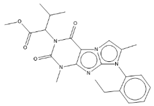
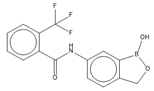
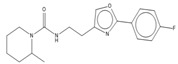
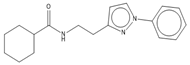
Abstract
Chagas disease caused by the protozoan Trypanosoma cruzi is endemic to 21 countries in the Americas, effects approximately 6 million people and on average results in 12,000 deaths annually. Human African Trypanosomiasis (HAT) is caused by the Trypanosoma brucei sub-species, endemic to 36 countries within sub-Saharan Africa. Treatment regimens for these parasitic diseases are complicated and not effective against all disease stages; thus, there is a need to find improved treatments. To identify new molecules for the drug discovery pipelines for these diseases, we have utilised in vitro assays to identify compounds with selective activity against both T. cruzi and T.b. brucei from the Medicines for Malaria Venture (MMV) Pathogen Box compound collection. To prioritise these molecules for further investigation, temporal and wash off assays were utilised to identify the speed of action and cidality of compounds. For translational relevance, compounds were tested against clinically relevant T.b. brucei subspecies. Compounds with activity against T. cruzi cytochrome P450 (TcCYP51) have not previously been successful in clinical trials for chronic Chagas disease; thus, to deprioritise compounds with this activity, they were tested against recombinant TcCYP51. Compounds with biological profiles warranting progression offer important tools for drug and target development against kinetoplastids.
1. Introduction
The kinetoplastid borne diseases, Human African Trypanosomiasis (HAT) and Chagas disease, threaten the lives of over 90 million people in 56 countries, spread over 3 continents. Issues associated with treatment regimens, toxicity and efficacy of drugs for the treatment of these diseases are considerable. Chagas disease is categorised by the World Health Organisation (WHO) as one of the world’s 20 most neglected tropical diseases (NTDs) [1]. The disease is endemic to 21 countries within the Americas, and causes more than 12,000 deaths per year, with a further 75 million people at risk of becoming infected with T. cruzi [2]. Current drugs used to treat Chagas disease, benznidazole and nifurtimox, lack efficacy in the chronic stage of disease and have associated side effects, resulting in reduced compliance [3]. In addition, treatment times for both drugs are lengthy, requiring 60–90 days [4]. With few compounds currently in clinical trials for Chagas disease, seeding the drug discovery pipeline with new and efficacious compounds is necessary in the search for new therapies with improved safety profiles and reduced treatment schedules.
HAT is caused by two subspecies, Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense, and presents in two stages, whereby parasites cross the blood–brain barrier in the second stage. None of the drugs used to treat HAT are effective against all disease stages and parasite species. However, the first oral drug, fexinidazole, was approved for treatment of both stages of HAT caused by T.b. gambiense in 2019 [5]. The Drugs for Neglected Diseases initiative (DNDi) is partnering with an African-based consortium (HAT-r-ACC) to undertake a 5-year study to assess the efficacy of fexinidazole against T.b. rhodesiense [6] and clinical trials are underway [7]. Currently, the only drug approved for stage-two HAT caused by T.b. rhodesiense is melarsoprol. Melarsoprol is very effective against HAT; however, post-treatment reactive encephalopathy is reported to occur in 5–10% of all treated cases and is fatal in 50% of those patients in which encephalopathy develops [8]. Whilst there is one other compound in the pre-clinical pipeline, acoziborole, it is again for the treatment of T.b. gambiense. Given that high attrition rates in drug discovery are well-documented, with lack of efficacy and safety issues as the main causes [9], new orally available compounds for the safe treatment of HAT are needed.
Medicines for Malaria venture (MMV) collated the Pathogen Box, containing 400 diverse, drug-like molecules active against the causative agents of various neglected tropical diseases (NTDs). This includes, but is not limited to, mycobacterial species causing tuberculosis and parasites causing schistosomiasis, HAT, Chagas disease and leishmaniasis [10]. This open-source collection was established to accelerate NTD drug discovery, especially those affecting low socioeconomic groups which disproportionally affect developing countries [10,11]. Open-source drug discovery using the Pathogen Box has successfully identified lead compounds for pathogens such as Shistosoma mansoni (schistosomiasis); Candida albicans (candidiasis), piroplasm parasites (including Babesia sp) and Fasciola hepatica (fascioliasis) [12,13,14,15]. In our previous in vitro study, the Pathogen Box was profiled against T. cruzi and T.b. brucei, in addition to Plasmodium falciparum and Leishmania donovani [16]. Forty-three compounds selectively active against either T. cruzi or T.b. brucei were identified that previously were not shown to be active against theses parasites; or if reported as active, have an undefined target [16]. From the 43 compounds, 93% had an undefined target and 65% had not been reported with activity against either one or both parasites. Sixty-eight percent of these have since been reported as active against T.b. brucei [17]; however, the target and dual activity against both T.b. brucei and T. cruzi was not reported. To confirm activity against T. cruzi and T.b. brucei, and prioritise compounds for drug discovery efforts, in vitro profiling to classify rapidly acting compounds with irreversible activity against T. cruzi and activity against the infective forms of HAT was undertaken with solid compound stocks. The speed of action and efficacy against both parasites were identified.
The speed of compound action is an important consideration in drug discovery, as this often reflects potential treatment duration, important from the perspective that elongated treatment regimens are an issue for both Chagas disease and HAT [2,18]. For Chagas disease, slow growing/persistent parasites are considered of particular clinical relevance [19,20]. A four-fold difference has been found in T. cruzi load in acute versus chronic infections in mice [21], suggesting that replication of the parasite may be reduced, limited, or prevented in vivo. In vivo analysis of mouse models of infection has recently shown T. cruzi parasites to persist in low numbers, in gut tissue [22]. Slow growing parasites may be resistant to treatment by compounds that do not rapidly kill; thus, we utilised an image-based rate of kill assay to assess the speed of action of compounds against T. cruzi [23]. Time-kill assays, that we have previously established for T.b. brucei [24], were utilised to assess the speed of action of compounds against T.b. brucei in this study.
A cidal mode of action (MOA) indicates the compound effect on the target cell is not reversible, or there is a commitment to apoptosis [25]. Current wash-out assays developed to identify the cidal nature of compounds against T. cruzi are lengthy and, considering the long incubation periods up to 60 days [26,27], the health of host cells may be compromised. We have developed a short-term wash off assay to identify compounds with an irreversible effect on T. cruzi parasites, by incubating in the presence of compound for 48 h [28], followed by incubation in the absence (wash off) of compound for 72 h [23]. This method enabled the rapid assessment of the cidality of compounds and thus the prioritisation of active compounds from the Pathogen Box, with a potentially irreversible MOA against T. cruzi.
T. cruzi cytochrome P450 (TcCYP51) inhibitors, such as posaconazole, have not been successful in clinical trials for the treatment of chronic Chagas disease [29,30]. Whilst the lack of efficacy of posaconazole may have been influenced by the concentration and duration of exposure in these clinical trials, currently posaconazole cannot be considered as a monotherapy [31]. As a result, many programs in T. cruzi drug discovery deprioritise CYP51 inhibitors [25,27]. Following 48 h exposure of T. cruzi infected cells to posaconazole there is incomplete clearance of T. cruzi parasites [23,32]. We have utilised temporal image-based assays to assess active compounds from the Pathogen Box collection against T. cruzi, to deprioritise a CYP51-like phenotype, which typically shows low efficacy following 48 h incubation and a slow-acting MOA [23]. In this current work, we have confirmed the lack of activity of prioritised compounds against T. cruzi CYP51, utilising the T. cruzi recombinant enzyme [33].
