Abstract
Antimicrobial peptides are potential alternatives to conventional antibiotics, primarily due to broad-spectrum activity and low propensity for inducing bacterial resistance. However, their clinical translation faces challenges, including peptide stability and potential mammalian cell toxicity. This study centers on DGL13K, an all D-amino acid peptide, which overcomes proteolytic susceptibility and demonstrates notable stability and broad-spectrum bactericidal activity without inducing de novo bacterial resistance. This work aimed to enhance the therapeutic properties of DGL13K by using targeted modifications to increase antimicrobial potency and decrease toxicity, as determined by hemolysis. DGL13K derivatives were synthesized and tested, involving amino acid substitutions, stereochemical alterations, and N-terminal functionalization with polyethylene glycol (PEG) or myristoylate. While some modifications altered bacterial specificity and reduced hemolytic activity, none of the tested alterations resulted in a substantial overall improvement compared to the parent DGL13K sequence. Furthermore, the antibacterial efficacy of DGL13K and its variants was significantly inhibited in the presence of 50% serum, suggesting limitations for systemic applications. The findings suggest that the DGL13K sequence, derived from an evolutionarily selected protein, is already highly optimized. Given its stability, broad-spectrum efficacy, in vivo activity, low resistance profile, and high safety margin, DGL13K is a promising therapeutic candidate for topical/localized infections.
1. Introduction
Antimicrobial peptides (AMPs) are a diverse group of host-defense molecules that are found throughout nature in organisms from bacteria and fungi to plants and animals, serving as an ancient component of innate immunity [1,2,3]. Combined with synthetic versions of natural peptides and de novo designed peptides, this class of antimicrobial molecules displays a wide variety of sequences, structures, and antimicrobial properties [4,5,6,7,8,9]. Cationic AMPs are typically amphipathic molecules that can disrupt the bacterial cell membrane by interacting with membrane lipids. This interaction can follow several models and depends on the peptide structure, the peptide/lipid ratio, and the properties of the lipid membrane, including membrane structure, topology, aggregation, and lipid interactions of AMPs; reviewed in [4]. AMPs are prominent in mucosal surfaces where they serve as a first line of defense against invading microorganisms. As an example, we have cataloged at least 45 distinct antimicrobial peptides and proteins in the oral cavity [10,11]. Interestingly, this rich environment of AMPs, allows the colonization by commensal organisms while invading microbes are effectively killed in this environment. Thus, it was already noted in the 1930’s that saliva allows the growth of oral bacteria while killing non-oral bacteria [12]. These properties suggest that human AMPs could be exploited as a new class of antimicrobial therapeutics with a low potential to induce pathogen resistance, while triggering fewer side effects by limiting the disruption of the commensal host microbiome.
Despite their promise as an alternative to traditional antibiotics, a number of challenges have been identified in the attempt to develop AMPs for clinical use [13,14,15]. For example, natural peptides are susceptible to proteolytic degradation, although this can be largely overcome by the use of unnatural and D-amino acids in synthetic peptides [16,17,18,19]. Many AMPs target the bacterial cell membrane [20,21] but, due to similarities with mammalian cell membranes, this is not always a specific target and mammalian cell toxicity has been cited as a concern for clinical development [22,23,24].
Thousands of AMPs have been identified and can be found in several online databases [7,8,9,25]. These peptides typically contain hydrophobic and cationic amino acids but no consensus sequence has been identified for antibacterial activity and the roles of intervening amino acids is poorly understood. Nevertheless, these databases can be queried for common themes that define AMPs [26]. In addition, recent machine learning and broader AI models have been developed to better predict novel AMP sequences [27,28,29,30], although the ultimate success of these approaches remains to be demonstrated [31].
Our laboratory has developed the AMP DGL13K, which was derived from the anti-inflammatory salivary protein BPIFA2 [32,33,34]. BPIFA2 is a Leu-rich protein [35] that is abundant in rodent [36,37] and dog [38] salivary glands/saliva and present in human saliva [39], albeit in relatively low amounts [40]. This protein belongs to a family of antibacterial and anti-inflammatory proteins that are found in multiple mucosal surfaces and secretions [41,42,43]. We noted that BPIFA2 facilitates bacterial aggregation and LPS binding [44,45,46]. To identify the active domain of BPIFA2, the protein was compared to active domains in the related proteins BPI and LBP, which also exhibit LPS-binding activity [32,47]. The initial peptide, GK7 was extended to develop GL13NH2 that shows anti-LPS and bacteria agglutinating activity [33,44,46].
Similar to BPIFA2, GL13NH2 causes bacterial agglutination, which is able to prevent the spread of Pseudomonas infection in a plant model [33]. However, GL13NH2 does not kill the bacteria. To achieve bactericidal activity, the positive charge of the peptide was increased by substituting three polar amino acids with Lys residues [34]. The resulting peptide, GL13K (now named LGL13K) exhibits bactericidal activity against most Gram-negative bacteria but is relatively inactive against Gram-positive bacteria [19,34,48,49,50,51].
LGL13K is susceptible to bacterial proteases, which led to the design of the stereo-isomer DGL13K [16]. DGL13K resists proteolytic degradation [16,19] and has antibacterial activity against all tested strains of both Gram-negative and Gram-positive bacteria, including A. baumanii (six strains) [49], Enterococcus faecalis (seven strains) [19], K. pneumoniae (seven strains) [49], Porphyromonas gingivalis (two strains) [50], P. aeruginosa (nine strains) [16,48,49], S. aureus (two strains) [48], and Streptococcus gordonii (three strains) [19]. Recent unpublished data also show efficacy against Bacteroides fragilis, Clostridioides difficile, Enterobacter cloacae, Enterococcus faecium, and Escherichia coli, thereby completing the ESKAPEE pathogens [52]. In addition, DGL13K shows activity against drug-resistant Gram-negative bacteria [49], including extended-spectrum beta-lactamase (ESBL)-producing and carbapenemase (KPC)-producing K. pneumoniae, multi-drug resistant and extensively drug-resistant P. aeruginosa, and extensively drug-resistant A. baumannii carrying metallo-beta-lactamases. Activity against drug-resistant Gram-positive bacteria includes methicillin-resistant S. aureus (MRSA) [48] and vancomycin-resistant E. faecalis (VRE) [19].
