Abstract
Non-typhoidal Salmonella (NTS) infection (salmonellosis) is one of the most prevalent gastrointestinal diseases throughout the world. Human infections caused by Salmonella Newport, Javiana, and Mississippi serotypes have been observed to occur at higher rates on an annual basis in western Tennessee. The reason for the increased rate of NTS infection by these three serotypes in this region is not known. We conducted a case-case analysis to identify potential risk factors associated with the three Salmonella serotypes using FoodNet data, obtained from the Tennessee Department of Health, consisting of 1578 culture-confirmed salmonellosis cases in Tennessee from 2013 through 2015. Among all the exposure variables tested (254 in total), we found contact with pet treats or chews in the seven days prior to illness was the factor that was significantly associated with these serotypes compared to other serotypes (odds ratio adjusted = 3.0 (95% confidence intervals 1.6, 5.5), P < 0.0005). This study highlights the need for further investigation of potential exposures (other than pet treats or chews), including several possible environmental sources of NTS infection in humans.
1. Introduction
Salmonellosis or non-typhoidal Salmonella (NTS) infections are common and significant public health concerns in the USA. The transmission of NTS in humans occurs through the ingestion of contaminated food and environmental exposures [1]. Common food sources of NTS infections include cheese made from unpasteurized milk, raw vegetables, undercooked beef, pork, eggs, and other poultry products [2,3,4,5]. Although rare, there are some reports of transmission of NTS infections via water [6]. In addition to food and water, several outbreaks suggest a strong association of NTS infections with direct or indirect contact with infected animals or their environment [7,8,9]. Many animals are asymptomatic carriers of Salmonella spp. and, thus, may serve as important reservoirs of NTS infections. Reptiles and amphibians are well-known carriers of several Salmonella serotypes, including, S. Newport and S. Javiana [7,10].
Recently, several studies established increased rates of NTS infections, particularly infections by S. Newport, S. Javiana, and S. Mississippi, in southern and southeastern United States each year [6,11,12,13,14]. In Tennessee, these infections have been observed to occur at higher rates in western counties during 2010–2014 [15]. Microbiological findings revealed an increased incidence of S. Newport, S. Javiana, and S. Mississippi in western Tennessee counties compared to other Tennessee counties [15]. Interestingly, the same trend was also reported in Louisiana, according to a report from the Louisiana Office of Public Health (LPH) [16]. Based on the similarity of these findings annually for these serotypes [15,16], we hypothesized that certain exposure signals in the geographical area of S. Newport, S. Javiana, and S. Mississippi clustering might be identified via case-case analysis. A common feature of these three serotypes is that historically they are associated with non-foodborne exposures, such as animals (pets and pests/rodent), birds (domestic or wild/migratory), amphibians, reptiles, and other aquatic organisms, or abiotic environmental components, such as soil, water, and dust [2,17,18,19]. Additionally, most of these infections are pan-susceptible which supports environmental exposure rather than exposure to food animal sources. This study analyzed existing data to assess associations of food, water, animal, and environmental exposures among salmonellosis cases in the state of Tennessee, USA, caused by S. Newport, S. Javiana, and S. Mississippi during 2013–2015.
2. Materials and Methods
2.1. Data Source
The data analyzed were collected from the Foodborne Diseases Active Surveillance Network (FoodNet) and the Tennessee Department of Health (TDH). These data included demographic and exposure data for Tennessee residents who were diagnosed with a culture-confirmed Salmonella infection and interviewed from January 2013 through December 2015.
2.2. Study Design
A case-case analysis was conducted. Case-case comparisons use existing case data as the “control” group. “Cases” were defined as a person diagnosed with a culture-confirmed NTS infection caused by any of the three Salmonella serotypes of interest, S. Newport, S. Javiana, and S. Mississippi, from January 2013 through December 2015 in Tennessee. The comparison group, referred to as the “control” group, was defined as a person diagnosed with culture-confirmed salmonellosis with serotypes other than S. Newport, S. Javiana, and S. Mississippi during the same time frame in Tennessee.
