Abstract
Metarhizium (M.) granulomatis and M. viride have previously been described as pathogens causing hyalohyphomycosis in various species of captive chameleons and bearded dragons (Pogona vitticeps). Previous studies yielded different genotypes of M. granulomatis and M. viride based on sequencing of the internal transcribed spacer 1-5.8S rDNA (ITS-1-5.8S) and a fragment of the large subunit of the 28S rDNA (LSU). The aim of this study was to clarify the relationships between these genotypes and obtain a more accurate phylogenetic classification by sequencing two different loci of the RNA polymerase II second largest subunit (NRPB2), referred to as RPB1 and RPB2, and the translation elongation factor 1 alpha (EF1α). A total of 23 frozen isolates from 21 lizards, including the first isolates of M. granulomatis and M. viride from Parson’s chameleons (Calumma parsonii), were available for phylogenetic analysis. A total of 13 isolates belonged to the M. granulomatis complex and 10 isolates belonged to the M. viride complex. Following the amplification and sequencing of the protein-coding genes, the resulting nucleotide sequences were analyzed, trimmed and assembled. These were further analyzed with regard to differences in single-nucleotide polymorphisms (SNPs) and amino acid structure. In consideration of the results of the present analyses, a phylogenetic reclassification is recommended. Three different genotypes of M. granulomatis can be distinguished, which can be phylogenetically addressed as subspecies. Six subspecies can be distinguished regarding M. viride.
1. Introduction
The importance of fungi within the family Clavicipitaceae, specifically the genus Metarhizium (M.), is constantly increasing. In regard to reptile medicine, diseases caused by Metarhizium have so far been described in several species of lizards, turtles, and tortoises, as well as in crocodiles [,,]. A pivotal element in the pathogenesis of these fungal species is the significant reliance of the immune system in poikilothermic animals on the prevailing husbandry conditions, including temperature, within their environment []. Humidity, diet, and UV light conditions have a constant impact on reptilian health [].
Metarhizium granulomatis and M. viride are known to cause hyalohyphomycosis in captive lizards [,]. In particular, M. granulomatis and M. viride have been described as primary pathogens, affecting chameleons and central bearded dragons and causing fungal dermatitis, granulomatous glossitis, pharyngitis, and disseminated visceral mycosis [,]. However, concurrent colonization and/or infection with both species in lizards and chameleons has also been described, emphasizing the importance of specifically identifying these fungal pathogens []. The most recent research results indicate that these keratinophilic fungi may constitute species complexes consisting of five different genotypes of M. granulomatis [] and four different genotypes for M. viride []. There appears to be a strong correlation between M. granulomatis genotype A and the occurrence of dermatitis []. Conversely, findings suggest that M. granulomatis genotypes B–E are linked to the development of pharyngitis and glossitis []. However, ribosomal genes of the internal transcribed spacer1-5,8s (ITS1-5,8S) and a fragment of the large subunit of the 28S rDNA (LSU) have been sequenced [,], constituting data that does not appear to be sufficient for phylogenetic studies on the family Clavicipitaceae [,]. Although ITS and LSU are extensively utilized for fungal barcoding, they frequently prove ineffective in resolving closely related taxa. In contrast, the utilization of single-copy, protein-coding genes such as NRPB2 and EF1α has been demonstrated to facilitate enhanced phylogenetic resolution and enable more precise discrimination of closely related genotypes [,].
The objective of this study was to determine whether the previously described genotypes of M. granulomatis and M. viride [,] can be underlined and differentiated more comprehensively through the integration of NRPB2 (RPB1, RPB2) and EF1α. A comprehensive evaluation of single-nucleotide polymorphisms (SNPs) was conducted, followed by a translation of nucleotides into amino acid patterns. This approach enabled the formulation of conclusions that contribute to a more profound comprehension of the phylogenesis inside the M. granulomatis and M. viride complexes. The significance of accurate phylogenetic classification, therefore, lies in its potential to reveal differences in host specificity, virulence, and pathogenesis, thereby potentially preventing infections.
