1. Introduction
Aquaculture, the fastest-growing food sector in the world, supplies nearly half of the total fish production for human consumption. China is the world’s largest aquaculture producer, accounting for about 60% of global aquaculture production [
1]. As the aquaculture industry transitions from extensive farming to intensive farming, this sector faces persistently high stocking densities combined with rampant misuse of pharmaceuticals and antibiotics, resulting in elevated disease incidence throughout the production cycle [
2,
3]. This context highlights the importance of developing natural immunostimulants as functional feed additives to improve innate immunity and reduce disease susceptibility of fish. Yeast culture (YC), as a member of the symbiotic family, is a natural product of yeast fermentation, which contains abundant nutritional elements, such as amino acids, peptides, organic acids, and vitamins, as well as immune stimulants such as β-glucan, mannan oligosaccharides, and nucleotides [
4,
5,
6]. It was previously reported that dietary β-glucan supplementation could improve both innate and adaptive immune responses [
7,
8,
9], while liver health benefited from dietary mannan oligosaccharide or nucleotide inclusion in many fish species [
10,
11]. At present, YC has gained widespread attention in aquaculture with proven benefits. In addition to being used as a protein source to replace fish meal [
6,
12], YC can also be used as an immunomodulator to facilitate feed ingestion and digestion, enhance immunity and disease resistance, regulate gut microbiota, and boost antioxidant capacity, thereby improving the growth performance of aquatic animals [
13,
14,
15,
16,
17]. It is noteworthy that the beneficial effects of dietary YC supplementation in aquatic animals are both dose-dependent and species-dependent [
17,
18].
Largemouth bass
Micropterus salmoides (LMB) has emerged as a vital freshwater economic fish species in China, owing to its exceptional traits, including rapid growth, high nutritional value, and lack of intermuscular spines [
19,
20]. In China, there are two primary farming modes for LMB: pond culture and recirculating aquaculture. In pond culture systems, the typical stocking density ranges from 3.0 to 6.0 kg/m
3, depending on management efficiency, while recirculating aquaculture systems can support densities up to 30 kg/m
3 [
21,
22,
23]. Generally, LMB fingerlings are introduced into water bodies for cultivation and must grow until they reach a weight of around 500 g to attain marketable size [
24]. With breakthroughs in feed processing and extensive promotion of compound feed, both the farming areas and scale of LMB have rapidly expanded in recent years. In 2023, the total production of LMB in China exceeded 800,000 tons [
25]. However, the application of compound feed is frequently associated with liver and intestine injuries in LMB [
26,
27], leading to disease outbreaks and enormous economic losses. To our knowledge, several studies have evaluated dietary yeast product supplementation on the growth performance and health status of LMB. For instance, Gong et al. reported that 15 or 30 g/kg dietary yeast hydrolysate improved antioxidant capacity and disease resistance of LMB without negative impact on growth performance [
28]. Dietary YC inclusion at 30 g/kg attenuated the negative effects on growth performance, liver function, intestinal barrier, and microbiota of LMB fed high-starch or high-plant protein diets [
15,
29]. However, the optimal level of dietary YC has not yet been determined in LMB. Therefore, this study was performed to evaluate dietary YC levels on the growth performance, feed utilization, digestive function, and intestinal microbiota composition of LMB.
2. Materials and Methods
2.1. Experimental Design and Diets
YC was supplied by Chongqing Changnuo Biotechnology Co., Ltd. (Chongqing, China). The proximate composition and selective bioactive elements of YC are shown in
Table 1. The formulation and proximate composition of the experimental diets are shown in
Table 2. Six isonitrogenous (i.e., 53.7% protein) and iso-lipidic (i.e., 12.1%) diets were prepared with graded levels of YC (0, 5, 10, 15, 20, and 30 g/kg) at the expense of cellulose inclusion, designated as CON, YC5, YC10, YC15, YC20, and YC30, respectively. Fish meal, soy protein concentrate, and chicken meal were used as the main protein sources. Cassava starch was maintained at 10% as the carbohydrate source [
18]. The feed ingredients were thoroughly pulverized and homogenously blended with water in the proportion of 40% by weight. Diet pellets were then processed through a twin-screw laboratory extruder (SG-YPYS-76, Xiamen, China) according to Chen et al. [
30]. The experimental diets were air-dried, sealed in plastic bags, and stored in a −20 °C refrigerator.
