Gut Bacteria Strategies of Hylurgus ligniperda F. (Coleoptera Scolytidae) in Adapting to Temperature Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Gut Sample Preparation and DNA Extraction
2.3. Metagenome Sequencing and Annotation
2.4. Screening of Culturable Gut Bacterial Strains
2.5. Impact of Gut Bacteria on Feed Nutrient Composition and RHB Development
2.6. Culture Media Formulations and Artificial Diet Formulations for RHBs
2.7. Statistical Analysis
3. Results
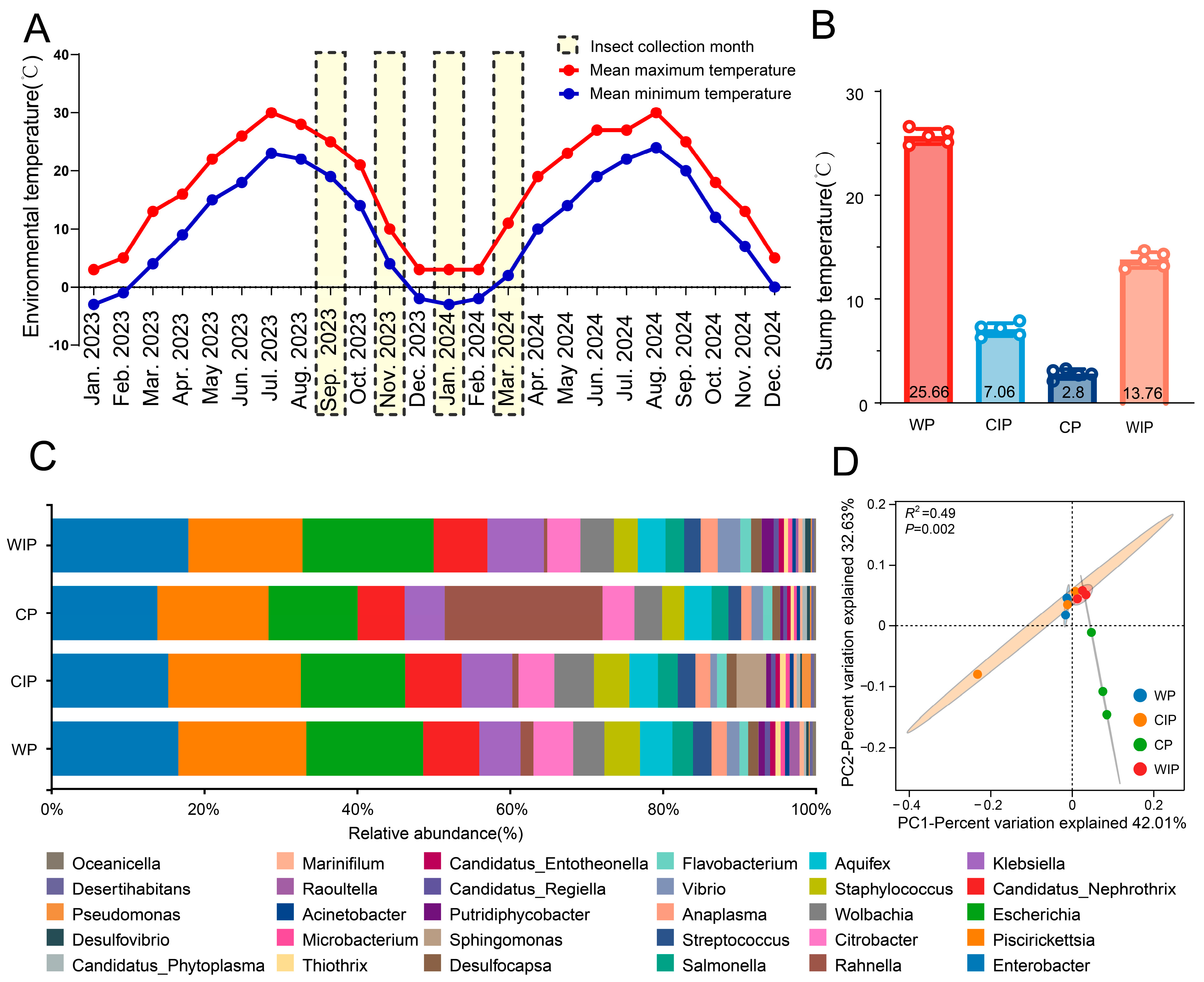
3.1. Gut Bacterial Community States in RHBs Under Different Temperature Conditions
3.2. Metabolic Function and Key Genera of the RHB Gut Bacterial Under Different Temperature Regimes
3.3. Screening for Culturable Gut Bacteria with Extracellular Protease and Lipase Activity
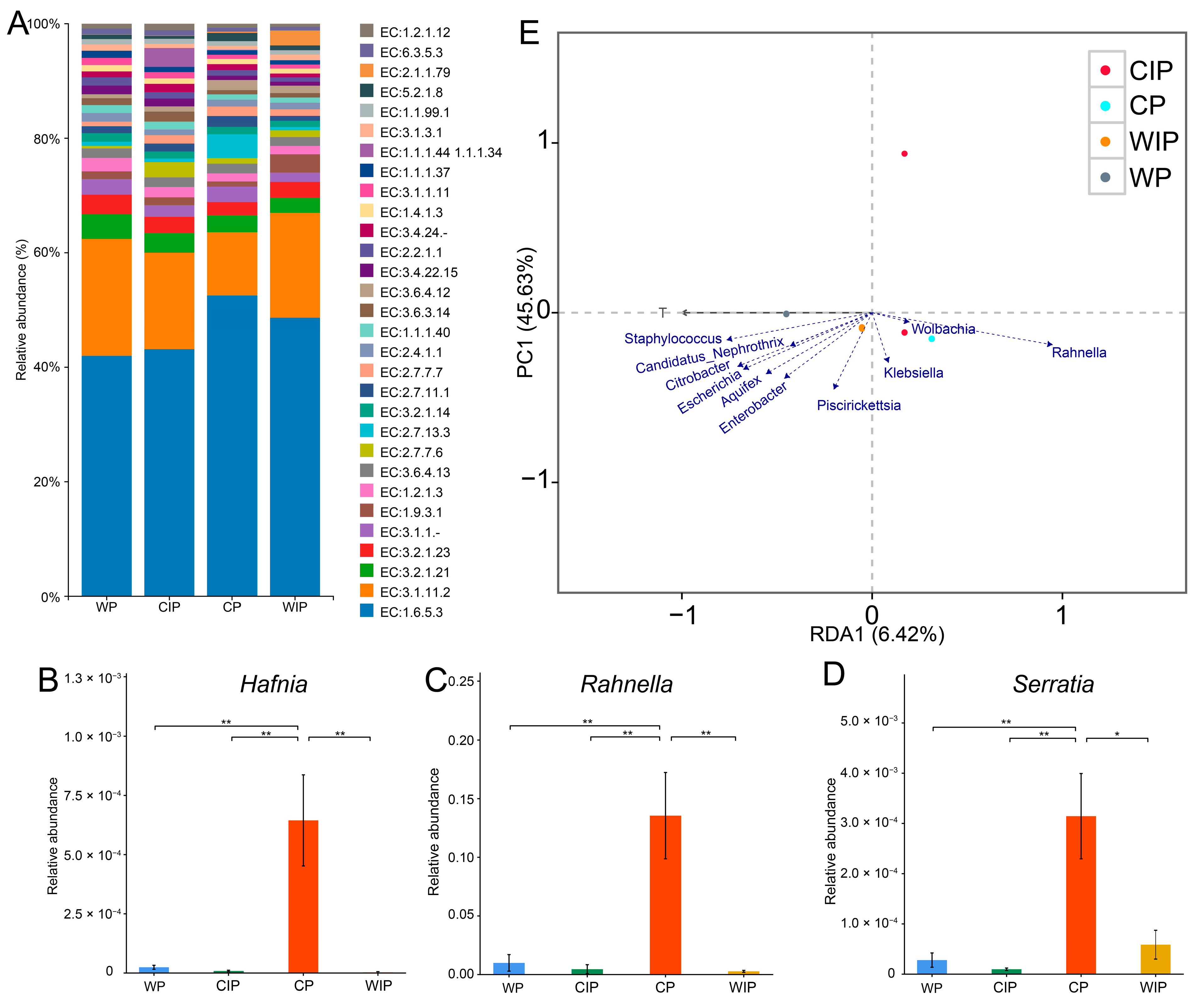

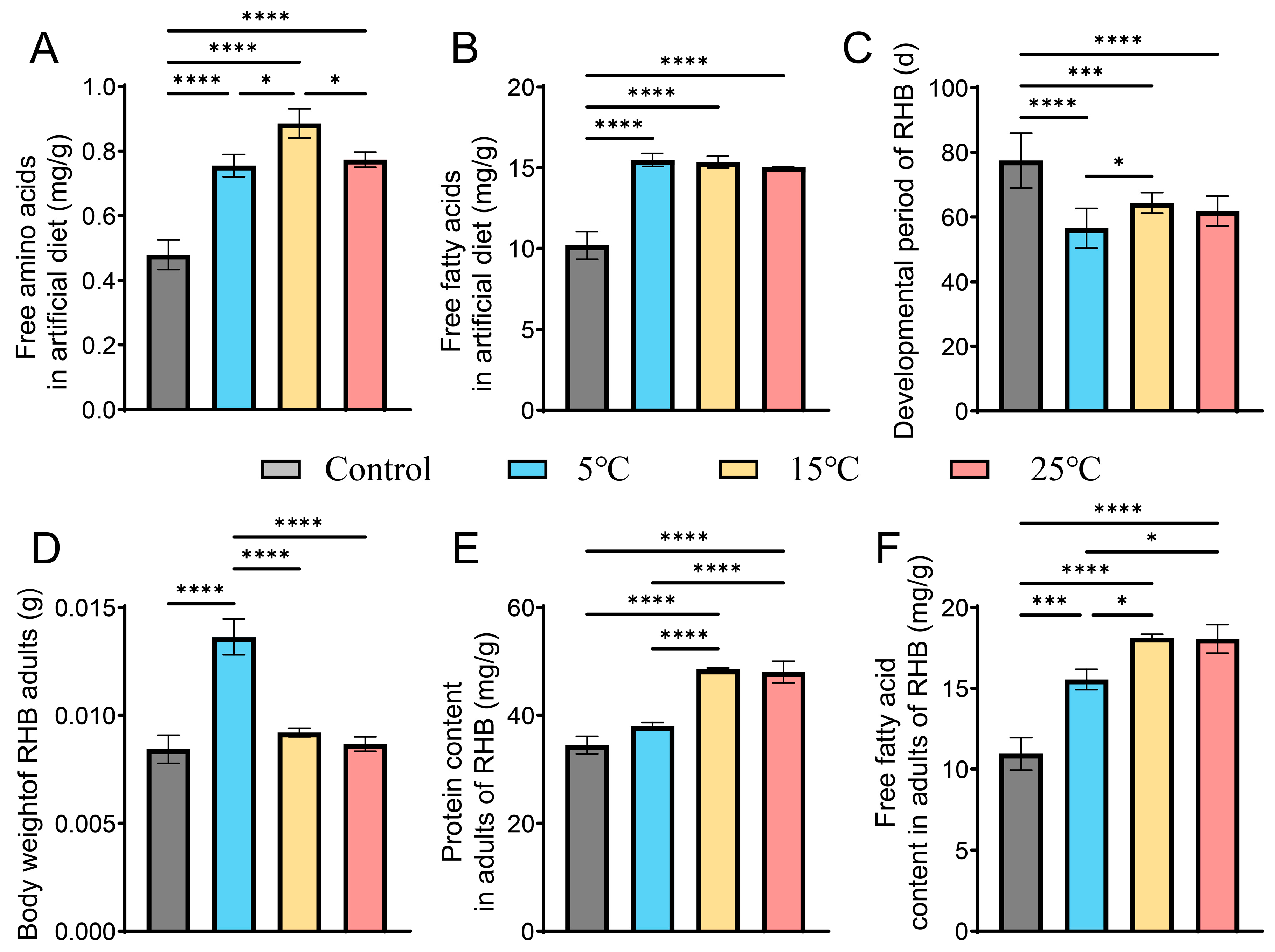
3.4. R. perminowiae Impact on Feed Nutritional Structure and RHB Development at Three Different Temperatures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Tihelka, E.; Cai, C.; Giacomelli, M.; Lozano-Fernandez, J.; Rota-Stabelli, O.; Huang, D.; Engel, M.S.; Donoghue, P.C.J.; Pisani, D. The evolution of insect biodiversity. Curr. Biol. 2021, 31, R1299–R1311. [Google Scholar] [CrossRef] [PubMed]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Gupta, A.; Nair, S. Dynamics of Insect–Microbiome Interaction Influence Host and Microbial Symbiont. Front. Microbiol. 2020, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into Diversity and Functionalities of Gut Microbiota in Insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef]
