Ameliorative Effect of Quercetin against Abamectin-Induced Hemato-Biochemical Alterations and Hepatorenal Oxidative Damage in Nile Tilapia, Oreochromis niloticus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Fish
2.3. Experimental Design
2.4. Experimental Diet
2.5. Blood Sampling
2.5.1. Hematological Examination
2.5.2. Biochemical Examination
2.6. Tissue Sampling and Antioxidant Status
2.7. Statistical Analyses
3. Results
3.1. Blood Performance
3.2. Biochemical Parameters
3.3. Liver and Kidney Functions
3.4. Antioxidant Status
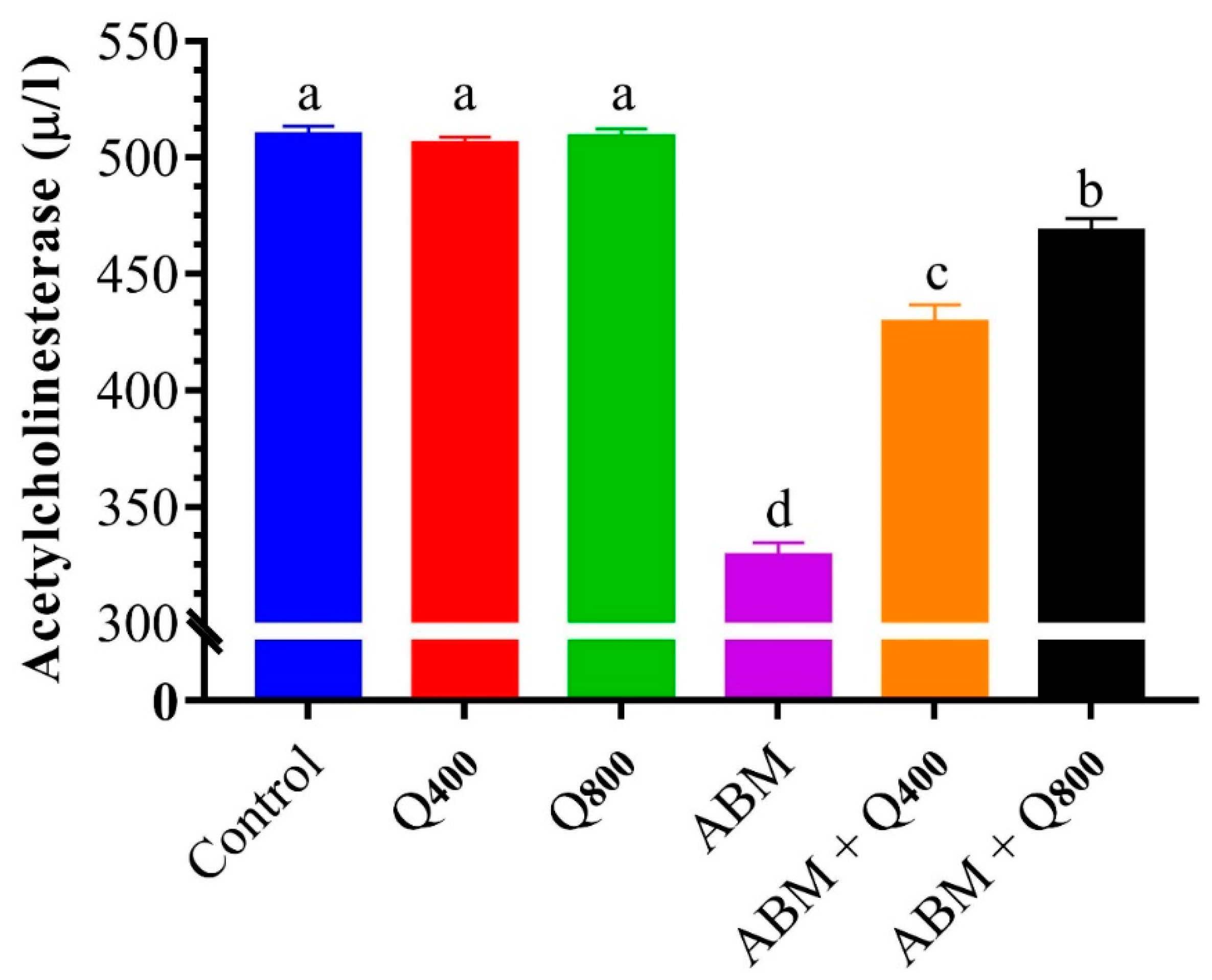
3.5. Acetylcholinesterase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, S.; Zuberi, A.; Alagawany, M.; Farag, M.R.; Dadar, M.; Karthik, K.; Tiwari, R.; Dhama, K.; Iqbal, H.M. Cypermethrin induced toxicities in fish and adverse health outcomes: Its prevention and control measure adaptation. J. Environ. Manag. 2018, 206, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Ogbourne, S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef]
- Luo, L.; Sun, Y.-J.; Wu, Y.-J. Abamectin resistance in Drosophila is related to increased expression of P-glycoprotein via the dEGFR and dAkt pathways. Insect Biochem. Mol. Biol. 2013, 43, 627–634. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Alvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; Crivellente, F. Peer review of the pesticide risk assessment of the active substance abamectin. EFSA J. 2022, 20, e07544. [Google Scholar]
- Brewer, B.N.; Armbrust, K.L.; Mead, K.T.; Holmes, W.E. Determination of abamectin in soil samples using high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Anastassiadou, M.; Arena, M.; Auteri, D.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; Crivellente, F. Peer review of the pesticide risk assessment of the active substance abamectin. EFSA J. 2020, 18, e06227. [Google Scholar] [PubMed]
- Kolar, L.; Eržen, N.K.; Hogerwerf, L.; van Gestel, C.A. Toxicity of abamectin and doramectin to soil invertebrates. Environ. Pollut. 2008, 151, 182–189. [Google Scholar] [CrossRef]
- Campbell, W.C. Ivermectin and Abamectin; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Ma, J.; Zhou, C.; Li, Y.; Li, X. Biochemical responses to the toxicity of the biocide abamectin on the freshwater snail, Physa acuta. Ecotoxicol. Environ. Saf. 2014, 101, 31–35. [Google Scholar] [CrossRef]
- Tišler, T.; Kožuh Eržen, N. Abamectin in the aquatic environment. Ecotoxicol. Environ. Saf. 2006, 15, 495–502. [Google Scholar] [CrossRef]
- Ahmed, F.A.G.; Reda, R.M. Comparative Acute Exposure Study of Abamectin Different Formulations Inducing Physiological and Oxidative Stress Biomarkers in Nile Tilapia,(Oreochromis niloticus). Egypt. Acad. J. Biol. Sci. B Zool. 2021, 13, 223–238. [Google Scholar] [CrossRef]
- Novelli, A.; Vieira, B.H.; Braun, A.S.; Mendes, L.B.; Daam, M.A.; Espíndola, E.L.G. Impact of runoff water from an experimental agricultural field applied with Vertimec® 18EC (abamectin) on the survival, growth and gill morphology of zebrafish juveniles. Chemosphere 2016, 144, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Thanomsit, C.; Wattanakornsiri, A.; Nanthanawat, P. Adverse effects of abamectin on hematological profile and histological alterations of hybrid catfish (Clarias macrocephalus x C. gariepinus). Burapha Sci. J. 2017, 22, 169–182. [Google Scholar]
