Abstract
The nematode family Aphelenchoididiae is considered fungal-feeding, predatory, or root hair feeders. Some members of this family are universally present in agricultural landscapes and are an integral part of soil health and conservation studies. In the present soil nematode biodiversity survey, we detected four species of the genera Aphelenchus, Aphelenchoides, and Robustodorus. Because fungal-feeding nematodes from southern Alberta have not previously been reported, we conducted a detailed morphological and molecular investigation, identifying these species as Aphelenchus avenae, Aphelenchoides limberi, Aphelenchoides prairiensis n. sp. and Robustodorus paramegadorus n. sp. The first two species we document as new records from southern Alberta, whereas A. prairiensis n. sp. and R. paramegadorus n. sp. we describe in detail as new taxa. Briefly, A. prairiensis n. sp. is an amphimictic species having 4 lateral lines; hemispherical anteriorly flattened lip region; delicate stylet and swelling-like stylet knobs; excretory pore at the posterior edge of nerve ring. Female tail conical, gradually tapering towards a truncated end with single mucro. Spicule 23.0 (20.0–25.0) µm long having elongated rounded condylus, small, blunt conical rostrum, and lamina that gradually tapers towards the rounded distal end; three pairs of caudal papillae were present on the male tail. Robustodorus paramegadorus n. sp., is a parthenogenetic species with 3 lines in the lateral fields; lip region rounded, anteriorly flattened; stylet robust, with knobs rounded to bean-shaped; excretory pore located posterior to nerve ring; reproductive components were quite indiscernible with a short 24.0 (18.0–27.0) µm post-vulval uterine sac; tail conical, ending with pointed to wedge-shaped tip. We performed molecular characterizations for each species and constructed phylogenetic trees to study the phylogenetic relationship of these aphelenchid species. The discovery of A. prairiensis n. sp. and R. paramegadorus n. sp. indicates that soil nematode diversity is relatively unexplored in southern Alberta. The findings of this study will significantly enhance the identification processes and may contribute towards future soil health and biodiversity efforts.
1. Introduction
Fungal- or root hair-feeding nematodes are universally present in agricultural landscapes worldwide, forming an important component of soil ecosystems [1,2,3,4]. Typically, nematodes within a genus tend to use similar food sources [5,6]. However, there are exceptions where some nematode species evolve to become significant parasites of plants. For instance, some members of Aphelenchoides Fischer [7] and Ditylenchus Filipjev [8] are benign and can be cultured on fungi, whereas several other species of these genera are present on plant quarantine lists [9,10,11]. Therefore, a precise and accurate identification is very important for nematode management, phytosanitary purposes or for the selection of resistant crops to the nematode.In the present study, a correct species delimitation between Aphelenchoides spp. was achieved by integrative taxonomical approaches that combined morphological, morphometrical, and molecular data. These methods constitute the basis for the development of posterior species-specific molecular probes, for example, the diagnostic recombinase polymerase amplification (RPA) assays and lateral flow dipsticks (LF), developed by Subbotin [12] for the separation of the strawberry foliar nematode Aphelenchoides fragariae from other important foliar pathogens, such as the summer crimp nematode of strawberry Aphelenchoides besseyi or the rice white tip nematode Aphelenchoides oryzae [12]. Control measures that have been successfully used to manage infestations of aphelenchid species, particularly Aphelenchoides spp., on key crops have been recently reviewed by de Souza et al. [13] and included the application of chemical nematicides, cultural practices, crop rotation with nonhost plants, biological control agents, induced resistance (silicon or endophytic microorganisms), and blocking parasitism genes (i.e., using RNA interference (RNAi) or genome editing (typically CRISPR/Cas) to disrupt the targets of the effectors in the plant). Here, we investigated both new and known species of Aphelenchus Bastian [14], Aphelenchoides, and Robustodorus (Goodey [15]) Andrássy [16], recovered from southern Alberta’s arable regions. Among these genera, Aphelenchoides is the most species-rich, with 181 known species [17], followed by Aphelenchus (12 species), and Robustodorus (4 species) [18,19,20,21]. Though these genera are known for their mycetophagy and plant-parasitic tendencies [22,23], none of the member species has previously been reported from southern Alberta. Consequently, when we discovered these species during our soil nematode inventory survey, we performed detailed characterization and identified them to the species level. Our thorough morphological and molecular analyses revealed their identities as Aphelenchus avenae Bastian [13], Aphelenchoides limberi Steiner [24], and two unique species of Aphelenchoides and Robustodorus, each displaying distinctive traits. Upon comparison with related species, it appeared that these species have a novel status. Therefore, we formally describe them here as A. prairiensis n. sp. and R. paramegadorus n. sp. Moreover, Aphelenchus avenae and A. limberi were documented as new records from southern Alberta. The discovery of A. prairiensis n. sp. and R. paramegadorus n. sp. indicate that the soil nematode diversity is relatively underexplored in southern Alberta. The findings of this study will significantly enhance identification processes and may contribute towards future soil health and biodiversity research.
2. Materials and Methods
2.1. Sampling, Nematode Extraction, and Morphological Studies
To investigate the diversity of plant-parasitic nematodes (PPNs) associated with natural vegetation in the marginal areas (uncultivated land on the edges of the fields) of irrigated fields, we conducted a nematode inventory assessment in selected fields of southern Alberta, Canada. Soil samples were collected from the fields using a pointed shovel, and each sample was carefully stored in labelled plastic bags for further analysis. The soil samples were stored in the cold storage facility of the University of Lethbridge (southern Alberta, AB, Canada) until processing. Nematodes were extracted from soil samples using the modified Cobb’s sieving and flotation–centrifugation method [25]. Among other soil nematodes, individual aphelenchid taxa were hand-picked and mounted on slides for observation and preservation. For preliminary examination, freshly isolated Aphelenchus, Aphelenchoides and Robustodorus taxa were transferred to a drop of distilled water, heat relaxed, and observed under a Zeiss Axioskope 40 microscope (Carl Zeiss, Jena, Germany). Morphological and morphometric characteristics are fundamental for the differential diagnosis of various genera and species. Therefore, we used the following de Man [26] body ratios and other characteristics [27] for the morphometric analysis of the species identified in this study: n, number of specimens on which the measurements are based; a, body length/maximum body diameter; b, body length/distance from anterior end to pharyngo-intestinal junction; b′ body length/distance from anterior end to posterior end of pharyngeal glands; c, body length/tail length; c′, tail length/tail diameter at anus; V, distance from the body anterior end to the vulva as a percentage (%) of the body length; V′, position of vulva from the anterior end expressed as percentage of distance from head to anal aperture. For morphometrical studies, the nematodes were fixed, and permanent slides were prepared as described by De Grisse [28]. Images of each specimen were acquired using a Zeiss Axioskope 40 microscope equipped with a Zeiss Axiocam 208 camera (Carl Zeiss, Jena, Germany). Measurements from the images were performed using ZEN blue 3.1 imaging software (Carl Zeiss).
2.2. DNA Extraction, PCR and Sequencing
After microscopic examination, single females of each unidentified species were transferred to a 0.2 mL PCR tube, and the DNA was extracted as described in Maria [29]. Three sets of DNA primers (Integrated DNA Technologies, Coralville, IA, USA) were used to amplify the partial 18S, 28S, and ITS ribosomal RNA (rRNA) genes. The partial 18S rRNA gene sequence was amplified with the 1813F/2646R primer pair [30]. The 28S rRNA gene was amplified using the D2A/D3B primer pair [31], and the ITS gene was amplified using the Vrain2F/Vrain2R primer pair [32]. For the 18S, ITS, and 28S genes, the PCR conditions were as described previously [30,31,32]. Amplified PCR products were resolved by electrophoresis in 1% agarose gels and visualized by staining with GelRed (Biotium, Fremont, CA, USA). The PCR products of appropriate size and sufficiently high quality were sent to Azenta Inc. for purification and sequencing (South Plainfield, NJ, USA). The newly obtained DNA sequences were aligned using the BioEdit sequence alignment tool (BioEdit v. 7.2.5) and compared for similarities with all known nematode species sequences in the GenBank database.
