Endophytic Bioactive Compounds for Wound Healing: A Review of Biological Activities and Therapeutic Potential
Abstract
1. Introduction
2. Pharmacological Potential of Compounds from Endophytic Microorganisms in Wound Healing
2.1. Phenolic Compounds
2.2. Anthraquinones
2.3. Alkaloids
2.4. Terpenoids
2.5. Proteins and Enzymes
2.6. Polysaccharides/Glycoconjugates
2.7. Other Compounds/Mixtures
| Endophyte | Host | Secondary Metabolites | The Effects on Wounds | References |
|---|---|---|---|---|
| Paecilomyces sp. AUMC 15510 | Cornulaca monacantha | Ethyl acetate crude extract | Wound closure activity | [20] |
| Neurospora crassa SSN01 | Lycium shawii | Benzoic acid | Burn wound infections | [48] |
| Talaromyces purpureogenus | Extracellular polysaccharides | Wound healing activity and cellular antioxidant | [77] | |
| Preussia africana | Aloe vera | Ethyl acetate crude extract | In vitro wound healing and anticancer effect | [93] |
| Phyllosticta fallopiae L67 | Aloe vera | Dichloromethane extract | Diabetic wound healing | [22] |
| Penicillium amestolkiae elv609 | Orthosiphon stamineus Benth | Ethyl acetate crude extract | Diabetic wound healing | [79] |
| Papiliotrema terrestris PT22AV | Olea europaea | Exopolysaccharide | Wound closure activity | [35] |
| Eurotium chevalieri MUT 2316 | Grantia compressa | Tetrahydroauroglaucin and dihydroglaucin | Wound closure effect | [23] |
| Penicillium porpurogenum | - | Purpurolide C | Diabetic wound healing | [68] |
| Penicillium rubens | Cucumis sativus L. | Ethyl acetate crude extract | Wound closure effect | [94] |
| Streptomyces parvulus GloL3 and Streptomyces lienomycini SK5 | Globba marantina L. and Selaginella kraussiana | Ethyl acetate crude extract | Antimicrobial and antioxidant | [95] |
3. Critical Analysis and Translational Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular Matrix |
| EPS | Extracellular Polysaccharide |
| HBA | Hydroxybenzaldehyde |
| MIC | Minimum Inhibitory Concentration |
| UPLC | Ultra-High-Performance Liquid Chromatography |
| TOF | Time of Flight |
| EtOH | Ethanol |
| EtAc | Ethyl Acetate |
| PDB | Potato Dextrose Broth |
| DICM | Dichloromethane |
| D | Direct Evidence |
| I | Indirect Evidence |
| GRADE | Recommendation Assessment, Development, and Evaluation |
| MS | Mass Spectrometry |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| TPH | Human monocytic cell line derived from an acute monocytic leukemia patient |
| ABTS | 2,2’-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid |
References
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Chohan, T.A.; Zaidi, S.H.H.; Hai, A.; Alzahrani, A.R.; Abida; Imran, M.; Saleem, H. A Systematic Review on Biochemical Perspectives on Natural Products in Wound Healing: Exploring Phytochemicals in Tissue Repair and Scar Prevention. Chem. Biodivers. 2024, 21, e202400615. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Extracellular Matrix as an Inductive Scaffold for Functional Tissue Reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-Enhanced Immunoregulation Hydrogel for Oxygenation and ROS Neutralization in Diabetic Foot Ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Gaba, A. Phyto-Extracts in Wound Healing. J. Pharm. Pharm. Sci. 2013, 16, 760. [Google Scholar] [CrossRef] [PubMed]