- Fırat, Ö.; Tutus, R. Comparative acute toxicity assessment of organophosphate and avermectin insecticides on a freshwater fish Oreochromis niloticus. Bull. Environ. Contam. Toxicol. 2020, 105, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.K.; Reda, F.M.; Alagawany, M.; Farag, M.R. The stress of abamectin toxicity reduced water quality, growth performance, immunity and antioxidant capacity of Oreochromis niloticus fish: Modulatory role of Simmondsia chinensis extract as a dietary supplement. Aquaculture 2021, 534, 736247. [Google Scholar] [CrossRef]
- Kushwaha, S.; Anerao, I.; Rajput, S.; Bhagriya, P.; Roy, H. Evaluation of abamectin induced hepatotoxicity in Oreochromis mossambicus. Cogent Biol. 2020, 6, 1761277. [Google Scholar] [CrossRef]
- Ogueji, E.; Nwani, C.; Mbah, C.; Nweke, F. Acute hematological toxicity of ivermectin to juvenile Clarias gariepinus. Toxicol. Environ. Chem. 2019, 101, 300–314. [Google Scholar] [CrossRef]
- Amaeze, N.H.; Komolafe, B.O.; Salako, A.F.; Akagha, K.K.; Briggs, T.-M.D.; Olatinwo, O.O.; Femi, M.A. Comparative assessment of the acute toxicity, haematological and genotoxic effects of ten commonly used pesticides on the African Catfish, Clarias gariepinus Burchell 1822. Heliyon 2020, 6, e04768. [Google Scholar] [CrossRef] [PubMed]
- Rohmah, M.K.; Salahdin, O.D.; Gupta, R.; Muzammil, K.; Qasim, M.T.; Al-Qaim, Z.H.; Abbas, N.F.; Jawad, M.A.; Yasin, G.; Mustafa, Y.F. Modulatory role of dietary curcumin and resveratrol on growth performance, serum immunity responses, mucus enzymes activity, antioxidant capacity and serum and mucus biochemicals in the common carp, Cyprinus carpio exposed to abamectin. Fish Shellfish. Immunol. 2022, 129, 221–230. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Rao, G.; Khachatryan, L.; Cormier, S.A.; Lomnicki, S. Environmentally persistent free radicals: Insights on a new class of pollutants. Environ. Sci. Technol. 2018, 52, 2468–2481. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Hamed, H.S.; Ismal, S.M.; Abdel-Tawwab, M. Modulatory effects of dietary cinnamon (Cinnamomum zeylanicum) against waterborne lead toxicity in Nile tilapia fingerlings: Growth performance, haemato-biochemical, innate immunity, and hepatic antioxidant indices. Aquac. Rep. 2022, 25, 101190. [Google Scholar] [CrossRef]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuroendocrinol. Lett. 2009, 30, 2. [Google Scholar] [PubMed]
- Zhang, Y.; Wu, J.; Xu, W.; Gao, J.; Cao, H.; Yang, M.; Wang, B.; Hao, Y.; Tao, L. Cytotoxic effects of Avermectin on human HepG2 cells in vitro bioassays. Environ. Pollut. 2017, 220, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Kadry, S.; Amer, A.; Marzouk, M.; Hanna, M.; Azmy, A.; Hamed, H. Vitamin E as antioxidant in female African catfish (Clarias gariepinus) exposed to chronic toxicity of atrazine. Egypt. J. Aquat. Biol. Fish. 2012, 16, 83–98. [Google Scholar] [CrossRef][Green Version]