2.2. Feeding Trial
LMB juveniles (Youlu No. 3 strain, about 4 g per fish) were purchased from Chongqing Three Gorges Ecological Fishery Co., Ltd. (Fuling, China). The fish were transported to our laboratory using a truck equipped with an insulated tank and an aeration system to maintain oxygenated water. Upon arrival, the fish were transferred into 2 circular plastic tanks (1500 L each) connected with a flow-through aquaculture system for 2 weeks of acclimatization. During that time, the fish were fed a commercial diet (Chongqing Haid Feed Co., Ltd., Chongqing, China) twice daily to apparent satiation. After the acclimatization, 480 LMB fingerlings (initial average weight, 8.11 ± 0.05 g) were selected and randomly distributed into 24 rectangular glass tanks (200 L each) operating as a recirculating system. Each diet was assigned to 4 replicate tanks of LMB with 20 fish per tank. During the next 8 weeks, the fish were fed twice a day (9:00 and 17:00) until apparent satiation, and feed intake was recorded accordingly.
The feeding trial was carried out under a controlled photoperiod (12 h: 12 h). During the experiment, the aquaculture system, including the filter materials (sponge and gravel) was cleaned once a week, while water quality parameters were monitored twice a week. Water quality indicators were maintained as follows: water temperature, 23.6 ± 1.8 °C; dissolved oxygen, 10.5 ± 0.4 mg/L, ammonia nitrogen, 0.10 ± 0.01 mg/L; nitrite, 0.12 ± 0.02 mg/L; pH, 7.40 ± 0.52.
2.3. Sample Collection
At the end of the feeding trial, the fish were fasted for 24 h before formal sampling. Twelve fish were randomly selected from each tank, and anesthetized with MS-222 (Sigma, Livonia, MI, USA) at a dose of about 75 mg/L. Three fish were used for the analysis of the proximate composition, including moisture, lipid, protein, and ash levels. Six fish were dissected for morphological measurement (including body weight, viscera weight, liver weight, and intraperitoneal fat weight). Then, gut samples of these fish were collected and immediately frozen in liquid nitrogen pending the analysis of digestive function indicators and microbiome composition. The remaining 3 fish were dissected to collect the intestine samples. Foregut and midgut were fixed in 4% paraformaldehyde solution for section preparation, while hindgut samples were immediately frozen in liquid nitrogen for real-time PCR analysis.
2.4. Proximate Composition Analysis
The proximate composition of the diets and whole fish was determined according to AOAC [
31]. The moisture was measured by drying at 105 °C in an oven for 24 h until constant weight was achieved. The crude protein (calculated as N × 6.25) was analyzed using the Kjeldahl method. Crude lipid was quantified via Soxhlet extraction. The ash was determined by incinerating samples in a muffle furnace at 550 °C.
2.5. Analysis of Digestive Enzyme Activity
Intestine samples were homogenized with a tissue homogenizer in 9 volumes of phosphate-buffered saline (PBS). The homogenates were centrifuged at 3500× g for 10 min, and the supernatant was then collected for the analysis of digestive enzyme activities. The protein level of the supernatant was quantified by the biuret method. The activities of amylase (AMS, Cat. No. C061-1-1), lipase (LPS, Cat. No. A054-1-1), and trypsin (TPS, Cat. No. A080-2) were determined by spectrophotometric assays with commercial kits (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). The activities of AMS and TPS were expressed as U/mg protein, while LPS activity was expressed as U/g protein.