2. Materials and Methods
2.1. Fungal Isolates
Fungal isolates were collected between 2010 and 2023 from lizards (n = 21) presented to the Clinic for Birds and Reptiles, University Leipzig, Germany. The isolates were derived from the following species: veiled chameleons (Chameleo calyptratus) (n = 11), panther chameleons (Furcifer pardalis) (n = 4), Parson’s chameleons (Calumma parsonii) (n = 3), central bearded dragons (Pogona vitticeps) (n = 2), and a carpet chameleon (Furcifer lateralis) (n = 1). Specifically, the isolates were obtained from the throat (n = 9), the cloaca (n = 7), the tongue (n = 4), liver (n = 2), fecal samples (n = 2), or the rectum (n = 1). The isolates were stored in ROTI®Store yeast cryotubes (Carl Roth GmbH & Co.KG, Karlsruhe, Germany) at a temperature of −32 °C until utilization.
Genomic DNA was purified using a DNeasy blood-and-tissue kit (Qiagen, Hilden, Germany), following the protocols given by the manufacturer. In the following steps, species affiliation was proven via ribosomal DNA sequencing [,,]. Regarding M. granulomatis, genotype A was obtained from Parson’s chameleons (n = 2) and a panther chameleon, while genotype B isolates were obtained from four chameleon species, including one Parson’s chameleon with co-isolation of genotype A. Genotypes C and D were only obtained from veiled chameleons (n = 2, each). One of these veiled chameleons exhibited genotypes B and C.
Regarding M. viride, genotype A was derived from veiled chameleons (n = 2); genotype B was obtained from a central bearded dragon and a panther chameleon (one each); and genotype C was obtained from one veiled chameleon, one panther chameleon, and one Parson’s chameleon. Genotype D (n = 1) was obtained from a central bearded dragon. In addition, two isolates with previously unpublished genotyping were obtained from veiled chameleons (n = 2). The purified genomic DNA of these isolates was frozen at −32 °C in Eppendorf tubes until further examination.
2.2. PCR and DNA Sequencing
The forward and reverse primers employed in this study were selected based on previous phylogenetic attempts to reclassify Metarhizium spp. and are listed in Table 1 [,,,].

Table 1.
Primers used for the amplification and sequencing of two different loci of NRPB2 (RPB1, RPB2) and the translation elongation factor 1 alpha (EF1α) of Metarhizium (M.) granulomatis and M. viride.
The DNA quality of the frozen samples was determined via nanodrop spectrophotometry using a NanoPhotometer® NP80 (Implen GmbH, Munich, Germany) (Table S1). DNA-free controls were used as negative samples. In addition, two to three PCR replicates were employed for each sample. In cases of co-occurring genotypes, separation was carried out during the cultivation process. Afterwards, the morphologically different colonies were frozen and labeled as required []. All of the samples were analyzed according to described protocols [], focusing on partial sequences of three gene loci: RPB1, RPB2, and EF1α [,,,]. There was a repetition in the PCR of RPB1 due to a change in the annealing temperature from 58 °C to 60 °C. After the samples were amplified, detection was carried out using gel electrophoresis. The PCR products were further processed via polyethylene glycol precipitation []. Sequencing was conducted via bidirectional Sanger sequencing, with subsequent sequence quality verification performed using the Phred base-calling program for DNA sequence traces, provided by a commercial DNA-sequencing service (Faculty of Medicine, Central Functional Area: DNA Technologies/Sequencing, Leipzig, Germany). A volume of 10 μL of the amplified sample, in conjunction with the primers, was employed in accordance with the previously delineated protocol.
2.3. Analyzing Data According to Phylogeny
The alignment lengths for each gene locus were 819 bp for RPB1 and 802 bp for RPB2. With respect to EF1α, these lengths were 729 bp and 723 bp for M. granulomatis and M. viride, respectively. As a next step, all sequences were edited into a multilocus dataset, combining EF1α (non-coding and CDS), RPB1, and RPB2 resulting in a total length of 2350 bp using MEGA12 (Molecular Evolutionary Genetics Analysis Version 12 for adaptive and green computing []). Comparison with already available data in the GenBank database was carried out according to the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/, accessed on 5 March 2025) [].
