Homocysteine—Potential Novel Diagnostic Indicator of Health and Disease in Horses
Abstract
Simple Summary
Abstract
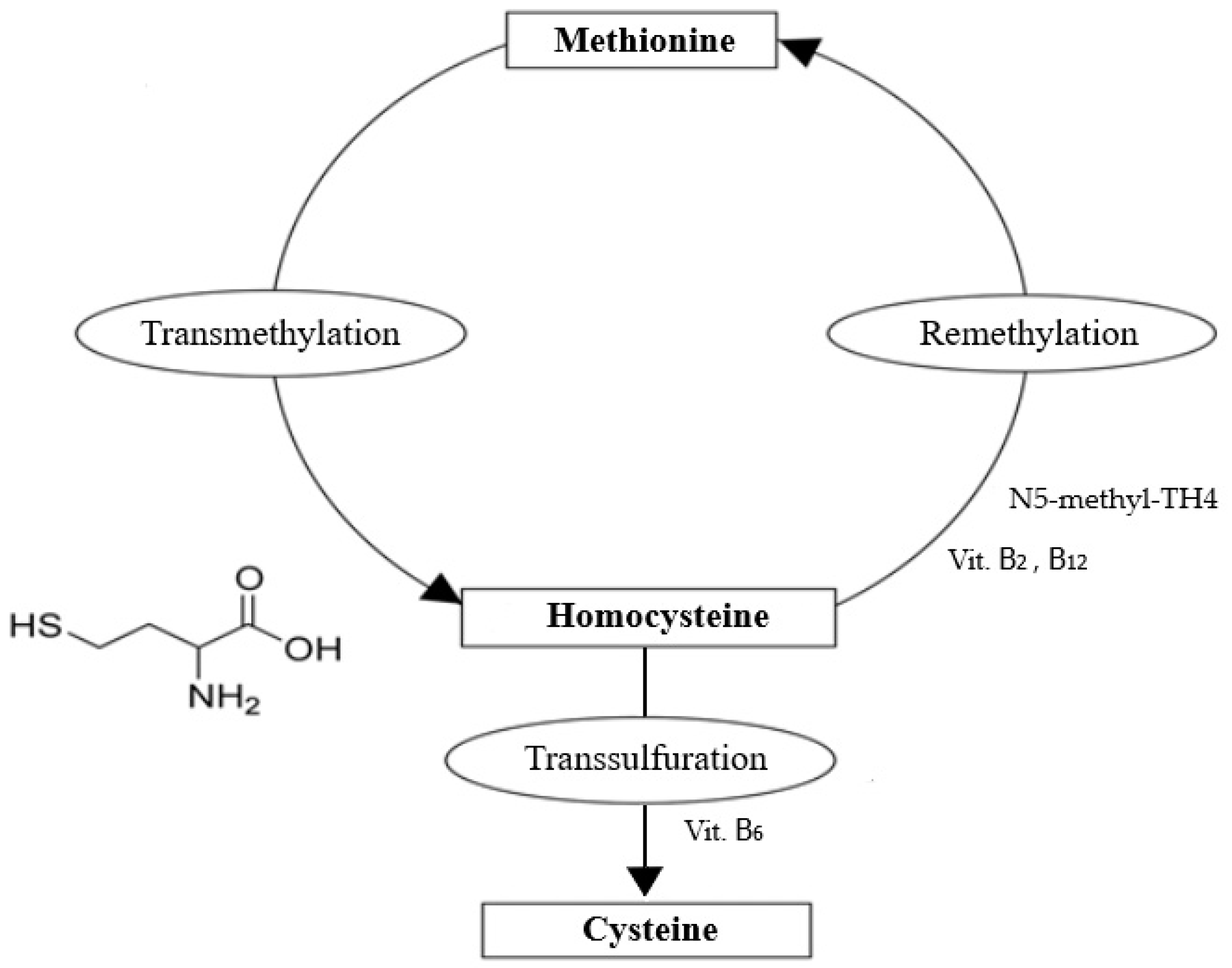
1. Introduction
2. Methods
3. Homocysteine in Pets and Farm Animals
4. Homocysteine in Horses
4.1. Cardiovascular Disease
4.2. Neurodegenerative Disease
4.3. Physical Activity
4.4. Oxidative Stress
4.5. Reference Values and Hyperhomocysteinemia
4.6. Determination Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed]
- Youssef-Saliba, S.; Milet, A.; Vallée, Y. Did Homocysteine Take Part in the Start of the Synthesis of Peptides on the Early Earth? Biomolecules 2022, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Riedijk, M.A.; Stoll, B.; Chacko, S.; Schierbeek, H.; Sunehag, A.L.; van Goudoever, J.B.; Burrin, D.G. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2007, 104, 3408–3413. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—Biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Tjong, E.; Dimri, M.; Mohiuddin, S.S. Biochemistry, Tetrahydrofolate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yoshitomi, R.; Nakayama, K.; Yamashita, S.; Kumazoe, M.; Lin, T.-A.; Mei, C.-Y.; Marugame, Y.; Fujimura, Y.; Maeda-Yamamoto, M.; Kuriyama, S.; et al. Plasma Homocysteine Concentration is Associated with the Expression Level of Folate Receptor 3. Sci. Rep. 2020, 10, 10283. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Peng, D.; Liu, C.; Huang, C.; Luo, J. Serum high concentrations of homocysteine and low levels of folic acid and vitamin B12 are significantly correlated with the categories of coronary artery diseases. BMC Cardiovasc. Disord. 2017, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- McMullin, M.; Young, P.; Bailie, K.; Savage, G.; Lappin, T.; White, R. Homocysteine and methylmalonic acid as indicators of folate and vitamin B12 deficiency in pregnancy. Clin. Lab. Haematol. 2001, 23, 161–165. [Google Scholar] [CrossRef]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef]
- Brattström, L.E.; Israelsson, B.; Jeppsson, J.O.; Hultberg, B.L. Folic acid--an innocuous means to reduce plasma homocysteine. Scand. J. Clin. Lab. Investig. 1988, 48, 215–221. [Google Scholar] [CrossRef]
- Shane, B. Folate and Vitamin B12 Metabolism: Overview and Interaction with Riboflavin, Vitamin B6, and Polymorphisms. Food Nutr. Bull. 2008, 29 (Suppl. S1), S5–S16. [Google Scholar] [CrossRef]
- Allen, L.H. Vitamin B-12. Adv. Nutr. 2012, 3, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Hustad, S.; Schneede, J.; Vollset, S.E.; Ueland, P.M. Plasma vitamin B-6 forms and their relation to transsulfuration metabolites in a large, population-based study. Am. J. Clin. Nutr. 2007, 86, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Tiwari, M.; Tiwari, R.K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic. Clin. Pharmacol. Toxicol. 2015, 117, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Furness, D.; Fenech, M.; Dekker, G.; Khong, T.Y.; Roberts, C.; Hague, W. Folate, Vitamin B12, Vitamin B6 and homocysteine: Impact on pregnancy outcome. Matern. Child. Nutr. 2013, 9, 155–166. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Homocysteine, vitamins, and vascular disease prevention. Am. J. Clin. Nutr. 2007, 86, 1563S–1568S. [Google Scholar] [CrossRef]
- Herrmann, W. The Importance of Hyperhomocysteinemia as a Risk Factor for Diseases: An Overview. Clin. Chem. Lab. Med. 2001, 39, 666–674. [Google Scholar] [CrossRef]
- Al-Maskari, M.Y.; Waly, M.I.; Ali, A.; Al-Shuaibi, Y.S.; Ouhtit, A. Folate and vitamin B12 deficiency and hyperhomocysteinemia promote oxidative stress in adult type 2 diabetes. Nutrition 2012, 28, e23–e26. [Google Scholar] [CrossRef]