- Hamed, H.S.; El-Sayed, Y.S. Antioxidant activities of Moringa oleifera leaf extract against pendimethalin-induced oxidative stress and genotoxicity in Nile tilapia, Oreochromis niloticus (L.). Fish Physiol. Biochem. 2019, 45, 71–82. [Google Scholar] [CrossRef]
- Hamed, H.S. Ameliorative effects of Spirulina platensis on deltamethrin-induced biochemical alterations and oxidative stress in the African catfish, Clarias gariepinus. Open J. Mar. Sci. 2016, 6, 62112. [Google Scholar] [CrossRef]
- Mansour, A.T.; Hamed, H.S.; El-Beltagi, H.S.; Mohamed, W.F. Modulatory Effect of Papaya Extract against Chlorpyrifos-Induced Oxidative Stress, Immune Suppression, Endocrine Disruption, and DNA Damage in Female Clarias gariepinus. Int. J. Environ. Res. Public 2022, 19, 4640. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Ibrahim, D.; Kishawy, A.T.; Khater, S.I.; Khalifa, E.; Ismail, T.A.; Mohammed, H.A.; Elnahriry, S.S.; Tolba, H.A.; Sherief, W.R.; Farag, M.F. Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2021, 119, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study. Int. J. Gen. Med. 2021, 14, 2359. [Google Scholar] [CrossRef] [PubMed]
- Shabana, M.; Taju, G.; Majeed, A.; Karthika, M.; Ramasubramanian, V.; AS, S.H. Preparation and evaluation of mesoporous silica nanoparticles loaded quercetin against bacterial infections in Oreochromis niloticus. Aquac. Rep. 2021, 21, 100808. [Google Scholar]
- El-Beltagi, H.S.; Ahmed, M.M. Assessment the protective role of quercetin on acrylamide-induced oxidative stress in rats. J. Food Biochem. 2016, 40, 715–723. [Google Scholar] [CrossRef]
- Zhai, S.-W.; Liu, S.-L. Effects of dietary quercetin on growth performance, serum lipids level and body composition of tilapia (Oreochromis niloticus). Ital. J. Anim. Sci. 2013, 12, e85. [Google Scholar] [CrossRef]
- Jia, E.; Yan, Y.; Zhou, M.; Li, X.; Jiang, G.; Liu, W.; Zhang, D. Combined effects of dietary quercetin and resveratrol on growth performance, antioxidant capability and innate immunity of blunt snout bream (Megalobrama amblycephala). Anim. Feed. Sci. Technol. 2019, 256, 114268. [Google Scholar] [CrossRef]
- Kong, Y.; Tian, J.; Niu, X.; Li, M.; Kong, Y.; Li, R.; Chen, X.; Wang, G. Effects of dietary quercetin on growth, antioxidant capacity, immune response and immune-related gene expression in snakehead fish, Channa argus. Aquac. Rep. 2022, 26, 101314. [Google Scholar] [CrossRef]
- Pês, T.S.; Saccol, E.M.; Ourique, G.M.; Londero, É.P.; Gressler, L.T.; Golombieski, J.I.; Glanzner, W.G.; Llesuy, S.F.; Gonçalves, P.B.; Neto, J.R. Quercetin in the diet of silver catfish: Effects on antioxidant status, blood parameters and pituitary hormone expression. Aquaculture 2016, 458, 100–106. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Javahery, S.; Van Doan, H. Effects of dietary vitamin C, thyme essential oil, and quercetin on the immunological and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2022, 553, 738053. [Google Scholar] [CrossRef]
- El Said, M.M. Evaluation of abamectin toxicity on some biochemical constituents and osmoregulation in freshwater fish Oreochromis niloticus. J. Egypt Soc. Toxicol. 2007, 37, 1–10. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2006. [Google Scholar]
