Pattern Recognition Receptors (PRRs) Expression and Activation in COVID-19 and Long COVID: From SARS-CoV-2 Escape Mechanisms to Emerging PRR-Targeted Immunotherapies
Abstract
1. Introduction
2. The Interferon (IFN) Response
2.1. Pattern Recognition Receptors
2.1.1. Toll-like Receptors
2.1.2. Cytoplasmatic RNA Sensors
2.1.3. DNA Sensors
2.2. The Interferon (IFN) System
| IFN Type | Members | Main Cellular Source | Receptor | Receptor Expression | Stimuli | Chromosomal Localization | References |
|---|---|---|---|---|---|---|---|
| Type I IFN | IFNα subtypes (n = 13), IFNβ, IFNε, IFNκ, IFNω. | pDCs, fibroblasts, macrophages | IFNAR (consisting of two transmembrane domains, IFNAR1 and IFNAR2) | Ubiquitous expression | Viral and microbial components | Chromosome 9 | [93,94,95,96,97,98,99] |
| Type II IFN | IFNγ | NK cells, NKT cells, Th1 CD4, Tc CD8 | IFNG (consisting of two transmembrane subunits R1 and R2) | Ubiquitous expression | IL-2, IL-12, IL-15, IL-18 | Chromosome 12 | [50,95,96, 100,101,102,103] |
| Type III IFN | IFNλ1, IFNλ2, IFNλ3, IFNλ4 | Epithelial cells, macrophages, pDCs, mDCs, neutrophils | IFNLR (consisting of two subunits, IFNLR1 and IL10Rβ) | Epithelial cells, endothelial cells, macrophages, DCs, neutrophils | Viral and microbial components | Chromosome 19 | [95,96,98, 104,105,106] |
2.3. Type I/III Interferon and Signaling Pathways
2.4. Interferon Stimulated Genes
3. SARS-CoV-2 Recognition by Pattern Recognition Receptors
3.1. SARS-CoV-2 Recognition by Toll-like Receptors
| TLRs | Immunopathogenesis and Clinical Outcomes | References |
| TLR2 |
| [36] |
| [147] | |
| [148] | |
| [36] | |
| TLR3 |
| [16] |
| [187] | |
| [150] | |
| [151] | |
| TLR4 |
| [147,153,154,155,156,157,158] |
| [167,168,169,170] | |
| [171] | |
| TLR7/TLR8 |
| [132,172,173,174,175,176,178] |
| TLR9 |
| [180] |
| TLRs | SNP | Clinical Outcome | References |
|---|---|---|---|
| TLR2 | rs5743708 | Higher risk of developing pneumonia and severe cases of COVID-19 | [188] |
| TLR3 | rs3775290 | Increased risk of pneumonia in individuals infected with SARS-CoV-2 | [189] |
| TLR4 | rs4986790 | Protective factor in COVID-19 | [190] |
| TLR7 | rs3853839 | Higher severity of COVID-19 | [191] |
| TLR9 | rs5743836 | Susceptibility to and severity of COVID-19 | [192] |
3.2. SARS-CoV-2 Recognition by RNA Sensors
3.2.1. RIG-I
3.2.2. MDA5
3.2.3. PKR and OAS Family
3.3. SARS-CoV-2 Recognition by Absent in Melanoma 2-like Receptors (ALRs)
3.3.1. IFI16/p204
3.3.2. AIM2
3.4. cGAS-STING Pathway
4. SARS-CoV-2 Evasion Strategies by PRRs
5. PRRs Agonists and Antagonists in SARS-CoV-2 Infection
5.1. TLRs Agonists and Antagonists
5.2. RLRs Agonists and Antagonists
5.3. Nucleotidyltransferase Family Agonists and Antagonists
| Compound | Targeted PRR | Effect of the PRR Modulation | Reference |
|---|---|---|---|
| Pam3CSK4 | TLR1/2 | Booster of anti-RBD antibody and cellular responses in immunized mice | [266] |
| oxPAPC | TLR2 | Reduction in cytokine and chemokine release in ACE2-expressing mice, lowering mortality compared to controls | [36] |
| poly IC | TLR3 | Its administration to K18-hACE2 transgenic mice during SARS-CoV-2 infection improves survival by reducing viral load and inflammation in both lung and brain tissue | [267] |
| Resatorvid | TLR4 | Suppression of TLR4/MyD88/NF-κB signaling and inhibition of NLRP3 inflammasome activation | [270] |
| IMQ | TLR7 | IMQ stimulation on PBMC from severe COVID-19 patients with rare LOF TLR7 variant demonstrated an insufficient induction of IRF7, IFNβ1, and ISG15, as well as a reduction in IFNγ production | [144] |
| Enpatoran | TLR7/8 | Enpatoran can reduce the uptake of SARS-CoV-2 RNA by RBCs | [173] |
| CpG-2722 | TLR9 | Booster of the immune response to SARS-CoV-2 vaccine | [186] |
| 3pRNA | RIG-1 | Improvement of survival, as evidenced by reduced viral loads in oropharyngeal swabs, lungs and brains of treated mice. | [273] |
| SLR14 | RIG-1 | Prevention of lower respiratory tract infections and severe COVID-19 disease progression through a type I IFN-dependent mechanism. | [274] |
| H-151 and VS-X4 | cGAS-STING | Inhibits STING reducing the level of TNF and IL-6 expression in SARS-CoV-2 infected cells in vitro | [239] |
| diABZI | cGAS–STING | Suppression of SARS-CoV-2 replication by stimulating ISGs production in transgenic mice expressing human ACE2, with a reduced lung inflammation and increased survival rates. | [231] |
| GA | STING | Ameliorated SARS-CoV-2 Omicron infection both inCalu-3 and in MEF cells and in mice. The transcription levels of Cxcl10, Ifnβ, Oas1, and Isg15 mRNA levels in the MEF cells were up regulated. | [277] |
6. Modulation of PRRs in Long COVID
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woldemeskel, B.A.; Kwaa, A.K.; Garliss, C.C.; Laeyendecker, O.; Ray, S.C.; Blankson, J.N. Healthy Donor T Cell Responses to Common Cold Coronaviruses and SARS-CoV-2. J. Clin. Investig. 2020, 130, 6631–6638. [Google Scholar] [CrossRef]
- Ogimi, C.; Kim, Y.J.; Martin, E.T.; Huh, H.J.; Chiu, C.-H.; Englund, J.A. What’s New With the Old Coronaviruses? J. Pediatr. Infect. Dis. Soc. 2020, 9, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Cheng, G. The Battle between Host and SARS-CoV-2: Innate Immunity and Viral Evasion Strategies. Mol. Ther. 2022, 30, 1869–1884. [Google Scholar] [CrossRef]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.-S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health Systems Resilience in Managing the COVID-19 Pandemic: Lessons from 28 Countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.-D. Innate Immunity, Cytokine Storm, and Inflammatory Cell Death in COVID-19. J. Transl. Med. 2022, 20, 542. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of Type I Interferon Responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 Infection: An Overview on Cytokine Storm and Related Interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chi, S.; Dmytruk, K.; Dmytruk, O.; Tan, S. Coronaviral Infection and Interferon Response: The Virus-Host Arms Race and COVID-19. Viruses 2022, 14, 1349. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Mutair, A.A.; Aljeldah, M.; Shammari, B.R.A.; Sulaiman, T.; Alshukairi, A.N.; Alfaresi, M.; Al-Jishi, J.M.; Al Bati, N.A.; Al-Mozaini, M.A.; et al. Genetic Variants and Protective Immunity against SARS-CoV-2. Genes 2022, 13, 2355. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.-D. Innate Immunity: The First Line of Defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Van der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; van den Heuvel, G.; Mantere, T.; Kersten, S.; van Deuren, R.C.; Steehouwer, M.; van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants Among Young Men with Severe COVID-19. JAMA 2020, 324, 663–673. [Google Scholar] [CrossRef]
- Asano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Renkilaraj, M.R.L.M.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-Linked Recessive TLR7 Deficiency in ~1\% of Men under 60 Years Old with Life-Threatening COVID-19. Sci. Immunol. 2021, 6, eabl4348. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Takaoka, A. Innate Immune Recognition against SARS-CoV-2. Inflamm. Regen. 2023, 43, 7. [Google Scholar] [CrossRef] [PubMed]
- Dhangadamajhi, G.; Rout, R. Association of TLR3 Functional Variant (Rs3775291) with COVID-19 Susceptibility and Death: A Population-Scale Study. Hum. Cell 2021, 34, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Gentili, V.; Rizzo, S.; Schiuma, G.; Beltrami, S.; Strazzabosco, G.; Fernandez, M.; Caccuri, F.; Caruso, A.; Rizzo, R. TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection. Microorganisms 2021, 9, 1820. [Google Scholar] [CrossRef]
- Poulas, K.; Farsalinos, K.; Zanidis, C. Activation of TLR7 and Innate Immunity as an Efficient Method Against COVID-19 Pandemic: Imiquimod as a Potential Therapy. Front. Immunol. 2020, 11, 1373. [Google Scholar] [CrossRef]
- Spiering, A.E.; de Vries, T.J. Why Females Do Better: The X Chromosomal TLR7 Gene-Dose Effect in COVID-19. Front. Immunol. 2021, 12, 756262. [Google Scholar] [CrossRef]
- Banday, A.R.; Stanifer, M.L.; Florez-Vargas, O.; Onabajo, O.O.; Papenberg, B.W.; Zahoor, M.A.; Mirabello, L.; Ring, T.J.; Lee, C.-H.; Albert, P.S.; et al. Genetic Regulation of OAS1 Nonsense-Mediated Decay Underlies Association with COVID-19 Hospitalization in Patients of European and African Ancestries. Nat. Genet. 2022, 54, 1103–1116. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Frasca, F.; Scordio, M.; Santinelli, L.; Gabriele, L.; Gandini, O.; Criniti, A.; Pierangeli, A.; Angeloni, A.; Mastroianni, C.M.; d’Ettorre, G.; et al. Anti-IFN-α/-ω Neutralizing Antibodies from COVID-19 Patients Correlate with Downregulation of IFN Response and Laboratory Biomarkers of Disease Severity. Eur. J. Immunol. 2022, 52, 1120–1128. [Google Scholar] [CrossRef]
- Scordio, M.; Frasca, F.; Santinelli, L.; Sorrentino, L.; Pierangeli, A.; Turriziani, O.; Mastroianni, C.M.; Antonelli, G.; Viscidi, R.P.; d’Ettorre, G.; et al. High Frequency of Neutralizing Antibodies to Type I Interferon in HIV-1 Patients Hospitalized for COVID-19. Clin. Immunol. 2022, 241, 109068. [Google Scholar] [CrossRef]
