Abstract
Our previous study reported that male university rugby players tended to have a gut with a dysbiotic environment, characterized by abundant pathobiont bacteria and an accumulation of succinate, when compared with age-matched, non-rugby playing healthy males. In the present study, we conducted a randomized, double-blinded, placebo-controlled experiment to evaluate the potential of blackcurrant extract and/or partially hydrolyzed guar gum (PHGG) to improve the gut environment of university rugby players. Participants were supplemented with blackcurrant extract and/or PHGG or a placebo for 4 weeks. Beneficial gut bacteria such as Megasphaera spp. tended to increase (p < 0.10) and Bifidobacterium spp. increased (p < 0.05) with the intake of blackcurrant extract and/or PHGG. A subgroup analysis further indicated that, unlike in those with a eubiotic gut environment, the dietary supplements also increased the number of beneficial gut bacteria such as Phascolarctobacterium spp. (p < 0.10) and Faecalibacterium spp. (p < 0.10) and fecal SCFA concentrations (p < 0.05) in participants with a possible dysbiotic gut environment. However, a synergistic effect between blackcurrant extract and PHGG was not clearly observed. Although further investigation is recommended, it was concluded that blackcurrant extract and PHGG can at least be used as functional materials to improve gut dysbiosis in university rugby players.
1. Introduction
Humans harbor dense and diverse microbial populations in their hindgut, and these gut microbes and their metabolites have been demonstrated to affect the various physiological functions of the host [1]. Therefore, the state of the gut microbiota and their metabolites seems to be associated with host health, including an abnormal state, known as dysbiosis, potentially leading to various gastrointestinal and systemic diseases [1]. Short-chain fatty acids (SCFAs) are well known to be key bacterial metabolites that contribute to regulating the absorption of water, minerals, and nutrients, inducing immune modulation, and mediating inflammation in the host hindgut [2,3,4]. Therefore, the concentrations of fecal SCFA are frequently regarded as indicators of gut health status. Indeed, a growing number of studies have reported that patients with gastrointestinal diseases, such as inflammatory bowel disease, show disturbances of the gut microbial population and significantly lower concentrations of fecal SCFA when compared with healthy controls [5,6,7,8].
Our previous study reported that male university rugby players tended to have a gut with a dysbiotic environment characterized by abundant pathobiont bacteria and an accumulation of succinate compared with age-matched, non-rugby-playing healthy males [9]. Although it was beyond the scope of the present study, a dysbiotic gut could have been attributed to an unbalanced diet and high-intensity exercise. Generally, to meet an optimal energy level and restore muscle and liver glycogen, athletes tend to consume large amounts of carbohydrates and protein while keeping the ingestion of dietary fiber low to prevent gastrointestinal disturbances such as gas and bloating [10,11]. However, such a dietary pattern is reported to reduce gut microbial diversity and functionality, leading to gut dysbiosis [10,11]. Exercise is also a factor affecting the gut environment. Although simple regular exercise has a positive effect on gut health [12,13], high-intensity exercise is reported to have an adverse effect on it. Specifically, high-intensity exercise can induce increased intestinal permeability and local inflammation resulting from ischemia–reperfusion, altered gut motility and transit, and site-specific oxidative stress, leading to bacterial translocation and an overgrowth of pathobiont bacteria [14,15,16].
In the present study, we focused on blackcurrant extract and partially hydrolyzed guar gum (PHGG) as candidates for functional materials to improve the gut environment of university rugby players. Blackcurrant contains abundant anthocyanins that are bioactive compounds with antioxidant and anti-inflammatory properties [17]. Studies have reported that blackcurrant anthocyanins exert beneficial effect on the host health via improvements to the gut microbial composition and SCFA production [18,19]. PHGG is a prebiotic dietary fiber obtained from the endosperm of the guar bean. Studies have reported that PHGG intake helps to increase the amount of beneficial butyrate-producing bacteria and promote SCFA production in animal and human guts [20,21,22]. Therefore, in the present work, we conducted a randomized, double-blinded, placebo-controlled experiment to evaluate the potential of blackcurrant extract and PHGG to improve the gut health of athletes.
2. Materials and Methods
2.1. Ethics Statements and Study Participants
The present study was registered in the UMIN Clinical Trial Registry (UMIN000044527) and approved by the ethical committee of Setsunan University, and conducted as per their guidelines (Approval Number: 2021-005, approval dates: 8 May 2021). Written informed consent was obtained from all participants. The enrollment of subjects and sample collection were conducted from June 2021 to August 2021.
