Abstract
The genus Trichoderma plays a pivotal role in sustainable agriculture through its multifaceted contributions to plant health and productivity. This review explores Trichoderma’s biological functions, including its roles as a biocontrol agent, plant growth promoter, and stress resilience enhancer. By producing various enzymes, secondary metabolites, and volatile organic compounds, Trichoderma effectively suppresses plant pathogens, promotes root development, and primes plant immune responses. This review details the evolutionary adaptations of Trichoderma, which has transitioned from saprotrophism to mycoparasitism and established beneficial symbiotic relationships with plants. It also highlights the ecological versatility of Trichoderma in colonizing plant roots and improving soil health, while emphasizing its role in mitigating both biotic and abiotic stressors. With increasing recognition as a biostimulant and biocontrol agent, Trichoderma has become a key player in reducing chemical inputs and advancing eco-friendly farming practices. This review addresses challenges such as strain selection, formulation stability, and regulatory hurdles and concludes by advocating for continued research to optimize Trichoderma’s applications in addressing climate change, enhancing food security, and promoting a sustainable agricultural future.
1. Introduction
Trichoderma, also known in its teleomorphic form as Hypocrea, is a genus of filamentous fungi renowned for its mycotrophic abilities, meaning it can parasitize other fungi [1]. This genus is globally distributed and thrives in a wide range of environments, including agricultural lands, forests, grasslands, deserts, and both freshwater and marine ecosystems. Trichoderma species are characterized by their rapid growth on various substrates and their prolific production of green conidia, which makes them easily identifiable [2].
Trichoderma species are considered opportunistic symbionts that are highly beneficial to plants. While the majority of Trichoderma species are non-pathogenic saprophytes or beneficial symbionts in soil and plant ecosystems, a few can act as opportunistic pathogens in specific contexts, such as in immunocompromised hosts or under rare environmental conditions [3,4]. They enhance plant health through multiple mechanisms. In terms of biological control of plant diseases, Trichoderma has direct antagonistic actions against pathogens. It can also indirectly strengthen plant defense systems via local and systemic responses. Moreover, Trichoderma is capable of stimulating root development and promoting plant growth. This dual-benefit situation is advantageous for both the host plant and the fungus itself, showing great potential in agriculture. The interactions between plants and Trichoderma are complex. Root colonization by Trichoderma and the bioactive compounds it produces activate biochemical and genetic pathways in plants [5]. These pathways play a crucial role in enhancing plants’ ability to defend against both biotic and abiotic stresses [6].
Recent advancements in omics technologies have further illuminated the ecological roles of Trichoderma species, providing insights into their evolution from common soil dwellers that decompose organic matter to mycoparasites that interact with other fungi, including those from closely related taxonomic groups [7,8]. These technological approaches have deepened our understanding of how Trichoderma benefits plants [9], particularly in the context of agricultural productivity. Molecular methods have significantly expanded Trichoderma’s taxonomy, increasing the number of recognized species from just nine fifty years ago to over four hundred today [10].
Research into Trichoderma has evolved to incorporate integrated, multidisciplinary approaches that explore its diverse roles as a plant-beneficial fungus [11]. As of 2025, the genus encompasses over 400 identified species, with ongoing discoveries driven by advanced genomic sequencing and phylogenetic analyses, revealing unprecedented diversity in habitats ranging from forest soils to agricultural fields (e.g., recent additions like T. cerradensis sp. nov. and Trichoderma egyptiacum sp. nov., identified through metagenomic studies in tropical ecosystems) [12,13]. This taxonomic expansion underscores Trichoderma’s adaptability and underscores its pivotal roles in sustainable agriculture development, including serving as a biocontrol agent to naturally suppress soilborne pathogens like Fusarium and Rhizoctonia, thereby reducing chemical pesticide use in field applications; acting as a biostimulant to enhance plant growth through improved nutrient uptake (e.g., phosphorus solubilization) and stress tolerance against drought, salinity, and heavy metals, leading to yield increases in crops such as maize and tomatoes; promoting soil health by boosting microbial diversity and organic matter decomposition; and facilitating bioremediation of contaminated soils by degrading pollutants like hydrocarbons and pesticides [3]. Consequently, Trichoderma is being recognized as a vital biotechnological tool in modern agricultural practices, enabling eco-friendly innovations like biofertilizers and microbiome engineering for resilient, low-input farming systems worldwide.
This review summarizes recent advancements in Trichoderma research, including its ecological physiology, adaptive behaviors leading to species diversification, and its dual roles in both direct biological control of pests and indirect stimulation of plant immune responses. We also explore its potential as a biostimulant for promoting plant growth and enhancing resilience to abiotic stress. Furthermore, this review discusses future strategies for improving Trichoderma applications, optimizing bioformulations, and considering policy implications. Ultimately, we explore how the use of Trichoderma could contribute to reducing chemical inputs in agriculture, fostering a more sustainable and environmentally friendly farming system.
2. Evolution and Ecological Adaptations of Trichoderma: From Saprotrophism to Plant Mutualism
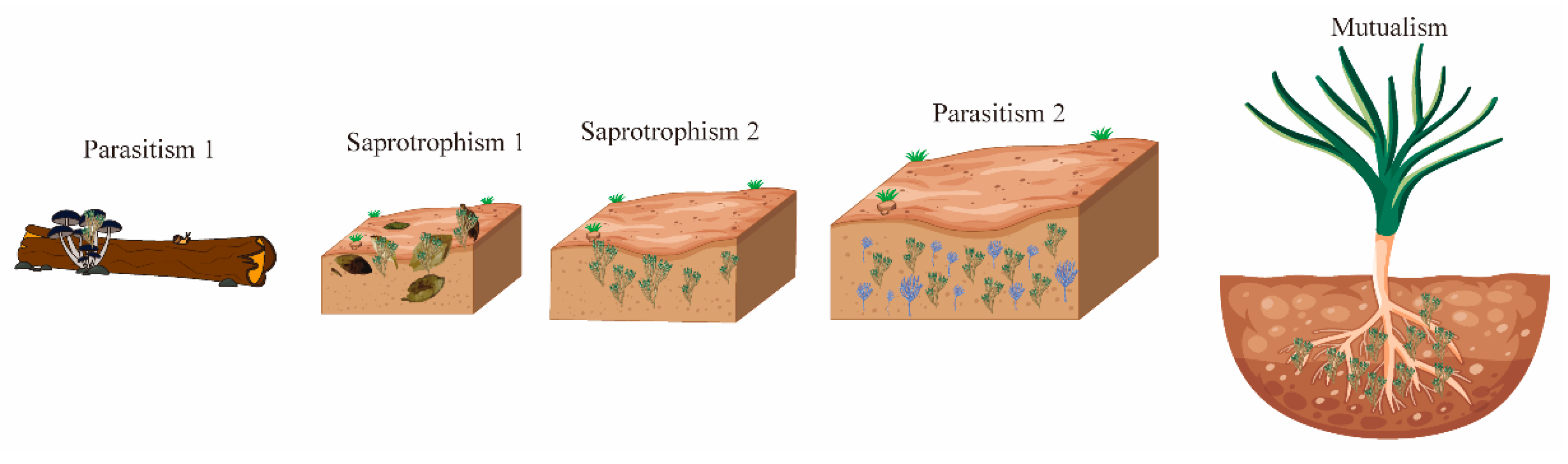
The Trichoderma genus exhibits notable morphological consistency and nutritional diversity, with a species count surpassing other fungi in similar ecological niches [10]. This diversity stems from four major evolutionary transitions that reshaped its nutritional strategies and ecological roles, leading to increased species diversification (Figure 1). Initially, Trichoderma shifted from parasitizing fungi-decomposing plants to saprotrophism, feeding on decaying plant matter. It then adapted to soil environments, developing saprotrophic, mycotrophic (fungus-feeding), and phytophagic (plant-feeding) behaviors. The third transition introduced mycoparasitism, where Trichoderma fed on living fungi, and the fourth established symbiotic relationships with living plants.