LGL13K is predominantly found in a random coil conformation in the absence of membranes; the peptide adopts an α-helical structure from residue K5 to K11 in the presence of dodecylphosphocholine micelles [53]. In the presence of negatively charged lipid bilayers, the peptide is predominantly found in a ß-sheet structure [53,54]. These ß-sheets can assemble into nanofibrils, which may be the active form of the peptide [55,56]. Rather than forming membrane pores, the relatively short peptide (13 amino acids) disrupts the structure of the bacterial membrane by removing lipid micelles [53,54].
No tested bacteria have developed resistance to DGL13K: Prolonged treatment with sub-inhibitory concentrations (0.5xMIC) of DGL13K does not lead to resistance in P. aeruginosa [49], S. aureus [51], E. faecalis, or S. gordonii [19]. Remarkably, when S. aureus are treated with the L- or D-isomer of GL13K, they gain resistance to LGL13K but not DGL13K, which remains effective against the selected bacteria [51].
The goal of this study was to use targeted modifications of the peptide sequence and modification by functional groups to test if the activity of the peptide can be increased while reducing toxicity to mammalian cells.
2. Materials and Methods
2.1. Bacteria
Pseudomonas aeruginosa Xen41 and Staphylococcus aureus Xen36 were obtained from Xenogen (Alameda, CA; now Revvity, Waltham, MA, USA) and Revvity, respectively, and stored at −80 °C in 10% glycerol. P. aeruginosa were cultured in Luria-Bertani broth while S. aureus were cultured in Todd-Hewitt Broth (Difco, Franklin Lakes, NJ, USA) overnight at 37 °C and shaking at 200 rpm. Cultures typically reached an optical density at 600 nm of 1.7 for P. aeruginosa and 1.3 for S. aureus.
2.2. Peptide Sequences
Synthetic peptides and N-terminally modified peptides were purchased from Bachem (Torrance, CA, USA) or Aaptec (Louisville, KY, USA) (Table 1). Peptide identity and purity were verified by the manufacturer by mass spectrometry and RP-HPLC, respectively. Unless otherwise noted, the peptides were C-terminally amidated and delivered as a TFA salt at >95% purity. Peptide stock solutions were prepared at 10 mg/mL in dH2O or 0.01% acetic acid and stored at 4 °C until use. We have recently reported that the stock solutions are stable for at least 2 years under these conditions [51].

Table 1.
Peptide modifications.
2.3. Heat Stability
Peptides were diluted to 1 mg/mL in sterile 10% PBS (1 part PBS, 9 parts dH2O) and heated for 60 min in a 0.5 mL microcentrifuge tube in a water bath set at 80 °C. Control samples were similarly incubated at room temperature. The samples were used for MIC assays, as described below.
2.4. Minimal Inhibitory Concentration
MIC assays were performed as previously described [51]. Briefly, overnight cultures of P. aeruginosa Xen41 were diluted to 105 CFU/mL in Mueller-Hinton Broth (Difco) while S. aureus Xen36 were similarly diluted in Todd-Hewitt Broth. Bacteria (100 µL) were added to 20 µL of 2-fold peptide dilutions in 10% PBS in 96-well polypropylene culture plates. The plates were incubated overnight at 37 °C with gentle rocking and then the optical density at 630 nm (OD630) was recorded in a BioTek Synergy HT plate reader (BioTek, Winooski, VT, USA; now Agilent, Santa Clara, CA, USA). Bioluminescence was recorded for quality control of the bioluminescent bacteria.
In some MIC assays, the culture medium was supplemented with 60% heat-inactivated fetal calf serum (final assay concentration: 50% serum).
2.5. Hemolysis
Lysis of human red blood cells (Innovative Research; Novi, MI, USA) was determined as previously described [48]. Briefly, red blood cells were incubated with 500 µg/mL peptide in PBS for 1 h at 37 °C. Control cells were incubated in dH2O to determine 100% lysis or PBS (background lysis). Samples were centrifuged for 10 min at 10,000× g and the OD at 405 nm of the supernatant determined as a measure of hemoglobin release due to cell lysis. Relative lysis of peptide-containing samples (% Lysis) was expressed as [(OD405 with peptide − OD405 without peptide)/OD405 in dH2O] × 100%.
2.6. Statistical Analysis
Assays were analyzed by either Student’s t-test (for two groups) or one-way ANOVA (for three or more groups), as stated in the figure legends, using Graphpad Prism 10.4 (Dotmatics, Boston, MA, USA). This study screened a diverse array of modified peptides. In some cases, a low number of replicates was employed for the initial screening. These data are included to provide a more complete picture of the possible changes.
3. Results and Discussion
3.1. Stability of DGL13K in Aqueous Solution
Peptides are typically considered inherently unstable in aqueous solutions [57]. However, an aqueous solution of DGL13K did not lose antibacterial activity after storage at 4 °C for more than 2 years [51]. Similarly, there is no loss of activity when a solution of DGL13K is heated at 80 °C for an hour (Figure 1). Together these results point to the robustness of DGL13K, which overcomes the frequent concern that AMPs are not sufficiently stable for therapeutic use [58].
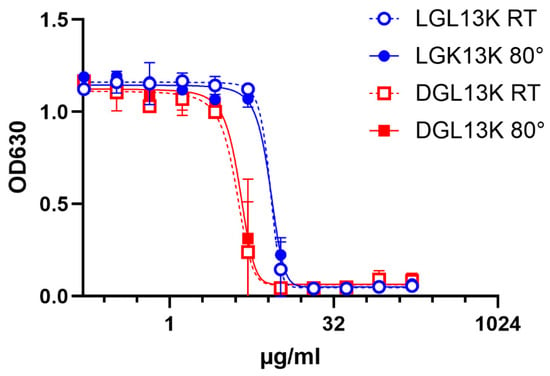
Figure 1.