- Sunder-Plassmann, G.; Winkelmayer, W.C.; Födinger, M. Therapeutic potential of total homocysteine-lowering drugs on cardiovascular disease. Expert. Opin. Investig. Drugs 2000, 9, 2637–2651. [Google Scholar] [CrossRef]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef]
- den Heijer, M.; Willems, H.P.; Blom, H.J.; Gerrits, W.B.; Cattaneo, M.; Eichinger, S.; Rosendaal, F.R.; Bos, G.M. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: A randomized, placebo-controlled, double-blind trial. Blood 2007, 109, 139–144. [Google Scholar] [CrossRef]
- Luo, J.J.; Zhang, L.; Dun, N.J. Homocysteine and Dementia in Parkinson Disease. In Dementia in Parkinson’s Disease: Everything You Need to Know; IntechOpen: London, UK, 2022; p. 41. [Google Scholar]
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of hyperhomocysteinemia and its pathological significance. Arch. Pharm. Res. 2018, 41, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Sauls, D.L.; Arnold, E.K.; Bell, C.W.; Allen, J.C.; Hoffman, M. Pro-thrombotic and pro-oxidant effects of diet-induced hyperhomocysteinemia. Thromb. Res. 2007, 120, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hoţoleanu, C.; Porojan-Iuga, M.; Rusu, M.L.; Andercou, A. Hyperhomocysteinemia: Clinical and therapeutical involvement in venous thrombosis. Rom. J. Intern. Med. 2007, 45, 159–164. [Google Scholar] [PubMed]
- van den Berg, M.; Stehouwer, C.D.; Bierdrager, E.; Rauwerda, J.A. Plasma homocysteine and severity of atherosclerosis in young patients with lower-limb atherosclerotic disease. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 165–171. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Malinow, M.R.; Willett, W.C.; Newcomer, L.M.; Upson, B.; Ullmann, D.; Tishler, P.V.; Hennekens, C.H. A Prospective Study of Plasma Homocyst(e)ine and Risk of Myocardial Infarction in US Physicians. JAMA 1992, 268, 877–881. [Google Scholar] [CrossRef]
- Perry, I.; Morris, R.; Ebrahim, S.; Shaper, A.; Refsum, H.; Ueland, P. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 1995, 346, 1395–1398. [Google Scholar] [CrossRef]
- Price, B.R.; Wilcock, D.M.; Weekman, E.M. Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front. Aging Neurosci. 2018, 10, 350. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and dementia: An international consensus statement. J. Alzheimer Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R.; Impellizzeri, D. Involvements of hyperhomocysteinemia in neurological disorders. Metabolites 2021, 11, 37. [Google Scholar] [CrossRef]
- Le Stunff, H.; Véret, J.; Kassis, N.; Denom, J.; Meneyrol, K.; Paul, J.-L.; Cruciani-Guglielmacci, C.; Magnan, C.; Janel, N. Deciphering the link between hyperhomocysteinemia and ceramide metabolism in Alzheimer-type neurodegeneration. Front. Neurol. 2019, 10, 807. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Sultan, S. Homocysteine, the cholesterol of the 21st century. Impact of hyperhomocysteinemia on patency and amputation-free survival after intervention for critical limb ischemia. J. Endovasc. Ther. 2008, 15, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Badri, S.; Vahdat, S.; Seirafian, S.; Pourfarzam, M.; Gholipur-Shahraki, T.; Ataei, S. Homocysteine-Lowering Interventions in Chronic Kidney Disease. J. Res. Pharm. Pract. 2021, 10, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Keskin, A.; Ustun, G.U.; Aci, R.; Duran, U. Homocysteine as a marker for predicting disease severity in patients with COVID-19. Biomark. Med. 2022, 16, 559–568. [Google Scholar] [CrossRef]
- Oksuz, M.; Yilmaz, E. C677T methylenetetrahydrofolate reductase polymorphism, folate and homocysteine levels and the risk of colorectal cancer. Ann. Med. Res. 2021, 28, 1620. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Wu, Y.; Ming, W.-K.; Wang, Z.; Kjaergaard, M.N.; Ellervik, C. Homocysteine and female fertility, pregnancy loss and offspring birthweight: A two-sample Mendelian randomization study. Eur. J. Clin. Nutr. 2022, 76, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed homocysteine metabolism is associated with cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fouda, E.M.; Wahba, N.S.; Elsharawy, A.I.M.; Ishak, S.R. Serum homocysteine level in pediatric patients with COVID-19 and its correlation with the disease severity. Pediatr. Pulmonol. 2022, 57, 1701–1708. [Google Scholar] [CrossRef]
