A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants
Abstract
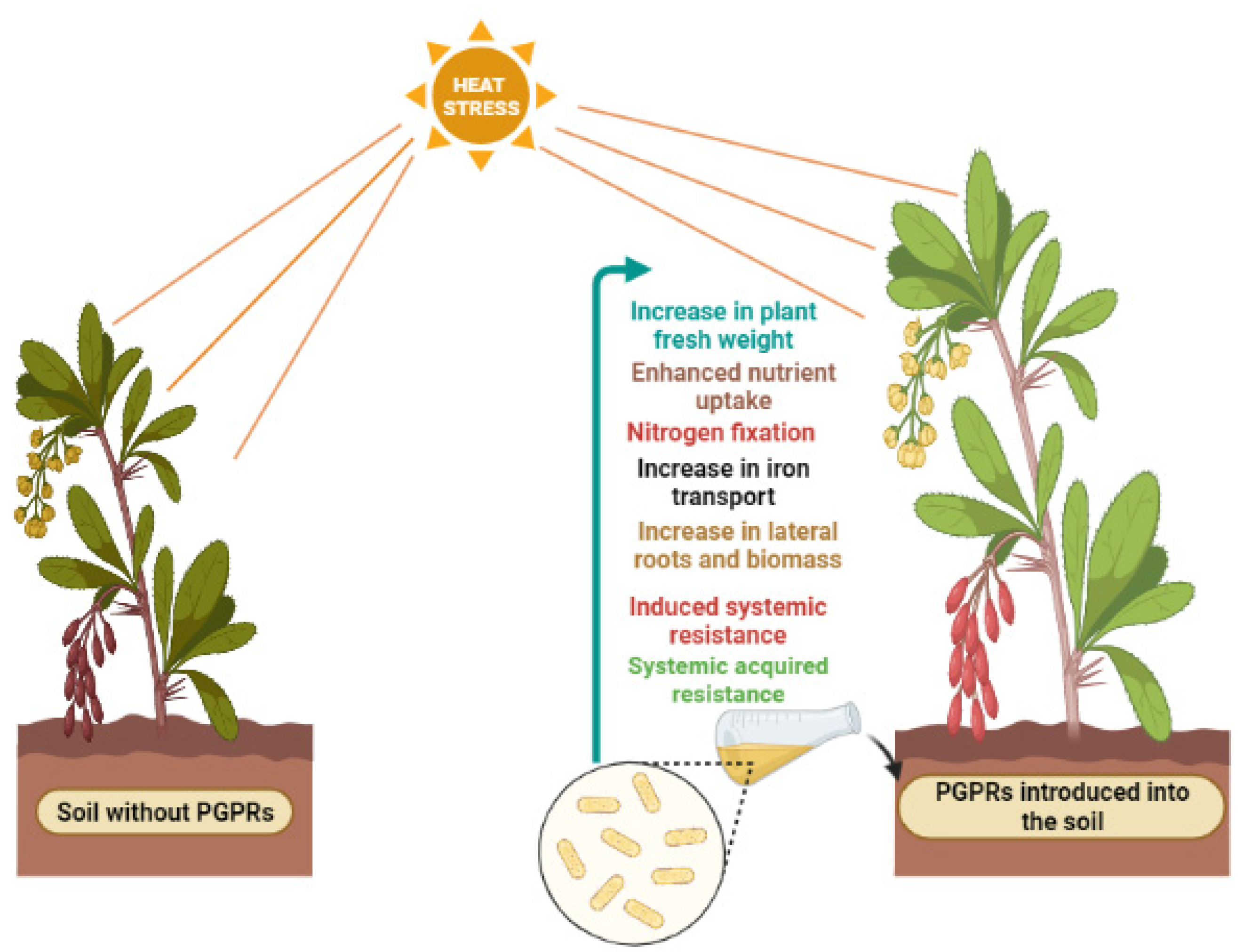
:1. Introduction
| References Country Year | Plants | Model/Approach | Heat Stress Regulators |
|---|---|---|---|
| Kotak et al. [22] 2007 | Arabidopsis | Omics | Phytohormone HS MBF1c HOT2 |
| Postgate et al. [36] 2013 | Arabidopsis | Microarray | HSP70 HSP60 APX |
| Peoples et al. [37] 2007 | NA | Appraisal | TATA box proximal 5′ flanking regions |
| Allahverdiyeva et al. [38] 2004 | Arabidopsis, Lycopersicon esculentum | Experimental | HsfA1,2 HsfB1 |
| Giller et al. [39] 2001 | Lycopersicon esculentum, Citrullus lanatus | Experimental | Phenolic components |
| Szymanska et al. [12] 2011 | NA | Appraisal | Dhn, Sag, Sgr |
| Ghosh et al. [17] 2004 | Fragaria × ananassa | Experimental | Antioxidant enzymes |
| Saha et al. [18] 2010 | Triticum aestivum | Experimental | Cellular, molecular and metabolic cascade |
| Glick et al. [40] 1999 | Soybean and Arabidopsis | In vivo and In vitro | HSP90 HSP60 HSP20 |
2. Role of Microorganisms in Thermotolerance
2.1. Endophytes
2.2. Plant Growth-Promoting Rhizobacteria (PGPR)
| References Country Year | Microbes | Model | Plant | Parameters | MOA | Stress | Effect |
|---|---|---|---|---|---|---|---|
| Abd El-Daim et al. [57] 2014 | Bacillus amyloliquefaciens UCMB5113 | Field | Triticum aestivum | ↑ Survival * rate | ↓ GR ↓ APX ↓ HPS17 | Short | Beneficial |
| Rana et al. [54] 2012 | Curvularia proturberata isolate Cp4666D, Burkholderia phytofirmans PsJN | Field experiment | Triticum aestivum, Dichanthelium lanuginosum, Solanum lycopersicum | Production of IAA, cytokines, protein and ↑ chlorophyll | ↓ Pathogen, ↓ ROS | Heat stress | Beneficial |
| Mitra et al. [59] 2021 | Bacillus cereus, Pseudomonas, Serratia liquefaciens, P. fluorescens and Pseudomonas putida | In vitro | S. lycopersicum L., Cajanus cajan, G. max, and Triticum spp. | ↑ ACC-deaminase, Production, ↑ phytohormone and ↑ antioxidant defense | Thermal tolerance | High temp | Sustainable |
| Maitra we al. [58] 2011 | Aeromonas hydrophilla Serratia liquefaciens Serratia proteamaculans | In vitro | Glycine max | ↑ Exopolysacchrides production | Thermotolerance | High temp | Remarkable |
| Kang et al. [53] 2019 | Bacillus tequilensis SSB07 | Experimental | Glycine max | ↑ Gibberellins ↑ IAA and ↑ ABA, jasmonic acid and salicylicacid contents | Thermotolerant | Moderate | Improvement |
| Ali et al. [55] 2011 | Pseudomonas putidaAKMP7 | Experimental | Triticum aestivum | ↑ Root and shoot length, ↑ biomass, ↑ SOD, ↑ CAT and APX | Thermotolerant | High | Improvement |
| Ali et al. [56] 2009 | Pseudomonas AKM-P6 | Experimental | Sorghum | ↑ Cellular metabolites | Thermotolerant | High | Improvement |
| Park et al. [47] 2017 | Bacillus aryabhattai SRB02 | Experimental | Glycine max | ↑ ABA ↑ IAA, JA, GAs contents, | Fertilizers+ thermotolerance | Medium | Improvement |
| Meena et al. [48] 2015 | Pseudomonas aeruginosa 2CpS1 | Net house experiment | Triticum aestivum cultivar (HUW-234) | ↑ Plant height and root length, ↑ chlorophyll content, | Mitigation | High | Improvement |
| 2018 | Bacillus amyloliquefaciens | Experimental | Oryza sativa | ↑ Proline, Total Soluble Sugar, ↑ Lipid Peroxidation and over expression of six stress-responsive of dehydrin (DHN), glutathione S- protein 6 (NRAMP6) genes | ↑ Modulated stress-responsive gene expressions ↑ phytohormone | High | Significant |