2.3. Phylogenetic Studies
The obtained 18S, ITS, and D2–D3 expansion segments of the 28S rRNA gene sequences, and selected aphelenchid species sequences, were used to perform phylogenetic analyses. Outgroup taxa for each dataset were chosen as described in previously published studies [17,20,21,33]. Multiple nucleotide sequence alignments of the different genes were produced using the FFT-NS-2 algorithm of MAFFT v7.450 [34]. Sequence alignments were manually visualized using BioEdit [35]. The sequences were edited manually, and poorly aligned positions were trimmed using a light filtering strategy (up to 20% of alignment positions). Phylogenetic analyses of the sequence datasets were based on Bayesian inference (BI) using MrBayes v3.1.2 [36]. The best-fit model of DNA evolution was obtained using JModelTest v2.1.7 [37] with the Akaike Information Criterion (AIC). The best-fit model, base frequency, proportion of invariable sites, and gamma distribution shape parameters and substitution rates in the AIC were then used in MrBayes for the phylogenetic analyses. The Tamura and Nei model with invariable sites and a gamma-shaped distribution (TrN + I + G) for the 18S, the transversional model with invariable sites and a gamma-shaped distribution (TVM + I + G) for the D2–D3 expansion segments of the 28S rRNA, and the transitional model with a gamma-shaped distribution (TIM1 + G) for the ITS rRNA, were performed with four chains for 4 × 106 generations. A combined analysis of the three ribosomal genes was not undertaken due to some sequences not being available for all species. The Markov chains were sampled at intervals of 100 generations. Two runs were conducted for each analysis. After discarding burn-in samples of 30% and evaluating convergence, the remaining samples were retained for in-depth analyses. The topologies were used to generate a 50% majority-rule consensus tree. Posterior probabilities (PP) were given on appropriate clades. Trees from all analyses were visualized using FigTree software v1.42 [38].
3. Results
3.1. Description of Aphelenchoides prairiensis n. sp.
Female: Body slender, cylindrical, straight to slightly arcuate ventrally with fine annulation. Lateral fields with four incisures. Lip region hemispherical, anteriorly flattened, slightly offset, labial framework lightly sclerotized. Stylet delicate, anterior part of stylet comprising 45–48% of total stylet length. Stylet knobs not well developed, rounded, more like swellings. Median bulb squarish to rounded, valve plates bean-shaped, situated centrally. Pharyngo–intestinal junction less than one stylet length posterior to median bulb. Pharyngeal glands extending dorsally and subdorsally over intestine. Nerve ring located about one body diam. posterior to median bulb. Excretory pore at the posterior edge of nerve ring. In mature females, ovary extending to pharyngeal glands, oocytes in one or two rows, spermatheca elongate oval, with sperm, crustaformeria indistinct, vagina anteriorly directed, vulva simple without any vulval flaps or protruding lips. Post-vulval uterine sac (PUS) conspicuous, large, tubular, occupying the entire body width, empty or containing sperm. Anus distinct, slit-like. Tail conical, gradually tapering towards a truncated end with a single mucro (Figure 1 and Figure 2).
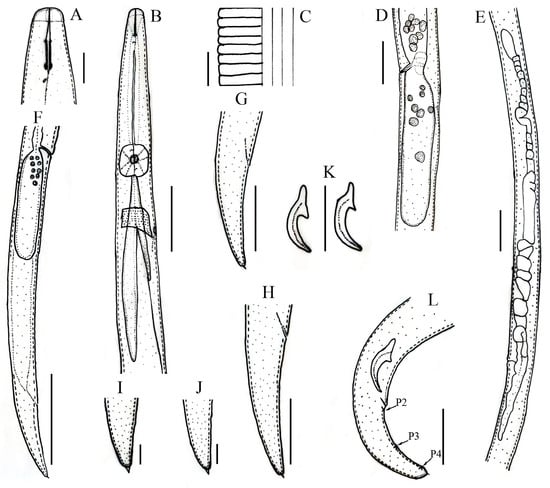
Figure 1.
Line drawings of Aphelenchoides prairiensis n. sp. male and female. (A) Anterior region; (B) pharyngeal region; (C) lateral field lines; (D) vulval region; (E) female gonad; (F) female posterior body region; (G,H) female tail regions; (I,J) female tail tips; (K) spicules; (L) male tail, arrows pointing towards P2, P3, P4, number of caudal papillae. Vertical lines are scale bars: (A,C,I,J) 5 μm; (F) 50 μm; (B,D,E,G,H,K,L) 20 μm.
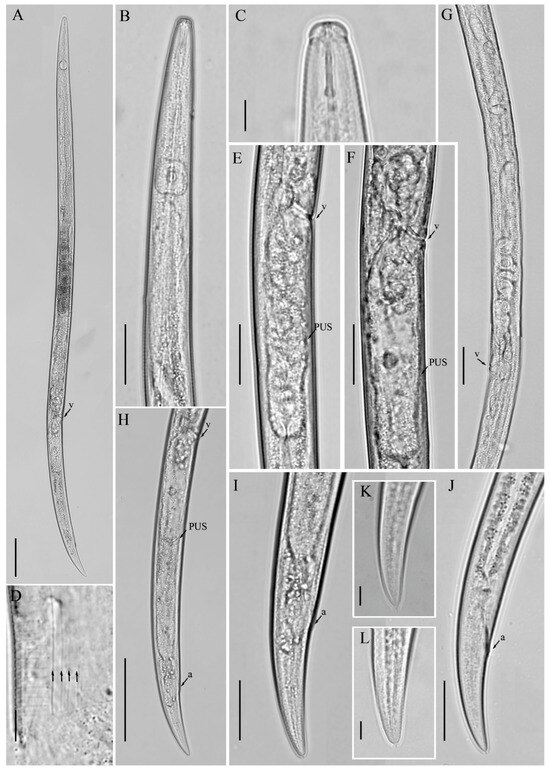
Figure 2.
Light micrographs of Aphelenchoides prairiensis n. sp. female. (A) Entire body; (B) pharyngeal region; (C) anterior region; (D) lateral field lines, arrows indicating the number of lines; (E,F) vulval region; (G) gonad; (H) posterior body region; (I–L) tail regions. Scale bars: (A) 50 μm; (B,E–I,J) 20 μm; (C,D,K,L) 5 μm. Arrows: (a) anus; (PUS) post-vulval uterine sac; (v) vulva.
Male: Less frequent than females (proportion 1:5). Body cylindrical attains J-shape after heat relaxed. Anterior end body morphology is similar to that of females. Reproductive system monorchic. Testis outstretched with spermatocytes arranged in single to two rows. Spicules ventrally arcuate, condylus elongated rounded, rostrum small, blunt conical, capitulum with slight to no depression, lamina less wide gradually tapers towards a rounded distal end. Three pairs of caudal papillae (single precloacal P1 absent), the first pair (P2) at cloacal level or slightly posterior to it, the second pair (P3) at about middle of tail, and the third pair (P4), close to the tail tip. Tail similar to that of females, ending with a single mucro (Figure 3).
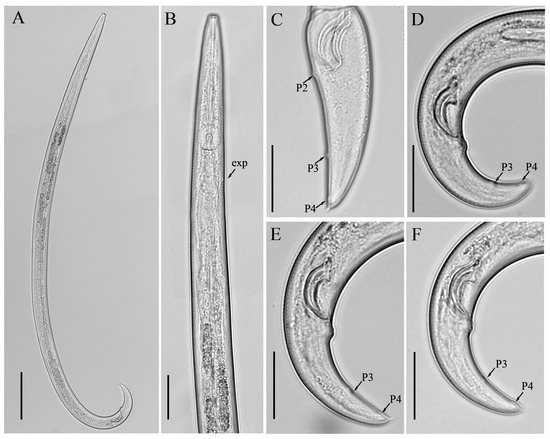
Figure 3.
Light micrographs of Aphelenchoides prairiensis n. sp. male. (A) Entire body; (B) pharyngeal region; (C–F) tail regions. Scale bars: (A) 50 μm; (B–F) 20 μm. Arrows: (exp) excretory pore; (P2, P3, P4) number of caudal papillae.
