Development of a Multiplex Real-Time PCR Assay for the Detection of Eight Pathogens Associated with Bovine Respiratory Disease Complex from Clinical Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Sample Collection
2.2. Primer and Probe Design
2.3. Design Multiplex qPCR Assay
2.4. Optimization of Reaction Conditions for Multiplex Fluorescence Quantitative PCR
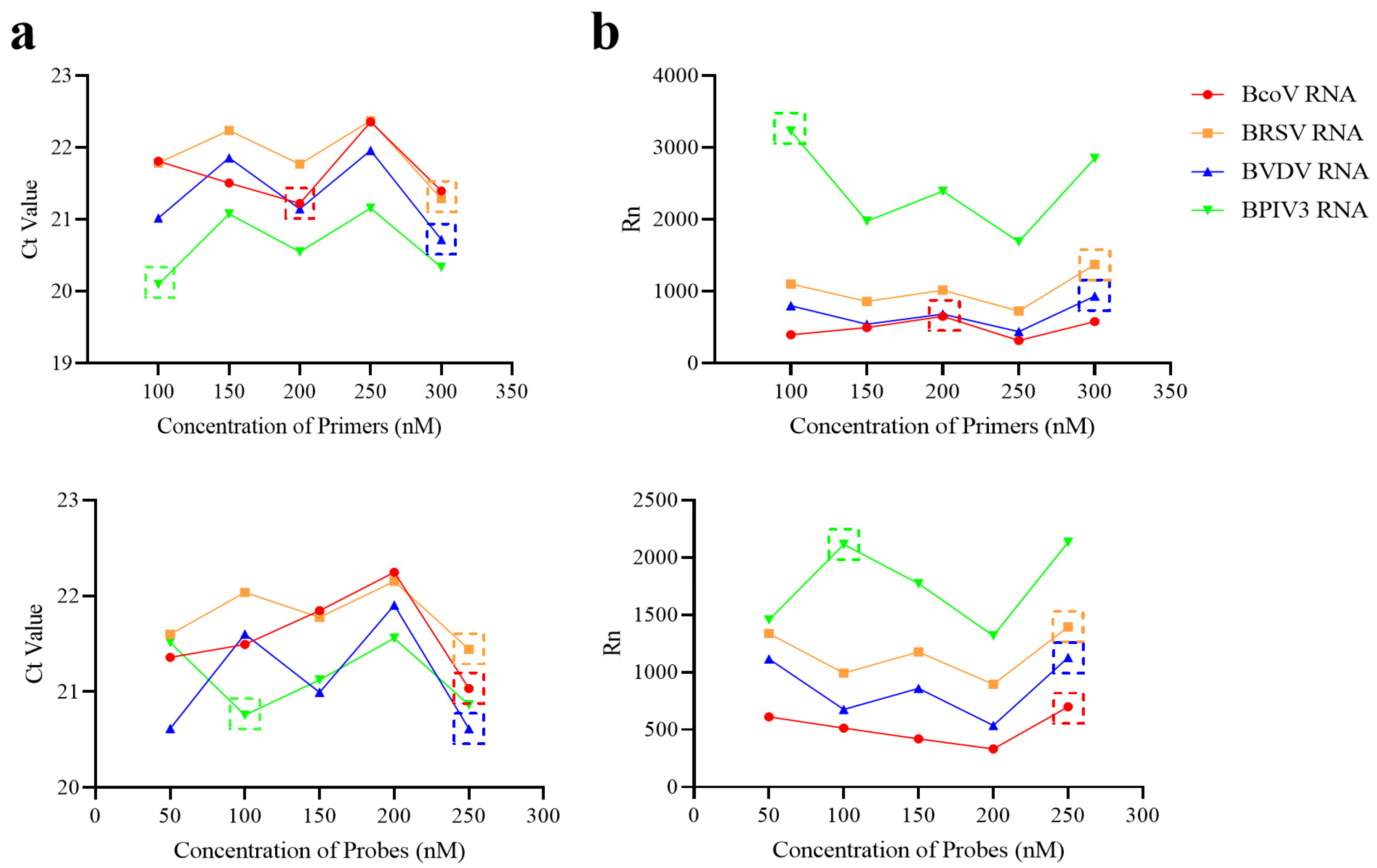
2.4.1. Optimization of Primer and Probe Concentrations in Two Reaction Pools
2.4.2. Determination of Optimal Annealing Temperature
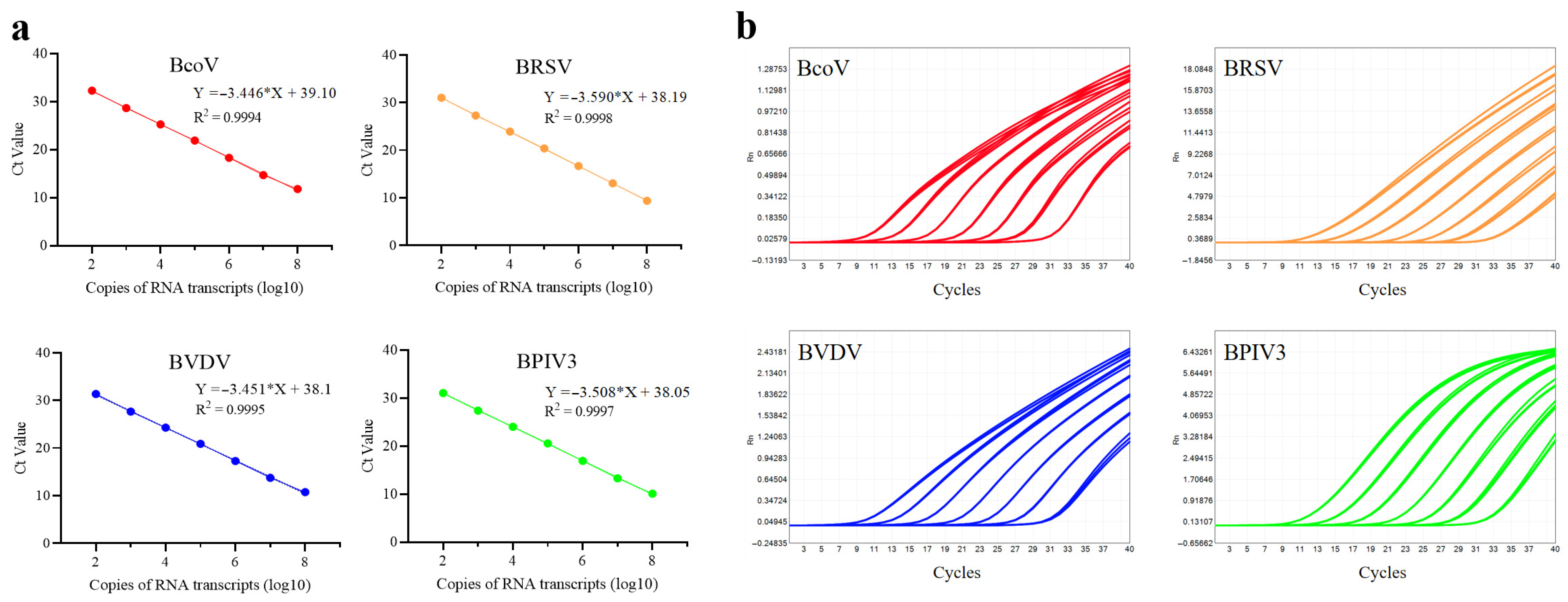
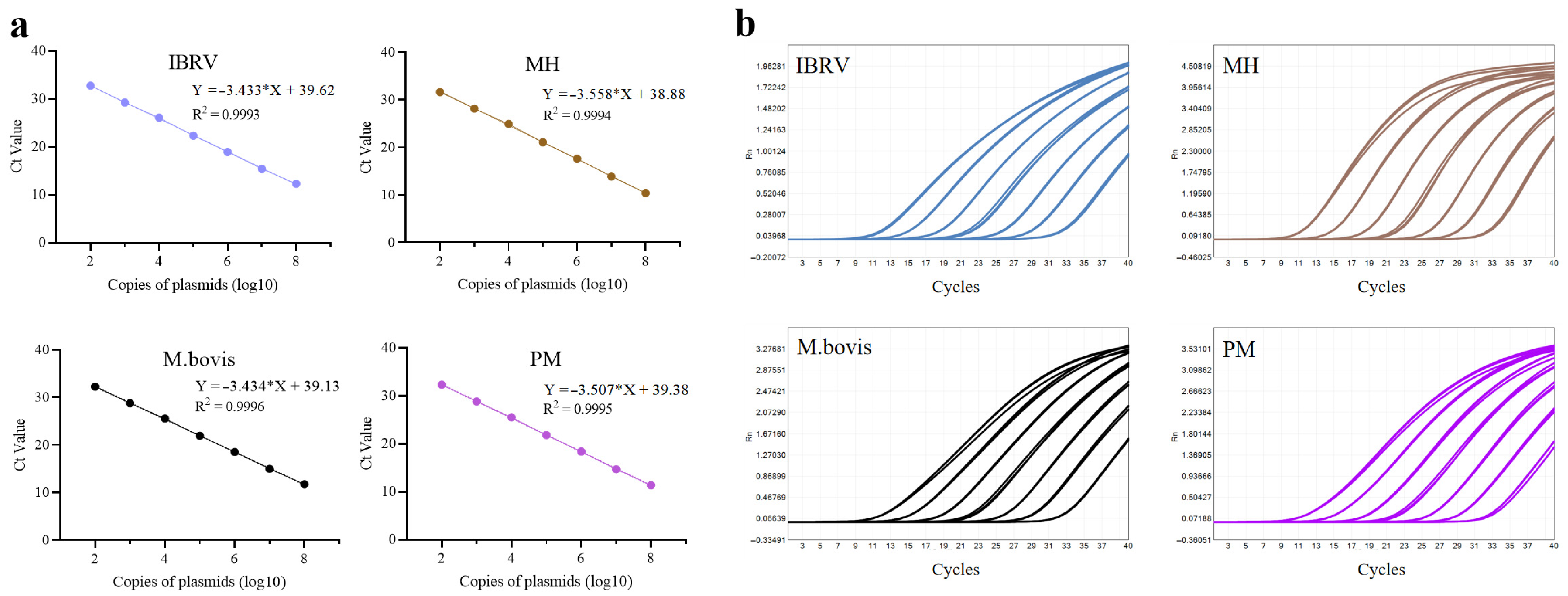
2.4.3. Development of Standard Curves for Eight Target Pathogens
2.5. Analytical Performance Evaluation
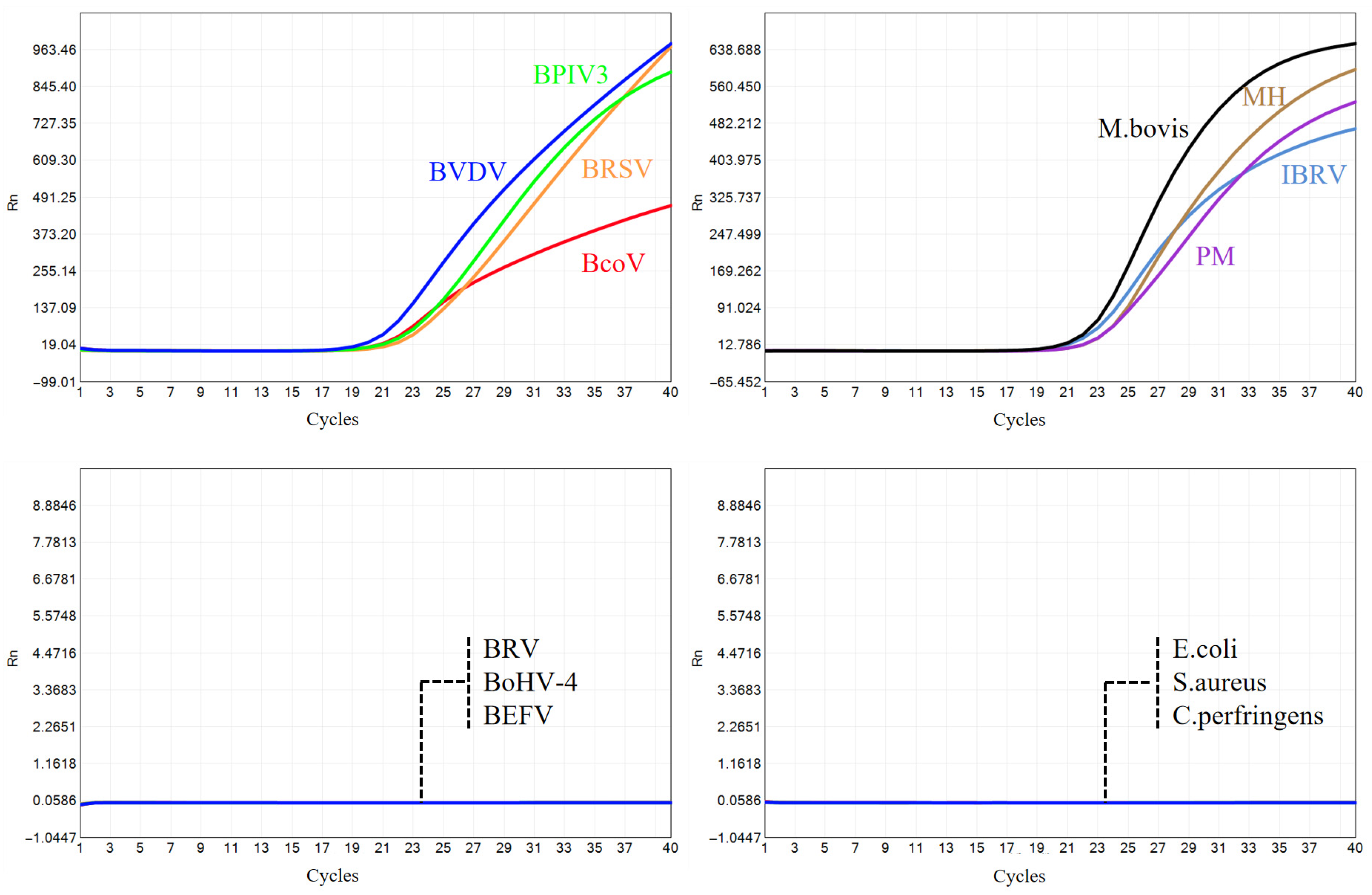
2.5.1. Specificity Testing
2.5.2. Sensitivity Testing
2.5.3. Reproducibility Testing
2.6. Analysis of Real-World Clinical Samples
2.7. Statistical Analysis
3. Results
3.1. Establishment of Multiplex qPCR Method
3.1.1. Validation of Primer Pool Performance
3.1.2. Optimization of Primer and Probe Concentrations in Primer Pools
3.1.3. Optimal Annealing Temperature
3.1.4. Construction of Standard Curves
3.2. Specificity Tests
3.3. Sensitivity Tests
3.4. Reproducibility Tests
3.5. Clinical Sample Testing
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRDC | Bovine respiratory disease complex |
| BVDV | Bovine viral diarrhea virus |
| BPIV3 | Bovine parainfluenza virus type 3 |
| BRSV | Bovine respiratory syncytial virus |
| BcoV | Bovine coronavirus |
| M.bovis | Mycoplasma bovis |
