Characterization of Extraintestinal Pathogenic Escherichia coli from Human Clinical and Poultry Samples
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
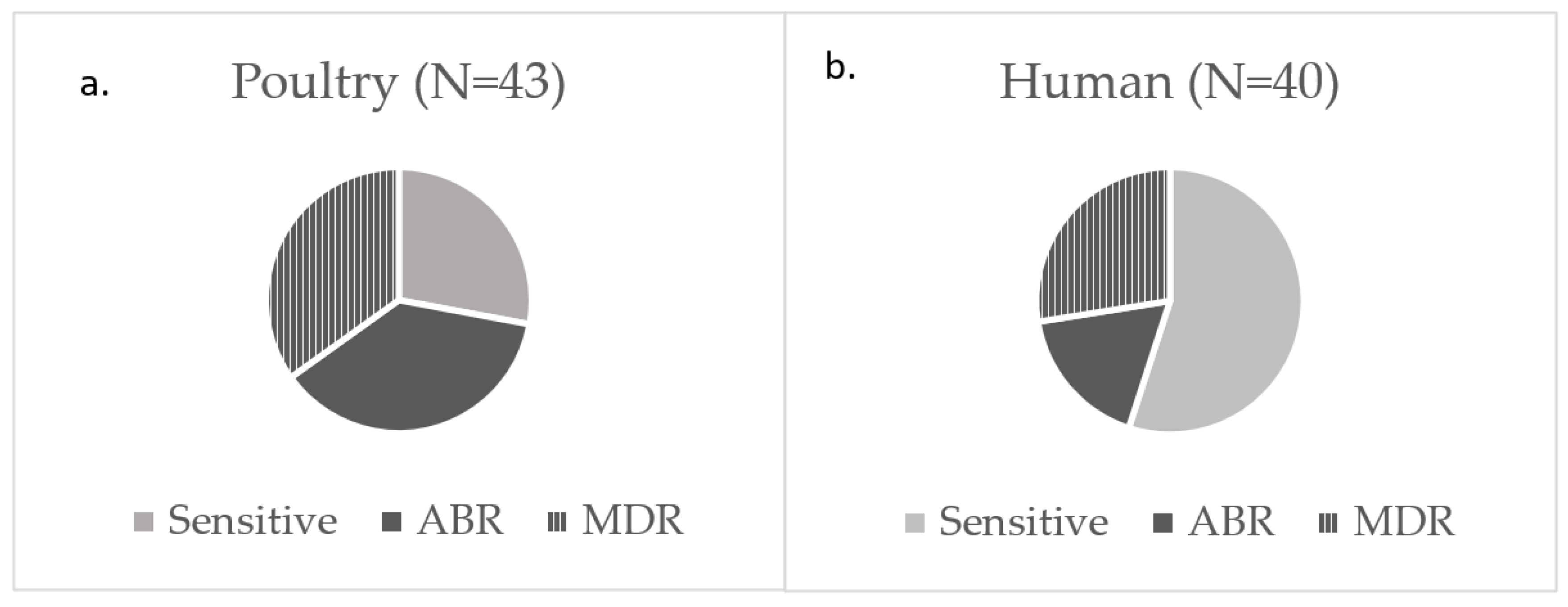
3.1. ExPEC Prevalence in Poultry Samples
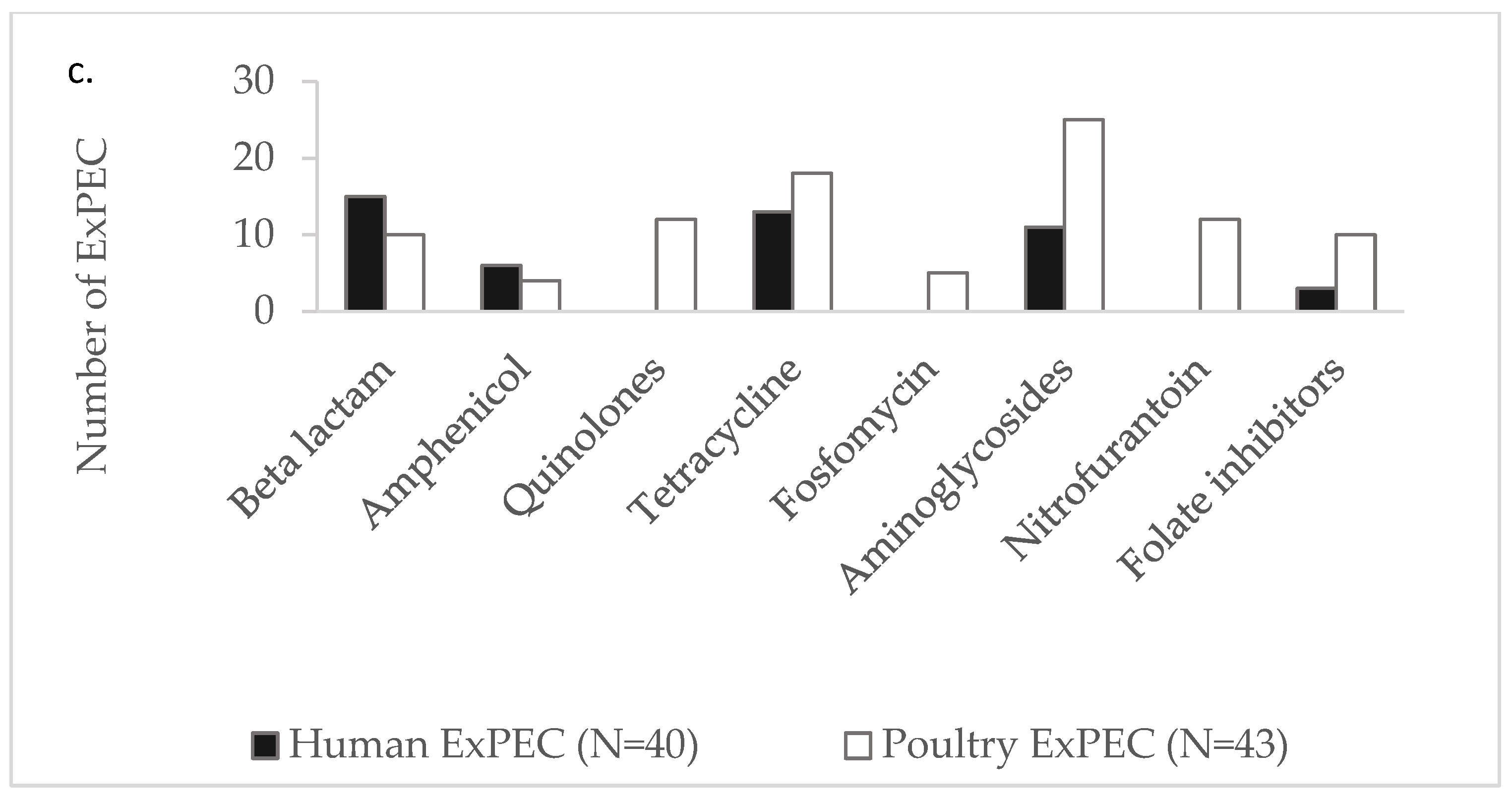
3.2. Antibiotic Susceptibility Among ExPEC
3.3. Sanitizer Tolerance of ExPEC
3.4. Biofilm Formation by ExPEC
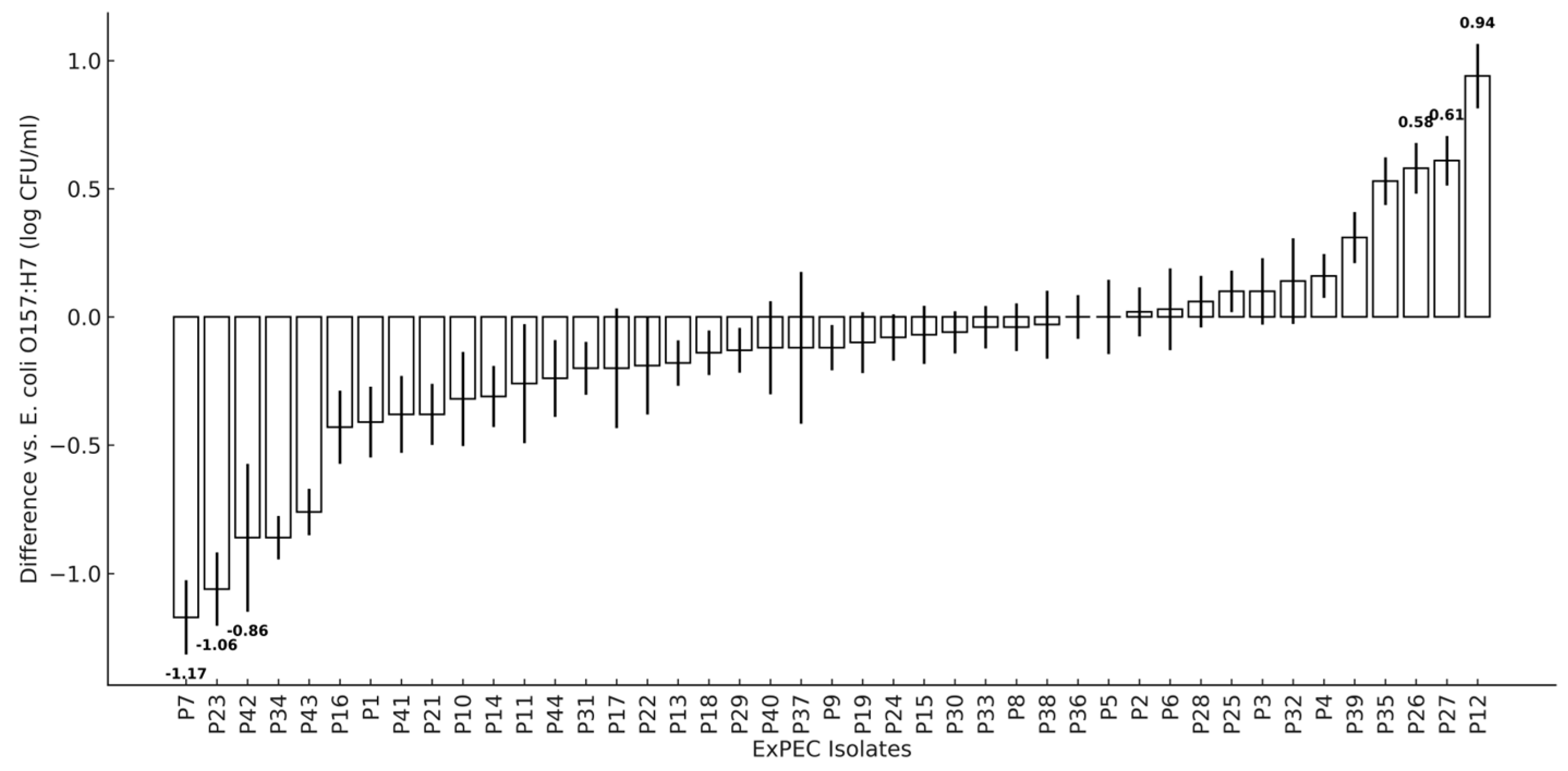
3.5. Adherence of Poultry ExPEC to Human Epithelial Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog. Dis. 2007, 4, 134–163. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef]
- Bonten, M.; Johnson, J.R.; van den Biggelaar, A.H.J.; Georgalis, L.; Geurtsen, J.; de Palacios, P.I.; Gravenstein, S.; Verstraeten, T.; Hermans, P.; Poolman, J.T. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin. Infect. Dis. 2021, 72, 1211–1219. [Google Scholar] [CrossRef]
- Gaschignard, J.; Levy, C.; Romain, O.; Cohen, R.; Bingen, E.; Aujard, Y.; Boileau, P. Neonatal Bacterial Meningitis: 444 Cases in 7 Years. Pediatr. Infect. Dis. J. 2011, 30, 212–217. [Google Scholar] [CrossRef]
- Ouchenir, L.; Renaud, C.; Khan, S.; Bitnun, A.; Boisvert, A.A.; McDonald, J.; Bowes, J.; Brophy, J.; Barton, M.; Ting, J.; et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics 2017, 140, e20170476. [Google Scholar] [CrossRef]
- Okike, I.O.; Johnson, A.P.; Henderson, K.L.; Blackburn, R.M.; Muller-Pebody, B.; Ladhani, S.N.; Anthony, M.; Ninis, N.; Heath, P.T.; neoMen Study Group. Incidence, etiology, and outcome of bacterial meningitis in infants aged < 90 days in the United kingdom and Republic of Ireland: Prospective, enhanced, national population-based surveillance. Clin. Infect. Dis. 2014, 59, e150–e157. [Google Scholar] [CrossRef]
- Zeng, Z.L.; Zhan, J.; Zhang, K.M.; Chen, H.L.; Cheng, S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: An analysis of the global burden of disease study 2019. World J. Urol. 2022, 40, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Ferraresso, J.; Apostolakos, I.; Fasolato, L.; Piccirillo, A. Third-generation cephalosporin (3GC) resistance and its association with Extra-intestinal pathogenic Escherichia coli (ExPEC). Focus on broiler carcasses. Food Microbiol. 2022, 103, 103936. [Google Scholar] [CrossRef] [PubMed]
