Simple Summary
Our study was designed for the Salmonella diagnosis from chickens and calves, to determine its resistance to antimicrobials’ phenotypic and genotypic characterization of integrons and β lactamase genes in the multidrug resistance of different Salmonella serotypes, and to detect the genetic relationship between isolated Salmonella using ERIC PCR. Isolated poultry Salmonella were identified as S. Typhimurium, S. Enteritidis, and S. Kentucky, but isolated cattle Salmonella were S. Enteritidis and S. Kentucky. An RT PCR was applied for identifying the accompanying class 1 integrons and ESBLs from MDR Salmonella isolates (two isolates of S. Kentucky were divided as one from calf and one from poultry). Our results detected a blaTEM and class 1 integron, but were negative for bla IMP, bla VIM, and bla SHV. An ERIC PCR was conducted for understanding the clonal relationship among various β-lactamase-producing MDR Salmonella isolates. Two isolates of S. Enteritidis isolated from poultry and calves had 100% similarity, despite indicating that there were interactions between broilers and calves living on the same farm that caused infection from the same Salmonella strains, while the other two isolates of S. Kentucky showed only 33% serovarities.
Abstract
A prevalent bacterial intestinal infection with severe economic damage is salmonellosis. Our study was carried out to diagnose Salmonella from chickens and calves, to determine its resistance to antimicrobials’ phenotypic and genotypic characterization of integrons and β lactamase genes in the multidrug resistance of different Salmonella serotypes, and to detect the genetic relationship between Salmonella isolates collected from different origins using an ERIC PCR. In total, 200 samples from diseased chicken and diarrheic calves were obtained from 50 various farms from Kafr El-sheikh, Egypt. Salmonella poultry isolates were characterized as S. Typhimurium (3/8), S. Enteritidis (3/8), and S. Kentucky (2/8), but Salmonella isolates from cattle were S. Enteritidis (1/2) and S. Kentucky (1/2). When antibiotic susceptibility testing was completed on all of the isolates, it showed that there was multidrug resistance present (MDR). A PCR was applied for identifying the accompanying class 1 integrons and ESBLs from MDR Salmonella isolates (two isolates of S. Kentucky were divided as one from calf and one from poultry). Our results detected blaTEM and class 1 integron, but were negative for bla IMP, bla VIM, and bla SHV. An ERIC PCR was conducted for understanding the clonal relation between various β-lactamase-producing MDR Salmonella isolates. The same four previously mentioned isolates were also tested. The two isolates of S. Enteritidis isolated from poultry and calves had 100% similarity despite indicating that there were interactions between broilers and calves living on the same farm that caused infection from the same Salmonella strains, while the other two isolates of S. Kentucky showed only 33% serovarities.
Keywords:
Salmonella; broilers; calf; antimicrobial resistance; β-lactamases; integrons; ERIC PCR; zoonosis 1. Introduction
A frequent bacterial intestinal infection caused by Salmonella serovars is salmonellosis [1]. It affects both humans and the majority of animals, which facilitates transmission and cross-contamination [2]. Enterobacteriaceae, a family of rod-shaped, Gram-negative bacteria, includes Salmonella. Clinical manifestations can include people with acute or chronic enteritis or septicemia as well as healthy chronic carriers. Poultry has been recognized as one of the main sources for disseminating Salmonella infection to man and animals [3]. Salmonella species can cause a wide range of clinical signs in calves, including diarrhea and possible dysentery, joint infections, chronic pneumonia, and sudden death from septicaemia. Direct contact with cattle and/or eating foods containing cow’s milk can both cause salmonellosis in humans (milk and uncooked beef meat) [4]. However, the isolation of S. Typhimurium and S. Enteridis from calves and poultry in the same area is probably indicative of fecal contamination at the source. Salmonella can persist for several months in the farm environment, including in water [5]. Serotyping, which offers details about the severity of the illness, the source of contamination, and the resistance pattern, is a crucial tool for understanding the epidemiology of Salmonella infections [6].
The widespread issue of multidrug resistance (MDR), which is encoded by resistance genes on integrons, is a serious one. Mobile DNA fragments known as integrons play a crucial part in the transmission of antibiotic resistance genes in Gram-negative bacteria [7]. The site-specific recombinase responsible for cassette insertion is encoded by the inte-grase gene (intI1), which is present in the majority of the class 1 integrons seen in clinical isolates [8,9]. The attI1 site on the integrase gene is where the cassettes are integrated, and Pc is the promoter that triggers the transcription of the cassette-encoded genes [8,10]. Whereas a number of the most recently discovered ESBL genes are typically figured out within integron-like structures including blaTEM or blaSHV, ESBL-encoding genes are typically figured out on conjugative plasmids (such as blaTEM or blaSHV) (blaCTX-M, blaGES, or blaVEB-1) [11,12]. Contrarily, isolates that produce ESBLs frequently exhibit resistant to certain other antibiotics, such as aminoglycosides, tetracyclines, chloramphenicol, trimethoprim, sulfonamides, or quinolones. A genetic structure created by the synergistic generation of diverse interactions includes different resistance genes on plasmids, transposons, or integrons as transferable elements or genetic structures [12,13]. The dissemination of these genetic elements can be aided by the existence of ESBL genes on integrons [11,14].
