Relationship between Bone Stability and Egg Production in Genetically Divergent Chicken Layer Lines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Note
2.2. Animals and Housing
2.3. Experimental Procedure
2.4. Statistical Analysis
3. Results
3.1. Basic Production Parameters
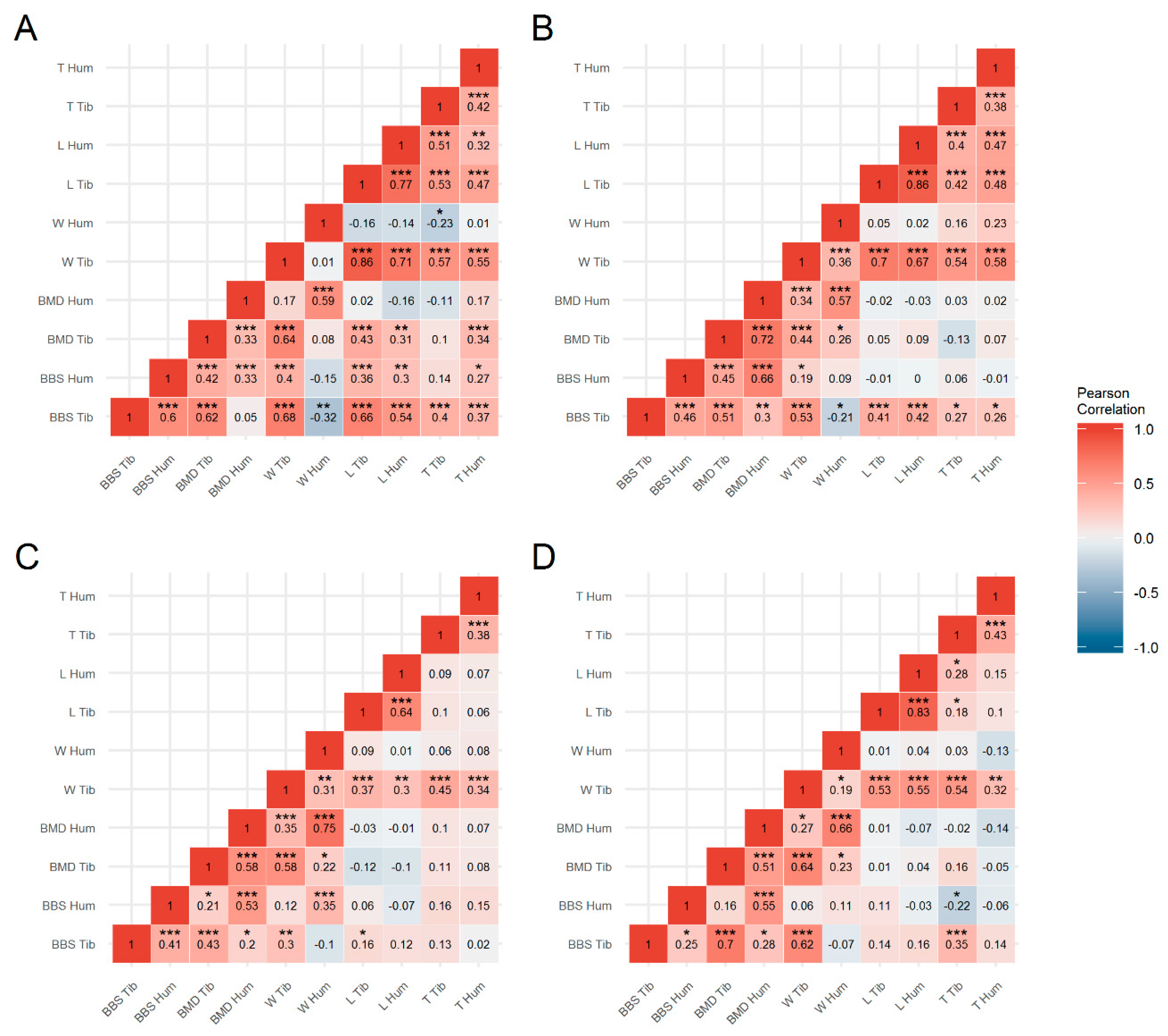
3.2. Bone Characteristics
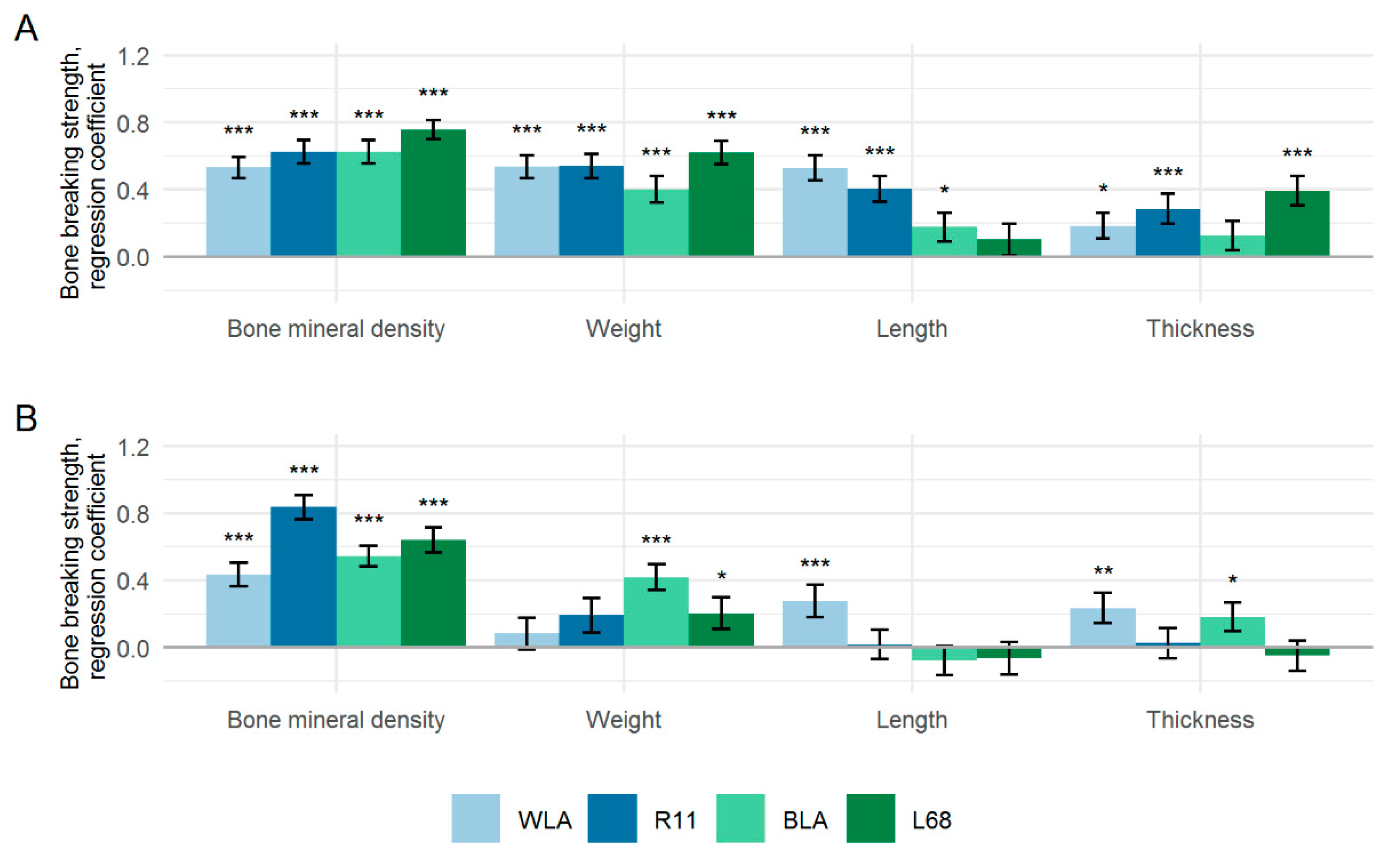
3.3. Factors Affecting Bone Strength
3.4. Genetic Parameters
4. Discussion
4.1. Phenotypic Characterization
4.2. Determinants of Bone Stability
4.3. Genetic Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Preisinger, R. Innovative layer genetics to handle global challenges in egg production. Br. Poult. Sci. 2018, 59, 1–6. [Google Scholar] [CrossRef]
- Roberts, J.R. Factors affecting egg internal quality and egg shell quality in laying hens. J. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Fleming, R.H.; McCormack, H.A.; McTeir, L.; Whitehead, C.C. Relationships between genetic, environmental and nutritional factors influencing osteoporosis in laying hens. Br. Poult. Sci. 2006, 47, 742–755. [Google Scholar] [CrossRef]
- Whitehead, C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dacke, C.G.; Arkle, S.; Cook, D.J.; Wormstone, I.M.; Jones, S.; Zaidi, M.; Bascal, Z.A. Medullary bone and avian calcium regulation. J. Exp. Biol. 1993, 184, 63–88. [Google Scholar]
- Wilson, S.; Thorp, B.H. Estrogen and cancellous bone loss in the fowl. Calcif. Tissue Int. 1998, 62, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C.; Fleming, R.H. Osteoporosis in cage layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef]
- Cransberg, P.H.; Parkinson, G.B.; Wilson, S.; Thorp, B.H. Sequential studies of skeletal calcium reserves and structural bone volume in a commercial layer flock. Br. Poult. Sci. 2001, 42, 260–265. [Google Scholar] [CrossRef]
- Webster, A.B. Welfare implications of avian osteoporosis. Poult. Sci. 2004, 83, 184–192. [Google Scholar] [CrossRef]
- Sandilands, V. The laying hen and bone fractures. Vet. Rec. 2011, 169, 411. [Google Scholar] [CrossRef]