- Awawdeh, L.; Turni, C.; Mollinger, J.L.; Henning, J.; Cobbold, R.N.; Trott, D.J.; Wakeham, D.L.; Gibson, J.S. Antimicrobial susceptibility, plasmid replicon typing, phylogenetic grouping, and virulence potential of avian pathogenic and faecal Escherichia coli isolated from meat chickens in Australia. Avian Pathol. 2022, 51, 349–360. [Google Scholar] [CrossRef]
- Aklilu, E.; Harun, A.; Singh, K.K.B. Molecular characterization of blaNDM, blaOXA-48, mcr-1 and blaTEM-52 positive and concurrently carbapenem and colistin resistant and extended spectrum beta-lactamase producing E. coli in chicken in Malaysia. BMC Vet. Res. 2022, 18, 190. [Google Scholar] [CrossRef]
- Liu, C.M.; Stegger, M.; Aziz, M.; Johnson, T.J.; Waits, K.; Nordstrom, L.; Gauld, L.; Weaver, B.; Rolland, D.; Statham, S.; et al. Escherichia coli ST131-H22 as a Foodborne Uropathogen. mBio 2018, 9, e00470-18. [Google Scholar] [CrossRef]
- Lee, S.; An, J.U.; Woo, J.; Song, H.; Yi, S.; Kim, W.H.; Lee, J.H.; Ryu, S.; Cho, S. Prevalence, Characteristics, and Clonal Distribution of Escherichia coli Carrying Mobilized Colistin Resistance Gene mcr-1.1 in Swine Farms and Their Differences According to Swine Production Stages. Front. Microbiol. 2022, 13, 873856. [Google Scholar] [CrossRef]
- Stromberg, Z.R.; Johnson, J.R.; Fairbrother, J.M.; Kilbourne, J.; Van Goor, A.; Curtiss, R.; Mellata, M. Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PLoS ONE 2017, 12, e0180599. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, R.; Friedman, C.R.; Rubin, J.; Suh, J.; Thys, E.; McDermott, P.; Hung-Fan, M.; Riley, L.W. A Population-Based Surveillance Study of Shared Genotypes of Escherichia coli Isolates from Retail Meat and Suspected Cases of Urinary Tract Infections. mSphere 2018, 3, e00179-18. [Google Scholar] [CrossRef]
- Meena, P.R.; Priyanka, P.; Rana, A.; Raj, D.; Singh, A.P. Alarming level of single or multidrug resistance in poultry environments-associated extraintestinal pathogenic pathotypes with potential to affect the One Health. Env. Microbiol. Rep. 2022, 14, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mao, L.; Li, J.; Hao, F.; Yang, L.; Zhang, W.; Sun, M.; Liu, M.; Wang, S.; Li, W. Molecular characterization and antimicrobial resistance profile of pathogenic Escherichia coli from goats with respiratory disease in eastern China. Microb. Pathog. 