Mycobacterium appelbergii sp. nov., a Novel Species Isolated from a Drinking Water Fountain in a Rural Community
Abstract
1. Introduction
2. Materials and Methods
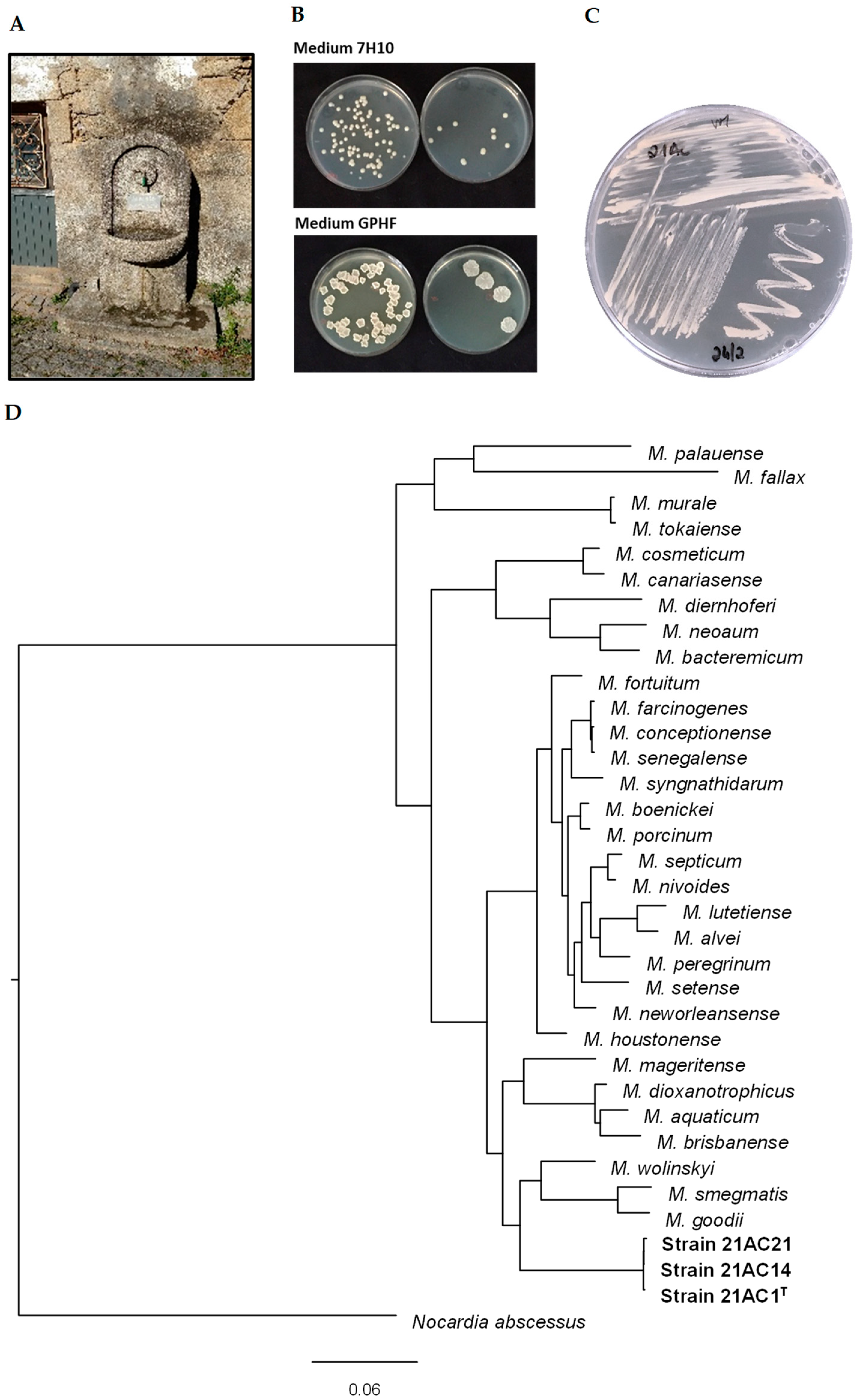
2.1. Water Sampling and Selective Isolation of Mycobacteria
2.2. 16S rRNA Gene Sequencing
2.3. Genomic Analysis and Genome Annotation
2.4. Phenotypic Analysis
2.5. Mycolic and Fatty Acid Analyses (MIDI/GC-MS) and MALDI-TOF MS
2.6. Antibiotic Susceptibility Testing
3. Results and Discussion
3.1. Sequence Identity and Phylogenetic Analysis
3.2. Nucleotide and Genome Sequence Accession Numbers
3.3. Chemical Analysis of the Water Source of Strain Isolation
3.4. Physiological and Chemotaxonomic Analysis
| 21AC1T | 21AC14 | 21AC21 | M. wolinskyi [31,48] | M. goodii [31] | M. smegmatis [31,48] | ||
|---|---|---|---|---|---|---|---|
| Optimal growth on solid media | 7H10 | 30–35 °C | 30–37 °C | 30–37 °C | 30–45 °C | 30–45 °C | 30–45 °C |
| GPHF | 25–30 °C | ND | ND | NA | NA | NA | |
| Growth on Middlebrook 7H9 | 20 °C | + | ND | ND | NA | NA | NA |
| 25 °C | + | ND | ND | NA | NA | NA | |
| 30 °C | + | ND | ND | + | + | NA | |
| 35 °C | + | ND | ND | + | + | NA | |
| 42 °C | - | ND | ND | + | + | NA | |
| 5% NaCl tolerance | 30 °C | + | ND | ND | + | + | + |
| Catalase | RT | + | ND | ND | NA | NA | + |
| t = 0, 68 °C | + | ND | ND | + | + | NA | |
| t = 20, 68 °C | + | ND | ND | + | + | NA | |
| Growth in McConkey agar (without crystal violet) | - | ND | ND | + | + | + | |
| Tween 80 hydrolysis | + | ND | ND | NA | NA | NA | |
| Arylsulfatase | + | ND | ND | - | - | - | |
| Iron uptake | - | ND | ND | + | + | + | |
| Tellurite reduction | + | ND | ND | NA | NA | NA | |
| Nitrate reductase | + | + | + | + | + | + | |
| Pyrazinamidase | + | + | + | NA | NA | NA | |
| Pyrrolidonylarylamidase | - | - | - | NA | NA | NA | |
| Alkaline phosphatase | + | + | + | NA | NA | NA | |
| β-Glucuronidase | - | - | - | NA | NA | NA | |
| β-Galactosidase | - | - | - | NA | NA | NA | |
| β-Glucosidase | + | + | + | NA | NA | NA | |
| N-Acetyl-β-glucosaminidase | - | - | - | NA | NA | NA | |
| Esculin | + | + | + | NA | NA | NA | |
| Urease | + | + | + | NA | NA | NA | |
| Gelatinase | - | - | - | NA | NA | - | |
| Arginine dihydrolase | - | - | - | NA | NA | NA | |
| Lysine decarboxylase | - | - | - | NA | NA | NA | |
| Ornithine decarboxylase | - | - | - | NA | NA | NA | |
| Citrate | - | - | - | + | - | +- | |
| H2S production | - | - | - | NA | NA | NA | |
| Tryptophan deaminase | - | - | - | NA | NA | NA | |
| Indole production | - | - | - | NA | NA | NA | |
| Acetoin production | + | + | + | NA | NA | NA | |
| Utilization of carbon sources | |||||||
| D-Glucose | - | - | - | NA | NA | + | |
| D-Mannitol | - | - | - | + | + | + | |
| Inositol | - | - | - | + | + | + | |
| D-Sorbitol | - | - | - | + | + | + | |
| L-Rhamnose | - | - | - | + | + | + | |