Furthermore, the isolates were evaluated in the four following datasets: RPB1, RPB2, EF1α, and the multilocus dataset. First, all the isolates were analyzed regarding single-nucleotide polymorphisms (SNPs) on a nucleotide base. The EF1α as well as the multilocus dataset also contained coding and non-coding regions of EF1α, as so including these can lead to a better analysis at the molecular genetic level. The phylogeny was inferred using the maximum likelihood method and the Jukes–Cantor model [] of nucleotide substitutions and the tree with the highest log likelihood (−3675.87) is shown. The percentage of replicate trees in which the associated taxa clustered together (1000 replicates) is shown next to the branches []. The analytical procedure encompassed 28 coding nucleotide sequences using 1st positions with 843 positions in the final dataset.
The isolates then were translated into amino acid patterns to detect any differences in branching. This led to a trimming of EF1α for M. granulomatis and M. viride in order to focus on CDS regions. The resulting length for EF1α was 257 bp, and the length of the multilocus dataset was 1878 bp. Evolutionary history and potential similarity were derived using the maximum likelihood method based on the Jones–Taylor–Thornton model []. The percentage of replicate trees in which the associated taxa clustered together (1000 replicates) is shown next to the branches []. The evolutionary-rate differences among sites were modeled using a discrete Gamma distribution across 5 categories (+G, parameter = 2.5938). The analytical procedure encompassed 28 coding nucleotide sequences using 1st positions, with 843 positions in the final dataset.
To create additional outgroup taxa, isolates of the Metarhizium anisopliae group (informally designated as the “PARB” clade) [], such as M. anisopliae, M. brunneum, and M. robertsii, were added. A member of the family Clavicipitaceae that was used was M. acridum. Also, Beauveria bassiana was added to act as a root. To create comparative isolates for the multilocus analysis, sequences of RPB1, RPB2, and EF1α from the outgroup taxa were taken from the NCBI GeneBank database (http://www.ncbi.nlm.nih.gov). They were then assembled as described earlier. M. anisopliae consisted of OK336701.1 [Du, 2021, unpublished []] for RPB1 and RPB2 and of DQ463996.2 [] for EF1α. M. brunneum consisted of EU248854.1 [] (RPB1, EF1α) and XM_014688283.1 [] (RPB2). M. robertsii consisted of DQ468368.1 [] (RPB1), XM_007819877.1 [] (RPB2), and DQ463994.2 [] (EF1α). M. acridum consisted of KM527862.1 [Zhang, 2014, Unpublished []] (RPB1), XM_066120576.1 [] (RPB2), and MK391183.1 [] (EF1α). Beauveria bassiana was assembled from MN401612.1 [] (RPB1), LC812020.1 [Yang et al., 2024, unpublished []] (RPB2), and MN026878.1 [Dalla Nora, 2019, Unpublished []] (EF1α).
To underline the results obtained using the maximum likelihood method, pairwise distance was computed via the MEGA program (v12) using the Maximum Composite Likelihood (MCL) approach.
3. Results
3.1. RNA Polymerase II Second Largest Subunit (NRPB2) of M. granulomatis
The sequencing of the first locus (RPB1) of NRPB2 revealed no differences between M. granulomatis isolate types A, C, and D, which exhibited 100% identity with respect to M. granulomatis [NCBI Acc.-Nr. KJ398688.1 []]. The sole discernible distinction between M. granulomatis type B and other types was a single-nucleotide polymorphism. However, translation into an amino acid sequence showed 100% identity of all the M. granulomatis isolates as well as to the additional outgroup taxa of M. anisopliae [NCBI Acc.-Nr. OK336701.1 [Du, 2021, unpublished []]], M. brunneum [NCBI Acc.-Nr. EU248934.1 []], M. robertsii [NCBI Acc-Nr. DQ468368.1 []], and M. acridum [NCBI Acc.-Nr. KM527862.1 [Zhang, 2014, Unpublished []]] (Figure S1).