2022, 166, 105501. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut. Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Tilman, S.; Wisser-Parker, K.; Scullen, O.J.; Sommers, C.H. Draft Genomic Sequences of Nine Extraintestinal Pathogenic Escherichia coli Isolates from Retail Chicken Skin. Microbiol. Resour. Announc. 2018, 7. [Google Scholar] [CrossRef]
- Harrison, L.; Tyson, G.H.; Strain, E.; Lindsey, R.L.; Strockbine, N.; Ceric, O.; Fortenberry, G.Z.; Harris, B.; Shaw, S.; Tillman, G.; et al. Use of Large-Scale Genomics to Identify the Role of Animals and Foods as Potential Sources of Extraintestinal Pathogenic Escherichia coli That Cause Human Illness. Foods 2022, 11, 1975. [Google Scholar] [CrossRef]
- Yamaji, R.; Rubin, J.; Thys, E.; Friedman, C.R.; Riley, L.W. Persistent Pandemic Lineages of Uropathogenic Escherichia coli in a College Community from 1999 to 2017. J. Clin. Microbiol. 2018, 56, e01834-17. [Google Scholar] [CrossRef]
- Mora, A.; Lopez, C.; Dabhi, G.; Blanco, M.; Blanco, J.E.; Alonso, M.P.; Herrera, A.; Mamani, R.; Bonacorsi, S.; Moulin-Schouleur, M.; et al. Extraintestinal pathogenic Escherichia coli O1:K1:H7/NM from human and avian origin: Detection of clonal groups B2 ST95 and D ST59 with different host distribution. BMC Microbiol. 2009, 9, 132. [Google Scholar] [CrossRef]
- Jakobsen, L.; Spangholm, D.J.; Pedersen, K.; Jensen, L.B.; Emborg, H.D.; Agerso, Y.; Aarestrup, F.M.; Hammerum, A.M.; Frimodt-Moller, N. Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int. J. Food. Microbiol. 2010, 142, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.; Garneau, P.; Bruant, G.; Harel, J.; Olsen, S.S.; Porsbo, L.J.; Hammerum, A.M.; Frimodt-Moller, N. Is Escherichia coli urinary tract infection a zoonosis? Proof of direct link with production animals and meat. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1121–1129. [Google Scholar] [CrossRef]
