Abstract
Proteases play key roles in many industrial processes and account for the majority of global enzyme sales. Geobacillus isolates from extreme environments such as marine hydrothermal vents are capable of producing high yields of proteases with thermophilic properties. Many proteases produced by Geobacillus species have been extensively studied, some of which have been purified and characterized. In addition, the high thermal stability largely depends on structural stability. Based on X-ray crystallography, several factors have been found to affect the structural stability of the thermostable proteases of Geobacillus. Moreover, the thermostable proteases of Geobacillus have a wide range of biotechnological applications, such as in detergent, food, bioremediation, leather-processing and textile industries. Therefore, this review focusses on the thermostable proteases of Geobacillus, including their characteristics, structural stability mechanisms and biotechnological applications. It will help the development of utilizing thermostable protease resources and enhancing their suitability for use in various industrial applications.
1. Introduction
Proteases, also known as peptidases or proteinases, are enzymes that catalyze the breakdown of proteins into short peptides or free amino acids by hydrolyzing peptide bonds [1]. Based on the role of amino acids in the active site, proteases are grouped into different types in the latest release (release 12.5) of the MEROPS database (https://www.ebi.ac.uk/merops, accessed on 9 October 2025): aspartic (A) protease, cysteine (C) protease, glutamic (G) protease, metallo (M) protease, asparagine (N) protease, mixed (P) protease, serine (S) protease, threonine (T) protease and unknown (U) protease [2]. Proteases have been determined to play crucial parts in the degradation of organic nitrogen compounds [3,4]. Proteases can be categorized into low-temperature, medium-temperature, and thermophilic proteases based on their optimal temperature.
Generally, thermophilic proteases have a maximum activity at high temperatures or exhibit sustained high activity at high temperatures for extended periods. Up to now, numerous thermophilic proteases have been produced, purified and characterized from thermophiles, which can thrive in temperatures above 50 °C [5]. Compared to mesophilic proteases, thermophilic proteases exhibit faster biological reactions, lower viscosity, and reduced susceptibility to contamination under high-temperature conditions [6]. The advantages have sparked curiosity about the extracellular protease activities of thermostable bacteria, making the thermostable proteases attractive to the biotechnology industries such as the detergent industry, food industry, and sludge biodegradation [7,8,9]. The most typical thermostable protease is thermolysin produced by Bacillus thermoproteolyticus, which has been utilized in the production of the artificial sweetener aspartame [10].
As the major sources of thermoactive proteases, members of the genus Geobacillus were frequently isolated and extensively studied during the last decades. Geobacillus was first proposed in 2001, which was transferred from many thermophilic members of the genus Bacillus on the basis of 16S rRNA gene sequence analysis [11]. This original genus description included six species: G. kaustophilus, G. thermocatenulatus, G. thermodenitrificans, G. thermoleovorans, G. thermoglucosidasius and the type species G. stearothermophilus. Subsequently, nine additional species, G. caldoxylosilyticus, G. galactosidasius, G. icigianus, G. jurassicus, G. lituanicus, G. thermantarcticus, G. toebii, G. uzenensis, and G. vulcani, have been validly described or transferred to the genus Geobacillus. More recently, Geobacillus has been re-assessed and sixty-three Geobacillus strains for which genome sequences are publicly available have been estimated for phylogenetic relatedness by whole genome approaches [12]. So far, 23 species of Geobacillus have been validly published (https://lpsn.dsmz.de/genus/geobacillus, accessed on 9 October 2025). The majority of Geobacillus strains grow in the temperature range 35–75 °C, with the optimum at 55–65 °C [13]. Hence, Geobacillus cultures have been isolated from diverse high temperature environments [14], such as compost [15], sewage sludge [16], hot springs [17,18,19,20], hot oil reservoir [21], natural gas wells [22], and geothermal effluents [23]. Notably, marine-derived Geobacillus strains have attracted increasing interest due to their unique adaptations to high salinity and pressure besides extreme temperatures. These microorganisms are widely distributed in shallow marine vents [24,25], and deep-sea hydrothermal vents [24,25,26,27,28]. They contribute to organic matter degradation and participate in biogeochemical cycling through the secretion of specific enzymes such as proteases, lipases, glucosidase, and amylase, which exhibit exceptional stability under extreme marine environments. G. thermodenitrificans strain V3 from the marine vents of the Aeolian Islands have been confirmed to degrade gelatin and casein at 60 °C, indicating its ability to produce thermophilic proteases [29]. Another protease-producing strain of Geobacillus isolated from undersea fumaroles, strain PLS A, has optimum activity at 60 °C [30]. A thermostable monoacylglycerol lipase from marine Geobacillus strain 12AMOR1 shows the highest hydrolysis activity at 60 °C, and the half-life was 60 min at 70 °C [31]. Geobacillus strain HTA-462 from a Mariana Trench sediment sample has been found to produce α-glucosidase, which shows optimal activity under alkaline (pH 9.0) and high-temperature (60 °C) conditions [32]. Two thermophilic bacteria from Geobacillus have been isolated from Likupang Marine Hydrothermal in Indonesia and they have an amylolytic index value at 55 °C [28]. The specific physiological properties make them promising candidates for marine biotechnology and extremophile enzyme applications.
To date, numerous thermophilic proteases from Geobacillus strains have been reported, mainly including enzymatic properties, structure and application potential. However, up to now, a holistic review of thermostable proteases of Geobacillus is still lacking. In this article, we summarize the studies on the thermostable proteases of Geobacillus species and industrial prospects of these proteases, by searching the literature with keywords ‘thermostable/thermophilic/thermoactive’, ‘protease/proteinase’ and ‘Geobacillus’ in the PubMed (https://pubmed.ncbi.nlm.nih.gov, accessed on 9 October 2025) and Web of Science (https://www.webofscience.com, accessed on 9 October 2025). This review covers the knowledge about characterization, structural stability mechanisms and biotechnological applications of thermostable proteases from Geobacillus. The review will help in making strategies for the exploitation of thermostable protease resources and improving their usability in different industrial applications.
2. Production, Purification and Characterization of Thermostable Proteases of Geobacillus
Thermostable proteases unusually exhibit remarkable stability under high temperature conditions owing to the fact that organisms producing them are adapted to thermal environments. The genus Geobacillus is a typical thermostable group and most of them can grow above 50 °C. Consequently, they are considered to be excellent sources to produce thermophilic proteases. Currently, there are two primary effective ways to produce thermostable proteases of Geobacillus spp. (Figure 1).
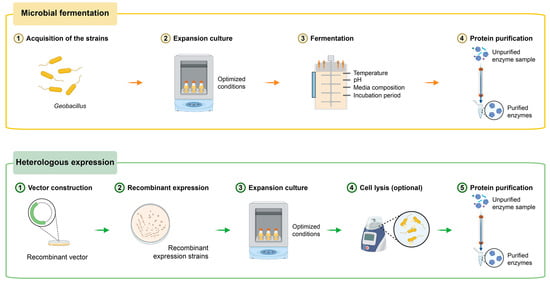
Figure 1.
Two primary effective ways to produce thermostable proteases of Geobacillus.
(1) Microbial fermentation: This method involves the cultivation of Geobacillus spp. in bioreactors under controlled conditions to produce and secrete thermostable proteases. The proteases are then harvested, purified, and utilized. The thermophiles secreting thermostable proteases need to grow under optimum fermentation conditions, including optimum temperature, pH, media compositions and incubation period. For instance, the protease-production capacity of a Geobacillus subterraneus C2-1 isolate from a hot spring was investigated and optimized using a Plackett–Burman experimental design. The highest protease activity of the strain C2-1 was observed using 14.75 g/L glucose as the carbon source, 7.51 g/L yeast extract as the nitrogen source, an inoculum amount of 3.56%, a temperature of 56.4 °C, and a reaction time of 168 h [32]. The effects of various parameters on the protease-production capacity showed that G. thermoglucosidasius SKF4 had the highest ability to produce protease in 1% NaCl at 60 °C at pH 7 for 24 h. It showed a maximum capacity to utilize casein and yeast as sources of nitrogen and sucrose and fructose as good sources of carbon [33]. This process requires high temperature conditions and substantial energy for the cultivation of Geobacillus strains. The target thermophilic proteases are purified by ammonium sulfate precipitation and subsequent ion exchange chromatography [34,35].