- Marulanda-Moreno, S.M.; Saldamando-Benjumea, C.I.; Gomez, R.V.; Cadavid-Restrepo, G.; Moreno-Herrera, C.X. Comparative analysis of Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae) corn and rice strains microbiota revealed minor changes across life cycle and strain endosymbiont association. PeerJ 2024, 12, e17087. [Google Scholar] [CrossRef] [PubMed]
- Provorov, N.A.; Onishchuk, O.P. Microbial Symbionts of Insects: Genetic Organization, Adaptive Role, and Evolution. Microbiology 2018, 87, 151–163. [Google Scholar] [CrossRef]
- Arora, A.K.; Douglas, A.E. Hype or opportunity? Using microbial symbionts in novel strategies for insect pest control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef]
- Shamjana, U.; Vasu, D.A.; Hembrom, P.S.; Nayak, K.; Grace, T. The role of insect gut microbiota in host fitness, detoxification and nutrient supplementation. Antonie Van Leeuwenhoek 2024, 117, 71. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Jing, T.-Z.; Qi, F.-H.; Wang, Z.-Y. Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef]
- Shao, Y.; Mason, C.J.; Felton, G.W. Toward an Integrated Understanding of the Lepidoptera Microbiome. Annu. Rev. Entomol. 2024, 69, 117–137. [Google Scholar] [CrossRef]
- Harwood, J.D.; Parajulee, M.N. Global impact of biological invasions: Transformation in pest management approaches. Biol. Invasions 2010, 12, 2855–2856. [Google Scholar] [CrossRef]
- Siegert, C.; Clay, N.; Pace, K.; Vissa, S.; Hofstetter, R.W.; Leverón, O.; Riggins, J.J. Bark beetle-driven community and biogeochemical impacts in forest ecosystems: A review. Ann. Entomol. Soc. Am. 2024, 117, 163–183. [Google Scholar] [CrossRef]