Heat stability of GL13K peptides. LGL13K (circles) or DGL13K (boxes) were incubated for 1 h at room temperature (open symbols) or 80 °C (closed symbols) and then used for an MIC assay against P. aeruginosa. Duplicate samples were tested and plotted as mean ± range, N = 2. The experiment was repeated with similar results.
3.2. Peptide Stereo Chemistry
Proteolytic processing of AMPs has been cited as a concern for their development for clinical use [58,59]. DGL13K was originally designed to overcome proteolytic processing of the L-enantiomer in cultures of P. aeruginosa [16]. Indeed, DGL13K is not degraded in cultures of Gram-negative P. aeruginosa [16] and also resists proteolytic degradation in cultures of Gram-positive Enterococcus faecalis [19].
In addition to its greater stability, DGL13K also exhibits different antibacterial properties from the L-enantiomer. LGL13K is mainly active against Gram-negative bacteria while DGL13K also shows strong activity against Gram-positive bacteria [19,48] (Table 2). This difference may be due to the preferential binding of DGL13K to peptidoglycan, a component of the Gram-positive cell wall [60]. In contrast, both LGL13K and DGL13K bind to lipopolysaccharide, a component of the Gram-negative cell envelope [60]. Thus, the stereochemistry of the amino acids affects not only the stability of the peptide but also directly affects bacterial selectivity.

Table 2.
MIC of peptide stereo-isomers.
In addition to the chiral centers at the alpha-carbon, GL13K contains two isoleucine residues that exhibit a second chiral center in the side chain. To test if this chiral center affects peptide activity, LGL13K and DGL13K were synthesized with two allo-Ile residues. MIC assays showed that for DGL13K and LGL13K for each bacterial species, the activity of the allo-Ile peptides matched that of the unmodified peptides (Table 2). These results suggest that only the alpha-carbon chiral center affects peptide activity.
3.3. Amino Acid Substitutions
The antimicrobial activity and toxicity profile of AMPs can be optimized by targeted amino acid substitutions. The antimicrobial activity of AMPs is typically defined by cationic and hydrophobic amino acids that target and disrupt the negatively charged bacterial membrane, respectively [30]. In this context, tryptophan and arginine have been identified as enabling peptide–membrane interactions and antibacterial activity [26,61,62,63,64] and were the focus of the tested modifications to DGL13K.
The two cationic amino acids lysine and arginine are often found in AMPs. DGL13K contains four Lys residues that contribute significant positive charge to the peptide (Table 1). In fact, without these Lys residues, the peptide does not exhibit bactericidal activity [34]. Lys can be substituted for the cationic amino acid Arg, which shows a linear relationship with hydrophobic residues in AMPs that does not exist for Lys [26]. Thus, Arg substitutions have been described to affect the antibacterial activity of AMPs [61,64]. Lys contains a four-carbon chain ending in a primary amine group with a pKa of 10.8 while Arg contains a 3-carbon aliphatic chain attached to a positively charged guanidinium group with a pKa of 12.5 [65]. To test if Arg substitution affected the activity of DGL13K, we designed DGL13R [66], which contains four Arg residues in place of the Lys residues in the parent peptide. The activity of DGL13R was not different from the parent peptide when tested against S. aureus (Table 3).

Table 3.
MIC of modified peptide sequences.
In addition to positively charged amino acids, hydrophobic amino acids play a role in membrane interaction. These can be grouped as aliphatic amino acids (e.g., Ala, Ile, Leu, Val) and aromatic amino acids (Trp, Phe). DGL13K contains seven of the former but none of the latter. Trp residues, in particular, have been introduced in AMPs [62,64] due to their preference for the interfacial region of lipid bilayers [61]. To examine the role of hydrophobic amino acids in peptide activity, DGL13K was redesigned by substituting one Ile and one Leu residue for Trp residues. To optimize the steric presentation of these amino acids, Lys residues were moved to generate a more amphipathic peptide [62] (Table 1). A helical wheel representation of the redesigned peptide, DGL12W, shows that charged and hydrophobic amino acids are now arranged on opposite sides of a predicted alpha-helix (Figure 2).
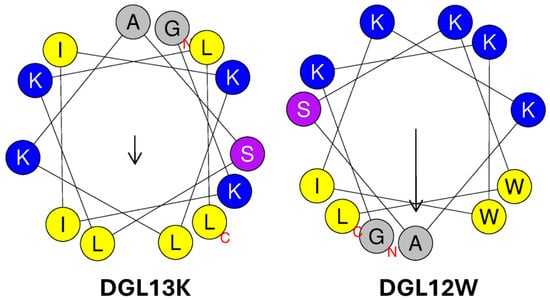
Figure 2.
Helical wheel representations of DGL13K and DGL12W. Amino acid residues are labeled with the single letter code, the N- and C-terminal are labeled with red letters. Cationic amino acids are colored blue, hydrophobic amino acids are colored yellow, and polar residues are purple. The arrows represent the hydrophobic moment of the helices (µH). The length is proportional to helix amphiphilicity and the arrow points to the center of each helix’s most hydrophobic face. The images were produced using Heliquest [67].
DGL12W showed a two-fold increase in the MIC against P. aeruginosa but had lost its activity against S. aureus with a mean MIC above the tested concentration range in some experiments (Table 3). Thus, re-arranging the location of the cationic residues and substituting two Ile/Leu residues with Trp created a peptide with increased specificity for the Gram-negative bacteria P. aeruginosa, compared to DGL13K. It is noted that these empirical changes are based on general rules for AMP design since no specific “AMP sequence motif” has been identified. As a result, each newly designed peptide sequence must be carefully tested to ensure that it exhibits the desired properties for stability, activity, specificity, toxicity, and resistance.