- Tivendale, K.A.; Logue, C.M.; Kariyawasam, S.; Jordan, D.; Hussein, A.; Li, G.; Wannemuehler, Y.; Nolan, L.K. Avian-pathogenic Escherichia coli strains are similar to neonatal meningitis E. coli strains and are able to cause meningitis in the rat model of human disease. Infect. Immun. 2010, 78, 3412–3419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, S.; Huan, H.; Xu, X.; Zhu, X.; Yang, W.; Gao, Q.; Liu, X. Comparison of virulence factors and expression of specific genes between uropathogenic Escherichia coli and avian pathogenic E. coli in a murine urinary tract infection model and a chicken challenge model. Microbiology 2009, 155, 1634–1644. [Google Scholar] [CrossRef]
- CDC. Summary of possible multistate enteric (intestinal) disease outbreaks in 2021. In Foodborne Outbreaks; Center for Diease Control: Atlanta, GA, USA, 2024. [Google Scholar]
- Guragain, M.; Schmidt, J.W.; Kalchayanand, N.; Dickey, A.M.; Bosilevac, J.M. Characterization of Escherichia coli harboring colibactin genes (clb) isolated from beef production and processing systems. Sci. Rep. 2022, 12, 5305. [Google Scholar] [CrossRef]
- DeLira-Bustillos, N.; Angulo-Zamudio, U.A.; Leon-Sicairos, N.; Flores-Villasenor, H.; Velazquez-Roman, J.; Tapia-Pastrana, G.; Martinez-Villa, F.A.; Velazquez-Cruz, R.; Salmeron, J.; Canizales-Quinteros, S.; et al. Distribution and virulence of Escherichia coli harboring cyclomodulins and supplementary virulence genes isolates from clinical and environmental samples. Microb. Pathog. 2024, 190, 106634. [Google Scholar] [CrossRef]
- Mora-Coto, D.; Moreno-Vélez, P.; Luna-Muñoz, J.; Moreno-Campuzano, S.; Ontiveros-Torres, M.A. Intestinal and Extraintestinal Pathotypes of Escherichia coli Are Prevalent in Food Prepared and Marketed on the Streets from the Central Zone of Mexico and Exhibit a Differential Phenotype of Resistance Against Antibiotics. Antibiotics 2025, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Meng, J.; Zhao, S.; Bodeis-Jones, S.; Gaines, S.A.; Ayers, S.L.; McDermott, P.F. Identification and antimicrobial resistance of extraintestinal pathogenic Escherichia coli from retail meats. J. Food. Prot. 2011, 74, 38–44. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Anti-Microbial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Chen, C.Y.; Nace, G.W.; Irwin, P.L. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods 2003, 55, 475–479. [Google Scholar] [CrossRef]
- EPA 712-C-07-091; Product Performance Test Guidelines OCSPP 810.2300: Sanitizers for Use on Hard Surfaces—Efficacy Data Recommendations. Environmental Protection Agency: Washington, DC, USA, 2012.
