Systematic Review and Meta-Analysis of Global Prevalence of HBsAg and HIV and HCV Antibodies among People Who Inject Drugs and Female Sex Workers
Abstract
1. Introduction
2. Results
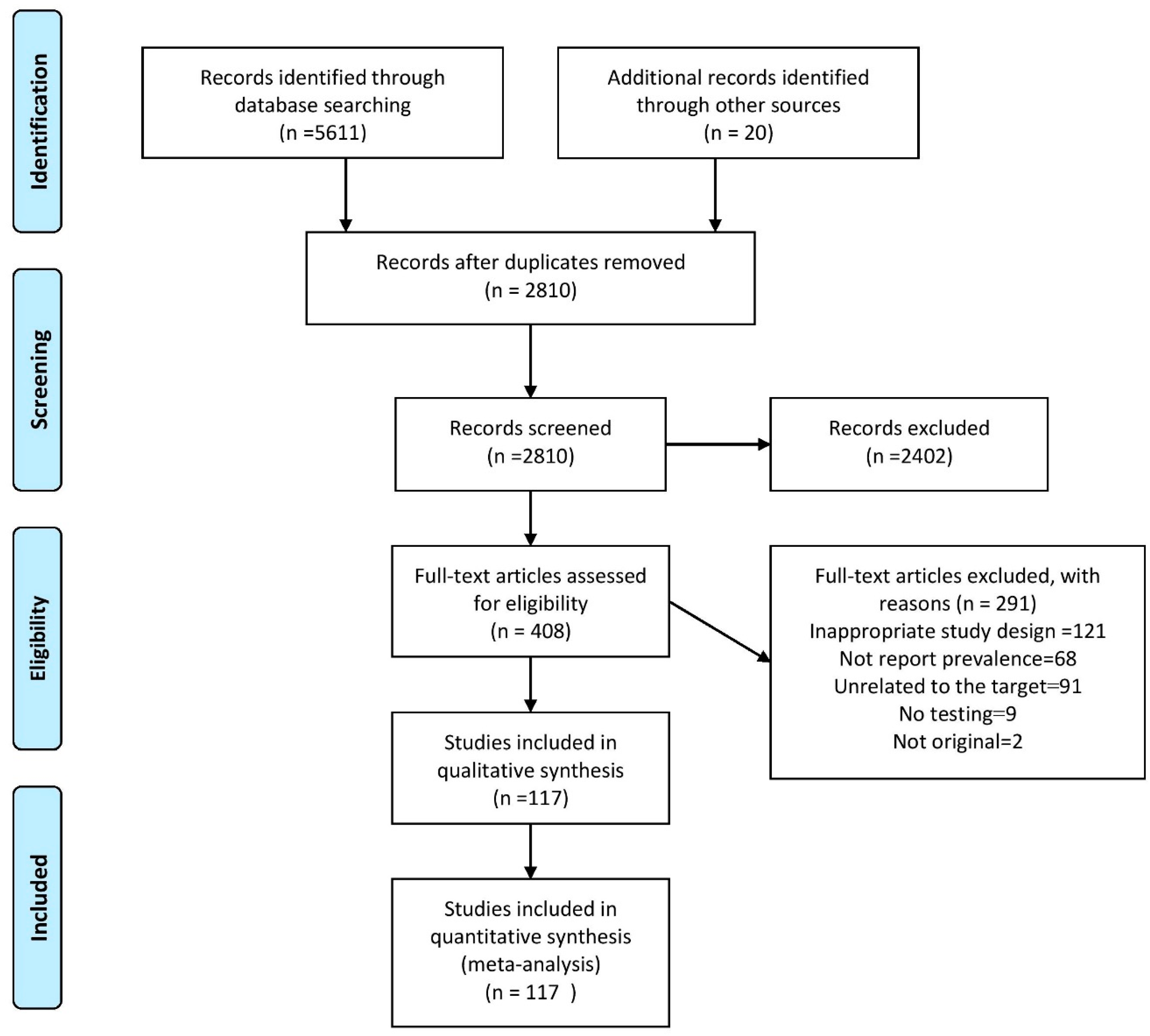
2.1. Search Results
2.2. Reports of Prevalence
2.2.1. Prevalence of HIV among PWID and FSWs
2.2.2. Prevalence of HCV among PWID and FSWs
2.2.3. Prevalence of HBV among PWID and FSWs
2.2.4. Prevalence of Co-infections of HIV, HCV and HBV among PWID and FSWs
2.3. Subgroup Analysis by Regions of WHO
2.4. Meta-Regression
2.5. Publication Bias
3. Discussion
Strength and Limitations
4. Materials and Methods
4.1. Search Strategy
4.2. Study Selection and Data Extraction
4.3. Quality Assessment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. HIV/AIDS. Available online: https://www.who.int/hiv/en/ (accessed on 19 February 2020).
- UNAIDS. 90-90-90: Treatment for all. Available online: https://www.unaids.org/en/resources/909090 (accessed on 19 February 2020).
- WHO. 90-90-90: Treatment for all. Available online: www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (accessed on 19 February 2020).
- Stanaway, J.D.; Flaxman, A.D.; Naghavi, M.; Fitzmaurice, C.; Vos, T.; Abubakar, I.; Abu-Raddad, L.J.; Assadi, R.; Bhala, N.; Cowie, B.; et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet 2016, 388, 1081–1088. [Google Scholar] [CrossRef]
- WHO. Hepatitis B. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b (accessed on 19 February 2020).
- World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Pourkarim, M.R.; Razavi, H.; Lemey, P.; Van Ranst, M. Iran’s hepatitis elimination programme is under threat. Lancet 2018, 392, 1009. [Google Scholar] [CrossRef]
- Thijssen, M.; Lemey, P.; Amini-Bavil-Olyaee, S.; Dellicour, S.; Alavian, S.M.; Tacke, F.; Verslype, C.; Nevens, F.; Pourkarim, M.R. Mass migration to Europe: An opportunity for elimination of hepatitis B virus? Lancet Gastroenterol. Hepatol. 2019, 4, 315–323. [Google Scholar] [CrossRef]
- Hesamizadeh, K.; Sharafi, H.; Rezaee-Zavareh, M.S.; Behnava, B.; Alavian, S.M. Next Steps Toward Eradication of Hepatitis C in the Era of Direct Acting Antivirals. Hepat. Mon. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- WHO. HIV/AIDS. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hiv-aids (accessed on 19 February 2020).
- Degenhardt, L.; Peacock, A.; Colledge, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, e1192–e1207. [Google Scholar] [CrossRef]
- WHO. Hepatitis C. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c (accessed on 19 February 2020).
- UNAIDS. Global HIV & AIDS Statistics—2019 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 19 February 2020).
- UNAIDS. AIDSinfo. Available online: http://aidsinfo.unaids.org/ (accessed on 19 February 2020).
- Medhi, G.K.; Mahanta, J.; Kermode, M.; Paranjape, R.S.; Adhikary, R.; Phukan, S.K.; Ngully, P. Factors associated with history of drug use among female sex workers (FSW) in a high HIV prevalence state of India. BMC Public Health 2012, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Desai Praseeda, S.; Anuradha, D. A Study on the HBV and the HCV Infections in Female Sex Workers and their Co-Infection with HIV. J. Clin. Diagn. Res. 2013, 7, 234–237. [Google Scholar] [CrossRef]
- WHO. Publication. Available online: https://www.unaids.org/en/resources/documents/2019/aids-by-the-numbers (accessed on 19 February 2020).
- Hu, J.; Liu, K.; Luo, J. HIV–HBV and HIV–HCV Coinfection and Liver Cancer Development. In HIV/AIDS-Associated Viral Oncogenesis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 231–250. [Google Scholar]
- Easterbrook, P.; Sands, A.; Harmanci, H. Challenges and Priorities in the Management of Hiv/Hbv and Hiv/Hcv Coinfection in Resource-Limited Settings; Seminars in Liver Disease, Thieme Medical Publishers: New York, NY, USA, 2012; pp. 147–157. [Google Scholar]
- French Network of Pharmacovigilance Centres; Bondon-Guitton, E.; Montastruc, J.L.; Lapeyre-Mestre, M. Influence of HCV or HBV coinfection on adverse drug reactions to antiretroviral drugs in HIV patients. Eur. J. Clin. Pharmacol. 2006, 62, 243–249. [Google Scholar] [CrossRef]
- Toro-Tobón, D.; Berbesi-Fernandez, D.; Mateu-Gelabert, P.; Segura-Cardona, Á.M.; Montoya-Vélez, L.P. Prevalence of hepatitis C virus in young people who inject drugs in four Colombian cities: A cross-sectional study using Respondent Driven Sampling. Int. J. Drug Policy 2018, 60, 56–64. [Google Scholar] [CrossRef]
- Rahman, M.; Hossain, M.E.; Afrad, M.H.; Hasan, R.; Rahman, M.; Sarker, S.; Azim, T. Hepatitis C virus infections among clients attending an HIV testing and counseling center in Dhaka, Bangladesh. J. Med. Virol. 2017, 90, 383–387. [Google Scholar] [CrossRef]
- Puga, M.A.M.; Bandeira, L.M.; Weis, S.M.D.S.; Fernandes, F.R.P.; Castro, L.S.; Tanaka, T.S.O.; De Rezende, G.R.; Teles, S.; Castro, V.D.O.L.D.; Murat, P.G.; et al. High-risk behaviors for hepatitis B and C infections among female sex workers. Rev. Soc. Bras. Med. Trop. 2018, 51, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Peach, E.; Cogger, S.; Byron, K.; Francis, P.; O’Keefe, D.; Higgs, P.; Stoove, M.; Elmore, K.; Dietze, P.; Hellard, M. Blood-borne virus transmission in an urban, culturally diverse neighbourhood: Results from a cross-sectional bio-behavioural survey using innovative outreach methods in a hard-to-reach population. Sex. Health 2018, 15, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.U.; Solomon, S.S.; Mcfall, A.M.; Srikrishnan, A.K.; Pradeep, A.; Nandagopal, P.; Laeyendecker, O.; Tobian, A.A.; Thomas, D.L.; Sulkowski, M.S. Hepatitis C care continuum and associated barriers among people who inject drugs in Chennai, India. Int. J. Drug Policy 2018, 57, 5–60. [Google Scholar] [CrossRef] [PubMed]
- Oyaro, M.; Wylie, J.; Chen, C.-Y.; Ondondo, R.O.; Kramvis, A. Human immunodeficiency virus infection predictors and genetic diversity of hepatitis B virus and hepatitis C virus co-infections among drug users in three major Kenyan cities. South. Afr. J. HIV Med. 2018, 19, 737. [Google Scholar] [CrossRef]
- Jõgeda, E.-L.; Avi, R.; Pauskar, M.; Kallas, E.G.; Karki, T.; Jarlais, N.D.; Uusküla, A.; Toompere, K.; Lutsar, I.; Huik, K. Association of IFNλ4 rs12979860 polymorphism with the acquisition of HCV and HIV infections among people who inject drugs. J. Med. Virol. 2018, 90, 1779–1783. [Google Scholar] [CrossRef]
- Jarlais, D.C.D.; Cooper, H.L.F.; Arasteh, K.; Feelemyer, J.; McKnight, C.; Ross, Z. Potential geographic “hotspots” for drug-injection related transmission of HIV and HCV and for initiation into injecting drug use in New York City, 2011–2015, with implications for the current opioid epidemic in the US. PLoS ONE 2018, 13, e0194799. [Google Scholar] [CrossRef]
- Iakunchykova, O.; Meteliuk, A.; Zelenev, A.; Mazhnaya, A.; Tracy, M.; Altice, F.L. Hepatitis C virus status awareness and test results confirmation among people who inject drugs in Ukraine. Int. J. Drug Policy 2018, 57, 11–17. [Google Scholar] [CrossRef]
- Haussig, J.; Nielsen, S.; Gassowski, M.; Bremer, V.; Marcus, U.; Wenz, B.; Bannert, N.; Bock, C.-T.; Zimmermann, R. A large proportion of people who inject drugs are susceptible to hepatitis B: Results from a bio-behavioural study in eight German cities. Int. J. Infect. Dis. 2018, 66, 5–13. [Google Scholar] [CrossRef]
- Ferreira-Júnior, O.D.C.; Guimarães, M.D.C.; Damacena, G.N.; Almeida, W.D.S.D.; De Souza-Júnior, P.R.B.; Szwarcwald, C.L. Prevalence estimates of HIV, syphilis, hepatitis B and C among female sex workers (FSW) in Brazil, 2016. Medicine 2018, 97, S3–S8. [Google Scholar] [CrossRef]
- Duong, H.T.; Jarlais, D.D.; Khuat, O.H.T.; Arasteh, K.; Feelemyer, J.; Khue, P.M.; Giang, H.T.; Laureillard, D.; Hai, V.V.; Drive Study Group; et al. Risk Behaviors for HIV and HCV Infection Among People Who Inject Drugs in Hai Phong, Viet Nam, 2014. AIDS Behav. 2017, 22, 2161–2171. [Google Scholar] [CrossRef]
- Demissie, M.; Johnston, L.G.; Muleta, M.; Desyebelew, D.; Belete, W.; G/egxiabehre, A.; Gezahegn, N.; Kassa, D.; Aseffa, Y. Prevalence of HIV and other infections and injection behaviours among people who inject drugs in Addis Ababa, Ethiopia. Afr. J. AIDS Res. 2018, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shengelia, N.; Chikovani, I.; Sulaberidze, L. Human immunodeficiency virus prevalence and risk determinants among people who inject drugs in the Republic of Georgia. J. Infect. Dev. Ctries. 2017, 11, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Sharhani, A.; Mehrabi, Y.; Noroozi, A.; Nasirian, M.; Higgs, P.; Hajebi, A.; Hamzeh, B.; Khademi, N.; Noroozi, M.; Shakiba, E. Hepatitis C virus seroprevalence and associated risk factors among male drug injectors in Kermanshah, Iran. Hepat. Mon. 2017, 17. [Google Scholar] [CrossRef]