| D-Saccharose | - | - | - | NA | NA | NA | |
| Amygdaline | - | - | - | NA | NA | NA | |
| L-Arabinose | - | - | - | + | + | + | |
| Ribose | - | - | - | NA | NA | NA | |
| Xylose | - | - | - | + | + | + | |
| Maltose | - | - | - | NA | NA | NA | |
| Lactose | - | - | - | NA | NA | NA | |
| Glycogen | - | - | - | NA | NA | NA | |
| Fatty Acid | (%) | Mycolic Acid | (%) |
|---|---|---|---|
| Saturated FAME | C62H122O3 | 7.17 | |
| C14:0 | 5.2 | C64H12603 | 3.2 |
| C16:0 | 34.1 | C75H14603 | 10.78 |
| C18:0 | 2.4 | C76H14803 | 9.02 |
| Unsaturated FAME | C77H15003 | 36.25 | |
| C16:1 ω6c | 16.3 | C78H15203 | 7.12 |
| C18:1 ω9c | 27.7 | C77H15004 | 8.57 |
| Tuberculostearic acid [TBSA] FAME | C79H15403 | 7.97 | |
| 10Me-18:0 | 14.3 | C79H15404 | 9.92 |
3.5. Antibiotic Susceptibility Profiles
4. Conclusions
5. Description of Mycobacterium appelbergii sp. nov.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nunes-Costa, D.; Alarico, S.; Dalcolmo, M.P.; Correia-Neves, M.; Empadinhas, N. The looming tide of nontuberculous mycobacterial infections in Portugal and Brazil. Tuberculosis 2016, 96, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.; Gaio, A.R.; Gomes, M.; Gonçalves, A.; Duarte, R. Mycobacterium avium infection in Portugal. Int. J. Tuberc. Lung. Dis. 2017, 21, 218–222. [Google Scholar] [CrossRef]
- Pavlik, I.; Ulmann, V.; Falkinham, J.O., III. Nontuberculous Mycobacteria: Ecology and Impact on Animal and Human Health. Microorganisms 2022, 10, 1516. [Google Scholar] [CrossRef]
- Falkinham, J.O., III. Nontuberculous mycobacteria in the environment. Tuberculosis 2022, 137, 102267. [Google Scholar] [CrossRef]
- Falkinham, J.O., III. Environmental Sources of Nontuberculous Mycobacteria. Clin. Chest. Med. 2015, 36, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Abebe, L.S.; Cronk, R.; Bartram, J. A systematic review of waterborne infections from nontuberculous mycobacteria in health care facility water systems. Int. J. Hyg. Environ. Health 2017, 220, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.M.; Tolson, C.; Carter, R.; Coulter, C.; Huygens, F.; Hargreaves, M. Isolation of Nontuberculous Mycobacteria (NTM) from Household Water and Shower Aerosols in Patients with Pulmonary Disease Caused by NTM. J. Clin. Microbiol. 2013, 51, 3006–3011. [Google Scholar] [CrossRef]
- Gopalaswamy, R.; Shanmugam, S.; Mondal, R.; Subbian, S. Of tuberculosis and non-tuberculous mycobacterial infections—A comparative analysis of epidemiology, diagnosis and treatment. J. Biomed. Sci. 2020, 27, 74. [Google Scholar] [CrossRef]
- Gardini, G.; Gregori, N.; Matteelli, A.; Castelli, F. Mycobacterial skin infection. Curr. Opin. Infect. Dis. 2022, 35, 79–87. [Google Scholar] [CrossRef]
- Timpe, A.; Runyon, E.H. The relationship of atypical acid-fast bacteria to human disease; a preliminary report. J. Lab. Clin. Med. 1954, 44, 202–209. [Google Scholar] [CrossRef]