Analyzing the nucleotide structure of the second locus (RPB2) of the NRPB2 of M. granulomatis isolates allowed us to observe 11 variable sites. In total, there were six SNPs regarding genotype B; four regarding genotypes C and D, which were 100% identical; and only one SNP regarding genotype A. Genotype A showed 99.12–99.38% of identity with respect to other isolates; isolates of genotype B showed an identity of 98.74–99.12% with respect to others. Isolates of genotype C and D showed an identity of 98.74–99.38%. None of these differences had an impact on amino acid sequences, so all the isolates of M. granulomatis had an amino acid identity of 100%. They also clustered with the additional outgroup taxa of M. anisopliae [NCBI Acc.-Nr. OK336701.1 [Du, 2021, unpublished []]], M. brunneum [NCBI Acc.-Nr. XM_014688283.1 []], M. robertsii [NCBI Acc.-Nr. XM_007819877.1 []], and M. acridum [NCBI Acc.-Nr. XM_066120576.1 []], also with an amino acid identity of 100% (Figure S2).
3.2. RNA Polymerase II Second Largest Subunit (NRPB2) of M. viride
Metarhizium viride was found to contain 11 variable sites in RPB1. The isolates of genotypes A and D and the two isolates of previously unpublished genotype differed by one single nucleotide each. Translation into amino acids, however, showed that they were 100% identical, with an identity of 100% with respect to M. viride [NCBI Acc.-Nr. KJ398717 []]. Genotype C could be differentiated from the other isolates via three SNPs. This resulted in a nucleotide identity of 99.71% to genotype A isolates and the ones of unpublished genotype, as well as 99.64% to the genotype D isolate. Furthermore, two isolates, which were labeled as M. viride genotype B, could be distinguished from each other based on four single-nucleotide polymorphisms, resulting in an identity of 99.71%. These differences were followed by one change in amino acid structure. One of these isolates [NCBI Acc.-Nr. PV231550] showed an identity of 98.18–99.41%, while the other isolate [NCBI Acc.-Nr. PV231551] had an identity of 98.34–99.49% compared to the remaining M. viride isolates. M. granulomatis [NCBI Acc.-Nr. KJ398688.1 []] exhibited an identity of 98.88% with respect to M. viride [NCBI Acc.-Nr. KJ398717 []]. Between the different genotypes of M. viride and M. granulomatis considered in this study, an identity of between 98.51 and 98.98% was observed.
There were a total of nine variable sides in the RPB2, resulting in an identity of 99.25–99.50% among all the isolates. Furthermore, no amino acid sequence differences were found, so there was an identity of 100% between all genotypes. Between the M. granulomatis and M. viride isolates, 97.15–97.71% identity was observed, while the amino acid sequences were 100% identical.
3.3. Translation Elongation Factor 1 alpha (EF1α)
The EF1α nucleotides of M. granulomatis revealed the presence of 26 variable sites, 22 of which were situated within non-coding regions. These SNPs resulted in a diverse presentation of identity regarding the M. granulomatis genotype B isolates, spanning from 98.75% [NCBI Acc.-Nr. PV231586] to over 99.17% [NCBI Acc.-Nr. PV231585, PV231588] and 100% [NCBI Acc.-Nr. PV231584, PV231587]. The M. granulomatis genotype A isolates showed an identity of 100%. Furthermore, as no SNPs were detected between the isolates of M. granulomatis genotype C and D, they were determined to have an identity of 100%.
Only four of the variable sites were located on the CDS regions. Out of these SNPs, one was observed in isolates that belonged to M. granulomatis genotype A. This also had an impact on the amino acid sequence, resulting in the M. granulomatis genotype A isolates clustering separately from others with an identity of 98.80%, and an identity of 100% with respect to M. granulomatis [NCBI Acc.-Nr. MH619511.1 []]. Furthermore, there were two SNPs in the CDS nucleotide sequence of the M. granulomatis genotype B isolates, two regarding VS14512 [NCBI Acc.-Nr. PV231585] and VS18409 [NCBI Acc.-Nr. PV231588] and one regarding VS15807 [NCBI Acc.-Nr. PV231586]. These SNPs did not translate into further changes, so all of the other M. granulomatis isolates clustered together with an identity of 100% (Figure S3).