- Salek, T.P.; Katz, A.R.; Lenze, S.M.; Lusk, H.M.; Li, D.; Jarlais, D.C.D. Seroprevalence of HCV and HIV infection among clients of the nation’s longest-standing statewide syringe exchange program: A cross-sectional study of Community Health Outreach Work to Prevent AIDS (CHOW). Int. J. Drug Policy 2017, 48, 34–43. [Google Scholar] [CrossRef]
- Niama, R.F.; Bongolo, N.C.L.; Mayengue, P.I.; Mboussou, F.F.; Bayonne, E.S.K.; Nzingoula, F.M.K.; Dossou-Yovo, L.R.; Louzolo, I.; Etoka-Beka, M.K.; Lanzy, A.; et al. A study on HIV, Syphilis, and Hepatitis B and C virus infections among female sex workers in the Republic of Congo. Arch. Public Health 2017, 75, 21. [Google Scholar] [CrossRef]
- Neaigus, A.; Reilly, K.H.; Jenness, S.M.; Hagan, H.; Wendel, T.; Gelpi-Acosta, C.; Marshall, D.M. Trends in HIV and HCV Risk Behaviors and Prevalent Infection Among People Who Inject Drugs in New York City, 2005–2012. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, S325–S332. [Google Scholar] [CrossRef]
- Mutagoma, M.; Nyirazinyoye, L.; Sebuhoro, D.; Riedel, D.J.; Ntaganira, J. Syphilis and HIV prevalence and associated factors to their co-infection, hepatitis B and hepatitis C viruses prevalence among female sex workers in Rwanda. BMC Infect. Dis. 2017, 17, 525. [Google Scholar] [CrossRef]
- Mmbaga, E.J.; Moen, K.; Makyao, N.; Leshabari, M. Prevalence and Predictors of Human Immunodeficiency Virus and Selected Sexually Transmitted Infections Among People Who Inject Drugs in Dar es Salaam, Tanzania. Sex. Transm. Dis. 2017, 44, 79–84. [Google Scholar] [CrossRef]
- McFall, A.M.; Solomon, S.S.; Lucas, G.M.; Celentano, D.D.; Srikrishnan, A.K.; Kumar, M.S.; Mehta, S.H. Epidemiology of HIV and hepatitis C infection among women who inject drugs in Northeast India: A respondent-driven sampling study. Addiction 2017, 112, 1480–1487. [Google Scholar] [CrossRef]
- Longo, J.D.D.; Simaleko, M.M.; Diemer, H.S.-C.; Grésenguet, G.; Brücker, G.; Bélec, L. Risk factors for HIV infection among female sex workers in Bangui, Central African Republic. PLoS ONE 2017, 12, e0187654. [Google Scholar] [CrossRef]
- Lambdin, B.H.; Lorvick, J.; Mbwambo, J.K.; Rwegasha, J.; Hassan, S.; Lum, P.; Kral, A.H. Prevalence and predictors of HCV among a cohort of opioid treatment patients in Dar es Salaam, Tanzania. Int. J. Drug Policy 2017, 45, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.; Matiko, E.; Khalid, F.; Welty, S.; Ali, A.; Othman, A.; Haji, S.; Dahoma, M.; Rutherford, G. HIV and hepatitis B and C co-infection among people who inject drugs in Zanzibar. BMC Public Health 2017, 17, 917. [Google Scholar] [CrossRef] [PubMed]
- Kåberg, M.; Hammarberg, A.; Lidman, C.; Weiland, O. Prevalence of hepatitis C and pre-testing awareness of hepatitis C status in 1500 consecutive PWID participants at the Stockholm needle exchange program. Infect. Dis. 2017, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jõgeda, E.-L.; Huik, K.; Pauskar, M.; Kallas, E.G.; Karki, T.; Jarlais, D.D.; Uusküla, A.; Lutsar, I.; Avi, R. Prevalence and genotypes of GBV-C and its associations with HIV infection among persons who inject drugs in Eastern Europe. J. Med. Virol. 2016, 89, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Tran, V.T.; Nguyen, C.H.; Tanimoto, T.; Hoang, H.T.T.; Pham, H.V.; Phan, C.T.T.; Bi, X.; Van Pham, T.; Ichimura, H. Discrepancies in prevalence trends for HIV, hepatitis B virus, and hepatitis C virus in Haiphong, Vietnam from 2007 to 2012. PLoS ONE 2017, 12, e0179616. [Google Scholar] [CrossRef] [PubMed]
- Handanagic, S.; Sevic, S.; Barbaric, J.; Dominkovic, Z.; Rode, O.D.; Begovac, J.; Bozicevic, I. Correlates of anti-hepatitis C positivity and use of harm reduction services among people who inject drugs in two cities in Croatia. Drug Alcohol Depend. 2017, 171, 132–139. [Google Scholar] [CrossRef]
- Gupta, D.; Saha, K.; Biswas, A.; Firdaus, R.; Ghosh, M.; Sadhukhan, P.C. Recombination in hepatitis C virus is not uncommon among people who inject drugs in Kolkata, India. Infect. Genet. Evol. 2017, 48, 156–163. [Google Scholar] [CrossRef]
- De Matos, M.A.; França, D.D.D.S.; Carneiro, M.; Martins, R.M.B.; Kerr, L.; Caetano, K.A.A.; Pinheiro, R.S.; De Araújo, L.A.; Mota, R.M.S.; De Matos, M.A.D.; et al. Viral hepatitis in female sex workers using the Respondent-Driven Sampling. Rev. Saúde Pública 2017, 51, s1518–s8787. [Google Scholar] [CrossRef]
- Iversen, J.; Grebely, J.; Catlett, B.; Cunningham, P.; Dore, G.J.; Maher, L. Estimating the cascade of hepatitis C testing, care and treatment among people who inject drugs in Australia. Int. J. Drug Policy 2017, 47, 77–85. [Google Scholar] [CrossRef]
- Parés-Badell, O.; Espelt, A.; Folch, C.; Roca, X.M.; González, V.; Casabona, J.; Brugal, M.T.; González, M.V. Undiagnosed HIV and Hepatitis C infection in people who inject drugs: From new evidence to better practice. J. Subst. Abuse Treat. 2017, 77, 13–20. [Google Scholar] [CrossRef]
- Wenz, B.; Nielsen, S.; Gassowski, M.; Santos-Hövener, C.; Cai, W.; Roß, S.; Bock, C.-T.; Ratsch, B.-A.; Kücherer, C.; Bannert, N.; et al. High variability of HIV and HCV seroprevalence and risk behaviours among people who inject drugs: Results from a cross-sectional study using respondent-driven sampling in eight German cities (2011-14). BMC Public Health 2016, 16, 927. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.S.; Srikrishnan, A.K.; McFall, A.M.; Kumar, M.S.; Saravanan, S.; Balakrishnan, P.; Solomon, S.; Thomas, D.L.; Sulkowski, M.S.; Mehta, S.H. Burden of Liver Disease among Community-Based People Who Inject Drugs (PWID) in Chennai, India. PLoS ONE 2016, 11, e0147879. [Google Scholar] [CrossRef] [PubMed]
- Skocibusic, S.; Martinac, M.; Arapovic, J.; Grgić, S.; Nikolic, J.; Hasanagic, D.; Bevanda, M.; Ravlija, J. HBV and HCV serological monitoring among injection drugs users in opiate substitution treatment in Bosnia and Herzegovina. J. Infect. Dev. Ctries. 2016, 10, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Gassowski, M.; Wenz, B.; Bannert, N.; Bock, C.-T.; Kücherer, C.; Roß, S.; Bremer, V.; Marcus, U.; Zimmermann, R.; et al. Concordance between self-reported and measured HIV and hepatitis C virus infection status among people who inject drugs in Germany. Hepatol. Med. Policy 2016, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Kermode, M.; Nuken, A.; Medhi, G.K.; Akoijam, B.S.; Sharma, H.U.; Mahanta, J. High burden of hepatitis C & HIV co-infection among people who inject drugs in Manipur, Northeast India. Indian J. Med. Res. 2016, 143, 348–356. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Hsieh, M.-Y.; Huang, C.-F.; Yeh, M.-L.; Wang, S.-C.; Yang, J.-F.; Chang, K.; Lin, W.-R.; Lin, C.-Y.; Chen, T.-C.; et al. Anti-HIV seropositivity was related to HBsAg seropositivity among injecting drug users in Taiwan. Kaohsiung J. Med. Sci. 2016, 32, 96–102. [Google Scholar] [CrossRef]
- Handanagic, S.; Bozicevic, I.; Civljak, M.; Dominkovic, Z.; Sevic, S.; Barbaric, J.; Blazic, T.N.; Rode, O.D.; Begovac, J. HIV and hepatitis C prevalence, and related risk behaviours among people who inject drugs in three cities in Croatia: Findings from respondent-driven sampling surveys. Int. J. Drug Policy 2016, 32, 57–63. [Google Scholar] [CrossRef]
- Fotiou, A.; Kanavou, E.; Antaraki, A.; Richardson, C.; Terzidou, M.; Kokkevi, A. HCV/HIV coinfection among people who inject drugs and enter opioid substitution treatment in Greece: Prevalence and correlates. Hepatol. Med. Policy 2016, 1, 9. [Google Scholar] [CrossRef]
- Folch, C.; Casabona, J.; Espelt, A.; Majó, X.; Meroño, M.; Gonzalez, V.; Wiessing, L.; Colom, J.; Brugal, M.T.; Group, R.S. High prevalence and incidence of HIV and HCV among new injecting drug users with a large proportion of migrants—Is prevention failing? Subst. Use Misuse 2016, 51, 250–260. [Google Scholar] [CrossRef]
- Fernàndez-López, L.; Folch, C.; Majó, X.; Gasulla, L.; Casabona, J. Implementation of rapid HIV and HCV testing within harm reduction programmes for people who inject drugs: A pilot study. AIDS Care 2016, 28, 1–5. [Google Scholar] [CrossRef]
