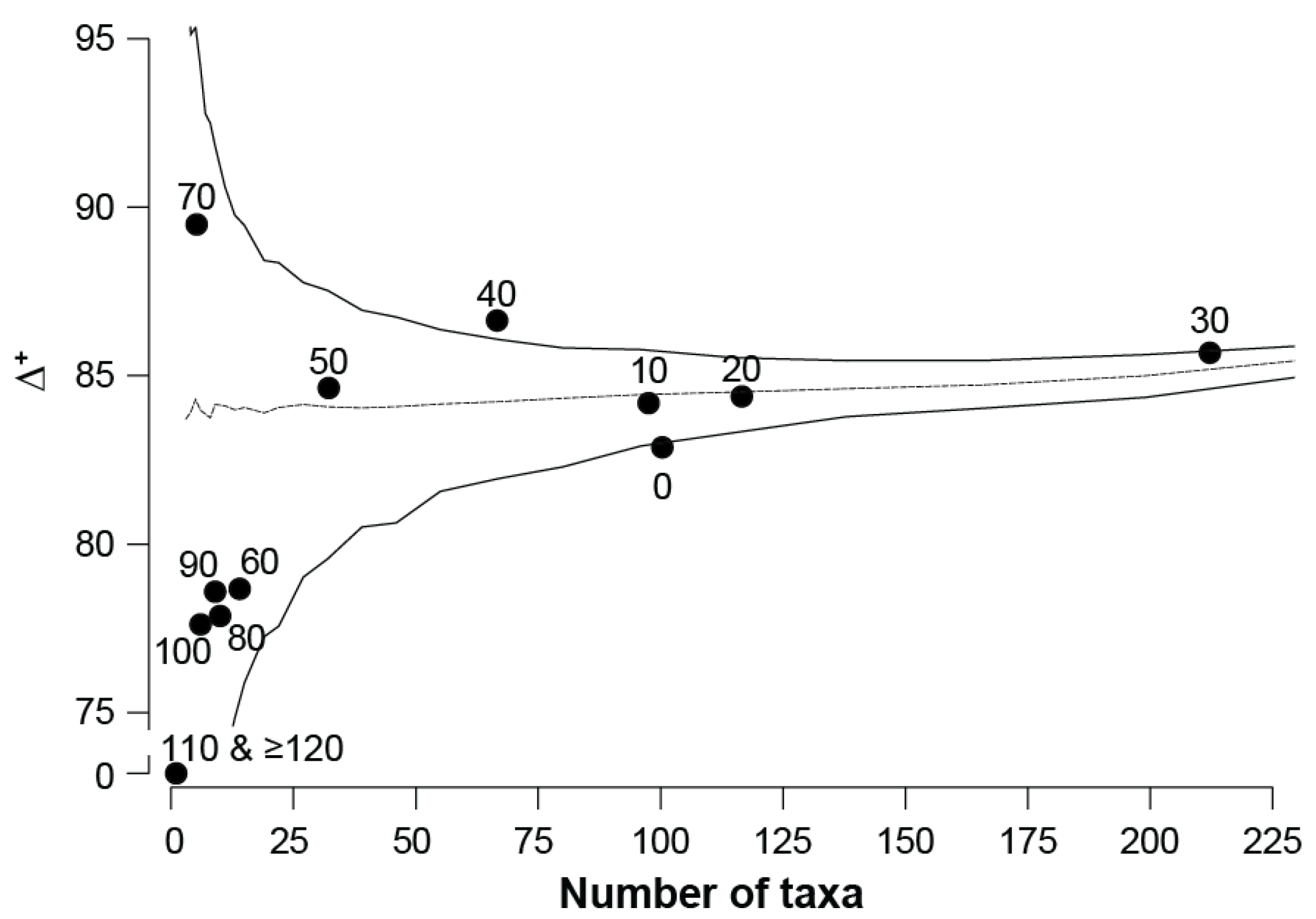
3.1. Univariate Diversity Measures
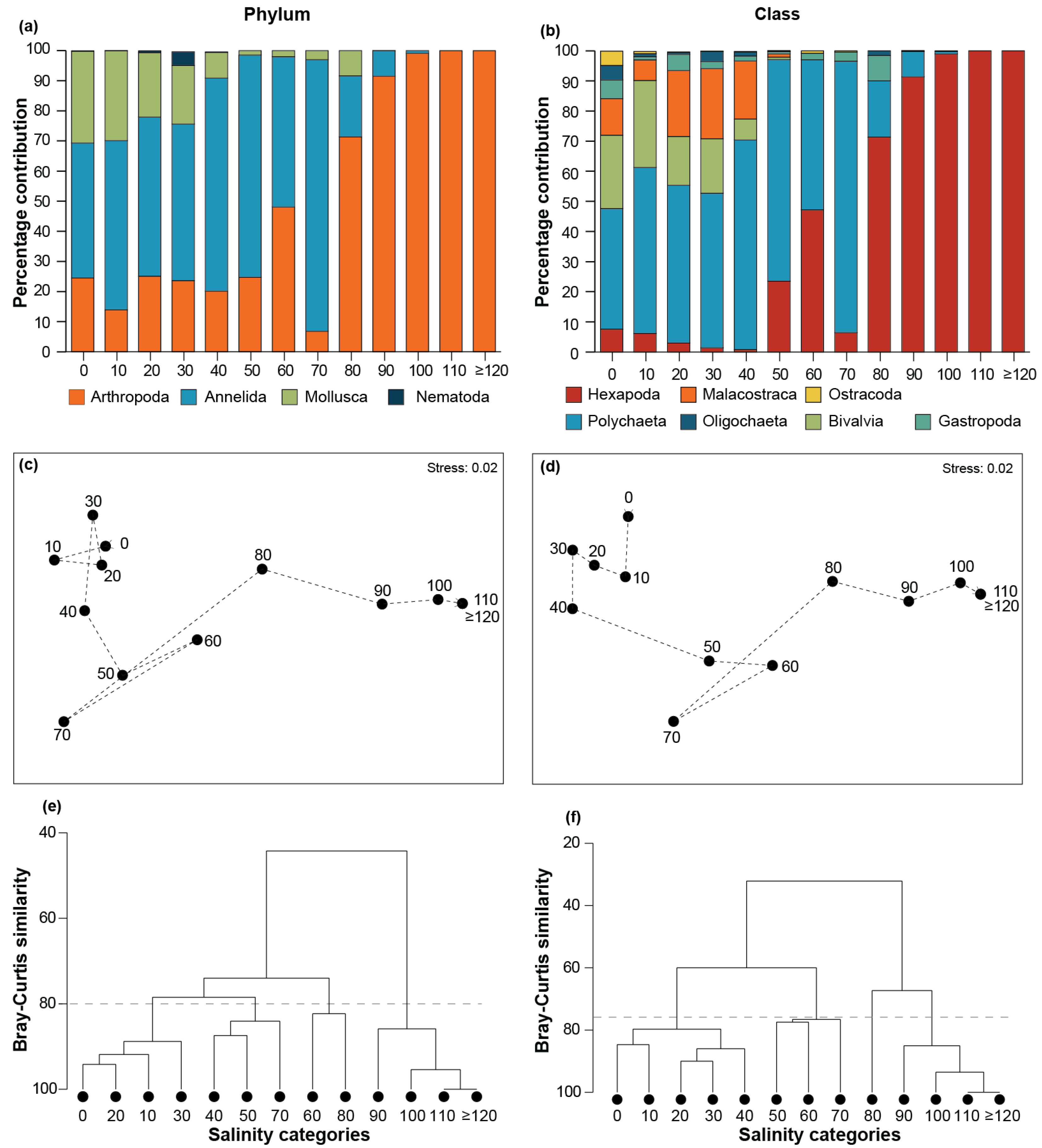
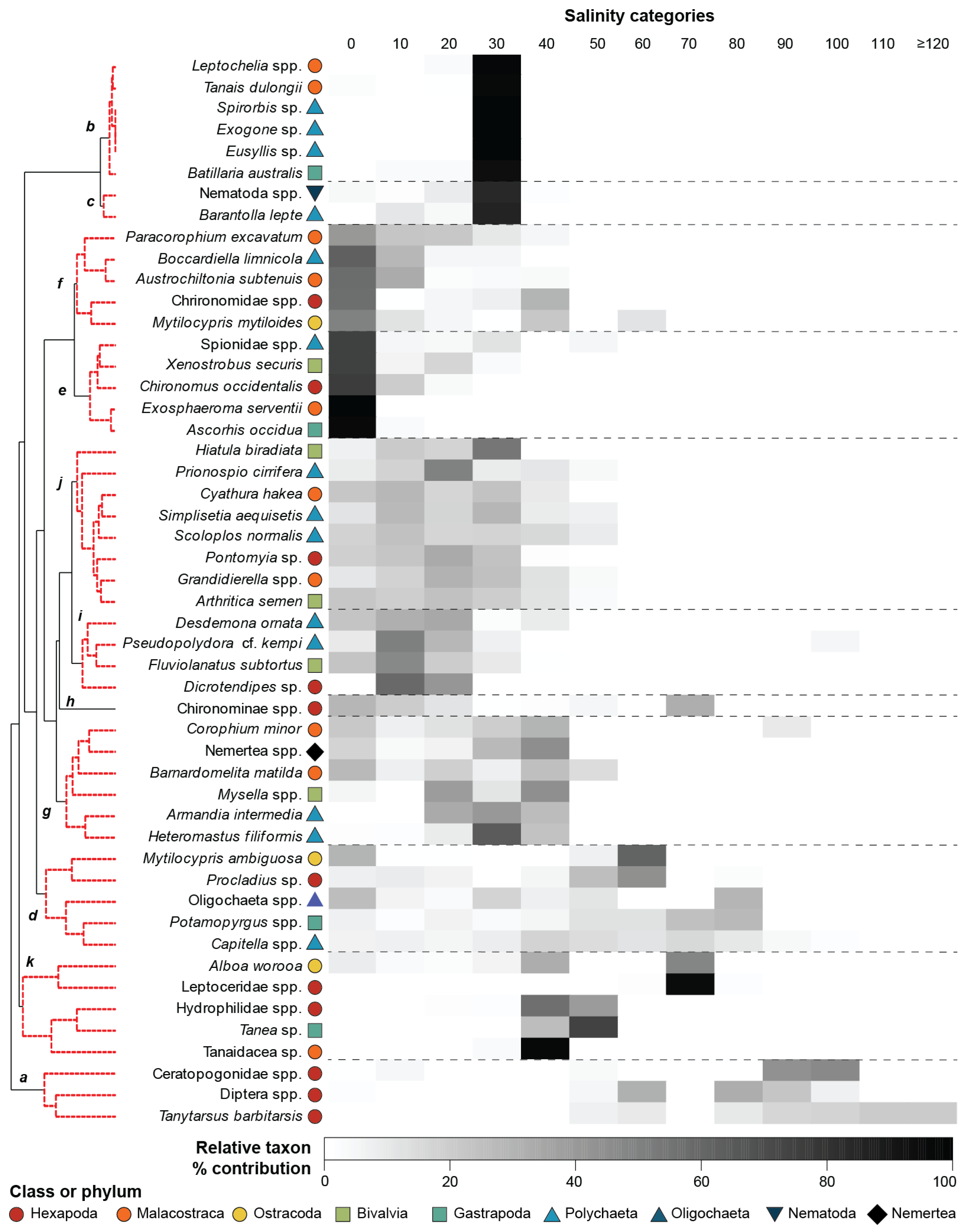
3.2. Community Composition
4. Discussion
4.1. Impacts of Hypersalinity on Diversity
4.2. Impacts of Hypersalinity on Community Composition
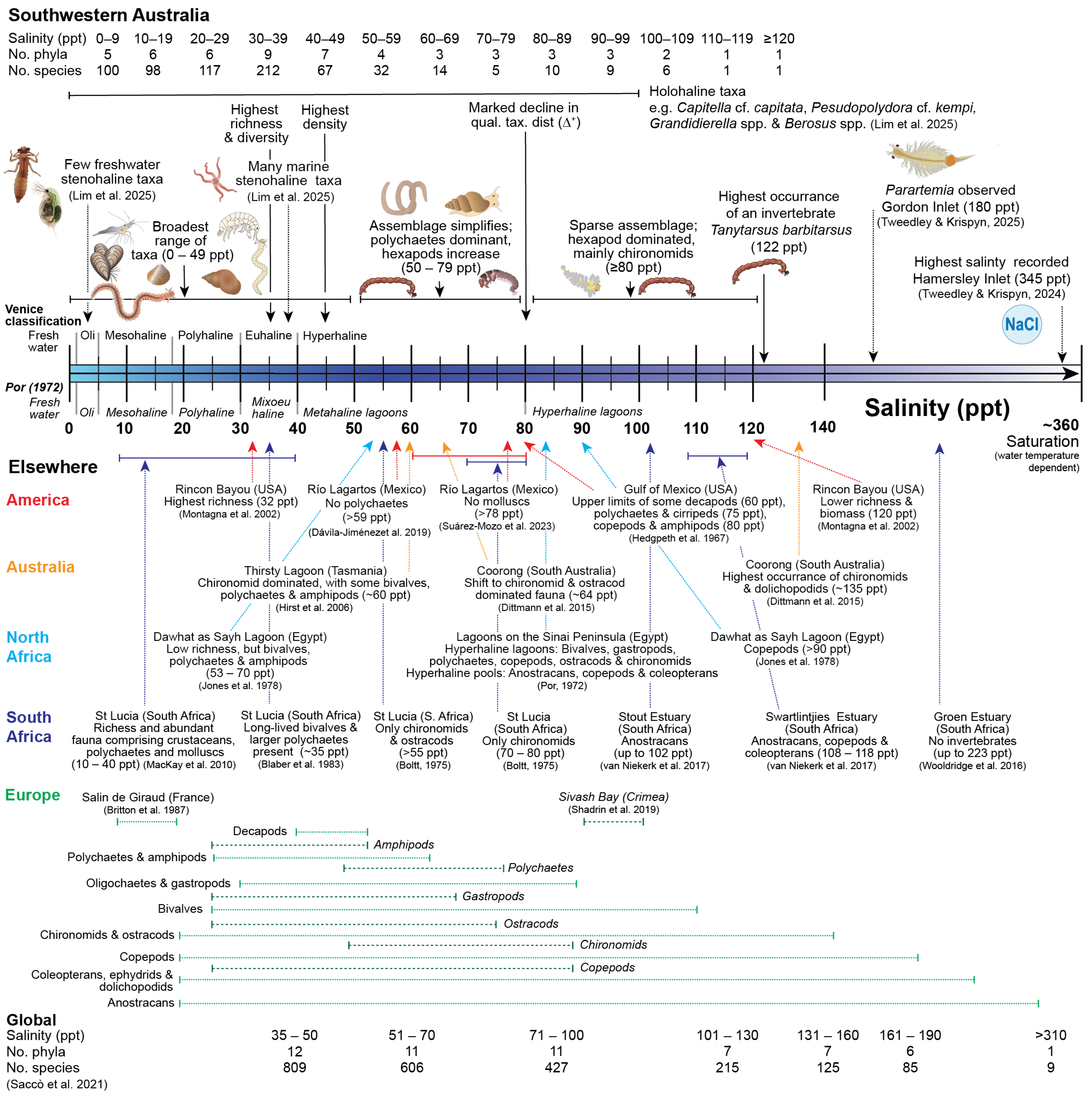
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennish, M.J. Environmental threats and environmental future of estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Warwick, R.M.; Tweedley, J.R.; Potter, I.C. Microtidal estuaries warrant special management measures that recognise their critical vulnerability to pollution and climate change. Mar. Pollut. Bull. 2018, 135, 41–46. [Google Scholar] [CrossRef]
- Potter, I.C.; Warwick, R.M.; Hall, N.G.; Tweedley, J.R. The physico-chemical characteristics, biota and fisheries of estuaries. Freshw. Fish. Ecol. 2015, 48–79. [Google Scholar]
- Cronin-O’Reilly, S.; Krispyn, K.N.; Maus, C.; Standish, R.J.; Loneragan, N.R.; Tweedley, J.R. Empirical evidence of alternative stable states in an estuary. Sci. Total Environ. 2024, 954, 176356. [Google Scholar] [CrossRef]
- Leal Filho, W.; Nagy, G.J.; Martinho, F.; Saroar, M.; Erache, M.G.; Primo, A.L.; Pardal, M.A.; Li, C. Influences of Climate Change and Variability on Estuarine Ecosystems: An Impact Study in Selected European, South American and Asian Countries. Int. J. Environ. Res. Public Health 2022, 19, 585. [Google Scholar] [CrossRef]
- Whitfield, A.; Elliott, M. Ecosystem and biotic classifications of estuaries and coasts. Treatise Estuar. Coast. Sci. 2011, 1, 99–124. [Google Scholar]
- Obolewski, K.; Glińska-Lewczuk, K.; Szymańska, M.; Mrozińska, N.; Bąkowska, M.; Astel, A.; Lew, S.; Paturej, E. Patterns of salinity regime in coastal lakes based on structure of benthic invertebrates. PLoS ONE 2018, 13, e0207825. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Anwar, T.N.; Das, K.; Paital, B. Influence of anthropogenic activities on redox regulation and oxidative stress responses in different phyla of animals in coastal water via changing in salinity. Water 2022, 14, 4026. [Google Scholar] [CrossRef]
