Simple Summary
Horses are high-level athletic athletes prone to musculoskeletal injuries. Tendon/ligament injuries are the most frequent types of injuries which that are very difficult to treat. Instead of tissue regeneration, usually, fibrous scar tissue develops which leads to decreased functionality of the injured area and threatens the participation of sport horses. The aim of regenerative medicine is to find a treatment that promotes tissue regeneration and that allows the equine patient to return to the same level of athletic performance in the shortest time period possible. In this study, we developed a solution of equine synovial membrane stem cells and autologous serum, to be injected at the lesion site to promote tissue regeneration. We describe the processes of tissue collection, preparation, isolation of synovial stem cells, expansion, culture, cryopreservation, and posterior preparation with autologous serum. The solution was tested in 16 tendons and ligaments of equines. After treatment, all equine patients underwent a physical rehabilitation program and were monitored with physical and ultrasonographic exams. The results were very promising, and thus, support the use of equine synovial stem cells and autologous serum in the treatment of tendonitis and desmitis.
Abstract
Tendon and ligament injuries are frequent in sport horses and humans, and such injuries represent a significant therapeutic challenge. Tissue regeneration and function recovery are the paramount goals of tendon and ligament lesion management. Nowadays, several regenerative treatments are being developed, based on the use of stem cell and stem cell-based therapies. In the present study, the preparation of equine synovial membrane mesenchymal stem cells (eSM-MSCs) is described for clinical use, collection, transport, isolation, differentiation, characterization, and application. These cells are fibroblast-like and grow in clusters. They retain osteogenic, chondrogenic, and adipogenic differentiation potential. We present 16 clinical cases of tendonitis and desmitis, treated with allogenic eSM-MSCs and autologous serum, and we also include their evaluation, treatment, and follow-up. The concerns associated with the use of autologous serum as a vehicle are related to a reduced immunogenic response after the administration of this therapeutic combination, as well as the pro-regenerative effects from the growth factors and immunoglobulins that are part of its constitution. Most of the cases (14/16) healed in 30 days and presented good outcomes. Treatment of tendon and ligament lesions with a mixture of eSM-MSCs and autologous serum appears to be a promising clinical option for this category of lesions in equine patients.
1. Introduction
Tendonitis and desmitis are defying clinical challenges in equine patients that require long recovery periods, and ineffective tendon repair can affect their sport careers. Tendons operate near their functional limits during maximal exercise, and their ability to adapt to stress and self-repair is limited. A controlled exercise program alone or in combination with a variety of conservative treatments, such as corrective shoeing and nonsteroidal anti-inflammatory drugs (NSAIDs), is still the gold standard therapy for equine tendon disease [1]. Current treatments often do not fully repair or regenerate the injured or affected tendon nor lead to its total functional recovery [1,2].
The aim of tendinopathy treatment is to achieve tissue regeneration and return to complete organ function and performance. Recently, tissue engineering approaches have attracted attention for tissue repair. Among the approaches, the use of mesenchymal stem cell-based therapy has increased, since it is a promising approach for tissue repair and regeneration including tendinopathy and desmitis [1,3,4,5,6].
Mesenchymal stem cells (MSCs) can be isolated from several tissue sources such as bone marrow, peripheral blood, dental pulp, umbilical cord, and amniotic fluid [7]. MSC characteristics have been defined by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT), and include plastic adherence when maintained in standard culture conditions; expressing clusters of differentiation (CDs), such as CD44, CD90, and CD105; and no expression of major histocompatibility complex (MHC)-class II markers and of hematopoietic-related markers (CD45 and CD34) [8]. Finally, MSCs must be able to differentiate in vitro into, at least, osteoblasts, adipocytes, and chondroblasts, in the presence of adequate differentiation culture media [8].
Synovial membrane mesenchymal stem cells (SM-MSCs) were initially isolated, in 2001, by De Bari et al. [9], from human knee joints and showed significant proliferative ability in culture, even after Passage 10 (P10), and multilineage differentiation potential in vitro [9]. These cells represent a good source of MSCs and a promising therapeutic tool mostly for musculoskeletal pathologies [10]. Sakagushi et al. compared the properties of different sources of human stem cells, i.e., bone marrow, synovium, periosteum, skeletal muscle, and adipose tissue, and observed the superiority of synovium as a source for MSCs for treatment of musculoskeletal pathologies as they had more ability to chondrogenesis. Pellets of synovium-derived stem cells were larger and expressed more intense staining for chondrogenic differentiation [11].
SM-MSCs have higher chondrogenic capacity than other studied sources of MSCs, such as bone marrow (BM-MSCs) [12,13]. Cartilage pellets from SM-MSCs have been reported to be significantly larger than those from BM-MSCs [12]. SM-MSCs have a higher production of uridine diphosphate glucose dehydrogenase (UDPGD) [13], an enzyme that converts UDP-glucose into UDP-glucuronate, one of the two substrates required by hyaluronan synthase for hyaluronan polymer assembly. In addition, Sox-9, collagen type II (Col-II), and aggrecan, specific markers for chondrogenesis, as well as cartilage-specific molecules such as cartilage oligomeric matrix protein (COMP) have also been found in high amounts in equine synovial fluid-derived MSCs and the extracellular matrix, respectively by reverse transcription polymerase chain reaction (RT-PCR) [13].
In a recent study, using a rabbit model, Bami et al. highlighted the superiority of SM-MSCs in terms of chondrogenesis, osteogenesis, myogenesis, and tenogenesis [14]. A study of xenogenic implantation of SM-MSCs in equine articular defects also confirmed better healing of the cartilage of affected knees as well as a higher expression of collagen type II, indicating the presence of hyaline cartilage in the healed defect [15].
SM-MSCs have been defined as MSCs due to their phenotypic profile and differentiation potential. Even though there are no specific antibody markers to identify these MSCs, there is general agreement that MSCs should be negative to hematopoietic markers CD34 and CD45 and positive to CD44, CD73, CD90, and CD105 [16]. Mochizuki et al. found that SM-MSCs maintained their proliferative ability, regardless of which region they were collected from in the synovium [17].
In 2003, Fickert et al. reported that the markers CD9, CD44, CD54, CD90, and CD166 could be used to identify MSCs isolated from the synovium of human patients with osteoarthritis (OA), and they also confirmed that CD9/CD90/CD166 triple-positive cell subgroups had obvious chondrogenic and osteogenic differentiation abilities [18].
Prado et al. confirmed the mesenchymal nature of equine synovial membrane and fluid-derived stem cells through the expression of significant hematopoietic (CD45, CD34, CD117, and CD133), mesenchymal (CD105, CD90), pluripotency (OCT3/4 and NANOG), embryonic (Tra-1-81), inflammatory, and angiogenesis (vascular endothelial growth factor (VEGF-R1) and LY6a) markers [19]. Although the presence of hematopoietic and inflammatory markers was not expected, variations may occur and must be considered to influence acute or chronic stages of osteochondrosis expression and/or inflammatory events [19,20].
Nevertheless, the immunophenotype characterization of equine MSCs (eMSCs), as well as in other veterinary species, has not yet been completely established [19]. This is a major challenge, since the expression of certain adult stem cell markers may differ between species. For that reason, it is a need to define a set of CD markers which can be uniformly applied for the identification of eMSCs [8,20].
Horses are high performance athletes prone to musculoskeletal diseases, i.e., osteoarticular, as well as tendon/ligament lesions and fractures of various degrees due to sport- and age-related injuries. These pathologies resemble human musculoskeletal conditions, and therefore, horses are a valuable animal model for assessing stem cell and cell-based therapies prior to the translation of results into humans [21]. The use of a therapy that can regenerate these structures and restore their complete functionality instead of ordinary healing is the aim of our study and of equine practitioners throughout the world.
Recent studies have suggested that MSCs can self-renew, migrate to injury sites (homing), perform multilineage differentiation, and secrete bioactive factors, thus, increasing proliferation and migration of tendon stem/progenitor cells via paracrine signaling and increasing the regeneration ability of tissues with poor aptitude [1,3,4,5,22,23].
In fact, the knowledge of the importance of this paracrine action has opened doors to cell-free therapeutic strategies in regenerative medicine. The soluble factors (cytokines, chemokines, and growth factors) and nonsoluble factors (extracellular vesicles and exosomes) released in the extracellular space by MSCs, commonly known as secretome, have become the focus of novel therapeutic approaches due to their key role in cell-to-cell communication, their active influence on immune modulation, and their pro-regenerative capacity both in vitro and in vivo [23]. Therefore, in this study, secretome was also analyzed with the prospect of being used therapeutically, in the future, in similar clinical cases.
In the present study, equines used as show jumping and dressage athletes as well as leisure horses with acute and chronic lesions were treated with intralesional administration of the considered combination, i.e., autologous serum and eSM-MSCs. The treatment consisted of two injections, 15 days apart. Pre- and post-treatment evaluations consisted of clinical, orthopedic, and tendon/ligament ultrasound exams. None of the selected equine patients had previously received any other regenerative treatment.
2. Materials and Methods
2.1. Study Design and Horse Selection
This prospective longitudinal study was performed in Portugal between February 2016 and January 2019. Sixteen horses, from 5 to 22 years old with acute and chronic signs of lameness were enrolled in this study (11 males and 5 mares), whose sport activities were distributed over show jumping (14), dressage (1), and leisure (1). The horses were all outpatients from an equine ambulatory clinic. This study included the treatment of 16 tendons, i.e., 14 superficial digital flexor tendons and 2 deep digital flexor tendons, and 4 suspensory ligaments.
Lameness was scored based on the American Association of Equine Practitioners (AAEP) scale (Table 1) and confirmed by using a positive regional nerve block. Flexion and pain to pressure tests were also evaluated [24].

Table 1.
Score systems used by the veterinary surgeon to assess lameness, and responses to flexion and pain to pressure tests [25,26].
2.2. Inclusion and Exclusion Criteria
In this study, horses with acute or chronic lameness (Table 2), with diagnosed tendonitis and/or desmitis and with no signs of systemic disease were accepted in the inclusion criteria. Injured horses were treated in acute stages of disease, except for two equine patients (Patients 3 and 6). Patient 3 had an injury the year before this treatment and laser therapy had been performed, without a complete recovery. After that, he had a re-injury and, at this time, this treatment was suggested. Patient 6 was referred by another clinician who tried, unsuccessfully, to treat this patient. Patient 6 was sent to the field for one year and then re-evaluated. At this time, and as its trainer wanted to improve its quality of life, this treatment was proposed by its clinician. The lameness grade of each equine patient is specified in Table 2. Considering the established exclusion criteria, the selected equine patients should not have been under any other medical treatment (including nonsteroidal anti-inflammatory drugs, intra-articular corticosteroids, hyaluronan, glycosaminoglycans, platelet-rich plasma (PRP), and other MSC preparations) for at least 2 months before the allogenic eSM-MSC treatment and did not receive any additional medical treatment (except for that described in the treatment plan) for at least 2 months post the cell-based treatment.