- Jarlais, D.C.D.; Huong, D.T.; Oanh, K.T.H.; Pham, M.K.; Giang, H.T.; Thanh, N.T.T.; Arasteh, K.; Feelemyer, J.; Hammett, T.; Peries, M.; et al. Prospects for ending the HIV epidemic among persons who inject drugs in Haiphong, Vietnam. Int. J. Drug Policy 2016, 32, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bussell, S.A.; Shen, Z.; Tang, Z.; Lan, G.; Zhu, Q.; Liu, W.; Tang, S.; Li, R.; Huang, W. Declining inconsistent condom use but increasing HIV and syphilis prevalence among older male clients of female sex workers: Analysis from sentinel surveillance sites (2010–2015), Guangxi, China. Medicine 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, N.A.; Patel, R.C.; Zibbell, J.E. Improving Screening Methods for Hepatitis C Among People Who Inject Drugs: Findings from the HepTLC Initiative, 2012–2014. Public Health Rep. 2016, 131, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Abadie, R.; Welch-Lazoritz, M.; Gelpi-Acosta, C.; Reyes, J.C.; Dombrowski, K. Understanding differences in HIV/HCV prevalence according to differentiated risk behaviors in a sample of PWID in rural Puerto Rico. Harm Reduct. J. 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Bouscaillou, J.; Evanno, J.; Prouté, M.; Inwoley, A.; Kabran, M.; N’Guessan, T.; Djé-Bi, S.; Sidibé, S.; Thiam-Niangoin, M.; N’Guessan, B.R.; et al. Prevalence and risk factors associated with HIV and tuberculosis in people who use drugs in Abidjan, Ivory Coast. Int. J. Drug Policy 2016, 30, 116–123. [Google Scholar] [CrossRef]
- Tun, W.; Sheehy, M.; Broz, D.; Okal, J.; Muraguri, N.; Raymond, H.F.; Musyoki, H.; Kim, A.A.; Muthui, M.; Geibel, S. HIV and STI Prevalence and Injection Behaviors Among People Who Inject Drugs in Nairobi: Results from a 2011 Bio-behavioral Study Using Respondent-Driven Sampling. AIDS Behav. 2014, 19, 24–35. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, C.; Li, X.; Liu, Y.; Su, S.; Zhou, Y.; Shen, Z. HIV risk among female sex workers with different patterns of drug use behaviors in Southwest China: A cross-sectional study. AIDS Care 2014, 27, 293–300. [Google Scholar] [CrossRef]
- Rosinska, M.; Sieroslawski, J.; Wiessing, L. High regional variability of HIV, HCV and injecting risks among people who inject drugs in Poland: Comparing a cross-sectional bio-behavioural study with case-based surveillance. BMC Infect. Dis. 2015, 15, 83. [Google Scholar] [CrossRef]
- Mwatelah, R.S.; Lwembe, R.M.; Osman, S.; Ogutu, B.R.; Aman, R.; Kitawi, R.C.; Wangai, L.N.; Oloo, F.A.; Kokwaro, G.O.; Ochieng, W. Co-Infection Burden of Hepatitis C Virus and Human Immunodeficiency Virus among Injecting Heroin Users at the Kenyan Coast. PLoS ONE 2015, 10, e0132287. [Google Scholar] [CrossRef]
- Kurth, A.E.; Cleland, C.M.; Jarlais, N.C.D.; Musyoki, H.; Lizcano, J.A.; Chhun, N.; Cherutich, P. HIV Prevalence, Estimated Incidence, and Risk Behaviors Among People Who Inject Drugs in Kenya. JAIDS J. Acquir. Immune Defic. Syndr. 2015, 70, 420–427. [Google Scholar] [CrossRef]
- Jordan, A.E.; Jarlais, N.C.D.; Arasteh, K.; McKnight, C.; Nash, D.; Perlman, D.C. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend. 2015, 152, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Iversen, J.; Dolan, K.; Ezard, N.; Maher, L. HIV and Hepatitis C Virus Infection and Risk Behaviors Among Heterosexual, Bisexual, and Lesbian Women Who Inject Drugs in Australia. LGBT Health 2015, 2, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-G.; Liu, J.-J.; Zhang, Y.-J.; Dai, S.-Y.; Li, M.; Ye, N.-Q. HIV, other sexually transmitted infections, and risk behaviors among female sex workers in Liuzhou, China. Int. J. Gynecol. Obstet. 2014, 128, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Collier, M.; Drobeniuc, J.; Cuevas-Mota, J.; Garfein, R.S.; Kamili, S.; Teshale, E.H. Hepatitis A and B among young persons who inject drugs—Vaccination, past, and present infection. Vaccine 2015, 33, 2808–2812. [Google Scholar] [CrossRef]
- Bugssa, G.; Dessalegn, B.; Dimtsu, B.; Berhane, Y. Prevalence and factors associated with HIV and hepatitis B virus infections among female commercial sex workers in Mekelle, Ethiopia: Cross sectional study. Int. J. Pharm. Sci. Res. 2015, 6, 135. [Google Scholar]
- Al-Tayyib, A.; Thiede, H.; Burt, R.; Koester, S. Unmet Health Care Needs and Hepatitis C Infection Among Persons Who Inject Drugs in Denver and Seattle, 2009. Prev. Sci. 2014, 16, 330–340. [Google Scholar] [CrossRef]
- Zibbell, J.E.; Hart-Malloy, R.; Barry, J.; Fan, L.; Flanigan, C. Risk Factors for HCV Infection Among Young Adults in Rural New York Who Inject Prescription Opioid Analgesics. Am. J. Public Health 2014, 104, 2226–2232. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, S.; Lu, W.; Pan, S.W.; Song, B.; Liu, Q.; Xu, Y.; Dong, H.; Xing, H.; Shao, Y.; et al. HIV, Syphilis, and Behavioral Risk Factors among Female Sex Workers before and after Implementation of Harm Reduction Programs in a High Drug-Using Area of China. PLoS ONE 2014, 9, e84950. [Google Scholar] [CrossRef]
- Wang, L.; Tang, W.; Qian, S.; Li, Y.-G.; Xing, J.; Li, D.; Ding, Z.; Babu, G.R.; Wang, N. The HIV, Syphilis, and HCV Epidemics Among Female Sex Workers in China: Results From a Serial Cross-Sectional Study Between 2008 and 2012. Clin. Infect. Dis. 2014, 59, e1–e9. [Google Scholar] [CrossRef]
- Ruiseñor-Escudero, H.; Wirtz, A.L.; Berry, M.; Mfochive-Njindan, I.; Paikan, F.; Yousufi, H.A.; Yadav, R.S.; Burnham, G.; Vu, A. Risky behavior and correlates of HIV and Hepatitis C Virus infection among people who inject drugs in three cities in Afghanistan. Drug Alcohol Depend. 2014, 143, 127–133. [Google Scholar] [CrossRef]
- Ramezani, A.; Amirmoezi, R.; Volk, J.E.; Aghakhani, A.; Zarinfar, N.; McFarland, W.; Banifazl, M.; Mostafavi, E.; Eslamifar, A.; Sofian, M. HCV, HBV, and HIV seroprevalence, coinfections, and related behaviors among male injection drug users in Arak, Iran. AIDS Care 2014, 26, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Palmateer, N.E.; Taylor, A.; Goldberg, D.J.; Munro, A.; Aitken, C.; Shepherd, S.J.; McAllister, G.; Gunson, R.; Hutchinson, S.J. Rapid Decline in HCV Incidence among People Who Inject Drugs Associated with National Scale-Up in Coverage of a Combination of Harm Reduction Interventions. PLoS ONE 2014, 9, e104515. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Assanangkornchai, S.; Duo, L.; McNeil, E.B.; Li, J. Risk Behaviors, Prevalence of HIV and Hepatitis C Virus Infection and Population Size of Current Injection Drug Users in a China-Myanmar Border City: Results from a Respondent-Driven Sampling Survey in 2012. PLoS ONE 2014, 9, e106899. [Google Scholar] [CrossRef] [PubMed]
- Javadi, A.; Ataei, B.; Kassaian, N.; Nokhodian, Z.; Yaran, M. Co-infection of human immunodeficiency virus, hepatitis C and hepatitis B virus among injection drug users in Drop in centers. J. Res. Med. Sci. 2014, 19, S17–S21. [Google Scholar] [PubMed]
- Hsieh, M.-H.; Tsai, J.-J.; Hsieh, M.-Y.; Huang, C.-F.; Yeh, M.-L.; Yang, J.-F.; Chang, K.; Lin, W.-R.; Lin, C.-Y.; Chen, T.-C.; et al. Hepatitis C Virus Infection among Injection Drug Users with and without Human Immunodeficiency Virus Co-Infection. PLoS ONE 2014, 9, e94791. [Google Scholar] [CrossRef] [PubMed]
- Broz, D.; Wejnert, C.; Pham, H.T.; DiNenno, E.; Heffelfinger, J.D.; Cribbin, M.; Krishna, N.; Teshale, E.H.; Paz-Bailey, G. HIV infection and risk, prevention, and testing behaviors among injecting drug users—National HIV Behavioral Surveillance System, 20 U.S. cities, 2009. MMWR. Surveill. Summ. 2014, 63, 1–51. [Google Scholar] [PubMed]
- Goswami, P.; Medhi, G.K.; Armstrong, G.; Setia, M.S.; Mathew, S.; Thongamba, G.; Ramakrishnan, L.; George, B.; Singh, R.K.; Paranjape, R.S.; et al. An assessment of an HIV prevention intervention among People Who Inject Drugs in the states of Manipur and Nagaland, India. Int. J. Drug Policy 2014, 25, 853–864. [Google Scholar] [CrossRef][Green Version]
- Seaberg, E.C.; Witt, M.D.; Jacobson, L.P.; Detels, R.; Rinaldo, C.R.; Young, S.; Phair, J.P.; Thio, C.L. Differences in hepatitis C virus prevalence and clearance by mode of acquisition among men who have sex with men. J. Viral Hepat. 2013, 21, 696–705. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Zhang, C.; Tan, G.; Stanton, B.; Zhang, X.; Cui, Y. Rates of HIV, syphilis, and HCV infections among different demographic groups of female sex workers in Guangxi China: Evidence from 2010 national sentinel surveillance data. AIDS Care 2013, 25, 1433–1441. [Google Scholar] [CrossRef]