- Brown, B. Routine hematology procedures. In Hematology. Principles and Procedures; Brown, B., Ed.; Leo and Fabiger: Philadelphia, PA, USA, 1988; pp. 7–122. [Google Scholar]
- Collier, H.B. Standardization of blood haemoglobin determinations. Can. Med. Assoc. J. 1944, 50, 550. [Google Scholar]
- Wintrobe, M.M. Variations in the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematol. 1934, 51, 32–49. [Google Scholar]
- Gupta, P.K. Haematological Techniques, 4th ed.; Syndicate: London, UK, 1977; p. 231. [Google Scholar]
- Reitman, S. Colorimetric determination of GPT activity according to the Reitman and Frankel method. Am. J. Clim. Path. 1957, 28, 56. [Google Scholar] [CrossRef]
- Young, D.S. Effects of disease on Clinical Lab. In Tests, 4th ed.; AACC Press: Washington, DC, USA, 2001. [Google Scholar]
- Henry, R.J. Clinical Chemistry, Principles and Technics; Hoeber Medical Division, Harper & Row: New York, NY, USA, 1964. [Google Scholar]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Frings, C.S.; Fendley, T.W.; Dunn, R.T.; Queen, C.A. Improved determination of total serum lipids by the sulfo-phospho-vanillin reaction. Clin. Chem. 1972, 18, 673–674. [Google Scholar] [CrossRef]
- Fabiny, D.L.; Ertingshausen, G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971, 17, 696–700. [Google Scholar] [CrossRef]
- Henry, J.; Todd, S.; Sanford, L.; Davidsohn, S. Clinical Diagnosis and Measurement by Laboratory Methods, 16th ed.; W.B. Saunders and Co.: Philadelphia, PA, USA, 1974. [Google Scholar]
- Turecky, L.; Kupcova, V.; Mojto, V.; Smutny, M.; Uhlikova, E.; Vozar, I. Serum cholinesterase activity and proteosynthetic function of liver in patients with diabetes mellitus. Bratisl. Lek. Listy 2005, 106, 266. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Colorimetric method for determination of total antioxidant capacity. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish–Not only for beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Esmaeili, M. Blood performance: A new formula for fish growth and health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef]
- Roth, M.; Rae, G.; McGill, A.S.; Young, K.W. Ivermectin depuration in Atlantic salmon (Salmo salar). J. Agric. Food Chem. 1993, 41, 2434–2436. [Google Scholar] [CrossRef]
- Pamila, D.; Subbaiyan, P.A.; Ramaswamy, M. Toxic effect of chromium and cobalt on Sartherodon mossambicus (peters). Indian J. Environ. Health 1991, 33, 218–224. [Google Scholar]
- Al-Kahtani, M.A. Effect of an insecticide abamectin on some biochemical characteristics of tilapia fish (Oreochromis niloticus). Am. J. Agric. Biol. Sci. 2011, 6, 62–68. [Google Scholar] [CrossRef]
- Nussey, G.; Van Vuren, J.H.J.; Du Preez, H.H. Effect of copper on the differential white blood cell counts of the Mozambique tilapia (Oreochromis mossambicus). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1995, 111, 381–388. [Google Scholar] [CrossRef]