- Zhang, Q.; Pizzorno, A.; Miorin, L.; Bastard, P.; Gervais, A.; Le Voyer, T.; Bizien, L.; Manry, J.; Rosain, J.; Philippot, Q.; et al. Autoantibodies against Type I IFNs in Patients with Critical Influenza Pneumonia. J. Exp. Med. 2022, 219, e20220514. [Google Scholar] [CrossRef] [PubMed]
- Isazadeh, A.; Heris, J.A.; Shahabi, P.; Mohammadinasab, R.; Shomali, N.; Nasiri, H.; Valedkarimi, Z.; Khosroshahi, A.J.; Hajazimian, S.; Akbari, M.; et al. Pattern-Recognition Receptors (PRRs) in SARS-CoV-2. Life Sci. 2023, 329, 121940. [Google Scholar] [CrossRef] [PubMed]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-Mediated Approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef]
- Frallonardo, L.; Segala, F.V.; Chhaganlal, K.D.; Yelshazly, M.; Novara, R.; Cotugno, S.; Guido, G.; Papagni, R.; Colpani, A.; De Vito, A.; et al. Incidence and Burden of Long COVID in Africa: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 21482. [Google Scholar] [CrossRef]
- Veronese, N.; Bonica, R.; Cotugno, S.; Tulone, O.; Camporeale, M.; Smith, L.; Trott, M.; Bruyere, O.; Mirarchi, L.; Rizzo, G.; et al. Interventions for Improving Long COVID-19 Symptomatology: A Systematic Review. Viruses 2022, 14, 1863. [Google Scholar] [CrossRef]
- Gewaid, H.; Bowie, A.G. Regulation of Type I and Type III Interferon Induction in Response to Pathogen Sensing. Curr. Opin. Immunol. 2024, 87, 102424. [Google Scholar] [CrossRef]
- Lee, H.-R.; Choi, U.Y.; Hwang, S.-W.; Kim, S.; Jung, J.U. Viral Inhibition of PRR-Mediated Innate Immune Response: Learning from KSHV Evasion Strategies. Mol. Cells 2016, 39, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-Sensing Receptors in Inflammation and Diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Innate Immunity to Virus Infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef]
- Carty, M.; Bowie, A.G. Recent Insights into the Role of Toll-like Receptors in Viral Infection. Clin. Exp. Immunol. 2010, 161, 397–406. [Google Scholar] [CrossRef]
- Oosenbrug, T.; van de Graaff, M.J.; Haks, M.C.; van Kasteren, S.; Ressing, M.E. An Alternative Model for Type I Interferon Induction Downstream of Human TLR2. J. Biol. Chem. 2020, 295, 14325–14342. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, H.; Zhao, M.; Lu, Q. TLR2 and TLR4 in Autoimmune Diseases: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2014, 47, 136–147. [Google Scholar] [CrossRef]
- Watters, T.M.; Kenny, E.F.; O’Neill, L.A.J. Structure, Function and Regulation of the Toll/IL-1 Receptor Adaptor Proteins. Immunol. Cell Biol. 2007, 85, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 Senses the SARS-CoV-2 Envelope Protein to Produce Inflammatory Cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of Adaptor TRIF in the MyD88-Independent Toll-Like Receptor Signaling Pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern Recognition Receptors TLR4 and CD14 Mediate Response to Respiratory Syncytial Virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Richez, C.; Blanco, P.; Rifkin, I.; Moreau, J.-F.; Schaeverbeke, T. Role for Toll-like Receptors in Autoimmune Disease: The Example of Systemic Lupus Erythematosus. Jt. Bone Spine 2011, 78, 124–130. [Google Scholar] [CrossRef]
- Chen, J.; Ng, M.M.-L.; Chu, J.J.H. Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection. PLoS Pathog. 2015, 11, e1005053. [Google Scholar] [CrossRef]
- Murawski, M.R.; Bowen, G.N.; Cerny, A.M.; Anderson, L.J.; Haynes, L.M.; Tripp, R.A.; Kurt-Jones, E.A.; Finberg, R.W. Respiratory Syncytial Virus Activates Innate Immunity through Toll-Like Receptor 2. J. Virol. 2009, 83, 1492–1500. [Google Scholar] [CrossRef]
- Nakao, Y.; Funami, K.; Kikkawa, S.; Taniguchi, M.; Nishiguchi, M.; Fukumori, Y.; Seya, T.; Matsumoto, M. Surface-Expressed TLR6 Participates in the Recognition of Diacylated Lipopeptide and Peptidoglycan in Human Cells. J. Immunol. 2005, 174, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Ferrao, R.; Zhou, H.; Shan, Y.; Liu, Q.; Li, Q.; Shaw, D.E.; Li, X.; Wu, H. IRAK4 Dimerization and Trans-Autophosphorylation Are Induced by Myddosome Assembly. Mol. Cell 2014, 55, 891–903. [Google Scholar] [CrossRef]
- Cushing, L.; Stochaj, W.; Siegel, M.; Czerwinski, R.; Dower, K.; Wright, Q.; Hirschfield, M.; Casanova, J.-L.; Picard, C.; Puel, A.; et al. Interleukin 1/Toll-like Receptor-Induced Autophosphorylation Activates Interleukin 1 Receptor-Associated Kinase 4 and Controls Cytokine Induction in a Cell Type-Specific Manner. J. Biol. Chem. 2014, 289, 10865–10875. [Google Scholar] [CrossRef]
- Brikos, C.; Wait, R.; Begum, S.; O’Neill, L.A.J.; Saklatvala, J. Mass Spectrometric Analysis of the Endogenous Type I Interleukin-1 (IL-1) Receptor Signaling Complex Formed after IL-1 Binding Identifies IL-1RAcP, MyD88, and IRAK-4 as the Stable Components*. Mol. Cell. Proteom. 2007, 6, 1551–1559. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like Receptor Recognizes Bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Kikkert, M. Innate Immune Evasion by Human Respiratory RNA Viruses. J. Innate Immun. 2019, 12, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Chattopadhyay, S.; Sen, G.C. No Love Lost Between Viruses and Interferons. Annu. Rev. Virol. 2015, 2, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhong, J.; Chung, J.; Chisari, F.V. Double-Stranded DNA and Double-Stranded RNA Induce a Common Antiviral Signaling Pathway in Human Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9035–9040. [Google Scholar] [CrossRef] [PubMed]
- Inn, K.-S.; Lee, S.-H.; Rathbun, J.Y.; Wong, L.-Y.; Toth, Z.; Machida, K.; Ou, J.-H.J.; Jung, J.U. Inhibition of RIG-I-Mediated Signaling by Kaposi’s Sarcoma-Associated Herpesvirus-Encoded Deubiquitinase ORF64. J. Virol. 2011, 85, 10899–10904. [Google Scholar] [CrossRef]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-Mediated Antiviral Responses to Single-Stranded RNA Bearing 5′-Phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Brisse, M.; Ly, H. Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front. Immunol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- Hovanessian, A.G. On the Discovery of Interferon-Inducible, Double-Stranded RNA Activated Enzymes: The 2′-5′oligoadenylate Synthetases and the Protein Kinase PKR. Cytokine Growth Factor Rev. 2007, 18, 351–361. [Google Scholar] [CrossRef]
- Schwartz, S.L.; Conn, G.L. RNA Regulation of the Antiviral Protein 2′-5′-Oligoadenylate Synthetase. Wiley Interdiscip. Rev. RNA 2019, 10, e1534. [Google Scholar] [CrossRef]
- Yang, K.; Dong, B.; Asthana, A.; Silverman, R.H.; Yan, N. RNA Helicase SKIV2L Limits Antiviral Defense and Autoinflammation Elicited by the OAS-RNase L Pathway. EMBO J. 2024, 43, 3876–3894. [Google Scholar] [CrossRef]
- McAllister, C.S.; Taghavi, N.; Samuel, C.E. Protein Kinase PKR Amplification of Interferon β Induction Occurs through Initiation Factor EIF-2α-Mediated Translational Control. J. Biol. Chem. 2012, 287, 36384–36392. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 Recognizes Cytosolic DsDNA and Forms a Caspase-1-Activating Inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Paludan, S.R. Reading the Viral Signature by Toll-like Receptors and Other Pattern Recognition Receptors. J. Mol. Med. 2005, 83, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Russo, A.J.; Shivcharan, S.; Rathinam, V.A. AIM2 in Health and Disease: Inflammasome and Beyond. Immunol. Rev. 2020, 297, 83–95. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Luan, Y.; Li, X.; Meng, X.; Liao, W.; Tang, J.; Wang, Z. CGAS-STING, Inflammasomes and Pyroptosis: An Overview of Crosstalk Mechanism of Activation and Regulation. Cell Commun. Signal. 2024, 22, 22. [Google Scholar] [CrossRef]
- Gray, E.E.; Winship, D.; Snyder, J.M.; Child, S.J.; Geballe, A.P.; Stetson, D.B. The AIM2-like Receptors Are Dispensable for the Interferon Response to Intracellular DNA. Immunity 2016, 45, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Grinberg, I.; Rappe, A.M. Intrinsic Ferroelectric Switching from First Principles. Nature 2016, 534, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jacobs, S.R.; West, J.A.; Stopford, C.; Zhang, Z.; Davis, Z.; Barber, G.N.; Glaunsinger, B.A.; Dittmer, D.P.; Damania, B. Modulation of the CGAS-STING DNA Sensing Pathway by Gammaherpesviruses. Proc. Natl. Acad. Sci. USA 2015, 112, E4306-15. [Google Scholar] [CrossRef]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. CGAS Produces a 2′-5′-Linked Cyclic Dinucleotide Second Messenger That Activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef]
- Chen, C.; Xu, P. Cellular Functions of CGAS-STING Signaling. Trends Cell Biol. 2023, 33, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhuang, Z.; Li, J.; Feng, Z. Significance of the CGAS-STING Pathway in Health and Disease. Int. J. Mol. Sci. 2023, 24, 13316. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jønsson, K.L.; Jakobsen, M.R.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-Canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-ΚB Signaling after Nuclear DNA Damage. Mol. Cell 2018, 71, 745–760.e5. [Google Scholar] [CrossRef]