(2) Heterologous expression: In this approach, the gene encoding the thermostable protease is isolated, cloned, and expressed in a suitable host organism, such as Escherichia coli or Bacillus subtilis [36,37,38,39]. By using recombinant DNA technology, researchers can produce large quantities of the thermostable protease in a mesophilic host organism, thereby conserving the energy for the cultivation of bacteria. After incubation, the recombinant cells are collected and disrupted by high pressure, and then the cell free crude enzyme extract is collected by centrifugation. For the recombinant proteases secreted outside the cell, the cell lysis step should be circumvented. Then the recombinant proteases are usually purified to homogeneity in a three-step procedure, including affinity chromatography, ion exchange chromatography and gel filtration chromatography [40].
The type of thermostable proteases of Geobacillus spp. is generally determined by measuring the enzyme activity after the addition of protease inhibitors such as PMSF (phenylmethylsulfonyl fluoride, an inhibitor of serine protease) and o-P (o-phenanthroline, an inhibitor of metalloprotease), a metal chelating agent such as EDTA (ethylene diamine tetraacetic acid) and EGTA (ethylene glycol tetraacetic acid), and some metal ions such as Ca2+, Mg2+, Fe2+, Zn2+. For instance, Zhu et al. [41] reported that the protease RH-1 of the Geobacillus strain YMTC 1049 was inhibited by 10 mM PMSF, which demonstrated that it belongs to the serine proteases family. Additionally, the keratinolytic proteinase RecGEOker derived from thermophilic bacterium G. stearothermophilus AD-11 was strongly inhibited by o-phenanthroline (29.5% residual activity), suggesting that RecGEOker is a kind of metalloproteinase [42].
Compared with mesophilic proteases, some thermophilic proteases isolated and characterized from thermophiles have advantages in thermostability. The biochemical characteristics of the thermostable proteases of Geobacillus spp. are summarized in Table 1. The studied thermostable proteases from Geobacillus mostly exhibited optimum activity at 55–70 °C and are stable at a wide range of pH values (6–10). In particular, the thermophilic protease from Geobacillus toebii strain LBT 77 is extremely stable and quite active up to 70 °C and pH 13, which indicates that the protease of the G. toebii strain LBT 77 is a thermostable alkaline protease. The protease can remain completely stable at 70 °C after 180 min of incubation, with a half-life of 70 min at 95 °C. These exceptional characteristics make it a promising candidate for various applications [43].

Table 1.
Characterization of thermostable proteases produced by Geobacillus a.
3. Structural Stability Mechanisms of Thermostable Proteases of Geobacillus
There are many factors that affect the thermal stability of enzymes, mainly including two aspects, intermolecular interaction and molecular conformation. Intermolecular interaction can make proteins fold more tightly and thus increase the rigidity. Common intermolecular interactions include ionic bond, hydrogen bond, hydrophobic interaction, and disulfide bond [49,50,51,52,53]. In addition, the conformation of the enzymes also plays an important role in thermal stability. The structural strength depends on the overall flexibility and rigidity of the enzyme, which are conducive to conformational stability.
The proteases of Geobacillus spp. are usually characterized by high thermal stability and heat resistance, which largely depend on structural stability. X-ray crystallography has been utilized to analyze the structures of the thermostable proteases of Geobacillus [54,55]. Research findings have revealed that the thermostable proteases of Geobacillus have special secondary structures and amino acid sequences, facilitating their ability to sustain catalytic activity in high-temperature environments. There are several important factors for the structural stability of the thermostable proteases of Geobacillus spp. (Figure 2).
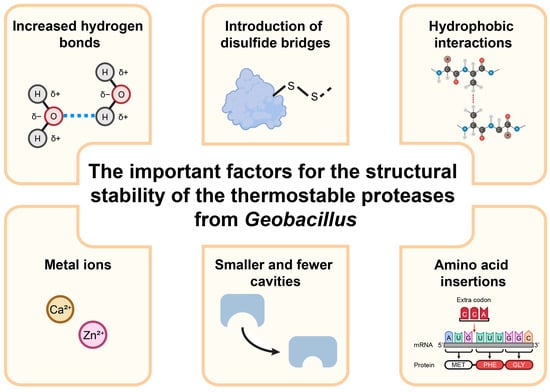
Figure 2.
The important factors for the structural stability of the thermostable proteases of Geobacillus.
3.1. Increased Hydrogen Bonds
Hydrogen bonds play a crucial role in the thermal stability of enzymes, which not only occur between protein molecules, but also form interactions with adjacent water molecules. The formation of hydrogen bonds should meet the following two conditions: first, the distance between the hydrogen bond donor and the acceptor should be less than 3 Å; second, the angle of the hydrogen bond donor–hydrogen atom–hydrogen bond acceptor should be between 90° and 180° [56]. The number of charged neutral hydrogen bonds (i.e., between a side chain atom of a charged residue and either a main chain atom of any residue or a side chain atom of a neutral residue) has also been demonstrated to have a strong correlation with the protein thermostability [57]. Several studies have revealed that enhancing the thermostability of industrial enzymes can be achieved by promoting the formation of hydrogen bonds through single substitutions [58,59,60,61,62]. Additionally, the buried Ala-170 of the neutral proteinase of G. stearothermophilus was replaced by serine (Ser), and hence an extra high-quality Ser-170-OH-Asn-241-Oδ hydrogen bond was formed. Molecular dynamics simulations indicated that the increased hydrogen bond could enhance the thermostability of the protease. The inclusion of the hydroxy group of Ser-170, which only functions as a donor but not as an acceptor in the folded mutant enzyme, offsets the favorable effect of the hydrogen-bonding potential of Asn-241-Oδ [63].
3.2. Introduction of Disulfide Bridges
The utilization of disulfide bridges is an effective strategy to enhance the stability of the thermophilic proteins, which stabilizes proteins by decreasing the entropy of the unfolded structure, primarily through entropic effects [56]. The entropic effect of the disulfide bridge grows in direct proportion to the logarithm of the number of residues separating the two bridged cysteines. Therefore, increasing the disulfide bridges can decrease the entropy of the protein unfolding state, thereby stabilizing the conformation of enzymes. So far, more than one report has shown that unspecific proteases from Geobacillus spp. can be stabilized dramatically by the introduction of disulfide bridges. The thermolysin-like protease produced by G. stearothermophilus possesses a crucial surface area in the N-terminal domain that is essential for thermal stability. The introduction of a disulfide bond into the N-terminal domain by the double mutation G8C/N60C resulted in an extraordinarily thermostable enzyme with a half-life of 35.9 min at 92.5 °C, which was increased more than 120-fold, from less than 0.3 to 35.9 min [64,65].
3.3. Hydrophobic Interactions
The hydrophobic interactions could be a prominent element for the thermostability of proteins. It was proposed that hydrophobic forces play a crucial role in thermostability and act as the primary driving force for protein folding [66,67]. Hydrophobic interactions between non-polar amino acid residues such as tryptophan, methionine, valine, leucine, isoleucine, alanine, phenylalanine, proline, and glycine significantly contribute to the conformational stability of proteins [68,69]. During the folding process, the hydrophobic residues of the enzymes are obscured within the protein structure to produce hydrophobic effects conducive to protein stability, which helps to maintain the thermal stability of the enzymes. Pace et al. [70] found that each additional buried methyl group during protein folding contributes an average stability increment of 1.3 (±0.5) kcal/mol. By constructing three-dimensional models of wild-type Npr-ste and its mutants, it was deduced that the cavity-filling mutations in the hydrophobic core, which could improve hydrophobic packing and Van der Waals interactions in the protein interior, had positive effects on the thermostability of the protease from G. stearothermophilus [71].