- García-Saura, M.F.; Galisteo, M.; Villar, I.C.; Bermejo, A.; Zarzuelo, A.; Vargas, F.; Duarte, J. Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol. Cell. Biochem. 2005, 270, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, C.; Li, B. Quercetin attenuates the progression of monocrotaline-induced pulmonary hypertension in rats. J. Biomed. Res. 2012, 26, 98–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mir, I.N.; Srivastava, P.P.; Bhat, I.A.; Jaffar, Y.D.; Sushila, N.; Sardar, P. Optimal dietary lipid and protein level for growth and survival of catfish Clarias magur larvae. Aquaculture 2020, 520, 734678. [Google Scholar] [CrossRef]
- Hamed, H.S.; Osman, A.G. Modulatory effect of lycopene against carbofuran toxicity in African catfish, Clarias gariepinus. Fish Physiol. Biochem. 2017, 43, 1721–1731. [Google Scholar] [CrossRef]
- Mastan, S.A.; Rammayya, P.J. Biochemical profile of Channa gachua (Ham) exposed to sublethal doses of Dichlorovas (DDVP). Internet J. Toxicol. 2010, 8, 27–32. [Google Scholar]
- Thanomsit, C. Evaluation of abamectin effect on some biochemical constituents and histological alterations in Asian sea bass (Lates calcarifer). Naresuan Univ. J. Sci. Technol. 2016, 24, 72–81. [Google Scholar]
- Kapoor, B.; Kaur, G.; Gupta, M.; Gupta, R. Indian medicinal plants useful in treatment of gout: A review for current status and future prospective. Asian J. Pharm. Clin. Res. 2017, 10, 407–416. [Google Scholar] [CrossRef]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.I.; Ha, T.Y. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef]
- Ouyang, W.W.; Yao, L.I.; Zhao, W.; Wang, M.H.; Jin, F. Effect of quercetin on cAMP signaling pathway in chicken adipocytes. Sci. Agric. Sin. 2013, 46, 2769–2776. [Google Scholar]
- Abdel-Tawwab, M.; Hamed, H.S. Antagonistic effects of dietary guava (Psidium guajava) leaves extract on growth, hemato-biochemical, and immunity response of cypermethrin-intoxicated Nile tilapia, Oreochromis niloticus, fingerlings. Aquaculture 2020, 529, 735668. [Google Scholar] [CrossRef]
- Mansour, A.T.; Espinosa, C.; García-Beltrán, J.M.; Miao, L.; Francisco, D.C.C.; Alsaqufi, A.S.; Esteban, M.Á. Dietary supplementation of drumstick tree, Moringa oleifera, improves mucosal immune response in skin and gills of seabream, Sparus aurata, and attenuates the effect of hydrogen peroxide exposure. Fish Physiol. Biochem. 2020, 46, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.; Bogé, G. Fish blood parameters as a potential tool for identification of stress caused by environmental factors and chemical intoxication. Mar. Environ. Res. 1996, 41, 27–43. [Google Scholar] [CrossRef]
- Katharios, P.; Iliopoulou-Georgudaki, J.; Kapata-Zoumbos, K.; Spiropoulos, S. Toxicity of intraperitoneally injected ivermectin in sea bream, Sparus aurata. Fish Physiol. Biochem. 2001, 25, 99–108. [Google Scholar] [CrossRef]
- Yan, S.X.; Li, X.; Sun, C.D.; Chen, K.S. Hypoglycemic and hypolipidemic effects of quercetin and its glycosides. China J. Chin. Mater. Med. 2015, 40, 4560–4567. [Google Scholar]
- Hamed, H.S.; Abdel-Tawwab, M. Dietary pomegranate (Punica granatum) peel mitigated the adverse effects of silver nanoparticles on the performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia fingerlings. Aquaculture 2021, 540, 736742. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Dabrowski, K.; Izquierdo, M.; Malinovskyi, O.; Kolářová, J.; Policar, T. Effects of Dietary Protein and Lipid Levels on Growth, Body Composition, Blood Biochemistry, Antioxidant Capacity and Ammonia Excretion of European Grayling (Thymallus thymallus). Front. Mar. Sci. 2021, 1016. [Google Scholar] [CrossRef]