Table 2.
Equine patient and lesion characterization. The left column characterizes the equine patients: Sex, male (M) or female (F); age measured in years old (yo); sports modality (SM), show jumping (SJ), dressage (Dre), and Leisure (Lsr); lameness score (AEEP score) pretreatment. The right column characterizes the lesions: Structure affected, superficial digital flexor tendon (SDFT), deep digital flexor tendon (DDFT), and suspensory ligament (SL) left branch (LB); affected limb, right frontlimb (RF), right hindlimb (RH), left frontlimb (LF) and left hindlimb (LH).
2.3. Ethics and Regulation
This study was carried out in accordance with the Organismo Responsável pelo Bem Estar Animal (ORBEA) from ICBAS-UP, project number P289/ORBEA/2018 recommendations and authorization. Treatments were performed with permission and signature of an informed consent from the equine patient’s legal trainer, following a thorough explanation on the procedure itself and possible risks and associated effects, in accordance with national regulations and project approval from the competent authorities. In addition, no animals were euthanized for this study.
2.4. Donor Selection and SM Collection
The eSM-MSC donor was a young and healthy foal, 7 months old, who died accidentally when running in the arena. The trainer authorized synovial membrane collection from the hocks, knees, and fetlocks. The synovial membrane was evaluated and its appearance was transparent, bright, and smooth; in addition, the presence of viscous and transparent synovial fluid confirmed its soundness. The skin covering the incisional field was surgically cleaned with chlorohexidine and alcohol. The skin and subcutaneous tissue were incised, debrided, the articular capsule was opened, and the synovial membrane was isolated and extracted into a Dulbecco′s phosphate buffered saline (DPBS) container. The samples were transported to the laboratory with ice packs for refrigerated temperatures. Figure 1a presents the fresh tissue arrival and Figure 1b shows the preparation at the laboratory. Figure 2 shows a schematic representation of the process from eSM-MSC collection to the administration of the combination, i.e., eSM-MSCs and autologous serum (1 × 106 cells/mL and 1 mL of autologous serum in a total volume of 2 mL).
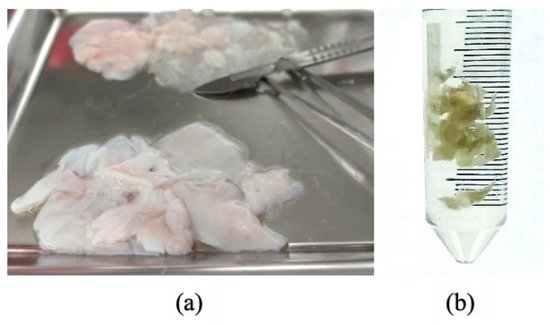
Figure 1.
Laboratory arrival and preparation of the fresh tissue to start digestion, isolation, and expansion: (a) Tissue collected in the field; (b) isolated synovial membrane.
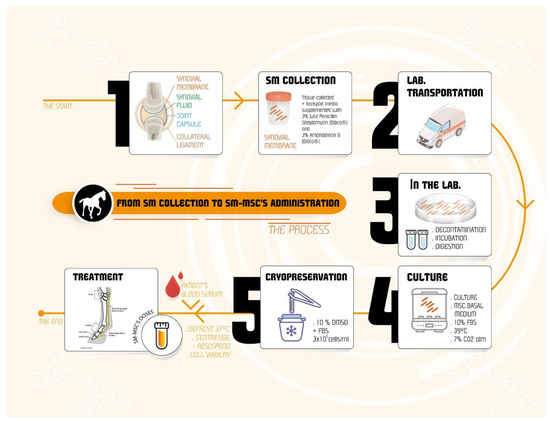
Figure 2.
Schematic representation of the event sequence from the collection of synovial membrane to the administration of the therapeutic combination. After the collection, the synovial membrane is transported to the laboratory where it is separated from the whole tissue, decontaminated, incubated, and digested. Then, cells are cultured and expanded and finally cryopreserved in a cell bank. When needed for treatment, cells are prepared with autologous serum, and then applied in the selected equine patient.
2.5. eSM-MSC Isolation
After collection, the equine synovial membrane was prepared at the Laboratory of Veterinary Cell-based Therapies from ICBAS-UP. The isolation protocol for eSM-MSCs was developed by patented proprietary technology Regenera® (PCT/IB2019/052006, WO2019175773, Compositions in use for the treatment of musculoskeletal conditions and methods for producing the same leveraging of the synergistic activity of two different types of mesenchymal stromal/stem cells, Regenera®). Fresh tissue was transported to the laboratory facilities in a hermetically sealed sterile container in transport media (supplemented with 3% (v/v) penicillin-streptomycin (Gibco®, Waltham, MA, USA) and 3% amphotericin B (Gibco®) and processed within a period of up to 48 h. The synovial tissue was digested using collagenase and the isolated cells were incubated in a static monolayer culture using standard MSC basal medium supplemented with 10% fetal bovine serum (FBS) and maintained in standard culture conditions (37 °C, 5% CO2, and humidified atmosphere) until they reached confluence. Cells from confluent cultures were cryopreserved in 10% dimethylsulphoxide (DMSO) and FBS, at a concentration of 3 × 10⁶ cells/mL, using a control rate temperature freezer (Sy-Lab Cryobiology, SY-LAB Geräte GmbH, Purkersdorf, Austria). For expansion optimization, cells were cryopreserved in passages (P) between P2 and P3 to generate suitable master cell banks (MCBs). Expansion, thereafter, was analyzed during a maximum of 20 cumulative population doublings (cCPDs). The range of cCPDs chosen allowed for enough expansion to maximize the number of cells in the working cell banks (WCB) but kept the cCPDs within the genomic stability range.
2.6. SM-MSC Characterization
2.6.1. Tri-Lineage Differentiation Protocols
For all the differentiation protocols, cells in P4 were used after thawing.
Adipogenic Differentiation and Oil Red O Staining
For the adipogenic differentiation protocol, 1 × 104 cells/cm2 were seeded in the wells of a 12-well plate (cell culture plates, 12-well, VWR®, Suwanee, Atlanta, GA, USA), with the addition of the standard culture medium. The plate was incubated under standard conditions for 4 days. After this period, the culture medium of 10 wells was replaced by complete adipogenesis differentiation medium (StemPro® Adipogenesis Differentiation Kit, Gibco®, Waltham, MA, USA), 2 wells were used as controls and maintained with the standard culture medium. Following the manufacturer’s instructions, the media were replaced every 3–4 days and the cells maintained in differentiation for 14 days. At the end of this period, the oil red O staining protocol was performed using a handmade solution. The culture differentiation medium was removed, and the wells were gently washed with PBS. Cells were fixed with 4% formaldehyde (3.7–4% buffered to pH 7, reference# 252931.1315, Panreac AppliChem®, Darmstadt, Germany) for 10 min at room temperature, and the wells were washed 3 additional times with phosphate-buffered saline (PBS). Oil red O solution was added to each well and the plate incubated for 10–20 min at room temperature. Oil red O was discarded, and any excess dye was removed by several washes with PBS. PBS was added to each well for visualization. The aim of this assay was the identification of rounded cells with intracytoplasmic lipid vacuoles and their red coloration due to the exposure to the oil red O solution.
Chondrogenic Differentiation and Alcian Blue Staining
Thawed eSM-MSCs were automatically counted, and cell viability determined (%). Then, the cells were centrifuged, supernatant removed, and the pellet resuspended in culture medium to generate a cell suspension with 1.6 × 107 viable cells/mL. To generate micro-mass cultures, 5 μL droplets of the cell suspension were placed in the center of 10 wells of a 96-well plate (cell culture plates, 96-well, VWR®, Suwanee, Atlanta, GA, USA) to induce chondrogenic differentiation. The plate was maintained under standard conditions for 2 h. After this time, chondrogenic differentiation medium (StemPro® Chondrogenesis Differentiation Kit, Gibco®, Waltham, MA, USA) was added to 8 wells; the other 2 wells were considered to be controls and to these, the standard culture medium was added. Following the manufacturer’s instructions, the media were replaced every 3–4 days and cells maintained in differentiation for 14 days. At the end of this period, the Alcian blue staining, pH 2.5, protocol was performed (Alcian Blue 8GX, Sigma-Aldrich®, St. Louis, MO, USA). The culture differentiation medium was removed, and the wells were gently washed with PBS. The cells were fixed with 4% formaldehyde for 20 min at room temperature, and the wells were washed 3 additional times with PBS. Alcian blue solution was added to each well and the plate incubated for 30 min at room temperature. Then, the Alcian blue was discarded, and the wells were rinsed 3 times with 3% acetic acid (v/v). For neutralization of acidity and for visualization by inverted phase contrast microscopy, distilled water was added to all wells. The aim of this assay was the identification of chondrogenic aggregates and their coloration in blue due to the exposure to Alcian blue solution.
Osteogenic Differentiation and Alizarin Red Staining
For osteogenic differentiation, 8 × 103 cells/cm2 were seeded into the wells of a 12-well plate. The plate was maintained under standard conditions for 4 days. After this period, the culture medium of 10 wells was replaced by complete osteogenic differentiation medium (StemPro® Osteogenic Differentiation Kit, Gibco®, Waltham, MA, USA), and 2 wells were used as controls and maintained with the standard culture medium. Following the manufacturer’s instructions, the media were replaced every 3–4 days and the cells maintained in differentiation for 21 days. At the end of this period, the alizarin red s staining protocol was performed using a commercial solution (alizarin red staining solution, Milllipore®, Burlington, MA, USA). The culture differentiation medium was removed, and the wells were gently washed with PBS. The cells were fixed with 4% formaldehyde for 30 min at room temperature, and the wells were washed twice with distilled water. One ml of 40 mM of alizarin red solution was added to each well and the plate incubated for 30 min. Then, the alizarin red solution was discarded, and the wells were rinsed 3 times with distilled water until the supernatant became clear. For visualization by inverted phase contrast microscopy, PBS was added to all the wells. The aim of this assay was to identify calcium-containing osteocytes stained in red after exposure to alizarin red solution.