- Uhlich, G.A.; Keen, J.E.; Elder, R.O. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2001, 67, 2367–2370. [Google Scholar] [CrossRef]
- Kondratyeva, K.; Wollman, A.; Gerlitz, G.; Navon-Venezia, S. Adhesion and invasion to epithelial cells and motility of extended-spectrum β-lactamase-producing Escherichia coli reveal ST131 superiority: A comparative in vitro study of extraintestinal pathogenic E. coli lineages. J. Med. Microbiol. 2017, 66, 1350–1357. [Google Scholar] [CrossRef]
- Johnson, J.R.; Delavari, P.; O’Bryan, T.T.; Smith, K.E.; Tatini, S. Contamination of retail foods, particularly turkey, from community markets (Minnesota, 1999–2000) with antimicrobial-resistant and extraintestinal pathogenic Escherichia coli. Foodborne Pathog. Dis. 2005, 2, 38–49. [Google Scholar] [CrossRef]
- Johnson, J.R.; Murray, A.C.; Gajewski, A.; Sullivan, M.; Snippes, P.; Kuskowski, M.A.; Smith, K.E. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob. Agents Chemother. 2003, 47, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: Early Implementation 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wu, D.; Ding, Y.; Yao, K.; Gao, W.; Wang, Y. Antimicrobial Resistance Analysis of Clinical Escherichia coli Isolates in Neonatal Ward. Front. Pediatr. 2021, 9, 670470. [Google Scholar] [CrossRef] [PubMed]
- Massella, E.; Giacometti, F.; Bonilauri, P.; Reid, C.J.; Djordjevic, S.P.; Merialdi, G.; Bacci, C.; Fiorentini, L.; Massi, P.; Bardasi, L.; et al. Antimicrobial Resistance Profile and ExPEC Virulence Potential in Commensal Escherichia coli of Multiple Sources. Antibiotics 2021, 10, 351. [Google Scholar] [CrossRef]
- Hines, M.C.; Al-Salamah, T.; Heil, E.L.; Mallemat, H.; Witting, M.D.; Johnson, J.K.; Winters, M.E.; Hayes, B.D. Resistance patterns of Escherichia coli in women with uncomplicated urinary tract infection do not correlate with emergency department antibiogram. J. Emerg. Med. 2015, 49, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Zhang, L.; Palin, K.; Tallman, P.; Marrs, C.F. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J. Infect. Dis. 1995, 171, 1514–1521. [Google Scholar] [CrossRef]
- Xu, A.; Hertrich, S.; Needleman, D.S.; Sheen, S.; Sommers, C. Draft Genome Sequences of Four Uropathogenic Escherichia coli Serotype O4:H5 Isolates (ATCC 700414, 700415, 700416, and 700417). Genome Announc. 2018, 6, e00134-18. [Google Scholar] [CrossRef]
- Jhonson, J.R.; Russo, T.A.; Scheutz, F.; Brown, J.J.; Zhang, L.X.; Palin, K.; Rode, C.; Bloch, C.; Marrs, C.F.; Foxman, B. Discovery of disseminated J96-like strains of uropathogenic Escherichia coli O4:H5 containing genes for both papG(J96) (class I) and prsG(J96) (class III) Gal(alpha 1-4)Gal-binding adhesins. J. Infect. Dis. 1997, 175, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Bortolussi, R.; Ferrieri, P.; Bjorksten, B.; Quie, P.G. Capsular K1 polysaccharide of Escherichia coli: Relationship to virulence in newborn rats and resistance to phagocytosis. Infect. Immun. 1979, 25, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Swenson, D.L.; Bukanov, N.O.; Berg, D.E.; Welch, R.A. Two pathogenicity islands in uropathogenic Escherichia coli J96: Cosmid cloning and sample sequencing. Infect. Immun. 1996, 64, 3736–3743. [Google Scholar] [CrossRef]
- Mobley, H.L.; Green, D.M.; Trifillis, A.L.; Johnson, D.E.; Chippendale, G.R.; Lockatell, C.V.; Jones, B.D.; Warren, J.W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: Role of hemolysin in some strains. Infect. Immun. 1990, 58, 1281–1289. [Google Scholar] [CrossRef]
- Johnson, J.R.; Manges, A.R.; O’Bryan, T.T.; Riley, L.W. A disseminated multidrug-resistant clonal group of uropathogenic Escherichia coli in pyelonephritis. Lancet 2002, 359, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Johnson, J.R.; Sheen, S.; Sommers, C. Draft Genome Sequences of Five Neonatal Meningitis-Causing Escherichia coli Isolates (SP-4, SP-5, SP-13, SP-46, and SP-65). Genome Announc. 2018, 6, e00091-18. [Google Scholar] [CrossRef]