A total of 106 male rugby players from Setsunan University were selected as participants based on following criteria: (1) healthy male belonging to the Setsunan university’s rugby team; (2) regularly participating in the team’s practices (at least 6 days per week, 3 h per day); and (3) with no previously diagnosed gastrointestinal disorders. The study was conducted in a randomized, double-blinded, placebo-controlled manner. The participants were randomly assigned into 4 groups: placebo group (PC, n = 26), blackcurrant group (BC, n = 27), guar gum group (GG, n = 27), and combination of blackcurrant and guar gum group (CO, n = 26) (Figure 1). Randomization was carried out with a permuted block method and group assignment to each block was conducted by a person who was unaware of the study details and backgrounds of the participants (Hiromi Ikeda, PhD at Setsunan University). The key (links between groups and blocks) was kept by H. Ikeda and blinded to all participants and researchers until all analyses were finalized.
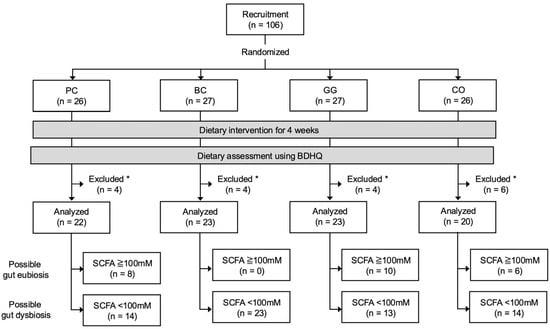
Figure 1.
Flow chart of study participants. PC, placebo group; BC, blackcurrant group; GG, guar gum group; CO, combination of blackcurrant and guar gum group; BDHQ, brief dietary history questionnaire; SCFA, short-chain fatty acid. * Participants who did not provide samples or questionnaires due to personal reasons were excluded from downstream analyses.
All participants were dietary-intervened for 4 weeks as follows. During the experiment, the PC group was supplemented with 6 g/d of maltodextrin as a placebo against PHGG (Sunfiber®; Taiyo Kagaku, Yokkaichi, Japan) [23] and 900 mg/d of placebo tablets (3 tablets) consisting of crystalline cellulose and maltitol as placebo blackcurrant extract [24]; the BC group was supplemented with 6 g/d of placebo powder and 900 mg/d of tablets containing 150 mg of blackcurrant extract; the GG group was supplemented with 6 g/d of PHGG powder and 900 mg/d of placebo tablets; and the CO group was supplemented with 6 g/d of PHGG powder and 900 mg/d of blackcurrant extract-containing tablet.
The participants collected their fecal samples with ad hoc scoop and container sets (Sarstedt K.K., Tokyo, Japan) at the beginning (week 0) and the end (week 4) of the experiment. The fecal samples were kept at 4 °C at all times and brought to the laboratory within 24 h after collection. After reception, the fecal samples were stored at −25 °C. Finally, a total of 88 participants (PC, n = 22; BC, n = 23; GG, n = 23; CO, n = 20) were included in the analyses in the study, effectively excluding those who did not provide samples or questionnaires due to personal reasons. For an additional subgroup analysis, 64 of 88 participants (PC, n = 14; BC, n = 23; GG, n = 13; CO, n = 14) were further identified as potentially having gut dysbiosis based on the concentrations of fecal SCFA at week 0 of the experiment (details in the Section 3).
2.2. Dietary Assessment
After the experiment, a survey regarding the dietary habits of participants was conducted using a brief dietary history questionnaire (BDHQ) [25] (Approval Number: 2021-029, approval dates: 15 September 2021). The BDHQ is a self-administered questionnaire that asks the frequency of consumption of foods commonly eaten in Japan so that daily energy and nutrient intake based on an algorithm that incorporates the nutrient composition of each food, derived from the Standard Tables of Food Composition in Japan, can be estimated. The BDHQ typically assesses dietary habits over the previous month. However, in the present study, participants were asked to recall and respond based on their dietary habits over the past three months, including the experiment period. In addition, participants were requested not to consider the blackcurrant and/or PHGG ingested during the experiment period in their response for the BDHQ.