2.6.2. Karyotype Analysis
The eSM-MSCs in two different passages (P4 and P7) were submitted to cytogenetic analysis to determine the genetic stability in terms of chromosome number and occurrence of neoplastic changes. For both passages, 70–80% confluence was reached. Then, the culture medium was changed and supplemented with 10 μg/mL colcemid solution (KaryoMAX® Colcemid™ Solution, Gibco®, Waltham, MA, USA). After 4 h, the eSM-MSCs were collected and resuspended in 8 mL of 0.075 M KCl solution, followed by incubation under standard conditions for 15 min. After centrifugation (1700 rpm), 8 mL of ice-cold fixative comprising methanol and glacial acetic at a proportion of 3:1 was added and mixed. Afterwards, the cells were centrifuged again. Three fixation rounds were carried out. After the last centrifugation, the suspension of eSM-MSCs was spread over glass slides. A karyotype analysis was performed by one scorer on Giemsa-stained cells. For the different passages, a specific number of cells in metaphase were evaluated depending on the number of cells with a normal karyotype identified, guaranteeing a better representation of the population under study.
2.6.3. Secretome Cell Conditioned Medium (CM) Analysis
The eSM-MSCs were harvested from equine synovial membrane and maintained in culture, as previously described. The cells in P4 were subjected to an analysis of their conditioned medium (CM) to identify cytokines and chemokines secreted after conditioning. When in culture, after reaching a confluence of around 70–80%, the culture medium was removed, and the culture flasks were gently washed with DPBS two to three times. Then, the culture flasks were further washed two to three times with the basal culture medium of each cell type, without any supplementation. To begin the conditioning, non-supplemented DMEM/F12 GlutaMAX™ (10565018, Gibco®, Thermo Fisher Scientific®, Waltham, MA, USA) culture medium was added to the culture flasks, which were then incubated under standard conditions. The culture medium rich in factors secreted by the cells (CM) was collected after 48 h. The collected CM was then concentrated five times. After collection, it was centrifuged for 10 min at 1600 rpm, and its supernatant collected and filtered with a 0.2 μm syringe filter (Filtropur S®, PES, Sarstedt, Nümbrecht, Germany). For the concentration procedure, Pierce™ Protein Concentrator, 3k MWCO, 5–20 mL tubes (88525, Thermo Scientific®, Waltham, MA, USA) were used. Initially, the concentrators were sterilized following the manufacturer’s instructions. Briefly, the upper compartment of each concentrator tube was filled with 70% ethanol (v/v) and centrifuged at 300× g for 10 min. At the end of the centrifugation, the ethanol was discarded, and the same procedure was carried out with DPBS. Each concentrator tube was subjected to two such centrifugation cycles, followed by a 10 min period in the laminar flow hood to complete drying. Finally, the upper compartment of the concentrator tubes was filled with plain CM (1 × concentration) and subjected to new centrifugation cycles, under the conditions described above, for the number of cycles necessary to obtain the desired CM concentration (5×). The concentrated CM was stored at −20 °C and subsequently subjected to a Multiplexing LASER Bead analysis (Eve Technologies, Calgary, AB, Canada) to identify a set of biomarkers present in the Equine Cytokine 8-Plex Assay (EQCYT-08-501). The list of searched biomarkers includes basic fibroblast growth factor (FGF-2), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), interleukins (IL) IL-6, IL-8, IL-17A, and human growth-regulated oncogene/keratinocyte chemoattractant (GRO/KC). All samples were analyzed in duplicate.
2.6.4. Immunohistochemistry
Early passages of eSM-MSCs-P0 and -P3 were maintained in culture until a confluence of 70–80% was reached, and then enzymatic detachment was performed with 0.25% trypsin-EDTA solution. A cytoblock was performed fixing the cells with Sure Thin® (Statlab®, Gerwig Ln Columbia, Columbia, MD, USA). Consecutive sections were cut at 2 μm, deparaffinized, hydrated, and submitted to immunohistochemical analysis using the Novolink™ Polymer Detection Systems (Leica Biosystems®, Vista, CA, USA) kit, according to the manufacturer’s instructions. Information regarding the primary antibodies and antigen retrieval recovery methods used in this study is summarized in Table 3.

Table 3.
List of antibodies investigated, dilutions, and antigen retrieval methods applied in the immunohistochemical analysis. Oct 4—Octamer-binding transcription factor 4; NANOG—Homeobox protein NANOG; GFAP—Anti-Glial Fibrillary Acidic Protein; CD31—Platelet endothelial cell adhesion molecule.
The antibodies were selected to confirm the pluripotent and mesenchymal origin of the eSM-MSCs’ octamer-binding transcription factor 4 (OCT4), homeobox protein (NANOG), proto-oncogene receptor tyrosine kinase or stem cell factor receptor (c-kit), synovial origin (lysozyme), and non-epithelial origin histogenesis (vimentin). Additionally, pan-cytokeratin (AE1 and AE3), synaptophysin, CD31, and glial fibrillary acidic protein (GFAP) were used to confirm there were no vascular, epithelial, neuronal, and neuroendocrine origins of cells, respectively. For each antibody, appropriate negative and positive controls were included, and all primary antibodies were incubated overnight.
The final step consisted of microscopic cell observation, evaluation, and photograph using the microscope Eclipse E600 (Nikon®, Tokyo, Japan) and the software Imaging Software NIS-Elements F Ver4.30.01 (Laboratory Imaging®, prague, mmun republic). A semi-quantitative score was used for mmunoexpression evaluation, consisting of the percentage of labeled cells (<5%, 5–80%, and >80%) and labeling intensity (0, negative; +, weak; ++, moderate; and +++, strong). Immunoreactivity was considered positive when distinct nuclear and cytoplasmic staining was recognized in at least 5% of the cells.
2.7. eSM-MSC Solution Preparation
The eSM-MSC solution for local clinical application in the 16 equine patients, was a combination of allogenic eSM-MSCs suspended in autologous serum. Prior to preparation of the final therapeutic combination, autologous serum was isolated from whole blood. Then, 10 mL samples of whole blood were collected into dry blood collection tubes, and after clotting, they were centrifuged at 2300 rpm for 10 min and their supernatant (serum) collected and transferred to a 15 mL falcon. Then, the serum was inactivated through a water bath at 56 °C for 20 min followed by cooling on ice. Finally, the serum was centrifuged and filtered using a 0.22 µm syringe filter and stored at −20 °C until further use. Cryopreserved P3 eSM-MSC batches were thawed in a 37 °C water bath, and the content was transferred to a 10 mL tube with autologous serum and slowly diluted, followed by the addition of sterile DPBS until reaching 10 mL. Then, the mixture was centrifuged at 1600 rpm for 10 min. The supernatant was discarded, and the cell pellet was re-suspended in a mixture of autologous serum at a ratio of 0.8:1. Cell counting and viability were determined by using the trypan blue exclusion dye assay (Invitrogen TM, Waltham, MA, USA) using an automatic counter (Countess II FL Automated Cell Counter, Thermo Fisher Scientific®, Waltham, MA, USA). Then, the cell number was adjusted to 5 × 10⁶ cells/mL, and then 2 mL of the solution of eSM-MSCs suspended in autologous serum was transferred to a perforable capped vial and preserved on ice until the time of administration.
2.8. Treatment Protocol
Twenty structures, tendons and ligaments, were treated with a mixture of allogenic eSM-MSCs and autologous serum. The same treatment protocol was used in every case. All equine patients were submitted to identification, anamnesis, physical examination (cardiac and respiratory frequency, body temperature, mucous membrane examination, inspection of the whole body, and palpation), orthopedic examination (evaluation of the limbs, gait inspection and movements (walk, trot and gallop), and flexion test of the main joints for 60 s followed by trot). Lameness was evaluated at a walk and a trot on hard surface and scored on a scale from 0 to 5, according to the AAEP parameters. Complementary diagnostic exams included regional nerve blocks (to identify the pain area), radiographs, and ultrasound image as reported in other studies [21,24,25,27,28,29,30,31,32].
Following the assumptions of the exclusion criteria, the horses did not receive any treatment before or after the administration of the therapy protocol. In the case of adverse events occurring, such as inflammatory/anaphylactic reactions or infections, the horses should be immediately evaluated and treated with anti-inflammatories or antibiotics, in accordance with their clinical status. The equine patients were monitored in the 48 h after treatment and any occurrences were registered. Following the treatment, the equine patients were assessed periodically to control the equine patient’s healing evolution and to provide valid comparative data among equine patients within the same study group. Table 4 presents the lesion type casuistic.

Table 4.
Lesion type casuistic.
2.8.1. Intralesional eSM-MSC Injection
Selected horses were sedated with detomidine (0.02 mg/kg), trichotomized, a regional nerve block was performed with lidocaine 2% (20 mg/mL, 2 mL/point), and the surgical skin was disinfected with chlorohexidine and alcohol. The therapeutic combination was aspired to a 2 mL syringe and homogenized, ultrasound was used to identify the lesion site, and an ultrasound guided injection was performed at the lesion over three different points. Finally, a bandage was applied to the limb. All equine patients were injected with phenylbutazone (2.2 mg/kg, IV, SID) at the end of the treatment. The established protocol included a second eSM-MSC administration 15 days after the first treatment using the same protocol.
2.8.2. Clinical Evaluation—Serial Evaluations
Tissue regeneration was estimated through a lameness evaluation, pain to pressure test, limb inflammation, sensitivity, and ultrasound image (reduction of hypoechoic area and fiber alignment). Lesion ultrasonographic evaluations were performed using a 7.5 MHz linear transductor probe (Sonoscape A5®, Shenzhen New Industries Biomedical Engineering Co Ltd., Shenzhen, China). For each assessment, a complete examination of the structure was conducted by means of longitudinal and transverse scans. The obtained images were evaluated at each examination for two parameters: lesion echogenicity and lesion longitudinal fiber alignment (FA). The contralateral healthy limb was used as comparison. The evaluation was performed on the treatment day (Day 1) as well as on Days 15, 30, and 45 post-treatments, as presented in Figure 3. According to the classification proposed by Guest et al., this is a short term period study [33].
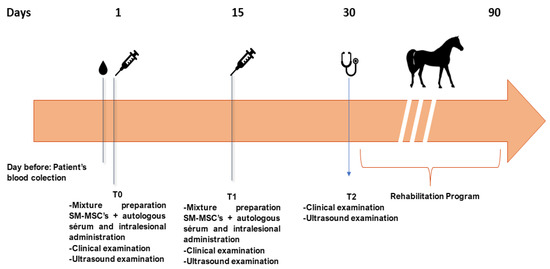
Figure 3.