- Boltt, R. The benthos of some southern African lakes Part V: The recovery of the benthic fauna of St Lucia Lake following a period of excessively high salinity. Trans. R. Soc. S. Afr. 1975, 41, 295–323. [Google Scholar] [CrossRef]
- Ridd, P.V.; Stieglitz, T. Dry Season Salinity Changes in Arid Estuaries Fringed by Mangroves and Saltflats. Estuar. Coast. Shelf Sci. 2002, 54, 1039–1049. [Google Scholar] [CrossRef]
- Hoeksema, S.D.; Chuwen, B.M.; Tweedley, J.R.; Potter, I.C. Factors influencing marked variations in the frequency and timing of bar breaching and salinity and oxygen regimes among normally-closed estuaries. Estuar. Coast. Shelf Sci. 2018, 208, 205–218. [Google Scholar] [CrossRef]
- Evans, D.H. Osmotic, ionic and nitrogenous-waste balance|Osmoregulation in Fishes: An Introduction. In Encyclopedia of Fish Physiology; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1348–1353. [Google Scholar]
- Danziger, J.; Zeidel, M.L. Osmotic homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.L. Osmoregulation in annelids: Cells and animals. In Osmotic and Ionic Regulation; CRC Press: Boca Raton, FL, USA, 2008; pp. 135–165. [Google Scholar]
- Wolcott, T.G.; Wolcott, D.L. Role of Behavior in Meeting Osmotic Challenges. Am. Zool. 2015, 41, 795–806. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Elsdon, T.S.; Halliday, I.A.; Jenkins, G.P.; Robins, J.B.; Valesini, F.J. Potential effects of climate change on Australian estuaries and fish utilising estuaries: A review. Mar. Freshw. Res. 2011, 62, 1115–1131. [Google Scholar] [CrossRef]
- Vargas, C.I.C.; Vaz, N.; Dias, J.M. An evaluation of climate change effects in estuarine salinity patterns: Application to Ria de Aveiro shallow water system. Estuar. Coast. Shelf Sci. 2017, 189, 33–45. [Google Scholar] [CrossRef]