- Gregory, N.G.; Wilkins, L.J. Broken bones in domestic fowl: Handling and processing damage in end-of-lay battery hens. Br. Poult. Sci. 1989, 30, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.T.; Guerin, M.T.; Widowski, T.M. On-farm comparison of keel fracture prevalence and other welfare indicators in conventional cage and floor-housed laying hens in Ontario, Canada. Poult. Sci. 2015, 94, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Riber, A.B.; Casey-Trott, T.M.; Herskin, M.S. The influence of keel bone damage on welfare of laying hens. Front. Vet. Sci. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.A.F.; Nicol, C.J.; Murrell, J.C. Do laying hens with keel bone fractures experience pain? PLoS ONE 2012, 7, e42420. [Google Scholar] [CrossRef]
- McCoy, M.A.; Reilly, G.A.C.; Kilpatrick, D.J. Density and breaking strength of bones of mortalities among caged layers. Res. Vet. Sci. 1996, 60, 185–186. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Murrell, J.; Nicol, C.J. The effect of keel fractures on egg production, feed and water consumption in individual laying hens. Br. Poult. Sci. 2013, 54, 165–170. [Google Scholar] [CrossRef]
- Candelotto, L.; Stratmann, A.; Gebhardt-Henrich, S.G.; Rufener, C.; van de Braak, T.; Toscano, M.J. Susceptibility to keel bone fractures in laying hens and the role of genetic variation. Poult. Sci. 2017, 96, 3517–3528. [Google Scholar] [CrossRef]
- Raymond, B.; Johansson, A.M.; McCormack, H.A.; Fleming, R.H.; Schmutz, M.; Dunn, I.C.; De Koning, D.J. Genome-wide association study for bone strength in laying hens. J. Anim. Sci. 2018, 96, 2525–2535. [Google Scholar] [CrossRef]
- Bishop, S.C.; Fleming, R.H.; McCormack, H.A.; Flock, D.K.; Whitehead, C.C. Inheritance of bone characteristics affecting osteoporosis in laying hens. Br. Poult. Sci. 2000, 41, 33–40. [Google Scholar] [CrossRef]
- Rufener, C.; Baur, S.; Stratmann, A.; Toscano, M.J. Keel bone fractures affect egg laying performance but not egg quality in laying hens housed in a commercial aviary system. Poult. Sci. 2019, 98, 1589–1600. [Google Scholar] [CrossRef]
- Habig, C.; Baulain, U.; Henning, M.; Scholz, A.; Sharifi, A.; Janisch, S.; Simianer, H.; Weigend, S. How bone stability in laying hens is affected by phylogenetic background and performance level. Eur. Poult. Sci. 2017, 81. [Google Scholar] [CrossRef]
- Hocking, P.M.; Bain, M.; Channing, C.E.; Fleming, R.; Wilson, S. Genetic variation for egg production, egg quality and bone strength in selected and traditional breeds of laying fowl. Br. Poult. Sci. 2003, 44, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Thorp, B.H. Skeletal disorders in the fowl: A review. Avian Pathol. 1994, 23, 203–236. [Google Scholar] [CrossRef] [PubMed]