3.4. N-Terminal Modifications
The addition of functional groups, including polyethylene glycol (PEG) or myristoylate, to the peptide sequence is known to affect peptide activity. For AMPs in particular, these modifications have been reported to increase antimicrobial activity and reduce peptide toxicity to mammalian cells [68,69,70,71]. To test the effect of these modifications on peptide activity, an N-terminally PEGylated version of LGL13K and N-terminally myristoylated or biotinylated (control) versions of DGL13K were prepared (Table 1) (Figure 3).
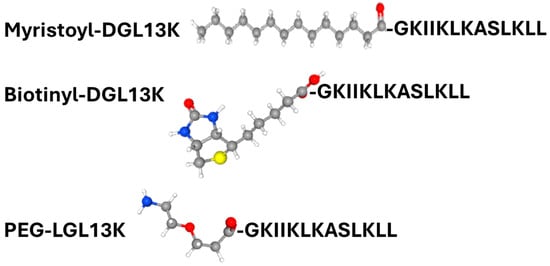
Figure 3.
Structure of N-terminal modifications are shown in ball and stick models. Atom color code: Carbon—grey; hydrogen—white; oxygen—red, nitrogen—blue; and sulfur—yellow. Peptide sequence is shown in one-letter code. Structures obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 4 September 2025)).
The N-terminal PEGylation of LGL13K caused a two- and three-fold increase in MIC for S. aureus and P. aeruginosa, respectively (Figure 4). Similarly, biotinylation increased the MIC for S. aureus and P. aeruginosa, two- and four-fold, respectively. In contrast, the addition of myristoylate to the N-terminus of DGL13K abolished its activity against both P. aeruginosa and S. aureus. Since the biotin molecule resembles the structure of PEG rather than that of myristate, these results suggest that the addition of a highly hydrophobic chain inactivates the antimicrobial activity of the peptide sequence whereas addition of more polar molecules, which include oxygen and NH groups has only a minimal effect on peptide activity.
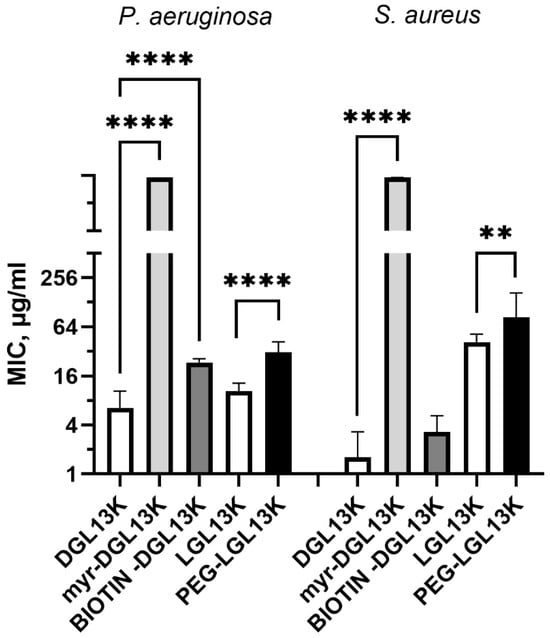
Figure 4.
MIC of N-terminally modified DGL13K. The MIC against P. aeruginosa and S. aureus were determined for myr-DGL13K, biotin-DGL13K, and compared to DGL13K in 2–6 independent experiments, which were analyzed by one-way ANOVA. PEG-LGL13K was compared to LGL13K in 3–5 independent experiments, which were analyzed by unpaired Students t-test. MIC values outside of the measured range were set at 1000 µg/mL for calculation purposes. **** p < 0.0001; ** p = 0.005.
3.5. Hemolysis
The selective activity of many AMPs exploits the compositional differences between prokaryotic and eukaryotic membranes to avoid mammalian cell toxicity. The selectivity of AMPs is typically determined as the ratio between the hemolytic concentration and the MIC of the AMP (therapeutic index) [72,73]. One measure of the hemolytic activity is the peptide concentration leading to 50% lysis of red blood cells (HC50 = hemolytic concentration 50) [74]. This assay is routinely used for screening purposes and the lack of red cell toxicity is an important consideration for IV delivery of an AMP. Dose response experiments with DGL13K and LGL13K had revealed that HC50 is 500–1000 µg/mL [48]. To compare multiple peptides, each peptide was incubated with human red blood cells at 500 µg/mL and hemolysis determined spectrophotometrically (Figure 5). DGL13K and LGL13K showed similar lysis as previously reported, while the Arg-modified peptide DGL13R exhibited a small increase in lysis.
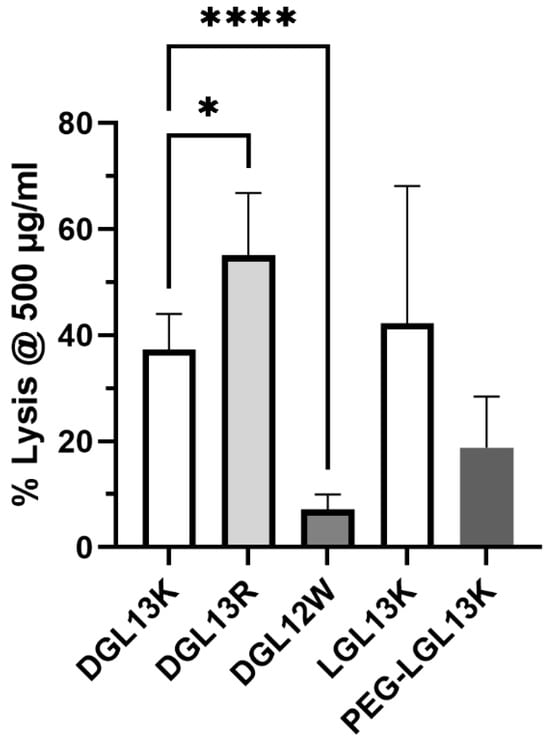
Figure 5.