- Valadez, J.J.; Berendes, S.; Jeffery, C.; Thomson, J.; Ben Othman, H.; Danon, L.; Turki, A.A.; Saffialden, R.; Mirzoyan, L. Filling the Knowledge Gap: Measuring HIV Prevalence and Risk Factors among Men Who Have Sex with Men and Female Sex Workers in Tripoli, Libya. PLoS ONE 2013, 8, e66701. [Google Scholar] [CrossRef]
- Taylor, A.; Munro, A.; Allen, E.; Dunleavy, K.; Cameron, S.; Miller, L.; Hickman, M. Low incidence of hepatitis C virus among prisoners in S cotland. Addiction 2013, 108, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Trevisol, D.J.; Custódio, G.; Da Silva, A.C.B.; De Oliveira, M.B.; Wolfart, A.; Trevisol, D.J. HIV, hepatitis B and C, and syphilis prevalence and coinfection among sex workers in Southern Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 493–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salter, M.L.; Lau, B.; Mehta, S.H.; Go, V.F.; Leng, S.; Kirk, G.D. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 64, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, A.A.; Dirgahayu, P.; Sari, Y.; Hudiyono, H.; Kageyama, S. Molecular epidemiology of HIV, HBV, HCV, and HTLV-1/2 in drug abuser inmates in central Javan prisons, Indonesia. J. Infect. Dev. Ctries. 2013, 7, 453–467. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Javadi, A.; Ataei, B.; Yaran, M.; Nokhodian, Z.; Kassaian, N.; Tayeri, K.; Meshkati, M.; Ali, Z. Prevalence of HIV infection and related risk factors in Isfahan Drop in Centers. Pak. J. Med Sci. 2013, 29. [Google Scholar] [CrossRef]
- Huik, K.; Avi, R.; Carrillo, A.; Harper, N.; Pauskar, M.; Sadam, M.; Karki, T.; Krispin, T.; Kongo, U.-K.; Jermilova, T.; et al. CCR5 Haplotypes Influence HCV Serostatus in Caucasian Intravenous Drug Users. PLoS ONE 2013, 8, e70561. [Google Scholar] [CrossRef] [PubMed]
- Hakre, S.; Arteaga, G.; Núñez, A.E.; Bautista, C.T.; Bolen, A.; Villarroel, M.; Peel, S.A.; Paz-Bailey, G.; Scott, P.T.; Pascale, J.M. Prevalence of HIV and other sexually transmitted infections and factors associated with syphilis among female sex workers in Panama. Sex. Transm. Infect. 2012, 89, 156–164. [Google Scholar] [CrossRef]
- Garfein, R.S.; Rondinelli, A.; Barnes, R.F.; Cuevas, J.; Metzner, M.; Velasquez, M.; Rodriguez, D.; Reilly, M.; Xing, J.; Teshale, E.H. HCV infection prevalence lower than expected among 18–40-year-old injection drug users in San Diego, CA. J. Urban Health 2013, 90, 516–528. [Google Scholar] [CrossRef][Green Version]
- Chalana, H.; Singh, H.; Sachdeva, J.K.; Sharma, S. Seroprevalence of human immunodeficiency virus, Hepatitis B surface antigen, and Hepatitis C in substance dependents admitted in a tertiary hospital at Amritsar, India. Asian J. Psychiatry 2013, 6, 552–555. [Google Scholar] [CrossRef]
- Bowring, A.L.; Luhmann, N.; Pont, S.; Debaulieu, C.; Derozier, S.; Asouab, F.; Toufik, A.; Van Gemert, C.; Dietze, P.M.; Stoové, M. An urgent need to scale-up injecting drug harm reduction services in Tanzania: Prevalence of blood-borne viruses among drug users in Temeke District, Dar-es-Salaam, 2011. Int. J. Drug Policy 2013, 24, 78–81. [Google Scholar] [CrossRef]
- Basu, D.; Kumar, V.; Sharma, A.K.; Barnwal, P.K.; Mattoo, S.K. Seroprevalence of anti-hepatitis C virus (anti-HCV) antibody and HCV-related risk in injecting drug users in northern India: Comparison with non-injecting drug users. Asian J. Psychiatry 2013, 6, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Alipour, A.; Haghdoost, A.A.; Sajadi, L.; Zolala, F. HIV prevalence and related risk behaviours among female partners of male injecting drugs users in Iran: Results of a bio-behavioural survey, 2010. Sex. Transm. Infect. 2013, 89, iii41–iii44. [Google Scholar] [CrossRef] [PubMed]
- Kotaki, T.; Khairunisa, S.Q.; Sukartiningrum, S.D.; Arfijanto, M.V.; Utsumi, T.; Normalina, I.; Handajani, R.; Widiyanti, P.; Rusli, M.; Rahayu, R.P.; et al. High Prevalence of HIV-1 CRF01_AE Viruses among Female Commercial Sex Workers Residing in Surabaya, Indonesia. PLoS ONE 2013, 8, e82645. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-A.; Yoon, Y.; Lee, H.J.; Choi, J.; Kwon, M.; Kim, K.; Lee, C.-U.; Kim, D.-J.; Yun, H. Prevalence and associated clinical characteristics of hepatitis B, C, and HIV infections among injecting drug users in Korea. J. Med. Virol. 2013, 85, 575–582. [Google Scholar] [CrossRef]
- Johnston, L.G.; Corceal, S. Unexpectedly High Injection Drug Use, HIV and Hepatitis C Prevalence Among Female Sex Workers in the Republic of Mauritius. AIDS Behav. 2012, 17, 574–584. [Google Scholar] [CrossRef]
- Yen, Y.-F.; Yen, M.-Y.; Su, L.-W.; Li, L.-H.; Chuang, P.; Jiang, X.-R.; Deng, C.-Y. Prevalences and associated risk factors of HCV/HIV co-infection and HCV mono-infection among injecting drug users in a methadone maintenance treatment program in Taipei, Taiwan. BMC Public Health 2012, 12, 1066. [Google Scholar] [CrossRef]
- Sofian, M.; Aghakhani, A.; Banifazl, M.; Azadmanesh, K.; Farazi, A.; McFarland, W.; Eslamifar, A.; Ramezani, A. Viral Hepatitis and HIV Infection Among Injection Drug Users in a Central Iranian City. J. Addict. Med. 2012, 6, 292–296. [Google Scholar] [CrossRef]
- Goldenberg, S.M.; Rangel, G.; Harvey-Vera, A.; Patterson, T.L.; Abramovitz, D.; Silverman, J.G.; Raj, A.; Strathdee, S.A. Exploring the Impact of Underage Sex Work Among Female Sex Workers in Two Mexico–US Border Cities. AIDS Behav. 2012, 16, 969–981. [Google Scholar] [CrossRef]
- Ghosh, I.; Ghosh, P.; Bharti, A.C.; Mandal, R.; Biswas, J.; Basu, P. Prevalence of human papillomavirus and co-existent sexually transmitted infections among female sex workers, men having sex with men and injectable drug abusers from eastern India. Asian Pac. J. Cancer Prev. 2012, 13, 799–802. [Google Scholar] [CrossRef]
- Dunford, L.; Carr, M.; Dean, J.; Waters, A.; Nguyen, L.T.; Thi, T.H.T.; Thi, L.A.B.; Do, H.D.; Thi, T.T.D.; Nguyen, H.T.; et al. Hepatitis C Virus in Vietnam: High Prevalence of Infection in Dialysis and Multi-Transfused Patients Involving Diverse and Novel Virus Variants. PLoS ONE 2012, 7, e41266. [Google Scholar] [CrossRef]
- Dunford, L.; Carr, M.; Dean, J.; Nguyen, L.T.; Thi, T.H.T.; Nguyen, B.T.; Connell, J.; Coughlan, S.; Nguyen, H.T.; Hall, W.W.; et al. A Multicentre Molecular Analysis of Hepatitis B and Blood-Borne Virus Coinfections in Viet Nam. PLoS ONE 2012, 7, e39027. [Google Scholar] [CrossRef]
- Barua, P.; Mahanta, J.; Medhi, G.K.; Dale, J.; Paranjape, R.S.; Thongamba, G. Sexual activity as risk factor for hepatitis C virus (HCV) transmission among the female sex workers in Nagaland. Indian J. Med. Res. 2012, 136, 30. [Google Scholar]
- Wu, N.; Ge, Q.; Feng, Q.; Zhang, J.; Liu, X.; Sun, C.; Xu, Y.; He, G.; Zhang, C. High Prevalence of Hepatitis C virus Among Injection Drug Users in Zhenjiang, Jiangsu, China. Indian J. Virol. 2011, 22, 77–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pilon, R.; Leonard, L.; Kim, J.; Vallée, D.; De Rubeis, E.; Jolly, A.M.; Wylie, J.; Pelude, L.; Sandstrom, P. Transmission Patterns of HIV and Hepatitis C Virus among Networks of People Who Inject Drugs. PLoS ONE 2011, 6, e22245. [Google Scholar] [CrossRef] [PubMed]
- Mir-Nasseri, M.M.; Mohammadkhani, A.; Tavakkoli, H.; Ansari, E.; Poustchi, H. Incarceration is a major risk factor for blood-borne infection among intravenous drug users. Zahedan J. Res. Med. Sci. 2011, 11, 19–22. [Google Scholar]
- Kassak, K.; Mahfoud, Z.; Kreidieh, K.; Shamra, S.; Afifi, R.; Ramia, S. Hepatitis B virus and hepatitis C virus infections among female sex workers and men who have sex with men in Lebanon: Prevalence, risk behaviour and immune status. Sex. Health 2011, 8, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Ataei, B.; Yaran, M.; Babak, A.; Shoaei, P. Hepatitis B and C among women with illegal social behavior in Isfahan, Iran: Seroprevalence and associated factors. Zahedan J. Res. Med. Sci. 2011, 11, 368–371. [Google Scholar]
- Johnston, L.G.; Saumtally, A.; Corceal, S.; Mahadoo, I.; Oodally, F. High HIV and hepatitis C prevalence amongst injecting drug users in Mauritius: Findings from a population size estimation and respondent driven sampling survey. Int. J. Drug Policy 2011, 22, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Yang, C.-L.; Ko, W.-S.; Liu, W.-C.; Lin, C.-Y.; Wu, C.-H.; Su, Y.-C.; Chen, M.-Y.; Sheng, W.-H.; Hung, C.; et al. Molecular Epidemiology of Hepatitis D Virus Infection among Injecting Drug Users with and without Human Immunodeficiency Virus Infection in Taiwan. J. Clin. Microbiol. 2010, 49, 1083–1089. [Google Scholar] [CrossRef]