- Eusemann, B.K.; Patt, A.; Schrader, L.; Weigend, S.; Thöne-Reineke, C.; Petow, S. The role of egg production in the etiology of keel bone damage in laying hens. Front. Vet. Sci. 2020, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.M.; Kanis, E.; Noordhuizen-Stassen, E.N.; Grommers, F.J. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Jensen, P.; Andersson, L. Genomics meets ethology: A new route to understanding domestication, behavior, and sustainability in animal breeding. Ambio 2005, 34, 320–324. [Google Scholar] [CrossRef]
- Hocking, P.M. Unexpected consequences of genetic selection in broilers and turkeys: Problems and solutions. Br. Poult. Sci. 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Mirkena, T.; Duguma, G.; Haile, A.; Tibbo, M.; Okeyo, A.M.; Wurzinger, M.; Sölkner, J. Genetics of adaptation in domestic farm animals: A review. Livest. Sci. 2010, 132, 1–12. [Google Scholar] [CrossRef]
- Van der Waaij, E.H. A resource allocation model describing consequences of artificial selection under metabolic stress. J. Anim. Sci. 2004, 82, 973–981. [Google Scholar] [CrossRef]
- Tixier-Boichard, M.; Bed’hom, B.; Rognon, X. Chicken domestication: From archeology to genomics. C. R. Biol. 2011, 334, 197–204. [Google Scholar] [CrossRef]
- Malomane, D.K.; Simianer, H.; Weigend, A.; Reimer, C.; Schmitt, A.O.; Weigend, S. The SYNBREED chicken diversity panel: A global resource to assess chicken diversity at high genomic resolution. BMC Genom. 2019, 20, 345. [Google Scholar] [CrossRef] [PubMed]
- Eusemann, B.K.; Baulain, U.; Schrader, L.; Thöne-Reineke, C.; Patt, A.; Petow, S. Radiographic examination of keel bone damage in living laying hens of different strains kept in two housing systems. PLoS ONE 2018, 13, e0194974. [Google Scholar] [CrossRef] [PubMed]
- Lieboldt, M.-A.; Halle, I.; Frahm, J.; Schrader, L.; Baulain, U.; Henning, M.; Preisinger, R.; Dänicke, S.; Weigend, S. Phylogenic versus selection effects on growth development, egg laying and egg quality in purebred laying hens. Eur. Poult. Sci. 2015, 79. [Google Scholar] [CrossRef]
- GfE. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Broiler des Ausschuss für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie; DLG-Verlag: Frankfurt am Main, Germany, 1999. [Google Scholar]
- European Union. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Commun. 2003, L268, 29–43. [Google Scholar]