Hemolysis. The ability of different peptides to lyse human red blood cells at 500 µg/mL. The data from 1–6 independent experiments were plotted as mean ± 95% confidence interval and analyzed by one-way ANOVA for modified DGL13K peptides compared to unmodified DGL13K (open bar) and unpaired Student’s t-test for LGL13K peptides. * p < 0.02; **** p < 0.0001; N = 2–13. A statistical outlier was removed using the ROUT method, Q-1% (Graphpad Prism 10.4).
DGL12W was created by introduction of Trp residues (Table 1). Since Trp has been reported to affect hemolytic activity [73], the peptide was simultaneously prepared without C-terminal amidation. Omitting this modification has been suggested to reduce hemolysis and increase the therapeutic index of AMPs in some cases [75]. Indeed, DGL12W caused less than 10% hemolysis at 500 µg/mL. Thus, the higher bacterial selectivity of this peptide also resulted in improved selectivity for bacterial membranes, i.e., less erythrocyte toxicity. The PEGylated LGL13K peptide appeared to show less hemolysis than the unmodified peptide but this difference did not reach statistical significance.
It is noted that the hemolytic concentration used here is about 100-fold higher than the MICs determined for several of these peptides (Table 2 and Table 3), suggesting a high safety margin for clinical application. Indeed, we have found that topical application of 1 mg/mL DGL13K does not cause acute skin toxicity [48].
3.6. Serum Activity
The relatively high safety margin for hemolysis, suggested that the peptides could have systemic applications. To explore this option, antibacterial activity of select peptides was compared in the presence and absence of 50% serum. Figure 6 shows that the antibacterial activity against P. aeruginosa is lost in the presence of 50% serum, suggesting that the interaction with the Gram-negative cell envelope is highly sensitive to serum components. We have previously formulated DGL13K with EDTA to enhance antibacterial activity against P. aeruginosa [48], suggesting that divalent cations, e.g., calcium, could play a role in this interaction. Interestingly, the effect of serum on the antibacterial activity was more modest for S. aureus (Figure 6). Several peptides, which showed low initial activity, were not affected by the presence of serum in the assay. These results support the view that the stereo-specific interactions of LGL13K and DGL13K with components of the Gram-negative and Gram-positive cell envelopes [60] are also differentially affected by serum.
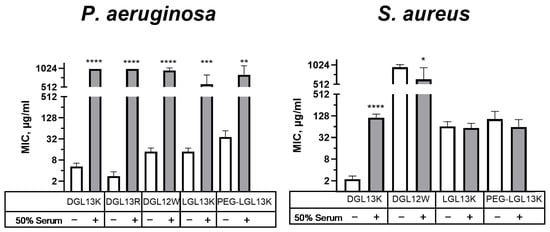
Figure 6.
Relative activity of peptides in 50% serum. MICs of selected peptides were determined in the absence (open bars) or presence (shaded bars) of 50% serum for P. aeruginosa or S. aureus. Each sample pair was analyzed via unpaired Student’s t-test with Welch’s correction for different variances, as needed. MIC values outside of the measured range were set to 1000 µg/mL for calculation purposes. P. aeruginosa: Data from 1–8 independent experiments are plotted as means ± 95% confidence intervals. ** p < 0.002; *** p = 0.0002; **** p < 0.0001 (N = 2–15). S. aureus: Data from 2–10 independent experiments are plotted as means ± 95% confidence intervals. * p < 0.05; **** p < 0.0001 (N = 4–21). Some values obtained in the absence of serum were also included in earlier figures to aid in the comparison between different experimental conditions.
4. Conclusions
The original design of the GL13 peptide family was based on the location of a potential anti-inflammatory peptide in the sequence of the salivary protein BPIFA2 [46]. Thus, peptide GL13NH2 exhibited anti-inflammatory activity that captures the activity of the parent protein since GL13NH2 blocks the binding of LPS to BPIFA2 [44]. The substitution of three polar amino acids in GL13NH2 for cationic amino acids (Lys) resulted in an antibacterial peptide, LGL13K (Formerly GL13K, [34]. The introduction of D-amino acids further optimized the properties of this peptide [16,19]. Modification of naturally occurring peptides has been used to optimize antibacterial properties and stability and reducing toxicity to human cells. The present study demonstrates that the sequence of DGL13K exhibits optimized properties, presumably derived from the natural evolution of the parent protein sequence. Thus, none of the introduced modifications were able to substantially alter the biological properties of GL13K. These results reinforce that “general rules” for AMP design are still empirical and must be balanced against the naturally evolved properties of AMPs. As an example, DGL12W exhibited low hemolytic activity, which was intended by design, while the selectivity for Gram-negative bacteria was not predicted by the design process. As far as AI models are trained on these data sets, it may still be challenging to design de novo peptides with fully predictable properties. The design process for DGL13K combined with the results of this study, suggest that naturally occurring proteins and peptides provide a robust platform for such peptide design projects.
The modified peptides showed no more than a two-fold increase in antibacterial activity, reduction in hemolytic activity, or activity in the presence of serum. The specific interaction of each peptide enantiomer with the cell envelopes of Gram-negative and Gram-positive bacteria deserve additional exploration as it may lead to the design of optimized AMPs with improved activity in serum [64].
The low serum activity is not unique to DGL13K and may be an inherent evolutionary aspect of these peptides, which are typically found in mucosal and skin surfaces [76]. Surprisingly the cationic peptide LL-37, which is found in circulating immune cells, also displays poor activity in the presence of serum; reviewed in [77]. Thus, it should be considered that in vitro assays with serum may not be a good approximation for in vivo activity in circulation. While the role of serum components in peptide inactivation is not fully elucidated, our unpublished data suggest that DGL13K binding to serum proteins (e.g., serum albumin) and the presence of divalent cations play a role. Indeed, we have previously reported that formulation of DGL13K with EDTA increases peptide activity [48].
Despite the low activity in the presence of serum, DGL13K shows activity in wound infections [48] and retains activity in synovial fluid (Gorr, unpublished) while LGL13K inactivates LPS in the peritoneum [34]. Given the stability and lack of bacterial resistance, in vivo activity and low in vivo toxicity of DGL13K, this peptide appears to be naturally optimized for the treatment of topical and localized infections.