Compounds with selective activity against both parasites, which do not target TcCYP51 with an irreversible MOA, and exhibit activity against the human infective forms of T. brucei serve as promising hits for Chagas disease and HAT drug discovery. These compounds provide tools to probe parasite biology via target identification. Recently, there was hope with proteasome inhibitors for developing treatments against multiple kinetoplastids [34], as there are similarities between DNA repair pathways for these parasites [35]. Fexinidazole, used to treat T.b. gambiense infection [36], has also recently been in clinical trials for Chagas disease [37], indicating the potential that compounds of the same compound class can be pursued as new chemical starting points for both parasites.
2. Methods
2.1. Growth and Maintenance of Parasites and Host Mammalian Cell Lines
T. cruzi Tulahuen strain parasites and 3T3 host cells (ATCC) were maintained as outlined previously [28]. Briefly, host 3T3 mouse fibroblasts (ATCC CCL-92) were grown in RPMI medium (ThermoFisher, Waltham, MA, USA) with no phenol red, supplemented with 10% FBS (ThermoFisher, Waltham, MA, USA) and incubated at 37 °C in 5% CO2. Host cells were harvested at 70% confluency and split every 2–3 days in 175 cm2 flasks. 3T3 cells are contact inhibited and thus were cultured up to passage 7 to minimise overgrowth of culture. Prior to infection with T. cruzi trypomastigotes, host cells were seeded at 1.2 × 106 cells in 75 cm2 or 4 × 105 cells in 25 cm2 flasks for 24 h before parasite addition. Parasites were added at a 10:1 multiplicity of infection (MOI), incubated for 24 h and extracellular trypomastigotes washed off the host cell bed with PBS supplemented with Ca2+ and Mg2+ (ThermoFisher, Waltham, MA, USA). Incubation was continued for a further 72 h, until tissue culture trypomastigotes initiated egress from host cells. Parasites were harvested from the flask supernatant and used for assays, or to continue the infective life cycle in vitro.
T.b. brucei bloodstream 427 strain parasites were sub-cultured as previously reported in HMI-9 medium [24], supplemented with 10% FBS. Parasites were maintained in the log phase of growth during sub-culturing for 48 or 72 h intervals, at 37 °C and 5% CO2 in 25 cm2 filtered tissue culture flasks (Corning, New York, NY, USA), under humidified conditions.
Human embryonic kidney cells (HEK293; ATCC) were grown in high glucose DMEM supplemented with 10% FBS at 37 °C and 5% CO2. Cells were sub-cultured every 3 or 4 days by seeding 1.5 × 106 or 1 × 106 cells, respectively, into 175 cm2 flasks. Human hepatocellular carcinoma cells (HEPG2; ATCC) were grown in high glucose DMEM supplemented with 10% FBS at 37 °C and 5% CO2. Cells were sub-cultured every 3 or 4 days by seeding 1.5 × 106 or 1 × 106 cells, respectively, into 175 cm2 flasks.
2.2. Compounds
All compounds were prepared in 100% DMSO and diluted in 14 log dilutions to determine IC50 values. Reference compounds for the inhibition of T.b. brucei growth following 24, 48 and 72 h were diminazene aceturate, with final assay concentrations ranging from 38 µM to 1.9 × 10−3 µM and puromycin, an inhibitor of eukaryotic protein synthesis [38] as a control for general cytotoxicity with final assay concentrations ranging from 36 µM to 1.8 × 10−3 µM (Sigma Aldrich, Saint Louis, MO, USA). The positive control, diminazene, was at a final assay concentration of 19 µM (EC100) and the negative control was 0.4% DMSO (vehicle only). Reference compounds for the inhibition of T. cruzi growth were benznidazole ranging from 127 µM to 6.3 × 10−3 µM (supplied by EpiChem Pty Ltd., Bentley, WA, Australia); nifurtimox ranging from 127 µM to 6.3 × 10−3 µM (isolated from Lampit tablets by Dr. Agatha Garavalis), posaconazole ranging from 1 µM to 5 × 10−5 µM (Sigma Aldrich, Saint Louis, MO, USA) and puromycin from 200 µM to 1 × 10−2 µM (Sigma Aldrich, Saint Louis, MO, USA) as a control of general cytotoxicity. The positive control, nifurtimox, was at a final assay concentration of 12 µM (EC100) and the negative control was 0.4% DMSO (vehicle only).
Forty-three compounds from the MMV Pathogen Box library were obtained as 1 mg solid stocks from MMV. These compounds previously identified with activity against T.b. brucei and T. cruzi in our previous publication [16]. Compounds were either potential hit molecules or probes for identification of new targets, for T. cruzi and T.b. brucei parasites. Prior to use, compounds were prepared in 100% DMSO at a stock concentration of 20 mM.
2.3. T.b. brucei 72 h REDOX-Based Viability Assay
The T.b. brucei 72 h in vitro viability assay was undertaken as previously described [16]. Briefly, parasites were inoculated into sterile polystyrene black/clear bottomed 384-well plates (Greiner, Monroe, NC, USA) at 1.2 × 103 cells/ mL in 55 µL of HMI-9, supplemented with 10% FBS. Parasites were incubated for 24 h prior to the addition of 5 µL of pre-diluted control or test compounds. Plates were incubated for 48 h before addition of 10 µL of 490 µM resazurin (Cayman Chemicals, Ann Arbor, MI, USA), to give a final concentration of 70 µM. Plates were incubated for 22 h at 37 °C, 5% CO2 followed by incubation at room temperature for 2 h. Total well fluorescence was determined using an EnSight plate reader (PerkinElmer, Waltham, MA, USA) at an excitation of 535 nm and emission of 590 nm. Compound additions to plates were performed with a MiniTrak liquid dispenser (PerkinElmer, Waltham, MA, USA). Compounds in 100% DMSO were pre-diluted in high glucose DMEM (ThermoFisher, Waltham, MA, USA) at a ratio of 1:21, before addition to assay plates. For dose response of control compounds, concentrations ranged from 38 to 1.9 × 10−3 µM for diminazene and 36 to 1.8 × 10−3 µM for puromycin. The positive control, diminazene, was used at a final assay concentration of 19 µM (EC100) and the negative control was 0.4% DMSO (vehicle only). A plate containing 14 doses of control compounds as described was included in every assay, with two biological replicates for each compound concentration series. In addition, negative (0.4% DMSO) and positive (puromycin and diminazene) in plate-controls (EC100) were included for each assay plate containing test compounds, with one column for each control. The Z′-factor was calculated from negative and positive controls to evaluate the reproducibility of the assay, with values > 0.5 indicating a reproducible assay [39].
2.4. T. cruzi 48 h Image-Based Assay
The T. cruzi 48 h image-based assay was performed as previously described [28]. Briefly, 3T3 mouse fibroblast cells were added at 1 × 103 cells/well in 50 μL of medium into 384-well Collagen I coated plates (CellCarrier, PerkinElmer, Waltham, MA, USA) using a Multidrop (ThermoFisher Scientific, Waltham, MA, USA). Plates were incubated for 24 h at 37 °C and 5% CO2 before addition of 10 μL of T. cruzi trypomastigotes at a MOI of 5:1 parasite–host cells. Plates were incubated for 24 h and then wells were washed three times with PBS containing magnesium and calcium, once with additions using a Multidrop (ThermoFisher Scientific, Waltham, MA, USA) and twice using a Bravo liquid handler (Agilent Technologies, Santa Clara, CA, USA), with 50 μL of media replaced after the third wash. The initial wash was carried out by discarding the supernatant into a reservoir containing bleach, under sterile conditions. Five microlitres of prediluted compounds were added to plates with a MiniTrak compound handing device (PerkinElmer, Waltham, MA, USA) and incubated for 48 h before fixing cells with paraformaldehyde and staining with fluorescent nuclear and cytoplasmic markers. Plates were imaged on an Opera image-based system (PerkinElmer, Waltham, MA, USA).