- Berthelot, J.-M.; Drouet, L.; Lioté, F. Kawasaki-like Diseases and Thrombotic Coagulopathy in COVID-19: Delayed over-Activation of the STING Pathway? Emerg. Microbes Infect. 2020, 9, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, J.-M.; Lioté, F. COVID-19 as a STING Disorder with Delayed over-Secretion of Interferon-Beta. EBioMedicine 2020, 56, 102801. [Google Scholar] [CrossRef]
- Isaacs, A.; Lindenmann, J. Virus Interference. I. The Interferon. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef]
- Le Page, C.; Génin, P.; Baines, M.G.; Hiscott, J. Interferon Activation and Innate Immunity. Rev. Immunogenet. 2000, 2, 374–386. [Google Scholar]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I Interferons in Infectious Disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Rusinova, I.; Forster, S.; Yu, S.; Kannan, A.; Masse, M.; Cumming, H.; Chapman, R.; Hertzog, P.J. Interferome v2.0: An Updated Database of Annotated Interferon-Regulated Genes. Nucleic Acids Res. 2013, 41, D1040–D1046. [Google Scholar] [CrossRef]
- Rong, L.; Perelson, A.S. Treatment of Hepatitis C Virus Infection with Interferon and Small Molecule Direct Antivirals: Viral Kinetics and Modeling. Crit. Rev. Immunol. 2010, 30, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, A.; Fujise, K.; Namiki, Y.; Tada, N. Peginterferon and Ribavirin Treatment for Hepatitis C Virus Infection. World J. Gastroenterol. 2011, 17, 419–432. [Google Scholar] [CrossRef]
- Rizza, P.; Moretti, F.; Belardelli, F. Recent Advances on the Immunomodulatory Effects of IFN-Alpha: Implications for Cancer Immunotherapy and Autoimmunity. Autoimmunity 2010, 43, 204–209. [Google Scholar] [CrossRef]
- Kotredes, K.P.; Gamero, A.M. Interferons as Inducers of Apoptosis in Malignant Cells. J. Interferon Cytokine Res. 2013, 33, 162–170. [Google Scholar] [CrossRef]
- Antonelli, G.; Scagnolari, C.; Moschella, F.; Proietti, E. Twenty-Five Years of Type I Interferon-Based Treatment: A Critical Analysis of Its Therapeutic Use. Cytokine Growth Factor Rev. 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Chelbi-Alix, M.K.; Wietzerbin, J. Interferon, a Growing Cytokine Family: 50 Years of Interferon Research. Biochimie 2007, 89, 713–718. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Il Jung, K.; McKenna, S.; Vijayamahantesh, V.; He, Y.; Hahm, B. Protective versus Pathogenic Type I Interferon Responses during Virus Infections. Viruses 2023, 15, 1916. [Google Scholar] [CrossRef]
- Hertzog, P.; Forster, S.; Samarajiwa, S. Systems Biology of Interferon Responses. J. Interf. Cytokine Res. Off. J. Int. Soc. Interf. Cytokine Res. 2011, 31, 5–11. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, Interferon-like Cytokines, and Their Receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- De Andrea, M.; Ravera, R.; Gioia, D.; Gariglio, M.; Landolfo, S. The Interferon System: An Overview. Eur. J. Paediatr. Neurol. 2002, 6 (Suppl. A), A41–A46; discussion A55–A58. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.C.; Sen, G.C.; Uze, G.; Silverman, R.H.; Ransohoff, R.M.; Foster, G.R.; Stark, G.R. Interferons at Age 50: Past, Current and Future Impact on Biomedicine. Nat. Rev. Drug Discov. 2007, 6, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Antonelli, G. Type I Interferon and HIV: Subtle Balance between Antiviral Activity, Immunopathogenesis and the Microbiome. Cytokine Growth Factor Rev. 2018, 40, 19–31. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. Overview of the Biology of Type I Interferons. Arthritis Res. Ther. 2010, 12 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Sen, G.C. Interferons and Viral Infections. Biofactors 2009, 35, 14–20. [Google Scholar] [CrossRef]
- Wells, A.I.; Coyne, C.B. Type III Interferons in Antiviral Defenses at Barrier Surfaces. Trends Immunol. 2018, 39, 848–858. [Google Scholar] [CrossRef]
- De Weerd, N.A.; Nguyen, T. The Interferons and Their Receptors--Distribution and Regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef]
- Shaw, A.E.; Hughes, J.; Gu, Q.; Behdenna, A.; Singer, J.B.; Dennis, T.; Orton, R.J.; Varela, M.; Gifford, R.J.; Wilson, S.J.; et al. Fundamental Properties of the Mammalian Innate Immune System Revealed by Multispecies Comparison of Type I Interferon Responses. PLoS Biol. 2017, 15, e2004086. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-F.; Gong, M.-J.; Zhao, F.-R.; Shao, J.-J.; Xie, Y.-L.; Zhang, Y.-G.; Chang, H.-Y. Type I Interferons: Distinct Biological Activities and Current Applications for Viral Infection. Cell. Physiol. Biochem. 2018, 51, 2377–2396. [Google Scholar] [CrossRef]
- Chin, K.L.; Anis, F.Z.; Sarmiento, M.E.; Norazmi, M.N.; Acosta, A. Role of Interferons in the Development of Diagnostics, Vaccines, and Therapy for Tuberculosis. J. Immunol. Res. 2017, 2017, 5212910. [Google Scholar] [CrossRef]
- Perry, A.K.; Chen, G.; Zheng, D.; Tang, H.; Cheng, G. The Host Type I Interferon Response to Viral and Bacterial Infections. Cell Res. 2005, 15, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Marié, I.J.; Durbin, J.E. Induction and Function of Type I and III Interferon in Response to Viral Infection. Curr. Opin. Virol. 2011, 1, 476–486. [Google Scholar] [CrossRef]
- Piehler, J.; Thomas, C.; Garcia, K.C.; Schreiber, G. Structural and Dynamic Determinants of Type I Interferon Receptor Assembly and Their Functional Interpretation. Immunol. Rev. 2012, 250, 317–334. [Google Scholar] [CrossRef]
- Ye, J.; Ortaldo, J.R.; Conlon, K.; Winkler-Pickett, R.; Young, H.A. Cellular and Molecular Mechanisms of IFN-Gamma Production Induced by IL-2 and IL-12 in a Human NK Cell Line. J. Leukoc. Biol. 1995, 58, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Gu, Q.; Robertson, D.L.; Hughes, J. Defining the Characteristics of Interferon-Alpha-Stimulated Human Genes: Insight from Expression Data and Machine Learning. Gigascience 2022, 11, giac103. [Google Scholar] [CrossRef]
- Takaoka, A.; Yanai, H. Interferon Signalling Network in Innate Defence. Cell. Microbiol. 2006, 8, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, S.; Klein, R.S. Type III Interferons: Emerging Roles in Autoimmunity. Front. Immunol. 2021, 12, 764062. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Durbin, J.E. Contribution of Type III Interferons to Antiviral Immunity: Location, Location, Location. J. Biol. Chem. 2017, 292, 7295–7303. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Langer, J.A. Type I Interferons BT—Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 5787–5794. ISBN 978-3-319-67199-4. [Google Scholar]
- Haque, S.J.; Williams, B.R. Signal Transduction in the Interferon System. Semin. Oncol. 1998, 25, 14–22. [Google Scholar]
- Li, M.M.H.; MacDonald, M.R.; Rice, C.M. To Translate, or Not to Translate: Viral and Host MRNA Regulation by Interferon-Stimulated Genes. Trends Cell Biol. 2015, 25, 320–329. [Google Scholar] [CrossRef]
- Stanton, G.J.; Weigent, D.A.; Fleischmann, W.R.J.; Dianzani, F.; Baron, S. Interferon Review. Investig. Radiol. 1987, 22, 259–273. [Google Scholar] [CrossRef]
- Kalvakolanu, D.V.; Borden, E.C. An Overview of the Interferon System: Signal Transduction and Mechanisms of Action. Cancer Investig. 1996, 14, 25–53. [Google Scholar] [CrossRef]
- Baum, A.; García-Sastre, A. Induction of Type I Interferon by RNA Viruses: Cellular Receptors and Their Substrates. Amino Acids 2010, 38, 1283–1299. [Google Scholar] [CrossRef]
- Lee, J.-H.; Koepke, L.; Kirchhoff, F.; Sparrer, K.M.J. Interferon Antagonists Encoded by SARS-CoV-2 at a Glance. Med. Microbiol. Immunol. 2023, 212, 125–131. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Rivera, A.; Parker, D.; Durbin, J.E. Type III IFNs: Beyond Antiviral Protection. Semin. Immunol. 2019, 43, 101303. [Google Scholar] [CrossRef] [PubMed]
- Odendall, C.; Dixit, E.; Stavru, F.; Bierne, H.; Franz, K.M.; Durbin, A.F.; Boulant, S.; Gehrke, L.; Cossart, P.; Kagan, J.C. Diverse Intracellular Pathogens Activate Type III Interferon Expression from Peroxisomes. Nat. Immunol. 2014, 15, 717–726. [Google Scholar] [CrossRef]
- Voigt, E.A.; Yin, J. Kinetic Differences and Synergistic Antiviral Effects Between Type I and Type III Interferon Signaling Indicate Pathway Independence. J. Interferon Cytokine Res. 2015, 35, 734–747. [Google Scholar] [CrossRef]
- Jilg, N.; Lin, W.; Hong, J.; Schaefer, E.A.; Wolski, D.; Meixong, J.; Goto, K.; Brisac, C.; Chusri, P.; Fusco, D.N.; et al. Kinetic Differences in the Induction of Interferon Stimulated Genes by Interferon-α and Interleukin 28B Are Altered by Infection with Hepatitis C Virus. Hepatology 2014, 59, 1250–1261. [Google Scholar] [CrossRef]
- Kohli, A.; Zhang, X.; Yang, J.; Russell, R.S.; Donnelly, R.P.; Sheikh, F.; Sherman, A.; Young, H.; Imamichi, T.; Lempicki, R.A.; et al. Distinct and Overlapping Genomic Profiles and Antiviral Effects of Interferon-λ and -α on HCV-Infected and Noninfected Hepatoma Cells. J. Viral Hepat. 2012, 19, 843–853. [Google Scholar] [CrossRef]
- Bolen, C.R.; Ding, S.; Robek, M.D.; Kleinstein, S.H. Dynamic Expression Profiling of Type I and Type III Interferon-Stimulated Hepatocytes Reveals a Stable Hierarchy of Gene Expression. Hepatology 2014, 59, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bogunovic, D.; Payelle-Brogard, B.; Francois-Newton, V.; Speer, S.D.; Yuan, C.; Volpi, S.; Li, Z.; Sanal, O.; Mansouri, D.; et al. Human Intracellular ISG15 Prevents Interferon-α/β over-Amplification and Auto-Inflammation. Nature 2015, 517, 89–93. [Google Scholar] [CrossRef] [PubMed]