Timeline of the eSM-MSC treatment protocol and rehabilitation program. The day before the first treatment (T0), blood from the equine patient was collected to prepare autologous serum. At T0, the mixture of autologous serum and eSM-MSCs was injected intralesionally after a clinical and ultrasound examination. After 15 days, the same procedure was repeated. At day 30 (T2), a clinical and ultrasound examination was performed and if a favorable outcome was considered, the horse progressed to a physical rehabilitation program. During the physical rehabilitation program, the equine patient was also re-evaluated at days 60 and 90.
The rehabilitation program consisted of an exercise-controlled program with stall confinement and increasing the amount of time for exercise. Early mobilization included weight-bearing activities, strengthening, and flexibility, and stall rest alone was used as infrequently as possible, as presented on Table 5 [34,35,36,37,38]. Regular ultrasound evaluations were also performed.

Table 5.
Physical rehabilitation program. After eSM-MSC treatment, all horses were submitted to a rehabilitation program consisting of two days of box rest followed by 13 days of 10 min of hand walking. The bandage applied on treatment day was removed 24 h after treatment. At Day 15, the second treatment was performed followed by another 15 days of rehabilitation, until Day 30. Between Days 30 and 45, the work consisted of 20 min hand walking; between Days 45 and 60, the work was 30 min of hand walking; between Days 60 and 75, the work was 30 min of hand walking plus 5 min trotting; and finally, between Days 75 and 90, each horse was submitted to 30 min of hand walking plus 10 min of trotting. After this, the horses could return to full work.
3. Results
3.1. eSM-MSC Isolation
eSM-MSCs were successfully isolated from equine synovial membrane samples and the average total number of cells isolated from the samples was 1.2 × 105 and 5.6 × 105 at Days 6 and 11, respectively, and expanded from the donor. Cells were observed radiating from the explants and those identified in culture showed clear plastic adherence and mostly fibroblast-like morphology, an essential feature to characterize cells as MSCs (Figure 4a,b).
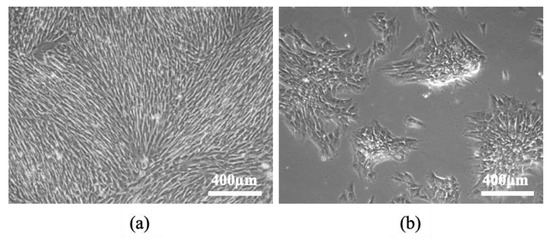
Figure 4.
eSM-MSCs in culture, isolated through enzymatic digestion: (a) Passage 0 (P0); (b) Passage 1 (P1). Plastic adherence, monolayer, and fibroblast-like shape of eSM-MSCs may be observed. Magnification, 100×.
3.2. eSM-MSC Characterization
3.2.1. Tri-lineage Differentiation
Tri-lineage differentiation was confirmed (Figure 5).
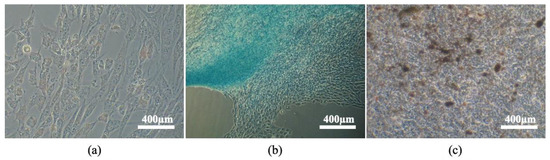
Figure 5.
Tri-lineage differentiation: (a) eSM-MSC’s adipogenic differentiation, cytoplasmatic lipid vacuoles stained in red (Oil red staining); (b) eSM-MSC’s chondrogenic differentiation, proteoglycans in extracellular matrix stained in blue (Alcian blue staining); (c) eSM-MSC’s osteogenic differentiation, extracellular calcium deposits stained in red (alizarin red staining). Magnification 100×.
Adipogenic Differentiation—Oil Red O Staining
Adipogenic differentiation was confirmed by the presence of large red stained lipid vacuoles in the cytoplasm due to exposure of oil red O staining.
Chondrogenic Differentiation—Alcian Blue Staining
Chondrogenic differentiation was confirmed by the presence of proteoglycans’ marked deposition in the extracellular matrix which stained blue, confirming the presence of chondrogenic aggregates.
Osteogenic Differentiation—Alizarin Red Staining
Osteogenic differentiation was demonstrated by the presence of extracellular calcium deposits stained red by alizarin red solution, which dyes chelate complexes with calcium.
3.2.2. Karyotype Analysis
The cytogenetic analysis revealed the presence of 36% normal cells in P4 and 32% normal cells in P7. Tetraploidy was present in 4% of P4 cells and 8% of P7 cells. Aneuploidy represented 60% of the cells in both passages, hypoploidy being the most representative (56%), as shown at Table 6 and Figure 6.

Table 6.
Cytogenetic analysis in Passages 4 and 7 (P4 and P7). Percentage of normal cells, tetraploid cells, and aneuploid cells.
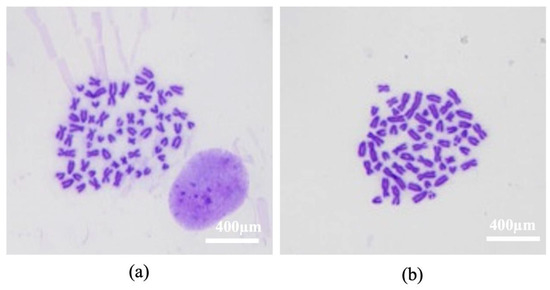
Figure 6.
Karyotype. Images of eSM-MSC cytogenetic analysis Passage 7 (P7): (a) Normal karyotype, 64 chromosomes, XY; (b) hypoploid cell, 59 chromosomes, 3 acro and 2 submeta. Magnification 100×.
3.2.3. Secretome Analysis
The analysis of CM revealed the production and secretion of several factors with immunomodulatory functions, capable of intervening beneficially in tissue regeneration. The results of the eSM-MSC CM analysis are shown in Figure 7. Seven biomarkers were identified: keratinocyte chemoattractant/growth regulated oncogene (KC/GRO), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), fibroblast growth factor (FGF-2), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), and interleukin-8 (IL-8). The most expressive were KC/GRO and MCP-1.
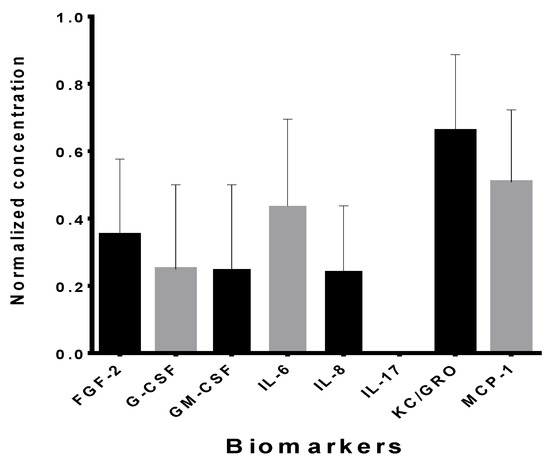
Figure 7.
Biomarkers, normalized concentration of each biomarker in the conditioned medium of eSM-MSCs P4 (mean ± SEM).
3.2.4. Immunohistochemistry
The eSM-MSCs showed strong expressions of OCT4/NANOG, vimentin, and lysozyme which confirmed marked stem cells, non-epithelial cells, and synovial cells, respectively; weak expression of GFAP; and no expression of CD31, synaptophysin, and pan-cytokeratin, as seen in Figure 8, which confirmed no vascular, neuronal, and epithelial origins of cells. Except for GFAP, in which a smaller number of cells exhibited weaker cytoplasmic immunolabeling in P3 as compared with in passage P0, there was preservation of immunoexpression of all the antibodies between passages P0 and P3. The combination of the positive and negative expressions of these different markers confirmed the expected mesenchymal origin of the cells. Figure 8 presents the immunolabeling of the eSM-MSCs.
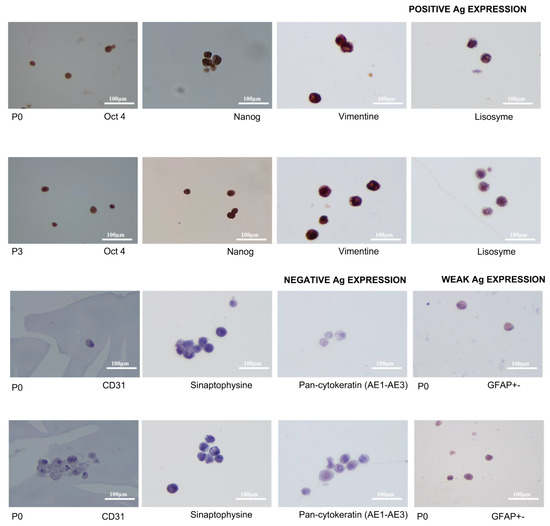
Figure 8.
Immunolabeling of eSM-MSCs P0 and P3. Magnification 600×. Images present positive Ag expression of OCT4 and NANOG confirming stem cells, positive expression of vimentin confirming non-epithelial cells, and positive expression of lysozyme confirming synovial cells. Positive expression was revealed by cytoplasmatic staining of the cells. CD31, synaptophysin and pan-cytokeratin had negative expressions, did not stain, and confirmed no vascular, neuronal, or epithelial origins of cells. GFAP represents a neuronal origin of cells and had a weak expression in P0, which reduced in P3. Magnification 600×.
3.3. Treatment Results
No horse had any adverse event that required study cessation, unplanned procedures, or additional treatments. All intra-tendinous injections and follow-up procedures had no adverse reactions (inflammation, infection, deterioration of the lesion, increased lameness), as shown by Godwin et al. (2012) [39]. No horse had abnormalities identified on the weeks following the injection.
Tendon/ligament regeneration occurred in a time frame of less than 30 days in 80% of the cases and between 30–90 days in 20% of the cases. In this study, eight horses had a lesion on the right front limb, six horses had a lesion on the left front limb, and two horses had a lesion on the right hind limb. There were 14 acute cases and two chronic cases. Chronic cases were diagnosed 6 months before our approach.
After Day 90, meaning they had completed the proposed rehabilitation physical program, the horses started cantering and started to return to their usual work plan. By Day 120 post the first treatment, 87.5% of the horses were back to full work, with the exception of the 12.5% who needed another 30 days to return to full work.
All horses returned to the same level of sport activity they had before injury. Table 2 and Table 7 summarize the recovery progress, with the respective ultrasound images in Figure 9 and Figure 10. At Day 30, the group that fully recovered demonstrated both a fulfilled ultrasound cross-sectional area and good fiber alignment. There was also no evidence of pain and lameness. Below, transversal and longitudinal ultrasound images of four cases on Day 1 and on Day 30 are presented. After the eSM-MSC treatment, all horses were submitted to a rehabilitation program, as explained in Table 4.

Table 7.
