Gills Just Want to Have Fun: Can Fish Play Games, Just like Us?
Abstract
Simple Summary
Abstract
1. Introduction
- The behavior is “incompletely functional” and does not contribute to immediate survival.
- The behavior is voluntary, spontaneous, intentional, and performed for its own sake.
- The behavior may resemble completely functional behaviors, but it differs in at least one respect, such as context, or is somehow incomplete, exaggerated, or awkward.
- The behavior is repeated consistently during at least a portion of the animal’s life but is not pathological.
- The behavior is begun in the absence of stress, hunger, predation, or circumstances that are otherwise unhealthy.
2. Materials and Methods
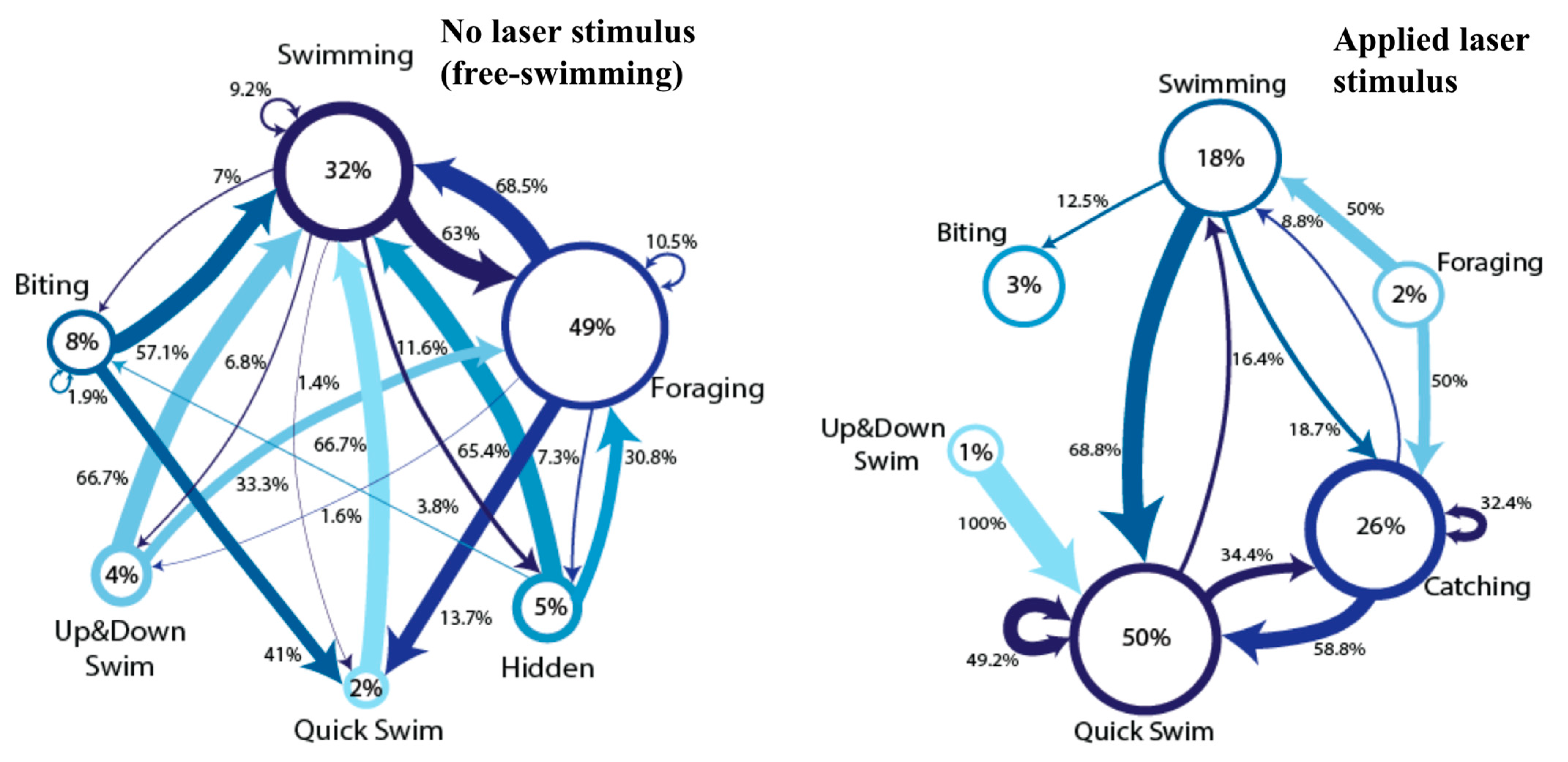
3. Results
4. Discussion
- Chasing, orienting toward, and attempting to catch the laser pointer dots is not likely to be a behavior that contributes to survival, and the repetitive nature of the behavior suggests that it is not intended to serve an immediate function. However, it could also be aggression toward the dot, as it is an unknown stimulus. We note that a cat chasing a laser spot could also be considered an aggressive behavior, but this behavior is considered play [19].