Acknowledging the clonal relationship between different lactamase-producing MDR Salmonella isolates using the enterobacteria repetitive intergenic consensus (ERIC) polymerase chain reaction (PCR) strain is crucial for both identifying the source of infection and preventing the spread of nosocomial pathogens. The cross-infection between animal and poultry species by Salmonella may be accrued through the ingestion of contaminated water and food [15]. Therefore, our investigation focused on the isolation and identification of Salmonella serovars from diseased chicken and diarrheic calves and declaring associated antimicrobial resistance profiles: the characterization of β-lactamase-encoding genes (blaTEM, bla IMP, bla VIM, and bla SHV), and class 1 integron (int) in addition to identifying the genetic relation among the same Salmonella serovar but from various origins using (ERIC PCR).
2. Materials and Methods
2.1. Samples Collection
Two hundred samples (one hundred fecal and rectal swabs from diarrheic calves aging up to 3 months, and one hundred samples from freshly dead broiler chickens suspected to be infected with Salmonella) were transported immediately in an ice box to the lab for bacteriological examination for Salmonella (Table 1). They were enriched in peptone water at 37 °C at 24 h for non-selective pre-enrichment then transferred onto 10 mL of Rappaport–Vassiliadis broth for selective enrichment. This broth was incubated aerobically at 42 °C for 18 h. A loopful from the culture was streaked into the surface of MacConkey agar for 24 h at 37 °C which was selective for Enterobacteriaceae. Colorless colonies on MacConkey agar were inoculated into the Xylose lysine deoxycholate (X.L.D.) surface and Salmonella-Shigella agar (S.S) media for selective plating. The inoculated plates were incubated at 37 °C for 24–48 h. Suspected colonies were characterized morphologically according to [16]. These colonies were picked up and sub-cultured, and a loopful of each pure culture was inoculated into the nutrient slope and semisolid agar tube for further biochemical and serological identification.

Table 1.
Samples taken for Salmonella isolation.
2.2. Biochemical Identification
It was done in accordance with [16,17,18,19]. All suspected Salmonella colonies were selected from the agar plates and inoculated onto the following biochemical test tubes for confirmation: the triple sugar iron (TSI) test (suspicious Salmonella colonies are thought to produce colonies that are black or have black centers and red medium on TSI agar; OXOID, Hampshire, UK), the citrate test (suspicious Salmonella colonies are thought to produce colonies that are blue in color for the citrate test), and the urease test (sus at 37 °C, plates were incubated for 24 or 48 h).
2.3. Serological Identification
A total of 10 Salmonella isolates (2 isolates from poultry and 8 from calves) were identified morphologically and biochemically as Salmonella were subjected to serological identification according to the Kauffman-white scheme [20] using the slide agglutination technique with the polyvalent somatic (O) and flagellar (H) antisera (Welcome Diagnostic, Birmingham, UK).
2.4. Antimicrobial Sensitivity Testing (Phenotypic Resistance)
The susceptibility of Salmonella isolates to different antimicrobial agents was discovered by the Kirby–Bauer method (Disc diffusion test) based on the criteria of the Clinical and Laboratory Standards Institute (CLSI, 2011). A total of 14 antimicrobials were chosen, according to their common usage for treatment and prevention of the Salmonella infection in poultry and humans, and they were used for sensitivity tests of 10 isolated S. enterica. The antimicrobial discs included ampicillin (AMP 10 Mg), Ciprofloxacin (CIP 5 Mg), cefotaxime (CTX 30 Mg), Chloramphenicol (C 30 Mg), Streptomycin (STR 10 Mg), nalidixic acid (NAL 30 Mg), trimethoprim-Sulfamethoxazole (SXT 25 Mg), and tetracycline (TE 30 Mg), Enrofloxacin (ENR 5 Mg), Vancomycin (VA 30 Mg), Kanamycin (KAN 30 Mg), ceftriaxone (CRO 30 Mg), Erthyomycin (E 15 Mg), and Amoxicillin (AMC 30 Mg).
2.5. Molecular Identification of B-Lactamase Encoding Genes, Class 1 Integron, and ERIC PCR of Isolated Salmonella
We modified the manufacturer’s instructions for the QIAamp DNA Mini kit (Qiagen, Hilden, Germany, GmbH) to extract DNA from the samples (Table 2). Briefly, three to five representative colonies of the same morphological type were taken from the slants of the previously isolated bacteria and enriched into a tube containing 2 mL of tryptic soya broth (TSB) for 18 h at 37 °C. Then, 200 µL of the sample suspension was incubated with 10 µL of proteinase K and 200 µL of lysis buffer at 56 °C for 10 min. After incubation, 200 µL of 100% ethanol was added to the lysate. Then, the sample was washed and centrifuged according to the manufacturer’s recommendations. Nucleic acid was eluted with 100 µL of elution buffer provided in the kit.