- Nunes-Vaz, R.A. The salinity response of an inverse estuary to climate change & desalination. Estuar. Coast. Shelf Sci. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- He, J.; Soden, B.J. A re-examination of the projected subtropical precipitation decline. Nat. Clim. Change 2017, 7, 53–57. [Google Scholar] [CrossRef]
- Silberstein, R.P.; Aryal, S.K.; Durrant, J.; Pearcey, M.; Braccia, M.; Charles, S.P.; Boniecka, L.; Hodgson, G.A.; Bari, M.A.; Viney, N.R.; et al. Climate change and runoff in south-western Australia. J. Hydrol. 2012, 475, 441–455. [Google Scholar] [CrossRef]
- Hodgkin, E.P.; Hesp, P. Estuaries to salt lakes: Holocene transformation of the estuarine ecosystems of south-western Australia. Mar. Freshw. Res. 1998, 49, 183–201. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Dittmann, S.R.; Whitfield, A.K.; Withers, K.; Hoeksema, S.D.; Potter, I.C. Hypersalinity: Global distribution, causes, and present and future effects on the biota of estuaries and lagoons. In Coasts and Estuaries; Wolanski, E., Day, J.W., Elliott, M., Ramachandran, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 523–546. [Google Scholar]
- Cottingham, A.; Bossie, A.; Valesini, F.; Maus, C.; Tweedley, J.R. Habitat compression of an estuarine mytilid following half a century of streamflow decline. Estuar. Coast. Shelf Sci. 2023, 282, 108253. [Google Scholar] [CrossRef]
- Hoeksema, S.; Chuwen, B.; Potter, I. Massive mortalities of the black bream Acanthopagrus butcheri (Sparidae) in two normally-closed estuaries, following extreme increases in salinity. J. Mar. Biol. Assoc. United Kingd. 2006, 86, 893–897. [Google Scholar] [CrossRef]
- Anufriieva, E.V.; Shadrin, N.V. Salinity as a factor limiting the potential taxonomic richness of crustaceans in Ecosystems of hypersaline reservoirs around the world (Review). Inland Water Biol. 2023, 16, 892–898. [Google Scholar] [CrossRef]
- Anufriieva, E.V.; Shadrin, N.V. General patterns of salinity influence on the energy balance of aquatic animals in hypersaline environment. Biol. Bull. Rev. 2023, 13, 420–430. [Google Scholar] [CrossRef]