- Ahmed, I.; Tiberi, S.; Farooqi, J.; Jabeen, K.; Yeboah-Manu, D.; Migliori, G.B.; Hasan, R. Non-tuberculous mycobacterial infections—A neglected and emerging problem. Int. J. Infect. Dis. 2020, 92, S46–S50. [Google Scholar] [CrossRef]
- Kunduracılar, H. Identification of mycobacteria species by molecular methods. Int. Wound J. 2020, 17, 245–250. [Google Scholar] [CrossRef]
- Ahmad, S.; Mokaddas, E. Diversity of Nontuberculous Mycobacteria in Kuwait: Rapid Identification and Differentiation of Mycobacterium Species by Multiplex PCR, INNO-LiPA Mycobacteria v2 Assay and PCR Sequencing of rDNA. Med. Princ. Pract. 2019, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.A.; Hall, G.S.; Miller, M.B.; Novak, S.M.; Rowlinson, M.-C.; Salfinger, M.; Somoskövi, A.; Warshauer, D.M.; Wilson, M.L. Practical Guidance for Clinical Microbiology Laboratories: Mycobacteria. Clin. Microbiol. Rev. 2018, 31, e00038-17. [Google Scholar] [CrossRef]
- Durão, V.; Silva, A.; Macedo, R.; Durão, P.; Santos-Silva, A.; Duarte, R. Portuguese in vitro antibiotic susceptibilities favor current nontuberculous mycobacteria treatment guidelines. Pulmonology 2019, 25, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Primm, T.P.; Lucero, C.A.; Falkinham III, J.O. Health Impacts of Environmental Mycobacteria. Clin. Microbiol. Rev. 2004, 17, 98–106. [Google Scholar] [CrossRef]
- Vaerewijck, M.J.M.; Huys, G.; Palomino, J.C.; Swings, J.; Portaels, F. Mycobacteria in drinking water distribution systems: Ecology and significance for human health. FEMS Microbiol. Rev. 2005, 29, 911–934. [Google Scholar] [CrossRef] [PubMed]
- Radomski, N.; Cambau, E.; Moulin, L.; Haenn, S.; Moilleron, R.; Lucas, F.S. Comparison of Culture Methods for Isolation of Nontuberculous Mycobacteria from Surface Waters. Appl. Environ. Microbiol. 2010, 76, 3514–3520. [Google Scholar] [CrossRef]
- Williams, M.D.; Falkinham, J.O., III. Effect of Cetylpyridinium Chloride (CPC) on Colony Formation of Common Nontuberculous Mycobacteria. Pathogens 2018, 7, 79. [Google Scholar] [CrossRef]
- Pereira, S.G.; Alarico, S.; Tiago, I.; Reis, D.; Nunes-Costa, D.; Cardoso, O.; Maranha, A.; Empadinhas, N. Studies of antimicrobial resistance in rare mycobacteria from a nosocomial environment. BMC Microbiol. 2019, 19, 62. [Google Scholar] [CrossRef]
- Alarico, S.; Nunes-Costa, D.; Silva, A.; Costa, M.; Macedo-Ribeiro, S.; Empadinhas, N. A genuine mycobacterial thermophile: Mycobacterium hassiacum growth, survival and GpgS stability at near-pasteurization temperatures. Microbiology 2020, 166, 474–483. [Google Scholar] [CrossRef]