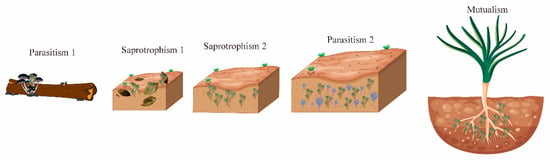
Figure 1.
Trichoderma and the plant microbiome: allies in stress, complexity in coexistence. The evolutionary trajectory of Trichoderma spp. reveals key ecophysiological shifts. Parasitism 1: ancient mycoparasitism targeting wood-decomposing Basidiomycota. Saprotrophism 1: transition to saprotrophism during the Cretaceous–Paleogene extinction, thriving on dead plant material via acquired carbohydrate hydrolytic enzymes. Saprotrophism 2: adaptation as soil-colonizing saprophytes. Parasitism 2: expanded parasitism to Ascomycota, oomycetes, basidiomycetes, and nematodes. Mutualism: rhizosphere-driven shift to mutualism with plants, culminating in endophytism as the latest development.
Genomic studies reveal that Trichoderma species have continually adapted their genomes to colonize new environments. Mycotrophy, an ancient trait, has been crucial for its success and beneficial plant interactions. Phylogenomic analyses suggest that Trichoderma shares a common ancestor with entomopathogenic fungi, with its earliest species evolving around the Cretaceous–Paleogene extinction event about 66 million years ago [14]. During this time, Trichoderma was mycoparasitic on Basidiomycota hosts, acquiring genes through horizontal gene transfer, enabling growth on decaying wood [15].
As Trichoderma evolved, it expanded its ecological niche to include mycoparasitism of related fungi like Ascomycota and parasitism of soilborne pathogens such as Phytophthora, Pythium, Rhizoctonia, and nematodes. It also formed mutualistic relationships with insects, protecting termites from entomopathogenic fungi [16]. In soil, Trichoderma increased enzyme production, including exochitinases and endochitinases, enhancing mycoparasitism and its competitive advantage. Many hydrolytic enzymes originated from lateral gene transfer from plant-associated fungi.
Trichoderma’s adaptation to various habitats led to rhizosphere colonization, attracted by fungal prey and plant root nutrients [17]. This eventually resulted in plant tissue colonization, evolving into endophytes that live within plants [18]. This transition marked Trichoderma’s evolution toward intimate plant interactions, acting as a non-pathogenic mutualist that promotes plant growth and provides protection against biotic and abiotic stresses [18].
Trichoderma exhibits significant opportunistic traits, allowing it to thrive in a variety of ecological niches by utilizing a range of strategies to compete for space and nutrients, withstand environmental stresses, and modify its habitat to its advantage. This includes detoxifying harmful substances, altering substrate pH, and inducing changes that promote its own survival [19]. Root-derived nutrients, such as pectin, xylan, and mucigel-released compounds, serve as attractants for Trichoderma, driving its colonization of plant roots [20,21]. The fungus also produces reactive oxygen species (ROS), which play a role in antagonizing phytopathogens, particularly those with cellulose-rich cell walls, such as Pythium ultimum [22]. Additionally, ROS and oxylipins in root exudates from plants under stress, such as pathogen attack or salt exposure, act as selective chemoattractants that further enhance Trichoderma’s growth [23,24,25].
Beyond its role in pathogen defense, Trichoderma contributes to plant health by modulating the plant’s antioxidant defense systems, reducing ROS levels, and limiting tissue damage under abiotic stress [26,27]. Unlike many other fungi, Trichoderma possesses a robust antioxidant system that supports both its own genomic stability and the plant’s resilience to oxidative damage. Moreover, the fungus can repair DNA damage caused by UV radiation and regulate metabolic processes in response to light, highlighting its versatile ability to adapt to environmental challenges [28].
Trichoderma also enhances its ecological role through the secretion of various compounds, such as siderophores [29], which facilitate its competition for iron in the rhizosphere, and volatile organic compounds (VOCs), like 6-pentyl-2H-pyran-2-one, which have antibiotic properties and influence plant growth, root development, and immune responses [30]. The ability to produce phytohormones such as auxins, gibberellins, and cytokinins further links Trichoderma to plant growth promotion [26,31], although excessive auxin accumulation can sometimes inhibit root development by acidifying the rhizosphere [32]. Additionally, non-secreted cell wall proteins, like QID74 in Trichoderma harzianum, increase root hair formation, enhancing nutrient absorption and contributing to plant biomass.
The endophytic colonization of plants by Trichoderma plays a crucial role in both disease control and plant growth enhancement [33]. By colonizing plant tissues, Trichoderma provides protection against pathogens, such as Verticillium dahliae, and helps improve photosynthetic capacity and stress tolerance [34,35]. However, the outcomes of endophytic colonization can be inconsistent, and further research is needed to understand the conditions under which these benefits are most pronounced.
Trichoderma species exhibit remarkable ecological adaptability, particularly in colonizing the rhizosphere and plant root systems. Their capacity for biocontrol and antagonism highlights their competitive and aggressive nature in occupying ecological niches. Given this, it is essential to evaluate the potential impacts of Trichoderma on non-target organisms, including plants and soil microbial communities. Notably, Trichoderma is often considered an indicator of healthy soil [36]. A core Trichoderma population appears to exist across diverse plant species worldwide, with endemic plants generally supporting a higher abundance of antagonistic strains [14]. Agricultural practices, including crop selection and cultivation methods, significantly influence soil characteristics and fungal diversity—affecting both harmful and beneficial fungi. Typically, microbial diversity peaks in bulk soil and declines in the rhizosphere and endosphere. However, Trichoderma inoculation alters both bacterial and fungal populations in all these zones [37]. Its application, either alone or combined with organic compost, has been shown to support plant growth and restructure rhizosphere microbial communities, particularly by enhancing phosphorus solubilization and encouraging beneficial microbial consortia [38,39].
Furthermore, different organic soil amendments variably affect the growth and disease-suppressive abilities of microorganisms, including Trichoderma, and promote greater root development [40]. Trichoderma can also help preserve microbial diversity under adverse conditions. For instance, it has been observed to increase the richness and abundance of beneficial bacteria in wheat rhizospheres that were negatively impacted by excessive nitrogen fertilizers [41]. Similar benefits were seen when Trichoderma was co-inoculated with endophytes in drought-stressed plants [42]. Despite some concerns regarding its compatibility with arbuscular mycorrhizal fungi (AMF)—mainly due to its potential for parasitism, its volatile compound emissions, and competition for resources—studies have shown that Trichoderma and AMF can be used together effectively [43,44]. Co-application has resulted in improved crop yields, successful colonization in tomato seedlings, and even enhanced AMF levels in wheat rhizospheres [45]. In some cases, Trichoderma has supported AMF interactions in non-mycorrhizal hosts, like rapeseed [46].
Nevertheless, compatibility assessments should be performed on a case-by-case basis, considering that Trichoderma generally colonizes roots more rapidly than mycorrhizal fungi. Some Trichoderma strains have been identified as harmful—for example, T. aggressivum, T. pleuroti, and T.pleuroticola have been reported to infect edible mushrooms [47] and T. brevicompactum produces trichodermin, a toxin with phytotoxic effects on tomato plants [48]. Moreover, T. longibrachiatum, which can thrive at elevated temperatures (37 °C), has been implicated in opportunistic infections in immunocompromised individuals. Importantly, such adverse traits are not found in the Trichoderma strains typically used in agriculture, as these undergo rigorous testing to ensure safety prior to commercialization. Interestingly, Trichoderma may also serve as a biological control agent against leaf-cutting ants by disrupting their fungal symbionts. Its endophytic presence in plant material transported by ants may function as a “Trojan horse”, ultimately benefiting the plant by reducing pest pressure [49].