Funding
This study was supported with research funds from the University of Minnesota School of Dentistry.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the author used Google Gemini (version 2.5) for the purposes to identify up-to-date references for statements in the text. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
Author Sven-Ulrik Gorr is the Chief Scientific Officer of and holds equity in Gavia BIO, LLC, which is developing the antimicrobial peptide Minneganan (DGL13K). These interests have been reviewed and managed by the University of Minnesota in accordance with its Conflict of Interests policies.
References
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Ganz, T.; Lehrer, R.I. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol. 1998, 10, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Brahmachary, M.; Krishnan, S.P.T.; Koh, J.L.Y.; Khan, A.M.; Seah, S.H.; Tan, T.W.; Brusic, V.; Bajic, V.B. ANTIMIC: A database of antimicrobial sequences. Nucleic Acids Res. 2004, 32, D586–D589. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef]
- Gawde, U.; Chakraborty, S.; Waghu, F.H.; Barai, R.S.; Khanderkar, A.; Indraguru, R.; Shirsat, T.; Idicula-Thomas, S. CAMPR4: A database of natural and synthetic antimicrobial peptides. Nucleic Acids Res. 2022, 51, D377–D383. [Google Scholar] [CrossRef]
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A review on antimicrobial peptides databases and the computational tools. Database 2022, 2022, baac011. [Google Scholar] [CrossRef]
- Gorr, S.U. Antimicrobial peptides in periodontal innate defense. Front. Oral Biol. 2012, 15, 84–98. [Google Scholar] [CrossRef]
- Gorr, S.U.; Abdolhosseini, M. Antimicrobial peptides and periodontal disease. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 126–141. [Google Scholar] [CrossRef] [PubMed]
- Bibby, B.G.; Hine, M.K.; Clough, O.W. The Antibacterial Action of Human Saliva. J. Am. Dent. Assoc. Dent. Cosm. 1938, 25, 1290–1302. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Y.; Li, B.; Qu, G.; Li, A.; Peng, X.; Li, S.; Shao, C. Antimicrobial peptide biological activity, delivery systems and clinical translation status and challenges. J. Transl. Med. 2025, 23, 292. [Google Scholar] [CrossRef]
- Garvey, M. Antimicrobial Peptides Demonstrate Activity against Resistant Bacterial Pathogens. Infect. Dis. Rep. 2023, 15, 454–469. [Google Scholar] [CrossRef]
- Hirt, H.; Gorr, S.U. Antimicrobial peptide GL13K is effective in reducing biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4903–4910. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides With Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Muhammad, I.; Cui, Q.; Zhang, H.; Jia, Y.; Xu, Q.; Kong, L.; Ma, H. An Antibacterial Peptide with High Resistance to Trypsin Obtained by Substituting d-Amino Acids for Trypsin Cleavage Sites. Antibiotics 2021, 10, 1465. [Google Scholar] [CrossRef]
- Hirt, H.; Hall, J.W.; Larson, E.; Gorr, S.U. A D-enantiomer of the antimicrobial peptide GL13K evades antimicrobial resistance in the Gram positive bacteria Enterococcus faecalis and Streptococcus gordonii. PLoS ONE 2018, 13, e0194900. [Google Scholar] [CrossRef] [PubMed]
- Felsztyna, I.; Galassi, V.V.; Wilke, N. Selectivity of membrane-active peptides: The role of electrostatics and other membrane biophysical properties. Biophys. Rev. 2025, 17, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B. Structure and Function of Membrane-Lytic Peptides. Crit. Rev. Plant Sci. 2004, 23, 271–292. [Google Scholar] [CrossRef]
- Bacalum, M.; Radu, M. Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther. 2015, 21, 47–55. [Google Scholar] [CrossRef]
- Rončević, T.; Puizina, J.; Tossi, A. Antimicrobial Peptides as Anti-Infective Agents in Pre-Post-Antibiotic Era? Int. J. Mol. Sci. 2019, 20, 5713. [Google Scholar] [CrossRef] [PubMed]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. The antimicrobial peptide database is 20 years old: Recent developments and future directions. Protein Sci. 2023, 32, e4778. [Google Scholar] [CrossRef]
- Lakshmaiah Narayana, J.; Mishra, B.; Lushnikova, T.; Wu, Q.; Chhonker, Y.S.; Zhang, Y.; Zarena, D.; Salnikov, E.S.; Dang, X.; Wang, F.; et al. Two distinct amphipathic peptide antibiotics with systemic efficacy. Proc. Natl. Acad. Sci. USA 2020, 117, 19446–19454. [Google Scholar] [CrossRef]
- Meher, P.K.; Sahu, T.K.; Saini, V.; Rao, A.R. Predicting antimicrobial peptides with improved accuracy by incorporating the compositional, physico-chemical and structural features into Chou’s general PseAAC. Sci. Rep. 2017, 7, 42362. [Google Scholar] [CrossRef]
- Musin, K.; Asyanova, E. How Machine Learning Helps in Combating Antimicrobial Resistance: A Review of AMP Analysis and Generation Methods. Int. J. Pept. Res. Ther. 2025, 31, 59. [Google Scholar] [CrossRef]
- Wang, B.; Lin, P.; Zhong, Y.; Tan, X.; Shen, Y.; Huang, Y.; Jin, K.; Zhang, Y.; Zhan, Y.; Shen, D.; et al. Explainable deep learning and virtual evolution identifies antimicrobial peptides with activity against multidrug-resistant human pathogens. Nat. Microbiol. 2025, 10, 332–347. [Google Scholar] [CrossRef]
- Verma, D.P.; Tripathi, A.K.; Thakur, A.K. Innovative Strategies and Methodologies in Antimicrobial Peptide Design. J. Funct. Biomater. 2024, 15, 320. [Google Scholar] [CrossRef]
- Kuchler, H.; Heikkilä, M. Why is AI Struggling to Discover New Drugs? Financial Times. 2025. Available online: https://www.ft.com/content/9a8aee4e-9cf6-4bb3-b7ea-d95ddd0d5e79 (accessed on 10 September 2025).