- Uritskiy, G.V.; DiRuggiero, J.; Taylor, J. MetaWRAP—A flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 2018, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.D.; Froula, J.; Egan, R.; Wang, Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 2015, 3, e1165. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Alarico, S.; Costa, M.; Sousa, M.S.; Maranha, A.; Lourenco, E.C.; Faria, T.Q.; Ventura, M.R.; Empadinhas, N. Mycobacterium hassiacum recovers from nitrogen starvation with up-regulation of a novel glucosylglycerate hydrolase and depletion of the accumulated glucosylglycerate. Sci. Rep. 2014, 4, 6766. [Google Scholar] [CrossRef]
- Bhalla, G.S.; Sarao, M.S.; Kalra, D.; Bandyopadhyay, K.; John, A.R. Methods of phenotypic identification of non-tuberculous mycobacteria. Pract. Lab. Med. 2018, 12, e00107. [Google Scholar] [CrossRef] [PubMed]
- Adékambi, T.; Stein, A.; Carvajal, J.; Raoult, D.; Drancourt, M. Description of Mycobacterium conceptionense sp. nov., a Mycobacterium fortuitum Group Organism Isolated from a Posttraumatic Osteitis Inflammation. J. Clin. Microbiol. 2006, 44, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes; Document M24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes; Supplement M24S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Brown-Elliott, B.A.; Woods, G.L. Antimycobacterial Susceptibility Testing of Nontuberculous Mycobacteria. J. Clin. Microbiol. 2019, 57, e00834-19. [Google Scholar] [CrossRef]
- Ankenbrand, M.J.; Keller, A. bcgTree: Automatized phylogenetic tree building from bacterial core genomes. Genome 2016, 59, 783–791. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Frederiksen, W.; Garrity, G.M.; Grimont, P.A.D.; Kämpfer, P.; Maiden, M.C.J.; Nesme, X.; Rosselló-Mora, R.; Swings, J.; Trüper, H.G.; et al. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 2002, 52, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Lipner, E.M.; French, J.; Bern, C.R.; Walton-Day, K.; Knox, D.; Strong, M.; Prevots, D.R.; Crooks, J.L. Nontuberculous Mycobacterial Disease and Molybdenum in Colorado Watersheds. Int. J. Environ. Res. Public Health 2020, 17, 3854. [Google Scholar] [CrossRef] [PubMed]
- Lipner, E.M.; French, J.P.; Falkinham, J.O., III; Crooks, J.L.; Mercaldo, R.A.; Henkle, E.; Prevots, D.R. Nontuberculous Mycobacteria Infection Risk and Trace Metals in Surface Water: A Population-based Ecologic Epidemiologic Study in Oregon. Ann. Am. Thorac. Soc. 2022, 19, 543–550. [Google Scholar] [CrossRef]
- Lipner, E.M.; French, J.P.; Mercaldo, R.A.; Nelson, S.; Zelazny, A.M.; Marshall, J.E.; Strong, M.; Falkinham III, J.O.; Prevots, D.R. The risk of pulmonary NTM infections and water-quality constituents among persons with cystic fibrosis in the United States, 2010–2019. Environ. Epidemiol. 2023, 7, e266. [Google Scholar] [CrossRef]
- Bahr, C.; Jekel, M.; Amy, G. Vanadium removal from drinking water by fixed-bed adsorption on granular ferric hydroxide. AWWA Water Sci. 2022, 4, e1271. [Google Scholar] [CrossRef]