- Jaime, L.; Batllori, E.; Ferretti, M.; Lloret, F. Climatic and stand drivers of forest resistance to recent bark beetle disturbance in European coniferous forests. Glob. Change Biol. 2022, 28, 2830–2841. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Escherich, K. Die Forstinsekten Mitteleuropas; Parey: Berlin, Germany, 1923; pp. 342–380. (In German) [Google Scholar]
- CABI. Hylurgus ligniperda (red-haired pine bark beetle). CABI Compend. 2022. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Schläfli, L.; Cornejo, C.; Kappeler, J.; Orbach, J.; Tiefenbacher, A.; Kupper, Q.; Avtzis, D.; Branco, M.; Carnegie, A.J.; et al. Worldwide spread of Hylurgus ligniperda (Coleoptera: Scolytinae), and the potential role of bridgehead invasions. bioRxiv 2025, 654641. [Google Scholar] [CrossRef]
- Ren, L.L.; Tao, J.; Wu, H.W.; Zong, S.X.; Zhen, W.C.; Hua, D.; Shi, J.; Liu, Y.Z.; Luo, Y.Q. The First Discovery and Infective Characteristics of a Major Invasive Pest Hylurgus ligniperda (Coleoptera: Scolytidae) in China. Sci. Silvae Sin. 2021, 57, 140–150. [Google Scholar] [CrossRef]
- Wang, B.-X.; Li, C.-J.; Zhou, Z.-F.; Yao, Y.-X.; Wang, X.-Y.; Zhong, K.; Yang, H.-Q.; Wei, J.-R.; Huai, W.-X. Forecasting the distribution range of Hylurgus ligniperda (Fabricius) (Coleoptera: Curculionidae) in the present and future under the influence of climate change. J. Econ. Entomol. 2025, 118, 132–144. [Google Scholar] [CrossRef]
- Ouyang, X.; Lu, T.; Pan, J.; Sun, Q. The role of climate change in shaping the distribution patterns of Hylurgus ligniperda and its key natural enemies. Pest Manag. Sci. 2025. [Google Scholar] [CrossRef]