2.6. Histological Examination
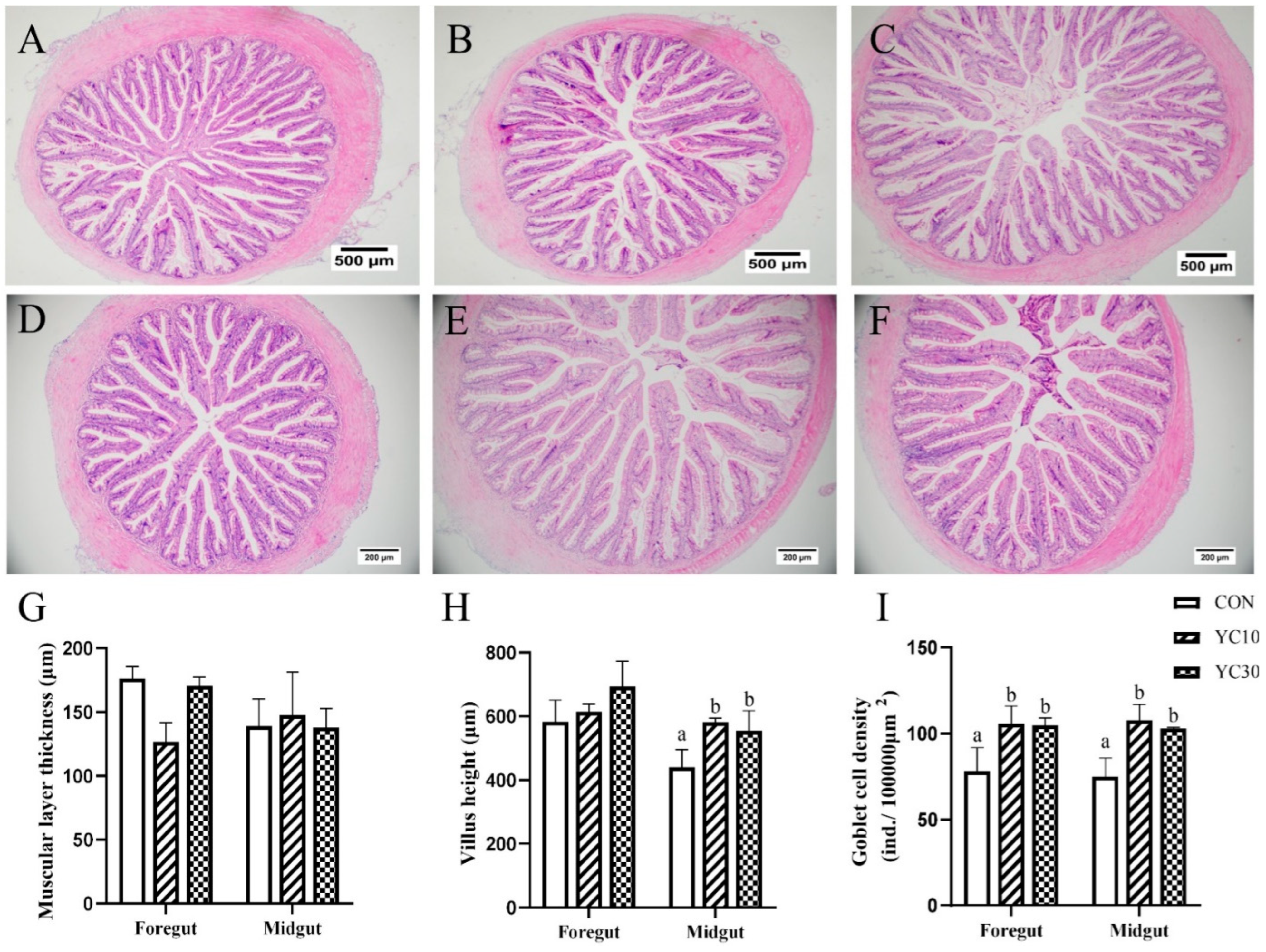
The intestine samples were fixed with 4% paraformaldehyde solution for at least 24 h and sent to Wuhan Servicebio Technology Co., Ltd. (Wuhan, China) for the preparation of paraffin sections. The samples were dehydrated in graded ethanol, embedded in paraffin, sliced into 5 μm thick sections, dewaxed, stained with hematoxylin-eosin (HE), and mounted with neutral resin. Then, the sections were observed and photographed using a microscope equipped with a computer image system (Olympus, DP73, Tokyo, Japan). The muscle layer thickness, the goblet cells count, and the villus height were quantified with the help of the ImageJ 23.0 software.