Table 2.
Sequencing of primers, target genes, sizes of amplicons, and cycling conditions.
PCR reaction primers were used in a 25 µL reaction and had 12.5 µL of EmeraldAmp Max PCR Master Mix (Takara, Shiga, Japan), 1 µL of each primer of 20 pmol concentrations, 5.5 µL of water, and 5 µL of the DNA template. The reaction was performed in an applied biosystem 2720 thermal cycler. The products of PCR were separated by electrophoresis on 1.5% agarose gel (Applichem GmbH, Darmstadt, Germany) in 1x TBE buffer at room temperature using gradients of 5V/cm. For the gel analysis, 20 µL of the multiplex PCR products were loaded in each gel slot. Gelpilot 100 bp DNA ladder (Qiagen GmbH, Hilden, Germany) and generuler 100 bp ladder (Fermentas, Thermo, Hamburg, Germany) were used to determine the fragment sizes. The gel was photographed by a gel documentation system (Alpha Innotech, Biometra, San Leandro, CA, USA), and the data were analyzed by computer software. ERIC fingerprinting data were transformed into a binary code depending on the presence or absence of each band. Dendrograms were generated by the unweighted pair group method with arithmetic average (UPGMA) and Ward’s hierarchical clustering routine. A similarity index (Jaccard/Tanimoto Coefficient and the number of intersecting elements) between all samples was calculated using the online tool (https://planetcalc.com/1664/, accessed on 21 May 2022).
3. Results
3.1. The Prevalence of Salmonella Isolated from Different Spp.
In this study, Salmonella enterica isolates were obtained from chickens and calves representing commonly isolated serotypes in Egypt. The overall incidence of Salmonella, in this investigation, was 2% in the chickens’ samples and 8% in the calves’ samples (Table 3).

Table 3.
Prevalence of Salmonella among the tested samples.
3.2. Serological Identification of Salmonella Isolates
Concerning serotyping, there were three Salmonella isolates identified which included S. Kentucky, S. Typhimurium, and S. Enteritidis from all isolates as shown in Table 4. Three Salmonella serovars were identified from poultry as (S. Kentucky (2/8), S.Typhimurium (3/8), and S. Enteritidis (3/8)), while in cattle there were two serovars identified serologically, S. Kentucky (1/2) and S. Enteritidis (1/2) as shown in Table 5.

Table 4.
Serological identification of S. Enterica.

Table 5.
The prevalence rates of isolated Salmonella serotypes from diseased poultry and calves.
3.3. The Antimicrobial Resistance
As shown in Table 6, the highest antibiotic resistances belonged to Macrolides antibiotics Erythromycin 100%, Vancomycin 90%, B-lactamase penicillin, Ampicillin 90%, Amoxicillin 80%, and quinolones nalidixic acid 90%, despite, the fact that it showed variable resistance to Aminoglycosides antibiotics Streptomycin 60%, Kanamycin 40%, Sulphonamides, antibiotics trimethoprim-Sulfamethoxazole 40%, and tetracycline 40%. Salmonella isolates showed sensitivity to cephalosporin ceftriaxone 60%, and Phenicols antibiotic Chloramphenicol 60%. In this study, most of the Salmonella isolates showed multidrug-resistance of 80% to ≥5 of the fourteen antimicrobials used.

Table 6.
Incidence of antimicrobial sensitivity test (AST) in S. Enterica.
3.4. Prevalence of β-Lactamase-Encoding Genes in Salmonella Isolation
A uniplex PCR was applied for the discovery of bla TEM, bla SHV, bla VIM, and bla IMP. It was exhibited that the four isolates (two S. Enteritidis and two S. Kentucky) tested genetically have obtained bla TEM amplicon (516 bp) with a percentage of 100%. As shown in (Figure 1), isolates 1, 2, 3, and 4 were positive for the blaTEM gene (516 bp) but negative for the blaSHV gene (392 bp) and blaVIM gene (280 bp).
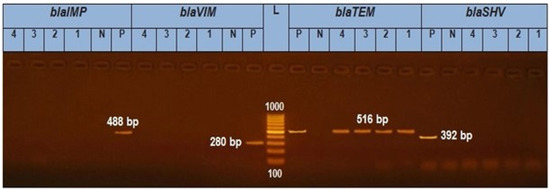
Figure 1.
In total, 1.5% Agarose gel electrophoresis for PCR results of blaTEM gene in Salmonella isolates (516 bp), blaVIM (280 bp), blaIMP (488), and blaSHV (392 bp). L is a 1000 bp ladder used as a size marker. P is controlled positively. N is controlled negatively.
3.5. Prevalence of Class 1 Integrons for MDR Salmonella
In our investigation, the PCR screening results detected amplified products of int1 amplicon (491 bp) with a percentage of 100%, as shown in Figure 2 and Figure 3.