- Pichler, T.; Koopmann, S. Should Monitoring of Molybdenum (Mo) in Groundwater, Drinking Water and Well Permitting Made Mandatory? Environ. Sci. Technol. 2020, 54, 1–2. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef]
- Koh, W.-J. Nontuberculous Mycobacteria—Overview. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Adékambi, T.; Raoult, D.; Drancourt, M. Mycobacterium barrassiae sp. nov., a Mycobacterium moriokaense Group Species Associated with Chronic Pneumonia. J. Clin. Microbiol. 2006, 44, 3493–3498. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Chedore, P.; Kasatiya, S. Use of Gas Chromatographic Fatty Acid and Mycolic Acid Cleavage Product Determination To Differentiate among Mycobacterium genavense, Mycobacterium fortuitum, Mycobacterium simiae, and Mycobacterium tuberculosis. J. Clin. Microbiol. 1998, 36, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Selvarangan, R.; Wu, W.-K.; Nguyen, T.T.; Carlson, L.D.C.; Wallis, C.K.; Stiglich, S.K.; Chen, Y.-C.; Jost, K.C.; Prentice, J.L.; Wallace, R.J.; et al. Characterization of a Novel Group of Mycobacteria and Proposal of Mycobacterium sherrisii sp. nov. J. Clin. Microbiol. 2004, 42, 52–59. [Google Scholar] [CrossRef]
- Zimhony, O.; Vilchèze, C.; Jacobs, W.R. Characterization of Mycobacterium smegmatis Expressing the Mycobacterium tuberculosis Fatty Acid Synthase I (fas1) Gene. J. Bacteriol. 2004, 186, 4051–4055. [Google Scholar] [CrossRef]
- Minnikin, D.E.; Minnikin, S.M.; Parlett, J.H.; Goodfellow, M. Mycolic Acid Patterns of Some Rapidly-Growing Species of Mycobacterium. Zentralblatt für Bakteriol. Mikrobiol. und Hygiene Ser. A Med. Microbiol. Infect. Dis. Virol. Parasitol. 1985, 259, 446–460. [Google Scholar] [CrossRef]
- Brown, B.A.; Springer, B.; Steingrube, V.A.; Wilson, R.W.; Pfyffer, G.E.; Garcia, M.J.; Menendez, M.C.; Rodriguez-Salgado, B.; Jost, K.C.; Chiu, S.H.; et al. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: A cooperative study from the International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Evol. Microbiol. 1999, 49, 1493–1511. [Google Scholar] [CrossRef]
- Butler, W.R.; Guthertz, L.S. Mycolic Acid Analysis by High-Performance Liquid Chromatography for Identification of Mycobacterium Species. Clin. Microbiol. Rev. 2001, 14, 704–726. [Google Scholar] [CrossRef] [PubMed]
- Jamet, S.; Slama, N.; Domingues, J.; Laval, F.; Texier, P.; Eynard, N.; Quémard, A.; Peixoto, A.; Lemassu, A.; Daffé, M.; et al. The Non-Essential Mycolic Acid Biosynthesis Genes hadA and hadC Contribute to the Physiology and Fitness of Mycobacterium smegmatis. PLoS ONE 2015, 10, e0145883. [Google Scholar] [CrossRef]
- Bouam, A.; Armstrong, N.; Levasseur, A.; Drancourt, M. Mycobacterium terramassiliense, Mycobacterium rhizamassiliense and Mycobacterium numidiamassiliense sp. nov., three new Mycobacterium simiae complex species cultured from plant roots. Sci. Rep. 2018, 8, 9309. [Google Scholar] [CrossRef]