3. The Multifaceted Role of Trichoderma in Enhancing Plant Growth and Stress Tolerance
Trichoderma stands as a crucial group of plant-growth-promoting fungi (PGPF) within rhizosphere soils, fundamentally contributing to enhanced plant development and stress tolerance [50]. As symbiotic microorganisms, Trichoderma coexist with plant root ecosystems, where they facilitate the solubilization of soil minerals, leading to improved nutrient availability and efficiency, thereby fostering plant growth [50]. These fungi induce systemic resistance in plants and produce a variety of growth-promoting compounds, significantly augmenting overall plant growth and development [51]. Their impact extends to multiple facets of plant physiology, including improvement in seed germination, viability, root development, and root structure, increased flowering, and enhanced yield quality—all crucial elements in optimizing the photosynthesis process [52].
In terms of species-specific effects, distinct strains such as Trichoderma reesei, Trichoderma longibrachiatum, and Trichoderma harzianum have been identified for their ability to expedite orchard grass growth and enhance its nutritional content, which points to their potential role in pasture and field applications [53]. The multifaceted role of Trichoderma is further exemplified by their production of volatile organic compounds (VOCs) like 2-heptanone, 2-pentyl furan (2-PF), and 6-pentyl-2H-pyran-2-one (6-PP), which function as crucial agents in improving various physiological and developmental aspects of plants. These compounds have been shown to enhance aerial and root dry weight, directly influencing the primary growth direction of species such as tomatoes and Arabidopsis thaliana [54,55,56].
Furthermore, Trichoderma produce secondary metabolites like harzianolide, demonstrated to promote growth in tomato seedlings across different growing conditions, including natural soil and hydroponic systems [57,58]. Beyond these growth enhancements, Trichoderma atroviride and Trichoderma virens have been revealed to generate indole acetic acid (IAA) and other auxin-related compounds [28,59]. IAA plays a pivotal role in early root development, notably during the initial stages of plant maturity. The interaction of Trichoderma with plant signaling compounds underlines its potential to act as an alternative biofertilizer that minimizes the ecological impact of conventional chemical fertilizers.
This expansive role in promoting plant growth is matched by the ability of Trichoderma to bolster plant tolerance to both biotic and abiotic stressors. Of particular note is their ability to mitigate the impacts of common agricultural stresses such as drought, salinity, extreme temperatures, and heavy metal deposition, all of which significantly affect crop output [52,60,61,62,63]. In adverse environmental conditions, Trichoderma can enhance antioxidant defense systems, reducing reactive oxygen species (ROS) accumulation and thereby mitigating tissue damage and maintaining cellular integrity [64,65]. The distinct antioxidant system of Trichoderma not only protects its own genomic stability by eliminating ROS but also supports plant resilience against environmental challenges [26,32].
Furthermore, studies have shown that application of Trichoderma enhances plant resistance through up-regulation of stress-responsive genes associated with hormonal pathways like ethylene and abscisic acid, which are crucial for plants coping with stress conditions [66,67]. Specific strains such as Trichoderma parareesei have been demonstrated to enhance rapeseed plant resistance to salinity and drought stress through these mechanisms, significantly boosting yield compared with non-inoculated plants [61]. This adaptive capability signifies Trichoderma’s potential in developing resilient crop varieties capable of thriving in challenging environmental conditions.
The exploration of Trichoderma in agricultural practices is increasingly prominent due to its effectiveness in enhancing plant growth and development, coupled with its ability to serve as a biological control agent against various pathogens. The discovery and application of Trichoderma-derived products as biofertilizers align with sustainable agriculture goals, offering a viable means to improve crop productivity while mitigating the environmental damage associated with traditional fertilizers [65,68].
4. Trichoderma Biocontrol in Crop Protection
Trichoderma is a versatile biocontrol agent (BCA) due to its ability to directly antagonize target organisms and stimulate plant defense responses against biotic stressors. While its direct biocontrol action has been well-documented, it is important to recognize that not all Trichoderma species or strains exhibit uniform efficacy [69]. Their effectiveness varies across crops, geographic locations, and environmental conditions. Strains of interest in agriculture belong primarily to two groups, ST (e.g., T. atroviride, T. gamsii, and T. viride) and HV (e.g., T. harzianum and T. afroharzianum) [70]. The success of these strains depends on their biological characteristics—such as rapid growth, prolific sporulation, and the ability to colonize diverse environments—as well as their biochemical abilities, including enzymes that degrade host cell walls, secondary metabolites, and VOCs such as methyl jasmonate that modulate the host plant’s physiological responses, shape the composition of microbial communities in the rhizosphere, and ultimately alter the efficacy of natural defenses against phytopathogens [54,71].
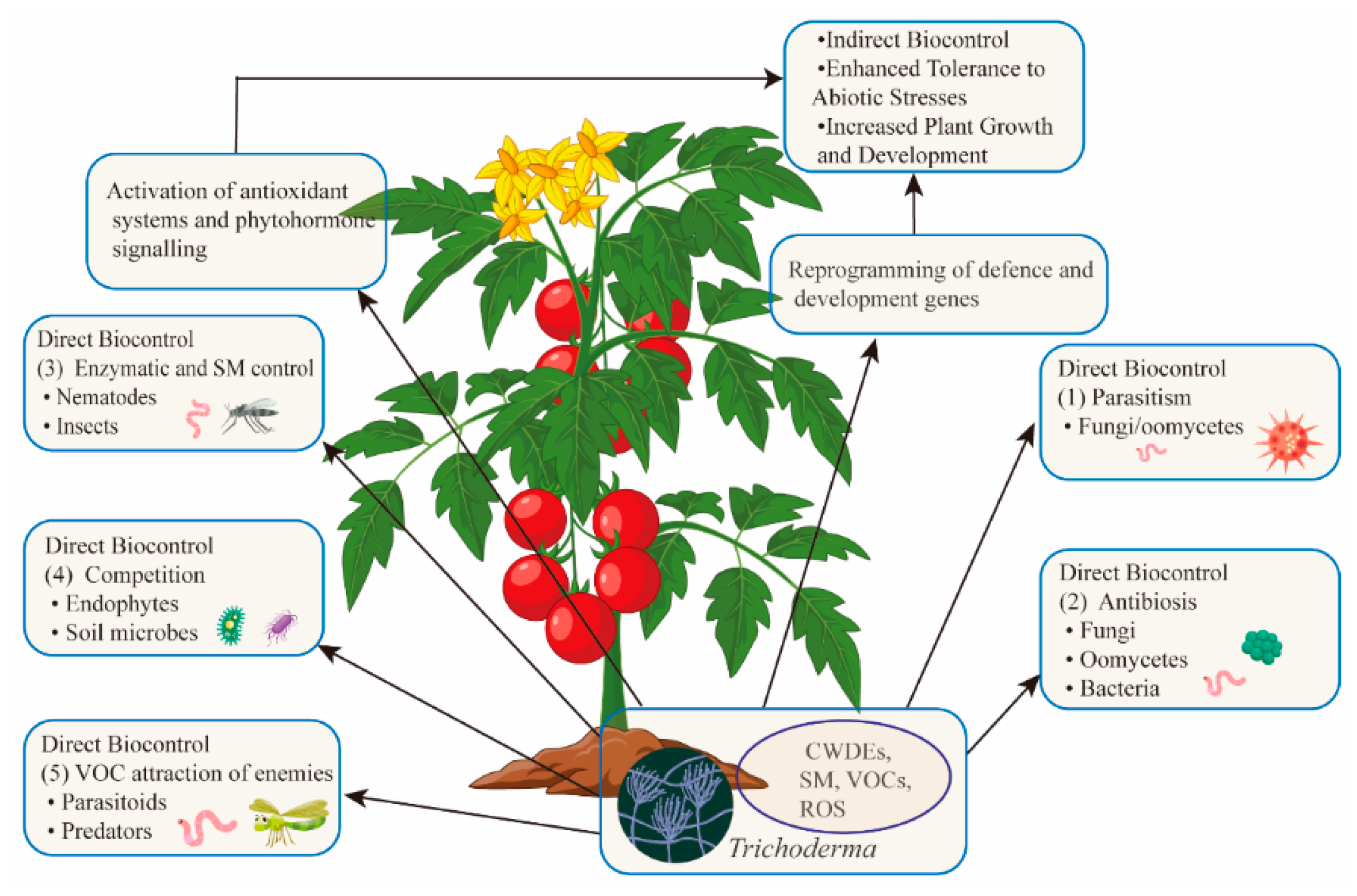
Trichoderma employs five primary mechanisms for direct antagonism (Figure 2) [72]: Parasitism (Trichoderma acts as a mycoparasite, feeding on fungal pathogens), Antibiosis (secondary metabolites inhibit pathogen growth and microbial competition), Enzymatic Activity (enzymes like chitinases target nematodes and pests), Competition (Trichoderma competes for resources like nutrients and space, promoting its colonization), and VOCs (VOCs attract natural predators of insect pests).