2.3. Measurement of Fecal Organic Acid Concentrations
For the measurement of fecal organic acids, including acetate, propionate, iso-butyrate, butyrate, iso-valerate, valerate, succinate, lactate, and formate, 0.3 g of feces was mixed with 600 µL of distilled water and 90 µL of 14% perchloric acid, and centrifuged at 13,000× g for 10 min at 4 °C. The supernatants were filtered through 0.45 μm cellulose acetate membrane filters (Cosmonice Filter W, Nakalai Tesque, Kyoto, Japan) and degassed by vacuum. The resulting supernatants were subjected to organic acid measurement using a high-performance liquid chromatography apparatus with an SIL-10 autoinjector (Shimadzu, Kyoto, Japan), as previously described [9].
2.4. Analysis of the Fecal Microbiota
Fecal microbial DNA extraction, library preparation, and MiSeq sequencing were conducted as previously described [26]. Briefly, microbial DNA was extracted and purified from 25 mg of feces using the QuickGene DNA Tissue kit SII (KURABO, Osaka, Japan). Microbial DNA was then used to amplify the V3–V4 region of the 16S rRNA gene using the primer sets 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) [27]. The PCR steps were conducted according to the following program: initial denaturation at 95 °C for 3 min, followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and a final extension step at 72 °C for 5 min. Amplicons were purified using NucleoFast96 PCR plates (TaKaRa bio, Kusatsu, Japan) and then subjected to a second PCR with unique dual indices primer sets for MiSeq sequencing. The resulting amplicons were purified using a SequalPrep Normalization Plate Kit (Life Technologies, Tokyo, Japan) and AMPure XP beads (Beckman-Coulter, Brea, CA, USA) and pooled, followed by 285 bp paired-end sequencing on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) with MiSeq Reagent Kit v3. Raw sequences were deposited in the NCBI Sequence Read Archive under BioProject ID PRJNA1256317 (available from 1 May 2026).
Data obtained from MiSeq sequencing were analyzed using the QIIME2 version 2022.2 [28]. To construct amplicon sequence variants (ASVs), paired-end reads were denoised using DADA2 via the q2-dada2 plugin [29]. The taxonomic classification of ASVs was carried out using the Naive Bayes classifier via the q2-classifier sklearn plugin against the SILVA 138 99% reference dataset. Singletons and ASVs assigned to mitochondria and chloroplasts were removed from downstream analyses. A phylogenetic tree was generated by SATé-enabled phylogenetic placement (SEPP) [30]. Alpha diversity indices were calculated by QIIME2 by setting the sampling depth at 5000. Outputs from QIIME2 were further analyzed with the R program using the bioconductor packages of Phyloseq [31] and MicrobiotaProcess [32]. Beta diversities were calculated based on weighted and unweighted UniFrac distances.
2.5. Statistical Analysis
Fecal organic acid concentrations, microbial alpha-diversity indices, and relative abundances of bacterial taxa in the respective groups were compared between weeks 0 and 4 using a paired t-test. In the two-group comparison between participants with gut eubiosis and dysbiosis, fecal microbial alpha-diversity indices and relative abundances of bacterial taxa were compared using Welch’s t-test. In the multiple comparison between dietary groups, the statistical differences were first evaluated using a Kruskal–Wallis test and then a Steel–Dwass test. Differences in fecal microbial beta-diversity were analyzed using permutational multivariate analysis of variance (PERMANOVA) with 9999 permutations. p-values of <0.05 and <0.10 were considered to be statistically significant and with a tendency to be significant, respectively.
3. Results
3.1. Characteristics of Participants
A total of 88 participants (PC, n = 22; BC, n = 23; GG, n = 23; CO, n = 20) were included in the analyses of the study, excluding those who did not provide samples or questionnaires due to personal reasons (Figure 1). There were no significant differences in the participants’ age, height, weight, and body mass index between groups (Table 1). The macronutrient intake estimated by BDHQ is shown in Table S1.

Table 1.
Information of participants included in the analyses.
3.2. Effects of Blackcurrant Extract and PHGG Intake on Fecal Organic Acid Concentrations and the Microbiota
Fecal organic acid concentrations measured at weeks 0 and 4 of the experiment are shown in Table 2. During the experiment, total SCFA concentrations did not change in the PC, GG, and CO groups, but significantly increased from week 0 to week 4 in the BC group (p < 0.001), although the initial concentrations in the BC group were considerably lower than in the other groups. Regarding each organic acid, a significant decrease in iso-butyrate concentration was observed in the PC group (p < 0.05). Significant increases in all SCFAs and formate concentrations were observed in the BC group (p < 0.05). During the experiment, the propionate concentration showed a tendency to increase in the CO group (p < 0.10).