| PM | Pasteurella multocida |
| MH | Mannheimia haemolytica |
| IBRV | Infectious bovine rhinotracheitis virus |
| qPCR | Quantitative polymerase chain reaction |
| LOD | Limit of detection |
| CV | Coefficient of variation |
References
- Fulton, R.W. Bovine Respiratory Disease Research (1983–2009). Anim. Health Res. Rev. 2009, 10, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Shallangwa, B.Z.; González Gordon, L.; Castro, L.E.H.; Cook, E.A.J.; de Clare Bronsvoort, B.M.; Kelly, R.F. The Epidemiology of Bovine Viral Diarrhea Virus in Low-and Middle-Income Countries: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2022, 9, 947515. [Google Scholar] [CrossRef] [PubMed]
- Raaperi, K.; Orro, T.; Viltrop, A. Epidemiology and Control of Bovine Herpesvirus 1 Infection in Europe. Vet. J. 2014, 201, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Tang, C.; Yue, H. Prevalence and Molecular Characterization of Bovine Parainfluenza Virus Type 3 in Cattle Herds in China. Animals 2023, 13, 793. [Google Scholar] [CrossRef]
- Makoschey, B.; Berge, A.C. Review on Bovine Respiratory Syncytial Virus and Bovine Parainfluenza-Usual Suspects in Bovine Respiratory Disease-a Narrative Review. BMC Vet. Res. 2021, 17, 261. [Google Scholar] [CrossRef]
- Geng, H.L.; Meng, X.Z.; Yan, W.L.; Li, X.M.; Jiang, J.; Ni, H.B.; Liu, W.H. Prevalence of Bovine Coronavirus in Cattle in China: A Systematic Review and Meta-Analysis. Microb. Pathog. 2023, 176, 106009. [Google Scholar] [CrossRef]