3.4. Metal Ions
It has long been known that a reason for the stability of thermophilic proteases is the presence of metal ions (Ca2+, Zn2+) to enhance molecular stability. For instance, calcium ions have been considered to contribute to the stability of thermolysin produced by G. stearothermophilus and are also crucial for the stability of thermolysin-like protease (TLP) [72]. The TLP from G. stearothermophilus (TLP-ste) binds four calcium ions, including one double (Ca1, 2) and two single (Ca3, Ca4) calcium-binding sites (Figure 3A). Since Ca3 and Ca4 are typically lacking in thermolabile TLPs, it is likely that they are critical for determining the stability of TLPs. The atomic details of the Ca3 and Ca4 binding sites in TLP-ste are shown in Figure 3B and Figure 3C, respectively. The mutations of Ca3 and Ca4 indeed reduced the thermal stability of TLP-ste. The unfolding of the TLP-ste region containing the Ca3 site is essential for thermal inactivation. It is not caused by the lack of calcium from the Ca3 site, but rather by unfolding of a region of TLP-ste whose stability depends on the occupancy of the Ca3 site [73].
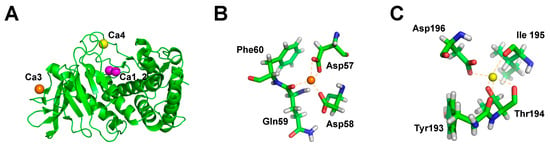
Figure 3.
(A) The overall structure of the thermolysin-like protease from Geobacillus stearothermophilus. The double calcium-binding site (Ca1, 2) is shown as a magenta sphere and the single calcium-binding sites (Ca3 and Ca4) are shown as an orange and yellow sphere, respectively. (B) The atomic details of the calcium-binding site 3 (Ca3). (C) The atomic details of the calcium-binding site 4 (Ca4).
3.5. Smaller and Fewer Cavities
Cavities are an inherent feature of protein structures, with research indicating that the majority of protein cores possess these voids [71,74]. Such cavities typically exhibit fewer Van der Waals interactions compared to locations in densely packed regions, rendering them energetically unfavorable. Filling the cavities by hydrophobic amino acids with bulkier side chains is considered as a thermostability factor [75]. Consequently, the thermophilic proteins known for their high stability tend to display smaller and fewer cavities [56,76]. The cavities in the hydrophobic core of the neutral protease from G. stearothermophilus were analyzed using a three-dimensional model that was inferred from the crystal structure of thermolysin, the highly homologous neutral protease of B. thermoproteolyticus. Site-directed mutagenesis was used to fill some of these cavities, producing slight effects on the thermostability. Those substitutions involving the larger side chains tended to result in more significant enhancement in the protease thermostability [71]. Additionally, Eijsink et al. [63] noted that the hydroxy group of Ser could improve stability by filling an internal cavity and an increase of 0.7 ± 0.1 °C in stability was obtained after the buried Ala was replaced by Ser.
3.6. Amino Acid Substitutions/Insertions
Protein amino acid composition has long been thought to be correlated to its thermostability and the thermophilic and mesophilic proteins have been found to have significantly different amino acid distributions. Based on the genome sequences of eight mesophilic and seven thermophilic organisms, it has been shown that more hydrophobic and more aromatic residues are found in thermophilic proteins than in mesophilic proteins [77]. Therefore, increasing the proportion of hydrophobic amino acids such as isoleucine and proline contributes to the tight packing of hydrophobic cores and helps to enhance the thermostability [69,78,79]. When prolines were inserted at the second position of β-turns or the N caps of α-helices, the stability enhancement was most noticeable [80]. In addition, due to the large side chains, amino acids like arginine and tyrosine may be beneficial in short-range local interactions and in long-range interactions. The guanidium group in arginine which can form salt bridges also contributes to enhancing the thermostability [56]. Veltman et al. [81] have reported that an arginine-rich three-residue insertion in the TLP produced by G. stearothermophilus CU21 appeared to make an important contribution to the stability of the protease.
Indeed, protein stability is not attributed to a single factor, but is often the result of multiple factors working together. Heat stable proteases of Geobacillus bacteria have attracted much attention due to their special structural properties. The studies of their structures and thermal stabilization mechanisms can provide a theoretical basis for the development and utilization of the thermostable proteases in the application of various fields. In the future, we need to study their structures and thermal stabilization mechanisms, which can provide theoretical guidance for biotechnological applications of the thermostable proteases of Geobacillus.
4. Biotechnological Applications of Thermostable Proteases of Geobacillus
Common proteases exhibit optimal activity between 25 and 40 °C, which is not ideal for all industrial applications. Most industrial biotechnology processes are carried out in high temperature environments, which could cause the denaturation of mesophilic proteases. Due to the special structural and catalytic properties, the proteases of Geobacillus are capable of thermal tolerance. The major benefit of thermophilic proteases is that they can remain active at a high temperature for a long duration of time and using thermophilic proteases can reduce the losses during preparation, storage and industrial production. Therefore, the thermostable proteases are generally suitable for industrial biotechnology processing, which have attracted much attention, and they have found potential applications in detergent, food, bioremediation, leather processing and textile industries (Figure 4).
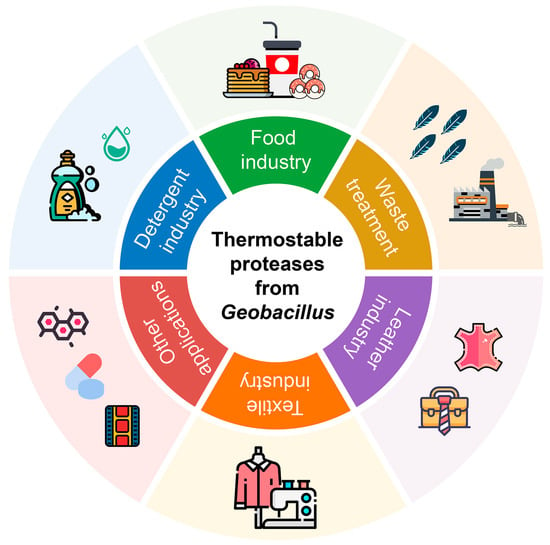
Figure 4.
The biotechnological applications of the thermostable proteases of Geobacillus.
4.1. Detergent Industry
Heat-stable proteases are able to break down proteins, including grime and stains on clothing. They can maintain activities at high temperatures, so even when washing clothes at high temperatures, thermostable proteases can work well. Therefore, adding thermostable proteases to laundry detergents has the potential to significantly improve cleaning efficiency [82]. Bayoumi et al. [7] reported that the thermostable protease from G. stearothermophilus B78 could effectively remove a variety of stains such as blood, chocolate, apple, mango, strawberry and pomegranate. This was achieved through a 15 min treatment at 55 °C, using the protease alone or with Rabso detergent (an Egyptian detergent product). Similarly, another report [83] indicated that the protease derived from G. stearothermophilus successfully removed stains from a blood sample at 50 °C, exhibiting promising potential as a viable candidate for commercial use in detergents. A serine protease from G. thermoglucosidasius SKF4 was successfully used to remove blood stains from cloth at 80 °C when combined with detergent [48]. Moreover, because of the high thermostability and stability to surfactants and metal ions, the serine protease from G. strain GS53 might have a potential effect in the detergent industry [35].