2.7. Identification of Target Genes
The complete cDNA sequence of target inflammatory genes, including interleukin-8 (
il-8),
il-1β, nuclear factor-κB (
nf-κb), tumor necrosis factor-α (
tnf-α), and transforming growth factor-β (
tgf-β), as well as
β-actin, was downloaded from the NCBI database (
http://blast.ncbi.nlm.nih.gov/, accessed on 8 January 2025). Primer Premier 6.0 software was used for the primer design, and the specific primer sequences are shown in
Table 3.
2.8. RNA Extraction, cDNA Synthesis, and Real-Time PCR
Total RNA was extracted from the intestine samples using RNAiso Plus reagent (TaKaRa, Kusatsu, Shiga, Japan). The RNA quality was verified through 1% agarose gel electrophoresis, and its concentration was quantified with an ultramicro spectrophotometer (Implen, Munich, Germany). Subsequently, total RNA was reverse transcribed into cDNA using the PrimeScript RT Master Mix Perfect Real-Time Kit (TaKaRa, Shiga, Japan). The cDNA products were stored at −20 °C pending real-time PCR analysis.
Real-time PCR analysis was performed using the fluorescent quantitative reagent SYBR
® Premix Ex Taq
TM II (TaKaRa, Shiga, Japan) on a Bio-Rad CFX96 real-time PCR system (Bio-Rad, Hercules, CA, USA), following the procedures described in our previous study [
32].
β-actin was selected as the reference gene, and the relative expression of target genes was calculated using the formula (R = 2