Exposure data were collected by interviews performed using a standard Salmonella questionnaire. In total, 254 exposure variables were included in the standard questionnaire. All patients were asked about food and water sources, person-to-person contact, and exposure to animals in the seven days prior to the onset of illness. Standard food histories included questions about restaurants and consumption of fruits, vegetables, meats, fish and seafood, frozen ready-to-eat foods, and dairy, as well as poultry products. Questions about sources of drinking water and recreational water exposures were routinely asked. Cases were asked about attendance at festivals, concerts, sporting events, reunions, and/or religious gatherings seven days prior to the onset of disease. Animal exposure questions included direct or indirect contact with live animals; and contact with pets, pet foods, manure, and compost.
2.3. Data Cleaning
TDH data were reviewed and cleaned by running range checks on dates, frequencies, text, categorical variables, and continuous variables using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
2.4. Statistical Analysis
To assess potential selection bias, the categorical demographic variables among case and control groups were compared descriptively and with chi-square tests. The continuous variable, age, was categorized following the standard categorization of Salmonella infection used by FoodNet [20]. Logistic regression (PROC LOGISTIC) was performed to determine the association between having one of the three selected serotype infections and various exposures. The crude odds ratio (OR), 95% confidence intervals (CI), and P-values are presented.
To adjust for potential confounding, multivariable logistic regression analyses were performed. The regions were classified as three greater divisions of East, Middle, and West Tennessee. Some exposure variables were created by combining multiple items from the questionnaire. For example, the consumption of “any tomatoes” was constructed by combining “cherry tomatoes,” “grape tomatoes,” “Roma tomatoes,” “other (e.g., beefsteak) tomatoes”, and “sold on vine tomatoes.” Similarly, the consumption of “any cheese” was constructed by combining all the cheese types mentioned in the questionnaire. Likewise, “contact with any animals” was constructed by combining all types of animal exposure information available in the questionnaire. Due to the very large number of exposures in the dataset, only those variables with P ≤ 0.2 were selected for potential inclusion in the model. To identify potential confounders, each demographic variable, such as gender, race, ethnicity, age, and region, was entered into the model with the exposure variable, one at a time. If the variable changed the OR by >10%, it remained in the model. Additional variables were added until the estimate no longer changed by >10%. The adjusted OR controlling for demographic variables, 95% CI, and P-value are presented. To identify those exposures associated with the three selected serotypes, even when adjusted for all other exposures, we created one final multivariable that included all risk factors with P ≤ 0.1 and all demographic variables. All data analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
A total of 2757 (92%) culture-confirmed salmonellosis cases were included and 237 (8%) suspected salmonellosis cases were excluded from the analysis (Figure 1). Demographic data were available for all 2757 culture-confirmed salmonellosis cases. The Salmonella-infected patients were in the age range of <1 year to 97 years. Exposure data were available for 1578 of 2757 culture-confirmed salmonellosis patients. Of the 2757 Salmonella-infected patients, 640 (23%) patients were infected with S. Newport, S. Javiana, or S. Mississippi, and, thus, were considered as a “case”. More cases were female (n = 333, 52%), white (n = 483, 84%), or non-Hispanic (n = 570, 98%) and the most common age group was adults aged 61 years and above (n = 135, 21%) (Table 1). A majority of the cases (n = 342, 53%) were reported from the West Grand Region of Tennessee. Among the cases, the most prevalent Salmonella serotype was S. Newport (n = 299, 47%), followed by S. Javiana (n = 239, 37%), and S. Mississippi (n = 102, 16%) (Table 1).
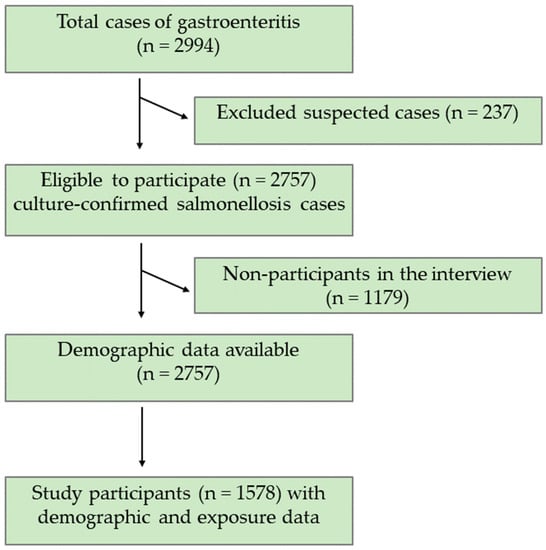
Figure 1.