- Dittmann, S.; Baring, R.; Baggalley, S.; Cantin, A.; Earl, J.; Gannon, R.; Keuning, J.; Mayo, A.; Navong, N.; Nelson, M.; et al. Drought and flood effects on macrobenthic communities in the estuary of Australia’s largest river system. Estuar. Coast. Shelf Sci. 2015, 165, 36–51. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Krispyn, K.N. Protracted bar closure temporarily transforms an estuary into a salt lake. Pac. Conserv. Biol. 2024, 30, PC24007. [Google Scholar] [CrossRef]
- Andrys, J.; Kala, J.; Lyons, T.J. Regional climate projections of mean and extreme climate for the southwest of Western Australia (1970–1999 compared to 2030–2059). Clim. Dyn. 2017, 48, 1723–1747. [Google Scholar] [CrossRef]
- Krispyn, K.N.; Loneragan, N.R.; Whitfield, A.K.; Tweedley, J.R. Salted mullet: Protracted occurrence of Mugil cephalus under extreme hypersaline conditions. Estuar. Coast. Shelf Sci. 2021, 261, 107533. [Google Scholar] [CrossRef]
- Hoeksema, S.D.; Chuwen, B.M.; Tweedley, J.R.; Potter, I.C. Ichthyofaunas of nearshore, shallow waters of normally-closed estuaries are highly depauperate and influenced markedly by salinity and oxygen concentration. Estuar. Coast. Shelf Sci. 2023, 291, 108410. [Google Scholar] [CrossRef]
- Shadrin, N.V.; Anufriieva, E.V. Structure and trophic relations in hypersaline environments. Biol. Bull. Rev. 2020, 10, 48–56. [Google Scholar] [CrossRef]
- Peer, N.; Stretch, D.; Ndabeni, L.; Ngcobo, S.M.; Madikizela, B. Review of the Scientific Basis for Breaching of the Mouth of Lake St Lucia Estuary; Department of Forestry, Fisheries and Environment: Pretoria, South Africa, 2022; p. 50. [Google Scholar]
- Hallett, C.S.; Hobday, A.J.; Tweedley, J.R.; Thompson, P.A.; McMahon, K.; Valesini, F.J. Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Reg. Environ. Change 2018, 18, 1357–1373. [Google Scholar] [CrossRef]
- Brearley, A. Ernest Hodgkin’s Swanland, 1st ed.; University of Western Australia Press: Crawley, Australia, 2005; p. 550. [Google Scholar]
