Selection of Non-Saccharomyces Yeasts from Extreme Oenological Environments for Potential Use in Winemaking
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Autochthonous Wine Yeasts
2.2. Culture Media and Enzymatic Screening Procedures
2.2.1. WL Nutrient Agar (Wallerstein Laboratory Medium)
2.2.2. Lysine Medium
2.2.3. Determination of Yeast Killer Activity
2.2.4. Medium for Detecting β-Glucosidase Activity
2.2.5. YPD Agar Medium
2.3. Yeast Identification
2.4. Alcoholic Fermentation Tests
2.4.1. Preparation of Pre-Inocula
2.4.2. Fermentation Monitoring
2.5. Determination of Microbiological Parameters
2.6. Determination of Oenological Parameters
2.7. Analytical Determinations of Major Aroma Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analysis
3.2. Yeast Identification and Selection
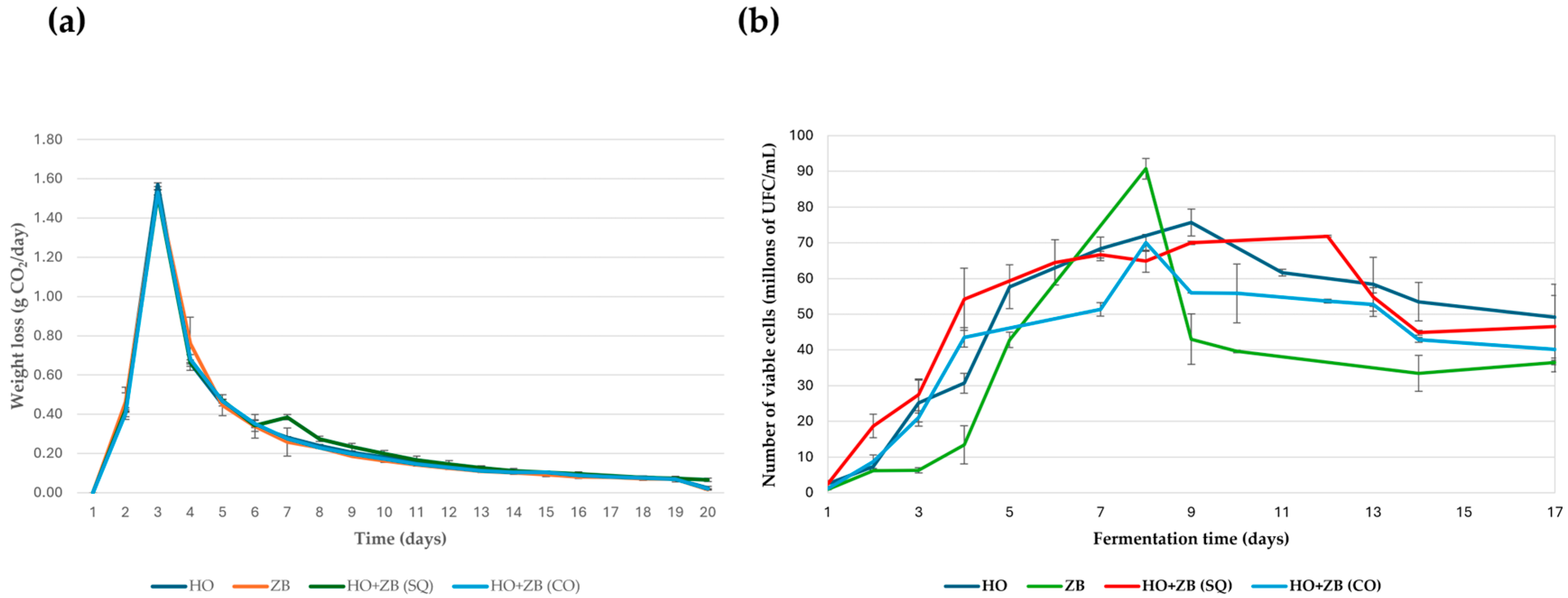
3.3. Fermentation Kinetics
3.4. Oenological Parameters
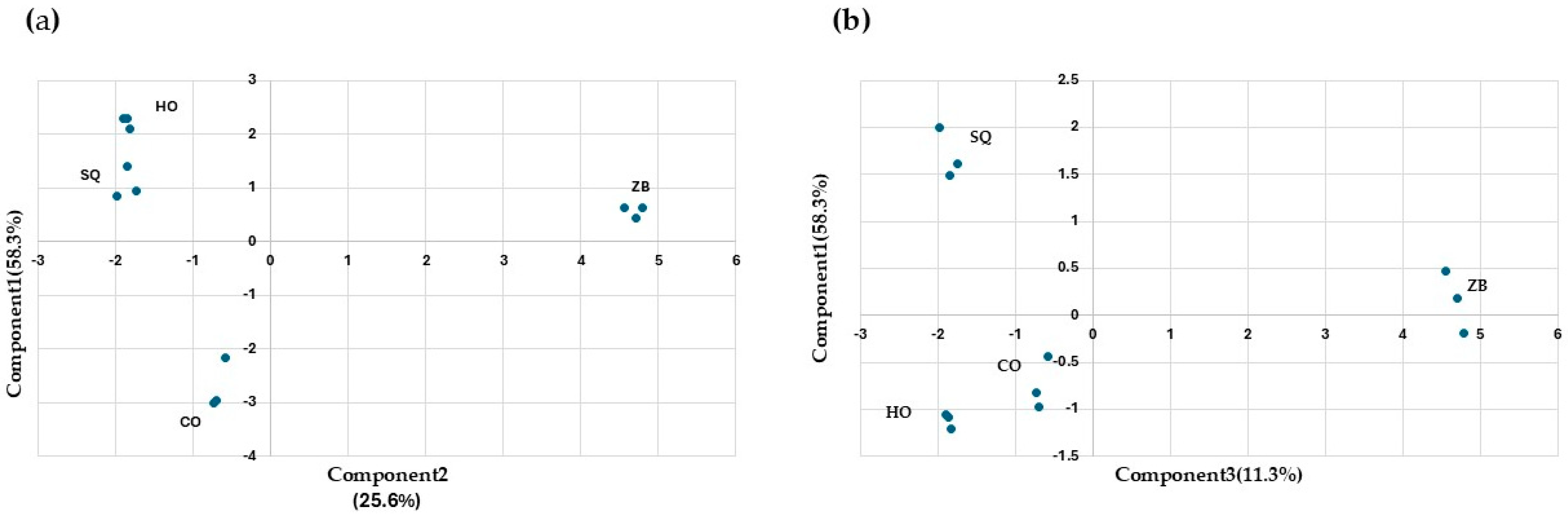
3.5. Major Aroma and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.Q.; Jiao, M.L.; Li, D.M.; Zhang, H.X.; Xie, Y.H.; Li, H.J.; Yang, M.-M.; Duan, X.-R.; Pang, X.-N.; Fan, X.-M. Microbial diversity during wine fermentation in Beijing region and its influence on wine flavor. LWT 2025, 215, 117209. [Google Scholar] [CrossRef]
- Gao, J.; Wang, M.; Huang, W.; You, Y.; Zhan, J. Indigenous Saccharomyces cerevisiae could better adapt to the physicochemical conditions and natural microbial ecology of prince grape must compared with commercial Saccharomyces cerevisiae FX10. Molecules 2022, 27, 6892. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, B.; Maimaitiyiming, R.; Wang, L.; Zhao, L.; Zhang, H.; Chen, K.; Aihaiti, A. The Effect of Non-Saccharomyces Cerevisiae Torulaspora delbrueckii on the aroma composition of munage grape base-wine and the mechanism of the effect. Fermentation 2024, 10, 266. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Fasoli, G.; Schirone, M.; Corsetti, A.; Suzzi, G. Biodiversity study of wine yeasts belonging to the “terroir” of Montepulciano d’Abruzzo “Colline Teramane” revealed Saccharomyces cerevisiae strains exhibiting atypical and unique 5.8S-ITS restriction patterns. Food Microbiol. 