- Hillman, P.F.; Lee, C.; Nam, S.-J. Microbial Natural Products with Wound-Healing Properties. Processes 2022, 11, 30. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Gao, H.; Zhao, Y.; Pang, Y.; Yao, Y.; Yang, Z.; Zhang, X.; Wang, Y.; Yang, S.; Ma, X.; et al. The Potential Application of Natural Products in Cutaneous Wound Healing: A Review of Preclinical Evidence. Front. Pharmacol. 2022, 13, 900439. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.; Babalola, O. Pharmacological Potential of Fungal Endophytes Associated with Medicinal Plants: A Review. J. Fungi 2021, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic Microbes: Biodiversity, Plant Growth-Promoting Mechanisms and Potential Applications for Agricultural Sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.H.; El-Maraghy, S.S.; Abdel-Mallek, A.Y.; Abdel-Rahman, M.A.A.; Hassanein, E.H.M.; Al-Bedak, O.A.; El-Aziz, F.E.-Z.A.A. The Antimicrobial, Antibiofilm, and Wound Healing Properties of Ethyl Acetate Crude Extract of an Endophytic Fungus Paecilomyces Sp. (AUMC 15510) in Earthworm Model. Sci. Rep. 2022, 12, 19239. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.A.; Tan, W.-N.; Chear, N.J.-Y.; Leong, C.-R.; Rashid, S.A.; Tong, W.-Y. Metabolites Characterisation of Endophytic Phyllosticta fallopiae L67 Isolated from Aloe vera with Antimicrobial Activity on Diabetic Wound Microorganisms. Nat. Prod. Res. 2023, 37, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Vitale, G.A.; Varese, G.C.; Barletta, E.; D’Auria, M.V.; De Pascale, D.; Degl’Innocenti, D. Dihydroauroglaucin Isolated from the Mediterranean Sponge Grantia compressa Endophyte Marine Fungus Eurotium chevalieri Inhibits Migration of Human Neuroblastoma Cells. Pharmaceutics 2022, 14, 616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and Chemistry of Endophytes. Nat. Prod. Rep. 2006, 23, 753. [Google Scholar] [CrossRef] [PubMed]
- Eshboev, F.; Karakozova, M.; Abdurakhmanov, J.; Bobakulov, K.; Dolimov, K.; Abdurashidov, A.; Baymirzaev, A.; Makhnyov, A.; Terenteva, E.; Sasmakov, S.; et al. Antimicrobial and Cytotoxic Activities of the Secondary Metabolites of Endophytic Fungi Isolated from the Medicinal Plant Hyssopus Officinalis. Antibiotics 2023, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Shurigin, V.; Alaylar, B.; Davranov, K.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. Diversity and Biological Activity of Culturable Endophytic Bacteria Associated with Marigold (Calendula officinalis L.). AIMS Microbiol. 2021, 7, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Shurigin, V.; Alaylar, B.; Wirth, S.; Bellingrath-Kimura, S.D. Bacterial Endophytes from Horseradish (Armoracia rusticana G. Gaertn.,B.Mey.&Scherb.) with Antimicrobial Efficacy against Pathogens. Plant Soil Environ. 2020, 66, 309–316. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Zou, W.X. Endophytes: A Rich Source of Functional Metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Turaeva, B.; Soliev, A.; Eshboev, F.; Kamolov, L.; Azimova, N.; Karimov, H.; Zukhritdinova, N.; Khamidova, K. The Use of Three Fungal Strains in Producing of Indole-3-Acetic Acid and Gibberelllic Acid. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 32–43. [Google Scholar]