- EFSA Panel on Additives Products or Substances used in Animal Feed. Scientific opinion on the safety and efficacy of vitamin D3 (cholecalciferol) as a feed additive for all animal species or categories based on a dossier submitted by Lohmann Animal Health GmbH. EFSA J. 2014, 12, 3568. [Google Scholar] [CrossRef]
- Asuero, A.G.; Sayago, A.; González, A.G. The correlation coefficient: An overview. Crit. Rev. Anal. Chem. 2006, 36, 41–59. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006. [Google Scholar]
- SAS Institute Inc. Chapter 47: The GLMSELECT Procedure. In SAS/STAT 13.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2013; pp. 3751–3752. [Google Scholar]
- Gilmour, A.R.; Gogel, B.J.; Cullis, B.R.; Welham, S.J.; Thompson, R. ASReml User Guide Release 4.1 Structural Specication; VSN International Ltd.: Hemel Hempstead, UK, 2015. [Google Scholar]
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef]
- Thiruvenkadan, A.K.; Panneerselvam, S.; Prabakaran, R. Layer breeding strategies: An overview. Worlds Poult. Sci. J. 2010, 66, 477–502. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kikuchi, H.; Hiyama, S.; Nishizawa, K.; Kusuhara, S. Expression and localisation of calbindin D28k in all intestinal segments of the laying hen. Br. Poult. Sci. 2007, 48, 233–238. [Google Scholar] [CrossRef]
- Riczu, C.M.; Saunders-Blades, J.L.; Yngvesson, A.K.; Robinson, F.E.; Korver, D.R. End-of-cycle bone quality in white- and brown-egg laying hens. Poult. Sci. 2004, 83, 375–383. [Google Scholar] [CrossRef]
- Schreiweis, M.; Orban, J.; Ledur, M.; Hester, P. The use of densitometry to detect differences in bone mineral density and content of live White Leghorns fed varying levels of dietary calcium. Poult. Sci. 2003, 82, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C.; Fleming, R.H.; Julian, R.J.; Sørensen, P. Skeletal problems associated with selection for increased production. In Poultry Genetics, Breeding and Biotechnology; Muir, W.M., Aggrey, S.E., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 29–52. [Google Scholar]
- Jendral, M.J.; Korver, D.R.; Church, J.S.; Feddes, J.J.R. Bone mineral density and breaking strength of White Leghorns housed in conventional, modified, and commercially available colony battery cages. Poult. Sci. 2008, 87, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. Bone strength: What are we trying to measure? Calcif. Tissue Int. 2001, 68, 205–210. [Google Scholar] [CrossRef]