- Rossi, S.; Rossi, G.; Giordano, A.; Paltrinieri, S. Homocysteine measurement by an enzymatic method and potential role of homocysteine as a biomarker in dogs. J. Vet. Diagn. Investig. 2008, 20, 644–649. [Google Scholar] [CrossRef]
- Giraldi, M.; Paltrinieri, S.; Curcio, C.; Scarpa, P. Serum concentration of homocysteine in spontaneous feline chronic kidney disease. Vet. J. 2019, 254, 105358. [Google Scholar] [CrossRef]
- Gołyński, M.; Lutnicki, K.; Krumrych, W.; Szczepanik, M.; Gołyńska, M.; Wilkołek, P.; Adamek, Ł.; Sitkowski, Ł.; Kurek, Ł. Relationship between Total Homocysteine, Folic Acid, and Thyroid Hormones in Hypothyroid Dogs. J. Vet. Intern. Med. 2017, 31, 1403–1405. [Google Scholar] [CrossRef]
- Benvenuti, E.; Pierini, A.; Gori, E.; Bottero, E.; Pietra, M.; Lippi, I.; Meucci, V.; Marchetti, V. Serum homocysteine concentration in dogs with immunosuppressant-responsive enteropathy. J. Vet. Sci. 2020, 21, e47. [Google Scholar] [CrossRef] [PubMed]
- Bamashmoos, S.A.; Al-Nuzaily, M.A.K.; Al-Meeri, A.M.; Ali, F.H.H. Relationship between total homocysteine, total cholesterol and creatinine levels in overt hypothyroid patients. SpringerPlus 2013, 2, 423. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska-Pawilojc, A.; Siekierska-Hellmann, M.; Syrenicz, A.; Sworczak, K. Homocysteine, folate, and cobalamin levels in hyperthyroid women before and after treatment. Endokrynol. Pol. 2009, 60, 443–448. [Google Scholar] [PubMed]
- Den Heijer, M.; Lewington, S.; Clarke, R. Homocysteine, MTHFR and risk of venous thrombosis: A meta-analysis of published epidemiological studies. J. Thromb. Haemost. 2005, 3, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Cao, X.; Liu, C.; Xie, Y. Association between plasma homocysteine status and hypothyroidism: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 4544–4553. [Google Scholar] [PubMed]
- Yang, R.; Pu, D.; Tan, R.; Wu, J. Association of methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms (C677T and A1298C) with thyroid dysfunction: A meta-analysis and trial sequential analysis. Arch. Endocrinol. Metab. 2022, 66, 551–581. [Google Scholar] [CrossRef]
- Moll, S.; Varga, E.A. Homocysteine and MTHFR Mutations. Circulation 2015, 132, e6–e9. [Google Scholar] [CrossRef]
- Morris, M.S.; Bostom, A.G.; Jacques, P.F.; Selhub, J.; Rosenberg, I.H. Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis 2001, 155, 195–200. [Google Scholar] [CrossRef]
- Audet, I.; Girard, C.; Lessard, M.; Lo Verso, L.; Beaudoin, F.; Matte, J. Homocysteine metabolism, growth performance, and immune responses in suckling and weanling piglets. J. Anim. Sci. 2015, 93, 147–157. [Google Scholar] [CrossRef]
- França, L.H.G.; Pereira, A.H.; Perini, S.C.; Aveline, C.C.; Argenta, R.; Mollerke, R.d.O.; Soares, M.E.; Nóbrega, F.; Ferreira, M.P. Atherogenesis in swine iliac artery with homocystinemia induced by methionine ingestion. J. Vasc. Bras. 2006, 5, 11–16. [Google Scholar] [CrossRef]
- Razavi, S.; Moghaddas, B.; Rakhshande, E.; Nazifi, S. Bovine Theileriosis: Effects on the Status of Thyroid Hormones, Homocystein, Serum Lipids and Lipoproteins. Res. J. Parasitol. 2015, 10, 151–159. [Google Scholar]