- Gershwin, L.J.; Van Eenennaam, A.L.; Anderson, M.L.; McEligot, H.A.; Shao, M.X.; Toaff-Rosenstein, R.; Taylor, J.F.; Neibergs, H.L.; Womack, J. Bovine Respiratory Disease Complex Coordinated Agricultural Project Research Team Single Pathogen Challenge with Agents of the Bovine Respiratory Disease Complex. PLoS ONE 2015, 10, e0142479. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, Z.; Dai, G.; Li, X.; Xiang, Y.; Jiang, S.; Zhang, Z.; Ren, Y.; Zhu, Z.; Fan, C.; et al. Pathogenic Infection Characteristics and Risk Factors for Bovine Respiratory Disease Complex Based on the Detection of Lung Pathogens in Dead Cattle in Northeast China. J. Dairy Sci. 2023, 106, 589–606. [Google Scholar] [CrossRef]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial Pathogens of the Bovine Respiratory Disease Complex. Vet. Clin. N. Am. Food. Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef]
- Bell, R.L.; Turkington, H.L.; Cosby, S.L. The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines 2021, 9, 337. [Google Scholar] [CrossRef]
- Srikumaran, S.; Kelling, C.L.; Ambagala, A. Immune Evasion by Pathogens of Bovine Respiratory Disease Complex. Anim. Health. Res. Rev. 2007, 8, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Horwood, P.F.; Schibrowski, M.I.; Fowler, E.V.; Gibson, J.S.; Barnes, T.S.; Mahony, T.J. Is Mycoplasma Bovis a Missing Component of the Bovine Respiratory Disease Complex in Australia? Aust. Vet. J. 2014, 92, 185–191. [Google Scholar] [CrossRef]