Table 2.
Effects of blackcurrant extract and/or PHGG intake on fecal organic acid concentrations.
Comparisons of fecal microbial alpha- and beta-diversities between weeks 0 and 4 are shown in Figure S1. During the experiment, Chao1 and Shannon indices did not change, irrespective of groups (Figure S1A). PCoA plots based on weighted UniFrac distances also did not show significant differences in the bacterial community structures between weeks 0 and 4, irrespective of groups (Figure S1B).
Bacterial taxa that showed statistical differences in the relative abundances between weeks 0 and 4 are shown in Table 3. The relative abundances of 10, 3, 6, and 9 bacterial taxa showed statistically significant changes in PC, BC, GG, and CO groups, respectively. For example, the relative abundance of Megasphaera spp. tended to increase (p < 0.10) from week 0 to week 4 in BC and GG groups and that of Bifidobacterium spp. significantly increased (p < 0.05) in the CO group.

Table 3.
Effects of blackcurrant extract and/or PHGG intake on fecal bacterial composition. Symbols “↑” and “↓” mean increase and decrease, respectively.
3.3. Identification of Participants with Gut Dysbiosis
Based on the results from the measurement of organic acids (Section 3.2, Table 2), with the exception of the BC group, the effects of the dietary interventions were less apparent. Increased SCFA concentrations were observed only in the BC group, which encompassed many participants with lower concentrations at week 0. Thus, we speculated that dietary interventions affected more participants with dysbiotic gut environments than with eubiotic gut environments. Therefore, to further evaluate the effects of the dietary interventions, a subgroup analysis focusing on participants with gut dysbiosis was conducted.
To identify the participants with gut dysbiosis, we grouped the participants with total fecal SCFA concentrations > 100 mM (n = 24) and <100 mM (n = 64) at baseline. This was carried out according to a meta-analysis report by Xu et al. [8], demonstrating that the total fecal SCFA concentrations of ulcerative colitis patients with gut dysbiosis ranged within 35–100 mM. We compared the bacterial diversity and composition between the above two groups and observed typical gut dysbiotic characteristics in participants with total fecal SCFA concentrations < 100 mM (details in Supplementary Text S1; Figure S2; Tables S2 and S3). Based on the results, at least in the present work, participants with total fecal SCFA concentrations > 100 mM and <100 mM were classified as participants with possible gut eubiosis and dysbiosis, respectively. Detailed differences in gut microbiota and dietary habits between these two groups are discussed in the Supplementary Text S1 because it was beyond the scope of the present study.
3.4. Subgroup Analysis of the Effects of Blackcurrant Extract and/or PHGG Intake on the Fecal SCFA Concentrations in Participants with Possible Gut Dysbiosis
The fecal organic acid concentrations of the participants with possible gut dysbiosis are shown in Table 4. During the experiment, the total SCFA did not change in the PC group but significantly increased in the BC (p < 0.001) and CO (p < 0.05) groups, and tended to increase in GG (p < 0.10). Regarding each organic acid, significant increases (p < 0.05) of all SCFAs and formate concentrations were observed in the BC group, and a tendency of the propionate concentration to increase (p < 0.10) was observed in the GG group. In addition, a significant increase in propionate (p < 0.05) and a tendency of the acetate concentration to increase (p < 0.10) were observed in the CO group.

Table 4.
Subgroup analysis of the effect of blackcurrant extract and/or PHGG intake on fecal organic acid concentrations, focusing on participants with possible gut dysbiosis.
3.5. Subgroup Analysis of the Effects of Blackcurrant Extract and PHGG Intake on the Fecal Microbiota in Participants with Possible Gut Dysbiosis
Results of alpha- and beta-diversity analyses in participants with possible gut dysbiosis are shown in Figure S3. There were no significant differences in any of the diversity analyses between weeks 0 and 4, irrespective of groups.