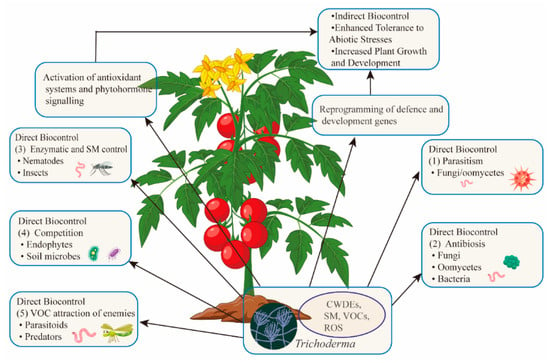
Figure 2.
Trichoderma: a multifunctional ally for crops. Trichoderma benefits plants by directly antagonizing pathogens and pests, altering soil conditions, and attracting natural enemies of pests. It also boosts plant defenses through immune activation and promotes growth. Overall, Trichoderma enhances plant health and yields.
In addition to these direct mechanisms, Trichoderma induces plant immune responses that help protect against both biotic and abiotic stresses. Trichoderma has proven effective in controlling economically important phytopathogens such as Botrytis, Fusarium, Rhizoctonia, Pythium, and Phytophthora. Secondary metabolites can also inhibit bacterial pathogens like Pseudomonas and Xanthomonas [73]. The genes linked to mycoparasitism and enzyme production have been shown to inhibit spore germination, hyphal growth, and fungal structures in numerous pathogens [74].
Trichoderma species are prolific producers of over 120 distinct secondary metabolites, encompassing diverse chemical classes such as terpenes (e.g., trichodermin and harzianopyridone), pyrones (e.g., 6-pentyl-2H-pyran-2-one), and nonribosomal peptides (e.g., gliotoxin and peptaibols), many of which exhibit potent antibiotic properties [30]. These compounds disrupt the growth and viability of a wide array of phytopathogens, including fungi (such as Fusarium and Rhizoctonia spp.), oomycetes (like Phytophthora and Pythium spp.), and bacteria (e.g., Pseudomonas and Erwinia spp.), by mechanisms such as inhibiting cell wall synthesis, permeabilizing membranes, or interfering with essential metabolic pathways like protein synthesis and respiration [71]. Notably, when purified and applied in isolation, these secondary metabolites can replicate the biocontrol efficacy observed in living Trichoderma cultures, often achieving pathogen inhibition rates comparable to whole-organism inoculants in controlled assays [75]. Despite being considered necrotrophic, Trichoderma may enter its prey via holes in the cell wall, utilizing a hemibiotrophic parasitic strategy that causes less extensive damage.
Trichoderma is also known for its suppressive effects on nematodes, such as Meloidogyne root-knot nematodes, and other types like Heterodera and Pratylenchus. It suppresses nematode eggs through proteases and chitinases or inhibits egg hatching with secondary metabolites [76]. Additionally, Trichoderma directly controls insects through enzymatic activity and inhibits cuticle formation, with secondary metabolites showing inhibitory effects on insect larvae [77].
As a competitor, Trichoderma counters pathogen strategies by secreting proteases that inhibit enzymes used by pathogens to invade plant tissues. Secondary metabolites can also downregulate pathogen virulence genes, such as those modulated by Trichoderma arundinaceum to reduce Botrytis cinerea’s virulence. Finally, Trichoderma produces VOCs, such as 6PP, which attract parasitoids and predators of insect pests, enhancing plant defense [78].
Trichoderma acts as an indirect biocontrol agent (BCA) by stimulating plant immune responses, leading to quicker and more robust activation of defense mechanisms upon subsequent stimuli (Figure 2). This process, known as “priming”, provides long-term protection by modulating various phytohormone-dependent pathways. Priming plays a crucial role in indirect biocontrol, as both types of responses share similar origins and development, despite being triggered by different stimuli. The interactions between Trichoderma and plants, and the mechanisms of signal activation and systemic transmission, have been extensively reviewed. Structural components of Trichoderma’s cell wall and membrane, such as chitin, β-glucans, and sterols, act as microorganism-associated molecular patterns (MAMPs). When recognized by plant pattern recognition receptors (PRRs), these MAMPs initiate MAMP-triggered immunity, which is more robust than pathogen-triggered immunity, thereby enhancing plant resistance. Upon reaching plant roots, Trichoderma prompts an increase in salicylic acid levels, a key phytohormone that controls early root colonization, confining Trichoderma to the apoplastic spaces of the epidermis and cortex. Trichoderma further enhances plant immunity through effector proteins and metabolites like xylanase EIX, LysM protein Tal6, ceratoplatanin Sm1, peptaibol alamethicin, and terpenes such as trichodiene and harzianum A, contributing to effector-triggered immunity even without PRR interactions. These secreted effectors, along with Trichoderma’s ability to tolerate reactive oxygen species (ROS), facilitate endophytic colonization and a non-harmful relationship with the plant, priming its defenses just below the threshold for effective resistance. The precise interactions between cytoplasmic nucleotide-binding site leucine-rich repeat (NLR) receptors and Trichoderma’s effectors remain only partially understood. In tomato plants, NLR receptors are notably more abundant in roots inoculated with T. atroviride and Rhizoctonia solani, with similar responses observed in other Trichoderma strains [79]. These interactions are under further investigation, including the potential role of small RNA trafficking across kingdoms. Salicylic-acid-dependent defenses that limit early Trichoderma growth can spread throughout the plant, creating systemic acquired resistance (SAR) effective against biotrophic pathogens [80]. Trichoderma suppresses salicylic acid defenses and induces jasmonic acid biosynthesis, activating jasmonic-acid-responsive genes in root cells. This triggers jasmonic acid–ethylene-dependent induced systemic resistance (ISR), particularly effective against necrotrophic pathogens and herbivore attacks. Trichoderma exploits the antagonistic relationship between salicylic acid and jasmonic acid to colonize roots, as seen in T. asperellum, which accumulates high levels of jasmonic acid and ethylene within 24 h, supporting the role of priming in induced systemic resistance. However, research indicates that Trichoderma-induced defenses against fungi and viruses are modulated by both jasmonic acid–ethylene and salicylic acid. Beyond enhancing plant fitness, Trichoderma helps prevent nematode access to roots [81]. In tomato roots affected by root-knot nematodes (RKNs), Trichoderma adjusts plant immunity by modulating salicylic-acid- and jasmonic-acid-dependent defenses based on the nematode infection stage [82]. Additionally, Trichoderma primes defenses in both leaves and roots through small-RNA-mediated gene silencing and by inducing transcription of genes involved in RNA-dependent DNA methylation, fine-tuning the expression of salicylic acid and jasmonic acid–ethylene defense genes [59]. Trichoderma also activates plant systemic defenses through the release of volatile organic compounds (VOCs), triggering an oxidative burst effective against aphids. It can enhance the expression of genes producing protective enzymes against moths and modify plant metabolic pathways to produce phytochemicals that deter herbivores or disrupt the insect gut proteome, reducing feeding [83,84,85]. VOCs released by Trichoderma can attract parasitoids and predators of aphids, further strengthening plant defense. By modulating plant defenses and growth, Trichoderma helps mitigate the effects of unfavorable environmental conditions. Abiotic-stress-related phytohormones share regulatory pathways with MAMPs and damage-associated molecular patterns (DAMPs). However, plants often face a combination of biotic and abiotic stresses, leading to varied sensitivity and signal transduction. In such cases, Trichoderma regulates calcium ion (Ca2+) signaling, crucial for plant immunity under stress, helping plants adapt to environmental changes by prioritizing growth and stress response functions [86]. Trichoderma has been shown to improve plant drought tolerance, enhance antioxidant defense, and delay water deficit responses. Studies report that Trichoderma can modulate ROS scavenging and influence ethylene levels, aiding plants in coping with waterlogging, salinity, and other stress factors [87]. Furthermore, Trichoderma increases plant growth and salt tolerance, either through direct contact or VOCs. However, combining Trichoderma with inorganic fertilizers in salt-stressed plants may disrupt the phytohormone network, hindering effective adaptation to conflicting signals. The interaction between Trichoderma and plants is dynamic, with the expression of salicylic acid and jasmonic acid–ethylene-dependent defense genes fluctuating in response to biotic and abiotic stresses [88]. Over time, the effects of Trichoderma priming may diminish, but plants retain a “transcriptional memory” of previous priming events, allowing for stronger defense responses upon subsequent stimuli. This priming ability can be passed on to the next generation, resulting in offspring with enhanced resistance to pests and diseases.