- Jamali, H.; Rezagholipour, M.; Fallah, S.; Dadrasnia, A.; Chelliah, S.; Velappan, R.D.; Wei, K.S.C.; Ismail, S. Prevalence, Characterization and Antibiotic Resistance of Pasteurella Multocida Isolated from Bovine Respiratory Infection. Vet. J. 2014, 202, 381–383. [Google Scholar] [CrossRef]
- Chai, J.; Capik, S.F.; Kegley, B.; Richeson, J.T.; Powell, J.G.; Zhao, J. Bovine Respiratory Microbiota of Feedlot Cattle and Its Association with Disease. Vet. Res. 2022, 53, 4. [Google Scholar] [CrossRef]
- Feider, L.A.; Stapley, E.D.; Burton, A.J. Unusual Diagnosis in a Recently Transported 11-Week-Old Dairy Calf with Acute Bovine Respiratory Disease in North America. J. Am. Vet. Med. Assoc. 2025, 263, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Tolone, M.; Jemaa, S.B.; Sottile, G.; Gerlando, R.D.; Cortés, O.; Senczuk, G.; Portolano, B.; Pilla, F.; Ciani, E. Refining the Genetic Structure and Relationships of European Cattle Breeds through Meta-Analysis of Worldwide Genomic SNP Data, Focusing on Italian Cattle. Sci. Rep. 2020, 10, 14522. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, J.; Chen, X.; Wei, X.; Wu, C.; Cui, Q.; Hao, Y. Investigation of Viral Pathogens in Cattle with Bovine Respiratory Disease Complex in Inner Mongolia, China. Microb. Pathog. 2021, 153, 104594. [Google Scholar] [CrossRef]
- Buczinski, S.; Pardon, B. Bovine Respiratory Disease Diagnosis: What Progress Has Been Made in Clinical Diagnosis? Vet. Clin. N. Am. Food. Anim. Pract. 2020, 36, 399–423. [Google Scholar] [CrossRef]
- Kamel, M.S.; Davidson, J.L.; Verma, M.S. Strategies for Bovine Respiratory Disease (BRD) Diagnosis and Prognosis: A Comprehensive Overview. Animals 2024, 14, 627. [Google Scholar] [CrossRef]