2.5. Pathogen Box IC50 Values and Parasite Selectivity
The 43 compounds from the Pathogen Box were tested over 14 log doses ranging from final assay concentrations of 79 µM to 4.0 × 10−3 µM against T.b. brucei and HEK 293 cells, and from 73 µM to 3.7 × 10−3 µM for T. cruzi and 3T3 host cells. Confirmed selective compounds to parasites in relation to HEK293 cells for either parasite progressed to temporal and wash-out assays and activity was determined against HEPG2 cells. Compounds were tested in two independent experiments (n = 2). Compounds with fast-acting activity against both parasites were prioritised for testing against T.b. brucei subspecies and TcCYP51.
2.6. T.b. brucei Temporal Viability Assays
Time-kill assays was undertaken as previously described, using the REDOX reagent PrestoBlue® [24]. To investigate the influence of 24 h incubation with compounds, plates were incubated for 15 h before the addition of 10 µL of 6× PrestoBlue® (ThermoFisher, Waltham, MA, USA), diluted in HMI-9 supplemented with 10% FBS, to give a final concentration of 1×. Plates were incubated for 9 h, giving a total incubation time of 24 h after addition of compounds. Control compounds were added at the same concentrations at outlined for the resazurin-based assays.
To investigate the effect of 48 h exposure to compounds on parasite viability, T.b. brucei were incubated with compound for 45 h followed by addition of Presto Blue (final 1× concentration) and further incubation for 3 h at 37 °C and 5% CO2. After either 24 h or 48 h incubation with compound and addition of Presto Blue, plates were read on an EnSight plate reader (PerkinElmer, Waltham, MA, USA), at Ex/Em 535/590 nm.
2.7. T. cruzi Temporal Image-Based Assays
Assays to assess the activity of compounds following 72 h exposure [23,28] and 24 h exposure [23] to T. cruzi infected host cells were undertaken as previously described. Briefly, the method for the 48 h assay [28] was modified for either 24 or 72 h. In the 72 h assay, the number of wash cycles was increased to 5 instead of 2 during PBS wash steps to ensure removal of parasites that had egressed from host cells.
2.8. T.b. brucei Subspecies Assays
The assays to determine the sensitivity of T. brucei human infective subspecies to prioritised Pathogen Box compounds were undertaken at the Swiss Tropical and Public Health Institute (Swiss TPH, Allschwil, Switzerland). The T.b. rhodesiense STIB900 and T.b. gambiense STIB930 assays were performed as previously described, with compound exposure for 72 h [24,40].
2.9. HEK293 Cytotoxicity Assay
The HEK293 cell cytotoxicity assay was carried out as described previously [24], modified slightly with the use of resazurin. Briefly, HEK293 cells (ATCC) were added to sterile polystyrene black/clear bottomed 384-well plates (Greiner, USA) at 4 × 103 cells per well, in 55 µL of high glucose DMEM supplemented with 10% FBS. Following 24 h incubation at 37 °C in 5% CO2, under humidified growth conditions, 5 µL of pre-diluted compound was added with a MiniTrak (PerkinElmer, Waltham, MA, USA). Final assay concentrations of Pathogen Box compounds ranged from 79.4 µM to 4.0 × 10−3 µM. Plates were incubated for 68 h, then 10 µL of 490 µM of resazurin was added to give a final concentration of 70 µM. Plates were incubated for a further 4 h under normal growth conditions giving a total compound incubation of 72 h. Fluorescence (Ex/Em 535/590 nm) was then determined on an EnSight plate reader (PerkinElmer, Waltham, MA, USA). Positive and negative controls used were as the same as those in the T.b. brucei assay; 4 µM puromycin was a positive control and 0.4% DMSO served as a negative control.
2.10. HEPG2 Cytotoxicity Assay
The assay was undertaken as described for the HEK293 assay, with a modification of the cell number used to 500 cells/well. Five microlitres of compounds pre-diluted 1:21 in DMEM were added to plates to give final assay concentrations between 79.4 µM to 4.0 × 10−3 µM. Plates were incubated for 68 h, before the addition of 10 µL of 490 µM of resazurin to give a final concentration of 70 µM. Plates were incubated for a further 4 h prior to being read on an EnSight plate reader (PerkinElmer, Waltham, MA, USA) at an excitation of 535 nm and emission of 590 nm. The concentration of the control compounds utilised for this assay were the same as those prepared for the T.b. brucei 72 h assay.
2.11. Compound Activity against T. cruzi Recombinant CYP51 Enzyme
The inhibitory properties of compounds against T. cruzi recombinant CYP51 (TcCYP51) was determined using an in vitro assay as previously described [33,41]. Compounds which inhibited T. cruzi in an image-based assay were tested against TcCYP51 in log concentrations (100, 33, 10, 3.3, 1.0, 0.33, 0.1 and 0 µM), with one replicate per dose, as previously described [41]. The control compound posaconazole was incubated at the same concentrations as the test compounds, over two replicates.
3. Results
3.1. Confirming Activity of Pathogen Box Solid Compound Stocks against T.b. brucei and T. cruzi
New solid stocks of the 43 compounds from the Pathogen Box were tested to determine activity against both parasites, with the selectivity of active compounds determined for HEK293 and HEPG2 cells (for those compounds with selectivity against HEK293), in addition to 3T3 host cells in the T. cruzi assay. Active compounds were defined as those with an IC50 value of <10 µM [16]. Of the 43 compounds tested, 19 were originally found selectively active against T.b. brucei; 14 compounds active against both T.b. brucei and T. cruzi and 10 active only against T. cruzi [16]. From the compounds active against T.b. brucei (either selectively, or active against both T.b. brucei and T. cruzi), 27 reconfirmed activities against T.b. brucei (82% reconfirmation: 27 from 33 compounds). From compounds that were active against T.b. brucei only from the original study [16], 8 compounds were found to also be active against T. cruzi upon testing the solid stocks. Table 1 shows that 22 compounds from the 43 compounds profiled demonstrated activity against both T.b. brucei and T. cruzi. Of these, MMV022029, MMV022478, MMV688514 and MMV21013 were not selective against either parasite in relation to HEK293 cells (SI < 10) and displayed activity against 3T3 cells (Table 1), and thus were deprioritised. MMV006901 did not display selectivity to T.b. brucei and showed variable activity against 3T3 cells. This compound was subsequently tested against HEPG2 and did not show selectivity for either parasite; therefore, this was deprioritised. MMV687706 had activity above the cut off of 10 µM against T. cruzi (11 µM) and was not selective to T.b. brucei (SI = 7.4) and thus was not progressed. MMV028694 demonstrated poor selectivity to T. cruzi in relation to HEK293 cells (SI = 1); however, it was selective against T.b. brucei, and T. cruzi to 3T3 cells, and thus was progressed to further in vitro profiling.

Table 1.