2014, 39, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, G.; Guan, X.; Li, A.; Tao, Y. Volatile aroma compound-based decoding and prediction of sweet berry aromas in dry red wine. Food Chem. 2025, 463, 141248. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Moreno-Arribas, M.V.; Tremonte, P.; Succi, M.; Sorrentino, E.; Macciola, V.; Di Renzo, M.; Coppola, R. Sequential inoculum of Hanseniaspora Guilliermondii and Saccharomyces Cerevisiae for winemaking campanino on an industrial Scale. World J. Microbiol. Biotechnol. 2018, 34, 161. [Google Scholar] [CrossRef]
- Wang, L.; Dou, G.; Guo, H.; Zhang, Q.; Qin, X.; Yu, W.; Jiang, C.; Xiao, H. Volatile organic compounds of Hanseniaspora Uvarum increase strawberry fruit flavor and defense during cold storage. Food Sci. Nutr. 2019, 7, 2625–2635. [Google Scholar] [CrossRef]
- Capozzi, V.; Berbegal, C.; Tufariello, M.; Grieco, F.; Spano, G. Impact of co-inoculation of Saccharomyces Cerevisiae, Hanseniaspora Uvarum and Oenococcus Oeni autochthonous strains in controlled multi starter grape must Fermentations. LWT 2019, 109, 241–249. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Rementeria, A.; Rodriguez, J.A.; Cadaval, A.; Amenabar, R.; Muguruza, J.R.; Hernando, F.L.; Sevilla, M.J. Yeast associated with spontaneous fermentations of white wines from the “Txakoli de Bizkaia” region (Basque Country, North Spain). Int. J. Food Microbiol. 2003, 86, 201–207. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Loira, I.; Manuel Del Fresno, J.M.; Vaquero, C.; Antonia Bañuelos, M.; Palomero, F.; López, C.; González, C. pH Control and Aroma Improvement Using the Non-Saccharomyces Lachancea thermotolerans and Hanseniaspora spp. Yeasts to improve wine freshness in warm areas. In Grapes Wine; Intechopen: London, UK, 2022. [Google Scholar] [CrossRef]