- Stangl, G.I.; Schwarz, F.J.; Müller, H.; Kirchgessner, M. Evaluation of the cobalt requirement of beef cattle based on vitamin B12, folate, homocysteine and methylmalonic acid. Br. J. Nutr. 2000, 84, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, M.; Batista, E.D.; Titgemeyer, E.C. Effect of post-ruminal guanidinoacetic acid supplementation on creatine synthesis and plasma homocysteine concentrations in cattle. J. Anim. Sci. 2020, 98, skaa072. [Google Scholar] [CrossRef] [PubMed]
- Denizhan, V.; Kozat, S.; Özkan, C. Evaluation of Cobalt, Vitamin B-12 and Homocystein levels in Cattle infected with Theileria annulata. J. Livest. Sci. 2017, 8, 72–76. [Google Scholar]
- Giammarino, A.; Robbe, D.; Dainese, E.; Minoia, R.; Sciorsci, R. Mare embryonic resorption and homocysteine. Vet. Res. Commun. 2003, 27, 607–609. [Google Scholar] [CrossRef]
- Berhane, Y.; Bailey, S.R.; Harris, P.A.; Griffiths, M.J.; Elliott, J. In vitro and in vivo studies of homocysteine in equine tissues: Implications for the pathophysiology of laminitis. Equine Vet. J. 2004, 36, 279–284. [Google Scholar] [CrossRef]
- Fazio, F.; Casella, S.; Giannetto, C.; Caola, G.; Piccione, G. Serum homocysteine and oxidative stress evaluation during exercise in horse. Pol. J. Vet. Sci. 2009, 12, 169–174. [Google Scholar]
- Fazio, F.; Piccione, G.; Casella, S.; Assenza, A.; Messina, V.; Caola, G. Influence of Acute Exercise on Serum Homocysteine in Horse. J. Equine Vet. Sci. 2010, 30, 39–43. [Google Scholar] [CrossRef]
- Mitchell, K.J.; De Clercq, D.; Stirn, M.; van Loon, G.; Schwarzwald, C.C. Plasma homocysteine concentrations in healthy horses and horses with atrial fibrillation. J. Vet. Cardiol. 2018, 20, 276–284. [Google Scholar] [CrossRef]
- Ahmadpour, S.; Esmaeilnejad, B.; Dalir-Naghadeh, B.; Asri-Rezaei, S. Alterations of cardiac and renal biomarkers in horses naturally infected with Theileria equi. Comp. Immunol. Microbiol. Infect. Dis. 2020, 71, 101502. [Google Scholar] [CrossRef]
- Arfuso, F.; Rizzo, M.; Giannetto, C.; Giudice, E.; Cirincione, R.; Cassata, G.; Cicero, L.; Piccione, G. Oxidant and Antioxidant Parameters’ Assessment Together with Homocysteine and Muscle Enzymes in Racehorses: Evaluation of Positive Effects of Exercise. Antioxidants 2022, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.W. Homocysteine and vitamins in cardiovascular disease. Clin. Chem. 1998, 44 Pt 2, 1833–1843. [Google Scholar] [CrossRef]
- Harpel, P.C.; Zhang, X.; Borth, W. Homocysteine and hemostasis: Pathogenic mechanisms predisposing to thrombosis. J. Nutr. 1996, 126 (Suppl. S4), 1285s–1289s. [Google Scholar] [CrossRef]
- Ling, Q.; Hajjar, K.A. Inhibition of endothelial cell thromboresistance by homocysteine. J. Nutr. 2000, 130 (Suppl. S2), 373s–376s. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liu, J.; Zhao, J.; Mao, J.; Zhang, X.; Feng, L.; Han, C.; Li, M.; Wang, S.; Wu, D. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014, 236, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.; Verdoia, M.; Cassetti, E.; Marino, P.; Suryapranata, H.; De Luca, G.; Group, N.A.S. Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb. Res. 2014, 134, 288–293. [Google Scholar] [CrossRef]
- Mangge, H.; Becker, K.; Fuchs, D.; Gostner, J.M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 2014, 6, 462. [Google Scholar] [CrossRef]
- Okura, T.; Miyoshi, K.-i.; Irita, J.; Enomoto, D.; Nagao, T.; Kukida, M.; Tanino, A.; Kudo, K.; Pei, Z.; Higaki, J. Hyperhomocysteinemia is one of the risk factors associated with cerebrovascular stiffness in hypertensive patients, especially elderly males. Sci. Rep. 2014, 4, srep05663. [Google Scholar] [CrossRef]
- Zhang, S.; Bai, Y.-Y.; Luo, L.-M.; Xiao, W.-K.; Wu, H.-M.; Ye, P. Association between serum homocysteine and arterial stiffness in elderly: A community-based study. J. Geriatr. Cardiol. JGC 2014, 11, 32. [Google Scholar]