−ΔΔCT), as described by Livak and Schmittgen [
33].
2.9. Intestinal Microbiome Composition
The gut samples of the CON, YC10, and YC30 fish (based on feed utilization) were sent to Beijing BioMarker Biotechnology Co., Ltd. (Beijing, China) for microbiome composition exploration through 16S rRNA sequencing, following the procedures described in our previous study [
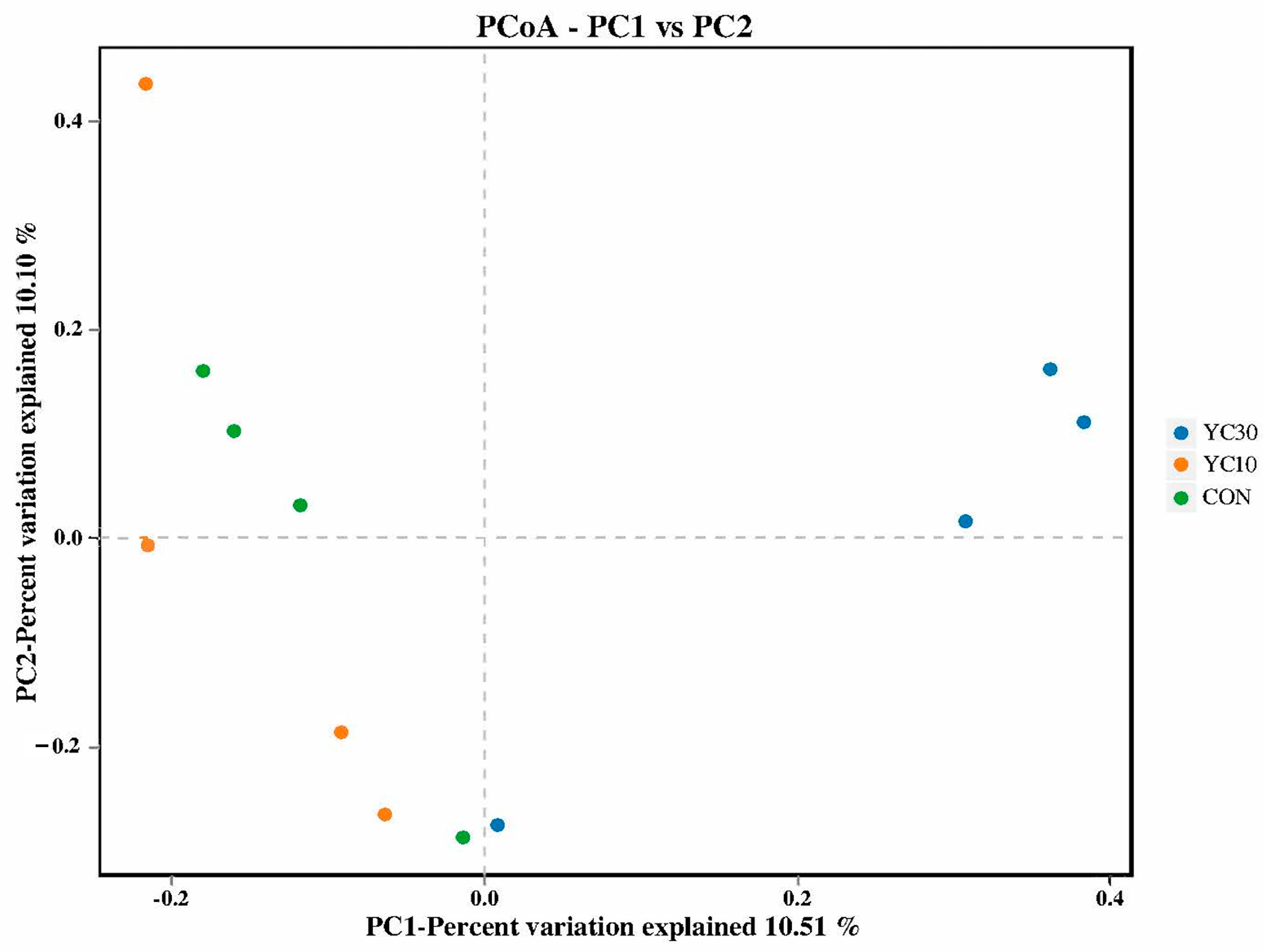
34]. Lima v1.7.0 and UCHIME v4.2 software were used to evaluate the quality of sequencing data. The sequences with similarity higher than 97% were defined as an operational taxonomic unit (OTU). The species classification information corresponding to the OTU was obtained by taxonomic annotation. The species composition and abundance were statistically analyzed at the level of phylum, genus, and species using QIIME 2. Alpha diversity indices (Chao1, ACE, Shannon, Simpson, and PD whole tree indices) were evaluated using mother vs. 1.30. Beta diversity was evaluated by principal component analysis (PCoA) based on unweighted UniFrac distance using QIIME.
2.10. Statistical Analyses
Data are expressed as mean ± SD (standard deviation) of four replicates. After normal distribution and variance homogeneity tests, the data were subject to one-way analysis of variance (ANOVA) and Duncan’s test. The significance level was set to 0.05. All the statistical analyses were performed using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA).