| Issa et al. [52] 2018 | Paraburkholderia phytofirmans PsJN | Green house experiment | Lycopersicon esculentum | ↑ Growth Biomass Chlorophyll content | ↑ Chlorophyll content, Photosystem II, ↑ Accumulations of sugars, total amino acids, proline, and Malate. | Thermotolerant | Improvement |
3. Physiological Changes Induced by Thermotolerant Microbes in Plants under Heat Stress
3.1. Photosynthesis
3.2. Changes in Respiration
3.3. Stomatal Closure
4. Molecular Mechanism of Action of Microbes in Mitigating Heat Stress in Plants
4.1. Nitrogen Fixation
4.2. Microbial Production of Siderophore
4.3. Microbial Production of 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase
4.4. Microbial Production of Phytohormone
4.5. Molecular Approaches
4.6. Microbial Production of Volatile Compounds
4.7. Organic Acid Production
5. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPCC | Intergovernmental Panel on Climate Change |
| HSPs | Heat shock proteins |
| DHN | Dehydrins |
| NRAMP6 | Natural resistance-associated macrophage protein 6 |
| PGPR | Plant growth-promoting rhizobacteria |
References
- Ali, M.G.; Ahmed, M.; Ibrahim, M.M.; El Baroudy, A.A.; Ali, E.F.; Shokr, M.S.; Aldosari, A.A.; Majrashi, A.; Kheir, A. Optimizing sowing window, cultivar choice, and plant density to boost maize yield under RCP8. 5 climate scenario of CMIP5. Int. J. Biometeorol. 2022, 66, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Abbas, G.; Fatima, Z.; Iqbal, P.; Raza, M.A.; Kheir, A.; Ahmed, M.; Kakar, K.M.; Ahmad, S. Modelling Climate Uncertainty and Adaptations for Soybean-Based Cropping System. Int. J. Plant Prod. 2022, 16, 235–250. [Google Scholar] [CrossRef]
- Ding, Z.; Ali, E.F.; Elmahdy, A.M.; Ragab, K.E.; Seleiman, M.F.; Kheir, A.M. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021, 244, 106626. [Google Scholar] [CrossRef]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Chang. Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Small heat shock proteins: Structural assembly and functional responses against heat stress in plants. In Plant Metabolites and Regulation under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 367–376. [Google Scholar]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Haider, S.; Raza, A.; Iqbal, J.; Shaukat, M.; Mahmood, T. Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: A brief appraisal. Mol. Biol. Rep. 2022, 1–15. [Google Scholar] [CrossRef]
- Perrella, G.; Bäurle, I.; van Zanten, M. Epigenetic regulation of thermomorphogenesis and heat stress tolerance. New Phytol. 2022, 234, 1144–1160. [Google Scholar] [CrossRef]
- Faizan, M.; Yu, F.; Rajput, V.D.; Minkina, T.; Hayat, S. Role of Brassinosteroids in Protein Folding Under High-Temperature Stress. In Brassinosteroids Signalling; Springer: Berlin/Heidelberg, Germany, 2022; pp. 259–268. [Google Scholar]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.-D. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Thakur, M.P.; van Der Putten, W.H.; Apon, F.; Angelini, E.; Vreš, B.; Geisen, S. Resilience of rhizosphere microbial predators and their prey communities after an extreme heat event. Funct. Ecol. 2021, 35, 216–225. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Malfanova, N.; Kamilova, F.; Berg, G. Plant growth promotion by microbes. Mol. Microb. Ecol. Rhizosphere 2013, 2, 561–573. [Google Scholar]