Ultrasonographic lesion characterization at Days 1, 15, and 30. Equine patient identification, structure affected, lesion ultrasonographic location, cross-sectional area, and longitudinal fiber pattern are characterized. Assessment outcome is also evaluated. Affected structure superficial digital flexor tendon (SDFT), deep digital flexor tendon (DDFT), suspensory ligament (SL), and left branch (LB). Lesion ultrasonographic location (zones 1A-1B, 2A-2B, 3A-3B) [27], cross-sectional area % (0, 0%; 1, <25%; 2, >25–50%; 3, >50–75%; 4, >75%), longitudinal fiber pattern (0, 0%; 1, <25%; 2, >25–50%; 3, >50–75%; 4, >75%) [27], and assessment outcome (full function, acceptable function, and unacceptable function) [33].
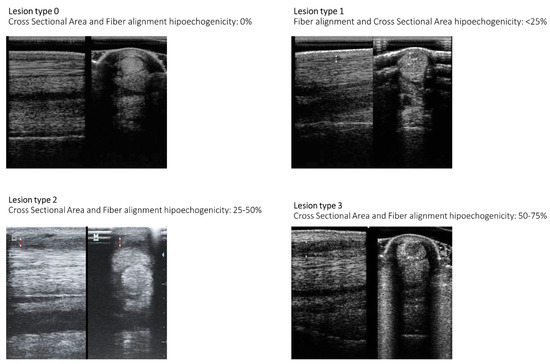
Figure 9.
Illustration of ultrasonographic lesion characterization summarized in Table 7. Longitudinal fiber alignment and cross-sectional area echogenicity loss is presented [27].

Figure 10.
Ultrasound images of Case numbers 4, 14, and 16 that represent the clinical cases concerning superficial digital flexor tendon (SDFT). Transversal and longitudinal images at Day 1 and at Day 30 after first treatment with eSM-MSCs. These ultrasounds are representative of the cases and very illustrative of good fiber alignment and cross-sectional area reduction, evidencing tissue regeneration.
Radiograph exams were performed to rule out the presence of other associated pathologies and regional nerve blocks were performed to better localize the injured region originating the pain.
Ultrasound images at Day 1 and at Day 30 clearly illustrate the evolution of tendon regeneration. Changes in echogenicity, fiber alignment, and cross-sectional area are evident, as seen in Figure 10.
4. Discussion
Recently, eSM-MSCs have become an interesting subject for those who study cellular and cell-based therapies due to their promising ability to promote tissue regeneration with high capacity of regeneration of articular structures, tendons, and ligaments. Regarding the collection, isolation, expansion, freezing, and thawing protocols used in this clinical trial, it was possible to use these cells in equine tendon regenerative treatments. The full characterization of eSM-MSCs presents a significant challenge since eSM-MSCs are not as well studied as MSCs from other species, namely human MSCs. However, in this study, their stemness and origins were confirmed through different processes: trilineage differentiation, karyotype, secretome, and immunohistochemistry. All the SM-MSC cultures presented monolayer culture, plastic adherence capacity, and fibroblast-like shape [40,41,42,43], accomplishing some of the minimal criteria defined by ISCT. Successful osteogenic, chondrogenic, and adipogenic differentiation was also demonstrated. De Bari et al. [9] were the first group of researchers to isolate MSCs from synovial tissues.
The karyotype presented some genomic variations when the number of passages was increased. That was consistent with some studies regarding genomic variations along cell passages [44,45,46,47,48]. DNA replication is a critical event for timely genome duplication. Errors in replication lead to genomic instability across evolution [49]. Prieto Gonzalez et al. considered that genomic instability, incurred during the process of stem cell isolation, culture expansion, and reprogramming, might be the most critical point of a stem cell-based therapeutic approach as a viable option from the clinical perspective [50]. Peterson et al. highlighted that there was very little evidence linking genomic abnormalities, for example, in human pluripotent stem cells (hPSCs) with tumorigeneses [44]. The frequency and effects of variations have increased with the development of even more sensitive methods for detecting genomic variation [45].
As reported by Simona Neri, the interpretation of genetic instability and senescence of cultured MSCs is controversial, but the increasing incidence of genetic alterations at advanced culture times clearly indicates that few culture passages correspond to a reduced chance to harbor dangerous alterations. Therefore, prudent behavior is desirable with a reduction in culture times as much as possible to avoid safety concerns [51]. More studies must be performed in this area.
During the last decade, it has been shown that the therapeutic effectiveness of MSCs is due mainly to the release of paracrine factors, namely CM, composed of soluble (cytokines, chemokines, and growth factors) and nonsoluble factors (extracellular vesicles) that are primarily secreted in the extracellular space by stem cells [52]. CM’s paracrine signaling can be considered to be the primary mechanism by which MSCs contribute to the healing process, and therefore, their study has become an interesting subject [53,54].
In our study, eSM-MSCs revealed a CM with a high level of KC/GRO, MCP-1, Il-6, FGF-2, G-CSF, GM-CSF, and IL-8. This highlights the intense activity of fibroblasts, producing KC/GRO that is chemotaxic for neutrophils during inflammation. MCP-1 is essential for reperfusion and the successful completion of musculoskeletal tissue after an ischemic injury [55]. Macrophages are tissue resident cells involved in tissue regeneration along with their inflammatory and infection responses [56]. IL-6 is a proinflammatory and angiogenic interleukin capable of increasing the expression of growth factors; reactivating, for example, intrinsic growth programs of neurons; promoting axonal regrowth; and creating a link between inflammation and tissue regeneration [57,58]. FGF-2 is a recognized GF responsible for proliferation of tenogenic stem cells. FGF-2 signaling has been reported to produce a tendon progenitor population that expressed scleraxis during somite development [59]. FGF-2 plays a crucial role in cell proliferation and collagen production, becoming a useful GF for tissue regeneration by promoting stem cell proliferation [60]. G-CSF is a cytokine that mobilizes bone marrow-derived cells (BM-DCs) to peripheral blood. A study suggested that injection of G-CSF to promote BM-DC release in the target area, i.e., rotator cuff, effectively enhanced rotator cuff healing by promoting tenocyte and cartilage matrix production [61]. Wright et al. presented a study that confirmed skeletal muscle damage, including muscle damage following strenuous exercise, induced an elevation in plasma G-CSF, implicating it as a potential mediator of skeletal muscle repair [62]. Recent human trials have shown the benefits of G-CSF administration as a treatment for neuromuscular diseases, considering that G-CSF affects skeletal muscle, leading to functional improvements [63,64,65,66,67,68]. GM-CSF is an hematopoietic growth factor with proinflammatory functions [69]. Major sources of GM-CSF are T and B cells, monocyte/macrophage endothelial cells, and fibroblasts. Neutrophils, eosinophils, epithelial cells, mesothelial cells, Paneth cells, chondrocytes, and tumor cells can also produce GM-CSF [70]. Paredes et al. evidenced that elevated levels of proinflammatory factors such as those found at these cells CM (GM-CSF, G-CSF, Il-6, IL-8 and IL-17), were implicated in the activation of resident tendon cells for effective healing, stimulating tendon cell proliferation [71,72]. IL-8 is one of the major mediators of inflammatory response and is a potent angiogenic factor. This is similar to IL-6, but IL-8 has a longer half-life [73].
A recent study highlighted that hematopoietic factor promoted tendon healing in aged mouse tendons. Histochemical results demonstrated that vascularization of the injury site was significantly elevated. It was concluded that vascular endothelial growth factor (VEGF) played an important role in decreasing adipocyte accumulation and also improved vascularization of the tendon during aged tendon healing. Active regulation of VEGF may improve the treatment of age-related tendon diseases and tendon injuries [74].
Studies with human BM-MSCs using a human-specific proteome profiler array with different angiogenic factors such as VEGF-A, IL-6, IL-8, platelet-derived growth factor A (PDGF-A), endothelin-1 (ET1), and urokinase plasminogen activator (uPA), which had not been previously reported in the CM of human MSCs, were also identified in an equine array, confirming what we found in this study [75]. This factor has been proposed as a modulator of the different neovascularization stages, through the enhancement of VEGF gene promotor activity [75,76]. Schokry et al. [77] reported that BM-MSC therapies have recovery times of 3–6 months and conservative therapeutic methods allow recovery in 12–18 months without regeneration but with formation of fibrous scar tissue. Retrospectively, no re-injuries of tendons have occurred in horses treated with this new approach, during the study frame time. In the literature [78], Smith et al. referred to a low percentage re-injury rate of 27% for SFD tendonitis treated with bone marrow stem cells. Horses returned to “full function” as defined by Cook et al. and modified by Guest et al. [33,79].
A study using a murine osteoarthritis (OA) model demonstrated that an injection of MSCs CM, similarly to injection of MSCs, resulted in early pain reduction and had a protective effect on the development of cartilage damage in a murine OA model, by using the regenerative capacities of the MSCs-secreted factors [80].
Interestingly, the results accumulated so far have provided evidence that veterinary patients affected by naturally occurring diseases should provide more reliable outcomes of cell therapy than laboratory animals, thus, allowing translating potential therapies to the human field. More recently, a cell-free therapy based on MSCs CM has been proposed. Even though there are very few clinical reports to refer to in veterinary medicine, recent acquisitions suggest that MSC-derived products may have major advantages compared to the related cells, for example, they are considered safer and less immunogenic [52]. As evidenced before, eSM-MSC CM factors are able to promote tendon healing by reducing inflammation and fatty infiltration, stimulating cell proliferation and tenogenic differentiation [81].
In this study we used a cell-based therapy instead of CM itself, but we were aware of its effect and potential on cell-based therapies; its advantages and therapeutic effects were the reason why this study was performed.
To better characterize the cells under study, we performed immunohistochemistry assays. The choice of markers was based on a previous work [8] and included several of the criteria used for humans, as determined by the ISCT. Results of our study demonstrated the presence of the embryonic stem cell markers OCT4 and NANOG. Detection of these markers has been previously described by Beltrami et al., in multipotent adult stem cells (HMASC) from human bone marrow [82], as well as, by Riekstina et al., who also demonstrated the presence of these markers in HMASC derived from bone marrow, adipose tissue, heart, and dermis [83]. Greco et al. also evidenced elevated expression of OCT4 in P3 MSCs and hypothesized OCT4 expression could be an indicator of MSC differentiation potential in clinical diagnostics [84]. In equine characterization of synovial fluid and membrane-derived MSCs, Prado et al. also evidenced the presence of NANOG and OCT4 markers [19]. In contrast, Fulber et al. had no positive results for these two markers in equine mesenchymal stem cells of synovial tissues [43]. Vimentin, a mesenchymal stem cell marker, was also detected, suggesting the mesenchymal origin of cells. The presence of lysozyme confirmed the synovial origin of cells, as stated by Fulber et al. [43].