- Sommers, C.H.; Scullen, O.J.; Sheen, S. Inactivation of Uropathogenic Escherichia coli in Ground Chicken Meat Using High Pressure Processing and Gamma Radiation, and in Purge and Chicken Meat Surfaces by Ultraviolet Light. Front. Microbiol. 2016, 7, 413. [Google Scholar] [CrossRef]
- Sommers, C.; Gunther, N.W.t.; Sheen, S. Inactivation of Salmonella spp., pathogenic Escherichia coli, Staphylococcus spp., or Listeria monocytogenes in chicken purge or skin using a 405-nm LED array. Food Microbiol. 2017, 64, 135–138. [Google Scholar] [CrossRef]
- Ajibola, A.T.; de Lagarde, M.; Ojo, O.E.; Balogun, S.A.; Vanier, G.; Fairbrother, J.M.; Shittu, O.B. Antimicrobial resistance and virulence gene profiles of Escherichia coli isolated from poultry farms using One Health perspective in Abeokuta, Nigeria. BMC Microbiol. 2025, 25, 440. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.; Wu, G.; Wu, Y.; Li, H.; Shao, B. Association analysis of antibiotic and disinfectant resistome in human and foodborne E. coli in Beijing, China. Sci. Total Environ. 2024, 944, 173888. [Google Scholar] [CrossRef]
- Sahin, S.; Mogulkoc, M.N.; Kürekci, C. Disinfectant and heavy metal resistance profiles in extended spectrum β-lactamase (ESBL) producing Escherichia coli isolates from chicken meat samples. Int. J. Food Microbiol. 2022, 377, 109831. [Google Scholar] [CrossRef]
- Al-Mustapha, A.I.; Alada, S.A.; Raufu, I.A.; Lawal, A.N.; Eskola, K.; Brouwer, M.S.M.; Adetunji, V.; Heikinheimo, A. Co-occurrence of antibiotic and disinfectant resistance genes in extensively drug-resistant Escherichia coli isolated from broilers in Ilorin, North Central Nigeria. J. Glob. Antimicrob. Resist. 2022, 31, 337–344. [Google Scholar] [CrossRef]
- Andersson, D.I. Persistence of antibiotic resistant bacteria. Curr. Opin. Microbiol. 2003, 6, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Oguadinma, I.C.; Mishra, A.; Juneja, V.K.; Dev Kumar, G. Antibiotic Resistance Influences Growth Rates and Cross-Tolerance to Lactic Acid in Escherichia coli O157:H7 H1730. Foodborne Pathog. Dis. 2022, 19, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Chapter seven—Metal resistance and its association with antibiotic resistance. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 70, pp. 261–313. [Google Scholar]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Lindsay, D.; von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar] [CrossRef]
- Martinez, J.J.; Mulvey, M.A.; Schilling, J.D.; Pinkner, J.S.; Hultgren, S.J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000, 19, 2803–2812. [Google Scholar] [CrossRef]
- Conover, M.S.; Hadjifrangiskou, M.; Palermo, J.J.; Hibbing, M.E.; Dodson, K.W.; Hultgren, S.J. Metabolic Requirements of Escherichia coli in Intracellular Bacterial Communities during Urinary Tract Infection Pathogenesis. mBio 2016, 7. [Google Scholar] [CrossRef]
- Gunathilaka, G.; Dewasmika, W.; Sandaruwan, U.M.; Neelawala, N.; Madhumali, G.E.D.; Dissanayake, B.N.; Priyantha, M.A.R.; Prasada, D.V.P.; Dissanayake, D.R.A. Biofilm-forming ability, antibiotic resistance and phylogeny of Escherichia coli isolated from extra intestinal infections of humans, dogs, and chickens. Comp. Immunol. Microbiol. Infect. Dis. 2024, 105, 102123. [Google Scholar] [CrossRef]
- Ballen, V.; Gabasa, Y.; Ratia, C.; Sánchez, M.; Soto, S. Correlation Between Antimicrobial Resistance, Virulence Determinants and Biofilm Formation Ability Among Extraintestinal Pathogenic E. coli Strains Isolated in Catalonia, Spain. Front. Microbiol. 2022, 12, 803862. [Google Scholar] [CrossRef]