4.2. Food Industry
For a considerable period, microbial proteases have played a significant role in the dairy, baking, and food-processing industries, including the production of some food additives [84]. The proteases produced by Geobacillus spp. have been extensively investigated for their applications in the food industry [85]. Thermolysin, a thermostable metalloendopeptidase produced by G. stearothermophilus, is used for the commercial synthesis of N-(benzyloxycarbonyl)-l-aspartyl-l-phenylalanine methyl ester, the precursor for the artificial sweetener aspartame [8]. The thermostable proteases are also involved in the hydrolysis of proteins for the preparation of hydrolysates which have bioactive potential, such as antioxidant, antidiabetic, and antihypertensive activities. The protein hydrolysates may be applied as nutraceuticals and functional food ingredients, potentially contributing to food quality and promoting human health [86]. Wu et al. [87] have reported that the protease secreted by G. stearothermophilus through high-temperature solid-state fermentation can improve the nutritional value and bioactivity of soybean meal. An alkaline serine protease from G. stearothermophilus CAU209 (GsProS8) displayed a good ability to hydrolyze whey protein at 50 °C to prepare antihypertensive hydrolysates. Therefore, it could be a potential candidate for the preparation of antihypertensive peptides. This property provided important insights for its applications in the food industry [88].
4.3. Waste Treatment
The thermostable proteases from Geobacillus have been used in the management of waste generated from various industries, such as the poultry industry. Several million tons of feather waste are generated worldwide by poultry-processing industries. The hydrolysis of insoluble feathers by the thermostable proteases into soluble peptides and amino acids is a cheaper and more effective way to produce valuable products which can be used as additives in fertilizers and feed. The keratinases (a type of protease) produced by G. stearothermophilus PTCC 1713 and G. thermodenitrificans PS41 could be used to degrade chicken feather at a high temperature. The introduction of these proteases secreted by Geobacillus in sustainable material development was a potential strategy for waste management, and has industrial applications in green technologies [89,90]. The thermostable keratinolytic protease RecGEOker from G. strain AD-11 is an effective biocatalyst for the environmentally friendly enzymatic biodegradation of substrates rich in keratin and high value hydrolysis products—small peptides obtained from keratin waste biodegradation could be suitable for industrial applications in white and green biotechnology [42]. Additionally, the thermophilic strains of Geobacillus are often used for sludge degradation by producing proteases. For example, the thermophilic protease was secreted by G. stearothermophilus TP-2, which resulted in a 23.2% reduction ratio in volatile suspended solids (VSS) after 12 h of sludge hydrolysis [9]. Inoculation with thermophilic strains (G. strain DX5, G. strain DX8, and G. strain DX11) at 65 °C reduced sludge VSS and the protease activity was 2584 U/L, which demonstrated that the protease of these thermophilic strains played important roles in the lysis of sewage sludge [91]. Similarly, the thermophilic bacterium G. kaustophilus X3 was shown to possess high relative protease activity, accelerating primary sludge hydrolysis [92,93]. G. thermodenitrificans DC8 screened from the compost could solubilize excess sludge to extract proteins for sludge reduction and resource recovery. Therefore, these works provide a potential strategy to realize high-efficient resource recovery in sludge waste treatment [94].
4.4. Leather-Processing Industry
Leather processing has traditionally depended on substantial amounts of water, alkali, and chemicals. This has led to the urgent requirement for process optimization and environmental pollution reduction in the leather manufacturing industry. Compared with the traditional method, the microbial proteases offer an environmentally friendly alternative [95]. The application of proteases can effectively enhance the water absorption in dehydrated skins, eliminate impurities and undesired proteins, and reduce the swelling of pelts. Furthermore, the proteases that possess the ability to break down elastin and keratin are helpful to generate high-quality leather with the desired attributes of strength, softness, cleanness and texture in a shorter period of time. The thermo-halostable protease produced by G. strain PLS A from undersea fumaroles has been reported to cause dehairing and produce soft leather, presenting a promising application in the leather-processing industry [38].
4.5. Textile Industry
Wool is a versatile natural fiber widely utilized in the textile industry, which is commonly used to make clothing, domestic textiles, and technical textiles. Wool is known for its capacity to absorb and expel moisture, which makes woolen clothing both warm and comfortable. Two-thirds of the wool fibers are utilized in the garment business, demonstrating the significance of the wool textile industry within the broader textile industry [96]. In the wool textile industry, wool processing involves oxidation of the wool surface by means of chlorination or softening agents. However, these chemicals are known to pose environmental hazards. To solve the problem, the proteases are implemented to reduce the felting propensity of wool and enhance the tactile qualities of fabrics by imparting a soft and smooth texture. The crude enzyme of the thermophilic Geobacillus strain has been successfully utilized to process wool fibers. The scanning electron microscope analysis revealed that the enzymatic treatment smoothed the outer layer of the wool fibers and improved the quality of the wool surface. Therefore, protease treatment is a promising eco-friendly alternative to chemical treatment in the wool textile industry, thereby aiding in the mitigation of environmental pollution [97].
4.6. Other Potential Applications
The thermostable proteases from Geobacillus are also used in clinical medicine and experimental reagents [8]. In addition, it was suggested that G. stearothermophilus protease could be used as a biocontrol agent due to its catalytic domains [30]. In addition, the thermostable proteases (Pz peptidases A and B) from G. collagenovorans MO-1 have been proved to contribute to collagen degradation [44]. Interestingly, the thermostable protease from G. thermoglucosidasius SKF has been used to hydrolyze the gelatin layer on X-ray film, facilitating the recovery of silver from X-ray film [48]. The thermostable protease produced by G. strain SBS-4S has been utilized as a supplement in poultry feed, and it has been shown to enhance weight gain, feed consumption and the feed conversion ratio in poultry [98,99]. In addition, many other thermostable proteases from Geobacillus with potential in various industries have been mined by genome and proteome analysis. For example, the proteases of G. thermoleovorans ARTRW1 with high melting point (Tm) value have been found by thermal proteome profiling analysis, and they have significant industrial and biomedical prospects to accelerate thermophilic enzyme research and innovation [100].
Therefore, the thermostable proteases of Geobacillus spp. have numerous biotechnological applications, including in the detergent, food, bioremediation, leather-processing and textile industries. Their ability to maintain their activity at high temperatures makes them particularly useful in these applications. Further research on the properties and applications of these proteases is needed to fully exploit their potential.
5. Conclusions and Perspectives
Numerous proteases of Geobacillus spp. from high temperature environments, such as marine geothermal sites and hot springs, exhibit high activity, and the thermophilic property makes them meet industrial requirements. This review emphasizes the production, purification, characterization, structural stability mechanisms and industrial application of proteases from a number of Geobacillus strains. It has been found that the thermostable proteases of Geobacillus mostly exhibited optimum activity at 55–70 °C. There are several important factors for the structural stability of the thermostable proteases from Geobacillus, such as an increased hydrogen bond, introduction of disulfide bridges, hydrophobic interactions, metal ions, smaller cavities, amino acid substitutions/insertion and so on. Due to the thermophilic property and structural stability, the thermostable proteases of Geobacillus have biotechnological and industrial applications in detergent, food, bioremediation, leather-processing and textile industries. However, most thermophilic proteases of Geobacillus have not yet been commercially applied, and the obstacle lies in the difficulty of constructing their high-efficiency expression vectors.
Despite recent advancements, the current understanding of the thermostable proteases of Geobacillus remains incomplete, necessitating further investigation. In particular, three future research areas should be noted. First, more new thermophilic proteases from Geobacillus need to be mined. Many thermostable proteases from Geobacillus with potential in various industries have been found by genome and proteome analysis, but they need to be further heterologously expressed and purified. Second, the regulatory mechanism of Geobacillus expressing thermostable proteases urgently needs to be explored, which will be conducive to the construction of chassis cells with efficient expression. The new catalytic mechanism, and thermal stability mechanism of protease also needs to be explored. Third, efficient and scalable strategies are needed to improve the feasibility of the use of thermostable proteases from Geobacillus in industrial applications. The immobilization of enzymes and other technologies are proposed in the industrial applications of thermostable proteases of Geobacillus, to improve the utilization of protease and reduce application costs.