4. Discussion
Throughout the entire experimental period, the stocking density (0.81~7.32 kg/m
3) was maintained much lower than the upper limit of the recirculating aquaculture system in China (30.0 kg/m
3) to avoid negatively affecting the growth performance and health status of LMB. At the end of the 8-week feeding trial, growth performance (specifically final body weight and specific growth rate) exhibited no statistically significant differences between the YC-supplemented and CON fish. However, it demonstrated a progressive elevation with increasing dietary YC concentrations, culminating in maximal values at the 15 g/kg supplementation level, beyond which a decline was evident. Growth performance data indicate that supplementing 15 g/kg YC in the diet for LMB offers substantial practical benefits in aquaculture. At an incremental cost of CNY 30 per metric ton compared to the YC-free feed, this formulation increases LMB production by approximately 79.3 kg per ton, generating additional revenue of CNY 2537. Feng et al. reported that the feeding ratio was not responsive to dietary YC inclusion at 10 or 30 g/kg in LMB fed a high-starch diet [
29]. In the present study, however, the YC-supplemented treatments obtained a lower feeding ratio than the CON fish. This indicated that the improvement in growth performance in LMB in response to dietary YC inclusion benefited from a higher feed efficiency ratio. Among the YC-supplemented treatments, the YC10 fish exhibited he best feed efficiency ratio, which was higher than that of the YC5, YC20, and YC30 fish. In line with our study, the improvement of growth performance and/or feed utilization in response to dietary YC inclusion was observed in the same fish species [
29] and many other fish species [
13,
17,
18,
35]. In this experiment, the viscerosomatic index and hepatosomatic index of LMB were not affected by dietary YC inclusion, which was similar to the results in gibel carp
Carassius auratus [
12] and Ussuri catfish
Pseudobagrus ussuriensis [
17].
Digestive enzymes are crucial when fish use a large amount of ingested nutrients for rapid growth and development [
36,
37]. The activity of digestive enzymes is indicative of the physiological status of fish and the quality of functional feed additives [
38,
39]. In this study, although amylase activity remained unaffected by dietary YC supplementation, intestinal lipase and trypsin activities of LMB were improved with 10 g/kg YC compared with those of the CON fish. The enhancement of digestive function was consistent with the feed efficiency ratio, which peaked in the YC10 fish. Notably, in hybrid grouper