Activity of compounds with activity against both T.b. brucei and T. cruzi parasites.
MMV028694 and MMV688283 were moderately activity against T. cruzi (9.4 ± 1.8 µM and 6.6 ± 1.2 µM, respectively, Table 1) and this activity may not have been identified with the lower concentration of 20 µM used in our previous testing [16], whilst MMV099637 and MMV688776 gave IC50 values of 2.7 µM and 3.2 µM, respectively (Table 1), despite not being identified as active against T. cruzi in our previous study using the liquid stocks provided. From the original Pathogen Box testing, 13 compounds were selectively active against T. cruzi and T.b. brucei parasites, with 13/14 confirming activity (93%) against T. cruzi. The compound that did not confirm activity was MMV687273 (SQ-109, Tuberculosis set), which was less active than the previous test, displaying 16 µM (results not shown) versus 5.4 µM in the previous studies [16].
T.b. brucei and HEK293 assays gave mean Z′ values of 0.87 and 0.84, respectively, and T. cruzi and 3T3 assays mean Z′-values of 0.56 and 0.75, respectively, indicating reproducible assays.
3.2. Time-Kill: Compounds Active against Both T. cruzi and T.b. brucei
Compounds selectively active against both parasites were tested for temporal activity against T. cruzi and T.b. brucei, and regrowth following the wash-out of compounds in the T. cruzi assay (Table 2). Compounds were categorised as fast-acting if they had an IC50 value of <10 µM following 24 h incubation, with a maximum inhibition on the plateau of activity (Emax) of ≥80%. The Emax was determined as previously described [23]. Compounds were categorised cidal if the Emax was >90% following compound wash off, and there was no decrease in the Emax between 48 h incubation (<10% difference in the % Emax) and following wash off of compound (48 h incubation, followed by wash off of compound, then a further 72 h incubation). Compounds were defined as efficacious if they cleared >90% T. cruzi parasites following 48 h exposure, following our previous criteria for definition of sub-efficacious compounds against T. cruzi. This profile may indicate TcCYP51 inhibition [23].

Table 2.
IC50 values and maximum activity (Emax) of compounds with activity against T. cruzi and T.b. brucei following 24–72 h exposure; in addition to compound wash off in the T. cruzi assay.
3.3. Fast-Acting Compound Activity against T.b. rhodesiense and T.b. gambiense
Compounds that were fast-acting and efficacious against both T. cruzi and T.b. brucei were tested against the human infective forms of T. brucei. The IC50 values of these compounds against human infective T. brucei spp. are shown in Table 3.

Table 3.
Activity against the human infective forms of HAT, T.b. gambiense and T.b. rhodesiense of fast-acting MMV Pathogen Box compounds active against T.b. brucei and T. cruzi.
3.4. Physicochemical Properties of Fast-Acting Compounds
The physicochemical properties of the fast-acting compounds identified with activity against T. cruzi and T.b. brucei are shown in Table 4. The molecular weight and LogP (XlogP) of test compounds were sourced from PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on 27 May 2022) and control compounds from DrugBank (https://www.drugbank.com/, accessed on 27 May 2022). All compounds have a LogP value of <5 and a molecular weight of <500 and thus are predicted to have favourable absorption and permeation in vivo, according to Lipinski [43].

Table 4.
Physicochemical properties of fast-acting Pathogen Box compounds with activity against T.b. brucei and T. cruzi.
3.5. TcCYP51 Biochemical Activity
Slow- and fast-acting compounds active against both T. cruzi and T.b. brucei were tested for their ability to inhibit TcCYP51 enzymatic activity (Figure 1). Of the fast-acting compounds, if there was more than one compound with activity against T. cruzi, one representative compound from each chemical class was selected. MMV652003 was not tested as benzoxaboroles have known activity against the parasite. Slow-acting compounds were also tested for activity against TcCYP51. The criteria defining a compound with TcCYP51 activity required that the IC50 value was within a log difference to posaconazole, as previously reported [33]. None of the compounds tested showed inhibition of TcCYP51, based on these criteria.
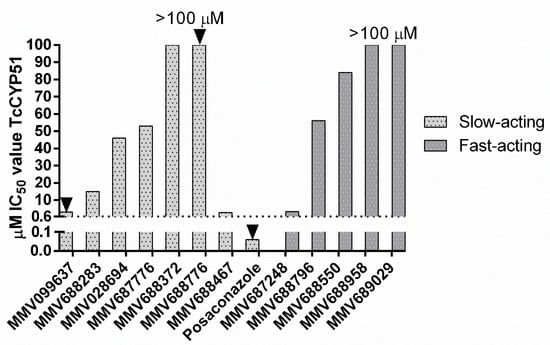
Figure 1.
IC50 values of slow and fast-acting compounds active against TcCYP51. Where IC50 values reach 100 µM, the IC50 value is >100 µM. Arrows denote compounds with a sub-efficacious Emax following 48 h incubation. MMV099637 = 70% Emax; MMV688776 = 88%, Posaconazole = 73% Emax. Dashed line = 10× the IC50 value of posaconazole as a cut off for CYP51 inhibition.
3.6. Compounds Recommended for Further Investigation
Prioritised compounds recommended for further investigation based on in vitro profiling and literature searches are shown in Table 5. Increased SAR exploration is required for the remainder of the compounds to identify compounds with improved selectivity, or solubility issues to be investigated, as outlined in the discussion.

Table 5.
Prioritised compounds from the Pathogen Box with activity against both T. cruzi and T. brucei recommended for progression.
4. Discussion
From more extensive in vitro profiling of hit compounds from the MMV Pathogen Box [16] with activity against T. cruzi and/or T.b. brucei parasites, we have identified selective, fast-acting, efficacious and cidal compounds with activity against both parasite species. These compounds possess promising in vitro profiles warranting target identification and/or progression as hit molecules (Table 5). This is the first study that has classified the compounds from the Pathogen Box with respect to speed of action against these kinetoplastids, in addition to their static/cidal action and TcCYP51 activity against T. cruzi. Compounds with activity against TcCYP51 are generally deprioritised [25,27], as TcCYP51 inhibitors have failed to clear chronic Chagas disease infection in clinical trials [29]. Compounds need to be effective against small numbers of persistent parasites in the chronic stage of Chagas disease; thus, we also investigated the ability of compounds to have an irreversible effect against T. cruzi using a compound wash off assay, utilising 48 h incubation of T. cruzi in the presence compounds, followed by compound wash off, then 72 h incubation. Whilst this is an initial indication of an irreversible action on T. cruzi parasites, comparative studies need to be undertaken with a longer incubation time, in concert with in vivo studies. It is important to develop longer-term assays, where consideration is given to the addition of fresh host cells [49], that divide slowly to prevent overgrowth [26], and to incorporate monitoring of host cell health. Prior to time-kill assays, the cytotoxicity of the Pathogen Box compounds was assessed. We previously had determined the selectivity of the Pathogen Box compounds for T. cruzi and T.b. brucei in relation to HEK293 [16]. To facilitate a more accurate determination of the selectivity of the compounds, we sourced solid samples, enabling an increase in the top concentration tested from 5 µM to 20 µM. Compounds exhibiting a SI > 10 were prioritised for further investigation, as recommended by the Drugs for Neglected Diseases initiative (DNDi) [25]. We have also identified slow-acting compounds against T. cruzi with activity against both parasites that are recommended for further investigation in the drug discovery pipeline.