- Berg, G.; Alavi, M.; Schmidt, C.S.; Zachow, C.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B. Biocontrol and osmoprotection for plants under salinated conditions. Mol. Microb. Ecol. Rhizosphere 2013, 1, 561–573. [Google Scholar]
- Naik, K.; Mishra, S.; Srichandan, H.; Singh, P.K.; Sarangi, P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol. 2019, 21, 101326. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore–A boon to agricultural sciences. Biol. Control. 2020, 144, 104214. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Houghton, J. Global warming. Rep. Prog. Phys. 2005, 68, 1343. [Google Scholar] [CrossRef]
- Harvey, L.D. Global Warming, The Hard Science; Routledge: Oxfordshire, UK, 2018; ISBN 978-0-582-38167-4. [Google Scholar]
- Kerr, R.A. Global warming is changing the world. Science 2007, 316, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.-D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Drake, F. Global Warming, The Science of Climate Change; Routledge: London, UK, 2014; ISBN 0-340-65302-7. [Google Scholar]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.R.; Van Houtan, K.S. The recent normalization of historical marine heat extremes. PLoS Clim. 2022, 1, e0000007. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Aamir, M.; Dubey, M.; Goutam, J.; Upadhyay, R. Beneficial Microbes for Disease Suppression and Plant Growth Promotion. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 395–432. [Google Scholar]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, G.; Yu, Z.; Ding, H.; Xu, Y.; Zhang, Z. Effect of Drought Stress and Developmental Stages on Microbial Community Structure and Diversity in Peanut Rhizosphere Soil. Int. J. Mol. Sci. 2019, 20, 2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapera, T.; Akhtar, S.; Ahmad, Z.; Ejah, w.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, A.; Labuschagne, M.; Rizwan Khan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018, 40, 80. [Google Scholar]
- Curá, J.A.; Franz, D.R.; Filosofía, J.E.; Balestrasse, K.B.; Burgueño, L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. Bacteria Increases the Tolerance of Maize to Drought Stress. Microorganisms 2017, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.B.; Li, C. Genetic Improvement of Heat Stress Tolerance in Cereal Crops. Agronomy 2022, 12, 1205. [Google Scholar] [CrossRef]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Bednarek, P.; Osbourn, A. Plant-microbe interactions: Chemical diversity in plant defense. Science 2009, 324, 746–748. [Google Scholar] [CrossRef]
- Thomas, A.S.S.; Pongprayoon, W.; Cheenkachorn, K.; Sriariyanun, M. Plant-Microbe Interactions-Insights and Views for Applications in Sustainable Agriculture. Appl. Sci. Eng. Prog. 2022. [Google Scholar] [CrossRef]
- Postgate, J.R. The Fundamentals of Nitrogen Fixation; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Peoples, M.B.; Herridge, D.F.; Ladha, J.K. Biological nitrogen fixation: An efficient source of nitrogen for sustainable agricultural production? In Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems; Springer: Berlin/Heidelberg, Germany, 1995; pp. 3–28. [Google Scholar]