Author Contributions
Conceptualization, J.-H.C.; Investigation, J.-H.C. and M.W.; Writing—Original Draft, M.W. and J.-W.W.; Visualization, M.W.; Writing—Review and Editing, J.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Provincial Natural Science Foundation (ZR2024QD146, ZR2025QC163); Qingdao Natural Science Foundation (24-4-4-zrjj-35-jch, 24-4-4-zrjj-50-jch); Shandong Postdoctoral Science Foundation (SDCX-ZG-202400154); China Postdoctoral Science Foundation (2024M761570).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Turk, B. Targeting Proteases: Successes, Failures and Future Prospects. Nat. Rev. Drug. Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Morton, F.R.; Kok, C.Y.; Kong, J.; Barrett, A.J. MEROPS: The Peptidase Database. Nucleic Acids Res. 2008, 36, D320–D325. [Google Scholar] [CrossRef]
- Ran, L.Y.; Su, H.N.; Zhou, M.Y.; Wang, L.; Chen, X.L.; Xie, B.B.; Song, X.Y.; Shi, M.; Qin, Q.L.; Pang, X.; et al. Characterization of a Novel Subtilisin-like Protease Myroicolsin from Deep Sea Bacterium Myroides profundi D25 and Molecular Insight into Its Collagenolytic Mechanism. J. Biol. Chem. 2014, 89, 6041–6053. [Google Scholar] [CrossRef]
- Li, H.J.; Tang, B.L.; Shao, X.; Liu, B.X.; Zheng, X.Y.; Han, X.X.; Li, P.Y.; Zhang, X.Y.; Song, X.Y.; Chen, X.L. Characterization of a New S8 serine Protease from Marine Sedimentary Photobacterium sp. A5-7 and the Function of Its Protease-Associated Domain. Front. Microbiol. 2016, 7, 2016. [Google Scholar] [CrossRef]
- Giddings, L.A.; Newman, D.J. Bioactive Compounds from Terrestrial Extremophiles. In Bioactive Compounds from Terrestrial Extremophiles; Springer: Cham, Switzerland, 2015; pp. 1–75. ISBN 978-3-319-13260-0. [Google Scholar]
- Bruins, M.E.; Janssen, A.E.M.; Boom, R.M. Thermozymes and Their Applications: A Review of Recent Literature and Patents. Appl. Biochem. Biotechnol. 2001, 90, 155–186. [Google Scholar] [CrossRef]
- Bayoumi, R.A.; Louboudy, S.S.; Sidkey, N.M.; Rahman, M.A.A.E. Biotechnological Application of Bacterial Alkaline Thermostable Enzymes in Bio-Detergent Industry. Egypt. J. Microbiol. 2009, 44, 29–46. [Google Scholar]
- Hussein, A.H.; Lisowska, B.K.; Leak, D.J. The Genus Geobacillus and Their Biotechnological Potential. Adv. Appl. Microbiol. 2015, 92, 1–48. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0065216415000064 (accessed on 9 October 2025).
- Guo, W.Q.; Zheng, H.S.; Li, S.; Ho, S.H.; Yang, S.S.; Feng, X.C.; Chang, J.S.; Wang, X.J.; Ren, N.Q. Promotion Effects of Ultrasound on Sludge Biodegradation by Thermophilic Bacteria Geobacillus stearothermophilus TP-12. Biochem. Eng. J. 2016, 105, 281–287. [Google Scholar] [CrossRef]
- Birrane, G.; Bhyravbhatla, B.; Navia, M.A. Synthesis of Aspartame by Thermolysin: An X-Ray Structural Study. ACS Med. Chem. Lett. 2014, 5, 706–710. [Google Scholar] [CrossRef]
- Nazina, T.N.; Tourova, T.P.; Poltaraus, A.B.; Novikova, E.V.; Isakov, A.Y. Taxonomic Study of Aerobic thermophilic Bacilli: Descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from Petroleum Reservoirs and Transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillus as the New Combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrifican. Int. J. Syst. Evol. Microbiol. 2001, 51, 433–446. Available online: https://www.microbiologyresearch.org/content/journal/ijsem/10.1099/00207713-51-2-433#tab2 (accessed on 9 October 2025).
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Phylogenomic Re-Assessment of the Thermophilic Genus Geobacillus. Syst. Appl. Microbiol. 2016, 39, 527–533, Erratum in: Syst. Appl. Microbiol. 2018, 41, 529–530. [Google Scholar] [CrossRef]
- Margaryan, A.; Shahinyan, G.; Hovhannisyan, P.; Panosyan, H.; Birkeland, N.K.; Trchounian, A. Geobacillus and Anoxybacillus spp. from Terrestrial Geothermal Springs Worldwide: Diversity and Biotechnological Applica-tions. In Extremophiles in Eurasian Ecosystems: Ecology, Diversity, and Applications; Nils-Kåre, B., Dilfuza, E., Eds.; Springer: Singapore, 2018; Volume 8, pp. 119–166. ISBN 978-981-13-0329-6. [Google Scholar]
- Zeigler, D.R. The Geobacillus Paradox: Why Is a Thermophilic Bacterial Genus so Prevalent on a Mesophilic Planet? Microbiology 2014, 160, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, D.; Li, J. Isolation and Application of Thermophilic and Psychrophilic Microorganisms in the Composting Process. Waste Biomass Valorization 2013, 5, 433–440. [Google Scholar] [CrossRef]
- Chen, X.G.; Stabnikova, O.; Tay, J.H.; Wang, J.Y.; Tay, S.T. Thermoactive Extracellular Proteases of Geobacillus caldoproteolyticus, sp. nov., from Sewage Sludge. Extremophiles 2004, 8, 489–498. [Google Scholar] [CrossRef]
- Hawumba, J.F.; Theron, J.; Brözel, V.S. Thermophilic Protease-Producing Geobacillus from Buranga Hot Springs in Western Uganda. Curr. Microbiol. 2002, 45, 144–150. [Google Scholar] [CrossRef]
- Atasoy, P.Y.; Inan, K.; Kaçağan, M.; Çanakçi, S.; Beldüz, A.O. Isolation and Characterization of Extracellular Protease Producing Geobacillus sp. from Various Hot Springs in Turkey. Curr. Opin. Biotechnol. 2011, 22, S89. [Google Scholar] [CrossRef]
- Cui, C.X.; Wei, S.L.; Niu, F.B.; Peng, Y.H.; Ming, H. Two Thermostable Xylanases with Different Acid-alkalinity Coexistence in One Bacterium Screened Using Lignocellulosic Biomass and Its Applications. J. Appl. Microbiol. 2025, 136, lxaf212. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Mukherjee, T.; Sen, U.; Roy, C.; Rameez, M.J.; Ghosh, W.; Mukhopadhyay, S.K. Genome Sequence of the Multiple-Protease-Producing Strain Geobacillus thermoleovorans N7, a Thermophilic Bacterium Isolated from Paniphala Hot Spring, West Bengal, India. Genome Announc. 2016, 4, e01202-16. [Google Scholar] [CrossRef] [PubMed]
- Kadnikov, V.V.; Mardanov, A.V.; Poltaraus, A.B.; Sokolova, D.S.; Semenova, E.M.; Ravin, N.V.; Tourova, T.P.; Nazina, T.N. Genome Sequencing and Annotation of Geobacillus sp. 1017, a Hydrocarbon-oxidizing Thermophilic Bacterium Isolated from a Heavy Oil Reservoir (China). Genom. Data 2017, 11, 95–97. [Google Scholar] [CrossRef]
- Struchtemeyer, C.G.; Davis, J.P.; Elshahed, M.S. Influence of the Drilling Mud Formulation Process on the Bacterial Communities in Thermogenic Natural Gas Wells of the Barnett Shale. Appl. Environ. Microbiol. 2011, 77, 4744–4753. [Google Scholar] [CrossRef]