Following in vitro profiling of the Pathogen Box collection, we identified 9 pan-active, selective, and fast-acting compounds (Table 5). These compounds did not inhibit TcCYP51, and 8 of these compounds showed no shift in the IC50 value against T. cruzi following compound wash off (<2-fold difference), thus suggesting no outgrowth following removal of compound pressure and a further incubation for 3 days. MMV652003 showed a 3.4-fold increase in the IC50 value following wash off, which may suggest outgrowth at some concentrations; however, this did not occur at the Emax, which remained constant. This compound is a boron containing molecule of the benzoxaborole (oxaborole) chemical class [50]; however, it has been identified with in vitro and in vivo activity against both T. cruzi and T.b. brucei, and thus is not a new class to pursue. Through a prior medicinal chemistry campaign, MMV652003 (AN3520) led to the development of SCYX-6759 (AN4169) [44], with curative activity in a stage 2 mouse model of HAT (utilising T.b. brucei) and is now an advanced lead for Chagas disease [45]. Subsequently, SCYX-7158 (AN5568) was developed with improved pharmacokinetic properties against HAT spp, which is currently in ongoing clinical trials as an oral treatment of HAT caused by T.b. gambiense [42] and has shown 100% cure of mice infected with T. cruzi when treated for 40 days [4]. It has recently been shown, using live-cell imaging, that SCYX-6759 demonstrated a 20-h lag phase of inhibition following exposure of T. cruzi infected H9C2 rat heart cells to 16 µM of compound, suggesting a slow MOA [46]. However, the study did not state the IC50 value, nor the EC100. It may be that the rate of inhibition is relative to concentration, as has been previously suggested for this compound [32]. For T. cruzi therefore, longer term wash off assays would be warranted for this class of compound. Whilst the target of benzoxaboroles is known to be mRNA processing endonuclease, CPSF3 in T.b. brucei [47] the target of oxaboroles remains unknown for T. cruzi. Thus, it would be beneficial to identify the MOA/ target, to facilitate further optimisation [32].
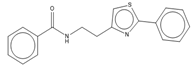
The 2-aryl oxazole class of compounds (MMV688797, MMV688958 and MMV688795) from the Pathogen Box collection were fast-acting against T.b. brucei and T. cruzi, with cidal activity demonstrated against T. cruzi. We have previously identified the activity of 2-aryl oxazole (2-phenylthiazole) chemical class against T.b. brucei in 2012 [24]. Follow up chemical modifications resulted in new, more active compounds, including MMV688958, of which a related compound was fast-acting against T. cruzi parasites [48]. A related compound to MMV688958 has also demonstrated activity in a mouse model of T. cruzi infection, following pre-treatment of mice with the nonselective CYP-inhibitor, 1-aminobenzotriazole, potentially mediated by the thiazole phenyl substituent resulting from rapid metabolism (compound 64a [48]). Pathogen Box associated data indicated that the aryl oxazoles were active against T.b. rhodesiense [10], and we have identified that these three compounds were also active against T.b. gambiense. Improving the metabolic stability of aryl oxazoles is ongoing [48]. The dual inhibitory nature of this chemical class against kinetoplastids further supports these efforts. Additionally, we have shown that MMV688958 does not inhibit TcCYP51, an important attribute for ongoing development in the drug discovery pipeline.
MMV688796 is 2,4-substituted furan, belonging to the kinetoplastid compound set in the Pathogen Box collection, previously demonstrated to be active against T.b. rhodesiense [51]. We have demonstrated that MMV688796 also has activity against T.b. gambiense and is a promising, fast-acting hit compound against both parasites. The in vitro DMPK data provided with the MMV Pathogen Box collection shows MMV688796 has favourable Log D, suggesting good lipophilicity, in addition to a favourable LogP (PubChem, https://pubchem.ncbi.nlm.nih.gov/, accessed on 27 May 2022) with a moderate half-life of 3.9 h, when dosed orally in mice [51]. Therefore, MMV688796 was recommended for progression to in vivo mouse models of infection for T.b. rhodesiense, T.b. gambiense and T. cruzi.
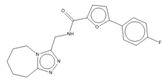
MMV688550 is an imidazo [1,2] purine which originated from the Pathogen Box kinetoplastid set, with activity against T.b. rhodesiense [51] and now with confirmed activity against both T.b. rhodesiense and T.b. gambiense in the current study. MMV688550 did not display activity against the mammalian cells at the concentrations and incubation times tested, nor against TcCYP51, with a favourable half-life and in vitro DMPK [51], and thus warrants in vivo investigation.
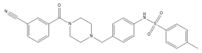
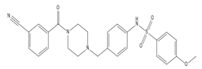
MMV689028 is a fast-acting benzyl piperazine, which showed no activity against HEK293 and HEPG2, nor exhibited TcCYP51 activity. Both MMV689028 and the related compound, MMV689029, induced CYP1A2 activity, resulting in either high clearance or low half lives in vivo, provided in the Pathogen Box in vitro DMPK data [10]. The in vitro profile of MMV689028, with an improved half-life in comparison to MMV689029 (6.8 and 2.4 h, respectively), should be investigated for in vivo activity against T. cruzi and T.b. rhodesiense/T.b. gambiense, potentially with the use of a CYP inhibitor as a proof of concept, as this enzyme contributes to elimination of xenobiotics, which may influence drug clearance [52].
All fast-acting compounds that were identified originated from the Pathogen Box kinetoplastid set, whilst MMV687248 was from the tuberculosis set. This compound showed moderate selectivity to T. cruzi in relation to HEPG2 cells (SI = 13). Thus, selectivity would need to be improved, by sourcing and/or synthesising analogues [10]. The remainder of the tuberculosis set of compounds were either cytotoxic or did not confirm activity for one or more of the parasites, as per example, MMV687273 (SQ-109), currently in clinical trials for the treatment of tuberculosis, has previously reported activity against T.b. brucei with an IC50 value of 5 µM [53]. This compound had poor activity against T.b. brucei in our original testing and did not reconfirm activity against T. cruzi, as we originally identified [16]. Following 48 h an IC50 value of 13 µM was demonstrated against T. cruzi in our studies, with 16 µM, following 72 h incubation (results not shown). Previous studies have suggested that this was a lead compound against T. cruzi [54], with an IC50 value of 0.5–1 µM, following 96 h incubation. The incubation time in the studies would need to be extended to 96 h and beyond to confirm this; however, it may be that the image-based format used for our analysis is more sensitive and thus identifies small numbers of parasites indicative of infected cells, in comparison to Giemsa staining [54]. This compound was therefore of limited interest for both parasites.
We identified whether some of the slower-acting compounds against T. cruzi, with efficacy against both parasites had potential with respect to further drug discovery efforts. The majority of these have lack of selectivity to one mammalian cell line, and therefore SAR is recommended to identify more selective molecules. Unlike many TcCYP51 inhibitors which are typically slow-acting against T. cruzi [23], MMV028694, MMV688372, MMV688283, MMV687776 and MMV688467 had high efficacy (>95%) against T. cruzi following 48 h incubation. The phenotypic profile suggests these compounds may not be TcCYP51 inhibitors [23], which was confirmed by testing against recombinant TcCYP51. MMV028694, a 2,4-disubstituted pyrimidine showed moderate and slow-acting activity against T. cruzi following 48 h incubation (9.4 µM) and 72 h incubation (16 µM) with poor selectivity toward T. cruzi in relation to HEK293 cells (SI = 1); however, it displayed selectivity to 3T3 fibroblasts (SI > 7.8). This compound showed moderate selectivity to T.b. brucei (SI = 12), as well as activity against both infective forms of T.b. brucei. However, medicinal chemistry efforts to improve the moderate activity and selectivity observed are required before further progression of this compound would be considered. MMV688467 was fast-acting against T.b. brucei but slow-acting against T. cruzi. This compound is a butyl sulphanilamide showed activity against HEPG2 cells, resulting in a SI to T. cruzi of 10 and for T.b. brucei, 74. This compound could be pursued for T.b. brucei but would need to be tested against the infective forms of the parasite. More selective analogues would be required to be considered for further studies against T. cruzi.