- Allahverdiyeva, Y.; Isojärvi, J.; Zhang, P.; Aro, E.-M. Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life 2015, 5, 716–743. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E. Nitrogen Fixation in Tropical Cropping Systems; Cabi: Wallingford, UK, 2001. [Google Scholar]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. New Perspect. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Rahou, Y.A. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Shekhawat, K.; Almeida-Trapp, M.; García-Ramírez, G.X.; Hirt, H. Beat the heat: Plant-and microbe-mediated strategies for crop thermotolerance. Trends Plant Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Khan, M.A.; Imran, M.; Park, Y.; Wani, S.H.; Lee, I.-J. Research progress in the field of microbial mitigation for drought stress in plants. Front. Plant Sci. 2022, 13, 870626. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; Ali, F.; Rafique, M.; Ali, J.; Afridi, M.S.; Smith, D.; Mehmood, S.; Souleimanov, A.; Jellani, G.; Sultan, T. Biochemical Characterization and Potential of Bacillus safensis Strain SCAL1 to Mitigate Heat Stress in Solanum lycopersicum L. J. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- Jia, H.; Xi, Z.; Ma, J.; Li, Y.; Hao, C.; Lu, M.; Zhang, Z.-Z.; Deng, W.-W. Endophytic bacteria from the leaves of two types of albino tea plants, indicating the plant growth promoting properties. Plant Growth Regul. 2022, 96, 331–343. [Google Scholar] [CrossRef]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022, 38, 79. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [Green Version]
- Meena, H.; Ahmed, M.A.; Prakash, P. Amelioration of heat stress in wheat, Triticum aestivum by PGPR (Pseudomonas aeruginosa strain 2CpS1). Biosci. Biotechnol. Res. 2015, 8, 171–174. [Google Scholar]
- Bisht, N.; Mishra, S.K.; Chauhan, P.S. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 2020, 143, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.; Reddy, M.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; El-Keblawy, A.; Ali, D.F.I.; Ibrahim, H.M.; El-Sheikh, M.A.; Sharma, A.; Alhaj Hamoud, Y.; Shaghaleh, H.; Brestic, M.; Skalicky, M. Arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria enhance soil key enzymes, plant growth, seed yield, and qualitative attributes of guar. Agriculture 2021, 11, 194. [Google Scholar] [CrossRef]
- Issa, A.; Esmaeel, Q.; Sanchez, L.; Courteaux, B.; Guise, J.-F.; Gibon, Y.; Ballias, P.; Clément, C.; Jacquard, C.; Vaillant-Gaveau, N. Impacts of Paraburkholderia phytofirmans strain PsJN on tomato (Lycopersicon esculentum L.) under high temperature. Front. Plant Sci. 2018, 9, 1397. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.-E.; Park, Y.-G.; Kim, A.-Y.; Khan, M.A.; You, Y.-H.; Lee, I.-J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Chauhan, R.; Walia, A.; Sharma, G.; Datt, N. Beneficial microbes in agriculture under abiotic stress conditions: An overview. Pharma Innov. 2021, 10, 360–368. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil 2014, 379, 337–350. [Google Scholar] [CrossRef]
- Maitra, S.; Pramanick, B.; Dey, P.; Bhadra, P.; Shankar, T.; Anand, K. Thermotolerant Soil Microbes and Their Role in Mitigation of Heat Stress in Plants. In Soil Microbiomes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2021; pp. 203–242. [Google Scholar]
- Mitra, D.; Rodríguez, A.M.D.; Cota, F.I.P.; Khoshru, B.; Panneerselvam, P.; Moradi, S.; Sagarika, M.S.; Anđelković, S.; de los Santos-Villalobos, S.; Mohapatra, P.K.D. Amelioration of thermal stress in crops by plant growth-promoting rhizobacteria. Physiol. Mol. Plant Pathol. 2021, 115, 101679. [Google Scholar] [CrossRef]