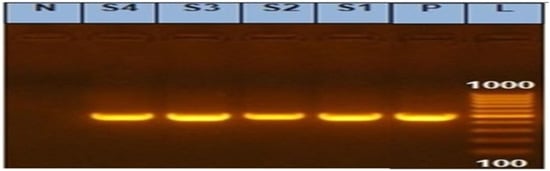
Figure 2.
Agarose gel showing amplified products of integrase gene (491 bp) in Salmonella isolates. N: Control Negative; P: Control Positive; L: DNA ladder 100 bp; and Lanes (S1, S2, S3, and S4) examined Salmonella isolates (S1: S. Kentucky from calf and S2: S. Kentucky from chicken) and (S3: S. Entertidis from chicken and S4: S. Enteritidis from the calf).
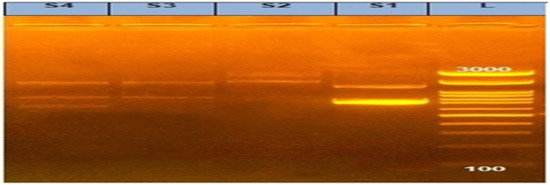
Figure 3.
Agarose gel showing amplified products of variable integrons in Salmonella isolates. L: DNA ladder 100 bp and Lanes (S1, S2, S3, and S4) examined Salmonella isolates (S1: S. Kentucky from calf and S2: S. Kentucky from chicken) and (S3: S. Entertidis from chicken and S4: S. Enteritidis from the calf).
3.6. ERIC PCR
It was found that two S. Enteritidis isolates (one from cattle and the other one from chicken) had 100% similarity according to (Jaccard/Tanimoto coefficient and the number of intersecting elements), despite the fact that two S. Kentucky isolates (one from cattle and the other one from chicken) had 33% similarities as shown in Table 7 and Figure 4 and Figure 5. Dendrograms were produced using 234 Ward’s hierarchical clustering procedure and the unweighted pair group technique with arithmetic average (UPGMA), as shown in Table 8 and Figure 6 and Figure 7.

Table 7.
The Jaccard/Tanimoto coefficient and the number of intersecting elements for every 2 samples with each other.
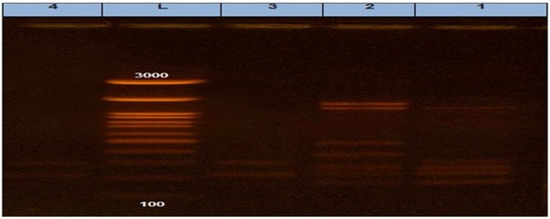
Figure 4.
ERIC PCR showing different bands in each isolate.

Figure 5.
ERIC bands (measured by BioDocAnalyze software for installation and image acquisition on the computer).

Table 8.
Agglomeration schedule of dendrograms showing genetic relationships among Salmonella serovars based on ERIC PCR. The similarity was calculated from the coefficient by the UPGMA.
Figure 6.
Dendrograms were generated by the unweighted pair group method with arithmetic average (UPGMA) and Ward’s hierarchical clustering routine. Similarity index (Jaccard/Tanimoto coefficient and the number of intersecting elements) between all samples were calculated using the online tool (https://planetcalc.com/1664/, accessed on 21 May 2022).
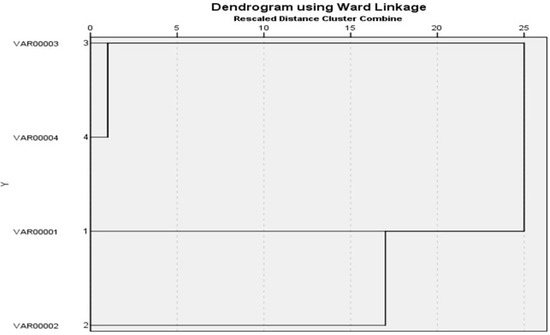
Figure 7.
Dendrograms were generated by the unweighted pair group method with arithmetic average (UPGMA) and Ward’s hierarchical clustering routine. Similarity index (Jaccard/Tanimoto coefficient and the number of intersecting elements) between all samples were calculated using the online tool (https://planetcalc.com/1664/, accessed on 21 May 2022).
4. Discussion
One of the most widely prevalent causes of both human and animal food-borne diarrheal illness is Salmonella [27]. The majority of the 2500 Salmonella serovars have a wide range of hosts and can infect a variety of hosts [28]. According to the nation, the type of manufacturing method, and the particular control mechanisms in place, the prevalence of Salmonella serovars in samples can change [29]. In the present study, 8 out 100 chicken samples were positive for S. Enterica representing (8%); this result is in good harmony with a study applied by [30,31], who reported (8.6% and 8%), respectively, in the chicken farms. Three serovars were identified in chicken samples which included S. Kentucky 25% (2/8), which was close to [32,33], and S. Typhimurium 37.5% (3/8) and S. Enteritidis 37.5% (3/8), which were quite similar to [33,34]. The prevalence of Salmonella from 100 fecal calves’ samples was 2% (2 isolates), which was close to that obtained from (1.5%) [35]. Subsequently, the isolation percentage among the diseased calves was lower than in chicken, which agreed with [29].