- Gu, Y.; Ge, S.; Li, J.; Ren, L.; Wang, C.; Luo, Y. Composition and Diversity of the Endobacteria and Ectobacteria of the Invasive Bark Beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in Newly Colonized Areas. Insects 2024, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Wong, A.C.-N.; Wang, Q.-P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut Microbiota Modifies Olfactory-Guided Microbial Preferences and Foraging Decisions in Drosophila. Curr. Biol. 2017, 27, 2397–2404.e2394. [Google Scholar] [CrossRef] [PubMed]
- Sivakala, K.K.; Jose, P.A.; Shamir, M.; Wong, A.C.-N.; Jurkevitch, E.; Yuval, B. Foraging behaviour of medfly larvae is affected by maternally transmitted and environmental bacteria. Anim. Behav. 2022, 183, 169–176. [Google Scholar] [CrossRef]
- Nie, S.; Liu, Y.-J.; Ge, Y. The host phylogeny and climate determine the gut bacteria of global insects. Sci. Total Environ. 2025, 966, 178812. [Google Scholar] [CrossRef]
- Liu, F.; Wickham, J.D.; Cao, Q.; Lu, M.; Sun, J. An invasive beetle–fungus complex is maintained by fungal nutritional-compensation mediated by bacterial volatiles. ISME J. 2020, 14, 2829–2842. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, L.; Wang, S.; Wang, B.; Lou, Q.; Lu, M.; Sun, J. Bacterial volatile ammonia regulates the consumption sequence of d-pinitol and d-glucose in a fungus associated with an invasive bark beetle. ISME J. 2017, 11, 2809–2820. [Google Scholar] [CrossRef]
- Ortiz, A.; Vega, N.M.; Ratzke, C.; Gore, J. Interspecies bacterial competition regulates community assembly in the C. elegans intestine. ISME J. 2021, 15, 2131–2145. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, K.; Chen, K.; Zhao, Z.; Ju, F. Symbiont community assembly shaped by insecticide exposure and feedback on insecticide resistance of Spodoptera frugiperda. Commun. Biol. 2024, 7, 1194. [Google Scholar] [CrossRef]