- Valenzuela, B.; Solís-Cornejo, F.; Araya, R.; Zamorano, P. Isolation of Thermophilic Bacteria from Extreme Environments in Northern Chile. Microorganisms 2024, 12, 473. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. A Polyphasic Taxonomic Study of Thermophilic Bacilli from Shallow, Marine Vents. Syst. Appl. Microbiol. 2001, 24, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. Three Novel Halotolerant and Thermophilic Geobacillus Strains from Shallow Marine Vents. Syst. Appl. Microbiol. 2002, 25, 450–455. [Google Scholar] [CrossRef]
- Kimura, H.; Asada, R.; Masta, A.; Naganuma, T. Distribution of Microorganisms in the Subsurface of the Manus Basin Hydrothermal Vent Field in Papua New Guinea. Appl. Environ. Microbiol. 2003, 69, 644–648. [Google Scholar] [CrossRef]
- Wissuwa, J.; Stokke, R.; Fedøy, A.E.; Lian, K.; Smalås, A.O.; Steen, I.H. Isolation and Complete Genome Sequence of the Thermophilic Geobacillus sp. 12AMOR1 from an Arctic Deep-Sea Hydrothermal Vent Site. Stand. Genomic Sci. 2016, 11, 16. [Google Scholar] [CrossRef]
- Ginting, E.L.; Wantania, L.L.; Moko, E.M.; Tumbol, R.A.; Siby, M.S.; Wullur, S. Isolation and Identification of Thermophilic Amylolytic Bacteria from Likupang Marine Hydrothermal, North Sulawesi, Indonesia. Biodiversitas 2021, 22, 3326–3332. [Google Scholar] [CrossRef]
- Lentini, V.; Gugliandolo, C.; Maugeri, T.L. Identification of Enzyme-producing Thermophilic Bacilli Isolated from Marine Vents of Aeolian Islands (Italy). Ann. Microbiol. 2007, 57, 355–361. [Google Scholar] [CrossRef]
- Iqbalsyah, T.M.; Malahayati; Atikah; Febriani. Purification and Partial Characterization of a Thermo-Halostable Protease Produced by Geobacillus sp. strain PLS A Isolated from Undersea Fumaroles. J. Taibah Univ. Sci. 2019, 13, 850–857. [Google Scholar] [CrossRef]
- Tang, W.; Lan, D.; Zhao, Z.; Li, S.; Li, X.; Wang, Y. A Thermostable Monoacylglycerol Lipase from Marine Geobacillus sp. 12AMOR1: Biochemical Characterization and Mutagenesis Study. Int. J. Mol. Sci. 2019, 20, 780. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Hung, V.S.; Akita, M.; Hatada, Y.; Ito, S.; Horikoshi, K. Crystallization and Preliminary X-ray Study of Alpha-glucosidase from Geobacillus sp. Strain HTA-462, One of The Deepest Sea Bacteria. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 1278–1279. [Google Scholar] [CrossRef]
- Akkır, E.Y.; Şahin, Y.B.; Gedikli, S.; Çelik, P.A.; Çabuk, A. Extremely Thermostable, EDTA-Resistant Alkaline Protease from a Thermophilic Geobacillus Subterraneus C2-1 Isolate. J. Microbiol. Biotechnol. 2017, 7, 50–56. Available online: https://office2.jmbfs.org/index.php/JMBFS/article/view/8636 (accessed on 9 October 2025).
- Suleiman, A.D.; Abdul Rahman, N.; Mohd Yusof, H.; Mohd Shariff, F.; Yasid, N.A. Effect of Cultural Conditions on Protease Production by a Thermophilic Geobacillus thermoglucosidasius SKF4 Isolated from Sungai Klah Hot Spring Park, Malaysia. Molecules 2020, 25, 2609. [Google Scholar] [CrossRef] [PubMed]
- Baykara, S.G.; Sürmeli, Y.; Şanlı-Mohamed, G. Purification and Biochemical Characterization of a Novel Thermostable Serine Protease from Geobacillus sp. GS53. Appl. Biochem. Biotechnol. 2021, 193, 1574–1584. [Google Scholar] [CrossRef]
- Iqbal, I.; Aftab, M.N.; Afzal, M.; Ur-Rehman, A.; Aftab, S.; Zafar, A.; Ud-Din, Z.; Khuharo, A.R.; Iqbal, J.; Ul-Haq, I. Purification and Characterization of Cloned Alkaline Protease Gene of Geobacillus stearothermophilus. J. Basic Microbiol. 2014, 55, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Jasilionis, A.; Kuisiene, N. Characterization of a Novel Thermostable Oligopeptidase from Geobacillus thermoleovorans DSM 15325. J. Microbiol. Biotechnol. 2015, 25, 1070–1083. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, C.; Du, L.; Lu, F.; Gao, C. Expression, Purification, and Characterization of a Thermophilic Neutral Protease from Bacillus stearothermophilus in Bacillus subtilis. Sci. China Ser. C 2008, 51, 52–59. [Google Scholar] [CrossRef]
- Özdemir, F.İ.; Asal, Z.; Camcı, H. Purification and Characterization of Recombinant Geobacillus kaustophilus Protein Lon from E. coli. New Biotechnol. 2012, 29, S85. [Google Scholar] [CrossRef]
- Cheng, J.H.; Zhao, W.X.; Cao, H.Y.; Wang, Z.; Wang, Y.; Sheng, Q.; Chen, Y.; Wang, P.; Chen, X.L.; Zhang, Y.Z. Mechanistic Insight into the Production of Collagen Oligopeptides by the S8 Family Protease A4095. J. Agr. Food Chem. 2023, 71, 603–614. [Google Scholar] [CrossRef]
- Zhu, W.; Cha, D.M.; Cheng, G.Y.; Peng, Q.; Shen, P. Purification and Characterization of a Thermostable Protease from a Newly Isolated sp. YMTC 1049. Enzyme Microb. Technol. 2007, 40, 1592–1597. [Google Scholar] [CrossRef]
- Gegeckas, A.; Gudiukaitė, R.; Debski, J.; Citavicius, D. Keratinous Waste Decomposition and Peptide Production by Keratinase from Geobacillus stearothermophilus AD-11. Int. J. Biol. Macromol. 2015, 75, 158–165. [Google Scholar] [CrossRef]
- Thebti, W.; Riahi, Y.; Belhadj, O. Purification and Characterization of a New Thermostable, Haloalkaline, Solvent Stable, and Detergent Compatible Serine Protease from Geobacillus toebii Strain LBT 77. Biomed Res. Int. 2016, 2016, 9178962. Available online: https://onlinelibrary.wiley.com/doi/10.1155/2016/9178962 (accessed on 9 October 2025). [CrossRef]
- Miyake, R.; Shigeri, Y.; Tatsu, Y.; Yumoto, N.; Umekawa, M.; Tsujimoto, Y.; Matsui, H.; Watanabe, K. Two Thimet Oligopeptidase-Like Pz Peptidases Produced by a Collagen- Degrading Thermophile, Geobacillus collagenovorans MO-1. J. Bacteriol. 2005, 187, 4140–4148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tayyab, M.; Rashid, N.; Angkawidjaja, C.; Kanaya, S.; Akhtar, M. Highly Active Metallocarboxypeptidase from Newly Isolated Geobacillus strain SBS-4S: Cloning and Characterization. J. Biosci. Bioeng. 2011, 111, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Tayyab, M.; Aftab, M.N.; Hashmi, A.S.; Awan, A.R. Optimization of Conditions for the Higher Level Production of Protease: Characterization of Protease from Geobacillus SBS-4S. Waste Biomass Valori. 2020, 11, 6613–6623. [Google Scholar] [CrossRef]
- Sookkheo, B.; Sinchaikul, S.; Phutrakul, S.; Chen, S.T. Purification and Characterization of the Highly Thermostable Proteases from Bacillus stearothermophilus TLS33. Protein Expr. Purif. 2000, 20, 142–151. [Google Scholar] [CrossRef]
- Allison, S.D.; AdeelaYasid, N.; Shariff, F.M.; Abdul Rahman, N. Molecular Cloning, Characterization, and Application of Organic Solvent-Stable and Detergent-Compatible Thermostable Alkaline Protease from Geobacillus thermoglucosidasius SKF4. J. Microbiol. Biotechnol. 2023, 34, 436–456. [Google Scholar] [CrossRef]