- François-Newton, V.; Magno de Freitas Almeida, G.; Payelle-Brogard, B.; Monneron, D.; Pichard-Garcia, L.; Piehler, J.; Pellegrini, S.; Uzé, G. USP18-Based Negative Feedback Control Is Induced by Type I and Type III Interferons and Specifically Inactivates Interferon α Response. PLoS ONE 2011, 6, e22200. [Google Scholar] [CrossRef]
- Walker, F.C.; Sridhar, P.R.; Baldridge, M.T. Differential Roles of Interferons in Innate Responses to Mucosal Viral Infections. Trends Immunol. 2021, 42, 1009–1023. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Wang, Y.-N.; Chang, Q.-Y.; Ma, P.; Hu, Y.; Cao, X. Type III Interferons in Viral Infection and Antiviral Immunity. Cell. Physiol. Biochem. 2018, 51, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Monteleone, K.; Selvaggi, C.; Pierangeli, A.; D’Ettorre, G.; Mezzaroma, I.; Turriziani, O.; Gentile, M.; Vullo, V.; Antonelli, G. ISG15 Expression Correlates with HIV-1 Viral Load and with Factors Regulating T Cell Response. Immunobiology 2016, 221, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, V.; Leaman, D.W.; Borden, E.C. Emerging Roles of FAM14 Family Members (G1P3/ISG 6-16 and ISG12/IFI27) in Innate Immunity and Cancer. J. Interferon Cytokine Res. 2011, 31, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Boasso, A. Type I Interferon at the Interface of Antiviral Immunity and Immune Regulation: The Curious Case of HIV-1. Scientifica 2013, 2013, 580968. [Google Scholar] [CrossRef]
- Brulois, K.; Jung, J.U. Interplay between Kaposi’s Sarcoma-Associated Herpesvirus and the Innate Immune System. Cytokine Growth Factor Rev. 2014, 25, 597–609. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Belardelli, F.; Gresser, I.; Maury, C.; Maunoury, M.T. Antitumor Effects of Interferon in Mice Injected with Interferon-Sensitive and Interferon-Resistant Friend Leukemia Cells. II. Role of Host Mechanisms. Int. J. Cancer 1982, 30, 821–825. [Google Scholar] [CrossRef]
- Davidson, S.; Maini, M.K.; Wack, A. Disease-Promoting Effects of Type I Interferons in Viral, Bacterial, and Coinfections. J. Interferon Cytokine Res. 2015, 35, 252–264. [Google Scholar] [CrossRef]
- Hussell, T.; Goulding, J. Structured Regulation of Inflammation during Respiratory Viral Infection. Lancet Infect. Dis. 2010, 10, 360–366. [Google Scholar] [CrossRef]
- Sorrentino, L.; Fracella, M.; Frasca, F.; D’Auria, A.; Santinelli, L.; Maddaloni, L.; Bugani, G.; Bitossi, C.; Gentile, M.; Ceccarelli, G.; et al. Alterations in the Expression of IFN Lambda, IFN Gamma and Toll-like Receptors in Severe COVID-19 Patients. Microorganisms 2023, 11, 689. [Google Scholar] [CrossRef]
- Pasrija, R.; Naime, M. The Deregulated Immune Reaction and Cytokines Release Storm (CRS) in COVID-19 Disease. Int. Immunopharmacol. 2021, 90, 107225. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine Release Syndrome in Severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef]
- Quan, C.; Li, C.; Ma, H.; Li, Y.; Zhang, H. Immunopathogenesis of Coronavirus-Induced Acute Respiratory Distress Syndrome (ARDS): Potential Infection-Associated Hemophagocytic Lymphohistiocytosis. Clin. Microbiol. Rev. 2020, 34, 10–1128. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptor and RIG-I-like Receptor Signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef]
- Ebermeyer, T.; Cognasse, F.; Berthelot, P.; Mismetti, P.; Garraud, O.; Hamzeh-Cognasse, H. Platelet Innate Immune Receptors and TLRs: A Double-Edged Sword. Int. J. Mol. Sci. 2021, 22, 7894. [Google Scholar] [CrossRef]
- Dajon, M.; Iribarren, K.; Cremer, I. Toll-like Receptor Stimulation in Cancer: A pro- and Anti-Tumor Double-Edged Sword. Immunobiology 2017, 222, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Harsini, S.; Beigy, M.; Akhavan-Sabbagh, M.; Rezaei, N. Toll-like Receptors in Lymphoid Malignancies: Double-Edged Sword. Crit. Rev. Oncol. Hematol. 2014, 89, 262–283. [Google Scholar] [CrossRef]
- Yokota, S.-I.; Okabayashi, T.; Fujii, N. The Battle between Virus and Host: Modulation of Toll-like Receptor Signaling Pathways by Virus Infection. Mediat. Inflamm. 2010, 2010, 184328. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Rezaei, N. Role of Toll-like Receptors in the Pathogenesis of COVID-19. J. Med. Virol. 2021, 93, 2735–2739. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Zheng, M.-H.; Meng, X.-T.; Ma, L.-W.; Liang, H.-Y.; Fan, H.-Y. Role of Toll-like Receptors in the Pathogenesis of COVID-19: Current and Future Perspectives. Scand. J. Immunol. 2023, 98, e13275. [Google Scholar] [CrossRef]
- Mantovani, S.; Oliviero, B.; Varchetta, S.; Renieri, A.; Mondelli, M.U. TLRs: Innate Immune Sentries against SARS-CoV-2 Infection. Int. J. Mol. Sci. 2023, 24, 8065. [Google Scholar] [CrossRef]
- Planès, R.; Bert, J.-B.; Tairi, S.; BenMohamed, L.; Bahraoui, E. SARS-CoV-2 Envelope (E) Protein Binds and Activates TLR2 Pathway: A Novel Molecular Target for COVID-19 Interventions. Viruses 2022, 14, 999. [Google Scholar] [CrossRef]
- Learnard, H.; Core, J.; Corkrey, H.; Sciaudone, A.; Rade, J.; Kornfeld, H.; Wang, J.P.; Freedman, J.E.; Tanriverdi, K.; Koupenova, M. Pattern Recognition Receptor-Associated Immuno-Thrombotic Transcript Changes in Platelets and Leukocytes with COVID19. PLoS Pathog. 2025, 21, e1013413. [Google Scholar] [CrossRef]
- Landolina, N.; Ricci, B.; Veneziani, I.; Alicata, C.; Mariotti, F.R.; Pelosi, A.; Quatrini, L.; Mortari, E.P.; Carsetti, R.; Vacca, P.; et al. TLR2/4 Are Novel Activating Receptors for SARS-CoV-2 Spike Protein on NK Cells. Front. Immunol. 2024, 15, 1368946. [Google Scholar] [CrossRef]
- Quagliariello, V.; Bonelli, A.; Caronna, A.; Lombari, M.C.; Conforti, G.; Libutti, M.; Iaffaioli, R.V.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 Infection: NLRP3 Inflammasome as Plausible Target to Prevent Cardiopulmonary Complications? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9169–9171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn Errors of Type I IFN Immunity in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.C.S.; Veiga, A.D.M.; Martins de Lima, T.; Kunimi Kubo Ariga, S.; Vieira Barbeiro, H.; de Lucena Moreira, C.; Pinto, A.A.S.; Brandao, R.A.; Marchini, J.F.; Alencar, J.C.; et al. Lower Peripheral Blood Toll-like Receptor 3 Expression Is Associated with an Unfavorable Outcome in Severe COVID-19 Patients. Sci. Rep. 2021, 11, 15223. [Google Scholar] [CrossRef]
- Chomel, L.; Vogt, M.; Demiselle, J.; Le Borgne, P.; Tschirhart, M.; Morandeau, V.; Merdji, H.; Miguet, L.; Helms, J.; Meziani, F.; et al. TLRs1-10 Protein Expression in Circulating Human White Blood Cells during Bacterial and COVID-19 Infections. J. Innate Immun. 2024, 16, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.; Kikkert, M.; van den Worm, S.H.E.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. SARS-Coronavirus Replication Is Supported by a Reticulovesicular Network of Modified Endoplasmic Reticulum. PLoS Biol. 2008, 6, e226. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In Silico Studies on the Comparative Characterization of the Interactions of SARS-CoV-2 Spike Glycoprotein with ACE-2 Receptor Homologs and Human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kuang, M.; Li, J.; Zhu, L.; Jia, Z.; Guo, X.; Hu, Y.; Kong, J.; Yin, H.; Wang, X.; et al. Publisher Correction: SARS-CoV-2 spike protein interacts with and activates TLR4. Cell Res. 2021, 31, 825, Erratum in Cell Res. Cell Res. 2021, 31, 818–820. https://doi.org/10.1038/s41422-021-00501-0. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirato, K.; Kizaki, T. SARS-CoV-2 Spike Protein S1 Subunit Induces pro-Inflammatory Responses via Toll-like Receptor 4 Signaling in Murine and Human Macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Richez, C.; Yasuda, K.; Watkins, A.A.; Akira, S.; Lafyatis, R.; van Seventer, J.M.; Rifkin, I.R. TLR4 Ligands Induce IFN-Alpha Production by Mouse Conventional Dendritic Cells and Human Monocytes after IFN-Beta Priming. J. Immunol. 2009, 182, 820–828. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bayry, J. The Yin and Yang of TLR4 in COVID-19. Cytokine Growth Factor Rev. 2024, 82, 70–85. [Google Scholar] [CrossRef]
- Zakeri, A.; Russo, M. Dual Role of Toll-like Receptors in Human and Experimental Asthma Models. Front. Immunol. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Durán-Méndez, A.; Aguilar-Arroyo, A.D.; Vivanco-Gómez, E.; Nieto-Ortega, E.; Pérez-Ortega, D.; Jiménez-Pérez, C.; Hernández-Skewes, K.Y.; Montiel-Bravo, G.; Roque-Reyes, O.J.; Romero-Lechuga, F.; et al. Tocilizumab Reduces COVID-19 Mortality and Pathology in a Dose and Timing-Dependent Fashion: A Multi-Centric Study. Sci. Rep. 2021, 11, 19728. [Google Scholar] [CrossRef]
- Franzetti, M.; Forastieri, A.; Borsa, N.; Pandolfo, A.; Molteni, C.; Borghesi, L.; Pontiggia, S.; Evasi, G.; Guiotto, L.; Erba, M.; et al. IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective, Observational Study. J. Immunol. 2021, 206, 1569–1575. [Google Scholar] [CrossRef]