Diagnosis and relationship: Aphelenchoides prairiensis n. sp. is an amphimictic species having four lateral lines; females having slender, cylindrical bodies; hemispherical anteriorly flattened lip region; delicate stylet and swelling-like stylet knobs; excretory pore at the posterior edge of the nerve ring. In mature females, ovary extending to pharyngeal glands; post-vulval uterine sac (PUS) conspicuous, large, tubular, occupying an entire body width, empty or containing sperm. Tail conical, gradually tapering towards a truncated end with a single mucro. Male with moderately long spicule 23.0 (20.0–25.0) µm; condylus elongated rounded, rostrum small, blunt conical, capitulum with slight to no depression, lamina less wide gradually tapers towards the rounded distal end. Caudal papillae are composed of three pairs, P1 absent, P2 at cloacal level, P3 at about the middle of the tail, and P4 close to the tail tip.
Aphelenchoides is a species-rich genus; it has more than 180 valid species [17]. For easy identification, the Aphelenchoides species were divided into four groups based on tail terminus (group 1: tail end without any outgrowth or mucro; group 2: tail end with one or sometimes two mucronate structures; group 3: star-shaped tail end with four mucronate structures; group 4; tail end with outgrowth other than spine or star). Another stable characteristic in this genus is the number of lateral lines; two to six lateral lines have been reported for Aphelenchoides species [39]. By following this scheme, our new species comes close to A. angusticaudatus Eroshenko [40], A. cyrtus Paesler [41], A. daubichaensis Eroshenko [40], and A. haguei Maslen [42].
The new species can be distinguished from A. angusticaudatus [39] by the presence of males vs. absence; a longer body length of females, 728.0 (564.0–978.0) µm vs. (550–610) µm; a lower b value of females, 9.0 (8.0–10.0) vs. (11–13); a higher c value of females 17.0 (15.0–21.0) vs. (12–18); and a lower c′ value of females, 3.4 (2.8–4.0) vs. 5.1. From A. cyrtus, by having an almost straight body habitus of females vs. arc-shaped; swelling type stylet knobs vs. distinctly knobbed; spicule shape (condylus elongated rounded, rostrum small, blunt conical, lamina less wide, gradually tapers towards the rounded distal end vs. (after drawings) condylus and rostrum appear pointed, lamina is wider, distal end appears pointed); the position of the tail mucro (central vs. appears ventrally located); habitat (post-harvest potato field vs. lichens, mushroom amended with flax and straw additives); longer body lengths of females, 728.0 (564.0–978.0) µm vs. (500–570) µm, and males, 720.0 (624.0–859.0) µm vs. (500–510) µm; a higher a value of females, 34.0 (31.0–38.0) vs. (24–28), and males, 33.5 (30.0–36.5) vs. (26–27). From A. daubichaensis, by having males vs. the absence; the shape of the median bulb (squarish rounded vs. heart shape); longer PUS length (about 3–5 vulval body width vs. 1 and half vulval body width); shape of tail terminus (truncated vs. peg-like); habitat (post-harvest potato field vs. soil, root system, leaves, and wheat straw); and a longer body length of females, 728.0 (564.0–978.0) µm vs. (467–594) µm. From A. haguei, by having simple vs. protrusive vulva lips; the shape of tail mucro (simple vs. multi-papillate); spicule morphology (condylus elongated rounded, rostrum small, blunt conical, lamina less wide, gradually tapers towards the rounded distal end vs. (after drawings) condylus very broad, rostrum small rounded, lamina wider with pointed distal ends); longer body lengths of females, 728.0 (564.0–978.0) µm vs. 624 (560–765) µm, and males, 720.0 (624.0–859.0) µm vs. 604 (525–740) µm; and a higher c value of females, 17.0 (15.0–21.0) vs. 13 (10.6–15.8) (Table 1).

Table 1.
Morphometrics of Aphelenchoides prairiensis n. sp. All measurements are in μm and in the form: mean ± standard deviation (range).
Type host and locality: The new species was found in a post-harvest potato field located in Bow Island, southern Alberta, Canada.
Type material: Holotype female, 13 paratype females, and 3 males (four slides, numbers UL-DY3-01 to UL-DY3-04) were deposited in the nematode collection of the University of Lethbridge, AB, Canada. Four paratype females and two paratype males were deposited in the nematode collection of the Institute for Sustainable Agriculture, CSIC, Córdoba, Spain.
Etymology: The species epithet is derived from the Latin word “prairiensis” meaning “of the prairie”. It indicates the origin or habitat of the species, suggesting that it is found in prairie regions of southern Alberta.
3.2. Description of Aphelenchoides limberi Steiner [24]
Female: Body slender, cylindrical, arc-shaped when heat relaxed. Lateral fields with four incisures. Lip region rounded, anteriorly truncated, slightly offset, labial framework slightly sclerotized. Stylet delicate, anterior part of stylet comprising 48–50% of total stylet length. Stylet knobs weakly developed, rounded, more like swellings. Median bulb rounded, valve plates bean-shaped, situated centrally. Pharyngo–intestinal junction slightly posterior to median bulb. Pharyngeal glands extend dorsally and subdorsally over intestine. Nerve ring located less than one body diam. posterior to median bulb. Excretory pore at the posterior edge of nerve ring. In mature females, ovary reflexed, oocytes in one or two rows, spermatheca and crustaformeria indistinct, vagina anteriorly directed, vulva with protruding lips. Post-vulval uterine sac (PUS) conspicuous, large, tubular, occupying the entire body width. Anus distinct, slit-like. Tail cylindrical, gradually tapering towards a truncated end without any mucro.
Male: Not found.
Remarks: The species was initially described in Juno plant examinations imported from Holland [24]. The species was sporadically mentioned in the literature, with records from Ottawa, Canada [43], Iran [44], and the Czech Republic [45]. However, none of these sources provided photographic evidence or comprehensive morphometric data, except for the latter. Our morphometric data align well with the characteristics observed in both the original description and the population from the Czech Republic. The host associations of A. limberi were reported as tulip bulbs (Ottawa, ON, Canada), vineyards (Markazi, Iran), and hop gardens (Senice na Hané, Czech Republic). In our study, we identified A. limberi in a post-harvest potato field of southern Alberta and performed detailed morpho-molecular analyses. Consequently, our data can be used in future integrated taxonomical studies (Figure 4; Table 2).
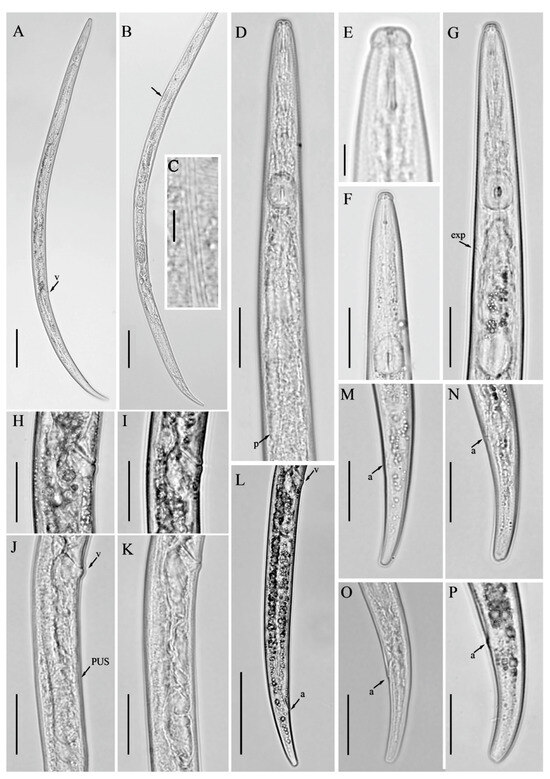
Figure 4.
Light micrographs of Aphelenchoides limberi Steiner [24], female. (A,B) Entire bodies, genital track arrowed; (C) lateral lines; (D,G) pharyngeal regions; (E,F) anterior regions; (H–K) vulval regions; (L) posterior body region; (M–P) tail regions. Scale bars: (A,B) 50 μm; (D,F–P) 20 μm; (C,E) 5 μm. Arrows: (a) anus; (exp) secretory–excretory pore; (p) pharyngeal lobe; (PUS) post-vulval uterine sac; (v) vulva.

Table 2.
Morphometrics of female Aphelenchoides limberi Steiner [24], reported in the present and previous studies. Our study’s measurements are in μm and in the form: mean ± standard deviation (range), whereas the original and Czech Republic population measurements are in μm and in the form: mean (range).