The EF1α of M. viride isolates revealed a total of 17 variable sites. This resulted in an identity of 98.29–100% of all the isolates with respect to M. viride [NCBI Acc.-Nr. MH619515.1 []]. Two M. viride genotype A isolates presented an identity of 98.73% to the M. viride genotype D isolate [NCBI Acc.-Nr. PV231592]. The two isolates designated as M. viride genotype B exhibited an identity of 99.16% to each other.
Only one of the variable sites mentioned was located in the CDS region of the EF1α. This SNP belonged to the sequence of the genotype A isolates. No amino acid sequence impact was observed, so all isolates clustered together. Maximum likelihood analyses confirmed a 100% identity with respect to M. viride [NCBI Acc.-Nr. MH619515.1 []].
A total of 85 variable sites were identified between the EF1α of M. granulomatis and M. viride, resulting in an identity of 94.24–96.08% regarding the different species. Eight of the single-nucleotide polymorphisms were located on the coding regions. By focusing on them, all the isolates apart from M. granulomatis genotype A, had an identity of 100%. Isolates of M. brunneum [NCBI Acc.-Nr. EU248854.1 []], M. robertsii [NCBI Acc.-Nr. DQ463994.2 []], M. anisopliae [NCBI Acc.-Nr. DQ463996.2 []], and M. acridum [NCBI Acc.-Nr. MK391183.1 []] clustered phylogenetically apart as additional outgroup taxa, resulting in an identity of 96.36–97.58%.
3.4. Multilocus Analysis
A total of 38 variable sites were identified through an evaluation of the single-nucleotide polymorphisms present within the multilocus dataset for M. granulomatis.
The isolates belonging to M. granulomatis genotype A were identical, as there were no differences in nucleotides among them. In addition, they revealed an identity of 99.25–99.46% with respect to the other isolates. One variable site in the isolates of M. granulomatis genotypes C and D resulted in an identity of 99.98%. As this SNP was located in the non-coding region of EF1α, no impact on amino acid sequence was observed. Furthermore, the isolates belonging to M. granulomatis genotype B showed eight variable sites, which were also located on EF1α. These variable sites facilitated differentiation within this genotype. VS15807 exhibited five SNPs; VS18415, VS17813 and VS17810 exhibited three SNPs; and VS14512 and VS18409 exhibited only one SNP. These results were confirmed by creating a phylogenetic tree and observing the result of branching. The identity among the isolates of genotype B was 99.83–100%.
However, only two of the variable sites were located in the CDS regions. These did not have any impact on the amino acid sequence, as isolates belonging to M. granulomatis genotype B had an amino acid identity of 99.90%. Concerning the amino acids, M. granulomatis genotype A showed an identity of 98.69–99.02% with respect to the other isolates, while M. granulomatis genotype C showed an identity of 98.35–99.02% (Figure 1).
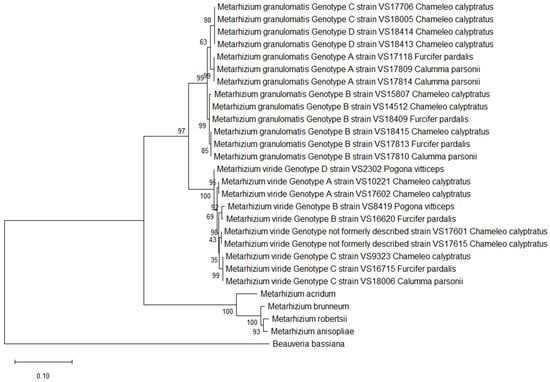
Figure 1.
Phylogeny inferred from the analysis of two loci of NRPB2 and coding, as well as non-coding regions of EF1α from 13 isolates of the Metarhizium (M.) granulomatis complex and 10 isolates of the M. viride complex, assembled into a multilocus dataset. Analysis was conducted based on nucleotide structures. Other isolates of Claviciptitaceae (Sordariomycetes: Hypocreales) and Beauveria bassiana were added as additional outgroup taxa. For this purpose, reference sequences of the two loci of NRPB2 and EF1α along with accession numbers were taken and assembled from the NCBI GeneBank database (http://www.ncbi.nlm.nih.gov).