5. Agricultural Applications of Trichoderma
Agricultural policies have evolved significantly since the 2015 UN Sustainable Development Goals, which emphasized the need for sustainable practices in food and agriculture, including pesticide and fertilizer management [89]. Climate change and intensive farming have led to biodiversity loss, pest spread, and chemical contamination of the environment, all of which impact both ecosystems and human health. In response, there has been a global shift toward reducing synthetic chemicals, increasing the role of beneficial microorganisms like Trichoderma. This shift has been reflected in Trichoderma’s growing use as a biological alternative in products such as biostimulants, bioprotectants, and biofertilizers.
Trichoderma is widely recognized as a biocontrol agent (BCA), used to control plant diseases through various mechanisms [65]. It has gained significant traction, with BCA registrations rising from 21 in 2014 to 144 globally in 2022, involving multiple strains across 40 countries [90]. Despite this, the registration process varies across nations, such as in India and China, where government research institutes test Trichoderma strains for controlling plant diseases. While some regions market Trichoderma as a biostimulant, it is not yet universally recognized as such, particularly in Europe. As the scientific community supports Trichoderma’s dual role as both a biocontrol agent and biostimulant, regulators must update policies to recognize its broad applications for more sustainable agricultural practices.
To boost the success and wider adoption of biological products in eco-sustainable agriculture, improving their shelf life, efficacy, and standards to match those of conventional chemicals is essential. Key strategies include enhancing the production of Trichoderma spores and improving their stress tolerance. An ideal Trichoderma product should select strains with strong biocontrol potential, the ability to colonize roots and promote plant growth or disease resistance.
Microbial consortia that include Trichoderma and other beneficial organisms, such as biocontrol bacteria and fungi, offer improved efficacy in agricultural applications [91]. These consortia may also include bioactive compounds from various microbial or botanical sources that enhance biocontrol and growth-promoting effects. Additionally, the development of new biofertilizers and probiotics using Trichoderma-based microbiomes can restore beneficial soil microbiota, especially in degraded agricultural areas.
As global agriculture increasingly shifts toward sustainable practices to minimize environmental impacts, reduce chemical residues in food chains, and combat issues like soil degradation and pesticide resistance, the careful selection of Trichoderma strains with high tolerance to residual agrochemicals—such as fungicides (e.g., triazoles or strobilurins), herbicides (e.g., glyphosate), and insecticides (e.g., neonicotinoids)—will be paramount. This tolerance ensures that Trichoderma can maintain its biocontrol efficacy and plant-growth-promoting activities even in transitional farming systems where chemical inputs are being phased out gradually, preventing disruptions to microbial community dynamics and avoiding unintended suppression of beneficial fungi [92,93]. Industrial processes and innovations like encapsulation and nanoparticle technology can improve the delivery and effectiveness of Trichoderma in large-scale applications.
Trichoderma is integral to agriculture’s transition toward a green and circular economy, focusing on reducing environmental impacts and recycling waste into valuable products. Biotechnological advancements may also enable Trichoderma gene expression in plants to improve resistance to pathogens and environmental stress, reducing the need for agrochemicals and enhancing crop resilience. Ongoing research into Trichoderma explores its applications in improving crop production in marginal lands, climate change resilience, bioremediation, and reducing greenhouse gas emissions.
6. Conclusions
In this review, we have explored Trichoderma’s standout roles as a natural biocontrol agent and plant growth booster, helping farmers protect crops and boost yields in real-world settings. Recent advances in classifying Trichoderma species are making it easier to pick the right ones for biotech applications, like developing targeted biofungicides. We have also gained better insights into how Trichoderma teams up with plant microbiomes and soil life, leading to healthier plants, improved soil quality, and even benefits for human and environmental health. Cutting-edge tools have revealed how it kickstarts plant defenses, providing built-in shields against diseases, pests, and stresses like drought or poor soil—practical perks that can cut down on chemical use.
Trichoderma’s adaptability lets it thrive in all sorts of environments, from fields to greenhouses. The big opportunity? Leveraging its diversity for smarter, long-lasting farming strategies that ramp up production while keeping things eco-friendly. For instance, in organic systems, Trichoderma formulations have shown promise in controlling pests like Fusarium in tomatoes or nematodes in bananas, potentially slashing pesticide needs by 30–50% based on field trials. It could also help tackle climate challenges, secure food supplies, and shift agriculture toward greener alternatives.
That said, rolling out Trichoderma products is not straightforward—regulatory hurdles, inconsistent performance in varying conditions, and gaps in collaboration between scientists, farmers, and regulators slow things down. Yet, with global policies pushing for sustainable farming and “One Health” approaches, there is momentum to overcome these.
Looking ahead, let us think big. Multidisciplinary efforts could engineer Trichoderma strains via CRISPR for supercharged traits, like enhanced drought tolerance in staple crops. Pairing it with precision tech, such as drone-applied bioinoculants or microbiome sensors, might revolutionize organic farming. By addressing scalability and policy barriers, we can turn Trichoderma into a go-to tool for resilient, chemical-free agriculture—ultimately leading to bountiful harvests, healthier ecosystems, and a better quality of life for all.