The relative abundances of each bacterial taxa in the respective groups were compared between weeks 0 and 4 (Table 5). In the PC group, while significant decreases were observed in the relative abundances of the Escherichia/Shigella group (p < 0.05), as well as tendencies to decrease in the abundances of Blautia spp. (p < 0.10), Phascolarctobacterium spp. (p < 0.10), and the putative Clostridium innocuum group (p < 0.10), tendencies to increase in the abundances of Megamonas spp (p < 0.10) and Flavonifracter spp. (p < 0.10) were detected. In the BC group, from week 0 to week 4, the relative abundances of Megasphaera spp. and Phascolarctobacterium spp. tended to increase (p < 0.10), while that of Subdoligranulum spp. significantly decreased (p < 0.05). In the GG group, while the relative abundances of Faecalibacterium spp. and Veillonella spp. tended to increase (p < 0.10), the relative abundance of the Eubacterium coprostanoligenes group tended to decrease (p < 0.10) and Tyzzerella spp. (p < 0.05) significantly decreased. In the CO group, from week 0 to week 4, while the relative abundance of unclassified Enterobacteriaceae tended to increase (p < 0.10), the relative abundances of unclassified Lachnospiraceae (p < 0.10), putative Ruminococcus torques group (p < 0.1), Lachnoclostridium spp. (p < 0.10), and Lachnospira spp. (p < 0.10) tended to decrease, and Blautia spp. (p < 0.05), Fusicatenibacter spp. (p < 0.05), the Lachnospiraceae ND 3007 group (p < 0.05), and Lachnospiraceae UCG-004 (p < 0.05) were observed to decrease.

Table 5.
Subgroup analysis of the effects of blackcurrant extract and/or PHGG intake on fecal microbiota, focusing on participants with possible gut dysbiosis. Symbols “↑” and “↓” mean increase and decrease, respectively.
4. Discussion
Our previous study reported that male university rugby players tended to experience gut dysbiosis [9]. In the present study, we evaluated blackcurrant extract and/or PHGG as functional materials to improve the gut environment of male university rugby players. First, we assessed the effects of blackcurrant extract and/or PHGG intake on the gut environment based on the results of all 88 participants. A microbiota analysis showed that dietary interventions did not alter the overall bacterial community structure, but did alter the relative abundance of some bacterial taxa. For example, an increase in Megasphaera spp., a beneficial microbe that produces butyrate, was observed in the BC and GG groups. In the CO group, Bifidobacterium spp., which is known to be a beneficial, acetate-producing microbe, increased. Although we observed an increased propionate concentration in the CO group, the total fecal SCFA concentration increased only in the BC group but not in the GG and CO groups. Participants in the BC group had considerably lower SCFA concentrations at week 0 compared with those of other groups. Therefore, we speculated that dietary interventions affected participants with a dysbiotic gut environment more than those with a eubiotic gut environment. We then attempted to conduct a subgroup analysis focusing on participants with gut dysbiosis to further evaluate the effects of the dietary interventions.
Gut dysbiosis is not quantitatively defined but is characterized by typical features such as decreased bacterial diversity, increased harmful bacteria, decreased beneficial bacteria, and decreased SCFA concentrations [8,33,34,35]. In the present study, we attempted to identify participants with possible gut dysbiosis based not on alpha-diversity indices but on fecal SCFA concentrations, because there is not an absolute scale; values tend to vary depending on several factors, such as the data analysis pipeline, and hence it is hard to establish a threshold based on published research. Xu et al. [8] reported that the total fecal SCFA concentrations of ulcerative colitis patients with gut dysbiosis ranged within 35–100 mM based on the meta-analysis of 11 studies. Indeed, in the present study, participants with total fecal SCFA < 100 mM also exhibited typical characteristics of gut dysbiosis (see Section 3.3). Hence, at least in the present work, participants with total fecal SCFA concentrations > 100 mM and <100 mM were classified as participants with possible gut eubiosis and dysbiosis, respectively. It should be noted that, in the present study, between participants with eubiotic and dysbiotic guts, there were differences in micronutrient intake, including several vitamins and minerals. The relation of these micronutrients to gut dysbiosis is of interest, and thus further investigation is needed to clarify this relationship.
For participants with possible gut dysbiosis, blackcurrant extract and/or PHGG intake significantly increased fecal SCFA concentrations, regardless of whether they were ingested solely or together. Specifically, the intake of blackcurrant and/or PHGG increased the propionate concentrations. This result suggests that blackcurrant extract and/or PHGG intake have different impacts on the dysbiotic gut microbiota. Because the intervention did not drastically alter the overall gut bacterial community structure, it is possible that blackcurrant extract and/or PHGG affected the metabolic activity of gut microbiota rather than inducing major shifts in microbial composition and increased SCFA concentrations. Further research is needed to clarify the underlying mechanisms.