- Graeber, J.E.; Slott, J.H.; Ulane, R.E.; Schulman, J.D.; Stuart, M.J. Effect of Homocysteine and Homocystine on Platelet and Vascular Arachidonic Acid Metabolism. Pediatr. Res. 1982, 16, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Mohan, I.V.; Jagroop, I.A.; Mikhailidis, D.P.; Stansby, G.P. Homocysteine Activates Platelets In Vitro. Clin. Appl. Thromb. Hemost. 2008, 14, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Selhub, J.; Miletich, J.P.; Malinow, M.R.; Stampfer, M.J. Interrelation of Hyperhomocyst(e)inemia, Factor V Leiden, and Risk of Future Venous Thromboembolism. Circulation 1997, 95, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Honda, G.; Suzuki, K. An atherogenic stimulus homocysteine inhibits cofactor activity of thrombomodulin and enhances thrombomodulin expression in human umbilical vein endothelial cells. Blood 1992, 79, 2930–2936. [Google Scholar] [CrossRef]
- Rodgers, G.; Kane, W. Activation of endogenous factor V by a homocysteine-induced vascular endothelial cell activator. J. Clin. Investig. 1986, 77, 1909–1916. [Google Scholar] [CrossRef]
- Lentz, S.; Sadler, J.E. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. J. Clin. Investig. 1991, 88, 1906–1914. [Google Scholar] [CrossRef]
- Palareti, G.; Coccheri, S. Lowered antithrombin III activity and other clotting changes in homocystinuria: Effects of a pyridoxine-folate regimen. Pathophysiol. Haemost. Thromb. 1989, 19 (Suppl. S1), 24–28. [Google Scholar] [CrossRef]
- Mandaviya, P.R.; Stolk, L.; Heil, S.G. Homocysteine and DNA methylation: A review of animal and human literature. Mol. Genet. Metab. 2014, 113, 243–252. [Google Scholar] [CrossRef]
- Jamaluddin, M.D.; Chen, I.; Yang, F.; Jiang, X.; Jan, M.; Liu, X.; Schafer, A.I.; Durante, W.; Yang, X.; Wang, H. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene. Blood 2007, 110, 3648–3655. [Google Scholar] [CrossRef]
- Barroso, M.; Handy, D.E.; Castro, R. The Link Between Hyperhomocysteinemia and Hypomethylation:Implications for Cardiovascular Disease. J. Inborn Errors Metab. Screen. 2017, 5, 2326409817698994. [Google Scholar] [CrossRef]
- Yi, P.; Melnyk, S.; Pogribna, M.; Pogribny, I.P.; Hine, R.J.; James, S.J. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J. Biol. Chem. 2000, 275, 29318–29323. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yoshizumi, M.; Lai, K.; Tsai, J.-C.; Perrella, M.A.; Haber, E.; Lee, M.-E. Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. J. Biol. Chem. 1997, 272, 25380–25385. [Google Scholar] [CrossRef] [PubMed]
- Bajic, Z.; Sobot, T.; Skrbic, R.; Stojiljkovic, M.P.; Ponorac, N.; Matavulj, A.; Djuric, D.M. Homocysteine, Vitamins B6 and Folic Acid in Experimental Models of Myocardial Infarction and Heart Failure-How Strong Is That Link? Biomolecules 2022, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- O’Suilleabhain, P.E.; Sung, V.; Hernandez, C.; Lacritz, L.; Dewey, R.B., Jr.; Bottiglieri, T.; Diaz-Arrastia, R. Elevated plasma homocysteine level in patients with Parkinson disease: Motor, affective, and cognitive associations. Arch. Neurol. 2004, 61, 865–868. [Google Scholar] [CrossRef] [PubMed]
- McCaddon, A. Homocysteine and cognitive impairment; a case series in a General Practice setting. Nutr. J. 2006, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Zanibbi, K. Homocysteine and cognitive function in elderly people. Can. Med. Assoc. J. 2004, 171, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Zoccolella, S.; Lamberti, P.; Armenise, E.; de Mari, M.; Lamberti, S.V.; Mastronardi, R.; Fraddosio, A.; Iliceto, G.; Livrea, P. Plasma homocysteine levels in Parkinson’s disease: Role of antiparkinsonian medications. Park. Relat. Disord. 2005, 11, 131–133. [Google Scholar] [CrossRef]
- Zoccolella, S.; Martino, D.; Defazio, G.; Lamberti, P.; Livrea, P. Hyperhomocysteinemia in movement disorders: Current evidence and hypotheses. Curr. Vasc. Pharmacol. 2006, 4, 237–243. [Google Scholar] [CrossRef]