Consort Diagram of study participants who completed the interview.

Table 1.
Characteristics of the Tennessee salmonellosis cases.
Of the 2757 lab-confirmed Salmonella-infected patients, 1578 (57%) patients had a completed interview that was performed using the standard Salmonella questionnaire (Table 2). The odds for participation were 60% higher in two age groups, (a) below 1 year and (b) 41–60 years, as compared to the participants aged 21–40 years. Likewise, the odds for participation was 50% higher in the age group, 61 years and above. The residents of West Grand Region, as well as the cases, were significantly less likely to participate in the interview (Table 2).

Table 2.
Comparing participants and non-participants that responded to the Salmonella questionnaire.
The demographics of the study participants are described in Table 3. Of the 1578 study participants, 331 (21%) were infected with one of the three Salmonella serotypes, namely, S. Newport, S. Javiana, and S. Mississippi. The majority (n = 157, 47%) of the cases were identified from the West Grand Region. S. Newport was identified as the predominant (n = 170, 51%) Salmonella serotype among the three selected case-associated serotypes (Table 3).

Table 3.
Demographics of the participants in the case-case study.
To identify the exposures associated with the three selected Salmonella serotypes, exposure status was compared between the case and comparison groups. Table 4 presents the crude and adjusted ORs, as well as 95% CI for those exposures with P-value ≤ 0.2. There was a significantly increased risk, adjusted for age, among those who consumed frozen pizza seven days before the onset of illness (OR age-adjusted = 1.4 (95% CI 1.0, 1.9), P = 0.02) and those who consumed powdered-formula baby food (OR age and gender-adjusted = 1.7 (95% CI 1.1, 2.4), P < 0.01) (Table 4). This study showed an increased risk for cases who had contact with dogs (OR = 1.3 (95% CI 1.0, 1.7), P = 0.02). In addition, this study found a significantly elevated odds ratio controlling for the region of residence for the exposure “visit to a farm a week prior to the onset of disease” (OR region-adjusted = 2.2 (95% CI 1.2, 3.7), P < 0.01). Exposure with pet treats or chews seven days before illness was associated with a significantly increased risk while controlling for age, race, and region of residence (OR = 1.7 (95% CI 1.2, 2.3), P < 0.01) (Table 4).

Table 4.
Selected exposures associated with the three Salmonella serotypes, S. Newport, S. Javiana, and S. Mississippi, from Tennessee, USA, from 2013 through 2015.
The final multivariable model identified that exposure to pet treats/chews (adjusted OR = 3.0 (95% CI 1.6, 5.5), P = 0.0005) was significantly associated with the cases (Supplementary Table S1). In this analysis, all other exposures were not found to be significantly associated (data presented in Supplementary Table S1). The consumption of powdered baby formula food and store-bought pureed baby food were risk factors in the previous model (Table 4); however, in the multivariable model (Supplementary Table S1), these exposures were not significantly associated with the cases after adjusting for all demographic and exposure variables.
4. Discussion
In this study, we extensively analyzed the exposure information of more than 250 exposure variables to identify potential exposures associated with S. Newport, S. Javiana, and S. Mississippi in NTS cases in Tennessee. Among all these variables, we found exposures to pet treats or chews as a significant risk factor for all of the case-associated NTS infections. Previous studies [21,22,23,24,25,26] have demonstrated dogs and pet treats as potential risk factors for human NTS infections. Pet foods were reported to be associated with an S. Newport outbreak in humans [26]. In addition to S. Newport, many other Salmonella serotypes, including S. Infantis, S. Typhimurium, and S. Derby, were isolated from contaminated pet treats [22,23,24,26]. Although there are several types of pet treats available commercially, most of them are made from animal body parts, such as pig ears and cow hooves; hence, contamination may occur if the pet treat is prepared from a contaminated animal origin.
There were other exposures that were found to have more than 10% OR changes, even though they did not result in statistical significance in our analysis. For example, the current study revealed that the consumption of frozen pizza and powdered baby formula food as risk factors for NTS infections caused by any of the case-associated serotypes. Frozen pizza has been previously reported to be associated with several foodborne pathogens, including Escherichia coli, Listeria, and Salmonella [27]. The present study identified that the consumption of powdered formula was associated with the cases. Although NTS infection in infants is observed frequently, [28] little is known about risk factors in this high-risk population. Risk factors associated with NTS infections in infants or children are more likely to be different from the other age group population since these risk factors are based on their food sources, eating habits, and the stage of their immune system development [29].