The immunohistochemistry analysis showed the absence of CD31, sinaptophysine, and pan-cytokeratin expressions, confirming no vascular, neuronal and epithelial origins of cells. GFAP was weakly expressed, being less expressed in P3 than in P0 cells. CD31 was performed to investigate the presence of hematopoietic cells in eSM-MSCs. The expression of VEGF was not found, these results being similar to those from Fulber et al., and to other authors that evidenced the absence of hematopoietic markers [43,85]. The absence of neuronal and dermal markers was also consistent with other studies [19,43].
In our clinical trial, we treated mainly early acute lesions; 87.5% of the cases were acute lesions of tendons or ligaments. Therefore, we created a master cell bank of allogenic eSM-MSCs suitable for treatments in early acute phases versus treatments with autologous cells where time of tissue collection, preparation, and cell culture need to be considered. Furthermore, cell harvesting for autologous treatment is an invasive procedure which is unnecessary with this new eSM-MSC solution. The possibility of having a master cell bank enables faster healing of the organ and a quicker return to sport life. Horses spend less time in recovery time and have a regenerated tissue instead of a fibrotic tissue. These are some advantages of the eSM-MSC solution. Another concern is that in the early stages of the lesion there is an inflammatory phase; however, the paracrine factors released by eSM-MSCs also have anti-inflammatory action, reducing inflammation.
Chronic cases represented 12.5% of the cases, involving four structures. Three of the horses recovered in 30 days and one of the horses had a delayed recovery time.
The delayed recovery time in 20% of the structures, meaning 12.5% of the horses, was due to, in Case 6, an increased number of involved structures (more than one tendon or ligament) and a foot conformation abnormality, as the horse had a fetlock hyperextension that was impairing correct tendon healing. This was corrected with special shoeing. Inappropriate rehabilitation program (Case 7) was another cause of delayed recovery time. As soon as the corrective shoeing was performed, ligament regeneration started.
We could also conclude that lameness grade was not directly correlated with lesion cross-sectional area. Horses with ultrasonographic cross-sectional grade 1, 2, and 3 lesions presented lameness grade 4/5, which was observed in 9 of 16 patients. Lameness grade 3/5 was presents in 4 of 16 of equine patients with ultrasonographic cross-sectional grade 1 and 2 lesions. Lameness grade 2/5 was present in 3 of 16 equine patients with ultrasonographic cross-sectional grade 1 lesions.
Kamm et al. (2021) concluded that based on the evidence to date, tendons appear to have improved healing when treated with allogeneic MSCs, and the use of these treatments in equine tendon and ligament lesions is warranted [86]. Colbath et al. (2020) claimed that some of the advantages of using allogenic stem cells include the ability to bank cells and to also reduce the treatment time, to collect MSCs from younger donor animals, and the ability to manipulate banked cells prior to administration [87]. Some of the disadvantages focused on the risk of immunological reactions. However, currently, there are several studies in horses accumulating evidence that allogeneic MSCs may be a safe alternative to autologous MSCs [87]. Nevertheless, the donor’s health must always be taken into consideration as well as the donor’s age [88].
5. Conclusions
To sum up, this study accomplishes the criteria for reporting veterinary and animal medicine research for MSCs in orthopedic applications [33] and the ISCT perspective on immune assays for MSC’s criteria for advanced phase clinical trials [89], confirmed by plastic adherence, tri-lineage differentiation, synovial membrane origin, spindle-shaped cells, as well as proliferative and immune modulatory capacity proven by immunohistochemistry and CM.
From a clinical point of view, the idea of having an allogenic eSM-MSC cell bank is very interesting. Therefore, the possibility of having a universal donor who can provide a large amount of eSM-MSCs, to culture and preserve non-immunogenic cells whose availability is immediate, allowing a quick and effective therapeutic answer in acute stages of musculoskeletal lesion is the paramount goal of orthopedic medicine.
From a “one-health” perspective, equines play an important role as a model for human musculoskeletal disorders; the high-level analogy between human and equine structures may have a great translational value for both species for future clinical aspects [28,90]. There are significant resemblances between equine SDFT and human Achilles tendon with respect to the size of anatomical structure and load, function (energy store), pathophysiology of tendon injury, and the healing response under activity or traumatic rupture compared to other species [90]. Moreover, considering the result of tendinopathy in equine species which reflects the conditions encountered in humans, the horse is accepted as an appropriate model in this area by the research community and by other authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Based on the clinical, ultrasonographic, and performance outcomes identified in the present study, the use of eSM-MSCs together with autologous serum solution has proven its efficiency for tendon and ligament repair and contributes to reduce the recovery period and subsequent rapid return to athletic activity. The therapy was demonstrated to be safe and had no adverse findings. The clinical results and athletic outcomes of the horses were very positive. Comparing our study with others, using for example BM-MSCs, it seems that our new approach has shorter recovery times and fewer re-injuries [39,77]. These results encourage the use of eSM-MSCs and autologous serum for the treatment of tendonitis and desmitis, since they can regenerate tendon and ligament tissue and regain organ function, enhancing the return to competition in excellent time frames.
Author Contributions
Conceptualization, I.L.R., B.L., P.S., L.A., J.M.S. and A.C.M.; methodology, I.L.R., B.L., P.S., A.C.S., M.B., A.R.C., S.S.P., A.R., B.P., I.A., R.A., J.M.S. and A.C.M.; software, I.L.R., A.C.S., M.B., R.A. and J.M.S.; validation, I.L.R., A.C.S., R.A., M.B., B.L., P.S., A.R., A.R.C., S.S.P., L.A., B.P., C.O., I.A., J.M.S. and A.C.M.; formal analysis, I.L.R.; investigation, I.L.R., B.L., P.S., A.C.S., M.B., A.R.C., S.S.P., A.R., L.A., B.P., C.O., I.A., R.A., J.M.S. and A.C.M.; resources, J.M.S. and A.C.M.; data curation, I.L.R., P.S. and B.L.; writing—original draft preparation, I.L.R., B.L. and P.S.; writing—review and editing, I.L.R., B.L. and P.S.; visualization, I.L.R., B.L., P.S., A.C.S., M.B., A.R.C., S.S.P., A.R., L.A., B.P., C.O., I.A., R.A., J.M.S. and A.C.M.; supervision, L.A., J.M.S. and A.C.M.; project administration, A.C.M.; funding acquisition, A.C.M. All authors reviewed the final work and approved its submission. Each authors agree to be personally accountable for the author’s own contributions, and for ensuring that questions related to the accuracy or integrity of any part of the work, even questions in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
Mariana Vieira Branquinho (SFRH/BD/146172/2019), Ana Catarina Sousa (SFRH/BD/146689/2019), and Bruna Lopes (2021.05265.BD) acknowledge Fundaçāo para a Ciência e Tecnologia (FCT) for financial support. Rui Damásio Alvites acknowledges the Animal Science Studies Centre (CECA), Agroenvironment, Technologies and Sciences Institute (ICETA), Porto University (UP), and FCT for the funding and availability of all technical, structural, and human resources necessary for the development of this work. The author Patrícia Sousa acknowledges Instituto Politécnico de Leiria–Center for Rapid and Sustainable Product Development (CDRSP), University of Porto (UP), Centro de Estudos de Ciêcia Animal (CECA), Instituto de Ciências, Tecnologias e Agroambiente (ICETA) for the funding (UIDB/04044/2020) and availability of all resources needed for this work. The work was supported through the project UIDB/00211/2020 funded by FCT/MCTES national funds. This research was funded by Projects PEst-OE/AGR/UI0211/2011 from FCT, and COMPETE 2020, from ANI–Projetos ID&T Empresas em Copromoçāo, and by the project “Print-on-Organs–Engineering bioinks and processes for direct printing on organs” with the reference POCI-01-0247-FEDER-033877, by the project “Bone2Move- Development of “in vivo” experimental techniques and modeling methodologies for the evaluation of 4D scaffolds for bone defect in sheepmodel: an integrative research approach” with the reference POCI-01-0145-FEDER-031146.
Institutional Review Board Statement
All procedures performed on animals were approved by the Organism Responsible for Animal Welfare (ORBEA) of the Abel Salazar Institute for Biomedical Sciences (ICBAS) from the University of Porto (UP) (project 289/ORBEA/2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.