- Liu, Y.-H.; Fang, B.-Z.; Dong, Z.-Y.; Li, L.; Mohamad, O.A.A.; Zhang, Y.-G.; Egamberdieva, D.; Xiao, M.; Li, W.-J. Croceibacterium Gen. Nov., with Description of Croceibacterium ferulae Sp. Nov., an Endophytic Bacterium Isolated from Ferula sinkiangensis K.M. Shen and Reclassification of Porphyrobacter mercurialis as Croceibacterium mercuriale Comb. Nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 2547–2554. [Google Scholar] [CrossRef] [PubMed]
- Musa, Z.; Ma, J.; Egamberdieva, D.; Abdelshafy Mohamad, O.A.; Abaydulla, G.; Liu, Y.; Li, W.-J.; Li, L. Diversity and Antimicrobial Potential of Cultivable Endophytic Actinobacteria Associated with the Medicinal Plant Thymus roseus. Front. Microbiol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Shurigin, V.; Davranov, K.; Wirth, S.; Egamberdieva, D.; Bellingrath-Kimura, S.D. Medicinal Plants with Phytotoxic Activity Harbour Endophytic Bacteria with Plant Growth Inhibitory Properties. Environ. Sustain. 2018, 1, 209–215. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Okoro, O.V.; Ianiri, G.; Jafari, H.; Rashidi, K.; Ghasemi, S.; Castoria, R.; Palmieri, D.; Delattre, C.; Pierre, G.; et al. Exopolysaccharide from the Yeast Papiliotrema terrestris PT22AV for Skin Wound Healing. J. Adv. Res. 2023, 46, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O. The Plant Endosphere-Hidden Treasures: A Review of Fungal Endophytes. Biotechnol. Genet. Eng. Rev. 2021, 37, 154–177. [Google Scholar] [CrossRef] [PubMed]
- Eshboev, F.; Mamadalieva, N.; Nazarov, P.; Hussain, H.; Katanaev, V.; Egamberdieva, D.; Azimova, S. Antimicrobial Action Mechanisms of Natural Compounds Isolated from Endophytic Microorganisms. Antibiotics 2024, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as Sources of Antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Gupta, P.; Keshri, P.K.; Verma, A.; Mishra, P.; Kumar, D.; Kumar, A.; Singh, S.K.; Gautam, V. Fungal Endophytes: An Accessible Source of Bioactive Compounds with Potential Anticancer Activity. Appl. Biochem. Biotechnol. 2022, 194, 3296–3319. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; Dey, Y.N.; Madhavan, Y.; Maity, A. Fungal Endophytes: A Storehouse of Bioactive Compounds. Mini-Rev. Med. Chem. 2023, 23, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Agarwal, S.; Verma, K.; Bhardwaj, R.; Mathur, V. Therapeutic Compounds from Medicinal Plant Endophytes: Molecular and Metabolic Adaptations. J. Appl. Microbiol. 2023, 134, lxad074. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Srivastava, Y.; Bae, H. Trends of Pharmaceutical Design of Endophytes as Anti-Infective. Curr. Top. Med. Chem. 2021, 21, 1572–1586. [Google Scholar] [CrossRef] [PubMed]