- Bain, P. Liver; Iowa State Press: Ames, IA, USA, 2003. [Google Scholar]
- Ibrahim, S.; Mahmoud, S. Effect of heavy metals accumulation on enzyme activity and histology in liver of some Nile fish in Egypt. Egypt. J. Aquat. Biol. Fish. 2005, 9, 203–219. [Google Scholar] [CrossRef][Green Version]
- El-Gendy, K.S.; Aly, N.M.; Mahmoud, F.H.; Abd Allah, D.M.; El-Sebae, A.K.H. Hepatotoxicity and nephrotoxicity in mice induced by abamectin and ameliorating effect of quercetin. Asian J. Agric. Food Sci. 2015, 3, 651–666. [Google Scholar]
- Eissa, F.; Zidan, N. Haematological, biochemical and histopathological alterations induced by abamectin and Bacillus thuringiensis in male albino rats. Acta Biol. Hung. 2010, 61, 33–44. [Google Scholar] [CrossRef]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Woźniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Hepatoprotective effect of quercetin: From chemistry to medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Aluani, D.; Tzankova, V.; Kondevaburdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Bols, N.C.; Brubacher, J.L.; Ganassin, R.C.; Lee, L.E. Ecotoxicology and innate immunity in fish. Dev. Comp. Immunol. 2001, 25, 853–873. [Google Scholar] [CrossRef]
- Al Ghais, S.M.; Varadharajulu, S.; Kumbhar, P. Effects of Abamectin on Tilapia mossambica peters changes in reduced glutathione (GSH) and protein content. Int. J. Fish. Aquat. Stud. 2019, 7, 280–284. [Google Scholar]
- Huang, Y.; Hong, Y.; Huang, Z.; He, H. Cytotoxicity induced by abamectin exposure in haemocytes of Chinese mitten crab, Eriocheir sinensis. Environ. Toxicol. Pharmacol. 2020, 77, 103384. [Google Scholar] [CrossRef]
- Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhang, J.; Xie, J.; Yang, L.; Xing, Y.; Li, Z. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2020, 46, 759–770. [Google Scholar] [CrossRef]
- Riceevans, C.; Miller, N.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lotti, M. Clinical toxicology of anticholinesterase agents in humans. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Oliveira, R.; Grisolia, C.K.; Monteiro, M.S.; Soares, A.M.; Domingues, I. Multilevel assessment of ivermectin effects using different zebrafish life stages. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 187, 50–61. [Google Scholar] [CrossRef]
- Olivares-Rubio, H.F.; Espinosa-Aguirre, J.J. Acetylcholinesterase activity in fish species exposed to crude oil hydrocarbons: A review and new perspectives. Chemosphere 2021, 264, 128401. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lin, Q.; Liang, Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Borah, A.; Das, S. Quercetin-induced amelioration of deltamethrin stress in freshwater teleost, Channa punctata: Multiple biomarker analysis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 227, 108626. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Quercetin Levels (g/kg Diet) | ||