The present study also identified some exposures that could be conceived as the so-called “protective factors” (used commonly in epidemiological studies) for S. Newport, S. Javiana, and S. Mississippi. In this case-case format, such exposures may be important transmission factors for the comparison group. We observed that most of the “protective” exposures for the case group were foodborne exposures. This finding is consistent with previous literature, indicating that many of the serotypes in the comparison group were largely foodborne Salmonella serotypes that were implicated in foodborne outbreak or illnesses [2,3,30]. In this study, the consumption of several types of fruits and vegetables was associated with the comparison group. The consumption of tomato and lettuce was described in the literature as risk factors for S. Newport [31,32] and S. Javiana [33,34,35] infections. In contrast to the previously reported studies, current findings demonstrated that the consumption of raw tomato and lettuce was less likely to be associated with S. Newport, S. Javiana, and S. Mississippi infections. Several studies identified the consumption of cheese [36,37] and eating out at a seafood restaurant [38] as risk factors for S. Javiana infection, whereas the present study contradicts these findings. The contradictory findings in our study do not necessarily mean that tomato, lettuce, and cheese are not potential carriers for S. Newport, S. Javiana, and S. Mississippi infections, given that any of the mentioned food items may carry diverse Salmonella serotypes.
The present study demonstrated that the cases were more likely to have been exposed to animals than the comparison group. This finding is expected since direct or indirect contact with an animal has previously been identified as a risk factor for NTS infections [7,8,9]. The current study identified that visiting a farm a week before the illness was associated with the cases. Several studies support the current study findings since cattle are considered as primary carriers of S. Newport [39,40]. Thus, direct contact with cattle or farm animals, as well as occupational exposure, is considered to be a threat to the transmission of S. Newport infection in humans [20,41]. Thus, touching and handling pet foods pose a risk for human NTS infections. Therefore, appropriate hand hygiene is needed to eliminate the chances of cross-contamination while handling pet foods. In addition, the ingestion of improperly cooked beef contaminated during the slaughtering process can cause foodborne salmonellosis [42] and contact with animal feces may also result in the contamination of fertilizers and, thus, the bacteria can spread to fresh produce [43].
The results of this study should be interpreted, keeping in mind the following limitations. Fundamentally, the comparison group is not a well-controlled group in population-based case-control studies. Both the case and the comparison groups were diagnosed with salmonellosis, but they differed by serotype. The controls were Salmonella-infected patients and they likely did not represent the exposure prevalence in the general population. Additionally, there were significant differences observed in the completion of interviews by region of the state. Lastly, the routine questionnaire used for Salmonella cases was lengthy, and the person answering might have lost interest in answering all the questions. This may lead to a non-differential misclassification bias since the exposure is equally misclassified in both cases and comparison groups. Similarly, both cases and comparison group may equally misclassify the foodborne exposure status in the interview, because, the gap between the onset of disease and the interview may sometimes be more than a week. Therefore, it is sometimes hard to remember what food they have had a week before disease onset, which is approximately two weeks before the interview, unless the same person is either sensitive to a particular food or developed a strong aversion against a food item. Therefore, this may cause information bias, more specifically, a non-differential misclassification in exposure status.
5. Conclusions
The results of the present study suggested that human NTS infections caused by any of the three serotypes, namely, S. Newport, S. Javiana, and S. Mississippi were significantly associated with pet treats or chews in adjusted univariate models. We also found that animal exposures, such as visit to a farm and contact with a dog, had a higher odds ratio of contracting NTS infections. In addition to animal exposures, this study pointed out that very few foodborne exposures were associated with the cases. This study agrees with previous studies which demonstrated that S. Newport, S. Javiana, and S. Mississippi infections are mostly associated with animal or environmental exposures. Educating the public about the modes of transmission of the disease could reduce risks.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/2/78/s1, Table S1: Multivariable analysis presenting adjusted OR controlling for all demographic and exposure variables.