- Firoozbahr, M.; Kingshott, P.; Palombo, E.A.; Zaferanloo, B. Recent Advances in Using Natural Antibacterial Additives in Bioactive Wound Dressings. Pharmaceutics 2023, 15, 644. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; Ferruzzi, M.G. Bioavailability and Metabolism of Bioactive Compounds from Foods. In Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 407–423. ISBN 978-0-12-391884-0. [Google Scholar]
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K.G.; Ren, X.; Qu, Y. In Vivo Wound Healing and in Vitro Antioxidant Activities of Bletilla striata Phenolic Extracts. Biomed. Pharmacother. 2017, 93, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Utpal, B.K.; Sutradhar, B.; Zehravi, M.; Sweilam, S.H.; Panigrahy, U.P.; Urs, D.; Fatima, A.F.; Nallasivan, P.K.; Chhabra, G.S.; Sayeed, M.; et al. Polyphenols in Wound Healing: Unlocking Prospects with Clinical Applications. Naunyn. Schmiedebergs Arch. Pharmacol. 2025, 398, 2459–2485. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Maffo, T.; Kapche, D.G.W.F.; Ngadjui, B.T.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, Antioxidant and Antibacterial Activity of Four Compounds Produced by an Endophytic Fungus Epicoccum nigrum Associated with Entada abyssinica. Rev. Bras. Farmacogn. 2017, 27, 251–253. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Ali, S.S.; Khalil, M.A.; Sun, J.; Nouh, H.S. Exploring the Potential of Benzoic Acid Derived from the Endophytic Fungus Strain Neurospora Crassa SSN01 as a Promising Antimicrobial Agent in Wound Healing. Microbiol. Res. 2022, 262, 127108. [Google Scholar] [CrossRef] [PubMed]
- Abonyi, D.O.; Eze, P.M.; Abba, C.C.; Ujam, N.T.; Proksch, P.; Okoye, F.B.C.; Esimone, C.O. Biologically Active Phenolic Acids Produced By Aspergillus sp., An Endophyte of Moringa oleifera. Eur. J. Biol. Res. 2018, 8, 157–167. [Google Scholar] [CrossRef]
- Kang, C.W.; Han, Y.E.; Kim, J.; Oh, J.H.; Cho, Y.H.; Lee, E.J. 4-Hydroxybenzaldehyde Accelerates Acute Wound Healing through Activation of Focal Adhesion Signalling in Keratinocytes. Sci. Rep. 2017, 7, 14192. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-5429-3. [Google Scholar]
- Hafez Ghoran, S.; Taktaz, F.; Ayatollahi, S.A.; Kijjoa, A. Anthraquinones and Their Analogues from Marine-Derived Fungi: Chemistry and Biological Activities. Mar. Drugs 2022, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Evidente, A. Fungal Bioactive Anthraquinones and Analogues. Toxins 2020, 12, 714. [Google Scholar] [CrossRef] [PubMed]
- Elawady, M.E.; Hamed, A.A.; Alsallami, W.M.; Gabr, E.Z.; Abdel-Monem, M.O.; Hassan, M.G. Bioactive Metabolite from Endophytic Aspergillus versicolor SB5 with Anti-Acetylcholinesterase, Anti-Inflammatory and Antioxidant Activities: In Vitro and In Silico Studies. Microorganisms 2023, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-T.; Wu, M.-F.; Ma, M.-H.; Zhao, L.; Zhu, J.-Y.; Nian, H.; Li, F.-L. Research on the Wound Healing Effect of Shengji Huayu Formula Ethanol Extract-Derived Fractions in Streptozotocin-Induced Diabetic Ulcer Rats. BMC Complement. Med. Ther. 2023, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Daley, S.; Cordell, G.A. Biologically Significant and Recently Isolated Alkaloids from Endophytic Fungi. J. Nat. Prod. 2021, 84, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Song, L.; Shen, S.; Fu, W.; Zhu, Y.; Liu, L. Bioactive Alkaloids as Secondary Metabolites from Plant Endophytic Aspergillus Genus. Molecules 2023, 28, 7789. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A.; Sharma, A. HPLC Analysis of Camptothecin Content in Various Parts of Nothapodytes Foetida Collected on Different Periods. Asian Pac. J. Trop. Biomed. 2012, 2, 389–393. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; ElSayed, A.I.; Wadan, K.M.; El-Saadany, S.S.; Abd El-Hady, N.A.A. Camptothecin Bioprocessing from Aspergillus terreus, an Endophyte of Catharanthus roseus: Antiproliferative Activity, Topoisomerase Inhibition and Cell Cycle Analysis. Microb. Cell Factories 2024, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Shanmuganathan, R.; Pugazhendhi, A. Vinblastine Production by the Endophytic Fungus Curvularia verruculosa from the Leaves of Catharanthus roseus and Its in Vitro Cytotoxicity against HeLa Cell Line. Anal. Biochem. 2020, 593, 113530. [Google Scholar] [CrossRef] [PubMed]
- Venkatadri, T.V.; Afzal Khan, A.K. Wound Healing Suppressant Effect of Vincristine Reversed by Vitamin A: An Experimental Study. Int. J. Basic Clin. Pharmacol. 2016, 5, 962–964. [Google Scholar] [CrossRef][Green Version]
- Radiastuti, N.; Mutea, D.; Sumarlin, L.O. Endophytic Colletrotrichum Spp. from Cinchona calisaya Wedd. and It’s Potential Quinine Production as Antibacterial and Antimalaria. In Proceedings of the The 1st International Conference on Mathematics, Science, and Computer Science (ICMSC) 2016: Sustainability and Eco Green Innovation in Tropical Studies for Global Future, East Kalimantan, Indonesia, 19–21 October 2016; p. 020022. [Google Scholar]
- Falkner, R. On the Use of Solutions of Quinine as a Dressing for Infected Wounds. BMJ 1916, 1, 11–12. [Google Scholar]
- Wolf, R.; Tufano, M.A.; Ruocco, V.; Grimaldi, E.; Ruocco, E.; Donnarumma, G.; Baroni, A. Quinine Sulfate Inhibits Invasion of Some Bacterial Skin Pathogens. Int. J. Dermatol. 2006, 45, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, H.; Liu, M.; Li, X.; Zhou, M.; Lyu, Y.; Huang, J.; Chen, S.; Wang, L. Therapeutic Effects of Quinine in a Mouse Model of Atopic Dermatitis. Mol. Med. Rep. 2021, 23, 313. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Solís, J.M.; Fernández, F.J. Endophytic Fungal Terpenoids: Natural Role and Bioactivities. Microorganisms 2022, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Słonimska, P.; Sachadyn, P.; Zieliński, J.; Skrzypski, M.; Pikuła, M. Chemotherapy-Mediated Complications of Wound Healing: An Understudied Side Effect. Adv. Wound Care 2024, 13, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, G.; Chen, Y.; Xia, H.; Xu, J.; Guo, L.; Lin, S.; Liu, Y. Purpurolide C-Based Microneedle Promotes Macrophage-Mediated Diabetic Wound Healing via Inhibiting TLR4-MD2 Dimerization and MYD88 Phosphorylation. Acta Pharm. Sin. B 2023, 13, 5060–5073. [Google Scholar] [CrossRef] [PubMed]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Baumann, P.; Luck, K.; Rothe, B.; Biedermann, P.H.W.