|---|---|---|---|
| 0 | 0.4 | 0.8 | |
| Fish meal (72% crude protein) | 85 | 85 | 85 |
| Soybean meal (45% crude protein) | 465 | 465 | 465 |
| Wheat bran | 183 | 183 | 183 |
| Yellow corn | 100 | 100 | 100 |
| Corn oil | 20 | 20 | 20 |
| Cod liver oil | 20 | 20 | 20 |
| Mineral mixture a | 30 | 30 | 30 |
| Vitamin mixture b | 30 | 30 | 30 |
| Starch | 67 | 66.6 | 66.2 |
| Quercetin | 0 | 0.4 | 0.8 |
| Chemical composition (g/kg) | |||
| Dry matter | 915 | ||
| Crude protein | 307 | ||
| Total lipids | 91 | ||
| Crude fiber | 48 | ||
| Total ash | 61 | ||
| Nitrogen free extract (NFE) c | 493 | ||
| Gross energy (kcal/kg diet) d | 4814 | ||
| Items | Red Blood Cells (106/mm3) | White Blood Cells (103/mm3) | Hemoglobin (g/dL) | Hematocrit (%) | Mean Cell Volume (fL) | Mean Corpuscular Hemoglobin (Pg/cell) | Mean Corpuscular Hemoglobin Concentrate (g/dL) |
|---|---|---|---|---|---|---|---|
| Control | 3.74 ± 0.07 a | 0.99 ± 0.05 c | 7.52 ± 0.57 a | 32.71 ± 1.46 a | 110.57 ± 3.21 a | 29.71 ± 0.97 a | 21.23 ± 0.67 a |
| Q400 | 3.71 ± 0.22 ab | 0.96 ± 0.04 d | 7.36 ± 0.86 a | 32.86 ± 1.53 a | 106.7 ± 2.61 b | 28.42 ± 1.03 ab | 20.59 ± 0.16 a |
| Q800 | 3.79 ± 0.13 a | 0.95 ± 0.12 d | 7.43 ± 0.29 a | 31.29 ± 1.15 b | 108.42 ± 2.72 a | 28.12 ± 1.18 ab | 21.12 ± 0.12 a |
| ABM | 1.99 ± 0.05 d | 2.64 ± 0.32 a | 4.22 ± 0.15 c | 20.80 ± 1.17 d | 90.80 ± 1.25 d | 17.87 ± 0.75 c | 12.07 ± 0.15 c |
| ABM + Q400 | 2.87 ± 0.35 c | 1.97 ± 0.24 b | 6.24 ± 0.41 b | 29.31 ± 1.72 c | 101.24 ± 2.16 c | 24.45 ± 1.26 b | 18.24 ± 1.46 b |
| ABM + Q800 | 3.11 ± 0.22 b | 0.97 ± 0.06 c | 7.01 ± 0.52 a | 32.89 ± 1.68 a | 109.22 ± 3.11 a | 29.34 ± 1.19 a | 21.8 ± 1.85 a |
| Items | Total Protein (g/dL) | Albumin (g/dL) | Globulin (g/dL) | Total Lipids (g/dL) | Cholesterol (g/dL) | Glucose (mg/dL) |
|---|---|---|---|---|---|---|
| Control | 5.22 ± 0.67 c | 3.64 ± 0.08 a | 1.58 ± 0.27 d | 33.14 ± 2.18 c | 41.08 ± 3.57 c | 52.02 ± 1.27 bc |
| Q400 | 6.25 ± 0.78 a | 3.39 ± 0.16 bc | 2.86 ± 0.14 b | 35.09 ± 2.23 c | 43.26 ± 3.18 c | 51.36 ± 1.34 c |
| Q800 | 6.56 ± 0.41 a | 3.14 ± 0.12 c | 3.42 ± 0.20 a | 36.04 ± 3.27 c | 45.40 ± 3.05 c | 52.47 ± 1.80 bc |
| ABM | 3.11 ± 0.13 d | 1.43 ± 0.09 d | 1.68 ± 0.25 c | 51.03 ± 2.69 a | 71.50 ± 3.10 a | 70.12 ± 0.86 a |
| ABM + Q400 | 5.89 ± 0.35 b | 3.45 ± 0.10 b | 2.44 ± 0.16 bc | 40.85 ± 2.39 b | 56.42 ± 3.75 b | 55.52 ± 0.98 b |
| ABM + Q800 | 5.20 ± 0.48 c | 3.60 ± 0.03 a | 1.60 ± 0.23 d | 32.80 ± 3.05 c | 42.21 ± 3.46 c | 49.98 ± 1.54 c |
| Items | AST (μ/L) | ALT (μ/L) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|
| Control | 22.17 ± 0.89 c | 63.26 ± 8.28 c | 19.22 ± 1.47 c | 0.25 ± 0.04 c |
| Q400 | 25.60 ± 2.79 c | 63.56 ± 3.48 c | 21.25 ± 1.28 c | 0.27 ± 0.08 c |
| Q800 | 24.43 ± 1.21 c | 62.82 ± 3.60 c | 22.36 ± 1.61 c | 0.27 ± 0.05 c |
| ABM | 73.82 ± 2.45 a | 129.43 ± 6.75 a | 35.43 ± 2.13 a | 0.80 ± 0.09 a |
| ABM + Q400 | 35.11 ± 5.62 b | 94.77 ± 5.20 b | 25.19 ± 0.44 b | 0.42 ± 0.02 b |
| ABM + Q800 | 23.28 ± 1.72 c | 62.29 ± 3.72 c | 18.43 ± 1.25 c | 0.24 ± 0.01 c |