- Zhang, B.; Coon, C. The relationship of various tibia bone measurements in hens. Poult. Sci. 1997, 76, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Ammann, P.; Rizzoli, R. Bone strength and its determinants. Osteoporos. Int. 2003, 14, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Dunn, I.C.; Andersson, B.; Fleming, R.H.; De Koning, D.J.; McCormack, H.M.; Rodriguez-Navarro, A.B.; Schmutz, M.; Wilson, P.W. Quality of the laying hen skeleton; insights and solutions from genetics. In Proceedings of the 17th International Conference on Production Diseases in Farm Animals, Bern, Switzerland, 27–29 June 2019; Bruckmaier, R.M., Gross, J.J., Eds.; Bern University: Bern, Switzerland, 2019; p. 50. [Google Scholar]
- Clark, W.D.; Cox, W.R.; Silversides, F.G. Bone fracture incidence in end-of-lay high-producing, noncommercial laying hens identified using radiographs. Poult. Sci. 2008, 87, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt-Henrich, S.G.; Fröhlich, E.K. Early onset of laying and bumblefoot favor keel bone fractures. Animals 2015, 5, 1192–1206. [Google Scholar] [CrossRef]
- Rennie, J.S.; Fleming, R.H.; McCormack, H.A.; McCorquodale, C.C.; Whitehead, C.C. Studies on effects of nutritional factors on bone structure and osteoporosis in laying hens. Br. Poult. Sci. 1997, 38, 417–424. [Google Scholar] [CrossRef] [PubMed]


| Effect | Laying Maturity (Weeks) | Total Number of Eggs 1 | Egg Weight (g) 2 | Eggshell Weight (g) 2 | Eggshell Proportion (%) 2 | Total Eggshell Production (g) 3 | Daily Feed Consumption (g) | Feed-to-Egg Conversion Rate | Feed-to-Eggshell Conversion Rate |
|---|---|---|---|---|---|---|---|---|---|
| Layer line (LL) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| WLA | 20.56 ± 0.15 c | 316.34 ± 2.57 a | 57.63 ± 0.41b | 6.38 ± 0.04 a | 11.10 ± 0.06 a | 2019.89 ± 16.23 a | 93.82 ± 0.97 b | 1.84 ± 0.02 c | 16.64 ± 0.29 d |
| R11 | 24.66 ± 0.15 a | 226.25 ± 2.53 b | 51.54 ± 0.41c | 5.29 ± 0.04 c | 10.28 ± 0.06 b | 1197.95 ± 16.00 c | 79.58 ± 0.96 c | 2.47 ± 0.02 b | 24.14 ± 0.29 b |
| BLA | 20.69 ± 0.15 c | 317.32 ± 2.55 a | 60.09 ± 0.41a | 6.14 ± 0.04 b | 10.25 ± 0.06 b | 1950.23 ± 16.08 b | 102.46 ± 0.98 a | 1.92 ± 0.02 c | 18.80 ± 0.29 c |