- Santos, J.; Sousa, M.J.; Cardoso, H.; Inacio, J.; Silva, S.; Spencer-Martins, I.; Leao, C. Ethanol tolerance of sugar transport, and the rectification of stuck wine fermentations. Microbiology 2008, 154, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, J.M.; Childs, B.C.; Edwards, C.G. Evaluation of Zygosaccharomyces bailii to metabolize residual sugar present in partially-fermented red wines. Fermentation 2015, 1, 3–12. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol 2017, 8, 995. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, J.; de Souza Schneider RD, C.; Mendes SD, C.; Welke, J.E.; Zini, C.A.; Caramão, E.B.; Valente, P. Evaluation of Zygosaccharomyces bailii BCV 08 as a co-starter in wine fermentation for the improvement of ethyl esters production. Microbial. Res. 2015, 173, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Solieri, L. The revenge of Zygosaccharomyces yeasts in food biotechnology and applied microbiology. World J. Microbiol. Biotechnol. 2021, 37, 96. [Google Scholar] [CrossRef]
- Alcalá-Jiménez, M.T.; García-Martínez, T.; Mauricio, J.C.; Moreno, J.; Peinado, R.A. Influence of terroir on microbial diversity and wine volatilome. Appl. Sci. 2025, 15, 3237. [Google Scholar] [CrossRef]
- Ramírez, M.; Vinagre, A.; Ambrona, J.; Molina, F.; Maqueda, M.; Rebollo, J.E. Genetic instability of heterozygous hybrid populations of natural wine yeasts. Appl. Environ. Microbiol. 2004, 70, 4686–4691. [Google Scholar] [CrossRef]
- Kaiser, C.; Michaelis, S.; Mitchell, A. Methods in Yeast Genetics; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1994. [Google Scholar]
- Lodder, J.; Kreger-van Rij, N.J.W. The Yeasts: A Taxonomic Study, 1st ed.; Elsevier: Cham, The Netherlands, 1952; pp. 84–85. [Google Scholar]
- Urtubia, A.; Franco, W.; De Lecco, C.C.; Benavides, S.; Durán, A. Impact of non-Saccharomyces autochthonous yeasts on ethanol reduction and chemical profile of Chilean Sauvignon Blanc wine. In Proceedings of the BIO Web of Conferences, Jember, Indonesia, 12–13 September 2023; EDP Sciences: Ulis, France, 2023; Volume 56, p. 02018. [Google Scholar]
- Spedding, G. Alcohol and its measurement. In Brewing Materials and Processes; Academic Press: Cambridge, MA, USA, 2016; pp. 123–149. [Google Scholar] [CrossRef]
- International Code of Oenological Practices; OIV International Organisation of Vine and Wine: Dijon, France, 2023.