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Volatile Emission and Biosynthesis in Endophytic Fungi Colonizing Black Poplar Leaves. Beilstein J. Org. Chem. 2021, 17, 1698–1711. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-Caryophyllene Enhances Wound Healing through Multiple Routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, V.H.S.; Cardoso, S.L.; Fonseca-Bazzo, Y.; Silveira, D.; Magalhães, P.O.; Souza, P.M. Protease Produced by Endophytic Fungi: A Systematic Review. Molecules 2021, 26, 7062. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Rhoden, S.A.; Mota, T.R.; Azevedo, J.L.; Pamphile, J.A.; De Souza, C.G.M.; Polizeli, M.D.L.T.D.M.; Bracht, A.; Peralta, R.M. Endophytic Fungi: Expanding the Arsenal of Industrial Enzyme Producers. J. Ind. Microbiol. Biotechnol. 2014, 41, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Orlandelli, R.C.; Almeida, T.T.D.; Alberto, R.N.; Polonio, J.C.; Azevedo, J.L.; Pamphile, J.A. Antifungal and Proteolytic Activities of Endophytic Fungi Isolated from Piper hispidum Sw. Braz. J. Microbiol. 2015, 46, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Sinclair And, R.D.; Ryan, T.J. Proteolytic Enzymes in Wound Healing: The Role of Enzymatic Debridement. Australas. J. Dermatol. 1994, 35, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Recent Advances in Endophytic Exopolysaccharides: Production, Structural Characterization, Physiological Role and Biological Activity. Carbohydr. Polym. 2017, 157, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Saravanakumar, K.; Park, S.; Han, K.; Wang, M.-H. Isolation, Characterization, Antioxidant, and Wound Healing Activities of Extracellular Polysaccharide from Endophytic Fungus Talaromyces purpureogenus. Appl. Biochem. Biotechnol. 2023, 195, 3822–3839. [Google Scholar] [CrossRef] [PubMed]
- Hakan Dogan, K. (Ed.) Wound Healing-Current Perspectives; IntechOpen: London, UK, 2019; ISBN 978-1-78985-537-1. [Google Scholar]
- Rozman, N.A.S.B.; Nor Hamin, N.S.B.M.; Ring, L.C.; Nee, T.W.; Mustapha, M.B.; Yenn, T.W. Antimicrobial Efficacy of Penicillium amestolkiae Elv609 Extract Treated Cotton Fabric for Diabetic Wound Care. Mycobiology 2017, 45, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.R.; Das, T.N. Septum Bleed during GC-MS Analysis: Utility of Septa of Various Makes. J. Chromatogr. Sci. 2013, 51, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin Promotes Cutaneous Wound Healing in Mice through Wnt/β-Catenin Signaling Pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Soberón, J.R.; Sgariglia, M.A.; Carabajal Torrez, J.A.; Aguilar, F.A.; Pero, E.J.I.; Sampietro, D.A.; Fernández De Luco, J.; Labadie, G.R. Antifungal Activity and Toxicity Studies of Flavanones Isolated from Tessaria dodoneifolia Aerial Parts. Heliyon 2020, 6, e05174. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.P.M.; Ferraz, A.B.F.; Albring, D.V.; Bordignon, S.A.L.; Schripsema, J.; Bridi, R.; Dutra-Filho, C.S.; Henriques, A.T.; Von Poser, G.L. Benzophenones from Hypericum c Arinatum. J. Nat. Prod. 2005, 68, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Holt, G.S.; Lodge, J.K.; McCarthy, A.J.; Graham, A.K.; Young, G.; Bridge, S.H.; Brown, A.K.; Veses-Garcia, M.; Lanyon, C.V.; Sails, A.; et al. Shigatoxin Encoding Bacteriophage ϕ24B Modulates Bacterial Metabolism to Raise Antimicrobial Tolerance. Sci. Rep. 2017, 7, 40424. [Google Scholar] [CrossRef] [PubMed]