- Ng, B.; Cash-Mason, T.; Wang, Y.; Seitzer, J.; Burchard, J.; Brown, D.; Dudkin, V.; Davide, J.; Jadhav, V.; Sepp-Lorenzino, L.; et al. Intratracheal Administration of SiRNA Triggers MRNA Silencing in the Lung to Modulate T Cell Immune Response and Lung Inflammation. Mol. Ther. Nucleic Acids 2019, 16, 194–205. [Google Scholar] [CrossRef]
- Asaba, C.N.; Ekabe, C.J.; Ayuk, H.S.; Gwanyama, B.N.; Bitazar, R.; Bukong, T.N. Interplay of TLR4 and SARS-CoV-2: Unveiling the Complex Mechanisms of Inflammation and Severity in COVID-19 Infections. J. Inflamm. Res. 2024, 17, 5077–5091. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, C.; He, C.; Ma, Q.; Li, J.; Wang, W.; Xu, Y.-T.; Wang, T. Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-KB Pathway and NLRP3 Inflammasome Activation. Front. Pharmacol. 2021, 12, 790072. [Google Scholar] [CrossRef] [PubMed]
- Girkin, J.L.N.; Maltby, S.; Bartlett, N.W. Toll-like Receptor-Agonist-Based Therapies for Respiratory Viral Diseases: Thinking Outside the Cell. Eur. Respir. Rev. 2022, 31, 210274. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.M.; Lee, S.G.; Kim, H.J.; Cheon, S.; Jeong, H.; Lee, J.; Kim, I.S.; Silwal, P.; Kim, Y.J.; Paik, S.; et al. COVID-19 Patients Upregulate Toll-like Receptor 4-Mediated Inflammatory Signaling That Mimics Bacterial Sepsis. J. Korean Med. Sci. 2020, 35, e343. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Guan, B.; Xu, J.; Zhang, H.; Yi, L.; Yang, Z. Role of Toll-like Receptor-Mediated Pyroptosis in Sepsis-Induced Cardiomyopathy. Biomed. Pharmacother. 2023, 167, 115493. [Google Scholar] [CrossRef]
- Kogan, E.A.; Berezovskiy, Y.S.; Blagova, O.V.; Kukleva, A.D.; Bogacheva, G.A.; Kurilina, E.V.; Kalinin, D.V.; Bagdasaryan, T.R.; Semeyonova, L.A.; Gretsov, E.M.; et al. Miocarditis in Patients with COVID-19 Confirmed by Immunohistochemical. Kardiologiia 2020, 60, 4–10. [Google Scholar] [CrossRef] [PubMed]
- van der Donk, L.E.H.; Bermejo-Jambrina, M.; van Hamme, J.L.; Volkers, M.M.W.; van Nuenen, A.C.; Kootstra, N.A.; Geijtenbeek, T.B.H. SARS-CoV-2 Suppresses TLR4-Induced Immunity by Dendritic Cells via C-Type Lectin Receptor DC-SIGN. PLoS Pathog. 2023, 19, e1011735. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Züst, R.; Weber, F.; Spiegel, M.; Lang, K.S.; Akira, S.; Thiel, V.; Ludewig, B. Control of Coronavirus Infection through Plasmacytoid Dendritic-Cell-Derived Type I Interferon. Blood 2007, 109, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, R.M.; Cham, L.B.; Gris-Oliver, A.; Gammelgaard, K.R.; Pedersen, J.G.; Idorn, M.; Ahmadov, U.; Hernandez, S.S.; Cémalovic, E.; Godsk, S.H.; et al. TLR2 and TLR7 Mediate Distinct Immunopathological and Antiviral Plasmacytoid Dendritic Cell Responses to SARS-CoV-2 Infection. EMBO J. 2022, 41, e109622. [Google Scholar] [CrossRef]
- Metthew Lam, L.K.; Oatman, E.; Eckart, K.A.; Klingensmith, N.J.; Flowers, E.; Sayegh, L.; Yuen, J.; Clements, R.L.; Meyer, N.J.; Jurado, K.A.; et al. Human Red Blood Cells Express the RNA Sensor TLR7. Sci. Rep. 2024, 14, 15789. [Google Scholar] [CrossRef]
- Bagheri-Hosseinabadi, Z.; Mohammadizadeh Ranjbar, F.; Nassiri, M.; Amiri, A.; Abbasifard, M. Nasopharyngeal Epithelial Cells from Patients with Coronavirus Disease 2019 Express Abnormal Levels of Toll-like Receptors. Pathog. Glob. Health 2023, 117, 401–408. [Google Scholar] [CrossRef]
- Bagheri-Hosseinabadi, Z.; Rezazadeh Zarandi, E.; Mirabzadeh, M.; Amiri, A.; Abbasifard, M. MRNA Expression of Toll-like Receptors 3, 7, 8, and 9 in the Nasopharyngeal Epithelial Cells of Coronavirus Disease 2019 Patients. BMC Infect. Dis. 2022, 22, 448. [Google Scholar] [CrossRef] [PubMed]
- Miquel, C.-H.; Abbas, F.; Cenac, C.; Foret-Lucas, C.; Guo, C.; Ducatez, M.; Joly, E.; Hou, B.; Guéry, J.-C. B Cell-Intrinsic TLR7 Signaling Is Required for Neutralizing Antibody Responses to SARS-CoV-2 and Pathogen-like COVID-19 Vaccines. Eur. J. Immunol. 2023, 53, e2350437. [Google Scholar] [CrossRef]
- Arefinia, N.; Banafi, P.; Zarezadeh, M.A.; Mousawi, H.S.; Yaghobi, R.; Farokhnia, M.; Sarvari, J. TLR3, TLR7, and TLR8 Genes Expression Datasets in COVID-19 Patients: Influences of the Disease Severity and Gender. Data Br. 2024, 54, 110498. [Google Scholar] [CrossRef]
- Chidambaram, V.; Kumar, A.; Sadaf, M.I.; Lu, E.; Al’Aref, S.J.; Tarun, T.; Galiatsatos, P.; Gulati, M.; Blumenthal, R.S.; Leucker, T.M.; et al. COVID-19 in the Initiation and Progression of Atherosclerosis: Pathophysiology During and Beyond the Acute Phase. JACC Adv. 2024, 3, 101107. [Google Scholar] [CrossRef]
- Costa, T.J.; Potje, S.R.; Fraga-Silva, T.F.C.; da Silva-Neto, J.A.; Barros, P.R.; Rodrigues, D.; Machado, M.R.; Martins, R.B.; Santos-Eichler, R.A.; Benatti, M.N.; et al. Mitochondrial DNA and TLR9 Activation Contribute to SARS-CoV-2-Induced Endothelial Cell Damage. Vascul. Pharmacol. 2022, 142, 106946. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, G.F.G.; Garssen, J. TLR9 and COVID-19: A Multidisciplinary Theory of a Multifaceted Therapeutic Target. Front. Pharmacol. 2020, 11, 601685. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.; Desquiret-Dumas, V.; Nagot, N.; Rapenne, C.; Van de Perre, P.; Reynier, P.; Molès, J.-P. Long-Term Persistence of Mitochondrial Dysfunctions after Viral Infections and Antiviral Therapies: A Review of Mechanisms Involved. J. Med. Virol. 2024, 96, e29886. [Google Scholar] [CrossRef] [PubMed]
- Romão, P.R.; Teixeira, P.C.; Schipper, L.; da Silva, I.; Santana Filho, P.; Júnior, L.C.R.; Peres, A.; Gonçalves da Fonseca, S.; Chagas Monteiro, M.; Lira, F.S.; et al. Viral Load Is Associated with Mitochondrial Dysfunction and Altered Monocyte Phenotype in Acute Severe SARS-CoV-2 Infection. Int. Immunopharmacol. 2022, 108, 108697. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-S.; Qi, H.-Y.; Boularan, C.; Huang, N.-N.; Abu-Asab, M.; Shelhamer, J.H.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-9b Suppresses Innate Immunity by Targeting Mitochondria and the MAVS/TRAF3/TRAF6 Signalosome. J. Immunol. 2014, 193, 3080–3089. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, K.V.; Yurchenko, K.A.; Marochkin, N.A.; Fomochkina, I.I.; Kubyshkin, A. V SARS-CoV-2 Will Constantly Sweep Its Tracks: A Vaccine Containing CpG Motifs in “lasso” for the Multi-Faced Virus. Inflamm. Res. 2020, 69, 801–812. [Google Scholar] [CrossRef]
- Yang, J.-X.; Tseng, J.-C.; Tien, C.-F.; Lee, C.-Y.; Liu, Y.-L.; Lin, J.-J.; Tsai, P.-J.; Liao, H.-C.; Liu, S.-J.; Su, Y.-W.; et al. TLR9 and STING Agonists Cooperatively Boost the Immune Response to SARS-CoV-2 RBD Vaccine through an Increased Germinal Center B Cell Response and Reshaped T Helper Responses. Int. J. Biol. Sci. 2023, 19, 2897–2913. [Google Scholar] [CrossRef]
- Han, L.; Zhuang, M.-W.; Deng, J.; Zheng, Y.; Zhang, J.; Nan, M.-L.; Zhang, X.-J.; Gao, C.; Wang, P.-H. SARS-CoV-2 ORF9b Antagonizes Type I and III Interferons by Targeting Multiple Components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and CGAS-STING Signaling Pathways. J. Med. Virol. 2021, 93, 5376–5389. [Google Scholar] [CrossRef]
- Bakaros, E.; Voulgaridi, I.; Paliatsa, V.; Gatselis, N.; Germanidis, G.; Asvestopoulou, E.; Alexiou, S.; Botsfari, E.; Lygoura, V.; Tsachouridou, O.; et al. Innate Immune Gene Polymorphisms and COVID-19 Prognosis. Viruses 2023, 15, 1784. [Google Scholar] [CrossRef] [PubMed]
- Alseoudy, M.M.; Elgamal, M.; Abdelghany, D.A.; Borg, A.M.; El-Mesery, A.; Elzeiny, D.; Hammad, M.O. Prognostic Impact of Toll-like Receptors Gene Polymorphism on Outcome of COVID-19 Pneumonia: A Case-Control Study. Clin. Immunol. 2022, 235, 108929. [Google Scholar] [CrossRef] [PubMed]
- Zacher, C.; Schönfelder, K.; Rohn, H.; Siffert, W.; Möhlendick, B. The Single Nucleotide Polymorphism Rs4986790 (C.896A>G) in the Gene TLR4 as a Protective Factor in Corona Virus Disease 2019 (COVID-19). Front. Immunol. 2024, 15, 1355193. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Wicke, P.; Fernández, S.; Roy-Vallejo, E.; Alegría-Carrasco, E.; Rodríguez-Serrano, D.A.; Lamana, A.; Montes, N.; Nicolao-Gómez, A.; Carracedo-Rodríguez, R.; Marcos-Jiménez, A.; et al. Genetic Variants Regulating the Immune Response Improve the Prediction of COVID-19 Severity Provided by Clinical Variables. Sci. Rep. 2024, 14. [Google Scholar] [CrossRef]
- Alhabibi, A.M.; Hassan, A.S.; Abd, M.; Eid, H.A.; Khalifa, A.; Wahab, M.A.; Althoqapy, A.A.; Abdou, A.E.; Zakaria, D.M.; Nassef, E.M.; et al. Impact of Toll-like Receptor 2 and 9 Gene Polymorphisms on COVID-19: Susceptibility, Severity, and Thrombosis. J. Inflamm. Res. 2023, 16, 665–675. [Google Scholar] [CrossRef]
- Kouwaki, T.; Nishimura, T.; Wang, G.; Oshiumi, H. RIG-I-Like Receptor-Mediated Recognition of Viral Genomic RNA of Severe Acute Respiratory Syndrome Coronavirus-2 and Viral Escape from the Host Innate Immune Responses. Front. Immunol. 2021, 12, 700926. [Google Scholar] [CrossRef]
- Thorne, L.G.; Reuschl, A.-K.; Zuliani-Alvarez, L.; Whelan, M.V.X.; Turner, J.; Noursadeghi, M.; Jolly, C.; Towers, G.J. SARS-CoV-2 Sensing by RIG-I and MDA5 Links Epithelial Infection to Macrophage Inflammation. EMBO J. 2021, 40, e107826. [Google Scholar] [CrossRef]