- Nielsen, D.W.; Klimavicz, J.S.; Cavender, T.; Wannemuehler, Y.; Barbieri, N.L.; Nolan, L.K.; Logue, C.M. The Impact of Media, Phylogenetic Classification, and E. coli Pathotypes on Biofilm Formation in Extraintestinal and Commensal E. coli From Humans and Animals. Front. Microbiol. 2018, 9, 902. [Google Scholar] [CrossRef]
- Liu, C.M.; Aziz, M.; Park, D.E.; Wu, Z.; Stegger, M.; Li, M.; Wang, Y.; Schmidlin, K.; Johnson, T.J.; Koch, B.J.; et al. Using source-associated mobile genetic elements to identify zoonotic extraintestinal E. coli infections. One Health 2023, 16, 100518. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, B.C.L.; van Hattem, J.M.; Penders, J.; Consortium, C.; Mende, D.R.; Schultsz, C. Extra-intestinal pathogenic lineages of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli are associated with prolonged ESBL gene carriage. Access Microbiol. 2024, 6, 000541.v4. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef] [PubMed]
| Isolate a | Source b | Geographical Location c | Reference d | Antibiotic Resistance e | Sanitizer Resistance | Biofilm Score g | |
|---|---|---|---|---|---|---|---|
| Untreated | 300 ppm QAC f | ||||||
| H1 | UTI | MW, USA | This study | ABR | 8.98 ± 0.04 | ND | 1 |
| H2 | UTI | PW, USA | This study | S | 8.70 ± 0.02 | ND | 1 |
| H3 | UTI | SE USA | This study | MDR | 8.90 ± 0.01 | ND | 1 |
| H4 | UTI | SE, USA | This study | MDR | 8.91 ± 0.08 | 4.44 ± 2.11 | 0 |
| H5 | UTI | MW, USA | This study | MDR | 8.92 ± 0.07 | 2.98 ± 1.14 | 0 |
| H6 | UTI | NE, USA | This study | S | 8.72 ± 0.06 | ND | 1 |
| H7 | UTI | NE, USA | This study | ABR | 8.77 ± 0.09 | ND | 0 |
| H8 | UTI | NE, USA | This study | S | 8.72 ± 0.06 | 4.09 ± 1.06 | 1 |
| H9 | UTI | NE, USA | This study | S | 8.83 ± 0.05 | ND | 0 |
| H10 | UTI | NE, USA | This study | ABR | 8.81 ± 0.02 | ND | 1 |
| H11 | UTI | NE, USA | This study | S | 8.83 ± 0.08 | ND | 1 |
| H12 | UTI | NE, USA | This study | S | 8.85 ± 0.02 | ND | 1 |
| H13 | UTI | NE, USA | This study | MDR | 8.90 ± 0.02 | 4.09 ± 0.68 I | 1 |
| H14 | UTI | NE, USA | This study | S | 8.86 ± 0.08 | ND | 0 |
| H15 | UTI | NE, USA | This study | S | 8.81 ± 0.07 | ND | 0 |
| H16 | UTI | NE, USA | This study | S | 8.78 ± 0.09 | 3.78 ± 0.88 | 1 |
| H17 | UTI | PA, USA | This study | S | 8.93 ± 0.05 | 6.72 ± 0.19 R | 1 |
| H18 | UTI | PA, USA | This study | S | 8.88 ± 0.05 | ND | 1 |
| H19 | UTI | NE, USA | This study | ABR | 8.77 ± 0.02 | ND | 0 |
| H20 | UTI | NE, USA | This study | S | 8.83 ± 0.04 | ND | 0 |
| H21 | NA | NA | MDR | 8.78 ± 0.05 | ND | 1 | |
| H22 | NA | NA | MDR | 8.87 ± 0.10 | ND | 0 | |
| H23 | NA | NA | MDR | 8.73 ± 0.07 | ND | 1 | |
| H24 | NA | NA | MDR | 8.84 ± 0.05 | ND | 1 | |
| H25 | NA | NA | MDR | 8.82 ± 0.10 | ND | 0 | |
| H26 | UTI | NA | [43] | S | 8.96 ± 0.09 | 3.41 ± 0.36 | 1 |
| H27 | UTI | PA, USA | [44] | ABR | 8.88 ± 0.11 | ND | 1 |
| H28 | UTI | PA, USA | [45] | ABR | 8.90 ± 0.01 | ND | 0 |
| H29 | UTI | PA, USA | [45] | ABR | 8.87 ± 0.09 | ND | 0 |
| H30 | UTI | NA | [45] | S | 8.73 ± 0.06 | ND | 0 |
| H31 | NM | NA | [46] | S | 8.86 ± 0.03 | ND | 1 |
| H32 | UTI | PW, USA | [47] | S | 8.90 ± 0.03 | ND | 2 |
| H33 | UTI | NE, USA | [48] | S | 8.92 ± 0.03 | ND | 0 |
| H34 | UTI | NA | This study | ABR | 8.83 ± 0.05 | 2.45 ± 0.78 | 0 |
| H35 | UTI | NA | This study | S | 8.59 ± 0.07 | 3.26 ± 0.59 | 1 |
| H36 | UTI | MW, USA | [49] | MDR | 8.99 ± 0.05 | ND | 0 |
| H37 | NM | Netherlands | [50] | S | 8.90 ± 0.05 | ND | 1 |
| H38 | NM | Netherlands | [50] | MDR | 8.90 ± 0.00 | ND | 1 |
| H39 | NM | Netherlands | [50] | S | 8.88 ± 0.03 | ND | 1 |
| H40 | NM | Netherlands | [50] | S | 8.88 ± 0.04 | 0.09 ± 0.12 | 1 |
| P1 | Chicken | Mexico | This study | MDR | 8.67 ± 0.20 | ND | 0 |
| P2 | Chicken | Mexico | This study | MDR | 8.83 ± 0.10 | ND | 0 |
| P3 | Chicken | Mexico | This study | MDR | 8.63 ± 0.17 | ND | 2 |
| P4 | Chicken | Mexico | This study | MDR | 8.85 ± 0.11 | ND | 1 |