- Evans, J.R. Improving photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef] [Green Version]
- Law, R.D.; Crafts-Brandner, S.J. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1999, 120, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Sakoda, K.; Fukayama, H.; Kondo, E.; Suzuki, Y.; Makino, A.; Terashima, I.; Yamori, W. Overexpression of both Rubisco and Rubisco activase rescues rice photosynthesis and biomass under heat stress. Plant Cell Environ. 2021, 44, 2308–2320. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- As, W.; Galani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Aliyari Rad, S.; Dehghanian, Z.; Asgari Lajayer, B.; Nobaharan, K.; Astatkie, T. Mitochondrial Respiration and Energy Production Under Some Abiotic Stresses. J. Plant Growth Regul. 2021, 1–15. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Fan, Y.; Posch, B.C.; Garcia, A.; Coast, O.; Atkin, O.K. Responses of leaf respiration to heatwaves. Plant Cell Environ. 2021, 44, 2090–2101. [Google Scholar] [CrossRef]
- Jagadish, S.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of heat stress on germination and growth in higher plants: Physiological, biochemical and molecular repercussions and mechanisms of defence. J. Biol. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Schöffl, F.; Prandl, R.; Reindl, A. Molecular responses to heat stress. Mol. Responses Cold Drought Heat Salt Stress High. Plants 1999, 83, 93. [Google Scholar]
- Zhu, L.; Li, H.; Thorpe, M.R.; Hocart, C.H.; Song, X. Stomatal and mesophyll conductance are dominant limitations to photosynthesis in response to heat stress during severe drought in a temperate and a tropical tree species. Trees 2021, 35, 1613–1626. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 324. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Crosstalk between abscisic acid and nitric oxide under heat stress: Exploring new vantage points. Plant Cell Rep. 2021, 40, 1429–1450. [Google Scholar] [CrossRef]
- Li, X.; Palta, J.A.; Liu, F. Modulation of Stomatal Response by Elevated CO2 in Plants Under Drought and Heat Stress. Front. Plant Sci. 2022, 13, 843999. [Google Scholar] [CrossRef]
- Kumar, M.; Kour, D.; Yadav, A.N.; Saxena, R.; Rai, P.K.; Jyoti, A.; Tomar, R.S. Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biologia 2019, 74, 287–308. [Google Scholar] [CrossRef]
- Mylona, P.; Pawlowski, K.; Bisseling, T. Symbiotic nitrogen fixation. Plant Cell 1995, 7, 869. [Google Scholar] [CrossRef]
- de Freitas, V.F.; Cerezini, P.; Hungria, M.; Nogueira, M.A. Strategies to deal with drought-stress in biological nitrogen fixation in soybean. Appl. Soil Ecol. 2022, 172, 104352. [Google Scholar] [CrossRef]
- Moynihan, M.A.; Goodkin, N.F.; Morgan, K.M.; Kho, P.Y.; Lopes dos Santos, A.; Lauro, F.M.; Baker, D.M.; Martin, P. Coral-associated nitrogen fixation rates and diazotrophic diversity on a nutrient-replete equatorial reef. ISME J. 2022, 16, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Schmidt, M.A.; Hynes, M.F. Molecular biology in the improvement of biological nitrogen fixation by rhizobia and extending the scope to cereals. Microorganisms 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Rädecker, N.; Pogoreutz, C.; Gegner, H.M.; Cárdenas, A.; Perna, G.; Geißler, L.; Roth, F.; Bougoure, J.; Guagliardo, P.; Struck, U. Heat stress reduces the contribution of diazotrophs to coral holobiont nitrogen cycling. ISME J. 2022, 16, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 2005, 251, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; He, L.