| L68 | 23.12 ± 0.15 b | 215.94 ± 2.57 c | 53.05 ± 0.41c | 5.29 ± 0.04 c | 9.95 ± 0.06 c | 1137.60 ± 16.27 d | 91.32 ± 0.98 b | 2.88 ± 0.02 a | 29.04 ± 0.29 a |
| Generation (Gen) | <0.0001 | 0.8384 | 0.0032 | <0.0001 | <0.0001 | <0.0001 | 0.0017 | 0.3673 | <0.0001 |
| Gen1 | 21.81 ± 0.11 | 269.23 ± 1.82 | 54.97 ± 0.29 | 5.94 ± 0.03 | 10.80 ± 0.05 | 1619.31 ± 11.53 | 90.25 ± 0.69 | 2.27 ± 0.02 | 21.14 ± 0.21 |
| Gen2 | 22.70 ± 0.11 | 268.70 ± 1.79 | 56.19 ± 0.29 | 5.62 ± 0.03 | 9.99 ± 0.05 | 1533.53 ± 11.30 | 93.33 ± 0.68 | 2.29 ± 0.02 | 23.17 ± 0.20 |
| LL × Gen | 0.4859 | 0.2202 | 0.0470 | 0.0028 | 0.4750 | 0.1823 | 0.6130 | 0.9523 | 0.2921 |
| WLA × Gen1 | 20.01 ± 0.22 | 319.87 ± 3.64 | 56.16 ± 0.59 b | 6.41 ± 0.06 a | 11.42 ± 0.09 | 2048.99 ± 22.99 | 91.57 ± 1.38 | 1.82 ± 0.03 | 16.00 ± 0.41 |
| WLA × Gen2 | 21.10 ± 0.22 | 312.80 ± 3.63 | 59.11 ± 0.58 a | 6.37 ± 0.06 a | 10.78 ± 0.09 | 1990.80 ± 22.91 | 96.06 ± 1.37 | 1.86 ± 0.03 | 17.27 ± 0.41 |
| R11 × Gen1 | 24.42 ± 0.21 | 224.59 ± 3.58 | 51.44 ± 0.58 c | 5.51 ± 0.06 c | 10.71 ± 0.09 | 1237.57 ± 22.66 | 78.85 ± 1.36 | 2.47 ± 0.03 | 23.09 ± 0.41 |
| R11 × Gen2 | 24.90 ± 0.22 | 227.92 ± 3.64 | 51.64 ± 0.58 c | 5.08 ± 0.06 d | 9.84 ± 0.09 | 1158.33 ± 22.58 | 80.30 ± 1.37 | 2.47 ± 0.03 | 25.20 ± 0.41 |
| BLA × Gen1 | 20.20 ± 0.22 | 319.53 ± 3.65 | 59.28 ± 0.59 a | 6.33 ± 0.06 a | 10.70 ± 0.09 | 2023.14 ± 23.10 | 101.39 ± 1.41 | 1.91 ± 0.04 | 17.85 ± 0.42 |
| BLA × Gen2 | 21.19 ± 0.21 | 315.12 ± 3.56 | 60.91 ± 0.58 a | 5.95 ± 0.06 b | 9.80 ± 0.09 | 1877.31 ± 22.38 | 103.53 ± 1.35 | 1.93 ± 0.03 | 19.74 ± 0.40 |
| L68 × Gen1 | 22.62 ± 0.22 | 212.90 ± 3.70 | 53.00 ± 0.59 c | 5.49 ± 0.06 c | 10.37 ± 0.09 | 1167.53 ± 23.45 | 89.21 ± 1.41 | 2.86 ± 0.04 | 27.62 ± 0.42 |
| L68 × Gen2 | 23.62 ± 0.22 | 218.99 ± 3.58 | 53.10 ± 0.58 c | 5.06 ± 0.06 d | 9.54 ± 0.09 | 1107.67 ± 22.56 | 93.43 ± 1.36 | 2.89 ± 0.03 | 30.46 ± 0.40 |
| Effect | Tibiotarsus | Humerus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bone Breaking Strength (N) | Bone Mineral Density (g/cm²) | Weight (g) | Length (mm) | Thickness (mm) | Bone Breaking Strength (N) | Bone Mineral Density (g/cm²) | Weight (g) | Length (mm) | Thickness (mm) | |
| Layer line (LL) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| WLA | 137.34 ± 3.62 b,c | 0.211 ± 0.005 d | 8.12 ± 0.11 c | 116.78 ± 0.44 c | 5.27 ± 0.04 c | 90.81 ± 3.43 c | 0.136 ± 0.003 d | 3.76 ± 0.10 c | 75.81 ± 0.25 c | 5.33 ± 0.03 c |