Author Contributions
Conceptualization, F.L.; methodology, X.L.; validation, F.L. and Y.L.; investigation, Y.G.; resources, Y.L.; writing—original draft preparation, X.C.; writing—review and editing, F.L.; visualization, X.C.; supervision, F.L.; funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Guizhou Province (Grant No. Qiankehejichu-ZK [2022] Zhongdian 033), the Forestry Research Project of Guizhou Province (Grant No. Qianlinkehe [2023]12), the 2022 Open Project of the State Key Laboratory of Microbiology Technology of Shandong University (grant no. M2022-15), and the Guizhou Normal University 2019 Academic New Talent Cultivation and Innovation Exploration Special Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atanasova, L.; Crom, S.L.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative Transcriptomics Reveals Different Strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.B.A.C.; Costa, C.J.M.; Ribeiro, E.J.; Zotarelli, M.F.; Santos, L.D. Evaluation of the Microencapsulation Process of Conidia of Trichoderma asperellum by Spray Drying. Braz. J. Microbiol. 2022, 53, 1871–1880. [Google Scholar] [CrossRef]
- Kredics, L.; Naeimi, S.; Hatvani, L.; Vágvölgyi, C.; Cai, F.; Druzhinina, I.S.; Manczinger, L. ‘The Good, the Bad and the Ugly’ in the Shades of Green: The Genus Trichoderma in the Spotlight. Indian Phytopathol. 2021, 74, 403–411. [Google Scholar] [CrossRef]
- Naeimi, S.; Hatvani, L.; Marik, T.; Balázs, D.; Dóczi, I.; Cai, F.; Vágvölgyi, C.; Druzhinina, I.S.; Kredics, L. Trichodermosis: Human Infections Caused by Trichoderma Species. In Advances in Trichoderma Biology for Agricultural Applications; Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 607–634. ISBN 978-3-030-91650-3. [Google Scholar]
- Agostini, R.B.; Ariel, F.; Rius, S.P.; Vargas, W.A.; Campos-Bermudez, V.A. Trichoderma Root Colonization in Maize Triggers Epigenetic Changes in Genes Related to the Jasmonic and Salicylic Acid Pathways That Prime Defenses against Colletotrichum graminicola Leaf Infection. J. Exp. Bot. 2023, 74, 2016–2028. [Google Scholar] [CrossRef]
- Morcuende, J.; Martín-García, J.; Velasco, P.; Sánchez-Gómez, T.; Santamaría, Ó.; Rodríguez, V.M.; Poveda, J. Effective Biological Control of Chickpea Rabies (Ascochyta rabiei) through Systemic Phytochemical Defenses Activation by Trichoderma Roots Colonization: From Strain Characterization to Seed Coating. Biol. Control 2024, 193, 105530. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez De Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the Plant Heritable Priming Responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational Research on Trichoderma: From ’Omics to the Field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Druzhinina, I.S. In Honor of John Bissett: Authoritative Guidelines on Molecular Identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Peixoto, G.H.S.; da Silva, R.A.F.; Zacaroni, A.B.; Silva, T.F.; Chaverri, P.; Pinho, D.B.; de Mello, S.C.M. Trichoderma Collection from Brazilian Soil Reveals a New Species: T. cerradensis sp. Nov. Front. Microbiol. 2025, 16, 1279142. [Google Scholar] [CrossRef] [PubMed]
- Rashad, Y.M.; Shabana, Y.M.; Natey, B.; Sleem, M.M.; Hafez, M.; Abd-ElGawad, A.M.; Deng, Q.; Deng, J.-X. Trichoderma Biodiversity from Egypt and a New Trichoderma Species, Trichoderma egyptiacum sp. Nov. (Hypocreaceae, Hypocreales). Mycol. Progress. 2025, 24, 33. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A.; et al. Evolution and Comparative Genomics of the Most Common Trichoderma Species. BMC Genom. 2019, 20, 485. [Google Scholar] [CrossRef] [PubMed]
- Ns, A.; Mp, B.; Ga, B.; Ml, C. Molecular Interactions of Trichoderma: From Microbial Competition to Soil Health Promotion. Int. J. Mol. Biol. Open Access 2024, 7, 135–138. [Google Scholar] [CrossRef]
- Wen, C.; Xiong, H.; Wen, J.; Wen, X.; Wang, C. Trichoderma Species Attract Coptotermes formosanus and Antagonize Termite Pathogen Metarhizium anisopliae. Front. Microbiol. 2020, 11, 653. [Google Scholar] [CrossRef]
- Rubio, M.B.; Quijada, N.M.; Pérez, E.; Domínguez, S.; Monte, E.; Hermosa, R. Identifying Beneficial Qualities of Trichoderma parareesei for Plants. Appl. Environ. Microbiol. 2014, 80, 1864–1873. [Google Scholar] [CrossRef]
- Scott, K.; Konkel, Z.; Gluck-Thaler, E.; Valero David, G.E.; Simmt, C.F.; Grootmyers, D.; Chaverri, P.; Slot, J. Endophyte Genomes Support Greater Metabolic Gene Cluster Diversity Compared with Non-Endophytes in Trichoderma. PLoS ONE 2023, 18, e0289280. [Google Scholar] [CrossRef]
- Klein, M.; Stewart, J.D.; Porter, S.S.; Weedon, J.T.; Kiers, E.T. Evolution of Manipulative Microbial Behaviors in the Rhizosphere. Evol. Appl. 2022, 15, 1521–1536. [Google Scholar] [CrossRef]
- Cai, F.; Chen, W.; Wei, Z.; Pang, G.; Li, R.; Ran, W.; Shen, Q. Colonization of Trichoderma Harzianum Strain SQR-T037 on Tomato Roots and Its Relationship to Plant Growth, Nutrient Availability and Soil Microflora. Plant Soil 2015, 388, 337–350. [Google Scholar] [CrossRef]
- Mehetre, S.T.; Mukherjee, P.K. Trichoderma Improves Nutrient Use Efficiency in Crop Plants. In Nutrient Use Efficiency: From Basics to Advances; Springer: New Delhi, India, 2015; Available online: https://link.springer.com/chapter/10.1007/978-81-322-2169-2_11 (accessed on 15 July 2025).
- Villalobos-Escobedo, J.M.; Esparza-Reynoso, S.; Pelagio-Flores, R.; López-Ramírez, F.; Ruiz-Herrera, L.F.; López-Bucio, J.; Herrera-Estrella, A. The Fungal NADPH Oxidase Is an Essential Element for the Molecular Dialog between Trichoderma and Arabidopsis. Plant J. 2020, 103, 2178–2192. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.A.; Tartoura, K.A.; Abdelraouf, G.A. Evaluation of Trichoderma harzianum and Serratia proteamaculans Effect on Disease Suppression, Stimulation of ROS-Scavenging Enzymes and Improving Tomato Growth Infected by Rhizoctonia solani. Biol. Control 2016, 100, 79–86. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, A.; Singh, S.P.; Singh, H.B. Trichoderma harzianum-Mediated Reprogramming of Oxidative Stress Response in Root Apoplast of Sunflower Enhances Defence against Rhizoctonia solani. Eur. J. Plant Pathol. 2011, 131, 121–134. [Google Scholar] [CrossRef]
- Chen, S.C.; Ren, J.J.; Zhao, H.J.; Wang, X.L.; Wang, T.H.; Jin, S.D.; Wang, Z.H.; Li, C.Y.; Liu, A.R.; Lin, X.M.; et al. Trichoderma harzianum Improves Defense Against Fusarium Oxysporum by Regulating ROS and RNS Metabolism, Redox Balance, and Energy Flow in Cucumber Roots. Phytopathology 2019, 109, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Pedrero-Méndez, A.; Insuasti, H.C.; Neagu, T.; Illescas, M.; Rubio, M.B.; Monte, E.; Hermosa, R. Why Is the Correct Selection of Trichoderma Strains Important? The Case of Wheat Endophytic Strains of T. harzianum and T. simmonsii. J. Fungi 2021, 7, 1087. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.B.; Hermosa, R.; Vicente, R.; Gómez-Acosta, F.A.; Morcuende, R.; Monte, E.; Bettiol, W. The Combination of Trichoderma harzianum and Chemical Fertilization Leads to the Deregulation of Phytohormone Networking, Preventing the Adaptive Responses of Tomato Plants to Salt Stress. Front. Plant Sci. 2017, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Pola-Sánchez, E.; Villalobos-Escobedo, J.M.; Carreras-Villaseñor, N.; Martínez-Hernández, P.; Beltrán-Hernández, E.B.; Esquivel-Naranjo, E.U.; Herrera-Estrella, A. A Global Analysis of Photoreceptor-Mediated Transcriptional Changes Reveals the Intricate Relationship Between Central Metabolism and DNA Repair in the Filamentous Fungus Trichoderma atroviride. Front. Microbiol. 2021, 12, 724676. [Google Scholar] [CrossRef]
- Sarkar, D.; Rakshit, A. Bio-Priming in Combination with Mineral Fertilizer Improves Nutritional Quality and Yield of Red Cabbage under Middle Gangetic Plains, India. Sci. Hortic. 2021, 283, 110075. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A Novel Role for Trichoderma Secondary Metabolites in the Interactions with Plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone Production Profiles in Trichoderma Species and Their Relationship to Wheat Plant Responses to Water Stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Rouina, H.; Groten, K.; Rajani, P.; Furch, A.C.U.; Reichelt, M.; Baldwin, I.T.; Nataraja, K.N.; Uma Shaanker, R.; Oelmüller, R. An Endophytic Trichoderma Strain Promotes Growth of Its Hosts and Defends Against Pathogen Attack. Front. Plant Sci. 2020, 11, 573670. [Google Scholar] [CrossRef]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum Is Effective for Biocontrol of Verticillium Wilt in Olive Caused by the Defoliating Pathotype of Verticillium dahliae. Crop Prot. 2016, 88, 45–52. [Google Scholar] [CrossRef]