Concerning the antibiotic sensitivity test, the highest antibiotic-resistant Salmonella isolates were Erythromycin 100%, Vancomycin 90%, Ampicillin 90%, Amoxicillin 80%, and nalidixic acid 90%, which agree with the previous study applied by [36,37]. In this study, most of the Salmonella isolates showed multidrug-resistance of 80% to ≥5 of the fourteen antimicrobials used, and the obtained results matched with the results reported by [36,38,39,40]. They were also similar to [41], which reported that numerous Salm. Typhi, Salm. Paratyphi A, Salm. Typhimurium, and Salm. Enteritidis isolates were MDR as they had a resistance to a minimum of four antibiotics. Salmonella typhimurium, Salmonella enteritidis, Salmonella typhi, and Salmonella paratyphi A isolates were all discovered to be susceptible to ciprofloxacin and ofloxacin.
The spread of antibiotic resistance 262 in Salmonella enterica species is greatly aided by the horizontal transmission of resistance genes. These resistance genes can be located on chromosome 263 of bacteria or on resistant plasmids. Generally, the most effective way to transfer resistance is by the horizontal transmission of genes via plasmids, which is happening frequently and involves multiple resistance genes at once [42]. Transposons, integrons, and plasmids that gain resistance genes have the potential to spread to various strains or species. Transposons, considered a genetic element, move about and have the ability to carry resistance genes. They also have the ability to recombine resistance genes with plasmids or chromosomes thanks to their transposase activity. Integrons are made up of a promoter, a recombination site that integrase can recognize, and the recombination enzyme integrase (encoded by the intI gene). Moreover, these components are required for the expression of the gene cassettes found within an integron [43]. These configurations, particularly on plasmids, effectively encourage the acquisition of external genes such as genes for antibiotic resistance in the bacterial genome. Additionally, conjugation events make it easier for resistance genes to move from plasmids to other strains or species via transposon or integrin [42]. Most Enterobacteriaceae produce extended spectrum-lactamases (ESBLs), which is a key mechanism for granting resistance. According to the substrate and inhibitor mechanisms, there are various types of ESBLs [44]. The first lactamase discovered was TEM-1, which was obtained from an E. coli strain that belonged to a patient in Greece by the name of Temoniera [45]. TEM-3 [44] was the first member of the TEM-type lactamase family to exhibit ESBL properties [44]. Salmonella spp. has been found to have TEM-type lactamases [45,46,47]. Another lactamase is SHV (sulphydryl variable), which is typically seen in Klebsiella pneumoniae and E. coli [45]. There are more than 90 different TEM, and more than 25 different SHV varieties of lactamases, which are the most prevalent and widely dispersed in nature [45,47]. SHV is plasmid-encoded. With more than 90 varieties of TEM and more than 25 types of SHV, the TEM and SHV types of lactamases are the most prevalent and extensively distributed in nature [46,48].
In this study, a PCR was performed on two S. Kentucky isolates and two S. Enteritidis isolates of various origins to figure out the extended spectrum β-lactamases genes (bla TEM, bla SHV, bla VIM, bla IMP), and finally class 1 integron encoding genes. The obtained results revealed the detection of blaTEM and class 1 integron, but were negative for bla IMP, bla VIM, and bla SHV. These results agreed with the work by Zhao and Moawad [49,50], and this result is similar to the result obtained by Bahatta et al. [41], which reported that research using PCR amplification and sequencing on all Salm. Enteritidis isolates with resistance illustrated the existence of the ESBL gene blaSHV; however, the genes blaTEM and blaCTX were missed.
Several methods exist for investigating the molecular diversity of bacteria. These strains’ random entire genomes were typed using the RAPD and ERIC PCR techniques. With a discriminating value (DI) of 0.9821 and 100% repeatability, ERIC PCR was discovered to be extremely effective. According to Nath et al. [51], RAPD had poor repeatability (40%) but was effective in differentiating the strains (DI = 0.8978). Regular epidemiological research can benefit from using ERIC PCR because of its shown discriminating power and ease of usage.
Salmonella and other pathogenic members of the Enterobacteriaceae family are studied genetically using the ERIC PCR method. This method depends on the creation of complex and repeatable fingerprints by amplifying a genomic DNA fragment using a single primer pair that is complementary to short repeat sequences. The same four previous mentioned isolates were also tested using the ERIC PCR. Another interesting finding was that two isolates of S.Enteritidis originating from poultry and calves had 100% similarity, despite the other two isolates of S. Kentucky showing only 33% serovarities. The current findings came in contact with those illustrated by Secundo de Souza et al. [52] which reported that some of the SG isolates improved from the various areas at certain times clustered with 100% similarity, proposing that the genotypes’ transportation among these various areas had occurred. According to our information, no research has used S. Enteritidis and S. Kentucky isolates from various origins such as calf and poultry for an ERIC PCR analysis. The goal of the current investigation was to evaluate how well the ERIC PCR could be used to analyze the genetic link between two strains. To our knowledge, there are no pervious data reported in Egypt at this point in the research. These results proved that infection by Salmonella may be acquired through contaminated water which was used for drinking or via contaminated food from wild birds, as reported by [15] about water, feed, and dust as a source for Salmonella infection. This is result is similar to the result of the authors of [53], who reported that poultry isolates had more similarities with cattle isolates than human isolates by the ERIC PCR.