- The fish were not forced, trained, or enticed to interact with the laser stimuli, proving the voluntary and spontaneous nature of the behavior.
- The incomplete behavior criterion is perhaps the most difficult to state as being concretely met in our studies. While laser pointer chasing behaviors performed by the fish did appear to be different from those present during displays of aggression toward other fish (behaviors were repeated in quicker conjunction and performed for longer with the laser stimulus), were the fish playing with the laser stimulus, or merely investigating it as being a potential threat or food? Since the interested fish could quickly determine that the laser stimulus was neither food nor a threat but continued to interact with the dot, we can state that this behavior does resemble functional behaviors but may differ in intent. Detailed experiments separating the motivation of the fish regarding the laser pointer stimulus would clarify this point.
- The observed behaviors were repeated by fish studied throughout multiple trials in our home laboratory. However, loss of interest was observed in our laboratory fish over time, indicating that the behaviors were not pathologically stereotyped.
- All fish tested were healthy, well-kept, and well-fed, so we can surmise that interactions with laser stimuli were not stress responses.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burghardt, G.M.; Dinets, V.; Murphy, J.B. Highly repetitive object play in a cichlid fish (Tropheus duboisi). Ethology 2015, 121, 38–44. [Google Scholar] [CrossRef]
- Gunter, G. Observations on fish turning flips over a line. Copeia 1953, 1953, 188–190. [Google Scholar] [CrossRef]
- Ladiges, W. Der Sterlet im Aquarium. Die Aquar. Und Terr. Z. 1954, 7, 200–202. [Google Scholar]
- Nilsson, G. Brain and body oxygen requirements of Gnathonemus petersii, a fish with an exceptionally large brain. J. Exp. Biol. 1996, 199, 603–607. [Google Scholar] [CrossRef]
- Meyer-Holzapfel, M. Über das Spiel bei Fischen, insbesondere beim Tapirrüsselfisch (Mormyrus kannume Forskal). Zool. Gart. 1960, 25, 189–202. [Google Scholar]
- Burghardt, G.M. The Genesis of Animal Play: Testing the Limits; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Bernardi, G. The use of tools by wrasses (Labridae). Coral Reefs. 2012, 31, 39. [Google Scholar] [CrossRef]
- Jones, A.M.; Brown, C.; Gardner, S. Tool use in the tuskfish Choerodon schoenleinii? Coral Reefs-J. Int. Soc. Reef Stud. 2011, 30, 865. [Google Scholar] [CrossRef]