- Pilone, G.J. Determination of ethanol in wine by titrimetric and spectrophotometric dichromate methods: Collaborative study. J. AOAC Int. 1985, 68, 188–190. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas chromatographic quantification of major volatile compounds and polyols in wine by direct injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef]
- Miranda, A.; Pereira, V.; Jardim, H.; Malfeito-Ferreira, M.; Marques, J.C. Impact of Non-Saccharomyces yeast fermentation in madeira wine chemical composition. Processes 2023, 11, 482. [Google Scholar] [CrossRef]
- Ruiz-Muñoz, M.; Hernández-Fernández, M.; Cordero-Bueso, G.; Martínez-Verdugo, S.; Pérez, F.; Cantoral, J.M. Non-Saccharomyces are also forming the veil of flor insherry wines. Fermentation 2022, 8, 456. [Google Scholar] [CrossRef]
- de Ovalle, S.; Cavello, I.; Brena, B.M.; Cavalitto, S.; González-Pombo, P. Production and characterization of a β-glucosidase from Issatchenkia terricola and its use for hydrolysis of aromatic precursors in Cabernet Sauvignon wine. LWT 2018, 87, 515–522. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Romo-Sánchez, S.; Arévalo-Villena, M.; García Romero, E.; Ramirez, H.L.; Briones Pérez, A. Immobilization of β-glucosidase and its application for enhancement of aroma precursors in muscat wine. Food Bioprocess Technol. 2014, 7, 1381–1392. [Google Scholar] [CrossRef]
- Englezos, V.; Jolly, N.P.; Di Gianvito, P.; Rantsiou, K.; Cocolin, L. Microbial interactions in winemaking: Ecological aspects and effect on wine quality. Trends Food Sci. Technol. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Lowes, K.F.; Shearman, C.A.; Payne, J.; MacKenzie, D.; Archer, D.B.; Merry, R.J.; Gasson, M.J. Prevention of yeast spoilage in feed and food by the yeast mycocin HMK. Appl. Environ. Microbiol. 2000, 66, 1066–1076. [Google Scholar] [CrossRef]
- Ansanay-Galeote, V.; Blondin, B.; Dequin, S.; Sablayrolles, J.-M. Stress effect of ethanol on fermentation kinetics by stationary-phase cells of Saccharomyces cerevisiae. Biotechnol. Lett. 2001, 23, 677–681. [Google Scholar] [CrossRef]
- Sottil, C.; Salor-Torregrosa, J.M.; Moreno-Garcia, J.; Peinado, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Using Torulaspora delbrueckii, Saccharomyces cerevisiae and Saccharomyces bayanus wine yeasts as starter cultures for fermentation and quality improvement of mead. Eur. Food Res. Technol. 2019, 245, 2705–2714. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Loira, I.; Escott, C.; Carrau, F.; González, C.; Cuerda, R.; Morata, A. Application of Hanseniaspora vineae yeast in the production of rosé wines from a blend of Tempranillo and Albillo grapes. Fermentation 2021, 7, 141. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, R. Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—A review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Guittin, C.; Maçna, F.; Picou, C.; Perez, M.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. New online monitoring approaches to describe and understand the kinetics of acetaldehyde concentration during wine alcoholic fermentation: Access to production balances. Fermentation 2023, 9, 299. [Google Scholar] [CrossRef]
- Wang, X.; Fan, G.; Peng, Y.; Xu, N.; Xie, Y.; Zhou, H.; Matallana, E.; Siesto, G.; Aranda, A. Mechanisms and effects of non-Saccharomyces yeast fermentation on the aromatic profile of wine. J. Food Compos. Anal. 2023, 124, 105660. [Google Scholar] [CrossRef]