| R11 | 149.40 ± 3.54 b | 0.231 ± 0.005 c | 8.23 ± 0.11 c | 116.63 ± 0.44 c | 5.17 ± 0.04 c | 109.94 ± 3.40 b | 0.156 ± 0.003 c | 4.07 ± 0.10 c | 75.38 ± 0.25 c | 5.19 ± 0.03 d |
| BLA | 124.23 ± 3.58 c | 0.265 ± 0.005 b | 10.90 ± 0.11 b | 119.41 ± 0.44 b | 5.94 ± 0.04 b | 138.64 ± 3.40 a | 0.197 ± 0.003 a | 6.53 ± 0.10 a | 79.69 ± 0.25 a | 5.59 ± 0.03 b |
| L68 | 211.57 ± 3.61 a | 0.327 ± 0.005 a | 12.03 ± 0.11 a | 121.37 ± 0.44 a | 6.19 ± 0.04 a | 146.02 ± 3.45 a | 0.180 ± 0.003 b | 4.93 ± 0.10 b | 76.85 ± 0.25 b | 5.71 ± 0.03 a |
| Generation (Gen) | <0.0001 | 0.0369 | 0.7077 | 0.0002 | 0.1231 | <0.0001 | <0.0001 | <0.0001 | 0.0021 | 0.0327 |
| Gen 1 | 139.23 ± 2.55 | 0.264 ± 0.004 | 9.80 ± 0.08 | 117.74 ± 0.31 | 5.61 ± 0.03 | 111.27 ± 2.43 | 0.174 ± 0.002 | 5.60 ± 0.07 | 76.54 ± 0.18 | 5.42 ± 0.02 |
| Gen 2 | 172.04 ± 2.53 | 0.253 ± 0.004 | 9.84 ± 0.07 | 119.36 ± 0.31 | 5.67 ± 0.03 | 131.43 ± 2.40 | 0.161 ± 0.002 | 4.05 ± 0.07 | 77.32 ± 0.18 | 5.49 ± 0.02 |
| LL × Gen | 0.0925 | 0.1725 | 0.0155 | 0.0892 | 0.4924 | 0.5249 | 0.8472 | 0.5376 | 0.2413 | 0.0665 |
| WLA × Gen1 | 113.51 ± 5.10 | 0.210 ± 0.007 | 7.84 ± 0.15 c | 115.03 ± 0.62 | 5.20 ± 0.05 | 77.13 ± 4.85 | 0.142 ± 0.004 | 4.51 ± 0.15 | 75.00 ± 0.36 | 5.27 ± 0.04 |
| WLA × Gen2 | 161.16 ± 5.15 | 0.214 ± 0.007 | 8.40 ± 0.15 c | 118.54 ± 0.62 | 5.35 ± 0.05 | 104.48 ± 4.85 | 0.131 ± 0.004 | 3.02 ± 0.15 | 76.61 ± 0.36 | 5.40 ± 0.04 |
| R11 × Gen1 | 132.92 ± 5.02 | 0.233 ± 0.007 | 8.25 ± 0.15 c | 116.22 ± 0.61 | 5.15 ± 0.05 | 103.33 ± 4.78 | 0.162 ± 0.004 | 4.89 ± 0.15 | 74.99 ± 0.35 | 5.21 ± 0.04 |
| R11 × Gen2 | 165.87 ± 5.01 | 0.229 ± 0.007 | 8.21 ± 0.15 c | 117.05 ± 0.62 | 5.20 ± 0.05 | 116.55 ± 4.83 | 0.151 ± 0.004 | 3.24 ± 0.14 | 75.77 ± 0.35 | 5.18 ± 0.04 |
| BLA × Gen1 | 112.38 ± 5.12 | 0.275 ± 0.007 | 11.10 ± 0.15 b | 119.07 ± 0.63 | 5.93 ± 0.05 | 127.87 ± 4.89 | 0.205 ± 0.004 | 7.20 ± 0.15 | 79.44 ± 0.36 | 5.59 ± 0.04 |
| BLA × Gen2 | 136.07 ± 5.01 | 0.255 ± 0.007 | 10.71 ± 0.15 b | 119.75 ± 0.61 | 5.94 ± 0.05 | 149.40 ± 4.72 | 0.190 ± 0.004 | 5.86 ± 0.14 | 79.94 ± 0.35 | 5.59 ± 0.04 |
| L68 × Gen1 | 198.11 ± 5.20 | 0.338 ± 0.007 | 12.02 ± 0.15 a | 120.62 ± 0.63 | 6.18 ± 0.05 | 136.75 ± 4.94 | 0.188 ± 0.004 | 5.79 ± 0.15 | 76.74 ± 0.36 | 5.62 ± 0.04 |
| L68 × Gen2 | 225.03 ± 5.03 | 0.315 ± 0.007 | 12.04 ± 0.15 a | 122.12 ± 0.62 | 6.20 ± 0.05 | 155.29 ± 4.83 | 0.171 ± 0.004 | 4.07 ± 0.14 | 76.97 ± 0.35 | 5.79 ± 0.04 |