| Items | Lipid Peroxidation (nmol/mg) | Catalase (Ug/mg) | Super Oxide Dismutase (Ug/mg) | Reduced Glutathione (nmol/mg) | Total Antioxidant Capacity (Umol/mg) |
|---|---|---|---|---|---|
| Control | 63.29 ± 2.58 c | 25.24 ± 2.25 a | 12.06 ± 0.74 a | 35.85 ± 2.57 a | 3.99 ± 0.12 a |
| Q400 | 60.43 ± 2.52 c | 24.32 ± 2.31 ab | 11.42 ± 0.69 ab | 34.16 ± 2.24 a | 3.15 ± 0.14 ab |
| Q800 | 64.38 ± 3.19 c | 24.84 ± 2.41 ab | 12.11 ± 0.51 a | 35.06 ± 1.86 a | 3.44 ± 0.13 a |
| ABM | 86.10 ± 2.30 a | 11.81 ± 0.60 c | 7.34 ± 0.15 c | 15.92 ± 0.49 c | 0.99 ± 0.54 d |
| ABM + Q400 | 69.22 ± 3.01 b | 18.35 ± 0.12 c | 10.68 ± 0.26 b | 29.91 ± 1.63 b | 2.85 ± 0.31 c |
| ABM + Q800 | 60.10 ± 3.13 c | 20.99 ± 1.16 b | 12.25 ± 0.60 a | 32.48 ± 1.63 ab | 3.02 ± 0.33 ab |
| Items | Lipid Peroxidation (nmol/mg) | Catalase (Ug/mg) | Super Oxide Dismutase (Ug/mg) | Reduced Glutathione (nmol/mg) | Total Antioxidant Capacity (Umol/mg) |
|---|---|---|---|---|---|
| Control | 72.09 ± 1.96 c | 16.21 ± 1.21 b | 15.74 ± 2.09 a | 28.65 ± 3.27 a | 12.21 ± 0.43 a |
| Q400 | 75.40 ± 1.69 c | 18.02 ± 1.34 a | 15.08 ± 2.74 a | 27.03 ± 3.05 a | 12.19 ± 0.35 a |
| Q800 | 75.30 ± 2.08 c | 18.51 ± 1.41 a | 15.55 ± 1.98 a | 26.58 ± 3.90 a | 13.65 ± 0.63 a |
| ABM | 98.23 ± 2.53 a | 8.26 ± 0.90 d | 9.47 ± 1.78 c | 17.02 ± 0.32 c | 5.53 ± 0.09 c |
| ABM + Q400 | 80.29 ± 1.87 b | 13.28 ± 0.29 c | 12.34 ± 1.46 b | 22.67 ± 2.07 b | 9.41 ± 0.36 b |
| ABM + Q800 | 71.29 ± 2.61 d | 16.30 ± 1.16 b | 15.01 ± 1.31 a | 26.05 ± 3.17 a | 11.18 ± 0.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, A.T.; Mahboub, H.H.; Amen, R.M.; El-Beltagy, M.A.; Ramah, A.; Abdelfattah, A.M.; El-Beltagi, H.S.; Shalaby, T.A.; Ghazzawy, H.S.; Ramadan, K.M.A.; et al. Ameliorative Effect of Quercetin against Abamectin-Induced Hemato-Biochemical Alterations and Hepatorenal Oxidative Damage in Nile Tilapia, Oreochromis niloticus. Animals 2022, 12, 3429. https://doi.org/10.3390/ani12233429
Mansour AT, Mahboub HH, Amen RM, El-Beltagy MA, Ramah A, Abdelfattah AM, El-Beltagi HS, Shalaby TA, Ghazzawy HS, Ramadan KMA, et al. Ameliorative Effect of Quercetin against Abamectin-Induced Hemato-Biochemical Alterations and Hepatorenal Oxidative Damage in Nile Tilapia, Oreochromis niloticus. Animals. 2022; 12(23):3429. https://doi.org/10.3390/ani12233429
Chicago/Turabian StyleMansour, Abdallah Tageldein, Heba H. Mahboub, Rehab M. Amen, Marwa A. El-Beltagy, Amany Ramah, Abdelfattah M. Abdelfattah, Hossam S. El-Beltagi, Tarek A. Shalaby, Hesham S. Ghazzawy, Khaled M. A. Ramadan, and et al. 2022. "Ameliorative Effect of Quercetin against Abamectin-Induced Hemato-Biochemical Alterations and Hepatorenal Oxidative Damage in Nile Tilapia, Oreochromis niloticus" Animals 12, no. 23: 3429. https://doi.org/10.3390/ani12233429
APA StyleMansour, A. T., Mahboub, H. H., Amen, R. M., El-Beltagy, M. A., Ramah, A., Abdelfattah, A. M., El-Beltagi, H. S., Shalaby, T. A., Ghazzawy, H. S., Ramadan, K. M. A., Alhajji, A. H. M., & Hamed, H. S. (2022). Ameliorative Effect of Quercetin against Abamectin-Induced Hemato-Biochemical Alterations and Hepatorenal Oxidative Damage in Nile Tilapia, Oreochromis niloticus. Animals, 12(23), 3429. https://doi.org/10.3390/ani12233429