- Chang, H.; Hou, P.; Wang, X.; Xiang, A.; Wu, H.; Qi, W.; Yang, R.; Wang, X.; Li, X.; He, W.; et al. CD97 Negatively Regulates the Innate Immune Response against RNA Viruses by Promoting RNF125-Mediated RIG-I Degradation. Cell. Mol. Immunol. 2023, 20, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Riva, L.; Pu, Y.; Martin-Sancho, L.; Kanamune, J.; Yamamoto, Y.; Sakai, K.; Gotoh, S.; Miorin, L.; De Jesus, P.D.; et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021, 34, 108628. [Google Scholar] [CrossRef] [PubMed]
- Rebendenne, A.; Valadão, A.L.C.; Tauziet, M.; Maarifi, G.; Bonaventure, B.; McKellar, J.; Planès, R.; Nisole, S.; Arnaud-Arnould, M.; Moncorgé, O.; et al. SARS-CoV-2 Triggers an MDA-5-Dependent Interferon Response Which Is Unable to Control Replication in Lung Epithelial Cells. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-M.; Geng, T.-T.; Harrison, A.G.; Wang, P.-H. Differential Roles of RIG-I like Receptors in SARS-CoV-2 Infection. Mil. Med. Res. 2021, 8, 49. [Google Scholar] [CrossRef]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-Activated Antiviral Innate Immunity in the Upper Airways Controls Early SARS-CoV-2 Infection in Children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef]
- Rice, M.; Tili, E.; Loghmani, H.; Nuovo, G.J. The Differential Expression of Toll like Receptors and RIG-1 Correlates to the Severity of Infectious Diseases. Ann. Diagn. Pathol. 2023, 63, 152102. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Sampaio, N.G.; Chauveau, L.; Hertzog, J.; Bridgeman, A.; Fowler, G.; Moonen, J.P.; Dupont, M.; Russell, R.A.; Noerenberg, M.; Rehwinkel, J. The RNA Sensor MDA5 Detects SARS-CoV-2 Infection. Sci. Rep. 2021, 11, 13638. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, N.; Xian, Y.; Tang, Y.; Ye, J.; Reza, F.; He, G.; Wen, X.; Jiang, X. The Multiple Roles of Interferon Regulatory Factor Family in Health and Disease. Signal Transduct. Target. Ther. 2024, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Sanchez David, R.Y.; Combredet, C.; Najburg, V.; Millot, G.A.; Beauclair, G.; Schwikowski, B.; Léger, T.; Camadro, J.-M.; Jacob, Y.; Bellalou, J.; et al. LGP2 Binds to PACT to Regulate RIG-I- and MDA5-Mediated Antiviral Responses. Sci. Signal. 2019, 12, eaar3993. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.R.; Bruns, A.M.; Horvath, C.M. MDA5 and LGP2: Accomplices and Antagonists of Antiviral Signal Transduction. J. Virol. 2014, 88, 8194–8200. [Google Scholar] [CrossRef]
- Liu, G.; Lee, J.-H.; Parker, Z.M.; Acharya, D.; Chiang, J.J.; van Gent, M.; Riedl, W.; Davis-Gardner, M.E.; Wies, E.; Chiang, C.; et al. ISG15-Dependent Activation of the Sensor MDA5 Is Antagonized by the SARS-CoV-2 Papain-like Protease to Evade Host Innate Immunity. Nat. Microbiol. 2021, 6, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, A.; Giannessi, F.; Sabatini, A.; Percario, Z.A.; Affabris, E. SARS-CoV-2 Evasion of the Interferon System: Can We Restore Its Effectiveness? Int. J. Mol. Sci. 2023, 24, 9353. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, J.; Kim, S.; Kim, M.; Kang, M.-G.; Kwak, C.; Kang, M.; Kim, B.; Rhee, H.-W.; Kim, V.N. PKR Senses Nuclear and Mitochondrial Signals by Interacting with Endogenous Double-Stranded RNAs. Mol. Cell 2018, 71, 1051–1063.e6. [Google Scholar] [CrossRef]
- Gal-Ben-Ari, S.; Barrera, I.; Ehrlich, M.; Rosenblum, K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2018, 11, 480. [Google Scholar] [CrossRef]
- Melchjorsen, J.; Kristiansen, H.; Christiansen, R.; Rintahaka, J.; Matikainen, S.; Paludan, S.R.; Hartmann, R. Differential Regulation of the OASL and OAS1 Genes in Response to Viral Infections. J. Interferon Cytokine Res. 2009, 29, 199–207. [Google Scholar] [CrossRef]
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Cardenas-Diaz, F.L.; Truitt, R.; Tan, L.H.; Dong, B.; Alysandratos, K.D.; et al. SARS-CoV-2 Induces Double-Stranded RNA-Mediated Innate Immune Responses in Respiratory Epithelial-Derived Cells and Cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2022643118. [Google Scholar] [CrossRef]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn Errors of OAS-RNase L in SARS-CoV-2-Related Multisystem Inflammatory Syndrome in Children. Science 2023, 379, eabo3627. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, J.; Han, L.; Zhuang, M.-W.; Xu, Y.; Zhang, J.; Nan, M.-L.; Xiao, Y.; Zhan, P.; Liu, X.; et al. SARS-CoV-2 NSP5 and N Protein Counteract the RIG-I Signaling Pathway by Suppressing the Formation of Stress Granules. Signal Transduct. Target. Ther. 2022, 7, 22. [Google Scholar] [CrossRef]
- Christ, W.; Klingström, J.; Tynell, J. SARS-CoV-2 Variant-Specific Differences in Inhibiting the Effects of the PKR-Activated Integrated Stress Response. Virus Res. 2024, 339, 199271. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Lundberg, V.; Le Pen, J.; Yuan, J.; Lee, D.; Pinci, F.; Volpi, S.; Nakajima, K.; Bondet, V.; Åkesson, S.; et al. SARS-CoV-2 Brainstem Encephalitis in Human Inherited DBR1 Deficiency. J. Exp. Med. 2024, 221, e20231725. [Google Scholar] [CrossRef] [PubMed]
- Ru, S.; Tang, S.; Xu, H.; Yin, J.; Guo, Y.; Song, L.; Jin, Z.; Lee, D.; Chan, Y.-H.; Chen, X.; et al. Human DBR1 Deficiency Impairs Stress Granule–Dependent PKR Antiviral Immunity. J. Exp. Med. 2024, 222, e20240010. [Google Scholar] [CrossRef]
- Wang, B.; Tian, Y.; Yin, Q. AIM2 Inflammasome Assembly and Signaling. Adv. Exp. Med. Biol. 2019, 1172, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wei, F.; Zhang, Y.; Wang, T.; Gao, W.; Yu, S.; Sun, H.; Pu, J.; Sun, Y.; Wang, M.; et al. IFI16 Directly Senses Viral RNA and Enhances RIG-I Transcription and Activation to Restrict Influenza Virus Infection. Nat. Microbiol. 2021, 6, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Hamldar, S.; Kiani, S.J.; Khoshmirsafa, M.; Nahand, J.S.; Mirzaei, H.; Khatami, A.; Kahyesh-Esfandiary, R.; Khanaliha, K.; Tavakoli, A.; Babakhaniyan, K.; et al. Expression Profiling of Inflammation-Related Genes Including IFI-16, NOTCH2, CXCL8, THBS1 in COVID-19 Patients. Biol. J. Int. Assoc. Biol. Stand. 2022, 80, 27–34. [Google Scholar] [CrossRef]
- Yang, C.-A.; Huang, Y.-L.; Chiang, B.-L. Innate Immune Response Analysis in COVID-19 and Kawasaki Disease Reveals MIS-C Predictors. J. Formos. Med. Assoc. 2022, 121, 623–632. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. AIM2 Inflammasome in Infection, Cancer, and Autoimmunity: Role in DNA Sensing, Inflammation, and Innate Immunity. Eur. J. Immunol. 2016, 46, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-Mediated SARS-CoV-2 Infection of Monocytes Activates Inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Schroder, K. Inflammasome Signaling and Regulation of Interleukin-1 Family Cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The CGAS-STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, M.; Yuan, C.; Ma, Z.; Li, W.; Zhang, Y.; Su, L.; Xu, J.; Liu, W. Progress of CGAS-STING Signaling in Response to SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 1010911. [Google Scholar] [CrossRef]
- Rui, Y.; Su, J.; Shen, S.; Hu, Y.; Huang, D.; Zheng, W.; Lou, M.; Shi, Y.; Wang, M.; Chen, S.; et al. Unique and Complementary Suppression of CGAS-STING and RNA Sensing- Triggered Innate Immune Responses by SARS-CoV-2 Proteins. Signal Transduct. Target. Ther. 2021, 6, 123. [Google Scholar] [CrossRef]
- Humphries, F.; Shmuel-Galia, L.; Jiang, Z.; Wilson, R.; Landis, P.; Ng, S.-L.; Parsi, K.-M.; Maehr, R.; Cruz, J.; Morales-Ramos, A.; et al. A Diamidobenzimidazole STING Agonist Protects against SARS-CoV-2 Infection. Sci. Immunol. 2021, 6, eabi9002. [Google Scholar] [CrossRef]
- Li, M.; Ferretti, M.; Ying, B.; Descamps, H.; Lee, E.; Dittmar, M.; Lee, J.S.; Whig, K.; Kamalia, B.; Dohnalová, L.; et al. Pharmacological Activation of STING Blocks SARS-CoV-2 Infection. Sci. Immunol. 2021, 6, eabi9007. [Google Scholar] [CrossRef]
- Di Domizio, J.; Gulen, M.F.; Saidoune, F.; Thacker, V.V.; Yatim, A.; Sharma, K.; Nass, T.; Guenova, E.; Schaller, M.; Conrad, C.; et al. The CGAS-STING Pathway Drives Type I IFN Immunopathology in COVID-19. Nature 2022, 603, 145–151. [Google Scholar] [CrossRef]
- Li, H.; Zhou, F.; Zhang, L. STING, a Critical Contributor to SARS-CoV-2 Immunopathology. Signal Transduct. Target. Ther. 2022, 7, 106. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, A. Involvement of the STING Signaling in COVID-19. Front. Immunol. 2022, 13, 1006395. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Zhang, B.; Schmitz, A.; Schwensen, H.V.; Reinert, L.S.; Paludan, S.R. STING Is Redundant for Host Defense and Pathology of COVID-19-like Disease in Mice. Life Sci. Alliance 2023, 6, e202301997. [Google Scholar] [CrossRef]