MMV688372 is a substituted 2-phenylimidazopyridine which is slow-acting against T. cruzi, with a 13-fold difference in the IC50 value from 24 to 72 h incubation. Whilst an IC50 value could be estimated following 24 h incubation, this was sub- efficacious (75% Emax against T. cruzi). This compound shows a lower SI against T. cruzi in relation to HEK293 and HEPG2 cells, with an SI of 14 and 129 for T. cruzi and T.b. brucei, respectively, in relation to HEK293 cells, and an SI of 28 for T. cruzi and 257 for T.b. brucei in relation to HEPG2 cells. MMV688372 has recently been shown to have activity against the protozoan parasite that causes East Coast fever in cattle, Theileria parva [55], with a low µM IC50 value (5.3 µM) against bovine peripheral blood mononuclear cells (PBMCs), following 24 h incubation [55]. MMV688372 also has associated CYP (1A2; 2C9 and 2D6) activity; thus, a CYP inhibitor may be required for in in vivo studies, to provide proof of concept. However, improving the current moderate selectivity against T. cruzi would need to be explored with generation of analogues. MMV688283 is a 4-amino quinoline, with moderate activity against both parasites, slow-acting activity against T. cruzi and limited selectivity. Synthesis of analogues is also required to ascertain structure activity relationships to improve moderate activity before proceeding further. The benzoxaborole MMV687776 was slow-acting against T. cruzi, with moderate activity displayed against T.b. brucei following 72 h incubation (4.3 µM) and low selectivity to T.b. brucei and T. cruzi to HEK293 (SI = 3.0, 8.1, respectively). Therefore, this was a different activity profile to the fast-acting benzoxaborole (MMV652003) identified. However, it had good selectivity for T.b. brucei in relation to HEPG2 (SI > 18), and T. cruzi in relation to 3T3 cells (SI > 46). Further profiling with other mammalian cells would be warranted and potentially SAR to improve potential cytotoxicity. However, benzoxaborole compounds are oxaborole pharmacophores [56], and oxaboroles are well-developed leads against both T.b. brucei and T. cruzi [50]. As this class is not novel, and the target is known for T.b. brucei, it would be beneficial to identify the biological target of this compound class in T. cruzi, rather than development of this compound for T.b. brucei. A recent study showed that carbonic anhydrases from T. cruzi and L. donovani chagasi are inhibited by benzoxaboroles. However, whilst there was low µM activity against L. donovani, there was less activity (13–88 µM) shown against the T. cruzi carbonic anhydrase [57]; thus, further work is needed to confirm the target. The activity of this and other slow-acting compounds would also need to be tested against the human infective forms of HAT.
The pyrazolopyridine MMV099637 with an IC50 value of 2.7 µM against T. cruzi (2.1 µM against T.b. brucei) was not previously active against T. cruzi at a final assay concentration of 20 µM [16]. Stability of some compounds in DMSO, due to various factors such as storage and freeze-thaw cycles [58], may have contributed to variation in activity. MMV099637 was slow-acting with low efficacy and whilst low efficacy is commonly associated with TcCYP51 activity [23], this was not the case with this compound. MMV099637 may present with this profile due to solubility issues noted during preparation of DMSO stock solutions (maximum 14 mM due to solubility issues in 100% DMSO); thus, in vitro solubility analysis, such as a nephelometry assay, to confirm if solubility is an issue, should be undertaken [59]. MMV688776 is a pyrazoloquinazoline with moderate activity against T. cruzi (3.1 µM) and sub-µM activity (0.79 µM) against T.b. brucei, which also was a sub-efficacious compound against T. cruzi. This compound did not inhibit TcCYP51 and may present with this profile due to solubility issues noted during preparation of DMSO stock solutions (maximum 14 mM due to solubility issues in 100% DMSO). Whilst pyrazoloquinazolines do not have reported activity against trypanosomes, compounds from this class have demonstrated inhibition of human topoisomerase I [60]. Topoisomerase has been identified as a target in trypanosomes and has recently been implicated in response to replication stress in T. cruzi [61].
Whilst we have identified compounds that are selective and pan-active against T. cruzi and T.b. brucei parasites, we also characterised these compounds based on their speed of action. While fast-acting compounds may be beneficial down the pipeline, this is not necessarily a prerequisite when considering the progression of compounds. However, a fast-acting and long-lasting compound is recommended by DNDi as optimal for Chagas disease, as it is questionable whether a slow-acting compound is desirable due to the clinical failure of slow-acting azoles [25]. Thus, fast-acting compounds are often prioritised in drug discovery campaigns [46]. Since the fast- and slow-activity compounds identified herein showed activity against T. cruzi and T.b. brucei, target-based studies could be undertaken with T.b. brucei parasites, as these do not require a host cell and thus are cultured axenically. Ultimately, thorough in vitro evaluation of these compounds provided a valuable resource for researchers and importantly, provided compounds for further pre-clinical and target-based studies to elucidate potentially new modes of action in both T. cruzi and T.b. brucei, thereby supporting the open-source initiative for the Pathogen Box collection.
Author Contributions
Conceptualization, M.L.S. and V.M.A.; methodology, M.L.S., E.K.K., K.D.R. and M.K.; performed experiments, E.K.K. and M.L.S.; analyzed data, M.L.S., E.K.K., K.D.R. and M.K.; writing—original draft preparation, M.L.S., V.M.A. and E.K.K.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was supported by a Griffith University Postdoctoral Fellowship (GUPF; MLS) and Drugs for Neglected Diseases initiative (DNDi). The authors would like to sincerely thank DNDi and their donors for financial support of this work, through support to MLS and VMA. For the work described in this paper, DNDi received financial support from the following donors: the Swiss Agency for Development and Cooperation (SDC), Switzerland; the Directorate General for International Cooperation (DGIS), The Netherlands; (DFID), UK and Médecins Sans Frontières, international. The donors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank the Medicines for Malaria Venture (MMV) for the provision of the solid samples of compounds from the Pathogen Box collection that were utilised in this study. The authors would like to thank Jennifer Riley from the University of Dundee who undertook compound testing against the recombinant Trypanosoma cruzi cytochrome P450 enzyme (TcCYP51).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organisation. Neglected Tropical Diseases. Available online: https://www.who.int/health-topics/neglected-tropical-diseases (accessed on 27 May 2022).
- World Health Organisation. Chagas Disease (American trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 27 May 2022).
- Castro, J.A.; de Mecca, M.M.; Bartel, L.C. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sales Junior, P.A.; Molina, I.; Fonseca Murta, S.M.; Sanchez-Montalva, A.; Salvador, F.; Correa-Oliveira, R.; Carneiro, C.M. Experimental and Clinical Treatment of Chagas Disease: A Review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First Global Approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Drugs for Neglected Diseases Initiative. Fexinidazole for T.b. rhodesiense. Available online: https://dndi.org/research-development/portfolio/fexinidazole-tb-rhodesiense/ (accessed on 27 May 2022).