- Martignoni, E.; Tassorelli, C.; Nappi, G.; Zangaglia, R.; Pacchetti, C.; Blandini, F. Homocysteine and Parkinson’s disease: A dangerous liaison? J. Neurol. Sci. 2007, 257, 31–37. [Google Scholar] [CrossRef]
- Zhou, H.; Zhong, X.; Chen, B.; Wang, Q.; Zhang, M.; Mai, N.; Wu, Z.; Huang, X.; Chen, X.; Peng, Q.; et al. Elevated homocysteine levels, white matter abnormalities and cognitive impairment in patients with late-life depression. Front. Aging Neurosci. 2022, 14, 951560. [Google Scholar] [CrossRef]
- Kocer, B.; Guven, H.; Comoglu, S.S. Homocysteine Levels in Parkinson’s Disease: Is Entacapone Effective? Biomed. Res. Int. 2016, 2016, 7563705. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Ladenheim, B.; Cutler, R.G.; Kruman, I.I.; Cadet, J.L.; Mattson, M.P. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem. 2002, 80, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Shcherbitskaia, A.D.; Vasilev, D.S.; Milyutina, Y.P.; Tumanova, N.L.; Mikhel, A.V.; Zalozniaia, I.V.; Arutjunyan, A.V. Prenatal Hyperhomocysteinemia Induces Glial Activation and Alters Neuroinflammatory Marker Expression in Infant Rat Hippocampus. Cells 2021, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef]
- Perna, A.; Ingrosso, D.; De Santo, N. Homocysteine and oxidative stress. Amino Acids 2003, 25, 409–417. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef]
- Su, J.H.; Anderson, A.J.; Cummings, B.J.; Cotman, C.W. Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport 1994, 5, 2529–2533. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Varshney, K.K.; Gupta, J.K.; Mujwar, S. Homocysteine induced neurological dysfunctions: A link to neurodegenerative disorders. Int. J. Med. Res. Health Sci. 2019, 8, 135–146. [Google Scholar]
- Wang, Q.; Zhao, J.; Chang, H.; Liu, X.; Zhu, R. Homocysteine and Folic Acid: Risk Factors for Alzheimer’s Disease—An Updated Meta-Analysis. Front. Aging Neurosci. 2021, 13, 665114. [Google Scholar] [CrossRef] [PubMed]
- Pi, T.; Liu, B.; Shi, J. Abnormal homocysteine metabolism: An insight of Alzheimer’s disease from DNA methylation. Behav. Neurol. 2020, 2020, 8438602. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.-M.; Wang, H.; Praticò, D. Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol. Sci. 2011, 32, 562–571. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Luzzi, S.; Papiri, G.; Viticchi, G.; Baldinelli, S.; Fiori, C.; Silvestrini, M.; Toraldo, A. Association between homocysteine levels and cognitive profile in Alzheimer’s Disease. J. Clin. Neurosci. 2021, 94, 250–256. [Google Scholar] [CrossRef]
- Tyagi, N.; Gillespie, W.; Vacek, J.C.; Sen, U.; Tyagi, S.C.; Lominadze, D. Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 activation by ERK pathway. J. Cell. Physiol. 2009, 220, 257–266. [Google Scholar] [CrossRef]
- Lominadze, D.; Tyagi, N.; Sen, U.; Ovechkin, A.; Tyagi, S.C. Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor. Mol. Cell. Biochem. 2012, 371, 89–96. [Google Scholar] [CrossRef]
- Jiménez-Balado, J.; Eich, T.S. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin. Cell. Dev. Biol. 2021, 116, 146–159. [Google Scholar] [CrossRef]
- Ghanizadeh, A. Increased glutamate and homocysteine and decreased glutamine levels in autism: A review and strategies for future studies of amino acids in autism. Dis. Markers 2013, 35, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Numata, S.; Kinoshita, M.; Tajima, A.; Nishi, A.; Imoto, I.; Ohmori, T. Evaluation of an association between plasma total homocysteine and schizophrenia by a Mendelian randomization analysis. BMC Med. Genet. 2015, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Gorgone, G.; Caccamo, D.; Pisani, L.R.; Currò, M.; Parisi, G.; Oteri, G.; Ientile, R.; Rossini, P.M.; Pisani, F. Hyperhomocysteinemia in patients with epilepsy: Does it play a role in the pathogenesis of brain atrophy? A preliminary report. Epilepsia 2009, 50, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A.; Singh, A.B.; Berk, M.; Torabi-Nami, M. Homocysteine as a potential biomarker in bipolar disorders: A critical review and suggestions for improved studies. Expert. Opin. Ther. Targets 2015, 19, 927–939. [Google Scholar] [CrossRef]