5. Conclusions
Salmonella isolates that produced both MDR and ESBL frequently included class 1 integrons. The high incidence of class 1 integrons provides evidence that antimicrobial gene cassettes mediated by integrons play a significant role in the Salmonella resistance profile. According to the results of the current investigation, there is a significant level of clonal heterogeneity in the strain typing of lactamase-producing MDR Salmonella obtained from distinct clinical samples. One explanation for the presence of a few clonally related groups among these isolates is either clonal transmission or the propagation of antibiotic resistance through the use of various lactamases.
Author Contributions
Conceptualization, R.G.T., M.F.G. and H.T.; methodology, R.G.T. and H.S.N.; software, M.M.A. and M.F.G.; validation, R.G.T., M.M.A. and H.S.N.; formal analysis, R.G.T.; investigation, M.M.A.; resources, M.M.A.A.; data curation, R.G.T.; writing—original draft preparation, R.G.T.; writing—review and editing, M.M.A.A.; visualization, H.T.; supervision, R.G.T.; project administration, H.S.N. and M.M.A.A.; funding acquisition, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors elect to not share the data.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Research Group Project under grant number (G.R.P.2. 72/43).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodriguez-Rivera, L.; Wright, E.; Siler, J.; Elton, M.; Cummings, K.; Warnick, L.; Wiedmann, M. Subtype analysis of Salmonella isolated from subclinically infected dairy cattle and dairy farm environments reveals the presence of both human-and bovine-associated subtypes. Vet. Microbiol. 2014, 170, 307–316. [Google Scholar] [CrossRef]
- Kebede, A.; Kemal, J.; Alemayehu, H.; Habte Mariam, S. Isolation, identification, and antibiotic susceptibility testing of Salmonella from slaughtered bovines and ovines in Addis Ababa Abattoir Enterprise, Ethiopia: A cross-sectional study. Int. J. Bacteriol. 2016, 2016, 714785. [Google Scholar] [CrossRef]
- Vandeplas, S.; Dauphin, R.D.; Beckers, Y.; Thonart, P.; Thewis, A. Salmonella in chicken: Current and developing strategies to reduce contamination at farm level. J. Food Prot. 2010, 73, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Habing, G.G.; Lombard, J.E.; Kopral, C.A.; Dargatz, D.A.; Kaneene, J.B. Farm-level associations with the shedding of Salmonella and antimicrobial-resistant Salmonella in US dairy cattle. Foodborne Pathog. Dis. 2012, 9, 815–821. [Google Scholar] [CrossRef]
- Huston, C.L.; Wittum, T.E.; Love, B.C.; Keen, J.E. Prevalence of fecal shedding of Salmonella spp in dairy herds. J. Am. Vet. Med. Assoc. 2002, 220, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Cliver, D.O.; Riemann, H.P. Foodborne Infections and Intoxications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ahmed, A.M.; Motoi, Y.; Sato, M.; Maruyama, A.; Watanabe, H.; Fukumoto, Y.; Shimamoto, T. Zoo animals as reservoirs of gram-negative bacteria harboring integrons and antimicrobial resistance genes. Appl. Environ. Microbiol. 2007, 73, 6686–6690. [Google Scholar] [CrossRef]
- Roy Chowdhury, P.; Ingold, A.; Vanegas, N.; Martínez, E.; Merlino, J.; Merkier, A.K.; Castro, M.; González Rocha, G.; Borthagaray, G.; Centrón, D. Dissemination of multiple drug resistance genes by class 1 integrons in Klebsiella pneumoniae isolates from four countries: A comparative study. Antimicrob. Agents Chemother. 2011, 55, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, T.; Partridge, S.R.; Iredell, J.R.; Stokes, H. Genetic context and structural diversity of class 1 integrons from human commensal bacteria in a hospital intensive care unit. Antimicrob. Agents Chemother. 2011, 55, 3939–3943. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Barraud, O.; Casellas, M.; Dagot, C.; Ploy, M.-C. Integron involvement in environmental spread of antibiotic resistance. Front. Microbiol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Bonnet, R. Growing group of extended-spectrum β-lactamases: The CTX-M enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Sutton, L. Properties of plasmids responsible for production of extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 1991, 35, 164–169. [Google Scholar] [CrossRef]