- Kong, W.-L.; Ni, H.; Wang, W.-Y.; Wu, X.-Q. Antifungal Effects of Volatile Organic Compounds Produced by Trichoderma koningiopsis T2 against Verticillium Dahliae. Front. Microbiol. 2022, 13, 1013468. [Google Scholar] [CrossRef]
- Oskiera, M.; Szczech, M.; Stępowska, A.; Smolińska, U.; Bartoszewski, G. Monitoring of Trichoderma Species in Agricultural Soil in Response to Application of Biopreparations. Biol. Control 2017, 113, 65–72. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.T.; Zhang, Y. Trichoderma Biofertilizer Links to Altered Soil Chemistry, Altered Microbial Communities, and Improved Grassland Biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; de Oliveira Simas, A.L.; Ribeiro, J.V.S.; de Alencar Guimarães, N.C.; Viana, T.F.C.; Masui, D.C.; Corrêa, B.O.; Giannesi, G.C.; de Lima, S.F.; da Silva Brasil, M.; et al. Phosphorus-Solubilizing Trichoderma Strains: Mechanisms to Promote Soybean Growth and Support Sustainable Agroecosystems. Plant Soil 2025. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.H.O.M.A.S.; Harman, G. Solubilization of Phosphates and Micronutrients by the Plant-Growth-Promoting and Biocontrol Fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Effect of Co-Application of Trichoderma spp. with Organic Composts on Plant Growth Enhancement, Soil Enzymes and Fungal Community in Soil. Arch. Microbiol. 2021, 203, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Illescas, M.; Rubio, M.B.; Hernández-Ruiz, V.; Morán-Diez, M.E.; Martínez de Alba, A.E.; Nicolás, C.; Monte, E.; Hermosa, R. Effect of Inorganic N Top Dressing and Trichoderma harzianum Seed-Inoculation on Crop Yield and the Shaping of Root Microbial Communities of Wheat Plants Cultivated Under High Basal N Fertilization. Front. Plant Sci. 2020, 11, 575861. [Google Scholar] [CrossRef]
- Estévez-Geffriaud, V.; Vicente, R.; Vergara-Díaz, O.; Narváez Reinaldo, J.J.; Trillas, M.I. Application of Trichoderma asperellum T34 on Maize (Zea mays) Seeds Protects against Drought Stress. Planta 2020, 252, 8. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Pascual, J.A.; Lloret, E.; Roldán, A. Interactions between Arbuscular Mycorrhizal Fungi and Trichoderma harzianum and Their Effects on Fusarium Wilt in Melon Plants Grown in Seedling Nurseries. J. Sci. Food Agric. 2009, 89, 1843–1850. [Google Scholar] [CrossRef]
- Fernandez-Gnecco, G.; Gégu, L.; Covacevich, F.; Consolo, V.F.; Bouffaud, M.-L.; Buscot, F.; Smalla, K.; Babin, D. Alone as Effective as Together: AMF and Trichoderma Inoculation Boost Maize Performance but Differentially Shape Soil and Rhizosphere Microbiota. J. Sustain. Agric. Environ. 2024, 3, e12091. [Google Scholar] [CrossRef]
- Baptista, J.S.P.; Rodrigues, P.; Declerck, S. Combinatorial Effect of Rhizophagus irregularis and Trichoderma harzianum on the Silicon Accumulation in Wheat and Maize, and the Improvement of Wheat Resistance Against Zymoseptoria tritic. Available online: https://bibliotecadigital.ipb.pt/entities/publication/7517eaae-2da9-42f5-b5f4-0c90c5385fdb (accessed on 15 July 2025).
- Poveda, J.; Hermosa, R.; Monte, E.; Nicolás, C. Trichoderma harzianum Favours the Access of Arbuscular Mycorrhizal Fungi to Non-Host Brassicaceae Roots and Increases Plant Productivity. Sci. Rep. 2019, 9, 11650. [Google Scholar] [CrossRef] [PubMed]
- Allaga, H.; Zhumakayev, A.; Büchner, R.; Kocsube, S.; Szűcs, A.; Vágvölgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum Species Complex with Mushroom Pathogenic Potential. Agronomy 2021, 11, 2434. [Google Scholar] [CrossRef]
- Tijerino, A.; Hermosa, R.; Cardoza, R.E.; Moraga, J.; Malmierca, M.G.; Aleu, J.; Collado, I.G.; Monte, E.; Gutierrez, S. Overexpression of the Trichoderma brevicompactum Tri5 Gene: Effect on the Expression of the Trichodermin Biosynthetic Genes and on Tomato Seedlings. Toxins 2011, 3, 1220–1232. [Google Scholar] [CrossRef]
- Seethapathy, P. Potential of Trichoderma in Combating Insect Pests. In The Role of Entomopathogenic Fungi in Agriculture; CRC Press: Boca Raton, FL, USA, 2025; Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003505228-9/potential-trichoderma-combating-insect-pests-parthasarathy-seethapathy (accessed on 15 July 2025).
- Zin, N.A.; Badaluddin, N.A. Biological Functions of Trichoderma spp. for Agriculture Applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, X.; Huo, Y.; Xiao, Y. Trichoderma-Inoculation and Mowing Synergistically Altered Soil Available Nutrients, Rhizosphere Chemical Compounds and Soil Microbial Community, Potentially Driving Alfalfa Growth. Front. Microbiol. 2019, 9, 3241. [Google Scholar] [CrossRef]
- Salem, A.; Khandaker, M.M.; Mahmud, K.; Alsufyani, S.J.; Majrashi, A.A.; Rashid, Z.M.; Alenazi, M.M.; Osman, N.; Badaluddin, N.A. Enhancing Photosynthesis and Root Development for Better Fruit Quality, Aroma, and Lessening of Radioactive Materials in Key Lime (Citrus aurantifolia) Using Trichoderma harzianum and Bacillus thuringiensis. Plant Physiol. Biochem. 2024, 206, 108295. [Google Scholar] [CrossRef]
- Ma, J.; Tsegaye, E.; Li, M.; Wu, B.; Jiang, X. Biodiversity of Trichoderma from Grassland and Forest Ecosystems in Northern Xinjiang, China. 3 Biotech 2020, 10, 362. [Google Scholar] [CrossRef]
- Jiménez-Bremont, J.F.; González-Pérez, E.; Ortega-Amaro, M.A.; Madrigal-Ortiz, S.; Duque-Ortiz, A.; Mendoza-Mendoza, A. Volatile Organic Compounds Emitted by Trichoderma: Small Molecules with Biotechnological Potential. Sci. Hortic. 2024, 325, 112656. [Google Scholar] [CrossRef]
- Rao, Y.; Zeng, L.; Jiang, H.; Mei, L.; Wang, Y. Trichoderma atroviride LZ42 Releases Volatile Organic Compounds Promoting Plant Growth and Suppressing Fusarium Wilt Disease in Tomato Seedlings. BMC Microbiol. 2022, 22, 88. [Google Scholar] [CrossRef]
- Midzi, J.; Jeffery, D.W.; Baumann, U.; Rogiers, S.; Tyerman, S.D.; Pagay, V. Stress-Induced Volatile Emissions and Signalling in Inter-Plant Communication. Plants 2022, 11, 2566. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The Effect of Trichoderma harzianum in Mitigating Low Temperature Stress in Tomato (Solanum lycopersicum L.) Plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Tripathi, R.; Keswani, C.; Tewari, R. Trichoderma koningii Enhances Tolerance against Thermal Stress by Regulating ROS Metabolism in Tomato (Solanum lycopersicum L.) Plants. J. Plant Interact. 2021, 16, 116–125. [Google Scholar] [CrossRef]
- Rebolledo-Prudencio, O.G.; Estrada-Rivera, M.; Dautt-Castro, M.; Arteaga-Vazquez, M.A.; Arenas-Huertero, C.; Rosendo-Vargas, M.M.; Jin, H.; Casas-Flores, S. The Small RNA-mediated Gene Silencing Machinery Is Required in Arabidopsis for Stimulation of Growth, Systemic Disease Resistance, and Suppression of the Nitrile-specifier Gene NSP4 by Trichoderma atroviride. Plant J. 2022, 109, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma Parareesei Favors the Tolerance of Rapeseed (Brassica napus L.) to Salinity and Drought Due to a Chorismate Mutase. Agronomy 2020, 10, 118. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Awal, M.A.; Mispan, M.S.; Saiman, M.Z.; Mohd-Yusuf, Y.; Suhaimi, N.S.M. Multi-Omics Tools for Understanding Trichoderma-Plant Symbiosis: Biotechnological Developments and Future Directions. Symbiosis 2024, 93, 125–138. [Google Scholar] [CrossRef]
- Lombardi, N.; Vitale, S.; Turrà, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; d’Errico, G.; et al. Root Exudates of Stressed Plants Stimulate and Attract Trichoderma Soil Fungi. Mol. Plant-Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Vergara, A.G.; López-Bucio, J. Trichoderma Modulates Stomatal Aperture and Leaf Transpiration Through an Abscisic Acid-Dependent Mechanism in Arabidopsis. J. Plant Growth Regul. 2015, 34, 425–432. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of Jasmonic Acid, Ethylene and Salicylic Acid Signaling Pathways behind the Systemic Resistance Induced by Trichoderma longibrachiatum H9 in Cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef]
- Riolo, M.; D’Opazo, V.; Cacciola, S.O. Trichoderma as a Source of Metabolites for Applications in Agriculture. In Fungal Metabolites for Agricultural Applications: Biostimulation and Crop Protection by Fungal Biotechnology; Springer: Cham, Switzerland, 2025; Available online: https://link.springer.com/chapter/10.1007/978-3-031-76587-2_8 (accessed on 16 July 2025).