- Fortin, J.S.; Hetak, A.A.; Duggan, K.E.; Burglass, C.M.; Penticoff, H.B.; Schott, H.C., 2nd. Equine pituitary pars intermedia dysfunction: A spontaneous model of synucleinopathy. Sci. Rep. 2021, 11, 16036. [Google Scholar] [CrossRef]
- Miller, M.M.; Collatos, C. Equine degenerative myeloencephalopathy. Vet. Clin. N. Am. Equine Pract. 1997, 13, 43–52. [Google Scholar] [CrossRef]
- De la Rua-Domenech, R.; Mohammed, H.; Cummings, J.; Divers, T.; De Lahunta, A.; Summers, B. Association between plasma vitamin E concentration and the risk of equine motor neuron disease. Vet. J. 1997, 154, 203–213. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Geor, R.J. The horse as an athlete: A physiological overview. Equine Exerc. Physiol. Sci. Exerc. Athl. Horse 2008, 454, 454. [Google Scholar]
- Goodwin, D. Horse Behaviour: Evolution, Domestication and Feralisation. In The Welfare of Horses; Waran, N., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–18. [Google Scholar]
- Art, T.; Lekeux, P. Ventilatory and arterial blood gas tension adjustments to strenuous exercise in standardbreds. Am. J. Vet. Res. 1995, 56, 1332–1337. [Google Scholar]
- Poole, D.C.; Erickson, H.H. Highly athletic terrestrial mammals: Horses and dogs. Compr. Physiol. 2011, 1, 1–37. [Google Scholar]
- Wagner, P.D.; Gillespie, J.R.; Landgren, G.L.; Fedde, M.R.; Jones, B.W.; DeBowes, R.M.; Pieschl, R.L.; Erickson, H.H. Mechanism of exercise-induced hypoxemia in horses. J. Appl. Physiol. 1989, 66, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.C.; Smith, N.C.; Casas, I.; Harris, P.; Harris, R.C.; Marlin, D.J. Effects of exercise intensity and environmental stress on indices of oxidative stress and iron homeostasis during exercise in the horse. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Marlin, D.J.; Fenn, K.; Smith, N.; Deaton, C.D.; Roberts, C.A.; Harris, P.A.; Dunster, C.; Kelly, F.J. Changes in circulatory antioxidant status in horses during prolonged exercise. J. Nutr. 2002, 132, 1622S–1627S. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, E.; Avellini, L.; Rueca, F.; Spaterna, A.; Porciello, F.; Antonioni, M.; Gaiti, A. Physical exercise, oxidative stress and muscle damage in racehorses. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1998, 119, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Tofas, T.; Draganidis, D.; Deli, C.K.; Georgakouli, K.; Fatouros, I.G.; Jamurtas, A.Z. Exercise-Induced Regulation of Redox Status in Cardiovascular Diseases: The Role of Exercise Training and Detraining. Antioxidants 2019, 9, 13. [Google Scholar] [CrossRef]
- Maroto-Sánchez, B.; Lopez-Torres, O.; Palacios, G.; González-Gross, M. What do we know about homocysteine and exercise? A review from the literature. Clin. Chem. Lab. Med. 2016, 54, 1561–1577. [Google Scholar] [CrossRef]
- Iglesias-Gutiérrez, E.; García-González, Á.; Montero-Bravo, A.; González-Medina, A.; Joglar, J.; Tomás-Zapico, C.; Fernández-García, B.; Fernández-Sanjurjo, M.; de Gonzalo-Calvo, D.; Díaz-Martínez, Á.E.; et al. Exercise-Induced Hyperhomocysteinemia Is Not Related to Oxidative Damage or Impaired Vascular Function in Amateur Middle-Aged Runners under Controlled Nutritional Intake. Nutrients 2021, 13, 3033. [Google Scholar] [CrossRef]
- Joubert, L.M.; Manore, M.M. The role of physical activity level and B-vitamin status on blood homocysteine levels. Med. Sci. Sport. Exerc. 2008, 40, 1923–1931. [Google Scholar] [CrossRef]