- Villa, L.; Pezzella, C.; Tosini, F.; Visca, P.; Petrucca, A.; Carattoli, A. Multiple-antibiotic resistance mediated by structurally related IncL/M plasmids carrying an extended-spectrum β-lactamase gene and a class 1 integron. Antimicrob. Agents Chemother. 2000, 44, 2911–2914. [Google Scholar] [CrossRef]
- Cantón, R.; Coque, T.M.; Baquero, F. Multi-resistant Gram-negative bacilli: From epidemics to endemics. Curr. Opin. Infect. Dis. 2003, 16, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Fagbamila, I.O.; Barco, L.; Mancin, M.; Kwaga, J.; Ngulukun, S.S.; Zavagnin, P.; Lettini, A.A.; Lorenzetto, M.; Abdu, P.A.; Kabir, J. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS ONE 2017, 12, e0173097. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.; Markey, B.K.; Carter, M.; Donnelly, W.; Leonard, F. Veterinary Microbiology and Microbial Disease; Blackwell Science: Oxford, UK, 2002. [Google Scholar]
- Krieg, N.; Holt, J.; Murray, R. Bergey’s Manual of Systematic Bacteriology; Williams and Wilkins: Baltimore, MD, USA, 1984; Volume 1, pp. 331–371. [Google Scholar]
- Topley, W.W.; Wilson, G.S.; Coller, L.; Sussman, M. Topley & Wilson’s Microbiology and Microbial Infections; John Wiley & Sons, Ltd.: New York, NY, USA, 1998. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Kauffmann, F. Das Kauffmann-White-Schema. In Ergebnisse der Mikrobiologie, Immunitätsforschung und Experimentellen Therapie; Springer: Berlin, Germany, 1957; pp. 160–216. [Google Scholar]
- Versalovic, J.; Koeuth, T.; and Lupski, R. Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial enomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- White, P.A.; McIver, C.J.; Deng, Y.; Rawlinson, W.D. Characterisation of two new gene cassettes, aadA5 and dfrA17. FEMS Microbiol. Lett. 2000, 182, 265–269. [Google Scholar] [CrossRef]
- Gassama Sow, A.; Aïdara-Kane, A.; Barraud, O.; Gatet, M.; Denis, F.; Ploy, M.C. High prevalence of trimethoprim-resistance cassettes in class 1 and 2 integrons in Senegalese Shigella spp isolates. J. Infect. Dev. Ctries. 2010, 4, 207–212. [Google Scholar] [CrossRef]
- Xia, Y.; Liang, Z.; Su, X.; Xiong, Y. Characterization of carbapenemase genes in Enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in a university hospital in Chongqing, China. Ann. Lab. Med. 2012, 32, 270–275. [Google Scholar] [CrossRef]
- Colom, K.; Pérez, J.; Alonso, R.; Fernández-Aranguiz, A.; Lariño, E.; Cisterna, R. Simple and reliable multiplex PCR assay for detection of bla TEM, bla SHV and bla OXA–1 genes in Enterobacteriaceae. FEMS Microbiol. Lett. 2003, 223, 147–151. [Google Scholar] [CrossRef]
- Carpenter, K.E.; Smith-Vaniz, W.F.; de Bruyne, G.; de Morais, L. Dicentrarchus punctatus. IUCN Red List of Threatened Species. 2015. Available online: https://www.iucnredlist.org/species/198671/21913001 (accessed on 21 May 2022).
- Beyene, T. Identification and antimicrobial susceptibility profile of Salmonella isolated from selected dairy farms, abattoir and humans at Asella town, Ethiopia. J. Vet. Sci. Technol. 2016, 7, 320. [Google Scholar] [CrossRef]
- Saroj, S.; Shashidhar, R.; Bandekar, J. Gamma radiation used as hygienization technique for foods does not induce viable but non-culturable state (VBNC) in Salmonella enterica subsp. enterica serovar Typhimurium. Curr. Microbiol. 2009, 59, 420–424. [Google Scholar] [CrossRef]
- Kagambèga, A.; Lienemann, T.; Aulu, L.; Traoré, A.; Barro, N.; Siitonen, A.; Haukka, K. Prevalence and characterization of Salmonella enterica from the feces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonellaisolates. BMC Microbiol. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Le Bouquin, S.; Allain, V.; Rouxel, S.; Petetin, I.; Picherot, M.; Michel, V.; Chemaly, M. Prevalence and risk factors for Salmonella spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 2010, 97, 245–251. [Google Scholar] [CrossRef]
- Aragaw, K.; Terefe, L.; Abera, M. Prevalence of Salmonella infection in intensive poultry farms in Hawassa and isolation of Salmonella species from sick and dead chickens. Ethiop. Vet. J. 2010, 14, 115–124. [Google Scholar] [CrossRef]
- Awad, A.; Gwida, M.; Khalifa, E.; Sadat, A. Phenotypes, antibacterial-resistant profile, and virulence-associated genes of Salmonella serovars isolated from retail chicken meat in Egypt. Vet. World 2020, 13, 440. [Google Scholar] [CrossRef]