- Queiroz, M.A.F.; Brito, W.R.D.S.; Pereira, K.A.S.; Pereira, L.M.S.; Amoras, E.d.S.G.; Lima, S.S.; Dos Santos, E.F.; da Costa, F.P.; de Sarges, K.M.L.; Cantanhede, M.H.D.; et al. Severe COVID-19 and Long COVID Are Associated with High Expression of STING, CGAS and IFN-α. Sci. Rep. 2024, 14, 4974. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, C.J.; Cerikan, B.; Cortese, M.; Frankish, J.; Lee, J.-Y.; Plociennikowska, A.; Heigwer, F.; Prasad, V.; Joecks, S.; Burkart, S.S.; et al. SARS-CoV-2 Infection Induces a pro-Inflammatory Cytokine Response through CGAS-STING and NF-ΚB. Commun. Biol. 2022, 5, 45. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Lei, X.; Xiao, X.; Jiao, T.; Ma, R.; Dong, X.; Jiang, Q.; Wang, W.; Shi, Y.; et al. Sensing of Cytoplasmic Chromatin by CGAS Activates Innate Immune Response in SARS-CoV-2 Infection. Signal Transduct. Target. Ther. 2021, 6, 382. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial Dysfunction: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Znaidia, M.; Demeret, C.; van der Werf, S.; Komarova, A. V Characterization of SARS-CoV-2 Evasion: Interferon Pathway and Therapeutic Options. Viruses 2022, 14, 1247. [Google Scholar] [CrossRef]
- Finkel, Y.; Mizrahi, O.; Nachshon, A.; Weingarten-Gabbay, S.; Morgenstern, D.; Yahalom-Ronen, Y.; Tamir, H.; Achdout, H.; Stein, D.; Israeli, O.; et al. The Coding Capacity of SARS-CoV-2. Nature 2021, 589, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, T.; Arya, S.; Chan, S.-H.; Qi, S.; Dai, N.; Misra, A.; Park, J.-G.; Oladunni, F.; Kovalskyy, D.; Hromas, R.A.; et al. Structural Basis of RNA Cap Modification by SARS-CoV-2. Nat. Commun. 2020, 11, 3718. [Google Scholar] [CrossRef]
- Daffis, S.; Szretter, K.J.; Schriewer, J.; Li, J.; Youn, S.; Errett, J.; Lin, T.-Y.; Schneller, S.; Zust, R.; Dong, H.; et al. 2′-O Methylation of the Viral MRNA Cap Evades Host Restriction by IFIT Family Members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef]
- Caobi, A.; Su, C.-M.; Beusch, C.M.; Kenney, D.; Darling, T.L.; Feng, S.; Semaan, M.; Wacquiez, A.; Sanders, N.L.; Tully, E.S.; et al. SARS-CoV-2 Nsp15 Enhances Viral Virulence by Subverting Host Antiviral Defenses. Proc. Natl. Acad. Sci. USA 2025, 122. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhuang, M.-W.; Han, L.; Zhang, J.; Nan, M.-L.; Zhan, P.; Kang, D.; Liu, X.; Gao, C.; Wang, P.-H. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Membrane (M) Protein Inhibits Type I and III Interferon Production by Targeting RIG-I/MDA-5 Signaling. Signal Transduct. Target. Ther. 2020, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Xiao, H.; Liao, F.; Shen, M.; Ge, W.; Ou, J.; Liu, Y.; Chen, L.; Zhao, Y.; et al. The R203M and D377Y Mutations of the Nucleocapsid Protein Promote SARS-CoV-2 Infectivity by Impairing RIG-I-Mediated Antiviral Signaling. PLOS Pathog. 2025, 21, e1012886. [Google Scholar] [CrossRef] [PubMed]
- Aloise, C.; Schipper, J.G.; van Vliet, A.; Oymans, J.; Donselaar, T.; Hurdiss, D.L.; de Groot, R.J.; van Kuppeveld, F.J.M. SARS-CoV-2 Nucleocapsid Protein Inhibits the PKR-Mediated Integrated Stress Response through RNA-Binding Domain N2b. PLoS Pathog. 2023, 19, e1011582. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, T.; Kuster, D.; Hyman, A.A. SARS-CoV-2 Nucleocapsid Protein Directly Prevents CGAS-DNA Recognition through Competitive Binding. Proc. Natl. Acad. Sci. USA 2025, 122, e2426204122. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Zhuang, Z.; Cai, S.; Zhao, Z.; Zhou, L.; Zhang, J.; Wang, P.-H.; Zhao, J.; Cui, J. Main Protease of SARS-CoV-2 Serves as a Bifunctional Molecule in Restricting Type I Interferon Antiviral Signaling. Signal Transduct. Target. Ther. 2020, 5, 221. [Google Scholar] [CrossRef]
- Jiang, H.-W.; Zhang, H.-N.; Meng, Q.-F.; Xie, J.; Li, Y.; Chen, H.; Zheng, Y.-X.; Wang, X.-N.; Qi, H.; Zhang, J.; et al. SARS-CoV-2 Orf9b Suppresses Type I Interferon Responses by Targeting TOM70. Cell. Mol. Immunol. 2020, 17, 998–1000. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, C.; Rao, Y.; Ngo, C.; Feng, J.J.; Zhao, J.; Zhang, S.; Wang, T.-Y.; Carriere, J.; Savas, A.C.; et al. SARS-CoV-2 Nsp5 Demonstrates Two Distinct Mechanisms Targeting RIG-I and MAVS To Evade the Innate Immune Response. MBio 2021, 12, e0233521. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Song, J.; Qian, W.; Gu, X.; Yang, C.; Shen, N.; Xue, F.; Tang, Y. SARS-CoV-2-Encoded MiRNAs Inhibit Host Type I Interferon Pathway and Mediate Allelic Differential Expression of Susceptible Gene. Front. Immunol. 2021, 12, 767726. [Google Scholar] [CrossRef]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.-C.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.-Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like Protease Regulates SARS-CoV-2 Viral Spread and Innate Immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef]
- Moustaqil, M.; Ollivier, E.; Chiu, H.-P.; Van Tol, S.; Rudolffi-Soto, P.; Stevens, C.; Bhumkar, A.; Hunter, D.J.B.; Freiberg, A.N.; Jacques, D.; et al. SARS-CoV-2 Proteases PLpro and 3CLpro Cleave IRF3 and Critical Modulators of Inflammatory Pathways (NLRP12 and TAB1): Implications for Disease Presentation across Species. Emerg. Microbes Infect. 2021, 10, 178–195. [Google Scholar] [CrossRef]
- Li, A.; Zhao, K.; Zhang, B.; Hua, R.; Fang, Y.; Jiang, W.; Zhang, J.; Hui, L.; Zheng, Y.; Li, Y.; et al. SARS-CoV-2 NSP12 Protein Is Not an Interferon-β Antagonist. J. Virol. 2021, 95, e0074721. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.; Swanson, S.E.; Negatu, S.G.; Dittmar, M.; Miller, J.; Ramage, H.R.; Cherry, S.; Jurado, K.A. SARS-CoV-2 Viral Proteins NSP1 and NSP13 Inhibit Interferon Activation through Distinct Mechanisms. PLoS ONE 2021, 16, e0253089. [Google Scholar] [CrossRef]
- Yuen, C.-K.; Lam, J.-Y.; Wong, W.-M.; Mak, L.-F.; Wang, X.; Chu, H.; Cai, J.-P.; Jin, D.-Y.; To, K.K.-W.; Chan, J.F.-W.; et al. SARS-CoV-2 Nsp13, Nsp14, Nsp15 and Orf6 Function as Potent Interferon Antagonists. Emerg. Microbes Infect. 2020, 9, 1418–1428. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Blanco, M.R.; Bruce, E.A.; Honson, D.D.; Chen, L.M.; Chow, A.; Bhat, P.; Ollikainen, N.; Quinodoz, S.A.; Loney, C.; et al. SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell 2020, 183, 1325–1339.e21. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Dzakah, E.E.; Wang, H.; Tang, S. The ORF8 Protein of SARS-CoV-2 Induced Endoplasmic Reticulum Stress and Mediated Immune Evasion by Antagonizing Production of Interferon Beta. Virus Res. 2021, 296, 198350. [Google Scholar] [CrossRef] [PubMed]
- Miorin, L.; Kehrer, T.; Sanchez-Aparicio, M.T.; Zhang, K.; Cohen, P.; Patel, R.S.; Cupic, A.; Makio, T.; Mei, M.; Moreno, E.; et al. SARS-CoV-2 Orf6 Hijacks Nup98 to Block STAT Nuclear Import and Antagonize Interferon Signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 28344–28354. [Google Scholar] [CrossRef]
- Konno, Y.; Kimura, I.; Uriu, K.; Fukushi, M.; Irie, T.; Koyanagi, Y.; Sauter, D.; Gifford, R.J.; Nakagawa, S.; Sato, K. SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant. Cell Rep. 2020, 32, 108185. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Choudhury, A.; Das, N.C.; Patra, R.; Mukherjee, S. In Silico Analyses on the Comparative Sensing of SARS-CoV-2 MRNA by the Intracellular TLRs of Humans. J. Med. Virol. 2021, 93, 2476–2486. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. An Overview of Recent Insights into the Response of TLR to SARS-CoV-2 Infection and the Potential of TLR Agonists as SARS-CoV-2 Vaccine Adjuvants. Viruses 2021, 13, 2302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-H.; Zhang, R.-Y.; Zhang, H.-W.; Liu, Y.-L.; Wen, Y.; Wang, J.; Li, Y.-T.; You, Z.-W.; Yin, X.-G.; Qiu, H.; et al. RBD Conjugate Vaccine with a Built-in TLR1/2 Agonist Is Highly Immunogenic against SARS-CoV-2 and Variants of Concern. Chem. Commun. 2022, 58, 2120–2123. [Google Scholar] [CrossRef]
- Cojocaru, E.; Cojocaru, C.; Antoniu, S.A.; Stafie, C.S.; Rajnoveanu, A.; Rajnoveanu, R.-M. Inhaled Interferons Beta and SARS-COV2 Infection: A Preliminary Therapeutic Perspective. Expert Rev. Respir. Med. 2022, 16, 257–261. [Google Scholar] [CrossRef]
- Tamir, H.; Melamed, S.; Erez, N.; Politi, B.; Yahalom-Ronen, Y.; Achdout, H.; Lazar, S.; Gutman, H.; Avraham, R.; Weiss, S.; et al. Induction of Innate Immune Response by TLR3 Agonist Protects Mice against SARS-CoV-2 Infection. Viruses 2022, 14, 189. [Google Scholar] [CrossRef]
- Kircheis, R. In Silico Analyses Indicate a Lower Potency for Dimerization of TLR4/MD-2 as the Reason for the Lower Pathogenicity of Omicron Compared to Wild-Type Virus and Earlier SARS-CoV-2 Variants. Int. J. Mol. Sci. 2024, 25, 5451. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, T.; Zhou, X.; Xiang, Y.; Gutierrez-Castrellon, P.; Ma, X. Inflammatory Pathways in COVID-19: Mechanism and Therapeutic Interventions. MedComm 2022, 3, e154. [Google Scholar] [CrossRef]
- McKinnon, J.E.; Santiaguel, J.; Murta de Oliveira, C.; Yu, D.; Khursheed, M.; Moreau, F.; Klopp-Schulze, L.; Shaw, J.; Roy, S.; Kao, A.H. Enpatoran in COVID-19 Pneumonia: Safety and Efficacy Results from a Phase II Randomized Trial. Clin. Transl. Sci. 2023, 16, 2640–2653. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, A.W.; Ahmad, B.; Kim, M.S.; Choi, S. Therapeutic Targeting of Innate Immune Receptors Against SARS-CoV-2 Infection. Front. Pharmacol. 2022, 13, 915565. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.; Kümmerer, B.M.; Grützner, C.; Kato, H.; Schlee, M.; Renn, M.; Bartok, E.; Hartmann, G. RIG-I-Induced Innate Antiviral Immunity Protects Mice from Lethal SARS-CoV-2 Infection. Mol. Ther. Nucleic Acids 2022, 27, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Israelow, B.; Lucas, C.; Vogels, C.B.F.; Gomez-Calvo, M.L.; Fedorova, O.; Breban, M.I.; Menasche, B.L.; Dong, H.; Linehan, M.; et al. A Stem-Loop RNA RIG-I Agonist Protects against Acute and Chronic SARS-CoV-2 Infection in Mice. J. Exp. Med. 2022, 219, e20211818. [Google Scholar] [CrossRef]