- Li, N.; Cai, Q.; Miao, Q.; Song, Z.; Fang, Y.; Hu, B. High-Throughput Metagenomics for Identification of Pathogens in the Clinical Settings. Small Methods 2021, 5, 2000792. [Google Scholar] [CrossRef]
- Clark, T.W.; Lindsley, K.; Wigmosta, T.B.; Bhagat, A.; Hemmert, R.B.; Uyei, J.; Timbrook, T.T. Rapid Multiplex PCR for Respiratory Viruses Reduces Time to Result and Improves Clinical Care: Results of a Systematic Review and Meta-Analysis. J. Infect. 2023, 86, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Zhu, X.; Huang, Y.; Yuan, W.; Wang, Z.; Zhu, H.; Jia, H. Development of a Multiplex RT-qPCR Method for the Identification and Lineage Typing of Porcine Reproductive and Respiratory Syndrome Virus. Int. J. Mol. Sci. 2024, 25, 13203. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- GB/T 18637-2018; Technical Specifications for Diagnosis of Bovine Viral Diarrhea/Mucosal Disease. Standards Press of China: Beijing, China, 2018.
- Liu, L.; Wang, J.; Li, R.; Zhang, X.; Sun, X.; Wang, J. Establishment and Preliminary Application of a Multiplex Real-Time PCR Platform for Detection of Common Pathogens Causing BRDC. Chin. Vet. Sci./Zhongguo Shouyi Kexue 2025, 55, 86–93. [Google Scholar] [CrossRef]
- GB/T 27530-2025; Diagnostic Techniques for Bovine Haemorrhagic Septicaemia. Standards Press of China: Beijing, China, 2025.
- GB/T 27981-2011; Real-time PCR Method for the Detection of Infectious Bovine Rhinotracheitis Virus. Standards Press of China: Beijing, China, 2011.