- Znazen, A.; Frikha-Gargouri, O.; Berrajah, L.; Bellalouna, S.; Hakim, H.; Gueddana, N.; Hammami, A. Sexually transmitted infections among female sex workers in Tunisia: High prevalence of Chlamydia trachomatis. Sex. Transm. Infect. 2010, 86, 500–505. [Google Scholar] [CrossRef]
- Telan, E.F.O.; Samonte, G.M.J.; Abellanosa-Tac-An, I.P.; Alesna, E.T.; Leaño, P.S.A.; Emphasis, Y.E.E.; Tsuneki, A.; Matsumoto, K.; Kageyama, S. The early phase of an HIV epidemic in a population exposed previously to HCV in the Philippines. J. Med. Virol. 2011, 83, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.S.; Nasir, A.; Stanekzai, M.R.; Bautista, C.; Botros, B.A.; Scott, P.T.; Strathdee, S.A.; Tjaden, J. HIV, hepatitis B, and hepatitis C prevalence and associated risk behaviors among female sex workers in three Afghan cities. AIDS 2010, 24, S69–S75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plitt, S.S.; Gratrix, J.; Hewitt, S.; Conroy, P.; Parnell, T.; Lucki, B.; Pilling, V.; Anderson, B.; Choudri, Y.; Archibald, C.P.; et al. Seroprevalence and Correlates of HIV and HCV among Injecting Drug Users in Edmonton, Alberta. Can. J. Public Health 2010, 101, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, Z.; Afifi, R.; Ramia, S.; El Khoury, D.; Kassak, K.; El Barbir, F.; Ghanem, M.; El-Nakib, M.; DeJong, J.; Afifi, R.A. HIV/AIDS among female sex workers, injecting drug users and men who have sex with men in Lebanon: Results of the first biobehavioral surveys. AIDS 2010, 24, S45–S54. [Google Scholar] [CrossRef]
- Iversen, J.; Wand, H.; Gonnermann, A.; Maher, L. Gender differences in hepatitis C antibody prevalence and risk behaviours amongst people who inject drugs in Australia 1998–2008. Int. J. Drug Policy 2010, 21, 471–476. [Google Scholar] [CrossRef]
- Alavi, S.M.; Behdad, F. Seroprevalence Study of Hepatitis C and Hepatitis B Virus among Hospitalized Intravenous Drug Users in Ahvaz, Iran (2002–2006). Zahedan J. Res. Med. Sci. 2010, 10, 101–104. [Google Scholar]
- Mulla, S.A.; Kosambiya, J.K.; Desai, V.K.; Shethwala, N.D. Sexually transmitted infections and reproductive tract infections in female sex workers. Indian J. Pathol. Microbiol. 2009, 52, 198. [Google Scholar] [CrossRef]
- Rehan, N.; Bokhari, A.; Nizamani, N.M.; Jackson, D.; Naqvi, H.R.; Qayyum, K.; Mansoor, S.; Muzaffar, R. National study of reproductive tract infections among high risk groups of Lahore and Karachi. J. Coll. Physicians Surg. Pak. 2009, 19, 228–231. [Google Scholar]
- Mahanta, J.; Borkakoty, B.; Das, H.K.; Chelleng, P.K. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care 2009, 21, 1420–1424. [Google Scholar] [CrossRef]
- Lidman, C.; Nordén, L.; Kåberg, M.; Kall, K.; Franck, J.; Aleman, S.; Birk, M. Hepatitis C infection among injection drug users in Stockholm Sweden: Prevalence and gender. Scand. J. Infect. Dis. 2009, 41, 679–684. [Google Scholar] [CrossRef]
- Dumchev, K.; Soldyshev, R.; Qian, H.-Z.; Zezyulin, O.O.; Chandler, S.D.; Slobodyanyuk, P.; Moroz, L.; Schumacher, J.E. HIV and hepatitis C virus infections among hanka injection drug users in central Ukraine: A cross-sectional survey. Harm. Reduct. J. 2009, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Davoodian, P.; Dadvand, H.; Mahouri, K.; Amoozandeh, A.; Salavati, A. Prevalence of selected sexually and blood-borne infections in Injecting drug abuser inmates of bandar abbas and roodan correction facilities, Iran, 2002. Braz. J. Infect. Dis. 2009, 13, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.-Y.; Chiang, S.-C.; Su, F.-H.; Chang, Y.-Y.; Cheng, S.-H. Prevalence of human immunodeficiency virus and its association with hepatitis B, C, and D virus infections among incarcerated male substance abusers in Taiwan. J. Med. Virol. 2009, 81, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Uusküla, A.; Fischer, K.; Raudne, R.; Kilgi, H.; Krylov, R.; Salminen, M.; Brummer-Korvenkontio, H.; Lawrence, J.S.; Aral, S. A study on HIV and hepatitis C virus among commercial sex workers in Tallinn. Sex. Transm. Infect. 2008, 84, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Tseng, F.-C.; Edlin, B.; Zhang, M.; Kral, A.; Busch, M.P.; Ortiz-Conde, B.A.; Welzel, T.M.; O’Brien, T.R. The inverse relationship between chronic HBV and HCV infections among injection drug users is associated with decades of age and drug use. J. Viral Hepat. 2008, 15, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Sunthornchart, S.; Linkins, R.W.; Natephisarnwanish, V.; Levine, W.C.; Maneesinthu, K.; Lolekha, R.; Tappero, J.W.; Trirat, N.; Muktier, S.; Chancharastong, P.; et al. Prevalence of hepatitis B, tetanus, hepatitis A, human immunodeficiency virus and feasibility of vaccine delivery among injecting drug users in Bangkok, Thailand, 2003–2005. Addiction 2008, 103, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.S.; Srikrishnan, A.K.; Mehta, S.H.; Vasudevan, C.K.; Murugavel, K.G.; Thamburaj, E.; Anand, S.; Kumar, M.S.; Latkin, C.; Solomon, S. High prevalence of HIV, HIV/hepatitis C virus co-infection and risk behaviors among IDUs in Chennai, India: A cause for concern. J. Acquir. Immune Defic. Syndr. 1999 2008, 49, 327. [Google Scholar] [CrossRef]
- Ngo, T.D.; Laeyendecker, O.; Li, C.; Tai, H.; Cui, M.; Lai, S.; Quinn, T.C. Herpes simplex virus type 2 infection among commercial sex workers in Kunming, Yunnan Province, China. Int. J. STD AIDS 2008, 19, 694–697. [Google Scholar] [CrossRef]
- Neaigus, A.; Zhao, M.; Gyarmathy, V.A.; Cisek, L.; Friedman, S.R.; Baxter, R.C. Greater Drug Injecting Risk for HIV, HBV, and HCV Infection in a City Where Syringe Exchange and Pharmacy Syringe Distribution are Illegal. J. Hered. 2008, 85, 309–322. [Google Scholar] [CrossRef][Green Version]
- Kuniholm, M.H.; Aladashvili, M.; Del Rio, C.; Stvilia, K.; Gabelia, N.; Chitale, R.A.; Tsertsvadze, T.; Nelson, K.E. Not All Injection Drug Users Are Created Equal: Heterogeneity of HIV, Hepatitis C Virus, and Hepatitis B Virus Infection in Georgia. Subst. Use Misuse 2008, 43, 1424–1437. [Google Scholar] [CrossRef][Green Version]
- Jindal, N.; Arora, U.; Singh, K. Prevalence of human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus in three groups of populations at high risk of HIV infection in Amritsar (Punjab), Northern India. Jpn. J. Infect. Dis. 2008, 61, 79. [Google Scholar]
- Baumbach, J.; Foster, L.N.; Mueller, M.; Cruz, M.F.; Arbona, S.; Melville, S.; Ramos, R.L.; Strathdee, S.A. Seroprevalence of select bloodborne pathogens and associated risk behaviors among injection drug users in the Paso del Norte region of the United States–Mexico border. Harm Reduct. J. 2008, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.D.; Carter, A.; Jahagirdar, D.; Biehl, M.H.; Douwes-Schultz, D.; Larson, S.L.; Arora, M.; Dwyer-Lindgren, L.; Steuben, K.M.; Abbastabar, H.; et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019, 6, e831–e859. [Google Scholar] [CrossRef]
- Ronen, K.; Sharma, A.; Overbaugh, J. HIV transmission biology: Translation for HIV prevention. AIDS 2015, 29, 2219–2227. [Google Scholar] [CrossRef]
- Jarlais, D.C.D. HIV/STDs and drug use. AIDS/STD Health Promot. Exch. 1997, 2, 1–3. [Google Scholar]
- Armstrong, G.; Humtsoe, C.; Kermode, M. HIV risk behaviours among injecting drug users in Northeast India following scale-up of a targeted HIV prevention programme. BMC Public Health 2011, 11, S9. [Google Scholar] [CrossRef]
- Scheibe, A.; Young, K.; Moses, L.; Basson, R.; Versfeld, A.; Spearman, W.; Sonderup, M.W.; Prabdial-Sing, N.; Manamela, J.; Puren, A.; et al. Understanding hepatitis B, hepatitis C and HIV among people who inject drugs in South Africa: Findings from a three-city cross-sectional survey. Harm Reduct. J. 2019, 16, 28. [Google Scholar] [CrossRef]
- Mumtaz, G.R.; Awad, S.F.; Feizzadeh, A.; Weiss, H.A.; Abu-Raddad, L.J. HIV incidence among people who inject drugs in the Middle East and North Africa: Mathematical modelling analysis. J. Int. AIDS Soc. 2018, 21, e25102. [Google Scholar] [CrossRef]
- Leung, J.; Peacock, A.; Colledge, S.; Grebely, J.; Cunningham, E.B.; Hickman, M.; Vickerman, P.; Stone, J.; Trickey, A.; Dumchev, K.; et al. A Global Meta-analysis of the Prevalence of HIV, Hepatitis C Virus, and Hepatitis B Virus Among People Who Inject Drugs—Do Gender-Based Differences Vary by Country-Level Indicators? J. Infect. Dis. 2019, 220, 78–90. [Google Scholar] [CrossRef]