-Y.; Chen, Z.-J.; Wang, Q.-Y.; Qian, M.; Sheng, X.-F. Characterization of ACC deaminase-producing endophytic bacteria isolated from copper-tolerant plants and their potential in promoting the growth and copper accumulation of Brassica napus. Chemosphere 2011, 83, 57–62. [Google Scholar] [CrossRef]
- Wong, W.; Tan, S.; Ge, L.; Chen, X.; Yong, J. The importance of phytohormones and microbes in biofertilizers. In Bacterial Metabolites in Sustainable Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2015; pp. 105–158. [Google Scholar]
- Frankenberger, W.T.; Arshad, M. Phytohormones in Soils: Microbial Production and Function; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Hirsch, A.M.; Fang, Y.; Asad, S.; Kapulnik, Y. The role of phytohormones in plant-microbe symbioses. Plant Soil 1997, 194, 171–184. [Google Scholar] [CrossRef]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Zhang, F.; Dashti, N.; Hynes, R.; Smith, D.L. Plant growth-promoting rhizobacteria and soybean [Glycine max (L.) Merr.] growth and physiology at suboptimal root zone temperatures. Ann. Bot. 1997, 79, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Ahmad, J.; Bashir, H.; Qureshi, M. Proteomics of nitrogen fixing nodules under various environmental stresses. Plant Omics 2012, 5, 167–176. [Google Scholar]
- Saha, B.N.; Roy, S.; Rakshit, R. Managing Abiotic Stressed Agriculture through Microbes. In Soil Management For Sustainable Agriculture; Apple Academic Press: Waretown, NJ, USA, 2022; pp. 123–141. [Google Scholar]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Kumar, R.; Min, D.; Krishna Jagadish, S. Genetic and molecular mechanisms underlying root architecture and function under heat stress-A hidden story. Plant Cell Environ. 2022, 45, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Kumar, S.; Bhati, S. Siderophore in Plant Nutritional Management: Role of Endophytic Bacteria. In Endophytes: Mineral Nutrient Management; Springer: Berlin/Heidelberg, Germany, 2021; Volume 3, pp. 315–329. [Google Scholar]
- Notununu, I.; Moleleki, L.; Roopnarain, A.; Adeleke, R. Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: A review. Pedosphere 2022, 32, 90–106. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.; Li, W.; Hu, T.; Wang, Q.; Yin, Y.; Liu, X.; He, S.; Zhang, M.; Liang, Y. Overexpression of SlBBX17 affects plant growth and enhances heat tolerance in tomato. Int. J. Biol. Macromol. 2022, 206, 799–811. [Google Scholar] [CrossRef]
- Prasad, P.; Staggenborg, S.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; American Society of Agronomy, Inc.; Crop Science Society of America, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 2008; Volume 1, pp. 301–355. [Google Scholar]
- Singh, R.P.; Ma, Y.; Shadan, A. Perspective of ACC-deaminase producing bacteria in stress agriculture. J. Biotechnol. 2022, 352, 36–46. [Google Scholar] [CrossRef]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum tuberosum Through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Choi, J.; Roy Choudhury, A.; Park, S.-Y.; Oh, M.-M.; Sa, T. Inoculation of ACC Deaminase-Producing Brevibacterium linens RS16 Enhances Tolerance against Combined UV-B Radiation and Heat Stresses in Rice (Oryza sativa L.). Sustainability 2021, 13, 10013. [Google Scholar] [CrossRef]