- Knapp, B.D.; Willis, L.; Gonzalez, C.; Vashistha, H.; Jammal-Touma, J.; Tikhonov, M.; Ram, J.; Salman, H.; Elias, J.E.; Huang, K.C. Metabolic rearrangement enables adaptation of microbial growth rate to temperature shifts. Nat. Microbiol. 2025, 10, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Spampinato, G.; Macavei, L.I.; Lugli, L.; Candeliere, F.; Rossi, M.; Maistrello, L.; Amaretti, A. Effect of Rearing Temperature on Growth and Microbiota Composition of Hermetia illucens. Microorganisms 2020, 8, 902. [Google Scholar] [CrossRef]
- Krieger, A.G.; Zhang, J.; Lin, X.N. Temperature regulation as a tool to program synthetic microbial community composition. Biotechnol. Bioeng. 2021, 118, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bao, C.; Shen, L.; Tian, C.; Zang, X.; Chen, G.; Zhang, S. Microbial Cold Shock Proteins: Overview of their Function and Mechanism of Action. Protein Pept. Lett. 2022, 29, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Galko, J.; Økland, B.; Kimoto, T.; Rell, S.; Zúbrik, M.; Kunca, A.; Vakula, J.; Gubka, A.; Nikolov, C. Testing temperature effects on woodboring beetles associated with oak dieback. Biologia 2018, 73, 361–370. [Google Scholar] [CrossRef]
- Rêgo, A.; Baur, J.; Girard-Tercieux, C.; de la Paz Celorio-Mancera, M.; Stelkens, R.; Berger, D. Repeatability of evolution and genomic predictions of temperature adaptation in seed beetles. Nat. Ecol. Evol. 2025, 9, 1061–1074. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, R.; Zhang, J.; Li, J.; Zhu, G.; Yao, M.; Sun, J.; Shen, Z. Isolation and identification of two Serratia marcescens strains from silkworm, Bombyx mori. Antonie Van Leeuwenhoek 2020, 113, 1313–1321. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, K.; Yin, Y.; Zhang, X.; Zhang, Q.; Kong, X.; Tang, L.; Zhang, R.; Zhang, Z. Serratia marcescens in the intestine of housefly larvae inhibits host growth by interfering with gut microbiota. Parasites Vectors 2023, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yao, Z.; Raza, M.F.; Cai, Z.; Zhang, H. Regulatory mechanisms of microbial homeostasis in insect gut. Insect Sci. 2021, 28, 286–301. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Xie, D.; Jiang, L.; Niu, F.; Wang, X.; Dai, Y.; Chi, D.; Yu, J. Gut Bacteria Strategies of Hylurgus ligniperda F. (Coleoptera Scolytidae) in Adapting to Temperature Changes. Microorganisms 2025, 13, 2502. https://doi.org/10.3390/microorganisms13112502
Chen H, Xie D, Jiang L, Niu F, Wang X, Dai Y, Chi D, Yu J. Gut Bacteria Strategies of Hylurgus ligniperda F. (Coleoptera Scolytidae) in Adapting to Temperature Changes. Microorganisms. 2025; 13(11):2502. https://doi.org/10.3390/microorganisms13112502
Chicago/Turabian StyleChen, Huanwen, Dan Xie, Lihong Jiang, Fang Niu, Xiaomei Wang, Yan Dai, Defu Chi, and Jia Yu. 2025. "Gut Bacteria Strategies of Hylurgus ligniperda F. (Coleoptera Scolytidae) in Adapting to Temperature Changes" Microorganisms 13, no. 11: 2502. https://doi.org/10.3390/microorganisms13112502
APA StyleChen, H., Xie, D., Jiang, L., Niu, F., Wang, X., Dai, Y., Chi, D., & Yu, J. (2025). Gut Bacteria Strategies of Hylurgus ligniperda F. (Coleoptera Scolytidae) in Adapting to Temperature Changes. Microorganisms, 13(11), 2502. https://doi.org/10.3390/microorganisms13112502