Epinephelus fuscoguttatus♀ ×
E. lanceolatus♂, intestinal amylase, lipase, and trypsin activities were enhanced with either 20 or 40 g/kg dietary YC supplementation [
18], suggesting species-specific responses to dietary YC administration.
Goblet cells play a crucial role in maintaining intestinal homeostasis and immune regulation through mucus secretion and bicarbonate provision in the intestine [
40,
41]. Villus height serves as a key physiological indicator of nutrient absorption capacity in animals [
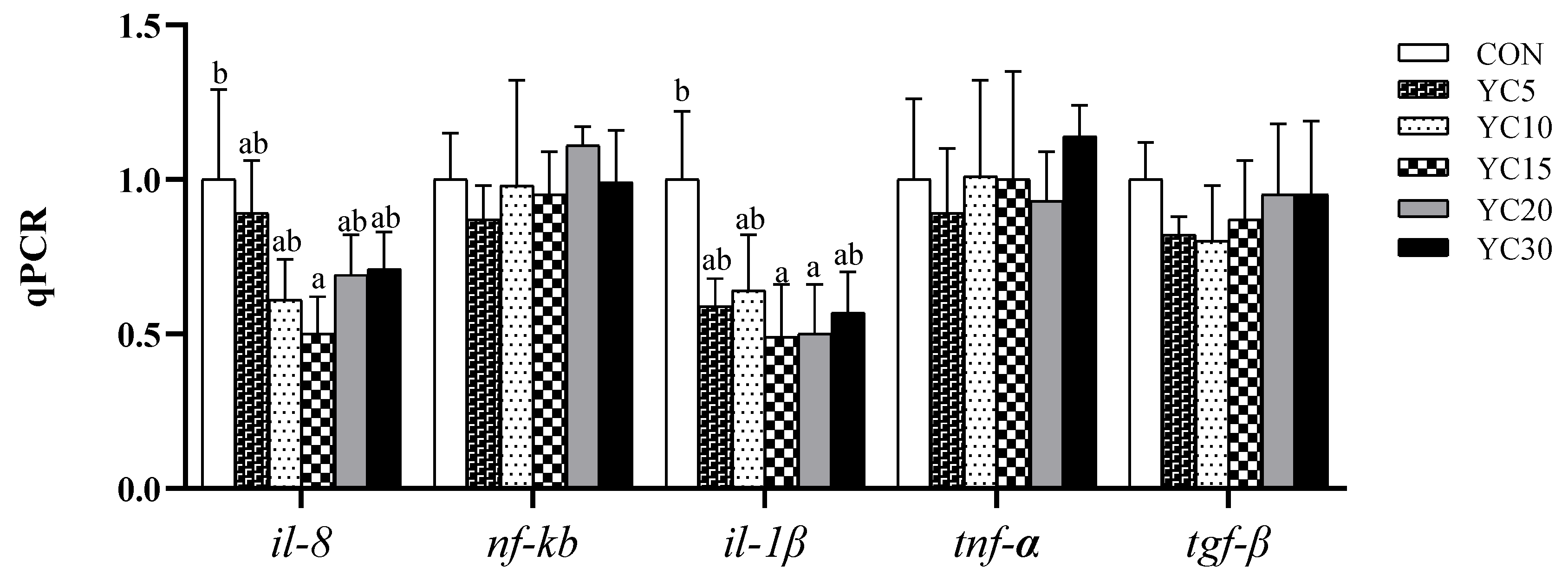
42]. Based on growth performance and feed efficiency ratio, intestine samples from the CON, YC10, and YC30 fish underwent further histological evaluation. HE staining revealed that the density of goblet cells in both foregut and midgut of the YC-supplemented treatments was higher than that of the CON fish. Furthermore, the YC10 and YC30 fish demonstrated higher villus height in the midgut than the CON fish. Interleukin-8 (
il-8) and
il-1β are pro-inflammatory cytokines that activate immune cells, induce cytokine release, and stimulate local/systemic inflammatory responses [
43,
44,
45]. In the present study, the mRNA level of
il-8 was lower in the hindgut of the YC10 fish, while the relative expression of
il-1β was downregulated in the hindgut of the YC10 and YC15 fish compared with the CON fish. These results suggested that dietary YC supplementation at 10~15 g/kg enhanced intestinal homeostasis and nutrient absorption, ultimately improving feed utilization and growth performance of LMB. In line with our results, dietary YC inclusion improved intestinal barriers and attenuated inflammatory response in the same species [
28] and other fish species [
4,
46].
Intestinal microbiota plays a vital role in host nutrition, physiology, and immune processes [
47]. Within this ecosystem, both pathogenic and beneficial bacteria coexist [
48]. Beneficial probiotics sustain microbial homeostasis while enhancing host nutrition through the synthesis of microbial-derived metabolites exceeding host requirements. This metabolic activity improves nutrient bioavailability and partitioning [
49,
50]. In the present study, 16S rRNA sequencing revealed the beneficial roles of microbiota in the intestinal health of LMB fed with 10 and 30 g/kg YC. Compared with the CON fish, PD whole tree of the intestinal microflora was increased in the YC10 fish, while the Chao 1, Shannon and Simpson indices were increased in both the YC10 and YC30 fish, suggesting that dietary YC inclusion increased both bacterial richness and diversity in LMB intestine, which is similar to the results of Zhou et al. [
51].