- Baba, T.; Kaneda, K.; Kusunose, E.; Kusunose, M.; Yano, I. Molecular species of mycolic acid subclasses in eight strains of Mycobacterium smegmatis. Lipids 1988, 23, 1132–1138. [Google Scholar] [CrossRef]
- Etemadi, A.-H. Sur l’intérêt taxinomique et la signification phylogénétique des acides mycoliques. Bull. Société Bot. Fr. 1965, 112, 47–74. [Google Scholar] [CrossRef]
- Gray, G.R.; Wong, M.Y.H.; Danielson, S.J. The major mycolic acids of Mycobacterium smegmatis. Prog. Lipid Res. 1982, 21, 91–107. [Google Scholar] [CrossRef]
- Laval, F.; Lanéelle, M.-A.; Déon, C.; Monsarrat, B.; Daffé, M. Accurate Molecular Mass Determination of Mycolic Acids by MALDI-TOF Mass Spectrometry. Anal. Chem. 2001, 73, 4537–4544. [Google Scholar] [CrossRef]
- Fujikura, H.; Kasahara, K.; Ogawa, Y.; Hirai, N.; Yoshii, S.; Yoshihara, S.; Ogawa, T.; Yonekawa, S.; Imakita, N.; Nishioka, Y.; et al. Mycobacterium wolinskyi Peritonitis after Peritoneal Catheter Embedment Surgery. Intern. Med. 2017, 56, 3097–3101. [Google Scholar] [CrossRef] [PubMed]
- Salas, N.M.; Klein, N. Mycobacterium goodii: An Emerging Nosocomial Pathogen: A Case Report and Review of the Literature. Infect. Dis. Clin. Pract. 2017, 25, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Waldron, R.; Waldron, D.; McMahon, E.; Reilly, L.; Riain, U.N.; Fleming, C.; O’Regan, A. Mycobacterium goodii pneumonia: An unusual presentation of nontuberculous mycobacterial infection requiring a novel multidisciplinary approach to management. Respir. Med. Case Rep. 2019, 26, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Meneses, M.; González-Martin, J.; Agüero, D.; Tolosana, J.M.; Sandoval, E.; Falces, C.; San Antonio, R.; Vidal, B.; Moreno, A.; Ambrosioni, J.; et al. Mycobacterium Wolinskyi: A New Non-Tuberculous Mycobacterium Associated with Cardiovascular Infections? Infect. Dis. Ther. 2021, 10, 1073–1080. [Google Scholar] [CrossRef]
- Pfeuffer-Jovic, E.; Heyckendorf, J.; Reischl, U.; Bohle, R.M.; Bley, T.; Buck, A.; Wilkens, H.; Schäfers, H.-J.; Langen, H.-J.; Held, M. Pulmonary vasculitis due to infection with Mycobacterium goodii: A case report. Int. J. Infect. Dis. 2021, 104, 178–180. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J.; Liu, Z.; Ye, J.; Yang, L.; Zhang, Z. Mixed infection of three nontuberculous mycobacteria species identified by metagenomic next-generation sequencing in a patient with peritoneal dialysis-associated peritonitis: A rare case report and literature review. BMC Nephrol. 2023, 24, 95. [Google Scholar] [CrossRef]
| Genome A | 21AC1T vs. | M. goodii vs. | M. wolinskyi vs. | |||
|---|---|---|---|---|---|---|
| Genome B | M. wolinskyi | M. smegmatis | M. goodii | M. wolinskyi | M. smegmatis | M. smegmatis |
| dDDH values in % * | 23.6 (30.4) | 22.1 (27) | 21.8 (26.6) | 23.8 (33.4) | 34.9 (65.9) | 23.7 (32.6) |
| OrthoANIu value (%) | 80.76 | 79.34 | 79.11 | 81.00 | 88.16 | 81.07 |