3.3. Description of Aphelenchus avenae Bastian [14]
Female: Body slender, cylindrical, slightly arched when heat relaxed. Lateral fields with twelve incisures. Lip region squarish rounded, anteriorly flattened, slightly offset. Stylet delicate, without stylet knobs. Median bulb oval rounded, valve plates bean-shaped, situated centrally. Pharyngo–intestinal junction less than one stylet length posterior to median bulb. Pharyngeal glands extend dorsally over intestine. Nerve ring located about one body diam. posterior to median bulb. Excretory pore at the anterior edge of nerve ring. Gonad monodelphic, anteriorly outstretched, oocytes in one or two rows, spermatheca and crustaformeria indistinct, vagina anteriorly directed, vulva lips simple with deep depression. Post-vulval uterine sac (PUS) conspicuous, large, occupying the entire body width. Anus distinct, slit-like. Tail cylindrical, gradually tapering towards a broadly rounded terminus (Figure 5; Table 3).
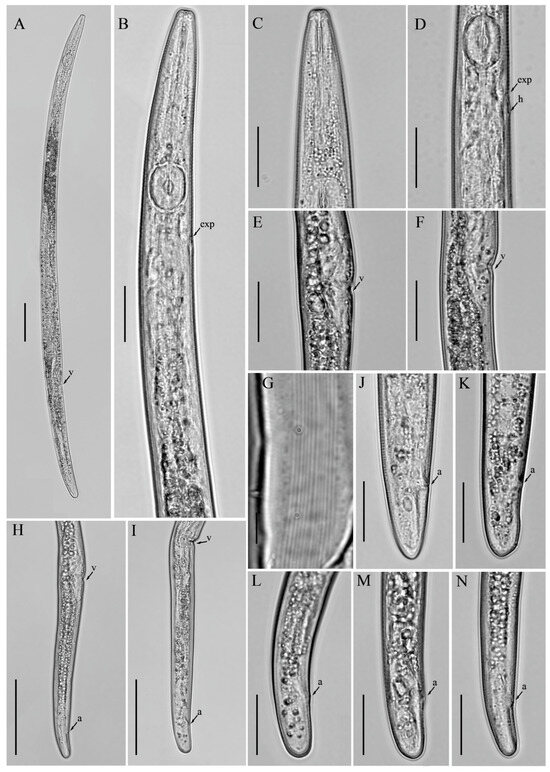
Figure 5.
Light micrographs of Aphelenchus avenae Bastian [14], female. (A) Entire body; (B) pharyngeal region; (C) anterior region; (D) posterior pharyngeal region; (E,F) vulval regions; (G) lateral lines; (H,I) posterior body regions; (J–N) tail regions. Scale bars: (A,H,I) 50 μm; (B–F,J–N) 20 μm; (G) 5 μm. Arrows: (a) anus; (exp) secretory–excretory pore; (h) hemizonid; (v) vulva.

Table 3.
Morphometrics of female Aphelenchus avenae Bastian [14], found in this study. Measurements are in μm and in the form: mean ± standard deviation (range).
Male: Not found.
Remarks: Aphelenchus avenae has global distribution and a wider host range [2]. This species was detected in several of our soil samples collected from cultivated and post-harvest potato fields. However, our focus in this study is to document its presence in southern Alberta. Morphometric or morphological comparisons across all reported populations of A. avenae are not within the purview of this research.
Fungal feeding tests: The Potato Research Laboratory at the University of Lethbridge is dedicated to studying major plant pathogens associated with potato diseases. Our laboratory maintains a diverse collection of fungal pathogens known to affect potatoes. Here, we selected two major fungal pathogens (Phytophthora infestans and Fusarium sambucinum) to study nematode reproduction. The rationale for selecting only two fungal pathogens was as follows: (i) the labor-intensive process of nematode extractions and the hand-picking of select Aphelenchoides species from soil nematode suspensions; (ii) the limited availability of nematodes; and (iii) this study’s focus, which was solely to provide taxonomic descriptions of these nematode species. For the fungal-feeding test, thirty individuals of A. limberi and A. prairiensis n. sp. were surface-sterilized using streptomycin sulphate and separately introduced to 14-day-old mycelial mats of the aforementioned fungal pathogens and incubated in the dark for two months, as described by Castillo et al. [10] and Munawar et al. [46] Microscopic observations revealed no evidence of nematode reproduction, indicating that P. infestans and F. sambucinum are not suitable hosts for A. limberi and A. prairiensis n. sp.
3.4. Description of Robustodorus paramegadorus n. sp.
Female: Body cylindrical, slightly arched when heat relaxed. Lateral fields with three incisures. Lip region rounded, anteriorly flattened, with deep constriction. Stylet robust, anterior part of stylet comprising 45–48% of total stylet length. Stylet knobs rounded to bean-shaped. Median bulb squarish to rounded, valve plates ellipsoidal-shaped, situated slightly posteriorly. The gland duct of the dorsal pharyngeal gland opens into the lumen of the median bulb just anterior to the valve; the sub-ventral glands ducts open immediately after the valve. Pharyngo–intestinal junction less than one stylet length posterior to median bulb. Pharyngeal glands extend dorsally and subdorsally over intestine. Nerve ring located 10–12 µm posterior to median bulb. Excretory pore located posterior to nerve ring. Hemizonid one median bulb length behind excretory pore. Reproductive components quite indiscernible, gonad outstretched, not extending to pharyngeal glands, oocytes in one or two rows, spermatheca and crustaformeria indistinct, vagina anteriorly directed, vulva simple without any vulval flaps, both vulval lips slightly protruding. Post-vulval uterine sac (PUS) conspicuous, hollow, tubular, occupying less than half of body width. Anus distinct, slit-like. Tail conical, ending with a pointed to wedge-shaped tip (Figure 6 and Figure 7; Table 4).
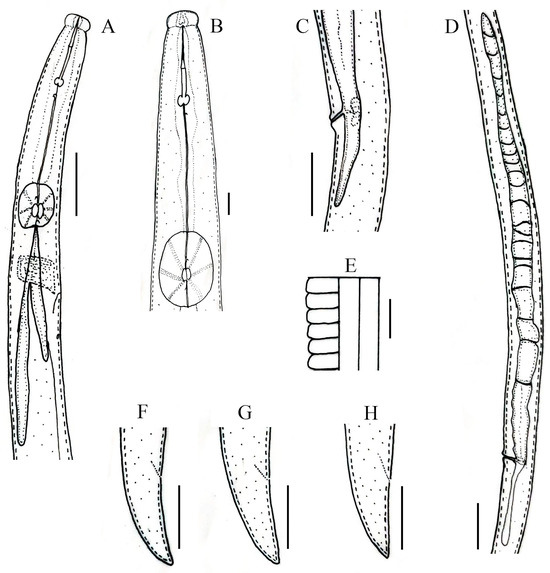
Figure 6.
Line drawings of Robustodorus paramegadorus n. sp. Female. (A) Pharyngeal region; (B) anterior region; (C) vulval regions; (D) gonad; (E) lateral lines; (F–H) tail regions. Scale bars: (A,C,D,F–H) 20 μm; (B,E) 5 μm.
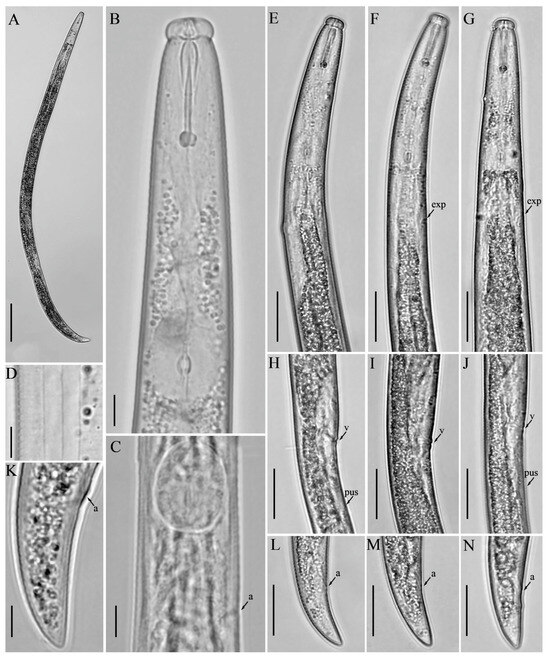
Figure 7.