Below, we discuss the bacterial taxa that changed in participants with possible gut dysbiosis. For those in the BC group, increases in Megasphaera spp. and Phascolarctobacterium spp. were observed. The former and latter consume lactate and succinate, respectively, and produce acetate and propionate [36,37]. Although the mechanism has not yet been clarified, increases in these bacteria by ingesting plant-derived anthocyanins were in line with previous reports [38]. Increases in these bacteria may be a possible cause of increased fecal acetate and propionate in the BC group. In addition, the accumulation of lactate and succinate is occasionally seen in athletes with gut dysbiosis, and it can lead to gut inflammation and diarrhea [9,16]. Although in the present study we did not observe an accumulation of lactate and succinate, increases in Megasphaera spp. and Phascolarctobacterium spp. seem to be beneficial for the gut health of athletes.
Similarly, for the participants with possible gut dysbiosis in the GG group, the relative abundance of Veillonella spp., which consume lactate and succinate and produce acetate and propionate [37], increased from weeks 0 to 4. In addition, PHGG intake increased the relative abundance of Faecalibacterium spp., as reported in a previous work [39]. Faecalibacterium spp. have been known to produce acetate and butyrate and have anti-inflammatory properties [40,41]. Numerical increases in acetate (1.26-fold) and butyrate (1.38-fold) were also observed in the GG group, although they were not statistically significant, probably due to large individual differences. Increases in Veillonella spp. and Faecalibacterium spp. due to PHGG intake can aid in improving gut dysbiosis via SCFA production.
In view of the CO group with possible gut dysbiosis, various uncharacterized Lachnospiraceae decreased. As genera belonging to Lachnospiraceae tend to produce SCFA, this change seems to be in conflict with the increase in fecal SCFA in this group. A detailed link between the taxonomical alteration in the gut microbiota and an increase in SCFA concentrations was beyond the scope of the present study, but, as discussed above, changes in the metabolic activity of the gut microbiota may be a possible mechanism explaining this contradiction. This point should be further investigated in the future using whole-genome shotgun metagenomics or metatranscriptomics techniques.
Regarding the synergistic effect of blackcurrant extract and PHGG, some unique effects that were not observed in the solo intake groups were observed in the combinational intake groups, regardless of gut dysbiosis (see the discussion above). However, when analyzed by two-way ANOVA, the statistical interaction was not confirmed for the beneficial changes observed, such as increases in SCFA concentrations and abundance of Bifidobacterium spp. Furthermore, additive effects such as the increases in butyrate, iso-butyrate, valerate, and iso-valerate observed in the BC group, along with increases in beneficial bacteria, were not observed even in the dysbiotic participants in the CO group. Although blackcurrant extract and PHGG are different types of functional ingredient—the former is an anthocyanin and the latter is a dietary fiber—their effects on the gut microbiota are likely similar. Both increase Bifidobacterium spp. and/or Bacteroides spp. in the human gut [42,43], and thus blackcurrant extract and PHGG may possibly have acted competitively in this study. Different dosages of each ingredient may result in a synergistic effect or an effect that leans more towards one ingredient. Further research is required regarding the combined use of these two ingredients.
Nevertheless, based on the results in this study, it is suggested that the beneficial impact on the gut microbiota of male university rugby players is somewhat expected due to the combinational intake of blackcurrant extract and PHGG; however, these are not necessarily taken together, as the intake of blackcurrant extract or PHGG alone exerted notably beneficial effects on the gut microbiota, comparable to—or even exceeding—those observed with their combined intake. The mechanisms underlying the effects of blackcurrant extract and/or PHGG ingestion are beyond the scope of this study, but, as mentioned above, both ingredients are reported to be fermented by beneficial gut bacteria, and thus prebiotic action would be one of the possible mechanisms [42,43]. Future research incorporating omics-based methodologies (e.g., metagenomics, metabolomics) will be crucial for elucidating the mode of actions for both ingredients.
There are several limitations in the present study. The present findings are derived from a specific cohort, namely male university rugby players in Japan. We believe that these findings may be applicable to other athletes who exhibit gut microbial disorders resulting from similar factors (e.g., endurance runners [16]), but their applicability to more or different populations compared to the current cohort (e.g., healthy non-athlete females) requires further research. Additionally, the effects of longer-term interventions should be investigated to determine whether more sustained changes in gut microbiota and host responses can be observed.