| P5 | Chicken | Mexico | This study | MDR | 8.90 ± 0.16 | ND | 3 |
| P6 | Chicken | Mexico | This study | MDR | 8.76 ± 0.12 | ND | 1 |
| P7 | Chicken | Mexico | This study | MDR | 8.42 ± 0.25 | ND | 0 |
| P8 | Chicken | Mexico | This study | MDR | 8.52 ± 0.23 | ND | 1 |
| P9 | Chicken | Mexico | This study | MDR | 8.56 ± 0.29 | ND | 1 |
| P10 | Chicken | Mexico | This study | MDR | 8.66 ± 0.20 | ND | 2 |
| P11 | Chicken | Mexico | This study | MDR | 8.90 ± 0.09 | ND | 1 |
| P12 | Turkey | PW, USA | This study | S | 8.80 ± 0.10 | 3.57 ± 0.46 | 1 |
| P13 | Chicken | MW, USA | This study | ABR | 8.63 ± 0.16 | ND | 0 |
| P14 | Chicken | PW, USA | This study | ABR | 8.69 ± 0.19 | ND | 1 |
| P15 | Chicken | MW, USA | This study | S | 8.71 ± 0.13 | ND | 0 |
| P16 | Chicken | SE, USA | This study | S | 8.71 ± 0.23 | ND | 1 |
| P17 | Chicken | PA, USA | This study | MDR | 8.71 ± 0.17 | ND | 0 |
| P18 | Chicken | PW, USA | This study | ABR | 8.70 ± 0.21 | ND | 0 |
| P19 | Chicken | NE, USA | This study | ABR | 8.76 ± 0.15 | ND | 0 |
| P21 | Chicken | NE, USA | This study | S | 8.82 ± 0.17 | ND | 0 |
| P22 | Chicken | NE, USA | This study | ABR | 8.43 ± 0.20 | ND | 1 |
| P23 | Chicken | NE, USA | This study | S | 8.77 ± 0.19 | ND | 0 |
| P24 | Chicken | PA, USA | This study | S | 8.82 ± 0.14 | ND | 1 |
| P25 | Chicken | SE, USA | This study | ABR | 8.84 ± 0.15 | 4.72 ± 0.71 | 0 |
| P26 | Chicken | NE, USA | This study | S | 8.59 ± 0.17 | ND | 1 |
| P27 | Chicken | SE, USA | This study | ABR | 8.78 ± 0.16 | 5.4 ± 0.8 I | 1 |
| P28 | Chicken | SE, USA | This study | S | 8.78 ± 0.15 | ND | 1 |
| P29 | Chicken | MW, USA | This study | ABR | 8.86 ± 0.10 | ND | 0 |
| P30 | Chicken | SE, USA | This study | MDR | 8.83 ± 0.14 | ND | 2 |
| P31 | Chicken | NE, USA | This study | S | 8.86 ± 0.14 | ND | 0 |
| P32 | Chicken | NE, USA | This study | S | 8.78 ± 0.18 | ND | 0 |
| P33 | Turkey | SE, USA | This study | ABR | 8.84 ± 0.39 | ND | 1 |
| P34 | Turkey | SE, USA | This study | MDR | 8.72 ± 0.18 | ND | 0 |
| P35 | Turkey | MW, USA | This study | S | 8.85 ± 0.15 | ND | 0 |
| P36 | Chicken | NE, USA | [19] | MDR | 8.78 ± 0.10 | ND | 1 |
| P37 | Chicken | NE, USA | [19] | ABR | 8.79 ± 0.05 | ND | 1 |
| P38 | Chicken | NE, USA | [19] | ABR | 8.73 ± 0.11 | ND | 0 |
| P39 | Chicken | NE, USA | [19] | S | 8.80 ± 0.07 | ND | 0 |
| P40 | Chicken | NE, USA | [19] | ABR | 8.82 ± 0.05 | ND | 1 |
| P41 | Chicken | NE, USA | [19] | ABR | 8.79 ± 0.01 | 3.17 ± 0.29 | 1 |
| P42 | Chicken | NE, USA | [19] | MDR | 8.86 ± 0.08 | ND | 1 |
| P43 | Chicken | NE, USA | [19] | ABR | 8.82 ± 0.06 | ND | 1 |
| P44 | Chicken | SE, USA | [19] | S | 8.77 ± 0.18 | ND | 1 |
| BHI | - | - | - | - | - | 0 | |
| 43894 OR 22/30 | Human O157:H7 | [35] | - | - | - | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guragain, M.; Bagi, L.; Liu, Y.; Bosilevac, J.M. Characterization of Extraintestinal Pathogenic Escherichia coli from Human Clinical and Poultry Samples. Microorganisms 2025, 13, 2603. https://doi.org/10.3390/microorganisms13112603
Guragain M, Bagi L, Liu Y, Bosilevac JM. Characterization of Extraintestinal Pathogenic Escherichia coli from Human Clinical and Poultry Samples. Microorganisms. 2025; 13(11):2603. https://doi.org/10.3390/microorganisms13112603
Chicago/Turabian StyleGuragain, Manita, Lori Bagi, Yanhong Liu, and Joseph M. Bosilevac. 2025. "Characterization of Extraintestinal Pathogenic Escherichia coli from Human Clinical and Poultry Samples" Microorganisms 13, no. 11: 2603. https://doi.org/10.3390/microorganisms13112603
APA StyleGuragain, M., Bagi, L., Liu, Y., & Bosilevac, J. M. (2025). Characterization of Extraintestinal Pathogenic Escherichia coli from Human Clinical and Poultry Samples. Microorganisms, 13(11), 2603. https://doi.org/10.3390/microorganisms13112603