- Ferraro, S.; Fecteau, G.; Dubuc, J.; Francoz, D.; Rousseau, M.; Roy, J.P.; Buczinski, S. Scoping Review on Clinical Definition of Bovine Respiratory Disease Complex and Related Clinical Signs in Dairy Cows. J. Dairy Sci. 2021, 104, 7095–7108. [Google Scholar] [CrossRef] [PubMed]
- Stokdyk, J.P.; Firnstahl, A.D.; Spencer, S.K.; Burch, T.R.; Borchardt, M.A. Determining the 95% Limit of Detection for Waterborne Pathogen Analyses from Primary Concentration to qPCR. Water Res. 2016, 96, 105–113. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, J.; Li, Z.; Zhang, A.; Cheng, Y. Establishment and Application of a Rapid Diagnostic Method for BVDV and IBRV Using Recombinase Polymerase Amplification-Lateral Flow Device. Front. Vet. Sci. 2024, 11, 1360504. [Google Scholar] [CrossRef]
- Mohamady, R.S.E.; Behour, T.S.; Rawash, Z.M. Concurrent Detection of Bovine Viral Diarrhoea Virus and Bovine Herpesvirus-1 in Bulls’ Semen and Their Effect on Semen Quality. Int. J. Vet. Sci. Med. 2020, 8, 106–114. [Google Scholar] [CrossRef]
- Drain, P.K. Point-of-Care Diagnostics (POCD) in Resource-Limited Settings. Diagnostics 2024, 14, 1926. [Google Scholar] [CrossRef]
- Horwood, P.F.; Mahony, T.J. Multiplex Real-Time RT-PCR Detection of Three Viruses Associated with the Bovine Respiratory Disease Complex. J. Virol. Methods 2011, 171, 360–363. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing Technologies-the next Generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global Trends in Antimicrobial Resistance in Animals in Low- and Middle-Income Countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- Renault, V.; Damiaans, B.; Humblet, M.F.; Jiménez Ruiz, S.; García Bocanegra, I.; Brennan, M.L.; Casal, J.; Petit, E.; Pieper, L.; Simoneit, C.; et al. Cattle Farmers’ Perception of Biosecurity Measures and the Main Predictors of Behaviour Change: The First European-Wide Pilot Study. Transbound Emerg. Dis. 2021, 68, 3305–3319. [Google Scholar] [CrossRef] [PubMed]
- Drewe, J.A.; Snary, E.L.; Crotta, M.; Alarcon, P.; Guitian, J. Surveillance and Risk Assessment for Early Detection of Emerging Infectious Diseases in Livestock. Rev. Sci. Tech. 2023, 42, 120–127. [Google Scholar] [CrossRef]
- Szenci, O.; Sassi, G.; Fodor, L.; Molnár, L.; Szelényi, Z.; Tibold, J.; Mádl, I.; Egyed, L. Co-Infection with Bovine Herpesvirus 4 and Histophilus Somni Significantly Extends the Service Period in Dairy Cattle with Purulent Vaginal Discharge. Reprod. Domest. Anim. 2016, 51, 143–149. [Google Scholar] [CrossRef]
- Andrés-Lasheras, S.; Zaheer, R.; Jelinski, M.; McAllister, T.A. Role of Biofilms in Antimicrobial Resistance of the Bacterial Bovine Respiratory Disease Complex. Front. Vet. Sci. 2024, 11, 1353551. [Google Scholar] [CrossRef]




| Primer/Probe 1 | Target Gene | Sequences (5′–3′) | Reference Strain Genbank No. |
|---|---|---|---|
| BVDV-F | 5′ UTR | AGCCATGCCCTTAGTAGGACTA | PP992316.2 |
| BVDV-R | GTCCACGTGGCATCTCGA | ||
| BVDV-P | FAM-CGACTACCCTGTACTCAG-MGB | ||
| BPIV3-F | N | TTTAGACAAGACGGGACAGTC | HQ530153.1 |
| BPIV3-R | TGCTGAGACCTCATGATCGCT | ||
| BPIV3-P | VIC-AATCCAGTCTGGTATTAAGTG-MGB | ||
| BRSV-F | N | AAGGAATCTTTGCAGGGTTATTC | MN316653.1 |
| BRSV-R | CTGGCATGACCAAGCATAATGT | ||
| BRSV-P | ROX-TGAATGCATATGGAGCAGGT-MGB | ||
| BcoV-F | N | ACCCAAGTAGCGATGAGGC | MK903505.1 |
| BcoV-R | AGGAGCAGACCTTCCTGAGC | ||
| BcoV-P | Cy5-TGGCACGGTACTCCCTCAG-BHQ2 | ||
| M.bovis-F | oppD | CTTTGCCTTAGAAATTGACTATGAA | CP045797.1 |
| M.bovis-R | GTCTTTGTATTTTTGGTGCATCAG | ||