5. Conclusions
In the present study, we observed that the intake of blackcurrant extract and/or PHGG increased beneficial gut bacteria such as Megasphaera spp. and Bifidobacterium spp. In addition, a subgroup analysis showed that the dietary interventions had greater beneficial impacts, including increased beneficial bacteria and SCFA concentrations, on participants with dysbiotic gut environments compared to those with eubiotic gut environments. Although further investigation is needed, blackcurrant extract and PHGG can be used at least as functional materials to improve gut dysbiosis in university rugby players.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13071561/s1: Table S1: Macronutrient intake of the university rugby players in the present study. Figure S1: Effects of blackcurrant extract and PHGG intake on the alpha- and beta-diversities of the fecal microbiota. Figure S2: Comparison of the alpha- and beta-diversities between participants with total fecal SCFA concentrations > 100 mM and <100 mM. Table S2. Bacterial taxa showing statistical differences in the relative abundances between participants with total fecal SCFA concentrations > 100 mM and <100 mM. Table S3: Comparison of dietary intake between participants with total fecal SCFA concentrations > 100 mM and <100 mM. Text S1: Results and Discussion of the comparison of the gut environment and dietary intake between participants with total fecal SCFA concentrations > 100 mM and <100 mM. Figure S3: Subgroup analysis for the effect of blackcurrant extract and PHGG intake on the alpha- and beta- diversities of the fecal microbiota, focusing on participants with possible gut dysbiosis.
Author Contributions
Conceptualization, M.F., T.S., T.T. (Tomohisa Takagi), Y.N., and R.I.; methodology, M.F., T.S., T.T. (Tomohisa Takagi), and R.I.; validation, H.M. and R.I.; formal analysis, H.M., M.O., K.A., and R.I.; resources, A.A. and N.U.; investigation, H.M., M.O., K.A., H.I., N.O., T.T. (Takamitsu Tsukahara), and R.I.; data curation, H.M. and R.I.; writing—original draft preparation, H.M.; writing—review and editing, R.I.; visualization, H.M.; supervision, R.I.; project administration, R.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Morishita Jintan Corporation and Taiyo Kagaku Corporation (collaboration research funds).
Institutional Review Board Statement
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethical committee of Setsunan University (approval numbers: 2021-005 and 2021-029; approval dates: 8 May 2021 and 15 September 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Raw sequences have been deposited in the DDBJ Sequence Read Archive under the BioProject ID PRJNA1256317 (available from 1 May 2026).
Acknowledgments
The authors gratefully thank Sachiko Furuno for her assistance in dietary assessment. The staff members of the Rugby Football Club at Setsunan University are acknowledged for their assistance with data collection.
Conflicts of Interest
N.U. is employed by Morishita Jintan Corporation, which sells blackcurrant extract commercially. A.A. is employed by Taiyo Kagaku Corporation, which sells PHGG commercially. R.I. received collaboration research funds from Morishita Jintan Corporation and Taiyo Kagaku Corporation. YN received scholarship funds from Taiyo Kagaku Co. Ltd., Morishita-Jintan Co. Ltd., and a collaboration research fund from Taiyo Kagaku Co. Ltd. The present research was partly supported by these funds. Neither the funding agency nor any outside organization has participated in the study design or have any competing interests. These companies have approved the final version of the manuscript. Other authors have no conflicts of interest.
References
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, H.; Matsuki, T.; Nakazawa, A.; Takada, T.; Kado, S.; Asahara, T.; Kamada, N.; Sakuraba, A.; Yajima, T.; Higuchi, H.; et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int. J. Méd Microbiol. 2008, 298, 463–472. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Sasso, G.L.; Khachatryan, L.; Kondylis, A.; Battey, J.N.D.; Sierro, N.; ADanilova, N.; Grigoryeva, T.V.; IMarkelova, M.; Khusnutdinova, D.R.; Laikov, A.V.; et al. Inflammatory Bowel Disease–Associated Changes in the Gut: Focus on Kazan Patients. Inflamm. Bowel Dis. 2020, 27, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-M.; Zhao, H.-L.; Guo, G.-J.; Xu, J.; Zhou, Y.-L.; Huang, H.-L.; Nie, Y.