- Lim, R.; Fourie, S.A.; Stout, E.J.; Roots, B.J.; Cronin-O’Reilly, S.; Rodgers, E.M.; Tweedley, J.R. Testing the Remane diagram: Occurrences of benthic macroinvertebrates in oligohaline to hyperhaline salinities. Water 2025, 17, 1642. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Hallett, C.S.; Warwick, R.M.; Clarke, K.R.; Potter, I.C. The hypoxia that developed in a microtidal estuary following an extreme storm produced dramatic changes in the benthos. Mar. Freshw. Res. 2016, 67, 327–341. [Google Scholar] [CrossRef]
- Lim, R. Multidecadal Changes in the Benthic Macroinvertebrates Assemblages of the Swan-Canning Estuary; Murdoch University: Perth, Australia, 2025. [Google Scholar]
- Stout, E. Benthic Macroinvertebrate Communities of the Swan Estuary Marine Park; Murdoch University: Perth, Australia, 2025. [Google Scholar]
- Cronin-O’Reilly, S. Benthic Community Structure, Health and Function of a Microtidal Estuary in South-Western Australia. Ph.D. Thesis, Murdoch University, Perth, Australia, 2021. [Google Scholar]
- Tweedley, J.R.; Cronin-O’Reilly, S.; Cottingham, A.; Beatty, S.J. Vasse-Wonnerup Integrated Monitoring Review of 2017-20: Benthic Macroinvertebrate Component; Report for the Department of Water and Environmental Regulation; Murdoch University: Perth, Australia, 2021; p. 65. [Google Scholar]
- Cronin-O’Reilly, S.; Cottingham, A.; Kalnejais, L.H.; Lynch, K.; Tweedley, J.R. Tidal exclusion barriers fragment an invertebrate community into taxonomically and functionally distinct estuarine and wetland assemblages. J. Mar. Sci. Eng. 2025, 13, 635. [Google Scholar] [CrossRef]
- Tweedley, J.R. The Relationships Between Habitat Types and Faunal Community Structure in Broke Inlet, Western Australia. Ph.D. Thesis, Murdoch University, Perth, Australia, 2011. [Google Scholar]
- Fourie, S.A. Benthic Macroinvertebrate Faunas of Microtidal Estuaries in Albany, South-Western Australia. Bachelor’s Thesis, Murdoch University, Perth, Australia, 2024. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 27 March 2025).