- Ball, T.; Monte, D.; Aidara-Kane, A.; Matheu, J.; Ru, H.; Thakur, S.; Ejobi, F.; Fedorka-Cray, P. International lineages of Salmonella enterica serovars isolated from chicken farms, Wakiso District, Uganda. PLoS ONE 2020, 15, e0220484. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Wang, Z.; Tian, Y.; Kang, X.; Meng, C.; Chen, X.; Pan, Z.; Jiao, X. Prevalence of Salmonella isolates and their distribution based on whole-genome sequence in a chicken slaughterhouse in Jiangsu, China. Front. Vet. Sci. 2020, 7, 29. [Google Scholar] [CrossRef]
- Cetin, E.; Temelli, S.; Eyigor, A. Salmonella prevalence and serovar distribution in healthy slaughter sheep and cattle determined by ISO 6579 and VIDAS UP Salmonella methods. J. Food Sci. Technol. 2019, 56, 5317–5325. [Google Scholar] [CrossRef] [PubMed]
- Halawa, M.; Moawad, A.; Eldesouky, I.; Ramadan, H. Detection of antimicrobial phenotypes, ß-lactamase encoding genes and class L INTEGRONS in Salmonella serovars isolated from broilers. Int. J. Poult. Sci. 2016, 15, 1–7. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Huang, J.; Zhang, Y.; Liu, S.; Chen, L.; Xiao, C.; Zeng, H.; Wei, X.; Gu, Q.; Li, Y.; et al. Prevalence, abundance, serovars and antimicrobial resistance of Salmonella isolated from retail raw poultry meat in China. Sci. Total Environ. 2020, 713, 136385. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Genetic analysis of multiple antimicrobial resistance in Salmonella isolated from diseased broilers in Egypt. Microbiol. Immunol. 2012, 56, 254–261. [Google Scholar] [CrossRef]
- Mihaiu, L.; Lapusan, A.; Tanasuica, R.; Sobolu, R.; Mihaiu, R.; Oniga, O.; Mihaiu, M. First study of Salmonella in meat in Romania. J. Infect. Dev. Ctries. 2014, 8, 050–058. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong, China. Front. Microbiol. 2018, 9, 2104. [Google Scholar] [CrossRef]
- Bhatta, D.; Bangtrakulnonth, A.; Tishyadhigama, P.; Saroj, S.; Bandekar, J.; Hendriksen, R.; Kapadnis., B. Serotyping, PCR, phage-typing and antibiotic sensitivity testing of Salmonella serovars isolated from urban drinking water supply systems of Nepal. Lett. Appl. Microbiol. 2007, 44, 588–594. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmid-mediated antimicrobial resistance in Salmonella enterica. Curr. Issues Mol. Biol. 2003, 5, 113–122. [Google Scholar]
- Domingues, S.; da Silva, G.; Nielsen, K. Integrons: Vehicles and pathways for horizontal dissemination in bacteria. Mob. Genet. Elem. 2012, 2, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.; Kamal, M. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Wu, Y.; Qiao, J.; Li, H.; Zheng, S.; Xia, X.; Cui, S.; Wang, X.; Xi, M.; et al. Emergence of β-lactamases and extended-spectrum β-lactamases (ESBLs) producing Salmonella in retail raw chicken in China. Foodborne Pathog. Dis. 2015, 12, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Hungerford, L.; Fedorka-Cray, P.; Headrick, M. Extended-spectrum-cephalosporin resistance in Salmonella enterica isolates of animal origin. Antimicrob. Agents Chemother. 2004, 48, 3179–3181. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S.; Perreten, V.; Johannes, S.; Droz, S.; Bodmer, T.; Endimiani, A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob. Agents Chemother. 2014, 58, 2446–2449. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Zhao, C.; Zhang, Y.; Li, L.; Qi, J.; Luo, Y.; Zhou, D.; Liu, Y. Prevalence and antimicrobial resistance of Salmonella isolated from broilers in Shandong, China. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Tomaso, H.; Neubauer, H.; Hafez, H.M.; El-Adawy, H. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog. 2017, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nath, G.; Maurya, P.; Gulati, A. ERIC PCR and RAPD based fingerprinting of Salmonella Typhi strains isolated over a period of two decades. Infect. Genet. Evol. 2010, 10, 530–536. [Google Scholar] [CrossRef]
- de Souza, A.; de Freitas Neto, O.; Batista, D.; Estupinan, A.; de Almeida, A.; Barrow, P.; Berchieri, A. ERIC-PCR genotyping of field isolates of Salmonella enterica subsp. enterica serovar Gallinarum biovars Gallinarum and Pullorum. Avian Pathol. 2015, 44, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shiroodi, A.; Jamshidian, M.; Salehi, T.; Boroujeni, G.; Amini, K. Genotyping of Salmonella enterica subsp. enterica serovar Entritidis, isolated from poultry, cattle and human in Iran by ERIC-PCR. Int. J. Biosci. 2014, 5, 147–153. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).