- Cohn, E.; Cole, P.; Haymaker, A.; Garner, A.M.; Londraville, R.L. Response to underwater laser pointer in the Orange-finned Anemonefish Amphiprion chrysopterus and three-spot damselfish Dascyllus trimaculatus. J. Fish. Biol. 2020, 96, 274–277. [Google Scholar] [CrossRef]
- Davies, P.; Sheehan, E.V. Laser chasing behaviour of wild fishes exploited as a tool to compare space use between size, sex and species. J. Appl. Ichthyol. 2019, 35, 1225–1233. [Google Scholar] [CrossRef]
- Frid, A.; McGreer, M.; Frid, T. Chasing the light: Positive bias in camera-based surveys of groundfish examined as risk-foraging trade-offs. Biol. Conserv. 2019, 231, 133–138. [Google Scholar] [CrossRef]
- Ioannou, C.C.; Guttal, V.; Couzin, I.D. Predatory fish select for coordinated collective motion in virtual prey. Science 2012, 337, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Hotta, T.; Kawasaka, K.; Satoh, S.; Kohda, M. Fish focus primarily on the faces of other fish. Sci. Rep. 2019, 9, 8377. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, G.M. Defining and recognizing play. In The Oxford Handbook of the Development of Play; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Cavraro, F.; Torricelli, P.; Malavasi, S. Quantitative ethogram of male reproductive behavior in the South European toothcarp Aphanius fasciatus. Biol Bull. 2013, 225, 71–78. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Hamilton, M.S.; Huang, T.; Kristan, W.B.; French, K.A. A hormone-activated central pattern generator for courtship. Curr Biol. 2010, 20, 487–495. [Google Scholar] [CrossRef][Green Version]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Kogan, L.R.; Grigg, E.K. Laser Light Pointers for Use in Companion Cat Play: Association with Guardian-Reported Abnormal Repetitive Behaviors. Animals 2021, 11, 2178. [Google Scholar] [CrossRef]
- Marshall, J. Vision and lack of vision in the ocean. Curr. Biol. 2017, 27, R494–R502. [Google Scholar] [CrossRef]
- Hunt, D.M.; Fitzgibbon, J.; Slobodyanyuk, S.J.; Bowmakers, J.K. Spectral tuning and molecular evolution of rod visual pigments in the species flock of cottoid fish in Lake Baikal. Vis. Res. 1996, 36, 1217–1224. [Google Scholar] [CrossRef]
- Kullander, S.O. Cichlids: Astronotus Ocellatus; Swedish Museum of Natural History: Stockholm, Sweden, 2007. [Google Scholar]