- Traore, I.T.; Hema, N.M.; Sanon, A.; Some, F.; Ouedraogo, D.; Some, R.; Niessougou, J.; Konate, I.; Mayaud, P.; Van De Perre, P.; et al. HIV risk and behaviour among part-time versus professional FSW: Baseline report of an interventional cohort in Burkina Faso: Table 1. Sex. Transm. Infect. 2016, 92, 550–553. [Google Scholar] [CrossRef]
- Aceijas, C.; Rhodes, T. Global estimates of prevalence of HCV infection among injecting drug users. Int. J. Drug Policy 2007, 18, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.K.; Mathers, B.M.; Cowie, B.; Hagan, H.; Jarlais, N.D.; Horyniak, D.; Degenhardt, L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet 2011, 378, 571–583. [Google Scholar] [CrossRef]
- Han, R.; Zhou, J.; François, C.; Toumi, M. Prevalence of hepatitis C infection among the general population and high-risk groups in the EU/EEA: A systematic review update. BMC Infect. Dis. 2019, 19, 655. [Google Scholar] [CrossRef]
- Mumtaz, G.R.; Weiss, H.A.; Thomas, S.L.; Riome, S.; Setayesh, H.; Riedner, G.; Semini, I.; Tawil, O.; Akala, F.A.; Wilson, D.; et al. HIV among People Who Inject Drugs in the Middle East and North Africa: Systematic Review and Data Synthesis. PLoS Med. 2014, 11, e1001663. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, H.; Alavian, S.M.; Behnava, B.; Pouryasin, A.; Keshvari, M. The Impact of IFNL4 rs12979860 Polymorphism on Spontaneous Clearance of Hepatitis C.; A Case-Control Study. Hepat Mon. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Aalaei-Andabili, S.H.; Behnava, B.; Salimi, S.; Sharafi, H.; Alavian, S.M. Mysterious Linkages Between Hepatitis C Virus Genotypes, Interleukin-28B Genotypes and Viral Clearance- A Meta-Analysis. Hepat Mon. 2014, 14. [Google Scholar] [CrossRef]
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Thio, C.L.; Hawkins, C. Hepatitis B Virus. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- André, F. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine 2000, 18, S20–S22. [Google Scholar] [CrossRef]
- Raven, S.F.; Urbanus, A.; De Gee, A.; Hoebe, C.; Van Steenbergen, J. Predictors of hepatitis B vaccination completion among people who use drugs participating in a national program of targeted vaccination. Vaccine 2018, 36, 5282–5287. [Google Scholar] [CrossRef]
- Larney, S.; Leung, J.; Grebely, J.; Hickman, M.; Vickerman, P.; Peacock, A.; Stone, J.; Trickey, A.; Dumchev, K.; Colledge, S.; et al. Global systematic review and ecological analysis of HIV in people who inject drugs: National population sizes and factors associated with HIV prevalence. Int. J. Drug Policy 2020, 77, 102656. [Google Scholar] [CrossRef]
- Mayaud, P.; McCartney, D.; Mabey, D. 7-Sexually Transmitted Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Content Repository Only!: London, UK, 2020; pp. 52–68. [Google Scholar] [CrossRef]
- Mücke, V.T.; Mücke, M.M.; Peiffer, K.-H.; Weiler, N.; Welzel, T.M.; Sarrazin, C.; Zeuzem, S.; Berger, A.; Vermehren, J. No evidence of hepatitis B virus reactivation in patients with resolved infection treated with direct-acting antivirals for hepatitis C in a large real-world cohort. Aliment. Pharmacol. Ther. 2017, 46, 432–439. [Google Scholar] [CrossRef] [PubMed]
| Authors (Reference) | Year of Publication | Year of Study | Sampling Location | Study Population | Age (Mean or Median) | Method of Sampling | n/N (Prevalence of HCV) | n/N (Prevalence of HBV) | n/N (Prevalence of HIV) | n/N (Prevalence of HCV/HBV) | n/N (Prevalence of HCV/HIV) | n/N (Prevalence of HIV/HBV) | n/N (Prevalence of HCV/HIV/HBV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toro-Tobón, D [21] | 2018 | 2014 | Colombia | PWID | 26 | RDS | 251/918 (27.3) | 47/918 (5.1) | |||||
| Rahman, M [22] | 2018 | 2011 | Bangladesh | PWID | 31.5 | 34/90 (37.8) | |||||||
| Rahman, M [22] | 2018 | 2011 | Bangladesh | FSWs | 31.5 | 0/15 (0) | |||||||
| Puga, M. A. M [23] | 2018 | 2009–2010 | Brazil | FSWs | 25 | RDS | 2/402 (0.5) | ||||||
| Peach, E [24] | 2018 | 2014 | Australia | PWID | 37 | 118/127 (92.9) | 4/127 (3.1) | 5/128 (3.9) | |||||
| Patel, E. U [25] | 2018 | 2015–2016 | India | PWID | 41 | 213/541 (39.4) | 35/541 (6.5) | 86/541 (15.9) | 61/541 (11.3) | ||||
| Oyaro, M [26] | 2018 | 2011–2012 | Kenya | PWID | RDS | 19/120 (15.8) | 13/120 (10.8) | 1/120 (0.8) | 1/120 (0.8) | ||||
| Jõgeda, E. L [27] | 2018 | 2011 | Estonia | PWID | 30 | RDS | 306/345 (88.7) | 172/345 (49.9) | 169/345 (49) | ||||
| Jarlais, D. C. D [28] | 2018 | 2011–2015 | USA | PWID | 40 | 493/796 (61.9) | 57/791 (7.2) | ||||||
| Iakunchykova, O [29] | 2018 | 2014–2015 | Ukraine | PWID | 36 | RDS | 1002/1613 (62.1) | 668/1613 (41.4) | 441/1613 (27.3) | ||||
| Haussig, J. M [30] | 2018 | 2011–2014 | German | PWID | RDS | 1361/2077 (65.5) | 22/2077 (1.1) | 100/2077 (4.8) | 20/2077 (1) | 3/2077 (0.1) | |||
| Ferreira, O. D [31] | 2018 | 2016 | Brazil | FSWs | RDS | 38/4154 (0.9) | 16/4154 (0.4) | 225/4154 (5.4) | 0/4154 (0) | 8/4154 (0.2) | 2/4154 (<0.1) | ||
| Duong, H. T [32] | 2018 | 2014 | Vietnam | PWID | 38 | RDS | 410/603 (68) | 151/603 (25) | |||||
| Demissie, M [33] | 2018 | 2015 | Ethiopia | PWID | 26 | RDS | 8/237 (3.4) | 12/237 (5.1) | 15/237 (6.3) | ||||
| Shengelia, N [34] | 2017 | 2014–2015 | Republic of Georgia | PWID | 39 | RDS | 44/2022 (2.2) | ||||||
| Sharhani, A [35] | 2017 | 2017 | Iran | PWID | 36.7 | snowball | 332/606 (54.8) | ||||||
| Salek, T. P [36] | 2017 | 2012 | USA | PWID (heroin) | 44 | randomly | 64/81 (79) | ||||||
| Salek, T. P [36] | 2017 | 2012 | USA | PWID (other narcotics) | 44 | randomly | 41/58 (70.7) | ||||||
| Niama, F. R [37] | 2017 | 2011–2012 | Republic of Congo | FSWs | 28.31 | RDS | 6/805 (0.7) | 34/805 (4.2) | 60/805 (7.5) | ||||
| Neaigus, A [38] | 2017 | 2005 | USA | PWID | 43 | RDS | 341/500 (68.2) | 90/500 (18) | |||||
| Neaigus, A [38] | 2017 | 2009 | USA | PWID | 41 | RDS | 390/514 (75.9) | 64/514 (12.5) | |||||
| Neaigus, A [38] | 2017 | 2012 | USA | PWID | 45 | RDS | 352/525 (67) | 64/525 (12.2) | |||||
| Mutagoma, M [39] | 2017 | 2015 | Rwanda | FSWs | 26 | Venue-Day-Time | 28/1978 (1.4) | 49/1978 (2.5) | 849/1978 (42.9) | ||||
| Mmbaga, E. J [40] | 2017 | 2015 | Tanzania | PWID | 32 | RDS | 7/610 (1.1) | 94/610 (15.4) | |||||
| McFall, A. M [41] | 2017 | 2014 | India | PWID (women) | 30 | RDS | 177/796 (22.2) | 421/796 (52.9) | 87/796 (10.9) | ||||
| McFall, A. M [41] | 2017 | 2014 | India | PWID (men) | 30 | RDS | 1721/5661 (30.4) | 985/5661 (17.4) | |||||
| Longo, J. D [42] | 2017 | 2013–2014 | Central African Republic | FSWs | 23 | 76/345 (22) | 66/345 (19.1) | 33/345 (9.6) | |||||
| Lambdin, B. H [43] | 2017 | 2011–2013 | Tanzania | PWID | 32 | 359/630 (57) | 187/630 (29.7) | 151/630 (24) | |||||
| Khatib, A [44] | 2017 | 2012 | Tanzania | PWID | 32 | RDS | 128/408 (31.4) | 25/408 (6.1) | 67/593 (16.4) | 13/408 (3.9) | 47/408 (11.5) | 7/408 (1.7) | 6/408 (1.5) |
| Kaberg, M [45] | 2017 | 2013–2014 | Sweden | PWID | 39 | 1139/1386 (82.2) | 29/1386 (2.1) | 93/1386 (6.7) | |||||
| Jõgeda, E. L [46] | 2017 | 2011 | Estonia | PWID | 30 | RDS | 306/345 (88.7) | 18/345 (5.2) | 172/345 (49.9) | ||||
| Ishizaki, A [47] | 2017 | 2007 | Vietnam | PWID | 34.1 | 81/760 (10.7) | 273/760 (35.9) | 8/760 (1.1) | |||||
| Ishizaki, A [47] | 2017 | 2008 | Vietnam | PWID | 32.5 | 34/302 (11.3) | 81/302 (26.8) | 4/302 (1.3) | |||||
| Ishizaki, A [47] | 2017 | 2012 | Vietnam | PWID | 32.2 | 43/389 (11.1) | 72/389 (18.5) | 4/389 (1) | |||||
| Ishizaki, A(47) | 2017 | 2007 | Vietnam | FSWs | 24.8 | 10/91 (11) | 21/91 (23.1) | 1/91 (1.1) | |||||
| Ishizaki, A [47] | 2017 | 2008 | Vietnam | FSWs | 30.1 | 2/63 (3.2) | 13/63 (20.6) | 0/63 (0) | |||||
| Ishizaki, A [47] | 2017 | 2012 | Vietnam | FSWs | 29.8 | 2/51 (3.9) | 5/51 (9.8) | 0/51 (0) | |||||
| Handanagic, S [48] | 2017 | 2014–2015 | Republic of Croatia (Rijeka) | PWID | 34 | RDS | 80/255 (31.4) | ||||||
| Handanagic, S [48] | 2017 | 2014–2015 | Republic of Croatia (Split) | PWID | 37 | RDS | 153/399 (38.3) | ||||||
| Gupta, D [49] | 2017 | 2013–2014 | India | PWID | 80/194 (41.2) | 125/194 (64.4) | 51/194 (26.3) | ||||||
| de Matos, M. A [50] | 2017 | 2009–2010 | Brazil | FSWs | RDS | 3/402 (0.7) | 6/402 (1.5) | ||||||
| Iversen, J [51] | 2017 | Australia | PWID | 40 | 1173/2054 (57.1) | ||||||||
| Pares-Badell, O [52] | 2017 | 2008–2012 | Spain | PWID | 1578/2243 (70.4) | 732/2243 (32.6) | 567/2243 (25.3) | ||||||
| Wenz, B [53] | 2016 | 2011–2014 | Germany | PWID | RDS | 1361/2077 (65.5) | 101/2077 (4.9) | 84/2077 (4) | |||||
| Solomon, S. S [54] | 2016 | 2005 | India | PWID | 39 | 355/998 (35.6) | 71/998 (7.1) | 148/998 (14.8) | 26/998 (2.6) | 103/998 (10.3) | |||