- Capece, A.; Pietrafesa, A.; Pietrafesa, R.; Garrigós, V.; Tedesco, F.; Romano, P.; Matallana, E.; Siesto, G.; Aranda, A. Impact of Starmerella bacillaris and Zygosaccharomyces bailii on ethanol reduction and Saccharomyces cerevisiae metabolism during mixed wine fermentations. Food Res. Int. 2022, 159, 111649. [Google Scholar] [CrossRef]
- Epifanio, S. La Influencia de la Tecnología de la Vinificación en la Microbiología y el Desarrollo de la Fermentación Alcohólica. Ph.D. Thesis, Universidad de la Rioja, Logroño, Spain, 2005. [Google Scholar]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Filippousi, M.E.; Chalvantzi, I.; Mallouchos, A.; Marmaras, I.; Banilas, G.; Nisiotou, A. The use of Hanseniaspora opuntiae to improve ‘Sideritis’ wine quality, a late-ripening Greek grape variety. Foods 2024, 13, 1061. [Google Scholar] [CrossRef]


| Oenological Parameters | H. opuntiae | Z. bailii | Co-Inoculation | Sequential |
|---|---|---|---|---|
| Volatile acidity (g/L) | 1.05 ± 0.00 b | 0.64 ± 0.06 a | 1.02 ± 0.00 b | 1.03 ± 0.06 b |
| Reducing sugar(g/L) | 3.92 ± 0.03 a | 4.11 ± 0.07 a | 0.83 ± 0.00 a | 0.70 ± 0.09 a |
| Ethanol (%, v/v) | 10.2 ± 0.1 a | 10.2 ± 0.1 a | 10.9 ± 0.1 c | 10.7 ± 0.2 b |
| Compounds (mg/L) | H. opuntiae | Z. bailii | Co-Inoculation | Sequential (SQ) |
|---|---|---|---|---|
| Alcohols | ||||
| 1-Propanol | 4.0 ± 0.3 a | 28 ± 1 b | 4.5 ± 0.4 a | 4.7 ± 0.3 a |
| Isobutanol | 72 ± 5 c | 20 ± 1 a | 66 ± 2 b | 73 ± 3 c |
| Isoamyl alcohols | 205 ± 13 bc | 130 ± 7 a | 189 ± 6 b | 211 ± 10 c |
| 2-Phenylethanol | 40 ± 2 c | 35 ± 1 b | 27 ± 2 a | 36 ± 3 bc |
| Carbonyl Compounds | ||||
| Acetaldehyde | 129 ± 14 a | 154 ± 7 b | 158 ± 12 b | 174 ± 18 b |
| Acetoin | 36 ± 3 b | 44 ± 3 c | 25 ± 2 a | 28 ± 2 a |
| Esters | ||||
| Ethyl lactate | 22 ± 2 b | 13.4 ± 0.9 a | 24 ± 2 b | 16 ± 2 b |
| Diethyl succinate | 19 ± 2 b | 18 ± 1 b | 7 ± 1 a | 9.30 ± 0.81 a |
| Ethyl acetate | 31 ± 3 c | 5.1 ± 0.2 a | 22 ± 3 b | 22 ± 3 b |
| Polyols | ||||
| 2,3-Butanediol (levo) | 286 ± 15 b | 187 ± 8 a | 208 ± 3 a | 272 ± 19 b |
| 2,3-Butanediol (meso) | 92 ± 6 b | 113 ± 8 c | 75 ± 2 a | 90 ± 8 b |
| Glycerol (g/L) | 4.60 ± 0.10 b | 2.6 ± 0.3 a | 2.80 ± 0.04 a | 4.6 ± 0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcalá-Jiménez, M.T.; García-García, J.C.; Mauricio, J.C.; Moreno, J.; Peinado, R.; García-Martínez, T. Selection of Non-Saccharomyces Yeasts from Extreme Oenological Environments for Potential Use in Winemaking. Microorganisms 2025, 13, 1260. https://doi.org/10.3390/microorganisms13061260
Alcalá-Jiménez MT, García-García JC, Mauricio JC, Moreno J, Peinado R, García-Martínez T. Selection of Non-Saccharomyces Yeasts from Extreme Oenological Environments for Potential Use in Winemaking. Microorganisms. 2025; 13(6):1260. https://doi.org/10.3390/microorganisms13061260
Chicago/Turabian StyleAlcalá-Jiménez, María Trinidad, Juan Carlos García-García, Juan Carlos Mauricio, Juan Moreno, Rafael Peinado, and Teresa García-Martínez. 2025. "Selection of Non-Saccharomyces Yeasts from Extreme Oenological Environments for Potential Use in Winemaking" Microorganisms 13, no. 6: 1260. https://doi.org/10.3390/microorganisms13061260
APA StyleAlcalá-Jiménez, M. T., García-García, J. C., Mauricio, J. C., Moreno, J., Peinado, R., & García-Martínez, T. (2025). Selection of Non-Saccharomyces Yeasts from Extreme Oenological Environments for Potential Use in Winemaking. Microorganisms, 13(6), 1260. https://doi.org/10.3390/microorganisms13061260