Author Contributions
Conceptualization, N.M., V.G.N., and P.B.; methodology, N.M. and V.G.N.; formal analysis, N.M.; resources, J.R.D. and P.B.; data source, J.R.D.; writing—original draft preparation, N.M.; writing—review and editing, V.G.N. and P.B.; supervision, V.G.N. and P.B.; funding acquisition, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by grants from the US Food & Drug Administration (1U54FD004330-01) and by funds from the FedEx Institute of Technology at the University of Memphis to P.B.
Acknowledgments
The authors thank Ms. Marcy McMillian and Ms. Katie Garman of the Tennessee Department of Health for their help on technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the united states—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- CDC. Multistate outbreak of salmonella serotype typhimurium infections associated with drinking unpasteurized milk—Illinois, Indiana, Ohio, and Tennessee, 2002–2003. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 613. [Google Scholar]
- CDC. Multistate outbreak of salmonella typhimurium infections associated with eating ground beef—United States, 2004. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 180. [Google Scholar]
- CDC. Multistate outbreak of salmonella infections associated with frozen pot pies—United States, 2007. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 1277. [Google Scholar]
- CDC. Outbreak of multidrug-resistant salmonella enterica serotype newport infections associated with consumption of unpasteurized mexican-style aged cheese—Illinois, March 2006–April 2007. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 432–435. [Google Scholar]
- Clarkson, L.S.; Tobin-D’Angelo, M.; Shuler, C.; Hanna, S.; Benson, J.; Voetsch, A.C. Sporadic salmonella enterica serotype javiana infections in georgia and tennessee: A hypothesis-generating study. Epidemiol. Infect. 2010, 138, 340–346. [Google Scholar] [CrossRef]
- Friedman, C.R.; Torigian, C.; Shillam, P.J.; Hoffman, R.E.; Heltzel, D.; Beebe, J.L.; Malcolm, G.; DeWitt, W.E.; Hutwagner, L.; Griffin, P.M. An outbreak of salmonellosis among children attending a reptile exhibit at a zoo. J. Pediatr. 1998, 132, 802–807. [Google Scholar] [CrossRef]
- Hale, C.R.; Scallan, E.; Cronquist, A.B.; Dunn, J.; Smith, K.; Robinson, T.; Lathrop, S.; Tobin-D’Angelo, M.; Clogher, P. Estimates of enteric illness attributable to contact with animals and their environments in the united states. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54 (Suppl. 5), S472–S479. [Google Scholar] [CrossRef]
- Hoelzer, K.; Moreno Switt, A.I.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 34. [Google Scholar] [CrossRef]
- de Jong, B.; Andersson, Y.; Ekdahl, K. Effect of regulation and education on reptile-associated salmonellosis. Emerg. Infect. Dis. 2005, 11, 398–403. [Google Scholar] [CrossRef]
- Boore, A.L.; Hoekstra, R.M.; Iwamoto, M.; Fields, P.I.; Bishop, R.D.; Swerdlow, D.L. Salmonella enterica infections in the united states and assessment of coefficients of variation: A novel approach to identify epidemiologic characteristics of individual serotypes, 1996–2011. PLoS ONE 2015, 10, e0145416. [Google Scholar] [CrossRef] [PubMed]
- Srikantiah, P.; Lay, J.C.; Hand, S.; Crump, J.A.; Campbell, J.; Van Duyne, M.S.; Bishop, R.; Middendor, R.; Currier, M.; Mead, P.S.; et al. Salmonella enterica serotype javiana infections associated with amphibian contact, mississippi, 2001. Epidemiol. Infect. 2004, 132, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N. Source Attribution, Antibiotic Resistance and Virulence Properties of Salmonella Serotypes Isolated from Clinically Disgnosed Human Salmonellosis Cases from Tennessee. Ph.D. Thesis, University of Memphis, Memphis, TN, USA, 2018. [Google Scholar]
- Mukherjee, N.; Nolan, V.G.; Dunn, J.R.; Banerjee, P. Sources of human infection by salmonella enterica serotype javiana: A systematic review. PLoS ONE 2019, 14, e0222108. [Google Scholar] [CrossRef] [PubMed]
- TDH. Interactive dashboard for selected reportable diseases and events. In Communicable and Environmental Diseases and Emergency Preparedness (CEDEP) 2010–2012 Annual Report. Available online: https://www.tn.gov/content/dam/tn/health/documents/cedep-weeklyreports/AnnualReport2010-12.pdf (accessed on 21 January 2020).