- Geetha, C.; Venkatesh, S.G.; Bingle, L.; Bingle, C.D.; Gorr, S.U. Design and validation of anti-inflammatory peptides from human parotid secretory protein. J. Dent. Res. 2005, 84, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Gorr, S.U.; Sotsky, J.B.; Shelar, A.P.; Demuth, D.R. Design of bacteria-agglutinating peptides derived from parotid secretory protein, a member of the bactericidal/permeability increasing-like protein family. Peptides 2008, 29, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Abdolhosseini, M.; Nandula, S.R.; Song, J.; Hirt, H.; Gorr, S.U. Lysine substitutions convert a bacterial-agglutinating peptide into a bactericidal peptide that retains anti-lipopolysaccharide activity and low hemolytic activity. Peptides 2012, 35, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Wallach, D.; Tessler, R.; Schramm, M. The proteins of the content of the secretory granules of the rat parotid gland. Biochim. Biophys. Acta 1975, 382, 552–564. [Google Scholar] [CrossRef]
- Gao, X.; Oei, M.S.; Ovitt, C.E.; Sincan, M.; Melvin, J.E. Transcriptional profiling reveals gland-specific differential expression in the three major salivary glands of the adult mouse. Physiol. Genom. 2018, 50, 263–271. [Google Scholar] [CrossRef]
- Owerbach, D.; Hjorth, J.P. Inheritance of a Parotid Secretory Protein in Mice and Its Use in Determining Salivary Amylase Quantitative Variants. Genetics 1980, 95, 129–141. [Google Scholar] [CrossRef]
- Torres, S.M.F.; Furrow, E.; Souza, C.P.; Granick, J.L.; de Jong, E.P.; Griffin, T.J.; Wang, X. Salivary proteomics of healthy dogs: An in depth catalog. PLoS ONE 2018, 13, e0191307. [Google Scholar] [CrossRef]
- Geetha, C.; Venkatesh, S.G.; Dunn, B.H.; Gorr, S.U. Expression and anti-bacterial activity of human parotid secretory protein (PSP). Biochem. Soc. Trans. 2003, 31, 815–818. [Google Scholar] [CrossRef]
- Ruhl, S. The scientific exploration of saliva in the post-proteomic era: From database back to basic function. Expert Rev. Proteom. 2012, 9, 85–96. [Google Scholar] [CrossRef]
- Bingle, C.D.; Gorr, S.U. Host defense in oral and airway epithelia: Chromosome 20 contributes a new protein family. Int. J. Biochem. Cell Biol. 2004, 36, 2144–2152. [Google Scholar] [CrossRef]
- Bingle, C.D.; Seal, R.L.; Craven, C.J. Systematic nomenclature for the PLUNC/PSP/BSP30/SMGB proteins as a subfamily of the BPI fold-containing superfamily. Biochem. Soc. Trans. 2011, 39, 977–983. [Google Scholar] [CrossRef]
- Leclair, E. Four BPI (bactericidal/permeability-increasing protein)-like genes expressed in the mouse nasal, oral, airway and digestive epithelia. Biochem. Soc. Trans. 2003, 31, 801–805. [Google Scholar] [CrossRef]
- Abdolhosseini, M.; Sotsky, J.B.; Shelar, A.P.; Joyce, P.B.; Gorr, S.U. Human parotid secretory protein is a lipopolysaccharide-binding protein: Identification of an anti-inflammatory peptide domain. Mol. Cell. Biochem. 2012, 359, 1–8. [Google Scholar] [CrossRef]
- Nandula, S.R.; Huxford, I.; Wheeler, T.T.; Aparicio, C.; Gorr, S.U. The parotid secretory protein BPIFA2 is a salivary surfactant that affects lipopolysaccharide action. Exp. Physiol. 2020, 105, 1280–1292. [Google Scholar] [CrossRef]
- Gorr, S.U.; Abdolhosseini, M.; Shelar, A.; Sotsky, J. Dual host-defence functions of SPLUNC2/PSP and synthetic peptides derived from the protein. Biochem. Soc. Trans. 2011, 39, 1028–1032. [Google Scholar] [CrossRef]
- Dankesreiter, S.; Hoess, A.; Schneider-Mergener, J.; Wagner, H.; Miethke, T. Synthetic endotoxin-binding peptides block endotoxin-triggered TNF-alpha production by macrophages in vitro and in vivo and prevent endotoxin-mediated toxic shock. J. Immunol. 2000, 164, 4804–4811. [Google Scholar] [CrossRef] [PubMed]
- Gorr, S.U.; Flory, C.M.; Schumacher, R.J. In vivo activity and low toxicity of the second-generation antimicrobial peptide DGL13K. PLoS ONE 2019, 14, e0216669. [Google Scholar] [CrossRef] [PubMed]
- Gorr, S.U.; Brigman, H.V.; Anderson, J.C.; Hirsch, E.B. The antimicrobial peptide DGL13K is active against drug-resistant gram-negative bacteria and sub-inhibitory concentrations stimulate bacterial growth without causing resistance. PLoS ONE 2022, 17, e0273504. [Google Scholar] [CrossRef] [PubMed]
- Gorr, S.U.; Chen, R.; Abrahante, J.E.; Joyce, P.B.M. The oral pathogen Porphyromonas gingivalis gains tolerance to the antimicrobial peptide DGL13K by synonymous mutations in hagA. PLoS ONE 2024, 19, e0312200. [Google Scholar] [CrossRef]
- Gorr, S.U. Resisting the resistance: The antimicrobial peptide DGL13K selects for small colony variants of Staphylococcus aureus that show increased resistance to its stereoisomer LGL13K, but not to DGL13K. J. Bacteriol. 2025, 207, e0050524. [Google Scholar] [CrossRef]
- Gorr, S.-U. DGL13K Kills the ESKAPEE Pathogens. Available online: https://www.linkedin.com/posts/activity-7290808720005939200-hXIx?utm_source=share&utm_medium=member_desktop&rcm=ACoAADf5m-wBy-WDhb5IOHuRQGcJtyAiIpso-uE (accessed on 30 July 2025).