- Lozhkov, A.A.; Plotnikova, M.A.; Egorova, M.A.; Baranovskaya, I.L.; Elpaeva, E.A.; Klotchenko, S.A.; Vasin, A. V Simultaneous Detection of RIG-1, MDA5, and IFIT-1 Expression Is a Convenient Tool for Evaluation of the Interferon-Mediated Response. Viruses 2022, 14, 2090. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Cao, X.; Lu, M.; Gao, Q.; Ma, T. The Intersection Molecule MDA5 in Cancer and COVID-19. Front. Immunol. 2022, 13, 963051. [Google Scholar] [CrossRef]
- Qi, H.; Ma, Q.-H.; Feng, W.; Chen, S.-M.; Wu, C.-S.; Wang, Y.; Wang, T.-X.; Hou, Y.-L.; Jia, Z.-H. Glycyrrhetinic Acid Blocks SARS-CoV-2 Infection by Activating the CGAS-STING Signalling Pathway. Br. J. Pharmacol. 2024, 181, 3976–3992. [Google Scholar] [CrossRef]
- Parums, D.V. Long COVID or Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) and the Urgent Need to Identify Diagnostic Biomarkers and Risk Factors. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e946512. [Google Scholar] [CrossRef]
- Fracella, M.; Mancino, E.; Nenna, R.; Virgillito, C.; Frasca, F.; D’Auria, A.; Sorrentino, L.; Petrarca, L.; La Regina, D.; Matera, L.; et al. Age-Related Transcript Changes in Type I Interferon Signaling in Children and Adolescents with Long COVID. Eur. J. Immunol. 2024, 54, e2350682. [Google Scholar] [CrossRef]
- Hope, A.A.; Evering, T.H. Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Infect. Dis. Clin. N. Am. 2022, 36, 379–395. [Google Scholar] [CrossRef]
- Umakanthan, S.; Katwaroo, A.R.; Bukelo, M.; Bg, S.; Boralingaiah, P.; Ranade, A.V.; Rangan, P.; Shashidhar, S.; Kini, J.R.; Kini, G. Post-Acute Sequelae of Covid-19: A System-Wise Approach on the Effects of Long-Covid-19. Am. J. Med. Open 2024, 12, 100071. [Google Scholar] [CrossRef]
- Konno, H.; Konno, K.; Barber, G.N. Cyclic Dinucleotides Trigger ULK1 (ATG1) Phosphorylation of STING to Prevent Sustained Innate Immune Signaling. Cell 2013, 155, 688–698. [Google Scholar] [CrossRef]
- Ablasser, A.; Chen, Z.J. CGAS in Action: Expanding Roles in Immunity and Inflammation. Science 2019, 363, eaat8657. [Google Scholar] [CrossRef]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and Psychiatric Risk Trajectories after SARS-CoV-2 Infection: An Analysis of 2-Year Retrospective Cohort Studies Including 1,284,437 Patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Damiano, R.F.; Rocca, C.C.d.A.; Serafim, A.d.P.; Loftis, J.M.; Talib, L.L.; Pan, P.M.; Cunha-Neto, E.; Kalil, J.; de Castro, G.S.; Seelaender, M.; et al. Cognitive Impairment in Long-COVID and Its Association with Persistent Dysregulation in Inflammatory Markers. Front. Immunol. 2023, 14, 1174020. [Google Scholar] [CrossRef]
- Fontes-Dantas, F.L.; Fernandes, G.G.; Gutman, E.G.; De Lima, E.V.; Antonio, L.S.; Hammerle, M.B.; Mota-Araujo, H.P.; Colodeti, L.C.; Araújo, S.M.B.; Froz, G.M.; et al. SARS-CoV-2 Spike Protein Induces TLR4-Mediated Long-Term Cognitive Dysfunction Recapitulating Post-COVID-19 Syndrome in Mice. Cell Rep. 2023, 42, 112189. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Iwasaki, A. The Neurobiology of Long COVID. Neuron 2022, 110, 3484–3496. [Google Scholar] [CrossRef] [PubMed]
- Mentor, G.; Farrar, D.S.; Di Chiara, C.; Dufour, M.-S.K.; Valois, S.; Taillefer, S.; Drouin, O.; Renaud, C.; Kakkar, F. The Effect of Age and Comorbidities: Children vs. Adults in Their Response to SARS-CoV-2 Infection. Viruses 2024, 16, 801. [Google Scholar] [CrossRef]
- Kuchitsu, Y.; Taguchi, T. Innate Immune Signals Triggered on Organelle Membranes. J. Biochem. 2025, 178, mvaf016. [Google Scholar] [CrossRef]
- Pascoal Ramos, M.I.; van der Vlist, M.; Meyaard, L. Inhibitory Pattern Recognition Receptors: Lessons from LAIR1. Nat. Rev. Immunol. 2025. [Google Scholar] [CrossRef]
- Helou, D.G.; Quach, C.; Hurrell, B.P.; Li, X.; Li, M.; Akbari, A.; Shen, S.; Shafiei-Jahani, P.; Akbari, O. LAIR-1 Limits Macrophage Activation in Acute Inflammatory Lung Injury. Mucosal Immunol. 2023, 16, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Sievers, B.L.; Cheng, M.T.K.; Csiba, K.; Meng, B.; Gupta, R.K. SARS-CoV-2 and Innate Immunity: The Good, the Bad, and the “Goldilocks”. Cell. Mol. Immunol. 2024, 21, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Minkoff, J.M.; tenOever, B. Innate Immune Evasion Strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 178–194. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L.-L. The NLRP3 Inflammasome and COVID-19: Activation, Pathogenesis and Therapeutic Strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Lazzaroni, M.G.; Piantoni, S.; Masneri, S.; Garrafa, E.; Martini, G.; Tincani, A.; Andreoli, L.; Franceschini, F. Coagulation Dysfunction in COVID-19: The Interplay between Inflammation, Viral Infection and the Coagulation System. Blood Rev. 2021, 46, 100745. [Google Scholar] [CrossRef]
- Eaton-Fitch, N.; Rudd, P.; Er, T.; Hool, L.; Herrero, L.; Marshall-Gradisnik, S. Immune Exhaustion in ME/CFS and Long COVID. JCI Insight 2024, 9, e183810. [Google Scholar] [CrossRef]
- Pal, S.; Rafiq, Z.; Kumari, R.; Al Aiyan, A.; Al-Ramadi, B.; Kishore, U.; Ponnachan, P. Trained Innate Immunity. In Innate Immunity: Pattern Recognition and Effector Mechanisms; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2025; pp. 275–296. [Google Scholar] [CrossRef]

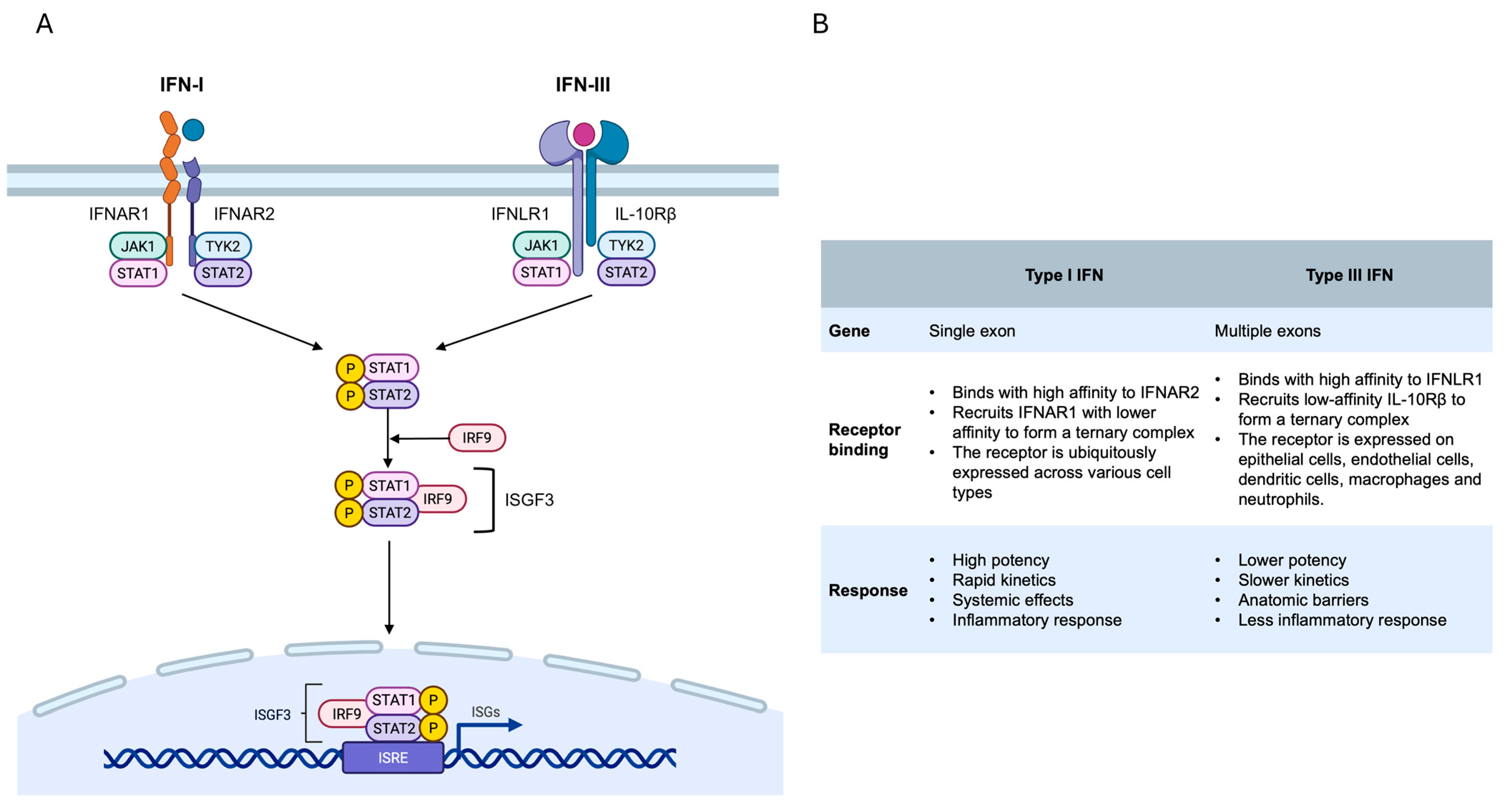
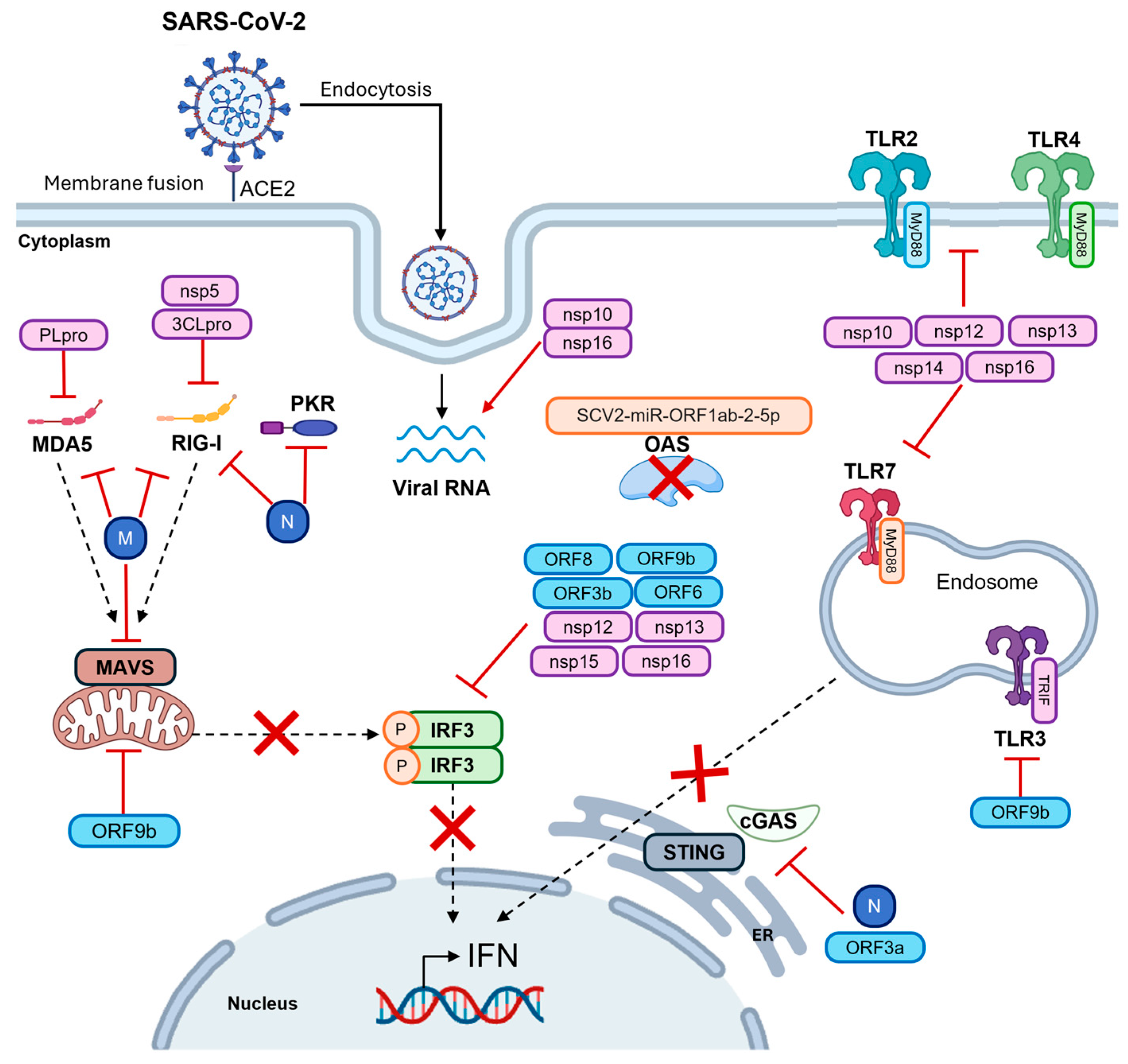
| PRR | Effect | Reference |
|---|---|---|
| cGAS-STING | Elevated levels lead to the onset of low-grade inflammatory diseases, including cardiomyopathy | [236] |
| TLR4 | Increased expression is associated with cognitive deficits and synapse loss | [287] |
| TLR2 and TLR4 | Elevated levels founded in the brains of died COVID-19 patients | [288] |
| OAS2 and MDA5 | Differential expression between healthy controls (HC), matched controls and LC cases | [279] |
| Knowledge Gaps | Future Directions | |
|---|---|---|
| Viral evasion mechanisms of SARS-CoV-2 and their impact on PRR and innate immune response in different human tissues | Development of targeted PRR agonists/antagonists to prevent severe COVID-19 and long COVID | |
| Long-term effects of PRR modulation on the development of long COVID (neurological, cardiovascular and metabolic symptoms) | Longitudinal studies on PRR and IFN-I/III expression to monitor long-term post-infection outcomes | |
| Impact of emerging variants on PRR functional relevance and treatment efficacy | Evaluation of immune responses and PRR modulation against emerging SARS-CoV-2 variants, including the efficacy of vaccines or combination therapies in preventing persistent long COVID symptoms | |
| Influence of age-, sex- and genetics-related differences (including TLR, OAS and MDA5 polymorphisms) on PRR activation and related immune responses | Integration of genomic and transcriptomic data to personalize antiviral and immunomodulatory therapies | |
| Combined effects of PRR-modulating drugs (agonists and/or antagonists) or vaccines on long COVID | Development of predictive biomarkers for long COVID based on PRRs and IFN | |
| Limitations of current studies: geographic bias, small sample sizes, heterogeneous protocols | International multicenter studies to validate PRR and IFN findings across distinctive populations and settings. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddaloni, L.; Bugani, G.; Fracella, M.; Bitossi, C.; D’Auria, A.; Aloisi, F.; Azri, A.; Santinelli, L.; Ben M’Hadheb, M.; Pierangeli, A.; et al. Pattern Recognition Receptors (PRRs) Expression and Activation in COVID-19 and Long COVID: From SARS-CoV-2 Escape Mechanisms to Emerging PRR-Targeted Immunotherapies. Microorganisms 2025, 13, 2176. https://doi.org/10.3390/microorganisms13092176
Maddaloni L, Bugani G, Fracella M, Bitossi C, D’Auria A, Aloisi F, Azri A, Santinelli L, Ben M’Hadheb M, Pierangeli A, et al. Pattern Recognition Receptors (PRRs) Expression and Activation in COVID-19 and Long COVID: From SARS-CoV-2 Escape Mechanisms to Emerging PRR-Targeted Immunotherapies. Microorganisms. 2025; 13(9):2176. https://doi.org/10.3390/microorganisms13092176
Chicago/Turabian StyleMaddaloni, Luca, Ginevra Bugani, Matteo Fracella, Camilla Bitossi, Alessandra D’Auria, Francesca Aloisi, Abir Azri, Letizia Santinelli, Manel Ben M’Hadheb, Alessandra Pierangeli, and et al. 2025. "Pattern Recognition Receptors (PRRs) Expression and Activation in COVID-19 and Long COVID: From SARS-CoV-2 Escape Mechanisms to Emerging PRR-Targeted Immunotherapies" Microorganisms 13, no. 9: 2176. https://doi.org/10.3390/microorganisms13092176
APA StyleMaddaloni, L., Bugani, G., Fracella, M., Bitossi, C., D’Auria, A., Aloisi, F., Azri, A., Santinelli, L., Ben M’Hadheb, M., Pierangeli, A., Frasca, F., & Scagnolari, C. (2025). Pattern Recognition Receptors (PRRs) Expression and Activation in COVID-19 and Long COVID: From SARS-CoV-2 Escape Mechanisms to Emerging PRR-Targeted Immunotherapies. Microorganisms, 13(9), 2176. https://doi.org/10.3390/microorganisms13092176