Light micrographs of Robustodorus paramegadorus n. sp. female. (A) Entire body; (B) anterior region; (C) median bulb region; (D) lateral lines; (E–G) pharyngeal regions; (H–J) vulval regions; (K–N) tail regions. Scale bars: (A) 50 μm; (E–J,L–N) 20 μm; (B–D,K) 5 μm. Arrows: (a) anus; (exp) secretory–excretory pore; (PUS) post-vulval uterine sac; (v) vulva.

Table 4.
Morphometrics of Robustodorus paramegadorus n. sp. All measurements are in μm and in the form: mean ± standard deviation (range).
Male: Not found.
Diagnosis and relationship: The new species appears to be parthenogenetic, because males were not found and no sperm was found in indistinct/irregular shaped spermatheca. It has a cylindrical, slightly arched body with three lines in the lateral fields. Lip region rounded, anteriorly flattened, with deep constriction. Stylet robust, with rounded to bean-shaped knobs. Median bulb squarish to rounded, valve plates ellipsoidal-shaped, situated slightly posteriorly. Nerve ring located 10–12 µm posterior to the median bulb. Excretory pore located posterior to nerve ring. Reproductive components are quite indiscernible, vagina anteriorly directed, vulva simple without any vulval flaps, both vulval lips slightly protruding. Post-vulval uterine sac (PUS), short 24.0 (18.0–27.0) µm, conspicuous, hollow, and tubular. Tail conical, ending with a pointed to wedge-shaped tip.
The genus has four nominal species, R. arachidis Bos [47], R. helicus Heyns [48], R. megadorus (Allen [49]) Andrássy [16], and R. subtenuis Cobb [50]. Robustodorus paramegadorus n. sp. is most similar to R. megadorus by Allen [45], and the R. megadorus’s Utah population by Ryss et al. [18]. Robustodorus paramegadorus n. sp. can be differentiated from R. megadorus by the shape of the stylet knobs (rounded to bean-shaped vs. C-shaped); the position of the excretory pore (posterior to nerve ring vs. between median bulb and nerve ring); position of the hemizonid (one median bulb length behind excretory pore vs. two–three annuli posterior to excretory pore); the shape of the median bulb (squarish rounded vs. rounded ovoid); the position of the median bulb valve plates (posteriorly located vs. central); protrusion of the vulval lips (both vs. anterior); tail tip shape (pointed to wedge-shaped vs. rounded); longer body length of females, 555 (466–623) µm vs. 496 (455–546) µm; longer stylet length of females, 19.5 (18–21) µm vs. 18 (17–19) µm; and longer tail length of females, 24 (21–26) µm vs. 19 (17–20) µm.
From R. arachidis [20], it can be differentiated by the absence of males vs. the presence; the shape of the lip region (rounded, anteriorly flattened vs. squarish round); the number of lateral lines (three vs. two–four); tail tip shape (pointed to wedge-shaped vs. broadly rounded with or without mucro); the smaller body length of females, 555.0 (466.0–623.0) µm vs. 690 (600–783) µm; the slimmer body of females; a value, 29.0 (26.0–32.0) vs. 38.5 (34.8–40.9), the longer stylet length of females, 19.5 (18–21) µm vs. 10.4 (10.0–10.6) µm; and a shorter PUS length, 24.0 (18.0–27.0) µm vs. 124 (106–144) µm.
From R. helicus, by the absence of males vs. the presence; stylet knob shape (rounded to bean-shaped vs. teardrop shape; the position of the excretory pore (posterior to nerve ring vs. between the median bulb and the anterior edge of the nerve ring); tail tip shape (pointed to wedge-shaped vs. rounded); the longer body length of females, 555.0 (466.0–623.0) µm vs. 464 (395–547) µm; the longer stylet length of females, 19.5 (18–21) µm vs. 10.0 (10.0–11.0) µm; a shorter PUS length, 24.0 (18.0–27.0) µm vs. 38 (22–55) µm; and posteriorly located vulva, 72.0 (70.0–76.0) vs. 69.3 (64.4–71.1).
From R. subtenuis [20,44], by the absence of males vs. the presence; the shape of the lip region (anteriorly flattened vs. anteriorly rounded); shape of stylet knobs (rounded to bean-shaped vs. swelling); the shape of the median bulb (squarish to rounded vs. oval); vulval lips (protruding vs. non-protruding); tail tip shape (pointed to wedge-shaped vs. hemispherical with mucro); the shorter body length of females, 555.0 (466.0–623.0) µm vs. 822 (694–927) µm; the longer stylet length of females, 19.5 (18–21) µm vs. 11.6 (11.0–13.0) µm; and a shorter PUS length 24.0 (18.0–27.0) µm vs. 115 (80–138) µm.
Type host and locality: The newly discovered species was identified in the natural vegetation (goldenrod plant) rhizosphere, growing in the marginal land of the Bow Island potato cultivated area in southern Alberta, Canada.
Type material: Holotype female, 14 paratype females (five slides, numbers UL-DY4-01 to UL-DY4-05) and an additional slide containing five females were deposited in the nematode collection of the University of Lethbridge, Alberta, Canada. Four paratype females were deposited in the nematode collection of the Institute for Sustainable Agriculture, CSIC, Córdoba, Spain.
Etymology: The species epithet refers to Gr. prep. para, alongside of, resembling; N.L. masc. n. megadorus, because of its close resemblance to Robustodorus megadorus.
3.5. Molecular Profiling and Phylogenetic Studies
In this study, the detected aphelenchid species were molecularly characterized using the partial 18S, D2–D3 expansion segments of 28S, and ITS rRNA genes. The newly obtained sequences were deposited in NCBI under the following accession numbers; 18S: PP718956–PP718960 for A. prairiensis n. sp.; PP718961–PP718963 for A. limberi; PP718964–PP718965 for A. avenae; and PP718966–PP718967 for R. paramegadorus n. sp.; the D2–D3 expansion segments of 28S: PP718968–PP718972 for A. prairiensis n. sp.; PP718973–PP718976 for A. limberi; PP718977–PP718978 for A. avenae; and PP718979–PP718980 for R. paramegadorus n. sp.; and ITS: PP729623–PP729625 for A. prairiensis n. sp.; PP729626–PP729628 for A. limberi; PP729629 for A. avenae; and PP729630–PP729632 for R. paramegadorus n. sp.
Phylogenetic trees were constructed for each gene to elucidate the phylogenetic relationships among the detected species and related aphelenchids. The 18S tree (Figure 8), incorporated 71 aphelenchid species sequences, with Aphelenchus avenae (JQ348399) serving as the outgroup taxa. Within this tree, A. prairiensis n. sp. and A. limberi are positioned in the uppermost clade. Aphelenchoides prairiensis n. sp. shares a branch with A. blastopthorus Franklin [51], A. fragariae (Ritzema Bos [52]) Christie [53], A. eldaricus Esmaeili, Heydari, Golhasan, and Kanzaki [54], and A. smolae Cai, Gu, Wang, Fang, and Li [55]. The sequence identity between A. prairiensis n. sp. and the clustered species was 97% (17–21 bp difference), while A. limberi formed a subclade with Aprutides guidetti Scognamiglio [54], an A. limberi population sequence from Iran, A. centralis Thorne and Malek [56], A. unisexus Jain and Singh [57], and A. paraxui Esmaeili, Heydari, Fang, and Li [58]. The sequence identity between A. limberi and the clustered species was 97% (15–17 bp difference). Aphelenchus avenae clustered with the A. avenae outgroup sequence, whereas R. paramegadorus n. sp. formed a monophyletic clade with R. arachidis, R. helicus, R. megadorus and R. subtenuis. The sequence identity between R. paramegadorus n. sp. and the clustered species ranged from 90% to 99% (2–61 bp difference).