- Carleton, K.L.; Kocher, T.D. Cone opsin genes of African cichlid fishes: Tuning spectral sensitivity by differential gene expression. Mol. Biol. Evol. 2001, 18, 1540–1550. [Google Scholar] [CrossRef]
- Marshall, N.J.; Vorobyev, M. The design of color signals and color vision in fishes. In Sensory Processing in Aquatic Environments; Springer: New York, NY, USA, 2003; pp. 194–222. [Google Scholar]
- Burghardt, G.M. Play in fishes, frogs and reptiles. Curr. Biol. 2015, 25, R9–R10. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Burghardt, G.M. Precocious courtship and play in emydid turtles. Ethology 1998, 104, 38–56. [Google Scholar] [CrossRef]
- Burghardt, G.M.; Ward, B.; Rosscoe, R. Problem of reptile play: Environmental enrichment and play behavior in a captive Nile soft-shelled turtle, Trionyx triunguis. Zoo Biol. Publ. Affil. Am. Zoo Aquar. Assoc. 1996, 15, 223–238. [Google Scholar] [CrossRef]
- Lazell, J.D., Jr.; Spitzer, N.C. Apparent play behavior in an American alligator. Copeia 1977, 16, 188. [Google Scholar] [CrossRef]
- Augustine, L.; MIller, K.; Burghardt, G.M. Crocodylus rhombifer (Cuban crocodile) play behavior. Herpetol. Rev. 2015, 46, 208–209. [Google Scholar]
- Barabanov, V.; Gulimova, V.; Berdiev, R.; Saveliev, S. Object play in thick-toed geckos during a space experiment. J. Ethol. 2015, 33, 109–115. [Google Scholar] [CrossRef]
- Kane, D.; Davis, A.C.; Michaels, C.J. Play behaviour by captive tree monitors, Varanus macraei and Varanus prasinus. Herpetol. Bull. 2019, 149, 28–31. [Google Scholar] [CrossRef]
- Zylinski, S. Fun and play in invertebrates. Curr. Biol. 2015, 25, R10–R12. [Google Scholar] [CrossRef]
- Kuba, M.J.; Byrne, R.A.; Meisel, D.V.; Mather, J.A. When do octopuses play? Effects of repeated testing, object type, age, and food deprivation on object play in Octopus vulgaris. J. Comp. Psychol. 2006, 120, 184. [Google Scholar] [CrossRef]
- Dapporto, L.; Turillazzi, S.; Palagi, E. Dominance interactions in young adult paper wasp (Polistes dominulus) foundresses: A playlike behavior? J. Comp. Psychol. 2006, 120, 394. [Google Scholar] [CrossRef]
- Pruitt, J.N.; Burghardt, G.M.; Riechert, S.E. Non-conceptive sexual behavior in spiders: A form of play associated with body condition, personality type, and male intrasexual selection. Ethology 2012, 118, 33–40. [Google Scholar] [CrossRef]
- Futuyma, D.; Kirkpatrick, M. Evolution; Sinauer: Sunderland, MA, USA, 2017. [Google Scholar]
- Johanson, Z.; Underwood, C.; Richter, M. (Eds.) Evolution and Development of Fishes; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