| M.bovis-P | FAM-AGTCATCATAAAGCAGCAACG-MGB | ||
| PM-F | kmt1 | GACATTACTGCTCTATCCGCTATT | CP003313.1 |
| PM-R | CCCACTCACAACGAGCCATA | ||
| PM-P | VIC-CCATTTCCCATTTCAAGTGG-MGB | ||
| MH-F | lktA | CGACCGAGTTCACTATAGCCGT | JQ423930.1 |
| MH-R | CGATTTACGGTATAACTACCTTGCTC | ||
| MH-P | ROX-CTTTAACTATTGATGCAACCA-MGB | ||
| IBRV-F | gB | GGCGGACGAAATGCTGCG | MK654723.1 |
| IBRV-R | GTGTGGCTGTCGCTCACA | ||
| IBRV-P | Cy5-TTCCGCTTCACGGCCCGCTCGC-BHQ2 |
| Primer Pool | Reagent 1 | Volume/Reaction |
|---|---|---|
| Primer Pool A | 2 × Hifair® Ⅲ P buffer | 10 μL |
| Hifair® UH Ⅲ Enzymes | 1 μL | |
| BVDV-F/BVDV-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| BVDV-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| BPIV3-F/BPIV3-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| BPIV3-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| BRSV-F/BRSV-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| BRSV-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| BcoV-F/BcoV-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| BcoV-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| RNA template | 3 μL | |
| nucleic acid-free water | Up to 20 μL | |
| Primer Pool B | 2 × Taq Pro HS U+ Probe Master Mix | 10 μL |
| M.bovis-F/M.bovis-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| M.bovis-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| PM-F/PM-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| PM-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| MH-F/MH-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| MH-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| IBRV-F/IBRV-R | 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL or 0.6 μL | |
| IBRV-P | 0.1 μL or 0.2 μL or 0.3 μL or 0.4 μL or 0.5 μL | |
| plasmid template | 3 μL | |
| nucleic acid-free water | Up to 20 μL |
| Nucleic Acid Template | Primer Pool A | Primer Pool B | ||||||
|---|---|---|---|---|---|---|---|---|
| BVDV | BPIV3 | BRSV | BcoV | M.bovis | PM | MH | IBRV | |
| BVDV RNA | 19.57 1 | − | − | − | − | − | − | − |
| BPIV3 RNA | − | 20.11 | − | − | − | − | − | − |
| BRSV RNA | − | − | 19.78 | − | − | − | − | − |
| BcoV RNA | − | − | − | 20.53 | − | − | − | − |
| M.bovis plasmid | − | − | − | − | 20.79 | − | − | − |
| PM plasmid | − | − | − | − | − | 21.82 | − | − |
| MH plasmid | − | − | − | − | − | − | 20.58 | − |
| IBRV plasmid | − | − | − | − | − | − | − | 21.42 |
| Mixture of eight Nucleic Acids 2 | 19.66 | 20.21 | 20.05 | 20.69 | 20.81 | 21.81 | 20.64 | 21.31 |
| Primer Pool | Template | Positive Rates (%) | |||
|---|---|---|---|---|---|
| (10 Copies/μL) | (5 Copies/μL) | (1 Copy/μL) | (0.5 Copies/μL) | ||
| A | BVDV RNA | 100 | 100 | 50 | 12.5 |
| BPIV3 RNA | 100 | 100 | 37.5 | 0 | |
| BRSV RNA | 100 | 100 | 37.5 | 12.5 | |
| BcoV RNA | 100 | 100 | 62.5 | 12.5 | |
| B | M.bovis plasmid | 100 | 100 | 25 | 12.5 |
| PM plasmid | 100 | 100 | 25 | 25 | |
| MH plasmid | 100 | 100 | 37.5 | 12.5 | |
| IBRV plasmid | 100 | 100 | 12.5 | 0 | |
| Primer Pool | Template | Copies of Template (Copies/μL) | Ct Value 1 | CI 2 | CVs (%) | |
|---|---|---|---|---|---|---|
| Inter-Assay | Intra-Assay | |||||
| A | BVDV RNA | 105 | 21.11 ± 0.05 | [21.09, 21.13] | 0.22 | 0.30 |
| BPIV3 RNA | 105 | 20.63 ± 0.07 | [20.59, 20.67] | 0.36 | 0.63 | |
| BRSV RNA | 105 | 20.35 ± 0.07 | [20.31, 20.38] | 0.37 | 0.76 | |
| BcoV RNA | 105 | 22.10 ± 0.04 | [22.08, 22.12] | 0.20 | 0.36 | |
| BVDV RNA | 102 | 31.37 ± 0.10 | [31.32, 31.42] | 0.32 | 0.53 | |
| BPIV3 RNA | 102 | 31.05 ± 0.12 | [30.98, 31.11] | 0.40 | 0.58 | |