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015; p. 296. [Google Scholar]
- Warwick, R.M.; Clarke, K.R. Practical measures of marine biodiversity based on relatedness of species. Oceanogr. Mar. Biol. Annu. Rev. 2001, 39, 207–231. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Clarke, K.R. Inverse analysis in non-parametric multivariate analyses: Distinguishing groups of associated species which covary coherently across samples. J. Exp. Mar. Biol. Ecol. 2013, 449, 261–273. [Google Scholar] [CrossRef]
- Veale, L.; Tweedley, J.R.; Clarke, K.R.; Hallett, C.S.; Potter, I.C. Characteristics of the ichthyofauna of a temperate microtidal estuary with a reverse salinity gradient, including inter-decadal comparisons. J. Fish Biol. 2014, 85, 1320–1354. [Google Scholar] [CrossRef]
- Clarke, K.R.; Tweedley, J.R.; Valesini, F.J. Simple shade plots aid better long-term choices of data pre-treatment in multivariate assemblage studies. J. Mar. Biol. Assoc. UK 2014, 94, 1–16. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology—A review of the Remane diagram with a suggested revised model for estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Saccò, M.; White, N.E.; Harrod, C.; Salazar, G.; Aguilar, P.; Cubillos, C.F.; Meredith, K.; Baxter, B.K.; Oren, A.; Anufriieva, E.; et al. Salt to conserve: A review on the ecology and preservation of hypersaline ecosystems. Biol. Rev. 2021, 96, 2828–2850. [Google Scholar] [CrossRef]
- Montagna, P.A.; Kalke, R.D.; Ritter, C. Effect of restored freshwater inflow on macrofauna and meiofauna in upper Rincon Bayou, Texas, USA. Estuaries 2002, 25, 1436–1447. [Google Scholar] [CrossRef]
- MacKay, F.; Cyrus, D.; Russell, K.L. Macrobenthic invertebrate responses to prolonged drought in South Africa’s largest estuarine lake complex. Estuar. Coast. Shelf Sci. 2010, 86, 553–567. [Google Scholar] [CrossRef]
- Shadrin, N.V.; Anufriieva, E.V.; Belyakov, V.P.; Bazhora, A.I. Chironomidae larvae in hypersaline waters of the Crimea: Diversity, distribution, abundance and production. Eur. Zool. J. 2017, 84, 61–72. [Google Scholar] [CrossRef]
- Shadrin, N.V.; Belyakov, V.P.; Bazhora, A.I.; Anufriieva, E.V. The role of salinity as an environmental filtering factor in the determination of the Diptera taxonomic composition in the Crimean waters. Knowl. Manage. Aquat. Ecosyst. 2019, 420, 3. [Google Scholar] [CrossRef]
- Wooldridge, T.; Adams, J.; Fernandes, M. Biotic responses to extreme hypersalinity in an arid zone estuary, South Africa. S. Afr. J. Bot. 2016, 107, 160–169. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Beatty, S.J.; Cottingham, A.; Morgan, D.L.; Lynch, K.; Lymbery, A.J. Spatial and temporal changes in the fish fauna of a low-inflow estuary following a mass mortality event and natural and artificial bar breaches. Coasts 2024, 4, 366–391. [Google Scholar] [CrossRef]
- Hedgpeth, J.W. Ecological aspects of the Laguna Madre, a hypersaline estuary. In Estuaries; Lauff, G.H., Ed.; AAAS Publication 83; American Association for the Advancement of Science: Washington, DC, USA, 1967; pp. 408–419. [Google Scholar]
- Lam-Gordillo, O.; Baring, R.; Dittmann, S. Taxonomic and Functional Patterns of Benthic Communities in Southern Temperate Tidal Flats. Front. Mar. Sci. 2021, 8, 723749. [Google Scholar] [CrossRef]
- Reiss, J.; Bridle, J.R.; Montoya, J.M.; Woodward, G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 2009, 24, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Por, F. A classification of hypersaline waters, based on trophic criteria. Mar. Ecol. 1980, 1, 121–131. [Google Scholar] [CrossRef]
- Cobbaert, D.; Wong, A.; Bayley, S.E. Precipitation-induced alternative regime switches in shallow lakes of the Boreal Plains (Alberta, Canada). Ecosystems 2014, 17, 535–549. [Google Scholar] [CrossRef]