| Skocibusic, S [55] | 2016 | Bosnia and Herzegovina | PWID | randomly | 63/120 (52.5) | 1/120 (0.8) | |||||||
| Nielsen, S [56] | 2016 | 2011–2014 | Germany | PWID | RDS | 854/2077 (41.1) | 100/2077 (4.8) | ||||||
| Kermode, M [57] | 2016 | 2009–2010 | India | PWID | 29.8 | RDS | 607/821 (73.9) | 253/821 (30.8) | 241/821 (29.4) | ||||
| Hsieh, M. H [58] | 2016 | 2008–2010 | Taiwan | PWID | 36.2 | 517/566 (91.3) | 87/566 (15.4) | 301/566 (53.2) | |||||
| Handanagic, S [59] | 2016 | 2014–2015 | Croatia (Zagreb) | PWID | RDS | 55/176 (31.3) | 1/176 (0.6) | ||||||
| Handanagic, S [59] | 2016 | 2014–2015 | Croatia (Rejika) | PWID | RDS | 85/254 (33.5) | 2/254 (0.8) | ||||||
| Handanagic, S [59] | 2016 | 2014–2015 | Croatia (Split) | PWID | RDS | 173/387 (44.7) | 1/390 (0.3) | ||||||
| Fotiou, A [60] | 2016 | 2013 | Greece | PWID | 36 | 447/563 (79.4) | 88/562 (15.7) | 81/541 (15) | |||||
| Folch, C [61] | 2016 | 2010–2011 | Spain | PWID | 548/761 (72) | 253/761 (33.2) | |||||||
| Fernandez-Lopez, L [62] | 2016 | 2011 | Spain | PWID | 35.6 | 35/172 (20.3) | 5/198 (2.5) | ||||||
| Des Jarlais, D. C [63] | 2016 | 2014 | Vietnam | PWID | 37 | RDS | 403/603 (66.8) | 152/603 (25.2) | |||||
| Chen, Yi M. D [64] | 2016 | 2010–2015 | China (high tier) | FSWs | 35 | 12/1832 (0.7) | 3/1832 (0.2) | ||||||
| Chen, Yi M. D [64] | 2016 | 2010–2015 | China (middle tier) | FSWs | 35 | 51/9938 (0.5) | 38/9938 (0.4) | ||||||
| Chen, Yi M. D [64] | 2016 | 2010–2015 | China (low tier) | FSWs | 35 | 79/10,686 (0.7) | 150/10,686 (1.4) | ||||||
| Blackburn, N. A [65] | 2016 | 2012–2014 | USA | PWID | 37 | 3495/15,274 (22.9) | 576/15,274 (3.8) | 166/15,274 (1.1) | |||||
| Abadie, R [66] | 2016 | 2015 | Puerto Rico | PWID | 41.8 | RDS | 19/315 (6) | ||||||
| Bouscaillou, J [67] | 2016 | 2014 | Ivory Coast | PWID | 33.5 | RDS | 4/73 (5.5) | ||||||
| Bouscaillou, J [67] | 2016 | 2014 | Ivory Coast | FSWs | 33.5 | RDS | 9/49 (18.4) | ||||||
| Tun, W [68] | 2015 | 2011 | Kenya | PWID | 31 | RDS | 50/269 (18.6) | ||||||
| Tang, Z. Z [69] | 2015 | 2010 | China | FSWs | 28 | 128/12,622 (1) | 125/12,622 (1) | ||||||
| Rosinska, M [70] | 2015 | 2004–2005 | Poland | PWID | 26 29 | snow-ball | 448/763 (58.7) | 137/763 (18) | 130/763 (17) | ||||
| Mwatelah, R. S [71] | 2015 | Kenya | PWID | 33 | 25/152 (16.4) | 159/186 (85.5) | 24/152 (15.8) | ||||||
| Kurth, A. E [72] | 2015 | 2012 | Kenya (Nairobi) | PWID | 31.71 | RDS | 96/663 (14.5) | ||||||
| Kurth, A. E [72] | 2015 | 2012 | Kenya (Coast region) | PWID | 30.40 | RDS | 230/1122 (20.5) | ||||||
| Jordan, A. E [73] | 2015 | 2006–2013 | USA | PWID | 41.2 | 1047/1535 (68.2) | 183/1535 (11.9) | 159/1535 (10.4) | |||||
| Iversen, J [74] | 2015 | 2004–2013 | Australia | PWID | 34 | 2879/5378 (53.5) | 29/5378 (0.5) | ||||||
| Fan, Y. G [75] | 2015 | 2012–2013 | China | FSWs | 44/622 (7.1) | 7/622 (1.1) | |||||||
| Collier, M. G [76] | 2015 | 2009–2010 | USA | PWID | 26 | 135/519 (26) | 7/519 (1.3) | ||||||
| Bugssa, G [77] | 2015 | 2013 | Ethiopia | FSWs | 32 | randomly | 19/319 (6) | 38/319 (11.9) | |||||
| Al-Tayyib, A. A [78] | 2015 | 2009 | USA (Colorado state) | PWID | RDS | 289/395 (73.2) | 18/395 (4.6) | 15/395 (3.8) | |||||
| Al-Tayyib, A. A [78] | 2015 | 2009 | USA (Washington state) | PWID | RDS | 189/260 (72.7) | 15/260 (5.8) | 11/260 (4.2) | |||||
| Zibbell, J. E [79] | 2014 | 2012 | USA | PWID | 30 | 34/100 (34) | |||||||
| Zhang, L [80] | 2014 | 2004 | China | FSWs | 24 | 2/343 (0.6) | |||||||
| Zhang, L [80] | 2014 | 2010 | China | FSWs | 26 | 6/404 (1.5) | |||||||
| Wang, L [81] | 2014 | 2008 | China | FSWs | randomly | 404/67,296 (0.6) | |||||||
| Wang, L [81] | 2014 | 2009 | China | FSW | randomly | 1328/147,528 (0.9) | 590/147,528 (0.4) | ||||||
| Wang, L [81] | 2014 | 2010 | China | FSWs | randomly | 1596/199,571 (0.8) | 599/199,571 (0.3) | ||||||
| Wang, L [81] | 2014 | 2011 | China | FSWs | randomly | 1434/204,873 (0.7) | 615/204,873 (0.3) | ||||||
| Wang, L [81] | 2014 | 2012 | China | FSWs | randomly | 1662/207,811 (0.8) | 623/207,811 (0.3) | ||||||
| Ruisenor-Escudero, H [82] | 2014 | 2009 | Afghanistan | PWID | 28 | RDS | 221/548 (40.3) | 39/548 (7.1) | 39/548 (7.1) | 37/548 (6.8) | |||
| Ramezani, A [83] | 2014 | 2012 | Iran | PWID | 33.3 | 56/100 (56) | 6/100 (6) | 19/100 (19) | 15/100 (15) | ||||
| Palmateer, N. E [84] | 2014 | 2008–2009 | Scotland | PWID | 33.6 | 1420/2629 (54) | |||||||
| Palmateer, N. E [84] | 2014 | 2010 | Scotland | PWID | 34.6 | 1774/3168 (56) | |||||||
| Palmateer, N. E [84] | 2014 | 2011–2012 | Scotland | PWID | 35.3 | 1142/2154 (53) | |||||||
| Li, L [85] | 2014 | 2012 | China | PWID | 36.2 | RDS | 154/370 (41.6) | 68/370 (18.4) | |||||
| Javadi, A [86] | 2014 | 2008–2009 | Iran | PWID | 35.3 | simple | 6/539 (1.1) | 6/539 (1.1) | |||||
| Hsieh, M. H [87] | 2014 | 2008–2010 | Taiwan | PWID | 36.1 | 513/562 (91.3) | 86/562 (15.3) | 297/562 (52.8) | 78/562 (13.9) | 293/562 (52.1) | 56/562 (10) | ||
| Broz, D [88] | 2014 | 2009 | USA | PWID | RDS | 397/9652 (4.1) | |||||||
| Goswami, P [89] | 2014 | 2008 | India (Manipour) | PWID | RDS | 565/839 (67.3) | 51/839 (6.1) | 233/839 (27.8) | |||||
| Goswami, P [89] | 2014 | 2009 | India (Manipour) | PWID | RDS | 601/860 (69.9) | 92/860 (10.7) | 251/860 (29.2) | |||||
| Goswami, P [89] | 2014 | 2008 | India (Nagaland) | PWID | RDS | 121/821 (14.7) | 46/821 (5.6) | 12/821 (1.5) | |||||
| Goswami, P [89] | 2014 | 2009 | India (Nagaland) | PWID | RDS | 125/829 (15.1) | 67/829 (8.1) | 13/829 (1.6) | |||||
| Seaberg, E. C [90] | 2014 | USA | PWID | 280/652 (42.9) | 43/640 (6.7) | 410/652 (62.9) | 13/640 (2) | 181/652 (27.8) | |||||
| Zhou, Y. J [91] | 2013 | 2010 | China | FSWs | 128/12,622 (1) | 125/12,622 (1) | |||||||
| Valadez, J. J [92] | 2013 | 2010 | Libya | FSWs | RDS | 5/69 (7.2) | 2/69 (2.9) | 7/69 (10.1) | 3/69 (4.3) | 0/69 (0) | |||
| Taylor, A [93] | 2013 | 2010–2011 | Scotland | PWID | 34 | 495/1625 (30.5) | |||||||
| Schuelter-Trevisol, F [94] | 2013 | 2009 | Brazil | FSWs | 28 | 13/147 (8.8) | 13/147 (8.8) | 3/147 (2) | |||||
| Salter, M. L [95] | 2013 | 2013 | USA | PWID | 47 | 1025/1191 (86.1) | 322/1191 (27) | ||||||
| Prasetyo, A. A [96] | 2013 | 2009 | Indonesia | PWID | 32.3 | 47/94 (50) | 1/94 (1.1) | 13/94 (13.8) | 0/94 (0) | 12/94 (12.8) | 0/94 (0) | ||
| Praseeda, S. D [16] | 2013 | 2007–2010 | India | FSWs | 31 | 7/250 (2.8) | 19/250 (7.6) | 105/250 (42.4) | 3/250 (1.2) | 7/250 (2.8) | |||
| Javadi, A [97] | 2013 | 2008–2009 | Iran | PWID | 35.3 | census | 6/539 (1.1) | ||||||
| Huik, K [98] | 2013 | 2006–2007 | Estonia | PWID | 26 | RDS | 281/373 (75.3) | 205/373 (55) | 174/373 (46.6) | ||||
| Hakre, S [99] | 2013 | 2009–2011 | Republic of Panama | FSWs | 29.4 | Venue-Day-Time | 2/999 (0.2) | 6/999 (0.6) | 7/999 (0.7) | ||||
| Garfein, R. S [100] | 2013 | 2009–2010 | USA | PWID | 28 | RDS | 137/510 (26.9) | 21/510 (4.1) | 6/510 (1.2) | ||||
| Chalana, H [101] | 2013 | 2009–2012 | India | PWID | 30.4 | 78/118 (66.1) | 6/118 (5.1) | 18/118 (15.2) | 2/118 (1.7) | 12/118 (10.2) | 2/118 (1.7) | ||
| Bowring, A. L [102] | 2013 | 2011 | Tanzania | PWID | 30 | snowball and targeted | 74/267 (27.7) | 93/267 (34.8) | 45/267 (16.9) | ||||
| Basu, D [103] | 2013 | 2008–2010 | India | PWID | 31.2 | 47/103 (45.6) | 2/103 (1.9) | ||||||
| Alipour, A [104] | 2013 | 2010 | Iran | PWID | 37 | convenience | 86/226 (38.1) | 8/226 (3.5) | 21/226 (9.3) | ||||
| Kotaki, T [105] | 2013 | 2012 | Indonesia | FSWs | 32 | randomly | 1/197 (0.5) | 8/197 (4.1) | 22/197 (11.2) | 0/197 (0) | 0/197 (0) | ||
| Min, J. A [106] | 2013 | 2007–2010 | Korea | PWID | 41.9 | 154/318 (48.4) | 21/318 (6.6) | 0/318 (0) | 13/318 (4.1) | ||||
| Johnston, L. G [107] | 2013 | 2012 | Republic of Mauritius | FSWs | 31 | RDS | 140/295 (47.5) | 0/295 (0) | 97/297 (32.7) | 84/295 (28.5) | |||
| Yen, Y. F [108] | 2012 | 2006–2010 | Taiwan | PWID | 40 | 1318/1447 (91.1) | 194/1447 (13.4) | 190/1447 (13.1) | |||||
| Sofian, M [109] | 2012 | 2009 | Iran | PWID | 30.7 | 91/153 (59.5) | 11/153 (7.2) | 9/153 (5.9) | 9/153 (5.9) | 8/153 (5.2) | 3/153 (2) | 2/153 (1.3) | |
| Goldenberg, S. M [110] | 2012 | 2008–2010 | Mexico | FSWs | 33 | 35/624 (5.6) | |||||||
| Ghosh, I [111] | 2012 | India | FSWs | 0/45 (0) | 7/45 (15.6) | ||||||||
| Ghosh, I [111] | 2012 | India | PWID | 2/58 (3.4) | 2/58 (3.4) | ||||||||
| Dunford, L [112] | 2012 | 2008–2009 | Vietnam | PWID | 45.7 | 556/1000 (55.6) | |||||||