| Effect | Tibiotarsus | Humerus | ||
|---|---|---|---|---|
| F Value | p-Value | F Value | p-Value | |
| Layer line (LL) | 9.10 | <0.0001 | 8.13 | <0.0001 |
| Generation (Gen) | 13.22 | 0.0003 | 8.92 | 0.0030 |
| LL × Gen | 1.75 | 0.1568 | 5.58 | 0.0009 |
| Bone mineral density (BMD) | 243.50 | <0.0001 | 281.92 | <0.0001 |
| BMD × LL | 24.71 | <0.0001 | 10.53 | <0.0001 |
| BMD × Gen | 33.96 | <0.0001 | 26.59 | <0.0001 |
| BMD × LL × Gen | 2.08 | 0.1025 | 7.23 | <0.0001 |
| Weight | 0.00 | 0.9927 | 3.42 | 0.0654 |
| Thickness | 23.33 | <0.0001 | 0.62 | 0.4319 |
| Length | 10.90 | 0.0011 | 0.09 | 0.7660 |
| Total eggshell production 1 | 0.13 | 0.7196 | 0.07 | 0.7879 |
| Layer Line | Tibiotarsus | Humerus | ||||
|---|---|---|---|---|---|---|
| h² BBS | h² BMD | rg | h² BBS | h² BMD | rg | |
| WLA | 0.58 ± 0.23 | 0.75 ± 0.23 | 0.93 ± 0.23 | 0.26 ± 0.22 | N.A. | N.A. |
| R11 | 0.29 ± 0.22 | N.A. | N.A. | 0.40 ± 0.20 | 0.73 ± 0.21 | 0.81 ± 0.18 |
| BLA | 0.17 ± 0.19 | 0.55 ± 0.20 | 0.16 ± 0.37 | 0.50 ± 0.26 | 0.25 ± 0.17 | 0.54 ± 0.30 |
| L68 | 0.46 ± 0.23 | 0.51 ± 0.23 | 0.74 ± 0.17 | 0.44 ± 0.21 | 0.46 ± 0.25 | 0.79 ± 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, S.; Baulain, U.; Habig, C.; Weigend, A.; Halle, I.; Scholz, A.M.; Simianer, H.; Sharifi, A.R.; Weigend, S. Relationship between Bone Stability and Egg Production in Genetically Divergent Chicken Layer Lines. Animals 2020, 10, 850. https://doi.org/10.3390/ani10050850
Jansen S, Baulain U, Habig C, Weigend A, Halle I, Scholz AM, Simianer H, Sharifi AR, Weigend S. Relationship between Bone Stability and Egg Production in Genetically Divergent Chicken Layer Lines. Animals. 2020; 10(5):850. https://doi.org/10.3390/ani10050850
Chicago/Turabian StyleJansen, Simon, Ulrich Baulain, Christin Habig, Annett Weigend, Ingrid Halle, Armin Manfred Scholz, Henner Simianer, Ahmad Reza Sharifi, and Steffen Weigend. 2020. "Relationship between Bone Stability and Egg Production in Genetically Divergent Chicken Layer Lines" Animals 10, no. 5: 850. https://doi.org/10.3390/ani10050850
APA StyleJansen, S., Baulain, U., Habig, C., Weigend, A., Halle, I., Scholz, A. M., Simianer, H., Sharifi, A. R., & Weigend, S. (2020). Relationship between Bone Stability and Egg Production in Genetically Divergent Chicken Layer Lines. Animals, 10(5), 850. https://doi.org/10.3390/ani10050850