- Gell, P.A.; Sluiter, I.R.; Fluin, J. Seasonal and interannual variations in diatom assemblages in Murray River connected wetlands in north-west Victoria, Australia. Mar. Freshw. Res. 2002, 53, 981–992. [Google Scholar] [CrossRef]
- Mosley, L.M.; Priestley, S.; Brookes, J.; Dittmann, S.; Farkaš, J.; Farrell, M.; Ferguson, A.J.; Gibbs, M.; Hipsey, M.; Huang, J.; et al. Extreme eutrophication and salinisation in the Coorong estuarine-lagoon ecosystem of Australia’s largest river basin (Murray-Darling). Mar. Pollut. Bull. 2023, 188, 114648. [Google Scholar] [CrossRef]
- Diehl, W.J. Osmoregulation in echinoderms. Comp. Biochem. Physiol. Part A Physiol. 1986, 84, 199–205. [Google Scholar] [CrossRef]
- Dávila-Jiménez, Y.; Papiol, V.; Hernández-Alcántara, P.; Enriquez, C.; Sauma-Castillo, L.; Chiappa-Carrara, X. Polychaete assemblages in a tropical hypersaline coastal lagoon of the southeastern Gulf of Mexico during the rainy season. Rev. Biol. Trop. 2019, 67, 136–156. [Google Scholar] [CrossRef]
- Britton, R.H.; Johnson, A.R. An ecological account of a Mediterranean salina: The Salin de Giraud, Camargue (S. France). Biol. Conserv. 1987, 42, 185–230. [Google Scholar] [CrossRef]
- Suárez-Mozo, N.Y.; Papiol, V.; Enriquez, C.; Brenner, M.; Simões, N. Molluscs along a salinity gradient in a hypersaline coastal lagoon, southern Gulf of Mexico. J. Mar. Biol. Assoc. UK 2023, 103, e19. [Google Scholar] [CrossRef]
- Davis, J.A.; Rosich, R.S.; Bradely, J.S.; Growns, J.E.; Schmidt, L.G.; Cheal, F. Wetland classification on the basis of water quality and invertebrate community data. In Wetlands of the Swan Coastal Plain; Water Authority of Western Australia and Environmental Protection Authority: Perth, Australia, 1993; Volume 6, p. 242. [Google Scholar]
- Lawrie, A.D.A.; Chaplin, J.; Pinder, A. Biology and conservation of the unique and diverse halophilic macroinvertebrates of Australian salt lakes. Mar. Freshw. Res. 2021, 72, 1553–1576. [Google Scholar] [CrossRef]
- Nhleko, J.; Cyrus, D.; Vivier, L. Diet of the demersal feeding Leiognathus equula in the Mfolozi–Msunduzi estuarine system, South Africa, in response to an impoverished macrobenthic invertebrate community. Afr. J. Aquat. Sci. 2012, 37, 175–182. [Google Scholar] [CrossRef]
- Breaux, N.; Lebreton, B.; Palmer, T.A.; Guillou, G.; Beseres Pollack, J. Ecosystem resilience following salinity change in a hypersaline estuary. Estuar. Coast. Shelf Sci. 2019, 225, 106258. [Google Scholar] [CrossRef]
- van Niekerk, L.; Adams, J.; Lamberth, S.J.; Taljaard, S. Determination of Ecological Water Requirements for Surface Water (River, Estuaries and Wetlands) and Groundwater in the Lower Orange WMA. Buffels, Swartlintjies, Spoeg, Groen and Sout Estuaries Ecological Water Requirement; RDM/WMA06/00/CON/COMP/0316; Council for Scientific and Industrial Research: Pretoria, South Africa, 2017. [Google Scholar]
- Tweedley, J.R.; Krispyn, K.N. Fish and salinities of low-inflow estuaries in the Fitzgerald Biosphere. FiSHMED Fishes Mediterr. Environ. 2025, 1, 1–14. [Google Scholar]
- Chuwen, B.M.; Platell, M.E.; Potter, I.C. Dietary compositions of the sparid Acanthopagrus butcheri in three normally closed and variably hypersaline estuaries differ markedly. Environ. Biol. Fishes 2007, 80, 363–376. [Google Scholar] [CrossRef]
- Hirst, A.; Alpine, J.; Crawford, C. Benthic macroinvertebrate communities of high-conservation value in Thirsty and Little Thirsty Lagoons, Cape Barren Island. Pap. Proc.-R. Soc. Tasman. 2006, 140, 17–23. [Google Scholar]
- Jones, D.A.; Price, A.R.G.; Hughs, R.N. Ecology of the high saline lagoons Dawhat as Sayh, Arabian Gulf, Saudi Arabia. Estuar. Coast. Mar. Sci. 1978, 6, 253–262. [Google Scholar] [CrossRef]