Acknowledgments
The authors greatly appreciate the animals’ proprietaries and caretakers for accepting to participate in the present study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAEP | American Association of Equine Practitioners |
| AE1/AE3 | Pan-cytokeratin |
| BM-MSC | Bone marrow mesenchymal stem cell |
| CD | Cluster differentiation |
| c-Kit | Proto-oncogene receptor tyrosine kinase or stem cell factor receptor |
| CM | Conditioned medium |
| cm2 | Square centimeter |
| Coll-II | Collagen type II |
| COMP | Cartilage oligomeric matrix protein |
| CPD | Cumulative population doublings |
| DMSO | Dimethylsulphoxide |
| DPBS | Dulbecco′s phosphate buffered saline |
| eSM-MSC | Equine synovial membrane mesenchymal stem cell |
| FBS | Fetal bovine serum |
| FGF-2 | Basic fibroblast growth factor |
| G-CSF | Granulocyte colony stimulating factor |
| GFAP | Glial fibrillary acidic protein |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| GRO/KC | Human growth-regulated oncogene/keratinocyte chemoattractant |
| ICBAS-UP | Instituto de Ciências Biomédicas Abel Salazar–Universidade do Porto |
| IL | Interleukins |
| ISCT | International Society for Cellular Therapy |
| IV | Endovenous |
| kg | Kilogram |
| MCB | Master cell banks |
| MCP-1 | Monocyte chemoattractant protein-1 |
| mg | milligram |
| MHC-II | Major histocompatibility complex |
| MHz | Megahertz |
| min | Minutes |
| mL | Milliliter |
| MSCs | Mesenchymal stem cell |
| NSAIDs | Nonsteroidal anti-inflamatory drugs |
| OA | Osteoarthritis |
| OCT4 | Octamer-binding transcription factor 4 |
| ORBEA | Organismo Responsável pelo Bem-estar Animal |
| P | Passage |
| PBS | Phosphate-buffered saline |
| PRP | Platelet-rich plasma |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SID | Once a day |
| SM-MSC | Synovial membrane mesenchymal stem cell |
| UDP | Uridine diphosphate |
| UDPGD | Uridine diphosphate glucose dehydrogenase |
| VEGF-R1 | Vascular endothelial growth factor |
| WCB | Working cell banks |
References
- Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative medicine for equine musculoskeletal diseases. Animals 2021, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, A.; Parham, A. Strategies of tenogenic differentiation of equine stem cells for tendon repair: Current status and challenges. Stem Cell Res. Ther. 2019, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Tuemmers, C.; Rebolledo, N.; Aguilera, R. Effect of the application of stem cells for tendon injuries in sporting horses. Arch. De Med. Vet. 2012, 44, 207–215. [Google Scholar] [CrossRef]
- Platonova, S.; Korovina, D.; Viktorova, E.; Savchenkova, I. Equine Tendinopathy Therapy Using Mesenchymal Stem Cells. KnE Life Sci. 2021, 533–541. [Google Scholar] [CrossRef]
- Chandra, V.; Mankuzhy, P.; Sharma G, T. Mesenchymal Stem Cells in Veterinary Regenerative Therapy: Basic Physiology and Clinical Applications. Curr. Stem Cell Res. Ther. 2022, 17, 237–251. [Google Scholar] [CrossRef]
- Depuydt, E.; Broeckx, S.Y.; Van Hecke, L.; Chiers, K.; Van Brantegem, L.; Van Schie, H.; Beerts, C.; Spaas, J.H.; Pille, F.; Martens, A. The evaluation of equine allogeneic tenogenic primed mesenchymal stem cells in a surgically induced superficial digital flexor tendon lesion model. Front. Vet. Sci. 2021, 8, 641441. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and clinical applications of mesenchymal stem cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264. [Google Scholar] [CrossRef] [PubMed]
- De Schauwer, C.; Meyer, E.; Van de Walle, G.R.; Van Soom, A. Markers of stemness in equine mesenchymal stem cells: A plea for uniformity. Theriogenology 2011, 75, 1431–1443. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Harvanová, D.; Tóthová, T.; Sarissky, M.; Amrichová, J.; Rosocha, J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol. (Praha) 2011, 57, 119–124. [Google Scholar]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, S.; Sekiya, I.; Sakaguchi, Y.; Yagishita, K.; Ichinose, S.; Muneta, T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem. 2006, 97, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Muneta, T.; Ju, Y.J.; Nagase, T.; Nimura, A.; Mochizuki, T.; Ichinose, S.; Von der Mark, K.; Sekiya, I. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells 2007, 25, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Bami, M.; Sarlikiotis, T.; Milonaki, M.; Vikentiou, M.; Konsta, E.; Kapsimali, V.; Pappa, V.; Koulalis, D.; Johnson, E.O.; Soucacos, P.N. Superiority of synovial membrane mesenchymal stem cells in chondrogenesis, osteogenesis, myogenesis and tenogenesis in a rabbit model. Injury 2020, 51, 2855–2865. [Google Scholar] [CrossRef]
- Zayed, M.; Newby, S.; Misk, N.; Donnell, R.; Dhar, M. Xenogenic implantation of equine synovial fluid-derived mesenchymal stem cells leads to articular cartilage regeneration. Stem Cells Int. 2018, 2018, 1073705. [Google Scholar] [CrossRef]
- Hermida-Gómez, T.; Fuentes-Boquete, I.; Gimeno-Longas, M.J.; Muiños-López, E.; Díaz-Prado, S.; Blanco, F.J. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J. Rheumatol. 2011, 38, 339–349. [Google Scholar] [CrossRef]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous synovium–and adipose synovium–derived cells compared with subcutaneous fat–derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006, 54, 843–853. [Google Scholar] [CrossRef]
- Fickert, S.; Fiedler, J.; Brenner, R. Identification, quantification and isolation of mesenchymal progenitor cells from osteoarthritic synovium by fluorescence automated cell sorting. Osteoarthr. Cartil. 2003, 11, 790–800. [Google Scholar] [CrossRef]
- Prado, A.A.F.; Favaron, P.O.; da Silva, L.C.L.C.; Baccarin, R.Y.A.; Miglino, M.A.; Maria, D.A. Characterization of mesenchymal stem cells derived from the equine synovial fluid and membrane. BMC Vet. Res. 2015, 11, 281. [Google Scholar] [CrossRef]
- Chen, Y.-W. Chondrogenic Capacities of Equine Synovial Progenitor Populations. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2012. [Google Scholar]
- Colbath, A.C.; Frisbie, D.D.; Dow, S.W.; Kisiday, J.D.; McIlwraith, C.W.; Goodrich, L.R. Equine models for the investigation of mesenchymal stem cell therapies in orthopaedic disease. Oper. Tech. Sport. Med. 2017, 25, 41–49. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef] [PubMed]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.J. Lameness evaluation of the athletic horse. Vet. Clin. Equine Pract. 2018, 34, 181–191. [Google Scholar] [CrossRef] [PubMed]
- AAEP Horse Show Committee. Guide to Veterinary Services for Horse Shows; American Association of Equine Practitioners: Lexington, KY, USA, 1999. [Google Scholar]
- Broeckx, S.Y.; Seys, B.; Suls, M.; Vandenberghe, A.; Mariën, T.; Adriaensen, E.; Declercq, J.; Van Hecke, L.; Braun, G.; Hellmann, K. Equine allogeneic chondrogenic induced mesenchymal stem cells are an effective treatment for degenerative joint disease in horses. Stem Cells Dev. 2019, 28, 410–422. [Google Scholar] [CrossRef]
- Alzola Domingo, R.; Riggs, C.M.; Gardner, D.S.; Freeman, S.L. Ultrasonographic scoring system for superficial digital flexor tendon injuries in horses: Intra-and inter-rater variability. Vet. Rec. 2017, 181, 655. [Google Scholar] [CrossRef]
- Melotti, L.; Carolo, A.; Elshazly, N.; Boesso, F.; Da Dalt, L.; Gabai, G.; Perazzi, A.; Iacopetti, I.; Patruno, M. Case Report: Repeated Intralesional Injections of Autologous Mesenchymal Stem Cells Combined With Platelet-Rich Plasma for Superficial Digital Flexor Tendon Healing in a Show Jumping Horse. Front. Vet. Sci. 2022, 9, 843131. [Google Scholar] [CrossRef]
- Johnson, S.A.; Donnell, J.R.; Donnell, A.D.; Frisbie, D.D. Retrospective analysis of lameness localisation in Western Performance Horses: A ten-year review. Equine Vet. J. 2021, 53, 1150–1158. [Google Scholar] [CrossRef]
- Ehrle, A.; Lilge, S.; Clegg, P.D.; Maddox, T.W. Equine flexor tendon imaging part 1: Recent developments in ultrasonography, with focus on the superficial digital flexor tendon. Vet. J. 2021, 278, 105764. [Google Scholar] [CrossRef]
- Iimori, M.; Tamura, N.; Seki, K.; Kasashima, Y. Relationship between the ultrasonographic findings of suspected superficial digital flexor tendon injury and the prevalence of subsequent severe superficial digital flexor tendon injuries in Thoroughbred horses: A retrospective study. J. Vet. Med. Sci. 2022, 84, 261–265. [Google Scholar] [CrossRef]
- Mitchell, R.; DaSilva, D.; Rosenbaum, C.; Blikslager, A.; Edwards, R.B., III. Ultrasound findings in tendons and ligaments of lame sport horses competing or training in South Florida venues during the winter seasons of 2007 through 2016. Equine Vet. Educ. 2021, 33, 306–309. [Google Scholar] [CrossRef]
- Guest, D.J.; Dudhia, J.; Smith, R.K.W.; Roberts, S.J.; Conzemius, M.; Innes, J.F.; Fortier, L.A.; Meeson, R.L. Position Statement: Minimal criteria for reporting veterinary and animal medicine research for mesenchymal stromal/stem cells in orthopaedic applications. Front. Vet. Sci. 2022, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Schils, S.; Turner, T. Review of Early Mobilization of Muscle, Tendon, and Ligament after Injury in Equine Rehabilitation. In Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners, Baltimore, MD, USA, 4–8 December 2010; pp. 374–380. [Google Scholar]
- Kaneps, A.J. Practical rehabilitation and physical therapy for the general equine practitioner. Vet. Clin. Equine Pract. 2016, 32, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.J. Controlled exercise in equine rehabilitation. Vet. Clin. Equine Pract. 2016, 32, 159–165. [Google Scholar] [CrossRef]
- Ortved, K.F. Regenerative medicine and rehabilitation for tendinous and ligamentous injuries in sport horses. Vet. Clin. Equine Pract. 2018, 34, 359–373. [Google Scholar] [CrossRef]
- Godwin, E.; Young, N.; Dudhia, J.; Beamish, I.; Smith, R. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef]
- Gale, A.L.; Linardi, R.L.; McClung, G.; Mammone, R.M.; Ortved, K.F. Comparison of the chondrogenic differentiation potential of equine synovial membrane-derived and bone marrow-derived mesenchymal stem cells. Front. Vet. Sci. 2019, 6, 178. [Google Scholar] [CrossRef]
- Zayed, M.; Adair, S.; Dhar, M. Effects of Normal Synovial Fluid and Interferon Gamma on Chondrogenic Capability and Immunomodulatory Potential Respectively on Equine Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 6391. [Google Scholar] [CrossRef]
- Murata, D.; Ishikawa, S.; Sunaga, T.; Saito, Y.; Sogawa, T.; Nakayama, K.; Hobo, S.; Hatazoe, T. Osteochondral regeneration of the femoral medial condyle by using a scaffold-free 3D construct of synovial membrane-derived mesenchymal stem cells in horses. BMC Vet. Res. 2022, 18, 53. [Google Scholar] [CrossRef]