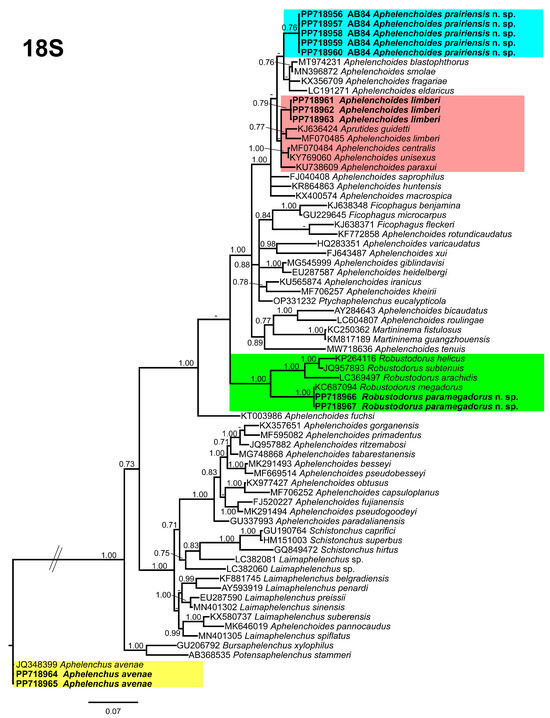
Figure 8.
Phylogenetic relationships of the Canadian population of aphelenchid species with the related species. Bayesian 50% majority rule consensus tree as inferred from 18S rRNA sequence alignment under the Tamura and Nei model with invariable sites and a gamma-shaped distribution (TrN + I + G). Posterior probabilities of greater than 0.70 are provided for in the corresponding appropriate clades. The sequences produced in this study are shown in bold, and the colored boxes indicate the clade association of the recovered aphelenchid species.
The 28S tree (Figure 9), was constructed with 67 aphelenchid species sequences, with Aphelenchus avenae (JQ348400) serving as the outgroup taxa. Once again, A. prairiensis n. sp. and A. limberi were positioned within the top clade of the tree. Aphelenchoides prairiensis n. sp. grouped independently with A. macrospica Golhasan, Heydari, Esmaeili, and Miraeiz [59]. The sequence identity between A. prairiensis n. sp. and A. macrospica was 92% (23 bp difference), whereas A. limberi was grouped with an unidentified Aphelenchoides sp. (MN702965) from Iran and shares a branch with A. parietinus (Bastian [14]) Steiner [60]. The sequence identity between A. limberi and the clustered species ranged from 96% to 99% (2–10 bp difference). Aphelenchus avenae clustered with the A. avenae outgroup sequence, whereas R. paramegadorus n. sp. formed a monophyletic clade with R. helicus, R. megadorus, and R. subtenuis. The sequence identity between R. paramegadorus n. sp. and the clustered species ranged from 77% to 96% (23–147 bp difference).
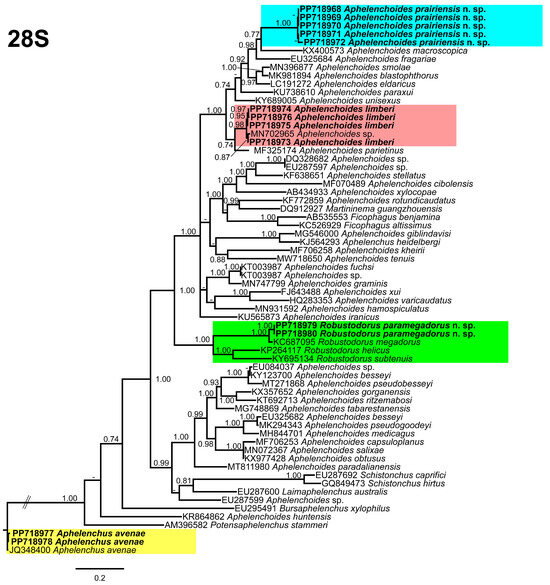
Figure 9.
Phylogenetic relationships of the Canadian population of aphelenchid species with the related species. Bayesian 50% majority rule consensus tree as inferred from the D2A–D3B segments of the 28S rRNA sequence alignment under the transversional model with invariable sites and a gamma-shaped distribution (TVM + I + G). Posterior probabilities of greater than 0.70 are provided for in the corresponding appropriate clades. The sequences produced in this study are shown in bold, and the colored boxes indicate the clade association of the recovered aphelenchid species.
The ITS tree (Figure 10), was constructed using 44 aphelenchid species sequences, with Aphelenchus avenae (JQ348400) as the outgroup taxa. Once again, A. prairiensis n. sp. and A. limberi were positioned within the top clade of the tree. A. prairiensis n. sp. grouped with A. fragariae, A. paraxui and our population of A. limberi. The sequence identity between A. prairiensis n. sp. and related species ranged from 80% to 88% (24–68 bp difference). Aphelenchus avenae clustered with the A. avenae outgroup sequence, whereas R. paramegadorus n. sp. formed a monophyletic clade with R. arachidis, R. megadorus, and R. subtenuis. The sequence identity between R. paramegadorus n. sp. and the clustered species ranged from 79% to 93% (22–63 bp difference).
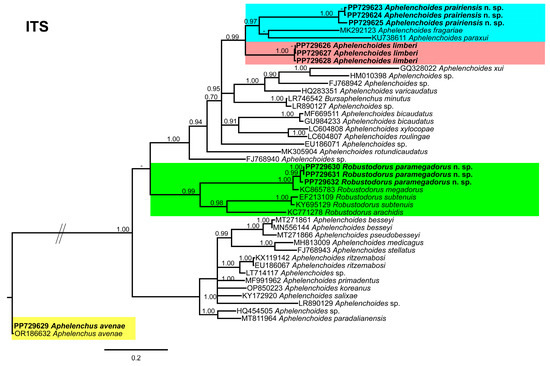
Figure 10.
Phylogenetic relationships of the Canadian population of aphelenchid species with the related species. Bayesian 50% majority rule consensus tree as inferred from the ITS rRNA sequence alignment under the transitional model with a gamma-shaped distribution (TIM1 + G). Posterior probabilities of greater than 0.70 are provided for in the corresponding appropriate clades. The sequences produced in this study are shown in bold, and the colored boxes indicate the clade association of the recovered aphelenchid species.
In our phylogenetic analyses, it is clear that both A. prairiensis n. sp. and R. paramegadorus n. sp. represent distinct species. Other than our population of A. limberi, we obtained a partial 18S sequence of A. limberi from the NCBI database. This sequence lacked accompanying measurements and photo-documentation, rendering validation of its true identity unfeasible. Because no other A. limberi sequence data are available in the NCBI, consequently, our A. limberi population data can be used for future phylogenetic studies.
4. Discussion
The soil-dwelling species of Aphelenchus, Aphelenchoides, and Robustodorus have received comparatively less scientific attention than aphelenchids inhabiting foliage or wood. When found in soil, species of these genera are generally categorized as fungal-feeding nematodes [1,61]. However, fungal-feeding activity has not been observed for Robustodorus. The genus remained monotypic for a significant duration, but with increased taxonomic studies and molecular characterization, three Aphelenchoides species have been reclassified under Robustodorus as R. arachidis, R. helicus, and R. subtenuis [18,19,20]. To date, all documented Robustodorus species have been discovered in the rhizosphere of various plants, with no reports of fungal feeding or insect association except for R. helicus, which was isolated from decaying wood samples and was capable of multiplying on Botrytis cinerea cultures [19,20]. Our R. paramegadorus n. sp. was discovered in natural vegetation growing on the marginal land adjacent to a potato-cultivated field. The field samples yielded no evidence of the presence of R. paramegadorus n. sp. Consequently, we speculate that this new species is primarily associated with natural plants rather than with cultivated crops.
Regarding Aphelenchus, the genus has 12 nominal species, with A. avenae receiving the most attention due to its global distribution and potential as a biocontrol agent against various fungal species [2]. Currently, there is no evidence indicating that it poses harm to higher plants. Since A. avenae is a well-studied species, our objective was solely to report its presence in southern Alberta; we do not speculate on its role in soil ecology or biodiversity. Concerning the genus Aphelenchoides, it comprises species that are associated with plants, fungi, and insects [62]. Some species were reported in the oviducts or elytra of insects [63,64]. The newly discovered and known Aphelenchoides species we report here were identified in the soil samples of post-harvest potato fields. We do not anticipate their association with insects; these species may engage in fungal feeding or root hair feeding. It is imperative to note that this study is part of our broader investigation into soil nematode diversity. Among all collected soil samples, this is the first instance of discovering Robustodorus sp., (50–70 individuals/500 g soil) from any of our soil samples, whereas A. limberi (30–40 individuals/500 g soil) and A. prairiensis n. sp., (45–55 individuals/500 g soil) were also relatively uncommon. However, A. avenae (20–50 individuals/500 g soil) was prevalent in the majority of soil samples. Overall, the species documented in this study were found in soil samples, and their fungal-feeding behavior and insect associations remain unknown.