- Efficacy and Safety of Fexinidazole in Patients with Human African Trypanosomiasis (HAT) Due to Trypanosoma brucei rhodesiense. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03974178 (accessed on 27 May 2022).
- World Health Organization. Control and surveillance of African trypanosomiasis. In Report of a WHO Expert Committee; World Health Organisation Technical Report Series: Geneva, Switzerland, 1998; Volume 881, pp. 1–114. [Google Scholar]
- Arrowsmith, J. Trial Watch Phase III and submission failures: 2007–2010. Nat. Rev. Drug Discov. 2011, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- MMV About the Pathogen Box. Available online: https://www.mmv.org/mmv-open/pathogen-box/about-pathogen-box (accessed on 27 May 2022).
- Hunter, P. Tropical diseases and the poor: Neglected tropical diseases are a public health problem for developing and developed countries alike. EMBO Rep. 2014, 15, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Maccesi, M.; Aguiar, P.H.N.; Pasche, V.; Padilla, M.; Suzuki, B.M.; Montefusco, S.; Abagyan, R.; Keiser, J.; Mourão, M.M.; Caffrey, C.R. Multi-center screening of the Pathogen Box collection for schistosomiasis drug discovery. Parasit Vectors 2019, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.B.; Tuvshintulga, B.; Guswanto, A.; Tayebwa, D.S.; Rizk, M.A.; Gantuya, S.; Batiha, G.E.-S.; Beshbishy, A.M.; Sivakumar, T.; Yokoyama, N.; et al. Screening the Medicines for Malaria Venture Pathogen Box against piroplasm parasites. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Lopez-Ribot, J.L. Screening the Pathogen Box for Identification of Candida albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 2017, 61, e02006-16. [Google Scholar] [CrossRef] [PubMed]
- Machicado, C.; Soto, M.P.; Timoteo, O.; Vaisberg, A.; Pajuelo, M.; Ortiz, P.; Marcos, L.A. Screening the Pathogen Box for Identification of New Chemical Agents with Anti-Fasciola hepatica Activity. Antimicrob. Agents Chemother. 2019, 63, e02373-18. [Google Scholar] [CrossRef]
- Duffy, S.; Sykes, M.L.; Jones, A.J.; Shelper, T.B.; Simpson, M.; Lang, R.; Poulsen, S.-A.; Sleebs, B.E.; Avery, V.M. Screening the Medicines for Malaria Venture Pathogen Box across Multiple Pathogens Reclassifies Starting Points for Open-Source Drug Discovery. Antimicrob. Agents Chemother. 2017, 61, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Veale, C.G.L.; Hoppe, H.C. Screening of the Pathogen Box reveals new starting points for anti-trypanosomal drug discovery. MedChemComm 2018, 9, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Human African Trypanosomiasis (Sleeping Sickness). Available online: https://www.who.int/health-topics/human-african-trypanosomiasis (accessed on 27 May 2022).
- Zrein, M.; Granjon, E.; Gueyffier, L.; Caillaudeau, J.; Liehl, P.; Pottel, H.; Cardoso, C.S.; Oliveira, C.D.L.; De Oliveira, L.C.; Lee, T.-H.; et al. A novel antibody surrogate biomarker to monitor parasite persistence in Trypanosoma cruzi-infected patients. PLoS Negl. Trop. Dis. 2018, 12, e0006226. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Konar, N. Translational challenges of animal models in Chagas disease drug development: A review. Drug. Des. Dev. Ther. 2015, 9, 4807–4823. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Cucunubá, Z.; Flórez, C.; Olivera, M.; Valencia, C.; Zambrano, P.; León, C.; Ramírez, J.D. Molecular Diagnosis of Chagas Disease in Colombia: Parasitic Loads and Discrete Typing Units in Patients from Acute and Chronic Phases. PLoS Negl. Trop. Dis. 2016, 10, e0004997. [Google Scholar] [CrossRef]
- Ward, A.I.; Lewis, M.D.; Khan, A.A.; McCann, C.J.; Francisco, A.F.; Jayawardhana, S.; Taylor, M.C.; Kelly, J.M. In Vivo Analysis of Trypanosoma cruzi Persistence Foci a Single-Cell Resolution. Mbio 2020, 11, e01242-20. [Google Scholar] [CrossRef]
- Sykes, M.L.; Avery, V.M. 3-pyridyl inhibitors with novel activity against Trypanosoma cruzi reveal in vitro profiles can aid prediction of putative cytochrome P450 inhibition. Sci. Rep. 2018, 8, 4901. [Google Scholar] [CrossRef]
- Sykes, M.L.; Baell, J.; Kaiser, M.; Chatelain, E.; Moawad, S.R.; Ganame, D.; Ioset, J.-R.; Avery, V.M. Identification of compounds with anti-proliferative activity against Trypanosoma brucei brucei strain 427 by a whole cell viability based HTS campaign. PLoS Negl. Trop. Dis. 2012, 6, e1896. [Google Scholar] [CrossRef]
- Chatelain, E. Chagas disease drug discovery: Toward a new era. J. Biomol. Screen. 2015, 20, 22–35. [Google Scholar] [CrossRef]
- Cal, M.; Ioset, J.R.; Fugi, M.A.; Maser, P.; Kaiser, M. Assessing anti-T. cruzi candidates in vitro for sterile cidality. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 165–170. [Google Scholar] [CrossRef]
- MacLean, L.M.; Thomas, J.; Lewis, M.D.; Cotillo, I.; Gray, D.W.; De Rycker, M. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’ disease drug discovery. PLoS Negl. Trop. Dis. 2018, 12, e0006612. [Google Scholar] [CrossRef]
- Sykes, M.L.; Avery, V.M. Development and application of a sensitive, phenotypic, high-throughput image-based assay to identify compound activity against Trypanosoma cruzi amastigotes. Int. J. Parasitol. Drug. 2015, 5, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Molina, I.; i Prat, J.G.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Bangher, M.D.C.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. Cruzi Carriers: The STOP-CHAGAS Trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Molina, I.; Salvador, F.; Sanchez-Montalva, A. The use of posaconazole against Chagas disease. Curr. Opin. Infect. Dis. 2015, 28, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.B.; Giardini, M.A.; Kim, H.; Franco, C.H.; Araujo, A.M.; Schenkman, S.; Chatelain, E.; Freitas, L.H. Nitroheterocyclic compounds are more efficacious than CYP, 51 inhibitors against Trypanosoma cruzi: Implications for Chagas disease drug discovery and development. Sci. Rep. 2014, 4, 4703. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.; Brand, S.; Voice, M.; Caballero, I.; Calvo, D.; Read, K.D. Development of a Fluorescence-based Trypanosoma cruzi CYP51 Inhibition Assay for Effective Compound Triaging in Drug Discovery Programmes for Chagas Disease. PLoS Neglect. Trop. D 2015, 9, e0004014. [Google Scholar] [CrossRef]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.; Myburgh, E.; Gao, M.-Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef]