- Bazghaleh, N.; Prashar, P.; Woo, S.; Vandenberg, A. Effects of Lentil Genotype on the Colonization of Beneficial Trichoderma Species and Biocontrol of Aphanomyces Root Rot. Microorganisms 2020, 8, 1290. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum Species Complex and the Re-Identification of Commercial Biocontrol Strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A Multipurpose, Plant-Beneficial Microorganism for Eco-Sustainable Agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological Control of Plant Diseases—What Has Been Achieved and What Is the Direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In Vitro Antibacterial, Antifungal, Nematocidal and Growth Promoting Activities of Trichoderma hamatum FB10 and Its Secondary Metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a Mechanism of Trichoderma-Mediated Suppression of Plant Diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Moreno-Velandia, C.A.; Garcia-Arias, F.L.; Dávila-Mora, L.; Rodríguez, E.; Villabona-Gélvez, A.; Revelo-Gómez, E.G.; Marcillo-Paguay, C.A.; Riascos-Ortiz, D.H.; Zuluaga, A.P. The Potential of PGPR and Trichoderma-Based Bioproducts and Resistant Cultivars as Tools to Manage Clubroot Disease in Cruciferous Crops. Front. Plant Sci. 2024, 14, 1323530. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Viterbo, A.; Bar-Eyal, M.; Nagan, H.; Samuels, G.J.; Spiegel, Y. Parasitism of Trichoderma on Meloidogyne javanica and Role of the Gelatinous matrix. Eur. J. Plant Pathol. 2007, 118, 247–258. [Google Scholar] [CrossRef]
- Da Silveira, A.A.; Andrade, J.S.P.; Guissoni, A.C.P.; Da Costa, A.C.; De Carvalho E Silva, A.; Da Silva, H.G.; Brito, P.; De Souza, G.R.L.; Fernandes, K.F. Larvicidal Potential of Cell Wall Degrading Enzymes from Trichoderma asperellum against Aedes aegypti (Diptera: Culicidae). Biotechnol. Progress. 2021, 37, e3182. [Google Scholar] [CrossRef] [PubMed]
- Seidl, V.; Song, L.; Lindquist, E.; Gruber, S.; Koptchinskiy, A.; Zeilinger, S.; Schmoll, M.; Martínez, P.; Sun, J.; Grigoriev, I.; et al. Transcriptomic Response of the Mycoparasitic Fungus Trichoderma atroviride to the Presence of a Fungal Prey. BMC Genom. 2009, 10, 567. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Lelio, I.D.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 Colonization of Tomato Plants Enhances Both Direct and Indirect Defense Barriers Against Insects. Front. Physiol. 2019, 10, 813. [Google Scholar] [CrossRef]
- Alonso-Ramírez, A.; Poveda, J.; Martín, I.; Hermosa, R.; Monte, E.; Nicolás, C. Salicylic Acid Prevents Trichoderma harzianum from Entering the Vascular System of Roots. Mol. Plant Pathol. 2014, 15, 823–831. [Google Scholar] [CrossRef]
- TariqJaveed, M.; Farooq, T.; Al-Hazmi, A.S.; Hussain, M.D.; Rehman, A.U. Role of Trichoderma as a Biocontrol Agent (BCA) of Phytoparasitic Nematodes and Plant Growth Inducer. J. Invertebr. Pathol. 2021, 183, 107626. [Google Scholar] [CrossRef]
- Medeiros, H.A.D.; Araújo Filho, J.V.D.; Freitas, L.G.D.; Castillo, P.; Rubio, M.B.; Hermosa, R.; Monte, E. Tomato Progeny Inherit Resistance to the Nematode Meloidogyne Javanica Linked to Plant Growth Induced by the Biocontrol Fungus Trichoderma atroviride. Sci. Rep. 2017, 7, 40216. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Smith, P.M.C.; Boughton, B.A.; Rupasinghe, T.W.T.; Natera, S.H.A.; Roessner, U. Inoculation of Barley with Trichoderma harzianum T-22 Modifies Lipids and Metabolites to Improve Salt Tolerance. J. Exp. Bot. 2021, 72, 7229–7246. [Google Scholar] [CrossRef]
- Praprotnik, E.; Lončar, J.; Razinger, J. Testing Virulence of Different Species of Insect Associated Fungi against Yellow Mealworm (Coleoptera: Tenebrionidae) and Their Potential Growth Stimulation to Maize. Plants 2021, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Díaz, C.E.; Chhipa, H.; Julio, L.F.; Andrés, M.F.; González-Coloma, A. Chemical Composition of an Aphid Antifeedant Extract from an Endophytic Fungus, Trichoderma sp. EFI671. Microorganisms 2020, 8, 420. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Méndez, W.; Obregón, M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma asperellum Biocontrol Activity and Induction of Systemic Defenses against Sclerotium Cepivorum in Onion Plants under Tropical Climate Conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- DeClerck, F.A.J.; Koziell, I.; Benton, T.; Garibaldi, L.A.; Kremen, C.; Maron, M.; Del Rio, C.R.; Sidhu, A.; Wirths, J.; Clark, M.; et al. A Whole Earth Approach to Nature-Positive Food: Biodiversity and Agriculture. In Science and Innovations for Food Systems Transformation; Von Braun, J., Afsana, K., Fresco, L.O., Hassan, M.H.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 469–496. ISBN 978-3-031-15702-8. [Google Scholar]
- Marchand, P. The Regulatory Obstacles to Bio Control Agents from Directive (EC) No 128/2009. J. Regul. Sci. 2024, 12, 1–9. [Google Scholar] [CrossRef]
- Pastor, N.; Palacios, S.; Torres, A.M. Microbial Consortia Containing Fungal Biocontrol Agents, with Emphasis on Trichoderma spp.: Current Applications for Plant Protection and Effects on Soil Microbial Communities. Eur. J. Plant Pathol. 2023, 167, 593–620. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Lanzuise, S.; Manganiello, G.; Guastaferro, V.M.; Vincenzo, C.; Vitaglione, P.; Ferracane, R.; Vecchi, A.; Vinale, F.; Kamau, S.; Lorito, M.; et al. Combined Biostimulant Applications of Trichoderma spp. with Fatty Acid Mixtures Improve Biocontrol Activity, Horticultural Crop Yield and Nutritional Quality. Agronomy 2022, 12, 275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).