- Liang, H.K.; Huang, C.M.; Ko, M.T.; Hwang, J.K. Amino acid Coupling Patterns in Thermophilic Proteins. Proteins 2005, 59, 58–63. [Google Scholar] [CrossRef]
- Osire, T.; Yang, T.; Xu, M.; Zhang, X.; Rao, Z. Lys-Arg Mutation Improved the Thermostability of Bacillus cereus Neutral Protease Through Increased Residue Interactions. World J. Microbiol. Biotechnol. 2019, 35, 173. [Google Scholar] [CrossRef]
- Nakayama, H.; Shimamura, T.; Imagawa, T.; Shirai, N.; Itoh, T.; Sako, Y.; Miyano, M.; Sakuraba, H.; Ohshima, T.; Nomura, N. Structure of a Hyperthermophilic Archaeal Homing Endonuclease, I-Tsp061I: Contribution of Cross-domain Polar Networks to Thermostability. J. Mol. Biol. 2007, 365, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Littlechild, J.A.; Guy, J.; Connelly, S.; Mallett, L.; Waddell, S.; Rye, C.A.; Line, K.; Isupov, M. Natural Methods of Protein Stabilization: Thermostable Biocatalysts. Biochem. Soc. Trans. 2007, 35, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Shirley, B.A.; Hendricks, M.N.; Iimura, S.; Gajiwala, K.; Scholtz, J.M. Contribution of Hydrophobic Interactions to Protein Stability. J. Mol. Biol. 2011, 408, 514–528. [Google Scholar] [CrossRef]
- Kawasaki, A.; Nakano, H.; Hosokawa, A.; Nakatsu, T.; Kato, H.; Watanabe, K. The Exquisite Structure and Reaction Mechanism of Bacterial Pz-Peptidase A Toward Collagenous Peptides. J. Biol. Chem. 2010, 285, 34972–34980. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Hosokawa, A.; Tagawa, R.; Inaka, K.; Ohta, K.; Nakatsu, T.; Kato, H.; Watanabe, K. Crystallization and Preliminary X-ray Crystallographic Analysis of Pz Peptidase B from Geobacillus collagenovorans MO-1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 38, 757–759. [Google Scholar] [CrossRef]
- Kumar, S.; Tsai, C.J.; Nussinov, R. Factors Enhancing Protein Thermostability. Protein Eng. Des. Sel. 2000, 13, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.J.; Hecht, R.M.; Krause, K.L. Determinants of Enzyme Thermostability Observed in the Molecular Structure of Thermus quaticus D-glyceraldehyde-3-phosphate Dehydrogenase at 2.5 Å resolution. Biochemistry 1996, 35, 2597–2609. [Google Scholar] [CrossRef]
- Rahimzadeh, M.; Khajeh, K.; Mirshahi, M.; Khayatian, M.; Schwarzenbacher, R. Probing the Role of Asparagine Mutation in Thermostability of Bacillus KR-8104 α-Amylase. Int. J. Biol. Macromol. 2012, 50, 1175–1182. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, R.; Kumar, R.; Mohammad, O.; Singh, R.; Kaur, J. Engineering of a Metagenome Derived Lipase Toward Thermal Tolerance: Effect of Asparagine to Lysine Mutation on the Protein Surface. Gene 2012, 491, 264–271. [Google Scholar] [CrossRef]
- Wang, K.; Luo, H.; Tian, J.; Turunen, O.; Huang, H.; Shi, P.; Hua, H.; Wang, C.; Wang, S.; Yao, B. Thermostability Improvement of a Streptomyces Xylanase by Introducing Proline and Glutamic Acid Residues. Appl. Environ. Microbiol. 2014, 80, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.M.; Krishnagopal, A.; Hussain, A.; Kastner, D.; Sayed, A.M.M.; Mok, Y.K.; Swaminathan, K.; Zeeshan, N. Engineering of Serine Protease for Improved Thermostability and Catalytic Activity Using Rational Design. Int. J. Biol. Macromol. 2019, 126, 229–237. [Google Scholar] [CrossRef]
- Zhang, H.T.; Sang, J.C.; Zhang, Y.; Sun, T.W.; Liu, H.; Yue, R.; Zhang, J.; Wang, H.K.; Dai, Y.J.; Lu, F.P. Rational Design of a Yarrowia lipolytica Derived Lipase for Improved Thermostability. Int. J. Biol. Macromol. 2019, 137, 1190–1198. [Google Scholar] [CrossRef]
- Eijsink, V.G.; Vriend, G.; van der Zee, J.R.; van den Burg, B.; Venema, G. Increasing the Thermostability of the Neutral Proteinase of Bacillus stearothermophilus by Improvement of Internal Hydrogen-Bonding. Biochem. J. 1992, 285, 625–628. [Google Scholar] [CrossRef]
- Mansfeld, J.; Vriend, G.; Dijkstra, B.W.; Veltman, O.R.; Van den Burg, B.; Venema, G.; Ulbrich-Hofmann, R.; Eijsink, V.G.H. Extreme Stabilization of a Thermolysin-Like Protease by an Engineered Disulfide Bond. J. Biol. Chem. 1997, 272, 11152–11156. [Google Scholar] [CrossRef]
- Dürrschmidt, P.; Mansfeld, J.; Ulbrich-Hofmann, R. Differentiation between Conformational and Autoproteolytic Stability of the Neutral Protease from Bacillus stearothermophilus Containing an Engineered Disulfide Bond. Eur. J. Biochem. 2001, 268, 3612–3618. [Google Scholar] [CrossRef]
- Camilloni, C.; Bonetti, D.; Morrone, A.; Giri, R.; Dobson, C.M.; Brunori, M.; Gianni, S.; Vendruscolo, M. Towards a Structural Biology of the Hydrophobic Effect in Protein Folding. Sci Rep. 2016, 6, 28285. [Google Scholar] [CrossRef]
- Nguyen, C.; Young, J.T.; Slade, G.G.; Oliveira, R.J.; McCully, M.E. A Dynamic Hydrophobic Core and Surface Salt Bridges Thermostabilize a Designed Three-Helix Bundle. Biophys. J. 2019, 116, 621–632. [Google Scholar] [CrossRef]
- Fakhravar, A.; Hesampour, A. Rational Design-Based Engineering of a Thermostable Phytase by Site-directed Mutagenesis. Mol. Biol. Rep. 2018, 45, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, N.G.; Rahman, R.N.Z.R.A.; Normi, Y.M.; Oslan, S.N.; Shariff, F.M.; Leow, T.C. Thermostability Engineering of Industrial Enzymes Through Structure Modification. Appl. Microbiol. Biotechnol. 2022, 106, 4845–4866, Erratum in Appl. Microbiol. Biotechnol. 2022, 106, 6363. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N. Contribution of the Hydrophobic Effect to Globular Protein Stability. J. Mol. Biol. 1992, 226, 29–35. [Google Scholar] [CrossRef]
- Eijsink, V.G.H.; Dijkstra, B.W.; Gerrit, V.; Rob, V.D.Z.J.; Vettman, O.R.; van der Vinne, B.; van den Burg, B.; Kempe, S.; Venema, G. The Effect of Cavity-Filling Mutations on the Thermostability of Bacillus stearothermophilus Neutral Protease. Protein Eng. 1992, 5, 421–426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eijsink, V.G.H.; Matthews, B.W.; Vriend, G. The Role of Calcium Ions in the Stability and Instability of a Thermolysin-like Protease. Protein Sci. 2011, 20, 1346–1355. [Google Scholar] [CrossRef]
- Veltman, O.R.; Vriend, G.; Berendsen, H.J.C.; Bertus, V.D.B.; Venema, G.; Eijsink, V.G.H. A Single Calcium Binding Site Is Crucial for the Calcium-Dependent Thermal Stability of Thermolysin-Like Proteases. Biochemistry 1998, 37, 5312. [Google Scholar] [CrossRef] [PubMed]