- Marrufo, T.; Nazzaro, F.; Mancini, E.; Fratianni, F.; Coppola, R.; De Martino, L.; Agostinho, A.; De Feo, V. Chemical Composition and Biological Activity of the Essential Oil from Leaves of Moringa oleifera Lam. Cultiv. Mozambique. Mol. 2013, 18, 10989–11000. [Google Scholar] [CrossRef]
- Mou, Y.; Meng, J.; Fu, X.; Wang, X.; Tian, J.; Wang, M.; Peng, Y.; Zhou, L. Antimicrobial and Antioxidant Activities and Effect of 1-Hexadecene Addition on Palmarumycin C2 and C3 Yields in Liquid Culture of Endophytic Fungus Berkleasmium sp. Dzf12. Molecules 2013, 18, 15587–15599. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Barros Cardoso, C.; Aparecida Souza, M.; Amália Vieira Ferro, E.; Favoreto, S.; Deolina Oliveira Pena, J. Influence of Topical Administration of N-3 and N-6 Essential and N-9 Nonessential Fatty Acids on the Healing of Cutaneous Wounds. Wound Repair Regen. 2004, 12, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kanetsuna, F. Bactericidal Effect of Fatty Acids on Mycobacteria, with Particular Reference to the Suggested Mechanism of Intracellular Killing. Microbiol. Immunol. 1985, 29, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Magdalon, J.; Vinolo, M.A.R.; Rodrigues, H.G.; Paschoal, V.A.; Torres, R.P.; Mancini-Filho, J.; Calder, P.C.; Hatanaka, E.; Curi, R. Oral Administration of Oleic or Linoleic Acids Modulates the Production of Inflammatory Mediators by Rat Macrophages. Lipids 2012, 47, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of Oleic and Linoleic Acids on the Inflammatory Phase of Wound Healing in Rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, Y.; Yang, J.; Xiao, Y.; Cai, Y.; Wan, Y.; Chen, H.; Yao, H.; Shan, Z.; Li, C.; et al. Isolation and Identification of Flavonoid-Producing Endophytic Fungi from Medicinal Plant Conyza blinii H.Lév That Exhibit Higher Antioxidant and Antibacterial Activities. PeerJ 2020, 8, e8978. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Stephenson, S.L.; AlNadhari, S.; Yassin, M.A. Isolation, Identification and Bioactivity Analysis of an Endophytic Fungus Isolated from Aloe Vera Collected from Asir Desert, Saudi Arabia. Bioprocess Biosyst. Eng. 2021, 44, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Eliwa, D.; El-Bouseary, M.M. Potential Antibacterial, Wound Healing and Anti-Inflammatory Activities of Penicillium Rubens, an Endophytic Fungus Isolated from the Leaves of Cucumis sativus L. Front. Sci. Res. Technol. 2025, 11, 71–77. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Bioactivities of Secondary Metabolites of Two Actinomycetes Streptomyces parvulus GloL3, and Streptomyces lienomycini SK5, Endophytes of Two Indian Medicinal Herbs- Globba marantina L. and Selaginella kraussiana (Kunze) A. Braun. Braz. J. Microbiol. 2025, 56, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Firoozbahr, M.; Palombo, E.A.; Kingshott, P.; Zaferanloo, B. Antibacterial and Antibiofilm Properties of Native Australian Plant Endophytes against Wound-Infecting Bacteria. Microorganisms 2024, 12, 1710. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Dai, K.; Gong, C.; Huang, C.; Jiao, S.; Zhang, J. Endophytic Fungus Umbelopsis Sp. TM01 as High-Activity Alternative to Tricholoma Matsutake. Bioresour. Technol. 2025, 422, 132216. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; deBeer, H. GRADE Guidelines: 1. Introduction—GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of Treatment Effects between Animal Experiments and Clinical Trials: Systematic Review. BMJ 2007, 334, 197. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Martins-Green, M. Animal Models for the Study of Acute Cutaneous Wound Healing. Wound Repair Regen. 2022, 31, 6–16. [Google Scholar] [CrossRef] [PubMed]
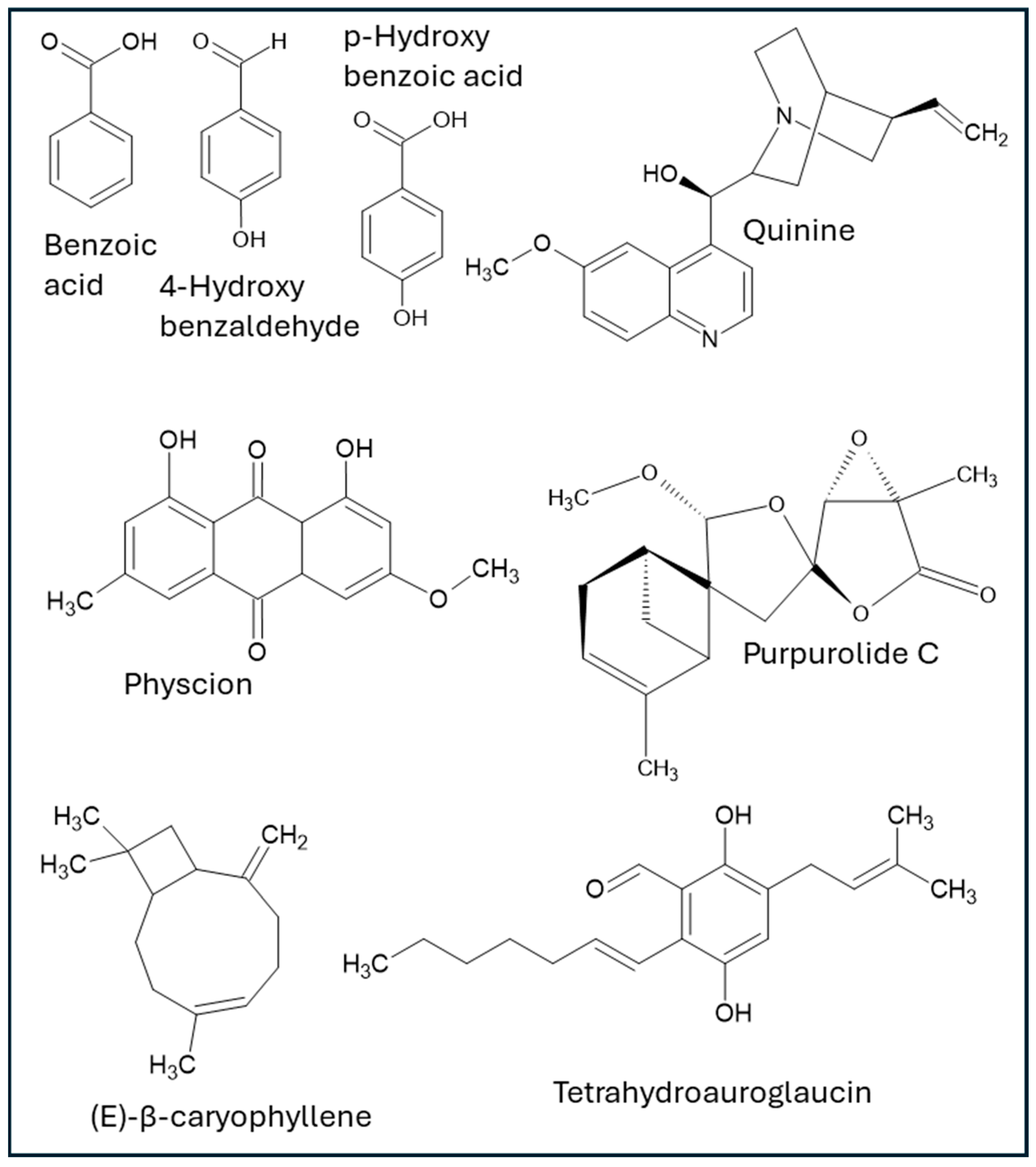
| Type of Compound | Compound/Extract | Reference | D/I | In Vivo Studies Regarding Wound Healing? | Reference for In Vivo Model | GRADE Rating |
|---|---|---|---|---|---|---|
| Phenolic | Benzoic acid | [48] | D | Yes | Low | |
| DICM extract | [22] | D | No | Very Low | ||
| 4-Hydroxybenzaldehyde | [47] | I | Yes (but indirect) | [50] | Very Low | |
| p-hydroxybenzoic acid | [49] | I | Yes (but indirect) | [46] | Very Low | |
| Anthraquinones | Physcion | [54] | D | Yes (but indirect) | [55] | Very Low |
| Alkaloids | Quinine | [62] | I | Yes (but indirect) | [65] | Very Low |
| Terpenoids | Purpurolide C | [68] | D | Yes | Low | |
| (E)-β-caryophyllene and others | [70] | I | Yes (but indirect) | [71] | Very Low | |
| Polysaccharides | EPS | [77] | D | No | Very Low | |
| EPS | [35] | D | Yes | Low | ||
| Other | EtOH extract | [79] | D | No | Very Low | |
| EtAc extract | [20] | D | Yes | Low | ||
| EtAc extract | [93] | D | No | Very Low | ||
| Tetrahydroauroglaucin | [23] | D | No | Very Low | ||
| EtAc extract | [94] | D | No | Very Low | ||
| EtAc extract | [95] | D | No | Very Low | ||
| EtAc extract | [96] | D | No | Very Low | ||
| PDB extract | [97] | D | No | Very Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Gomez, O.; Eshboev, F.; Mullaiarova, K.; Egamberdieva, D. Endophytic Bioactive Compounds for Wound Healing: A Review of Biological Activities and Therapeutic Potential. Microorganisms 2025, 13, 1691. https://doi.org/10.3390/microorganisms13071691
Calvo-Gomez O, Eshboev F, Mullaiarova K, Egamberdieva D. Endophytic Bioactive Compounds for Wound Healing: A Review of Biological Activities and Therapeutic Potential. Microorganisms. 2025; 13(7):1691. https://doi.org/10.3390/microorganisms13071691
Chicago/Turabian StyleCalvo-Gomez, Octavio, Farkhod Eshboev, Kamilla Mullaiarova, and Dilfuza Egamberdieva. 2025. "Endophytic Bioactive Compounds for Wound Healing: A Review of Biological Activities and Therapeutic Potential" Microorganisms 13, no. 7: 1691. https://doi.org/10.3390/microorganisms13071691
APA StyleCalvo-Gomez, O., Eshboev, F., Mullaiarova, K., & Egamberdieva, D. (2025). Endophytic Bioactive Compounds for Wound Healing: A Review of Biological Activities and Therapeutic Potential. Microorganisms, 13(7), 1691. https://doi.org/10.3390/microorganisms13071691