- Mukhtar, T.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Ali, S.; Chaudhary, H.J.; Solieman, T.H.; Ibrahim, A.A.; Saad, M.A. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef] [Green Version]
- Shekhawat, K.; Saad, M.M.; Sheikh, A.; Mariappan, K.; Al-Mahmoudi, H.; Abdulhakim, F.; Eida, A.A.; Jalal, R.; Masmoudi, K.; Hirt, H. Root endophyte induced plant thermotolerance by constitutive chromatin modification at heat stress memory gene loci. EMBO Rep. 2021, 22, e51049. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Cosoveanu, A.; Chowdhary, K.; Cabrera, R.; Sharma, S. Role of Phytohormones-Producing Fungal Endophytes in Plant–Microbial Interactions Under Stress. In Endophytes; Springer: Singapore, 2021; pp. 195–223. [Google Scholar]
- Choudhary, D.K.; Kasotia, A.; Jain, S.; Vaishnav, A.; Kumari, S.; Sharma, K.P.; Varma, A. Bacterial-Mediated Tolerance and Resistance to Plants Under Abiotic and Biotic Stresses. J. Plant Growth Regul. 2016, 35, 276–300. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzorn, R.; Crozier, A.; Wheeler, C.; Sandberg, G. Production of gibberellins and indole-3-acetic acid by Rhizobium phaseoli in relation to nodulation of Phaseolus vulgaris roots. Planta 1988, 175, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.; Belford, R.; Tennant, D. Root: Shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil 1990, 121, 89–98. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Ito, T. JMJ histone demethylases balance H3K27me3 and H3K4me3 levels at the HSP21 locus during heat acclimation in Arabidopsis. Biomol. 2021, 11, 852. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, C.; Jiang, L.; Zhang, J.; Ren, Q. Genome-wide identification, classification and expression analysis of the JmjC domain-containing histone demethylase gene family in maize. BMC Genom. 2019, 20, 256. [Google Scholar] [CrossRef]
- Oberkofler, V.; Bäurle, I. Inducible epigenome editing probes for the role of histone H3K4 methylation in Arabidopsis heat stress memory. Plant Physiol. 2022, 189, 703–714. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Matsubara, S.; Yoshimizu, K.; Seki, M.; Hamada, K.; Kamitani, M.; Kurita, Y.; Inagaki, S.; Suzuki, T.; Gan, E.-S. Removal of repressive histone marks creates epigenetic memory of recurring heat in Arabidopsis. BioRxiv 2020. [Google Scholar] [CrossRef]
- He, K.; Cao, X.; Deng, X. Histone methylation in epigenetic regulation and temperature responses. Curr. Opin. Plant Biol. 2021, 61, 102001. [Google Scholar] [CrossRef]
- Zhongming, Z.; Wei, L. How to Beat the Heat: Memory Mechanism Allows Plants to Adapt to Heat Stress; Nara Institute of Science and Technology: Nara, Japan, 2021. [Google Scholar]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Kumar, P.; Verma, V.; Sharma, R.; Bhargava, B.; Irfan, M. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol. Biochem. 2022, 179, 10–24. [Google Scholar] [CrossRef]
- Cellini, A.; Spinelli, F.; Donati, I.; Ryu, C.-M.; Kloepper, J.W. Bacterial volatile compound-based tools for crop management and quality. Trends Plant Sci. 2021, 26, 968–983. [Google Scholar] [CrossRef]