Single-nucleotide polymorphisms of M. viride resulted in a total of 41 variable sites. Consequently, six different branches were built by constructing maximum likelihood trees. M. viride genotype A isolates VS17602 and VS10221, which were 100% identical, differed from VS2302, formerly named M. viride genotype D. This conclusion was based on 11 changes in nucleotide structure and therefore resulted in an identity of 99.52%. As four of these SNPs were located in the coding regions, VS2302 therefore contained three different amino acids. Furthermore, this specific M. viride genotype showed an identity of 98.18–98.85% with respect to the remaining isolates and clustered separately. The two isolates formerly named M. viride genotype B (VS8419, VS16620) revealed a total of 12 variable sites, resulting in an identity of 99.49%. As four of these SNPs were located in the coding regions, the isolates could be distinguished from each other based on three amino acids. This was also proven by the clustering in the phylogenetic tree.
The isolates belonging to M. viride genotype C revealed an identity of 98.97–99.45% among each other. The ones with previously unpublished genotypes, which were 100% identical to each other, showed an identity of 99.10–99.49% with respect to the other isolates. Regarding amino acid sequences, differentiation within six different branches was once again possible. This was a result of the 24 variable sites, which were located in the coding regions and could be translated into 20 differences in amino acid structure among the isolates. (Figure 2).
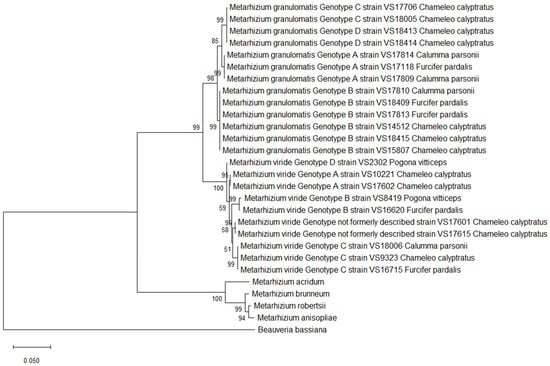
Figure 2.
Phylogeny inferred from the analysis of two loci of the NRPB2 and CDS regions of EF1α from 13 isolates of Metarhizium (M.) granulomatis–complex and 10 isolates of M. viride–complex, assembled into a multilocus dataset. Analysis was conducted based on amino acid sequences. Other isolates of Claviciptitaceae (Sordariomycetes: Hypocreales) and Beauveria bassiana were added as additional outgroup taxa. For this purpose, reference sequences of the two loci of NRPB2 and EF1α along with accession numbers were taken and assembled from the NCBI GeneBank database (http://www.ncbi.nlm.nih.gov).
All the M. granulomatis isolates could be distinguished from the M. viride isolates with a nucleotide identity of 96.50–96.93%. Once more, including M. granulomatis or M. viride isolates from the BLAST dataset for comparison was rendered unfeasible, by the paucity of extant published data. A maximum likelihood analysis further confirmed that the earlier described PARB clade is distinct from the M. granulomatis and M. viride isolates, with the M. granulomatis isolates having an identity of 87.38–88.08%. The M. viride isolates showed an identity of 86.94–87.79% with respect to the outgroup taxa.
3.5. Isolates and Associated Findings
Further evaluation concerning the source of isolation revealed that the isolates belonging to M. granulomatis genotypes A and B could all be obtained from cloacal swabs (n = 7). In addition, two isolates of M. granulomatis genotype B were found in the throat of the lizards sampled. When examining the isolates of M. granulomatis genotype C and M. viride, the origin of isolation was found to exhibit more diversity. An overview of isolates, host species, origin of isolation and associated pathological findings is shown in Table 2.

Table 2.
Summary of Metarhizium (M.) granulomatis and M. viride genotypes (GTs) and isolates identified via sequence analysis of ribosomal DNA, host species, origins of isolates, associated pathological findings, and/or additional disease.