| Dunford, L [113] | 2012 | 2008–2009 | Vietnam | PWID | 174/1000 (17.4) | 49/1000 (4.9) | 44/1000 (4.4) | ||||||
| Barua, P [114] | 2012 | 2006 | India | FSWs | RDS | 41/426 (9.6) | 57/426 (13.4) | 21/426 (4.9) | |||||
| Wu, N [115] | 2011 | 2009 | China | PWID | 115/141 (81.6) | ||||||||
| Pilon, R [116] | 2011 | 2007 | Canada | PWID | chain-referral | 246/407 (60.4) | 41/407 (10.1) | 40/407 (9.8) | |||||
| Mir-Nasseri, M. M [117] | 2011 | 2001–2002 | Iran | PWID | 35.24 | randomly | 359/518 (69.3) | 19/518 (3.7) | 70/452 (15.5) | 16/518 (3.1) | 58/452 (12.8) | 3/452 (0.7) | 3/452 (0.7) |
| Kassak, K [118] | 2011 | 2007–2008 | Lebanon | FSWs | RDS | 0/103 (0) | 0/103 (0) | 0/103 (0) | |||||
| Kassaian, N [119] | 2011 | 2009–2010 | Iran | FSWs | 30.84 | snowball | 9/91 (9.9) | 1/91 (1.1) | |||||
| Johnston, L [120] | 2011 | 2009 | Mauritius | PWID | 31 | RDS | 495/511 (96.9) | 39/511 (7.6) | 230/511 (45) | ||||
| Chang, S. Y [121] | 2011 | 2006–2009 | Taiwan | PWID | 37 | 36/211 (17.1) | |||||||
| Znazen, A [122] | 2011 | 2007 | Tunisia | FSWs | 34 | 2/183 (1.1) | 1/183 (0.5) | 0/183 (0) | |||||
| Telan, E. F. O [123] | 2011 | 2007 | Philippines | PWID | RDS | 219/250 (87.6) | 1/250 (0.4) | ||||||
| Telan, E. F. O [123] | 2011 | 2009 | Philippines | PWID | RDS | 323/341 (94.7) | 2/341 (0.6) | ||||||
| Telan, E. F. O [123] | 2011 | 2010 | Philippines | PWID | RDS | 59/59 (100) | 44/59 (74.6) | ||||||
| Todd, C. S [124] | 2010 | 2006–2008 | Afghanistan | FSWs | 29 | 10/520 (1.9) | 34/520 (6.5) | 1/520 (0.2) | |||||
| Plitt, S. S [125] | 2010 | 2005 | Canada | PWID | 38 | 181/274 (66.1) | 65/272 (23.9) | 62/272 (22.8) | |||||
| Mahfoud, Z [126] | 2010 | 2007–2008 | Lebanon | FSWs | RDS | 0/95 (0) | |||||||
| Mahfoud, Z [126] | 2010 | 2007–2008 | Lebanon | PWID | RDS | 43/81 (53.1) | 2/81 (2.5) | 0/81 (0) | |||||
| Iversen, J [127] | 2010 | 1998–2008 | Australia | PWID | 31 | 8100/15,583 (52) | |||||||
| Alavi, S. M [128] | 2010 | 2002–2006 | Iran | PWID | 24.8 | 103/333 (30.9) | 12/333 (3.6) | 60/333 (18) | |||||
| Shethwala, N. D [129] | 2009 | India | FSWs | 10/300 (3.3) | 35/300 (11.7) | 2/300 (0.7) | |||||||
| Rehan, N [130] | 2009 | 2004 | Pakistan (Karachi) | PWID | RDS | 347/399 (87) | |||||||
| Rehan, N [130] | 2009 | 2004 | Pakistan (Lahore) | PWID | RDS | 348/380 (91.6) | |||||||
| Mahanta, J [131] | 2009 | 2004–2006 | India | PWID | 26 | 190/398 (47.7) | 15/397 (3.8) | 43/398 (10.8) | 8/398 (2) | 34/398 (8.5) | 3/398 (0.8) | ||
| Lidman, C [132] | 2009 | 2004–2006 | Sweden | PWID | 35.6 | 268/310 (86.5) | 8/310 (2.6) | 3/310 (1) | |||||
| Dumchev, K. V [133] | 2009 | 2005 | Ukraine | PWID | 28.9 | snowball | 230/315 (73) | 44/315 (14) | 38/315(12.1) | ||||
| Davoodian, P [134] | 2009 | 2002 | Iran | PWID | 35.4 | randomly | 163/249 (65.5) | 12/249 (4.8) | 38/249 (15.3) | 7/249 (2.8) | 36/249 (14.5) | 3/249 (1.2) | 3/249 (1.2) |
| Chu, F. Y [135] | 2009 | 2005 | Taiwan | PWID | 32.4 | 172/192 (89.6) | 32/192 (16.7) | 49/192 (25.5) | |||||
| Uuskula, A [136] | 2008 | 2005–2006 | Estonia | FSWs | 29.5 | RDS | 15/191 (7.9) | 16/206 (7.8) | 5/185 (2.7) | ||||
| Tseng, F. C [137] | 2008 | 1998–2000 | USA | PWID | 45 | 2092/2296 (91.1) | 73/2296 (3.2) | 273/2296 (11.9) | |||||
| Sunthornchart, S [138] | 2008 | 2003–2005 | Thailand | PWID | 551/1535 (35.9) | ||||||||
| Solomon, S. S [139] | 2008 | 2004–2005 | India | PWID | 35 | convenience | 566/912 (62.1) | 101/912 (11.1) | 271/912 (29.7) | 235/912 (25.8) | 25/912 (2.7) | ||
| Ngo, T. D [140] | 2008 | 2004 | China | FSWs | 26 | 24/310 (7.7) | 12/310 (3.9) | 8/310 (2.6) | |||||
| Neaigus, A [141] | 2008 | 2004–2006 | USA (Newark) | PWID | 32.8 | 169/205 (82.4) | 52/199 (26.1) | ||||||
| Neaigus, A [141] | 2008 | 2004–2006 | USA (NYC) | PWID | 32.8 | 151/282 (53.5) | 15/288 (5.2) | ||||||
| Kuniholm, M. H [142] | 2008 | 1997–1998 | Georgia | PWID | 539/926 (58.2) | 67/926(7.2) | 5/926 (0.5) | 4/926 (0.4) | |||||
| Jindal, N [143] | 2008 | India | PWID | 53/157 (33.8) | 28/157(17.8) | 26/157 (16.6) | 2/157 (1.3) | 11/157 (7) | 2/157 (1.3) | 2/157 (1.3) | |||
| Baumbach, J. P [144] | 2008 | 2005 | USA (Mexico) | PWID | 38.3 | RDS | 194/203 (95.6) | 6/203 (3) | |||||
| Baumbach, J. P [144] | 2008 | 2005 | USA (Texas) | PWID | 42 | RDS | 122/147 (83) | 9/155 (5.8) | |||||
| Baumbach, J. P [144] | 2008 | 2005 | USA (New Mexico) | PWID | 42 | RDS | 76/95 (80) | 1/100 (1) |
| Prevalence | Americas, % (95% CI) | Africa, % (95% CI) | South-East Asia, % (95% CI) | Europe, % (95% CI) | Eastern Mediterranean, % (95% CI) | Western Pacific, % (95% CI) | Total, % (95% CI) |
|---|---|---|---|---|---|---|---|
| PWID | |||||||
| HIV | 10 (7–14) | 24 (16–34) | 22 (16–28) | 12 (6–20) | 8 (3–13) | 11 (2–26) | 15 (12–18) |
| HCV | 64 (51–77) | 38 (10–72) | 54 (43–66) | 59 (53–65) | 60 (46–73) | 75 (68–82) | 60 (55–64) |
| HBV | 3 (1–6) | 5 (2–9) | 9 (7–11) | 3 (1–5) | 5 (4–6) | - | 6 (5–8) |
| HIV/HCV | 9 (3–18) | 16 (11–22) | 17 (11–24) | 16 (16–28) | 8 (4–14) | - | 13 (9–18) |
| HIV/HBV | - | 1 (1–3) | 2 (1–5) | - | 1 (0–2) | - | 2 (1–3) |
| HBV/HCV | - | - | 3 (1–6) | - | 3 (2–5) | - | 3 (1–5) |
| HIV/HCV/HBV | - | 1 (0–2) | 2 (1–4) | - | 1 (0–2) | - | 2 (1–3) |
| FSWs | |||||||
| HIV | 4 (2–9) | 19 (8–34) | 18 (10–27) | - | 0 (0–0) | 1 (0–1) | 5 (4–5) |
| HCV | 1 (0–2) | 9 (0–29) | 3 (0–8) | - | 2 (0–5) | 1 (1–1) | 1 (1–2) |
| HBV | 1 (0–1) | 5 (1–10) | 4 (2–7) | - | 1 (0–6) | - | 3 (1–5) |
| HIV/HCV | 0 (0–0) | 23 (18–27) | 1 (0–6) | - | - | - | 3 (0–9) |
| HIV/HBV | - | 7 (5–10) | 1 (0–2) | - | - | - | 1 (0–3) |
| Study Population | Coefficient | [95% Confidence Interval] | Standard Error | t | p > t | ||
|---|---|---|---|---|---|---|---|
| PWID | HIV | ||||||
| age | 0.27 | −0.12 | 0.67 | 0.19 | 1.39 | 0.17 | |
| Sample size | 0.19 | 0.15 | 0.23 | 0.02 | 9.47 | 0.00 | |
| HCV | |||||||
| age | 0.07 | −0.29 | 0.45 | 0.18 | 0.43 | 0.67 | |
| Sample size | 0.59 | 0.54 | 0.64 | 0.02 | 23.96 | 0.00 | |
| HBV | |||||||
| age | 0.05 | −0.12 | 0.24 | 0.08 | 0.69 | 0.5 | |
| Sample size | 0.06 | 0.04 | 0.09 | 0.01 | 6.26 | 0.00 | |
| HIV/HCV | |||||||
| age | 0.18 | −0.30 | 0.66 | 0.22 | 0.81 | 0.43 | |
| Sample size | 0.16 | 0.12 | 0.20 | 0.02 | 7.76 | 0.00 | |
| HIV/HBV | |||||||
| age | −0.15 | −0.58 | 0.27 | 0.17 | −0.90 | 0.40 | |
| Sample size | 0.02 | 0.00 | 0.04 | 0.01 | 2.02 | 0.06 | |
| HBV/HCV | |||||||
| age | 0.00 | −0.51 | 0.50 | 0.18 | −0.01 | 0.99 | |
| Sample size | 0.04 | 0.00 | 0.07 | 0.01 | 2.75 | 0.02 | |
| HIV/HCV/HBV | |||||||
| age | 0.04 | −0.89 | 0.98 | 0.61 | 0.7 | 0.65 | |
| Sample size | 0.00 | −0.01 | 0.01 | 0.00 | 0.03 | 0.97 | |
| FSWs | HIV | ||||||
| age | −0.38 | −1.16 | 0.38 | 0.35 | −1.10 | 0.29 | |
| Sample size | 0.10 | 0.06 | 0.14 | 0.02 | 5.27 | 0.00 | |
| HCV | |||||||
| age | −0.01 | −0.38 | 0.34 | 0.15 | −0.11 | 0.91 | |
| Sample size | 0.05 | 0.01 | 0.08 | 0.01 | 2.71 | 0.01 | |
| HBV | |||||||
| age | 0.23 | −0.10 | 0.57 | 0.14 | 1.66 | 0.14 | |
| Sample size | 0.05 | 0.02 | 0.08 | 0.01 | 3.65 | 0.00 | |
| HIV/HCV | |||||||
| age | 0.12 | −1.55 | 1.80 | 0.13 | 0.96 | 0.51 | |
| Sample size | 0.06 | −0.02 | 0.16 | 0.03 | 1.73 | 0.13 | |
| HIV/HBV | |||||||
| Sample size | 0.03 | −0.03 | 0.10 | 0.02 | 1.61 | 0.20 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashti, R.; Sharafi, H.; Alavian, S.M.; Moradi, Y.; Mohamadi Bolbanabad, A.; Moradi, G. Systematic Review and Meta-Analysis of Global Prevalence of HBsAg and HIV and HCV Antibodies among People Who Inject Drugs and Female Sex Workers. Pathogens 2020, 9, 432. https://doi.org/10.3390/pathogens9060432
Rashti R, Sharafi H, Alavian SM, Moradi Y, Mohamadi Bolbanabad A, Moradi G. Systematic Review and Meta-Analysis of Global Prevalence of HBsAg and HIV and HCV Antibodies among People Who Inject Drugs and Female Sex Workers. Pathogens. 2020; 9(6):432. https://doi.org/10.3390/pathogens9060432
Chicago/Turabian StyleRashti, Roya, Heidar Sharafi, Seyed Moayed Alavian, Yousef Moradi, Amjad Mohamadi Bolbanabad, and Ghobad Moradi. 2020. "Systematic Review and Meta-Analysis of Global Prevalence of HBsAg and HIV and HCV Antibodies among People Who Inject Drugs and Female Sex Workers" Pathogens 9, no. 6: 432. https://doi.org/10.3390/pathogens9060432
APA StyleRashti, R., Sharafi, H., Alavian, S. M., Moradi, Y., Mohamadi Bolbanabad, A., & Moradi, G. (2020). Systematic Review and Meta-Analysis of Global Prevalence of HBsAg and HIV and HCV Antibodies among People Who Inject Drugs and Female Sex Workers. Pathogens, 9(6), 432. https://doi.org/10.3390/pathogens9060432