Since many soil samples from agricultural and natural systems may comprise mixed aphelenchid species, applying integrative taxonomical approaches (molecular barcoding of single individuals and morphometric analyses) can accurately identify and separate these species. Nevertheless, further studies are required to define the exact distribution patterns and co-occurrence of the four encountered species with each other and/or with co-detected species. For this reason, additional samplings are needed for optimizing nematode sampling and extraction methods to define distribution patterns of these aphelenchids. In this scenario, the recent review by Abd-Elgawad [65] may help to design the most appropriate sampling programs to decipher the pattern distributions of these nematodes and to hypothesize about their active or indirect dispersal process [65].
Our phylogenetic studies provide robust evidence supporting the distinct status of both A. prairiensis n. sp. and R. paramegadorus n. sp. Across all phylogenetic trees analyzed, A. prairiensis n. sp. consistently grouped with various Aphelenchoides species, thereby confirming its taxonomic association within the genus. The clustered species exhibit no significant morphological resemblance to A. prairiensis n. sp. Based on sequence similarity we propose Aphelenchoides sp. sH1 (MN702965) as a population of A. limberi. However, no morphological or morphometrical data are available for this unidentified Aphelenchoides sp. Concerning Robustodorus, our findings align with previous studies [18,19,20], supporting the monophyletic status of Robustodorus species.
5. Conclusions
Soil nematodes play crucial roles in decomposition and nutrient cycling [61]. Previous studies in southern Alberta were primarily focused on plant-parasitic species [3,4,11]. Our study expands this knowledge by reporting the presence of nematodes falling within a broader category of fungal or root hair feeders. Here, we have provided the detailed identification of four aphelenchid species, supported by detailed photo documentation. Our findings not only provide data that may enhance soil health assessments, but also describe two new aphelenchid species, suggesting an increase in the known soil nematode diversity of southern Alberta. Further research is required to elucidate the exact food sources, dispersal modes, and distribution patterns of these nematodes for a comprehensive understanding of their biodiversity and ecological roles.
Author Contributions
Conceptualization, M.M. and D.P.Y. methodology, M.M., P.C. and D.P.Y.; software, M.M., P.C. and D.P.Y.; validation, M.M., P.C. and D.P.Y.; formal analysis, M.M., P.C. and D.P.Y.; investigation, M.M., P.C. and D.P.Y.; resources, P.C. and D.P.Y.; data curation, M.M. and P.C.; writing—original draft preparation, M.M., P.C. and D.P.Y.; writing—review and editing, M.M., P.C. and D.P.Y.; visualization, M.M., P.C. and D.P.Y.; supervision, M.M., P.C. and D.P.Y.; project administration, D.P.Y.; funding acquisition, D.P.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Results Driven Agricultural Research (RDAR) grant 2023F292R to D.P.Y., and a collaboration with the Potato Growers of Alberta (Taber, AB, Canada).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We express our sincere gratitude to the potato growers of Alberta, Canada, for generously providing access to their fields. Special appreciation to Carolina Cantalapiedra-Navarrete from the Institute for Sustainable Agriculture (IAS), CSIC, Córdoba, Spain, for sequence analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar] [PubMed]
- Haraguchi, S.; Yoshiga, T. Potential of the fungal feeding nematode Aphelenchus avenae to control fungi and the plant parasitic nematode Ditylenchus destructor associated with garlic. Biol. Control 2020, 143, 104203. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Palomares-Rius, J.E.; Castillo, P. Species diversity of pin nematodes (Paratylenchus spp.) from potato growing regions of southern Alberta, Canada. Plants 2021, 10, 188. [Google Scholar] [CrossRef]
- Munawar, M.; Castillo, P.; Yevtushenko, D.P. Description of Filenchus species from agroecosystem of southern Alberta, Canada. Agronomy 2022, 12, 690. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T. Nematode indicators of organic enrichment. J. Nematol. 2006, 38, 3. [Google Scholar] [PubMed]
- Fischer, M. Über eine Clematis-Krankheit; Physiological Laboratory of the Agricultural Institute of the University of Halle: Halle, Germany, 1894; Volume 3. [Google Scholar]
- Filipjev, I. On the classification of the Tylenchinae. Proc. Helminthol. Soc. Wash. 1936, 3, 80–82. [Google Scholar]
- Devran, Z.; Tülek, A.; Mıstanoğlu, İ.; Çiftçiğil, T.H.; Özalp, T. A rapid molecular detection method for Aphelenchoides besseyi from rice tissues. Australas. Plant Pathol. 2017, 46, 43–48. [Google Scholar] [CrossRef]
- Castillo, P. Ditylenchus gigas (giant stem and bulb nematode). CABI Compend. Datasets 2022, 1–12. Available online: https://www.cabi.org/isc/datasheet/118252 (accessed on 30 March 2023).
- Munawar, M.; Rahman, A.U.; Castillo, P.; Yevtushenko, D.P. New records of Ditylenchus species from southern Alberta, Canada. Plants 2023, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Subbotin, S.A. Rapid Detection of the Strawberry Foliar Nematode Aphelenchoides fragariae Using Recombinase Polymerase Amplification Assay with Lateral Flow Dipsticks. Int. J. Mol. Sci. 2024, 25, 844. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.H.M.; Philadelphi, S.M.; Galbieri, R.; Sonawala, U.; Eves-van den Akker, S. An emergent plant-parasitic nematode in Brazil: Aphelenchoides besseyi. Current status and research perspectives. Plant Pathol. 2024, 73, 478–491. [Google Scholar] [CrossRef]
- Bastian, H.C., II. Monograph on the Anguillulidae, or free nematoids, Marine, Land, and Freshwater; with descriptions of 100 new species. Trans. Linn. Soc. Lond. 1865, 25, 73–184. [Google Scholar] [CrossRef]
- Goodey, J.B. The classification of the Aphelenchoidea Fuchs, 1937. Nematologica 1960, 5, 111–126. [Google Scholar] [CrossRef]
- Andrássy, I. Free-Living Nematodes of Hungary (Nematoda errantia); Andrássy, I., Ed.; Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Science: Budapest, Hungary, 2007. [Google Scholar]
- Handoo, Z.; Kantor, M.; Carta, L. Taxonomy and identification of principal foliar nematode species (Aphelenchoides and Litylenchus). Plants 2020, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Ryss, A.Y.; McClure, M.A.; Nischwitz, C.; Dhiman, C.; Subbotin, S.A. Redescription of Robustodorus megadorus with molecular characterization and analysis of its phylogenetic position within the family Aphelenchoididae. J. Nematol. 2013, 45, 237–252. [Google Scholar] [PubMed]
- Aliramaji, F.; Pourjam, E.; Álvarez-Ortega, S.; Afshar, F.J.; Pedram, M. Description of Aphelenchoides giblindavisi n. sp. (Nematoda: Aphelenchoididae), and proposal for a new combination. J. Nematol. 2018, 50, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, N.; Shokoohi, E.; Fourie, H.; Swart, A.; Muller, L.; Giblin-Davis, R.M. On the morphology and phylogeny of Robustodorus Andrássy, 2007 and two ‘Aphelenchoides’ species (Nematoda: Aphelenchoidinae). Nematology 2018, 20, 601–615. [Google Scholar] [CrossRef]
- Jianfeng, G.; Maria, M.; Fang, Y.; Lele, L.; Chen, X.; Cai, B. Aphelenchus yinyuensis n. sp. (Tylenchina: Aphelenchoididae) found in Terminalia sp. in China. J. Nematol. 2020, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.J. A checklist of the Aphelenchoidea (Nematoda: Tylenchina). J. Nematode Morphol. Syst. 2007, 10, 99–135. [Google Scholar]
- Kanzaki, N.; Tanaka, R.; Ikeda, H.; Taki, H.; Sugiura, S.; Matsumoto, K. Phylogenetic status of insect parasitism in the subfamily Entaphelenchinae Nickle with description of Peraphelenchus orientalis n. sp. (Tylenchomorpha: Aphelenchoididae). J. Parasitol. 2013, 99, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G. Opuscula miscellanea nematologica, IV. Proc. Helminthol. Soc. Wash. 1936, 3, 74–80. [Google Scholar]
- Jenkins, W.R.B. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- de Man, J.G. Die Einheimischen, Frei in der Reinen Erde und Im Süssen Wasser Lebende Nematoden; Brill: Leiden, The Netherlands, 1880; Volume 1. [Google Scholar]
- Siddiqi, M.R. Tylenchida: Parasites of Plants and Insects; CABI Publishing: Wallingford, UK, 2000; Volume 2. [Google Scholar]
- Grisse, D. Redescription ou modification de quelques techniques utilisées dans l’étude des nématodes phytoparasitaires. Meded. Rijks Fac. Landbouwwet. Gent 1969, 34, 351. [Google Scholar]
- Maria, M.; Powers, T.; Tian, Z.; Zheng, J. Distribution and description of criconematids from Hangzhou, Zhejiang Province. China. J. Nematol. 2018, 50, 183–206. [Google Scholar]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- De Ley, P.; Felix, M.-A.; Frisse, L.; Nadler, S.; Sternberg, P.; Thomas, W.K. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar] [CrossRef]
- Vrain, T.; Wakarchuk, D.; Levesque, A.; Hamilton, R. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Cai, J.; Gu, J.; Wang, X.; Fang, Y.; Li, H. Aphelenchoides smolae n. sp. (Tylenchina: Aphelenchoididae) found in Lilium orientalis imported into China from the Netherlands. Nematology 2020, 22, 799–813. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; pp. 95–98. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and high-performance computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1. 4.2, a Graphical Viewer of Phylogenetic Trees. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/rightanglebracket (accessed on 19 April 2024).