- Passos-Silva, D.G.; Rajão, M.A.; de Aguiar, P.H.N.; Vieira-Da-Rocha, J.P.; Machado, C.R.; Furtado, C. Overview of DNA Repair in Trypanosoma cruzi, Trypanosoma brucei, and Leishmania major. J. Nucleic Acids 2010, 2010, 840768. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Chappuis, F.; Seixas, J.; Kazumba, L.; Barrett, M.P.; Mwamba, E.; Erphas, O.; A Akl, E.; Villanueva, G.; et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: Substantial changes for clinical practice. Lancet Infect. Dis. 2020, 20, e38–e46. [Google Scholar] [CrossRef]
- Watson, J.A.; Strub-Wourgraft, N.; Tarral, A.; Ribeiro, I.; Tarning, J.; White, N.J. Pharmacokinetic-Pharmacodynamic Assessment of the Hepatic and Bone Marrow Toxicities of the New Trypanoside Fexinidazole. Antimicrob. Agents Chemother. 2019, 63, e02515-18. [Google Scholar] [CrossRef]
- Azzam, M.E.; Algranati, I.D. Mechanism of Puromycin Action—Fate of Ribosomes after Release of Nascent Protein Chains from Polysomes. Proc. Natl. Acad. Sci. USA 1973, 70, 3866–3869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Bray, M.A.; Cal, M.; Trunz, B.B.; Torreele, E.; Brun, R. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob. Agents Chemother. 2011, 55, 5602–5608. [Google Scholar] [CrossRef]
- Brand, S.; Ko, E.J.; Viayna, E.; Thompson, S.; Spinks, D.; Thomas, M.; Sandberg, L.; Francisco, A.F.; Jayawardhana, S.; Smith, V.C.; et al. Discovery and Optimization of 5-Amino-1,2,3-triazole-4-carboxamide Series against Trypanosoma cruzi. J. Med. Chem. 2017, 60, 7284–7299. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.T.; Nare, B.; Wring, S.A.; Orr, M.D.; Chen, D.; Sligar, J.M.; Jenks, M.X.; Noe, R.A.; Bowling, T.S.; Mercer, L.T.; et al. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl. Trop. Dis. 2011, 5, e1151. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug. Deliv. Rev. 2016, 101, 34–41. [Google Scholar] [CrossRef]
- Nare, B.; Wring, S.; Bacchi, C.; Beaudet, B.; Bowling, T.; Brun, R.; Chen, D.; Ding, C.; Freund, Y.; Gaukel, E.; et al. Discovery of Novel Orally Bioavailable Oxaborole 6-Carboxamides That Demonstrate Cure in a Murine Model of Late-Stage Central Nervous System African Trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 4379–4388. [Google Scholar] [CrossRef]
- Bustamante, J.M.; Craft, J.M.; Crowe, B.D.; Ketchie, S.A.; Tarleton, R.L. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J. Infect. Dis. 2014, 209, 150–162. [Google Scholar] [CrossRef]
- Svensen, N.; Wyllie, S.; Gray, D.W.; De Rycker, M. Live-imaging rate-of-kill compound profiling for Chagas disease drug discovery with a new automated high-content assay. PLoS Negl. Trop. Dis. 2021, 15, e0009870. [Google Scholar] [CrossRef]
- Wall, R.J.; Rico, E.; Lukac, I.; Zuccotto, F.; Elg, S.; Gilbert, I.H.; Freund, Y.; Alley, M.R.K.; Field, M.C.; Wyllie, S.; et al. Clinical and veterinary trypanocidal benzoxaboroles target CPSF. Proc. Natl. Acad. Sci. USA 2018, 115, 9616–9621. [Google Scholar] [CrossRef]
- Russell, S.; Rahmani, R.; Jones, A.J.; Newson, H.L.; Neilde, K.; Cotillo, I.; Khajouei, M.R.; Ferrins, L.; Qureishi, S.; Nguyen, N.; et al. Hit-to-Lead Optimization of a Novel Class of Potent, Broad-Spectrum Trypanosomacides. J. Med. Chem. 2016, 59, 9686–9720. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valdez, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. eLife 2018, 7, e34039. [Google Scholar] [CrossRef] [PubMed]
- Veale, C.G.L. Unpacking the Pathogen Box-An Open Source Tool for Fighting Neglected Tropical Disease. ChemMedChem 2019, 14, 386–453. [Google Scholar] [CrossRef] [PubMed]
- MMV Pathogen Box Supporting Information. Available online: https://www.mmv.org/mmv-open/pathogen-box/pathogen-box-supporting-information (accessed on 27 May 2022).
- Parmentier, Y.; Pothier, C.; Hewitt, N.; Vincent, L.; Caradec, F.; Liu, J.; Lin, F.; Trancart, M.-M.; Guillet, F.; Bouaita, B.; et al. Direct and quantitative evaluation of the major human CYP contribution (fmCYP) to drug clearance using the in vitro Silensomes (TM) model. Xenobiotica 2019, 49, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Y.; Yang, G.; Byun, S.; Rao, G.; Shoen, C.; Yang, H.; Gulati, A.; Crick, D.C.; Cynamon, M.; et al. Oxa, Thia, Heterocycle, and Carborane Analogues of SQ109: Bacterial and Protozoal Cell Growth Inhibitors. ACS Infect. Dis. 2015, 1, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Santos, P.; Li, K.; Lameira, L.; de Carvalho, T.M.U.; Huang, G.; Galizzi, M.; Shang, N.; Li, Q.; Gonzalez-Pacanowska, D.; Hernandez-Rodriguez, V.; et al. SQ109, a New Drug Lead for Chagas Disease. Antimicrob. Agents Chemother. 2015, 59, 1950–1961. [Google Scholar] [CrossRef]
- Nyagwange, J.; Awino, E.; Tijhaar, E.; Svitek, N.; Pelle, R.; Nene, V. Leveraging the Medicines for Malaria Venture malaria and pathogen boxes to discover chemical inhibitors of East Coast fever. Int. J. Parasitol. Drug 2019, 9, 80–86. [Google Scholar] [CrossRef]
- Vanveller, B.; Aronoff, M.R.; Raines, R.T. A Divalent Protecting Group for Benzoxaboroles. RSC Adv. 2013, 44, 21331–21334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nocentini, A.; Cadoni, R.; Dumy, P.; Supuran, C.T.; Winum, J.Y. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J. Enzym. Inhib. Med. Chem. 2017, 33, 286–289. [Google Scholar] [CrossRef]
- Cheng, X.; Hochlowski, J.; Tang, H.; Hepp, D.; Beckner, C.; Kantor, S.; Schmitt, R. Studies on repository compound stability in DMSO under various conditions. J. Biomol. Screen. 2003, 8, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Kerns, E.H.; Di, L.; Carter, G.T. In vitro solubility assays in drug discovery. Curr. Drug Metab. 2008, 9, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Taliani, S.; Pugliesi, I.; Barresi, E.; Salerno, S.; Marchand, C.; Agama, K.; Simorini, F.; La Motta, C.; Marini, A.M.; Di Leva, F.S.; et al. Phenylpyrazolo[1,5-a]quinazolin-5(4H)-one: A suitable scaffold for the development of noncamptothecin topoisomerase I (Top1) inhibitors. J. Med. Chem. 2013, 56, 7458–7462. [Google Scholar] [CrossRef][Green Version]
- Costa-Silva, H.M.; Resende, B.C.; Umaki, A.C.S.; Prado, W.; da Silva, M.S.; Virgílio, S.; Macedo, A.M.; Pena, S.D.J.; Tahara, E.B.; Tosi, L.R.O.; et al. DNA Topoisomerase 3alpha Is Involved in Homologous Recombination Repair and Replication Stress Response in Trypanosoma cruzi. Front. Cell Dev. Biol. 2021, 9, 633195w. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).