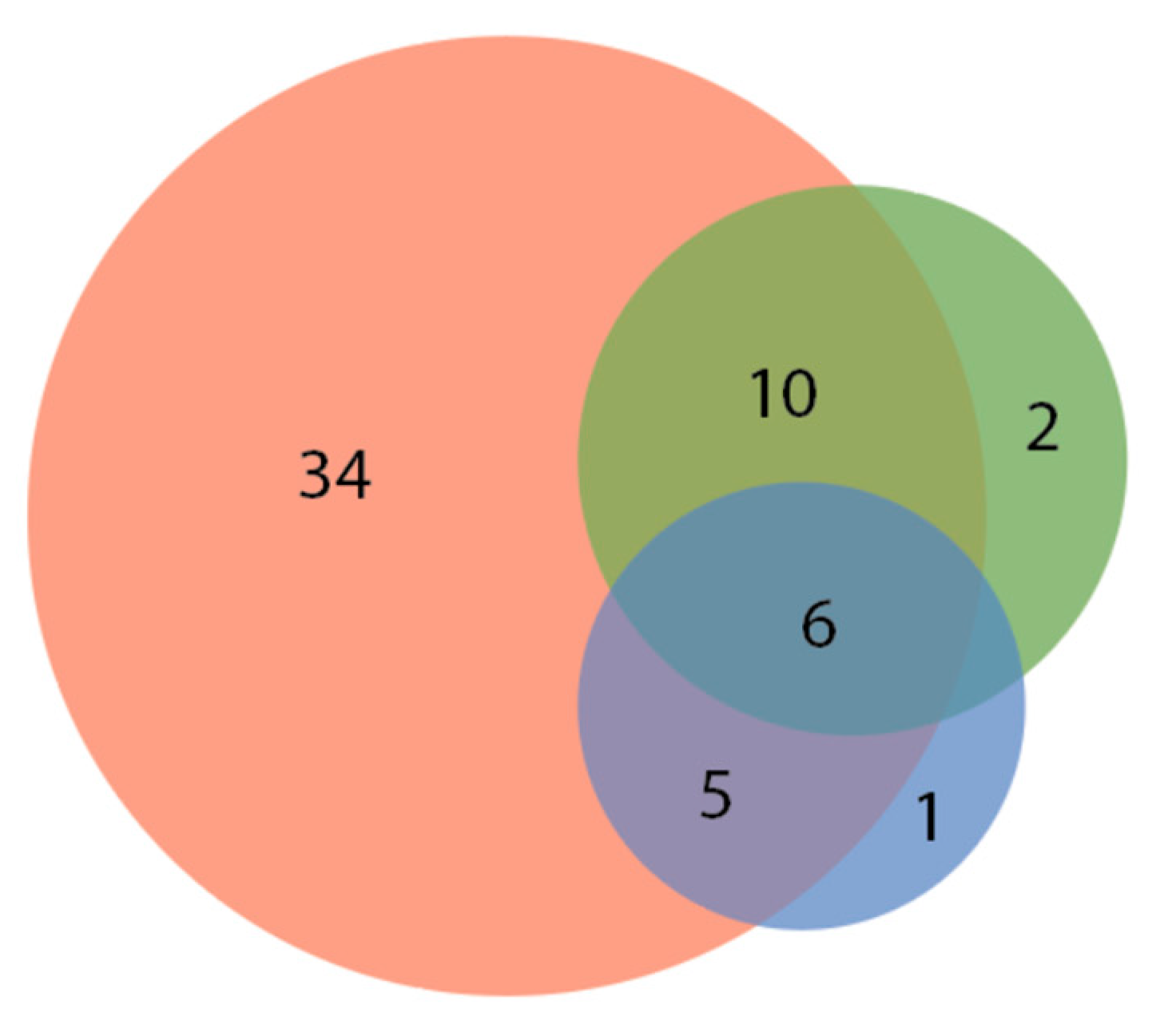

| No Response 12.1% (8 of 66) Aulonocara Betta splendens Carassius auratus Carassius stratus species Cichlidae (REG assorted) Hemigrammus rhodostomus Phoxinus phoxinus Rocio octofasciata | Some Response: brief change in swimming direction / orienting towards the laser pointer. 22.7% (15 of 66) Amphiprion ocellaris Astronotus ocellatus Aulonocara Carassius auratus (2 varieties) Cyprinus rubrofuscus Elacatinus evelynae Epalazeorhychnos frenatum Gymnocorymbus ternetzi Pethia padamya Poecilia reticulata Pterophyllum Sudotropheus demasoni Trichopodus trichopterus Siphophorus maculatus | Moderate Response: following and investigating laser pointer for up to five seconds. 42.4% (28 of 66) Desmopuntius johorensis Amblyglyphidodon aureus Chromis viridis Cichlidae (SM assorted) Cichlidae (MD assorted) Coilsa lalia Corydoras panda Danio rerio Gymnocorymbus ternetzi Hyphessobrycon eques (2 varieties) Hyphessobrycon flammeus Labidochromis cichlid Melanochromis johanni Nimbochromis venustus Poecilla latipinna Poecilla reticulata (4 varieties) Pseudotropheus crabro Sphaeramia nematoptera Tanichthys albonubes Trichogaster lalius Xiphophorus maculatus (4 varieties) | High Response: interacting with the laser pointer for at least five or more seconds. 22.7% (15 of 66) Amphiprion ocellaris Devario aequipinnatus * Epalzeorhynchos frenatum Gramma loreto Gymnocorymbus ternetzi Melanotaenildae Phenacrogrammus interruptus Pseucochromis fridmani Puntius aurilius Puntigrus tetrazona (5 varieties) Xiphophorus maculatus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenbeiser, S.; Serbe-Kamp, É.; Gage, G.J.; Marzullo, T.C. Gills Just Want to Have Fun: Can Fish Play Games, Just like Us? Animals 2022, 12, 1684. https://doi.org/10.3390/ani12131684
Eisenbeiser S, Serbe-Kamp É, Gage GJ, Marzullo TC. Gills Just Want to Have Fun: Can Fish Play Games, Just like Us? Animals. 2022; 12(13):1684. https://doi.org/10.3390/ani12131684
Chicago/Turabian StyleEisenbeiser, Sofia, Étienne Serbe-Kamp, Gregory J. Gage, and Timothy C. Marzullo. 2022. "Gills Just Want to Have Fun: Can Fish Play Games, Just like Us?" Animals 12, no. 13: 1684. https://doi.org/10.3390/ani12131684
APA StyleEisenbeiser, S., Serbe-Kamp, É., Gage, G. J., & Marzullo, T. C. (2022). Gills Just Want to Have Fun: Can Fish Play Games, Just like Us? Animals, 12(13), 1684. https://doi.org/10.3390/ani12131684