- Harmouche, N.; Aisenbrey, C.; Porcelli, F.; Xia, Y.; Nelson, S.E.D.; Chen, X.; Raya, J.; Vermeer, L.; Aparicio, C.; Veglia, G.; et al. Solution and Solid-State Nuclear Magnetic Resonance Structural Investigations of the Antimicrobial Designer Peptide GL13K in Membranes. Biochemistry 2017, 56, 4269–4278. [Google Scholar] [CrossRef]
- Balhara, V.; Schmidt, R.; Gorr, S.U.; Dewolf, C. Membrane selectivity and biophysical studies of the antimicrobial peptide GL13K. Biochim. Biophys. Acta 2013, 1828, 2193–2203. [Google Scholar] [CrossRef]
- Ye, Z.; Zhu, X.; Acosta, S.; Kumar, D.; Sang, T.; Aparicio, C. Self-assembly dynamics and antimicrobial activity of all l- and d-amino acid enantiomers of a designer peptide. Nanoscale 2018, 11, 266–275. [Google Scholar] [CrossRef]
- Ye, Z.; Aparicio, C. Modulation of supramolecular self-assembly of an antimicrobial designer peptide by single amino acid substitution: Implications on peptide activity. Nanoscale Adv. 2019, 1, 4679–4682. [Google Scholar] [CrossRef] [PubMed]
- Nugrahadi, P.P.; Hinrichs, W.L.J.; Frijlink, H.W.; Schöneich, C.; Avanti, C. Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review. Pharmaceutics 2023, 15, 935. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tan, P.; Tang, Q.; Wang, T.; Ding, Y.; Fu, H.; Zhang, Y.; Zhou, C.; Song, M.; Tang, Q.; et al. Enhancing the stability of antimicrobial peptides: From design strategies to applications. Chem. Eng. J. 2023, 475, 145923. [Google Scholar] [CrossRef]
- Lai, Z.; Yuan, X.; Chen, H.; Zhu, Y.; Dong, N.; Shan, A. Strategies employed in the design of antimicrobial peptides with enhanced proteolytic stability. Biotechnol. Adv. 2022, 59, 107962. [Google Scholar] [CrossRef]
- Ye, Z.; Aparicio, C. Interactions of two enantiomers of a designer antimicrobial peptide with structural components of the bacterial cell envelope. J. Pept. Sci. 2022, 28, e3299. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef]
- Deslouches, B.; Phadke, S.M.; Lazarevic, V.; Cascio, M.; Islam, K.; Montelaro, R.C.; Mietzner, T.A. De novo generation of cationic antimicrobial peptides: Influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 2005, 49, 316–322. [Google Scholar] [CrossRef]
- Deslouches, B.; Islam, K.; Craigo, J.K.; Paranjape, S.M.; Montelaro, R.C.; Mietzner, T.A. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: Implications for systemic applications. Antimicrob. Agents Chemother. 2005, 49, 3208–3216. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Lushnikova, T.; Golla, R.M.; Wang, X.; Wang, G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017, 49, 316–328. [Google Scholar] [CrossRef]
- Peptideweb. Amino Acid Properties. Available online: https://www.peptideweb.com/amino-acid-properties (accessed on 20 August 2025).
- Gorr, S.-U.; Aparicio, C.; Ye, Z. Peptides, Hydrogel Compositions and Methods of Use Thereof. U.S. Patent 12,037,374 B2, 2024. [Google Scholar]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Morris, C.J.; Beck, K.; Fox, M.A.; Ulaeto, D.; Clark, G.C.; Gumbleton, M. Pegylation of antimicrobial peptides maintains the active peptide conformation, model membrane interactions, and antimicrobial activity while improving lung tissue biocompatibility following airway delivery. Antimicrob. Agents Chemother. 2012, 56, 3298–3308. [Google Scholar] [CrossRef]
- Brunetti, J.; Falciani, C.; Roscia, G.; Pollini, S.; Bindi, S.; Scali, S.; Arrieta, U.C.; Gomez-Vallejo, V.; Quercini, L.; Ibba, E.; et al. In vitro and in vivo efficacy, toxicity, bio-distribution and resistance selection of a novel antibacterial drug candidate. Sci. Rep. 2016, 6, 26077. [Google Scholar] [CrossRef]
- Lei, R.; Yang, C.; Sun, Y.; Li, D.; Hao, L.; Li, Y.; Wu, S.; Li, H.; Lan, C.; Fang, X. Turning cationic antimicrobial peptide KR-12 into self-assembled nanobiotics with potent bacterial killing and LPS neutralizing activities. Nanoscale 2024, 16, 887–902. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Shen, T.; Chen, L.; Zhou, J.; Shi, S.; Wang, Y.; Zhao, Z.; Liao, C.; Wang, C. N-terminal Myristoylation Enhanced the Antimicrobial Activity of Antimicrobial Peptide PMAP-36PW. Front. Cell. Infect. Microbiol. 2020, 10, 450. [Google Scholar] [CrossRef]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.C.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid selectivity in novel antimicrobial peptides: Implication on antimicrobial and hemolytic activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef]
- Blondelle, S.E.; Simpkins, L.R.; Pérez-Payá, E.; Houghten, R.A. Influence of tryptophan residues on melittin’s hemolytic activity. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1993, 1202, 331–336. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kumar, N.; Choudhury, S.; Mehta, N.K.; Raghava, G.P.S. Prediction of hemolytic peptides and their hemolytic concentration. Commun. Biol. 2025, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Tiltak, D.; Ieronimo, M.; Kanithasen, N.; Wadhwani, P.; Ulrich, A.S. Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides. Pure Appl. Chem. 2007, 79, 717–728. [Google Scholar] [CrossRef]
- Hancock, R.E.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef] [PubMed]
- Durr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).