| Average aligned length (bp) | 3,170,245 | 2,901,392 | 2,802,236 | 3,105,041 | 3,862,094 | 2,991,255 |
| Genome A coverage (%) | 41.62 | 38.09 | 36.79 | 46.06 | 57.29 | 39.88 |
| Genome B coverage (%) | 42.26 | 41.48 | 41.57 | 41.39 | 55.22 | 42.77 |
| Type strains | 21AC1T | M. wolinskyi ATCC 700010 | M. goodii ATCC 700504 | M. smegmatis NCTC 8159 | ||
| Genome length (bp) | 7,617,360 | 7,501,080 | 6,741,281 | 6,994,140 | ||
| Parameters/Method * | Result | Unit | U (%) | MAC |
|---|---|---|---|---|
| pH | 6.5 at 20 °C | Sorensen Scale | ±0.2; ±0.1 | ∙∙∙ |
| Alkalinity | 33 | mg CaCO3 L−1 | ±11; ±1.6 | ∙∙∙ |
| Calcium | 11 | mg Ca L−1 | ±16; ±2.9 | ∙∙∙ |
| Conductivity | 136 | µS/cm, at 20 °C | ±4; ±1.1 | ∙∙∙ |
| Total hardness | 33 | mg CaCO3 L−1 | ∙∙∙ | ∙∙∙ |
| Molybdenum | <10 | μg Mo L−1 | ±20; ±7.5 | 50–70 |
| Vanadium | <0.010 | mg V L−1 | ∙∙∙ | ∙∙∙ |
| Nitrate | 19 | mg NO3 L−1 | ±27; ±(m) | 50 |
| Nitrite | <0.010 | mg NO2 L−1 | ±25; ±(m) | 0.5 |
| Chloride | 8.6 | mg Cl− L−1 | ±26; ±(m) | 250 |
| Antimicrobial | MIC (µg/mL) | ||
|---|---|---|---|
| Strain 21AC1T | Strain 21AC14 | Strain 21AC21 | |
| Amikacin | 0.25 S | 0.5 S | 0.25 S |
| Cefoxitin | 0.25 S | 2 S | 2 S |
| Ciprofloxacin | 0.5 S | 2 I | 2 I |
| Clarithromycin | <0.125 S | <0.125 S | <0.125 S |
| Imipenem | 16 I | 16 I | 32 R |
| Linezolid | 1 S | 0.25 S | 0.25 S |
| Meropenem | 0.25 S | 0.125 S | 0.25 S |
| Minocycline | 0.25 S | 0.5 S | 0.5 S |
| Moxifloxacin | 0.25 S | 0.5 S | 0.25 S |
| Tobramycin | 1 S | 4 I | 4 I |
| Doxycycline | 2 I | 2 I | 2 I |
| TMP-SMX | 0.5–9.5 S | <0.125–2.375 S | <0.125–2.375 S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roxo, I.C.; Alarico, S.; Fonseca, A.; Machado, D.; Maranha, A.; Tiago, I.; Duarte, R.; Empadinhas, N. Mycobacterium appelbergii sp. nov., a Novel Species Isolated from a Drinking Water Fountain in a Rural Community. Microorganisms 2025, 13, 1259. https://doi.org/10.3390/microorganisms13061259
Roxo IC, Alarico S, Fonseca A, Machado D, Maranha A, Tiago I, Duarte R, Empadinhas N. Mycobacterium appelbergii sp. nov., a Novel Species Isolated from a Drinking Water Fountain in a Rural Community. Microorganisms. 2025; 13(6):1259. https://doi.org/10.3390/microorganisms13061259
Chicago/Turabian StyleRoxo, Inês Cravo, Susana Alarico, Ana Fonseca, Daniela Machado, Ana Maranha, Igor Tiago, Raquel Duarte, and Nuno Empadinhas. 2025. "Mycobacterium appelbergii sp. nov., a Novel Species Isolated from a Drinking Water Fountain in a Rural Community" Microorganisms 13, no. 6: 1259. https://doi.org/10.3390/microorganisms13061259
APA StyleRoxo, I. C., Alarico, S., Fonseca, A., Machado, D., Maranha, A., Tiago, I., Duarte, R., & Empadinhas, N. (2025). Mycobacterium appelbergii sp. nov., a Novel Species Isolated from a Drinking Water Fountain in a Rural Community. Microorganisms, 13(6), 1259. https://doi.org/10.3390/microorganisms13061259