| BRSV RNA | 102 | 30.84 ± 0.15 | [30.77, 30.91] | 0.49 | 0.91 | |
| BcoV RNA | 102 | 32.40 ± 0.12 | [32.35, 32.46] | 0.36 | 0.48 | |
| B | M.bovis plasmid | 105 | 22.06 ± 0.12 | [22.00, 22.12] | 0.54 | 0.94 |
| PM plasmid | 105 | 21.98 ± 0.14 | [21.92, 22.05] | 0.62 | 1.27 | |
| MH plasmid | 105 | 21.21 ± 0.10 | [21.15, 21.26] | 0.49 | 0.92 | |
| IBRV plasmid | 105 | 22.53 ± 0.08 | [22.49, 22.57] | 0.37 | 0.74 | |
| M.bovis plasmid | 102 | 32.40 ± 0.29 | [32.26, 32.55] | 0.88 | 1.58 | |
| PM plasmid | 102 | 32.37 ± 0.39 | [32.17, 32.56] | 1.19 | 2.00 | |
| MH plasmid | 102 | 31.82 ± 0.33 | [31.65, 31.99] | 1.05 | 1.52 | |
| IBRV plasmid | 102 | 32.95 ± 0.32 | [32.79, 33.11] | 0.97 | 1.79 | |
| Farm | Co-Infection Level | Co-Infected Pathogens (Details) 1 | Counts |
|---|---|---|---|
| A | Single infection | BcoV | 31 |
| BVDV | 28 | ||
| PM | 19 | ||
| MH | 10 | ||
| Dual infection | BcoV + PM | 33 | |
| BVDV + PM | 24 | ||
| BVDV + BcoV | 22 | ||
| PM + MH | 22 | ||
| Triple infection | BcoV + PM + MH | 18 | |
| BVDV + BcoV + PM | 9 | ||
| BVDV + BcoV + MH | 6 | ||
| BVDV + PM + MH | 4 | ||
| Quadruple infection | BVDV + BcoV + PM + MH | 16 | |
| B | Single infection | MH | 59 |
| IBRV | 6 | ||
| BcoV | 1 | ||
| PM | 1 | ||
| Dual infection | IBRV + MH | 15 | |
| BcoV + PM | 4 | ||
| BcoV + IBRV | 4 | ||
| Triple infection | BcoV + IBRV + PM | 2 | |
| BcoV + IBRV + MH | 1 |
| Reference Method No. | Pathogen | Kappa Test | Developed Method | Total | Sensitivity (%) | Specificity (%) | Agreement Rate (%) | |
|---|---|---|---|---|---|---|---|---|
| + | − | |||||||
| [24] | BVDV | + | 103 | 4 | 107 | 94.50 | 96.00 | 95.22 |
| − | 6 | 96 | 102 | |||||
| Total | 109 | 100 | 209 | |||||
| [25] | BcoV | + | 132 | 3 | 135 | 89.79 | 97.00 | 92.71 |
| − | 15 | 97 | 112 | |||||
| Total | 147 | 100 | 247 | |||||
| [26] | PM | + | 148 | 1 | 149 | 97.37 | 99.00 | 98.02 |
| − | 4 | 99 | 103 | |||||
| Total | 152 | 100 | 252 | |||||
| [25] | MH | + | 145 | 3 | 148 | 96.03 | 97.00 | 96.41 |
| − | 6 | 97 | 103 | |||||
| Total | 151 | 100 | 251 | |||||
| [27] | IBRV | + | 27 | 0 | 27 | 96.43 | 100 | 99.22 |
| − | 1 | 100 | 101 | |||||
| Total | 28 | 100 | 128 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, F.; Tao, C.; Xiao, R.; Huang, Y.; Yuan, W.; Wang, Z.; Jia, H. Development of a Multiplex Real-Time PCR Assay for the Detection of Eight Pathogens Associated with Bovine Respiratory Disease Complex from Clinical Samples. Microorganisms 2025, 13, 1629. https://doi.org/10.3390/microorganisms13071629
Hao F, Tao C, Xiao R, Huang Y, Yuan W, Wang Z, Jia H. Development of a Multiplex Real-Time PCR Assay for the Detection of Eight Pathogens Associated with Bovine Respiratory Disease Complex from Clinical Samples. Microorganisms. 2025; 13(7):1629. https://doi.org/10.3390/microorganisms13071629
Chicago/Turabian StyleHao, Fuxing, Chunhao Tao, Ruilong Xiao, Ying Huang, Weifeng Yuan, Zhen Wang, and Hong Jia. 2025. "Development of a Multiplex Real-Time PCR Assay for the Detection of Eight Pathogens Associated with Bovine Respiratory Disease Complex from Clinical Samples" Microorganisms 13, no. 7: 1629. https://doi.org/10.3390/microorganisms13071629
APA StyleHao, F., Tao, C., Xiao, R., Huang, Y., Yuan, W., Wang, Z., & Jia, H. (2025). Development of a Multiplex Real-Time PCR Assay for the Detection of Eight Pathogens Associated with Bovine Respiratory Disease Complex from Clinical Samples. Microorganisms, 13(7), 1629. https://doi.org/10.3390/microorganisms13071629