- LPH. Salmonella annual report 2018. In Louisiana Office of Public Health—Infectious Disease Epidemiology Section; 2018. Available online: http://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Annuals/Salmonella_LaIDAnnual_2018.docx.pdf (accessed on 21 January 2020).
- Harris, J.R.; Neil, K.P.; Behravesh, C.B.; Sotir, M.J.; Angulo, F.J. Recent multistate outbreaks of human salmonella infections acquired from turtles: A continuing public health challenge. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 50, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Hofacre, C.L.; Lee, M.D.; Maurer, J.J.; Doyle, M.P. Animal sources of salmonellosis in humans. J. Am. Vet. Med. Assoc. 2002, 221, 492–497. [Google Scholar] [CrossRef]
- Stam, F.; Römkens, T.E.H.; Hekker, T.A.M.; Smulders, Y.M. Turtle-associated human salmonellosis. Clin. Infect. Dis. 2003, 37, e167–e169. [Google Scholar] [CrossRef][Green Version]
- Cummings, K.J.; Warnick, L.D.; Davis, M.A.; Eckmann, K.; Gröhn, Y.T.; Hoelzer, K.; MacDonald, K.; Root, T.P.; Siler, J.D.; McGuire, S.M. Farm animal contact as risk factor for transmission of bovine-associated salmonella subtypes. Emerg. Infect. Dis. 2012, 18, 1929. [Google Scholar] [CrossRef]
- Amadi, V.A.; Hariharan, H.; Arya, G.; Matthew-Belmar, V.; Nicholas-Thomas, R.; Pinckney, R.; Sharma, R.; Johnson, R. Serovars and antimicrobial resistance of non-typhoidal salmonella isolated from non-diarrhoeic dogs in grenada, west indies. Vet. Med. Sci. 2018, 4, 26–34. [Google Scholar] [CrossRef]
- Behravesh, C.B.; Ferraro, A.; Deasy, M., 3rd; Dato, V.; Moll, M.; Sandt, C.; Rea, N.K.; Rickert, R.; Marriott, C.; Warren, K.; et al. Human salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics 2010, 126, 477–483. [Google Scholar] [CrossRef]
- Clark, C.; Cunningham, J.; Ahmed, R.; Woodward, D.; Fonseca, K.; Isaacs, S.; Ellis, A.; Anand, C.; Ziebell, K.; Muckle, A.; et al. Characterization of salmonella associated with pig ear dog treats in canada. J. Clin. Microbiol. 2001, 39, 3962–3968. [Google Scholar] [CrossRef]
- Finley, R.; Reid-Smith, R.; Weese, J.S.; Angulo, F.J. Human health implications of salmonella-contaminated natural pet treats and raw pet food. Clin. Infect. Dis. 2006, 42, 686–691. [Google Scholar] [CrossRef] [PubMed]
- KuKanich, K.S. Update on salmonella spp contamination of pet food, treats, and nutritional products and safe feeding recommendations. J. Am. Vet. Med. Assoc. 2011, 238, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Reisbig, M.D.; Mulvey, M.; Chui, L.; Louie, M.; Crowe, L.; Church, D.L.; Elsayed, S.; Gregson, D.; Ahmed, R.; et al. Association between handling of pet treats and infection with salmonella enterica serotype newport expressing the ampc beta-lactamase, cmy-2. J. Clin. Microbiol. 2003, 41, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Cho, S.Y.; Park, B.K.; Chung, D.H.; Oh, D.H. Incidence and characterization of listeria spp. From foods available in korea. J. Food Prot. 2001, 64, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Rushdy, A.A.; Stuart, J.M.; Ward, L.R.; Bruce, J.; Threlfall, E.J.; Punia, P.; Bailey, J.R. National outbreak of salmonella senftenberg associated with infant food. Epidemiol. Infect. 1998, 120, 125–128. [Google Scholar] [CrossRef]
- Sockett, P.N.; Rodgers, F.G. Enteric and foodborne disease in children: A review of the influence of food- and environment-related risk factors. Paediatr. Child Health 2001, 6, 203–209. [Google Scholar] [CrossRef]
- CDC. Outbreak of salmonella serotype saintpaul infections associated with multiple raw produce items—United States, 2008. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 929–934. [Google Scholar]