- Shahina, F. A diagnostic compendium of the genus Aphelenchoides Fischer, 1894 (Nematoda: Aphelenchida) with some new records of the group from Pakistan. Pak. J. Nematol. 1996, 14, 1–32. [Google Scholar]
- Eroshenko, A. Five new species of Aphelenchoides Fischer, 1894 (Nematoda: Aphelenchoididae). Otd. Akad. Nauk. SSSR 1968, 26, 58–66. [Google Scholar]
- Paesler, F. Beschreibung Einiger Nematoden Aus Champignonbeeten 1) 2). Nematologica 1957, 2, 314–328. [Google Scholar] [CrossRef]
- Maslen, N.R. Six new nematode species from the maritime Antarctic. Nematologica 1979, 25, 288–308. [Google Scholar] [CrossRef]
- Sewell, R. Plant-parasitic nematodes from Canada and abroad, 1971. Can. Plant Dis. Surv. 1973, 53, 34–35. [Google Scholar]
- Mohammad-Deimi, A.; Maafi, Z.T.; Rius, J.P.; Castillo, P. Aphelenchoides subtenuis (Cobb, 1926) Steiner & Buhrer, 1932 (Nematoda: Aphelenchoididae) from Iran with morphological and morphometric characterisation. Nematology 2006, 8, 903–908. [Google Scholar]
- Čermák, V.; Háněl, L.; Gaar, V.; Douda, O. First description of the males of Aphelenchoides limberi Steiner, 1936 (Nematoda: Aphelenchina). Helminthologia 2012, 49, 181–186. [Google Scholar] [CrossRef]
- Munawar, M.; Konschuh, M.; Castillo, P.; Yevtushenko, D.P. Morpho-Anatomy, In Vitro Culture, and Phylogenetic Studies of Two Helicotylenchus Species from Southern Alberta, Canada. Microorganisms 2024, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Bos, W.S. Aphelenchoides arachidis n. sp. (Nematoda: Aphelenchoidea), an endoparasite of the testa of groundnuts in Nigeria. Z. Pflanzenkrankh. Pflanzenschutz 1977, 84, 95–99. [Google Scholar]
- Heyns, J. Aphelenchoides helicus n. sp. and Ditylenchus equalis n. sp. two new soil inhabiting nematodes. S. Afr. J. Agric. Sci. 1964, 7, 147–150. [Google Scholar]
- Allen, M. Aphelenchoides megadorus, a new species of Tylenchoidea (Nematoda). Proc. Helminthol. Soc. Wash. 1941, 8, 21–23. [Google Scholar]
- Cobb, N.A. Nemic Diseases of Narcissus; Official Records; USDA: Washington, DC, USA, 1926; Volume 5.
- Franklin, M.T. A disease of Scabiosa caucasica caused by the nematode Aphelenchoides blastophthorus n. sp. Ann. Appl. Biol. 1952, 39, 54–60. [Google Scholar] [CrossRef]
- Bos, J.R. Zwei neue Nematodenkrankheiten der Erdbeerpflanze. Z. Pflanzenkrankh. 1891, 1, 1–16. [Google Scholar]
- Christie, J.R. Recent observations on the strawberry dwarf nematode in Massachusetts. Plant Dis. Rep. 1932, 16, 113–114. [Google Scholar]
- Esmaeili, M.; Heydari, R.; Golhasan, B.; Kanzaki, N. Aphelenchoides eldaricus n. sp. (Nematoda: Aphelenchoididae) isolated from Pinus eldarica in western Iran. Nematology 2017, 19, 605–616. [Google Scholar] [CrossRef]
- Scognamiglio, A. Aprutides guidettii n. sp.(Nematoda: Aphelenchoididae). Bull. Agric. Entomol. Lab. Filippo Silvestri 1974, 31, 17–21. [Google Scholar]
- Thorne, G.; Malek, R.B. Nematodes of the Northern Great Plains: Part 1 Tylenchida [Nemata Secernentra]; Technical Bulletins; Agricultural Experiment Station, South Dakota State University: Brookings, South Dakota, 1968; pp. 1–111. [Google Scholar]
- Jain, V.K.; Singh, S.P. Two new species of Aphelenchoides (Nematoda: Aphelenchoididae) from India. Bull. Entomol. 1984, 25, 135–142. [Google Scholar]
- Esmaeili, M.; Heydari, R.; Fang, Y.; Li, H. Molecular and morphological characterisation of Aphelenchoides paraxui n. sp. (Nematoda: Aphelenchoididae) isolated from Quercus brantii in western Iran. Eur. J. Plant Pathol. 2017, 149, 625–637. [Google Scholar] [CrossRef]
- Golhasan, B.; Heydari, R.; Esmaeili, M.; Miraeiz, E. Description of Aphelenchoides macrospica n. sp. (Nematoda: Aphelenchoididae) from northwestern Iran. J. Nematol. 2017, 49, 67–76. [Google Scholar] [CrossRef]
- Steiner, G.; Buhrer, E.M. The nonspecificity of the brown-ring symptoms in narcissus attacked by nematodes. Phytopathology 1932, 22, 927–928. [Google Scholar]
- Ruess, L.; Zapata, E.J.G.; Dighton, J. Food preferences of a fungal-feeding Aphelenchoides species. Nematology 2000, 2, 223–230. [Google Scholar] [CrossRef]
- Sanchez-Monge, A.; Flores, L.; Salazar, L.; Hockland, S.; Bert, W. An updated list of the plants associated with plant-parasitic Aphelenchoides (Nematoda: Aphelenchoididae) and its implications for plant-parasitism within this genus. Zootaxa 2015, 4013, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, N. Description of Aphelenchoides xylocopae n. sp. (Nematoda: Aphelenchoididae), the first observed association between nematodes and carpenter bees. Nematology 2006, 8, 555–562. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Moser, J.C.; Klepzig, K.D.; Raffa, K.F. Multipartite symbioses among fungi, mites, nematodes, and the spruce beetle, Dendroctonus rufipennis. Environ. Entomol. 2008, 37, 956–963. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M. Optimizing sampling and extraction methods for plant-parasitic and entomopathogenic nematodes. Plants 2021, 10, 629. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).