- Karpusas, M.; Baase, W.A.; Matthews, M.B.W. Hydrophobic Packing in T4 Lysozyme Probed by Cavity-filling Mutants. Proc. Natl. Acad. Sci. USA 1989, 86, 8237–8241. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Moon, S.Y.; Kim, Y.R.; Kim, K.W.; Lee, B.J.; Kong, I.S. Improvement of Thermostability and Halostability of β-1,3-1,4-Glucanase by Substituting Hydrophobic Residue for Lys 48. Int. J. Biol. Macromol. 2017, 94, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Yoon, J.; An, Y.J.; Lee, S.; Cha, H.G.; Pandey, A.; Yoo, Y.J.; Joo, J.C. Statistical Analysis of the Role of Cavity Flexibility in Thermostability of Proteins. Polymers 2024, 16, 291. [Google Scholar] [CrossRef]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhai, L.; Meng, D.; Tian, Q.; Guan, Z.; Cai, Y.; Liao, X. Improving the Catalytic Thermostability of Bacillus altitudinis W3 ω-Transaminase by Proline Substitutions. 3 Biotech 2020, 10, 323. [Google Scholar] [CrossRef]
- Cao, L.; Chen, R.; Xie, W.; Liu, Y. Enhancing the Thermostability of Feruloyl Esterase EstF27 by Directed Evolution and the Underlying Structural Basis. J. Agric. Food Chem. 2015, 63, 8225–8233. [Google Scholar] [CrossRef]
- Watanabe, K.; Masuda, T.; Ohashi, H.; Mihara, H.; Suzuki, Y. Multiple Proline Substitutions Cumulatively Thermostabilize Bacillus cereus ATCC7064 Oligo-1,6-glucosidase. Eur. J. Biochem. 1994, 226, 277–283. [Google Scholar] [CrossRef]
- Veltman, O.R.; Gert, V.; Middelhoven, P.J.; Bertus, V.D.B.; Gerard, V.; Eijsink, V.G.H. Analysis of Structural Determinants of the Stability of Thermolysin-Like Proteases by Molecular Modelling and Site-Directed Mutagenesis. Protein Eng. 1996, 9, 1181–1189. [Google Scholar] [CrossRef]
- Arya, P.S.; Yagnik, S.M.; Rajput, K.N.; Panchal, R.R.; Raval, V.H. Understanding the Basis of Occurrence, Biosynthesis, and Implications of Thermostable Alkaline Proteases. Appl. Biochem. Biotechnol. 2021, 193, 4113–4150. [Google Scholar] [CrossRef]
- Iqbal, I.; Aftab, M.N.; Afzal, M.S.; Zafar, A.; Kaleem, A. Characterization of Geobacillus stearothermophilus Protease for Detergent Industry. Rev. Mex. Ing. Quim. 2020, 19, 267–279. [Google Scholar] [CrossRef]
- Matkawala, F.; Nighojkar, S.; Kumar, A.; Nighojkar, A. Microbial Alkaline Serine Proteases: Production, Properties and Applications. World J. Microbiol. Biotechnol. 2021, 37, 63. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.; Condón, S.; Gayán, E. Parageobacillus and Geobacillus spp.: From Food Spoilage to Beneficial Food Applications. Foods 2025, 14, 2775. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and Functional Properties of Food Protein-Derived Antioxidant Peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Wu, P.; Guo, Y.; Golly, M.K.; Ma, H.; He, R.; Luo, S.; Zhang, C.; Zhang, L.; Zhu, J. Feasibility Study on Direct Fermentation of Soybean Meal by Bacillus stearothermophilus under Non-Sterile Conditions. J. Sci. Food Agric. 2019, 99, 3291–3298. [Google Scholar] [CrossRef]
- Chang, C.; Gong, S.; Liu, Z.; Yan, Q.; Jiang, Z. High Level Expression and Biochemical Characterization of an Alkaline Serine Protease from Geobacillus stearothermophilus to Prepare Antihypertensive Whey Protein Hydrolysate. BMC Biotechnol. 2021, 21, 21. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Sharifi, S.D.; Tabandeh, F.; Honarbakhsh, S.; Ghazanfari, S. Bioconversion of Chicken Feather Wastes by Keratinolytic Bacteria. Process Saf. Environ. Protect. 2020, 135, 171–178. [Google Scholar] [CrossRef]
- Siddharthan, N.; Balagurunathan, R.; Hemalatha, N. A Novel Feather-Degrading Bacterial Isolate Geobacillus thermodenitrificans PS41 Isolated from Poultry Farm Soil. Arch. Microbiol. 2022, 204, 565. [Google Scholar] [CrossRef]
- Chen, A.; Huang, Y.; Chi, B.; Tan, J.; Duan, X.; Ruan, X. Research on Enhancing Sewage Sludge Lysis and Bioreactor Initiation Through Composite Thermophilic Strains. Biochem. Eng. J. 2024, 206, 109298. [Google Scholar] [CrossRef]
- Shao, Y.; Li, S.; Wang, H.; Jin, C.; Zhao, Y.; Zhao, J.; Guo, L. Effect of Rhamnolipid on the Performance of Compound Thermophilic Bacteria Agent Pretreatment System for Waste Sludge Hydrolysis. Sci. Total Environ. 2024, 957, 177513. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Xing, D.; Jin, C.; Zhao, Y.; Zhao, J.; Guo, L. Performance of Four Thermophilic Bacteria for Primary Sludge Hydrolysis: Sludge Disintegration and Hydrolase Activities. Bioresour. Technol. 2025, 420, 132123. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Ma, C.; Zhong, W.; Liu, C.; Niu, J.; Wang, C.; Wang, Z. Compound Mutation by Ultraviolet and Diethyl sulfate of Protease Producing Thermophilic Bacteria to Hydrolyze Excess Sludge. Bioresour. Technol. 2024, 395, 130330. [Google Scholar] [CrossRef]
- Hammami, A.; Fakhfakh, N.; Abdelhedi, O.; Nasri, M.; Bayoudh, A. Proteolytic and Amylolytic Enzymes from a Newly Isolated Bacillus mojavensis SA: Characterization and Applications as Laundry Detergent Additive and in Leather Processing. Int. J. Biol. Macromol. 2018, 108, 56–68. [Google Scholar] [CrossRef]
- Cai, S.B.; Huang, Z.H.; Zhang, X.Q.; Cao, Z.J.; Zhou, M.H.; Hong, F. Identification of a Keratinase-Producing Bacterial Strain and Enzymatic Study for Its Improvement on Shrink Resistance and Tensile Strength of Wool- and Polyester-Blended Fabric. Appl. Biochem. Biotechnol. 2010, 163, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Amro, A.; Serour, E.A. Wool Quality Improvement Using Thermophilic Crude Proteolytic Microbial Enzymes. American-Eurasian J. Agric. Environ. Sci. 2008, 3, 554–560. Available online: https://www.idosi.org/aejaes/jaes3(4)/7.pdf (accessed on 9 October 2025).
- Ahmad, W.; Tayyab, M.; Muneer, B.; Hashmi, A.S.; Ahmad, M.D.; Saeed, S.; Aftab, M.N.; Firyal, S.; Wasim, M.; Azam, M.; et al. Impact of Locally Characterized Protease from Geobacillus SBS 4S on the Growth of Poultry Bird. Pak. J. Zool. 2023, 56, 17–23. [Google Scholar] [CrossRef]
- Oztug, M.; Kilinc, E.; Akgoz, M.; Karaguler, N.G. Thermal Proteome Profiling and Meltome Analysis of a Thermophilic Bacterial Strain, Geobacillus thermoleovorans ARTRW1: Toward Industrial Applications. OMICS J. Integr. Biol. 2020, 24, 756–765. [Google Scholar] [CrossRef]
- Lai, R.; Lin, M.; Yan, Y.; Jiang, S.; Zhou, Z.; Wang, J. Comparative Genomic Analysis of a Thermophilic Protease-Producing Strain Geobacillus stearothermophilus H6. Genes 2023, 14, 466. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).