- Shadrin, N.; Kolesnikova, E.; Revkova, T.; Latushkin, A.; Chepyzhenko, A.; Dyakov, N.; Anufriieva, E. Macrostructure of benthos along a salinity gradient: The case of Sivash Bay (the Sea of Azov), the largest hypersaline lagoon worldwide. J. Sea Res. 2019, 154, 101811. [Google Scholar] [CrossRef]
- Por, F.D. Hydrobiological notes on the high-salinity waters of the Sinai Peninsula. Mar. Biol. 1972, 14, 111–119. [Google Scholar] [CrossRef]
- Blaber, S.J.M.; Kure, N.F.; Jackson, S.; Cyrus, D.P. The benthos of South Lake, St Lucia following a period of stable salinities. S. Afr. J. Zool. 1983, 18, 311–319. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A. Food-web modification by an invertebrate predator in the Great Salt Lake (USA). Oecologia 1992, 89, 168–175. [Google Scholar] [CrossRef]
- Herbst, D.B.; Medhurst, R.B.; Roberts, S.W.; Jellison, R. Substratum associations and depth distribution of benthic invertebrates in saline Walker Lake, Nevada, USA. Hydrobiologia 2013, 700, 61–72. [Google Scholar] [CrossRef]
- Pinder, A.M.; Halse, S.; McRae, J.; Shiel, R. Aquatic invertebrate assemblages of wetlands and rivers in the wheatbelt region of Western Australia. Rec. West. Aust. Mus. Suppl. 2004, 67, 37. [Google Scholar] [CrossRef][Green Version]
- Shadrin, N.V.; Anufriieva, E.V.; Amat, F.; Eremin, O.Y. Dormant stages of crustaceans as a mechanism of propagation in the extreme and unpredictable environment in the Crimean hypersaline lakes. Chin. J. Oceanol. Limnol. 2015, 33, 1362–1367. [Google Scholar] [CrossRef]
- Bonifazi, A.; Galli, S.; Gravina, M.F.; Ventura, D. Macrozoobenthos structure and dynamics in a Mediterranean hypersaline ecosystem with implications for wetland conservation. Water 2023, 15, 1411. [Google Scholar] [CrossRef]
- Choy, E.J.; An, S.; Kang, C.-K. Pathways of organic matter through food webs of diverse habitats in the regulated Nakdong River estuary (Korea). Estuar. Coast. Shelf Sci. 2008, 78, 215–226. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, B.; Zhang, Z.; Jiang, X. Salinity-oriented environmental flows for keystone species in the Modaomen Estuary, China. Front. Earth Sci. 2017, 11, 670–681. [Google Scholar] [CrossRef]
- Palmer, T.A.; Breaux, N.; Lebreton, B.; Guillou, G.; Beseres Pollack, J. Importance of serpulid reef to the functioning of a hypersaline estuary. Estuaries Coasts 2022, 45, 603–618. [Google Scholar] [CrossRef]
- McLeod, I.; Parsons, D.; Morrison, M.; Van Dijken, S.; Taylor, R. Mussel reefs on soft sediments: A severely reduced but important habitat for macroinvertebrates and fishes in New Zealand. New Zealand J. Mar. Freshw. Res. 2014, 48, 48–59. [Google Scholar] [CrossRef]
- Schroder, L.; Lam-Gordillo, O.; Dittmann, S. Classification, density, and spatial distribution of polychaete reefs in the Coorong, South Australia. Estuar. Coast. Shelf Sci. 2024, 306, 108905. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roots, B.J.; Lim, R.; Fourie, S.A.; Rodgers, E.M.; Stout, E.J.; Cronin-O’Reilly, S.; Tweedley, J.R. Hypersalinity Drives Dramatic Shifts in the Invertebrate Fauna of Estuaries. Animals 2025, 15, 1629. https://doi.org/10.3390/ani15111629
Roots BJ, Lim R, Fourie SA, Rodgers EM, Stout EJ, Cronin-O’Reilly S, Tweedley JR. Hypersalinity Drives Dramatic Shifts in the Invertebrate Fauna of Estuaries. Animals. 2025; 15(11):1629. https://doi.org/10.3390/ani15111629
Chicago/Turabian StyleRoots, Ben J., Ruth Lim, Stephanie A. Fourie, Essie M. Rodgers, Emily J. Stout, Sorcha Cronin-O’Reilly, and James R. Tweedley. 2025. "Hypersalinity Drives Dramatic Shifts in the Invertebrate Fauna of Estuaries" Animals 15, no. 11: 1629. https://doi.org/10.3390/ani15111629
APA StyleRoots, B. J., Lim, R., Fourie, S. A., Rodgers, E. M., Stout, E. J., Cronin-O’Reilly, S., & Tweedley, J. R. (2025). Hypersalinity Drives Dramatic Shifts in the Invertebrate Fauna of Estuaries. Animals, 15(11), 1629. https://doi.org/10.3390/ani15111629