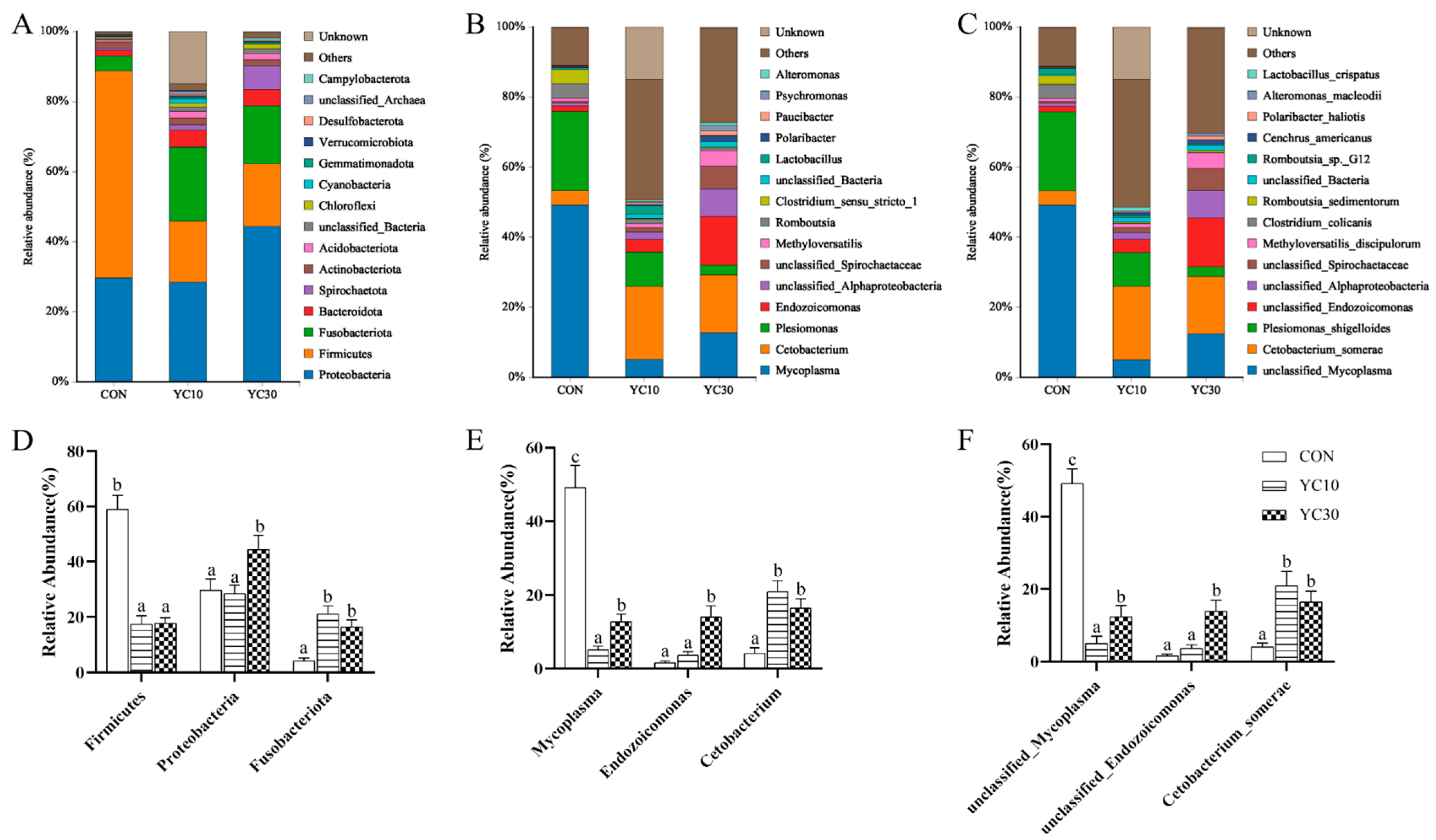
Proteobacteria,
Fusobacteria, and
Firmicutes dominated the microbiota in the LMB intestine in this study. The relative abundance of
Mycoplasma increases when the host is suffering from metabolic disorders or diseases [
52,
53]. In this experiment, the decrease in
Mycoplasma drove the dramatic change in
Firmicutes abundance, while the increase in
Cetobacterium (specifically
C. somerae) abundance explained
Fusobacteria changes in the gut microbiota of the YC-supplemented fish compared with the CON fish.
C. somerae abundance is positively correlated with the production of acetic acid, propionic acid, and vitamin B12 in the body [
54].
Endozoicomonas could not only act as the center of protein transformation and production that could be beneficial for the fish [
55] but also play a role in the host health or disease, such as competitive exclusion of pathogenic bacteria [
56,
57]. In this study, the abundance of
Endozoicomonas was increased in the YC30 fish compared with the CON fish, which was the main reason for the Proteobacteria changes. These findings demonstrate that dietary YC supplementation not only enhances nutrient production/absorption via metabolite synthesis but also inhibits detrimental microbiota proliferation, ultimately improving digestive efficiency and growth performance in LMB.