- Angelo, K.M.; Chu, A.; Anand, M.; Nguyen, T.-A.; Bottichio, L.; Wise, M.; Williams, I.; Seelman, S.; Bell, R.; Fatica, M. Outbreak of salmonella newport infections linked to cucumbers—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 144–147. [Google Scholar]
- Greene, S.K.; Daly, E.R.; Talbot, E.A.; Demma, L.J.; Holzbauer, S.; Patel, N.J.; Hill, T.A.; Walderhaug, M.O.; Hoekstra, R.M.; Lynch, M.F.; et al. Recurrent multistate outbreak of salmonella newport associated with tomatoes from contaminated fields, 2005. Epidemiol. Infect. 2008, 136, 157–165. [Google Scholar] [CrossRef]
- CDC. Outbreaks of salmonella infections associated with eating roma tomatoes United States and Canada, 2004. MMWR Morb. Mortal. Wkly. Rep. 2005, 54, 325–328. [Google Scholar]
- Hedberg, C.W.; Angulo, F.J.; White, K.E.; Langkop, C.W.; Schell, W.L.; Stobierski, M.G.; Schuchat, A.; Besser, J.M.; Dietrich, S.; Helsel, L.; et al. Outbreaks of salmonellosis associated with eating uncooked tomatoes: Implications for public health. The investigation team. Epidemiol. Infect. 1999, 122, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Srikantiah, P.; Bodager, D.; Toth, B.; Kass-Hout, T.; Hammond, R.; Stenzel, S.; Hoekstra, R.M.; Adams, J.; Van Duyne, S.; Mead, P.S. Web-based investigation of multistate salmonellosis outbreak. Emerg. Infect. Dis. 2005, 11, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Alley, R.D.; Pijoan, M. Salmonella javiana food infection. Yale J. Biol. Med. 1942, 15, 229–239. [Google Scholar] [PubMed]
- Hedberg, C.W.; Korlath, J.A.; D’Aoust, J.Y.; White, K.E.; Schell, W.L.; Miller, M.R.; Cameron, D.N.; MacDonald, K.L.; Osterholm, M.T. A multistate outbreak of salmonella javiana and salmonella oranienburg infections due to consumption of contaminated cheese. JAMA 1992, 268, 3203–3207. [Google Scholar] [CrossRef] [PubMed]
- Venkat, H.; Matthews, J.; Lumadao, P.; Caballero, B.; Collins, J.; Fowle, N.; Kellis, M.; Tewell, M.; White, S.; Hassan, R.; et al. Salmonella enterica serotype javiana infections linked to a seafood restaurant in maricopa county, arizona, 2016. J. Food Prot. 2018, 81, 1283–1292. [Google Scholar] [CrossRef]
- Gupta, A.; Fontana, J.; Crowe, C.; Bolstorff, B.; Stout, A.; Van Duyne, S.; Hoekstra, M.P.; Whichard, J.M.; Barrett, T.J.; Angulo, F.J. Emergence of multidrug-resistant salmonella enterica serotype newport infections resistant to expanded-spectrum cephalosporins in the united states. J. Infect. Dis. 2003, 188, 1707–1716. [Google Scholar] [CrossRef]
- Karon, A.E.; Archer, J.R.; Sotir, M.J.; Monson, T.A.; Kazmierczak, J.J. Human multidrug-resistant salmonella newport infections, wisconsin, 2003–2005. Emerg. Infect. Dis. 2007, 13, 1777–1780. [Google Scholar] [CrossRef]
- Su, C.-P.; de Perio, M.A.; Fagan, K.; Smith, M.L.; Salehi, E.; Levine, S.; Gruszynski, K.; Luckhaupt, S.E. Occupational distribution of campylobacteriosis and salmonellosis cases—maryland, ohio, and virginia, 2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 850. [Google Scholar] [CrossRef]
- Wells, S.J.; Fedorka-Cray, P.J.; Dargatz, D.A.; Ferris, K.; Green, A. Fecal shedding of salmonella spp. By dairy cows on farm and at cull cow markets. J. Food Prot. 2001, 64, 3–11. [Google Scholar] [CrossRef]
- Hanning, I.B.; Nutt, J.D.; Ricke, S.C. Salmonellosis outbreaks in the united states due to fresh produce: Sources and potential intervention measures. Foodborne Pathog. Dis. 2009, 6, 635–648. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).