- Borrione, P.; Rizzo, M.; Spaccamiglio, A.; Salvo, R.A.; Dovio, A.; Termine, A.; Parisi, A.; Fagnani, F.; Angeli, A.; Pigozzi, F. Sport-related hyperhomocysteinaemia: A putative marker of muscular demand to be noted for cardiovascular risk. Br. J. Sport. Med. 2008, 42, 894–900. [Google Scholar] [CrossRef]
- Hodgson, D.R.; Rose, R.J. The Athletic Horse: Principles and Practice of Equine Sports Medicine; Saunders: Philadephia, PA, USA, 1994. [Google Scholar]
- Mattson, M.P. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997, 77, 1081–1132. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, L.; Li, H.; Chen, G.; Qi, G.; Ma, X.; Jin, Y. Role of homocysteine in the development and progression of Parkinson’s disease. Ann. Clin. Transl. Neurol. 2020, 7, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Siow, Y.L.; Isaak, C.K.; Karmin, O. Downregulation of Glutathione Biosynthesis Contributes to Oxidative Stress and Liver Dysfunction in Acute Kidney Injury. Oxid. Med. Cell. Longev. 2016, 2016, 9707292. [Google Scholar] [CrossRef] [PubMed]
- Lawrence de Koning, A.B.; Werstuck, G.H.; Zhou, J.; Austin, R.C. Hyperhomocysteinemia and its role in the development of atherosclerosis. Clin. Biochem. 2003, 36, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.J.; Kirkby, K.; Malalana, F.; McGowan, C.M. Elevations in serum muscle enzyme activities in racehorses due to unaccustomed exercise and training. Vet. Rec. 2014, 174, 145. [Google Scholar] [CrossRef]
- Mann, S.; Ramsay, J.D.; Wakshlag, J.J.; Stokol, T.; Reed, S.; Divers, T.J. Investigating the pathogenesis of high-serum gamma-glutamyl transferase activity in Thoroughbred racehorses: A series of case-control studies. Equine Vet. J. 2022, 54, 39–51. [Google Scholar] [CrossRef]
- Welch, G.N.; Loscalzo, J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998, 338, 1042–1050. [Google Scholar] [CrossRef]
- Williams, K.T.; Schalinske, K.L. Homocysteine metabolism and its relation to health and disease. Biofactors 2010, 36, 19–24. [Google Scholar] [CrossRef]
- Demuth, K.; Ducros, V.; Michelsohn, S.; Paul, J.L. Evaluation of Advia Centaur automated chemiluminescence immunoassay for determining total homocysteine in plasma. Clin. Chim. Acta 2004, 349, 113–120. [Google Scholar] [CrossRef]
| Keywords | Publication Date | Reference |
|---|---|---|
| Embryonic resorption; mare | 2003 | [57] |
| Vascular endothelium; laminitis | 2004 | [58] |
| Reactive oxidant species (dROMs); antioxidant barrier (Oxy-adsorbent); thiol antioxidant barrier (SHp) | 2009 | [59] |
| Acute exercise; workload; serum lactate | 2010 | [60] |
| Cardiovascular; arrythmia; atrial fibrillation | 2018 | [61] |
| Cardio-renal biomarkers; parasitemia; Theileria equi | 2020 | [62] |
| Aspartate aminotransferase; creatine kinase; lactate dehydrogenase; oxidative stress | 2022 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołyński, M.; Metyk, M.; Ciszewska, J.; Szczepanik, M.P.; Fitch, G.; Bęczkowski, P.M. Homocysteine—Potential Novel Diagnostic Indicator of Health and Disease in Horses. Animals 2023, 13, 1311. https://doi.org/10.3390/ani13081311
Gołyński M, Metyk M, Ciszewska J, Szczepanik MP, Fitch G, Bęczkowski PM. Homocysteine—Potential Novel Diagnostic Indicator of Health and Disease in Horses. Animals. 2023; 13(8):1311. https://doi.org/10.3390/ani13081311
Chicago/Turabian StyleGołyński, Marcin, Michał Metyk, Jagoda Ciszewska, Marcin Paweł Szczepanik, Gareth Fitch, and Paweł Marek Bęczkowski. 2023. "Homocysteine—Potential Novel Diagnostic Indicator of Health and Disease in Horses" Animals 13, no. 8: 1311. https://doi.org/10.3390/ani13081311
APA StyleGołyński, M., Metyk, M., Ciszewska, J., Szczepanik, M. P., Fitch, G., & Bęczkowski, P. M. (2023). Homocysteine—Potential Novel Diagnostic Indicator of Health and Disease in Horses. Animals, 13(8), 1311. https://doi.org/10.3390/ani13081311