- Fülber, J.; Maria, D.A.; Silva, L.C.L.C.d.; Massoco, C.O.; Agreste, F.; Baccarin, R.Y.A. Comparative study of equine mesenchymal stem cells from healthy and injured synovial tissues: An in vitro assessment. Stem Cell Res. Ther. 2016, 7, 35. [Google Scholar] [CrossRef]
- Peterson, S.E.; Loring, J.F. Genomic instability in pluripotent stem cells: Implications for clinical applications. J. Biol. Chem. 2014, 289, 4578–4584. [Google Scholar] [CrossRef]
- Lupski, J.R. Genome mosaicism—One human, multiple genomes. Science 2013, 341, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Rohrback, S.; Siddoway, B.; Liu, C.S.; Chun, J. Genomic mosaicism in the developing and adult brain. Dev. Neurobiol. 2018, 78, 1026–1048. [Google Scholar] [CrossRef]
- Thorpe, J.; Osei-Owusu, I.A.; Avigdor, B.E.; Tupler, R.; Pevsner, J. Mosaicism in human health and disease. Annu. Rev. Genet. 2020, 54, 487. [Google Scholar] [CrossRef] [PubMed]
- Vattathil, S.; Scheet, P. Extensive hidden genomic mosaicism revealed in normal tissue. Am. J. Hum. Genet. 2016, 98, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, M.; Tsaniras, S.C.; Taraviras, S.; Lygerou, Z. Replication licensing aberrations, replication stress, and genomic instability. Trends Biochem. Sci. 2019, 44, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Prieto González, E.; Haider, K.H. Genomic Instability in Stem Cells: The Basic Issues. In Stem Cells: From Potential to Promise; Springer: Berlin/Heidelberg, Germany, 2021; pp. 107–150. [Google Scholar]
- Neri, S. Genetic stability of mesenchymal stromal cells for regenerative medicine applications: A fundamental biosafety aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef]
- Mocchi, M.; Dotti, S.; Del Bue, M.; Villa, R.; Bari, E.; Perteghella, S.; Torre, M.L.; Grolli, S. Veterinary regenerative medicine for musculoskeletal disorders: Can mesenchymal stem/stromal cells and their secretome be the new frontier? Cells 2020, 9, 1453. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Al Naem, M.; Bourebaba, L.; Kucharczyk, K.; Röcken, M.; Marycz, K. Therapeutic mesenchymal stromal stem cells: Isolation, characterization and role in equine regenerative medicine and metabolic disorders. Stem Cell Rev. Rep. 2020, 16, 301–322. [Google Scholar] [CrossRef]
- Shireman, P.K.; Contreras-Shannon, V.; Ochoa, O.; Karia, B.P.; Michalek, J.E.; McManus, L.M. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J. Leukoc. Biol. 2007, 81, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Marine, T.; Marielle, S.; Graziella, M.; Fabio, R. Macrophages in skeletal muscle dystrophies, an entangled partner. J. Neuromuscul. Dis. 2021, 9, 1–23. [Google Scholar]
- Yang, P.; Wen, H.; Ou, S.; Cui, J.; Fan, D. IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Exp. Neurol. 2012, 236, 19–27. [Google Scholar] [CrossRef]
- Friese, N.; Gierschner, M.B.; Schadzek, P.; Roger, Y.; Hoffmann, A. Regeneration of damaged tendon-bone junctions (entheses)—TAK1 as a potential node factor. Int. J. Mol. Sci. 2020, 21, 5177. [Google Scholar] [CrossRef]
- Brent, A.E.; Tabin, C.J. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 2004, 131, 3885–3896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Chen, X.; Li, G.; Chan, K.-M.; Heng, B.C.; Yin, Z.; Ouyang, H.-W. Concise review: Stem cell fate guided by bioactive molecules for tendon regeneration. Stem Cells Transl. Med. 2018, 7, 404–414. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kida, Y.; Kabuto, Y.; Morihara, T.; Sukenari, T.; Nakagawa, H.; Onishi, O.; Oda, R.; Kida, N.; Tanida, T. Healing Effect of Subcutaneous Administration of Granulocyte Colony-Stimulating Factor on Acute Rotator Cuff Injury in a Rat Model. Tissue Eng. Part A 2021, 27, 1205–1212. [Google Scholar] [CrossRef]
- Wright, C.R.; Ward, A.C.; Russell, A.P. Granulocyte colony-stimulating factor and its potential application for skeletal muscle repair and regeneration. Mediat. Inflamm. 2017, 2017, 7517350. [Google Scholar] [CrossRef]
- Sakuma, T.; Yamazaki, M.; Okawa, A.; Takahashi, H.; Kato, K.; Hashimoto, M.; Hayashi, K.; Furuya, T.; Fujiyoshi, T.; Kawabe, J. Neuroprotective therapy using granulocyte colony–stimulating factor for patients with worsening symptoms of thoracic myelopathy: A multicenter prospective controlled trial. Spine 2012, 37, 1475–1478. [Google Scholar] [CrossRef]
- Yamazaki, M.; Sakuma, T.; Kato, K.; Furuya, T.; Koda, M. Granulocyte colony-stimulating factor reduced neuropathic pain associated with thoracic compression myelopathy: Report of two cases. J. Spinal Cord Med. 2013, 36, 40–43. [Google Scholar] [CrossRef]
- Kato, K.; Koda, M.; Takahashi, H.; Sakuma, T.; Inada, T.; Kamiya, K.; Ota, M.; Maki, S.; Okawa, A.; Takahashi, K. Granulocyte colony-stimulating factor attenuates spinal cord injury-induced mechanical allodynia in adult rats. J. Neurol. Sci. 2015, 355, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Koda, M.; Furuya, T.; Kato, K.; Takahashi, H.; Sakuma, T.; Inada, T.; Ota, M.; Maki, S.; Okawa, A. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: A comparison with high-dose methylprednisolone as a historical control. Eur. Spine J. 2015, 24, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yamazaki, M.; Okawa, A.; Furuya, T.; Sakuma, T.; Takahashi, H.; Kamiya, K.; Inada, T.; Takahashi, K.; Koda, M. Intravenous administration of granulocyte colony-stimulating factor for treating neuropathic pain associated with compression myelopathy: A phase I and IIa clinical trial. Eur. Spine J. 2013, 22, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Okurowska-Zawada, B.; Kułak, W.; Sienkiewicz, D.; Paszko-Patej, G.; Dmitruk, E.; Kalinowska, A.; Wojtkowski, J.; Korzeniecka–Kozerska, A. Safety and efficacy of granulocyte colony stimulating factor in a patient with tetraplegia caused by cervical hyperextension injury: A case report. Prog. Health Sci. 2014, 4, 181–184. [Google Scholar]
- Shiomi, A.; Usui, T.; Mimori, T. GM-CSF as a therapeutic target in autoimmune diseases. Inflamm. Regen. 2016, 36, 8. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, A.; Usui, T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediat. Inflamm. 2015, 2015, 568543. [Google Scholar] [CrossRef]
- Paredes, J.; Marvin, J.C.; Vaughn, B.; Andarawis-Puri, N. Innate tissue properties drive improved tendon healing in MRL/MpJ and harness cues that enhance behavior of canonical healing cells. FASEB J. 2020, 34, 8341–8356. [Google Scholar] [CrossRef]
- Al-Sadi, O.; Schulze-Tanzil, G.; Kohl, B.; Lohan, A.; Lemke, M.; Ertel, W.; John, T. Tenocytes, pro-inflammatory cytokines and leukocytes: A relationship? Muscles Ligaments Tendons J. 2011, 1, 68. [Google Scholar]
- Ohls, R.K.; Maheshwari, A. Hematology, Immunology and Infectious Disease: Neonatology Questions and Controversies: Expert Consult-Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lai, F.; Wang, J.; Tang, H.; Huang, P.; Liu, J.; He, G.; Zhou, M.; Tao, X.; Tang, K. VEGF promotes tendon regeneration of aged rats by inhibiting adipogenic differentiation of tendon stem/progenitor cells and promoting vascularization. FASEB J. 2022, 36, e22433. [Google Scholar] [CrossRef]
- Bussche, L.; Van de Walle, G.R. Peripheral Blood-Derived Mesenchymal Stromal Cells Promote Angiogenesis via Paracrine Stimulation of Vascular Endothelial Growth Factor Secretion in the Equine Model. Stem Cells Transl. Med. 2014, 3, 1514–1525. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Shokry, M.; Mostafo, A.; Tohamy, A.; El-Sharkawi, M. Autologous mesenchymal stem cells for treatment of acute superficial digital flexor tendonitis in athletic horses-A clinical study of 1 5 cases. Pferdeheilkunde 2020, 36, 43–48. [Google Scholar] [CrossRef]
- Smith, R.K.; Cauvin, E.R. Ultrasonography of the Metacarpus and Metatarsus. In Atlas of Equine Ultrasonography; Wiley: Hoboken, NJ, USA, 2014; pp. 73–105. [Google Scholar]
- Cook, J.L.; Evans, R.; Conzemius, M.G.; Lascelles, B.D.X.; McIlwraith, C.W.; Pozzi, A.; Clegg, P.; Innes, J.; Schulz, K.; Houlton, J. Proposed definitions and criteria for reporting time frame, outcome, and complications for clinical orthopedic studies in veterinary medicine. Vet. Surg. 2010, 39, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Khatab, S.; van Osch, G.; Kops, N.; Bastiaansen-Jenniskens, Y.; Bos, K.; Verhaar, J.; Bernsen, M.; Buul, G. Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur. Cells Mater. 2018, 36, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-N.; Rong, X.; Yang, L.-M.; Hua, W.-Z.; Ni, G.-X. Advances in Stem Cell Therapies for Rotator Cuff Injuries. Front. Bioeng. Biotechnol. 2022, 10, 866195. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Cesselli, D.; Bergamin, N.; Marcon, P.; Rigo, S.; Puppato, E.; D’Aurizio, F.; Verardo, R.; Piazza, S.; Pignatelli, A. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood J. Am. Soc. Hematol. 2007, 110, 3438–3446. [Google Scholar] [CrossRef]
- Riekstina, U.; Cakstina, I.; Parfejevs, V.; Hoogduijn, M.; Jankovskis, G.; Muiznieks, I.; Muceniece, R.; Ancans, J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. Rep. 2009, 5, 378–386. [Google Scholar] [CrossRef]
- Greco, S.J.; Liu, K.; Rameshwar, P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells 2007, 25, 3143–3154. [Google Scholar] [CrossRef]
- Zhang, S.; Muneta, T.; Morito, T.; Mochizuki, T.; Sekiya, I. Autologous synovial fluid enhances migration of mesenchymal stem cells from synovium of osteoarthritis patients in tissue culture system. J. Orthop. Res. 2008, 26, 1413–1418. [Google Scholar] [CrossRef]
- Kamm, J.L.; Riley, C.B.; Parlane, N.; Gee, E.K.; McIlwraith, C.W. Interactions between allogeneic mesenchymal stromal cells and the recipient immune system: A comparative review with relevance to equine outcomes. Front. Vet. Sci. 2021, 7, 617647. [Google Scholar] [CrossRef]
- Colbath, A.C.; Dow, S.W.; McIlwraith, C.W.; Goodrich, L.R. Mesenchymal stem cells for treatment of musculoskeletal disease in horses: Relative merits of allogeneic versus autologous stem cells. Equine Vet. J. 2020, 52, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Krampera, M.; Barrett, J.; Dazzi, F.; Deans, R.J.; DeBruijn, J.; Dominici, M.; Fibbe, W.E.; Gee, A.P.; Gimble, J.M. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016, 18, 151–159. [Google Scholar] [CrossRef]
- Lui, P.; Maffulli, N.; Rolf, C.; Smith, R. What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sport. 2011, 21, 3–17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).