-Q. Characterization of short-chain fatty acids in patients with ulcerative colitis: A meta-analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Morishima, S.; Oda, N.; Ikeda, H.; Segawa, T.; Oda, M.; Tsukahara, T.; Kawase, Y.; Takagi, T.; Naito, Y.; Fujibayashi, M.; et al. Altered Fecal Microbiotas and Organic Acid Concentrations Indicate Possible Gut Dysbiosis in University Rugby Players: An Observational Study. Microorganisms 2021, 9, 1687. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Jang, L.-G.; Choi, G.; Kim, S.-W.; Kim, B.-Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary Running Exercise Alters Microbiota Composition and Increases n-Butyrate Concentration in the Rat Cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M.; et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Oliveira EPde Burini, R.C.; Jeukendrup, A. Gastrointestinal Complaints During Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Nadatani, Y.; Watanabe, T.; Shimada, S.; Otani, K.; Tanigawa, T.; Fujiwara, Y. Microbiome and intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2018, 63, 17–137. [Google Scholar] [CrossRef]
- Morishima, S.; Aoi, W.; Kawamura, A.; Kawase, T.; Takagi, T.; Naito, Y.; Tsukahara, T.; Inoue, R. Intensive, prolonged exercise seemingly causes gut dysbiosis in female endurance runners. J. Clin. Biochem. Nutr. 2020, 68, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Park, Y.; Lee, S.; Kim, D.-O. Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.). Appl. Sci. 2021, 11, 1863. [Google Scholar] [CrossRef]
- Molan, A.; Liu, Z.; Plimmer, G. Evaluation of the Effect of Blackcurrant Products on Gut Microbiota and on Markers of Risk for Colon Cancer in Humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef]
- Cao, L.; Gil Lee, S.; Melough, M.M.; Sakaki, J.R.; Maas, K.R.; Koo, S.I.; Chun, O.K. Long-Term Blackcurrant Supplementation Modified Gut Microbiome Profiles in Mice in an Age-Dependent Manner: An Exploratory Study. Nutrients 2020, 12, 290. [Google Scholar] [CrossRef]
- Slavin, J.L.; Greenberg, N.A. Partially hydrolyzed guar gum Clinical nutrition uses. Nutrition 2003, 19, 549–552. [Google Scholar] [CrossRef]
- Ohashi, Y.; Sumitani, K.; Tokunaga, M.; Ishihara, N.; Okubo, T.; Fujisawa, T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef. Microbes 2014, 6, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Morishima, S.; Kapoor, M.P.; Inoue, R.; Tsukahara, T.; Naito, Y.; Ozeki, M. Partially hydrolyzed guar gum is associated with improvement in gut health, sleep, and motivation among healthy subjects. J. Clin. Biochem. Nutr. 2023, 72, 189–197. [Google Scholar] [CrossRef]
- Yasukawa, Z.; Inoue, R.; Ozeki, M.; Okubo, T.; Takagi, T.; Honda, A.; Naito, Y. Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial. Nutrients 2019, 11, 2170. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public. Heal. Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Inoue, R.; Sakaue, Y.; Sawai, C.; Sawai, T.; Ozeki, M.; Romero-Pérez, G.A.; Tsukahara, T. A preliminary investigation on the relationship between gut microbiota and gene expressions in peripheral mononuclear cells of infants with autism spectrum disorders. Biosci. Biotechnol. Biochem. 2016, 80, 1–9. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems 2018, 3, e00021-18. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhan, L.; Tang, W.; Wang, Q.; Dai, Z.; Zhou, L.; Feng, T.; Chen, M.; Wu, T.; Hu, E.; et al. MicrobiotaProcess: A comprehensive R package for deep mining microbiome. Innovation 2023, 4, 100388. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.; Hua, M.; Miao, X.; Su, Y.; Chi, Y.; Li, Y.; Sun, R.; Niu, H.; Wang, J. Analysis of the alleviating effect of black bean peel anthocyanins on type 2 diabetes based on gut microbiota and serum metabolome. J. Funct. Foods 2023, 102, 105456. [Google Scholar] [CrossRef]
- Reider, S.J.; Moosmang, S.; Tragust, J.; Trgovec-Greif, L.; Tragust, S.; Perschy, L.; Przysiecki, N.; Sturm, S.; Tilg, H.; Stuppner, H.; et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota-Results from the PAGODA Trial. Nutrients 2020, 12, 1257. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, S.; Claesen, J. Mechanisms of gut bacterial metabolism of dietary polyphenols into bioactive compounds. Gut Microbes 2024, 16, 2426614. [Google Scholar] [CrossRef] [PubMed]
- Morishima, S.; Abe, A.; Okamoto, S.; Kapoor, M.P.; Matsuura, S.; Kuriya, K.; Ozeki, M.; Nishio, M.; Miura, H.; Inoue, R. Partially hydrolyzed guar gum ingestion suppresses atopic dermatitis-like symptoms through prebiotic effect in mice. J. Clin. Biochem. Nutr. 2025, 76, 280–288. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).