- Gámez-Arcas, S.; Baroja-Fernández, E.; García-Gómez, P.; Muñoz, F.J.; Almagro, G.; Bahaji, A.; Sánchez-López, Á.M.; Pozueta-Romero, J. Action mechanisms of small microbial volatile compounds in plants. J. Exp. Bot. 2022, 73, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Mburu, S.; Koskey, G.; Njeru, E.; Maingi, J. Revitalization of bacterial endophytes and rhizobacteria for nutrients bioavailability in degraded soils to promote crop production. AIMS Agric. Food 2021, 6, 496–524. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 2020, 15, e0232228. [Google Scholar] [CrossRef]
- Onwe, R.O.; Onwosi, C.O.; Ezugworie, F.N.; Ekwealor, C.C.; Okonkwo, C.C. Microbial trehalose boosts the ecological fitness of biocontrol agents, the viability of probiotics during long-term storage and plants tolerance to environmental-driven abiotic stress. Sci. Total Environ. 2022, 806, 150432. [Google Scholar] [CrossRef]
- Elnahal, A.S.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]

| References Country Year | Microbes | Model | Plant | Parameters | MOA | Stress | Effect |
|---|---|---|---|---|---|---|---|
| Park et al. [47] 2021 | Enterobacter SA187 | Vitro Experimental field | Arabidopsis thaliana, Wheat plant | ↑ Biomass, ↑ 10–14% height,↑ 40% grain yield and seed weight 12%. | Chromatin modification | Long term | Beneficial |
| Meena et al. [48] 2018 | Septoglomus deserticola and Septoglomus constrictu | In vitro | Solanum lycopersicum | Improved Stomal conductance, water content and leaf water content | ↓ Oxidative stress | Heat +drought | Improved |
| Anli et al. [41] 2011 | Pseudomonas fluorescens, Pantoea agglomerans, Mycobacterium sp., Bacillus amyloliquefaciens, Pantoea agglomerans | Appraisail | Triticum aestivum | HSP90 Antioxidant enzymes, HSTP | Thermoregulation | High temp | Significant |
| Anli et al. [41] 2011 | B. phytofirmans | NA | Solanum tuberosum | ↑ Proline and glycine betaine | Thermotolerance | High temperature | Good biocontrol |
| Bisht et al. [49] 2020 | B. cereus SA1 | Experimental | Soybean | ↑ Chlorophyll a and b ↑ Carotenoid, ↑ Chlorophyll florescence | Thermotolerant | Medium to high temp | Bio fertilizer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaffique, S.; Khan, M.A.; Wani, S.H.; Pande, A.; Imran, M.; Kang, S.-M.; Rahim, W.; Khan, S.A.; Bhatta, D.; Kwon, E.-H.; et al. A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants. Microorganisms 2022, 10, 1286. https://doi.org/10.3390/microorganisms10071286
Shaffique S, Khan MA, Wani SH, Pande A, Imran M, Kang S-M, Rahim W, Khan SA, Bhatta D, Kwon E-H, et al. A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants. Microorganisms. 2022; 10(7):1286. https://doi.org/10.3390/microorganisms10071286
Chicago/Turabian StyleShaffique, Shifa, Muhammad Aaqil Khan, Shabir Hussain Wani, Anjali Pande, Muhammad Imran, Sang-Mo Kang, Waqas Rahim, Sumera Afzal Khan, Dibya Bhatta, Eun-Hae Kwon, and et al. 2022. "A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants" Microorganisms 10, no. 7: 1286. https://doi.org/10.3390/microorganisms10071286
APA StyleShaffique, S., Khan, M. A., Wani, S. H., Pande, A., Imran, M., Kang, S.-M., Rahim, W., Khan, S. A., Bhatta, D., Kwon, E.-H., & Lee, I.-J. (2022). A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants. Microorganisms, 10(7), 1286. https://doi.org/10.3390/microorganisms10071286