A pathological examination was performed on 7 of the 23 lizards (30.4%) sampled. One isolate of M. granulomatis genotype A and one of M. granulomatis genotype B were isolated from a Parson’s chameleon with diphtheroid colorectitis. However, due to severe bacterial overgrowth, it remains unclear whether the fungi were pathogens or accidental findings. Nevertheless, granulomatous colorectitis caused by M. granulomatis genotype C was diagnosed in a veiled chameleon (Figure S4). Three isolates of M. granulomatis genotype B were the cause of petechial hemorrhages on the tongue of three different chameleon species. Furthermore, M. granulomatis genotype C led to the same finding in veiled chameleons (n = 2). M. viride genotype A and one isolate of M. viride genotype C were found to be the causes of granulomatous glossitis in two veiled chameleons. Furthermore, one isolate belonging to M. viride genotype D and one belonging to M. viride genotype B were the cause of granulomatous mycotic pharyngitis in central bearded dragons (n = 2). Associated pathological findings were lacking for five of the animals presented (21.7%) (Table 2).
4. Discussion
In the present study, the evolutionary relationships between fungal isolates of M. granulomatis and M. viride were investigated. These isolates were obtained from five different lizard species and six different sampling sites. This study presents the first isolates of M. granulomatis and M. viride obtained from Parson’s chameleons. In addition, the presence of petechiae on the tongue, or necrosis of the caudal tip, was observed in panther chameleons and a carpet chameleon infected with M. granulomatis. These findings suggest that M. granulomatis may have a broader host range and pathogenicity, as previously described [].
4.1. Phylogenetic Analysis of M. granulomatis Using a Multilocus Approach
By sequencing of the SSU, ITS1-5.8S, and LSU, Schmidt et al. [] yielded five different genotypes of M. granulomatis. In contrast, the multilocus dataset presented here yielded six different genotypes of M. granulomatis, which resulted in three different amino acid sequences. The amino acid sequences of these isolates exhibited a similarity of less than 98.7%. A threshold for species separation for bacterial species of ≥98.7–99% was proposed by Stackebrandt and Ebers []. No such threshold value has been defined for fungi. Nevertheless, the available data can be used as a guide. Therefore, it may be appropriate to categorize M. granulomatis A-C as three distinct subspecies of M. granulomatis. Even though the nucleotide sequences of the M. granulomatis B isolates showed differences, especially in the non-coding regions, their identity was >98.7%. As a result of the inability to distinguish between M. granulomatis subspecies C and D described herein, reclassifying these isolates as a single subspecies is recommended.
4.2. Phylogenetic Analysis of M. viride Using a Multilocus Approach
By sequencing of the SSU, ITS1-5.8S and LSU, Schmidt et al. [] yielded four different genotypes of M. viride. In contrast, the multilocus dataset presented here yielded six genotypes of M. viride. The isolate formerly labeled as M. viride genotype D (VS2302) is genetically very similar to the isolates of genotype A. At this point, one should speak of genetic divergence owing the results of the Maximum Composite Likelihood (MCL) approach. However, since the percentage of similarity to the other isolates of M. viride was below 98.7%, it is suggested to reclassify the isolate VS2302. Potentially being renamed as M. viride genotype A1, it would appear to be a genetic strain within genotype A. The same applies to the previously mentioned isolates of M. viride genotype B (VS8419, VS16620). The results of the multilocus approach also underscore that they are genetically distinct and therefore support a subdivision within M. viride genotype B. Furthermore, the isolates belonging to M. viride genotype C clustered together in phylogenetic trees built on nucleotides as well as amino acids. Regarding amino acids, their similarity to the other isolates was below the threshold value used for comparison. In conclusion, they comprised one group of subspecies. The isolates of the previously unpublished genotype (VS17601, VS17615) also clustered separately from the others regarding nucleotide and amino acid sequences. The proposal is to refer to them as M. viride genotype E.
4.3. Evaluation of the Phylogenetic Analysis Using NRPB2 (RPB1)
The first locus (RPB1) of NRPB2 was not target-orientated to yield different genotypes of M. granulomatis. No major differences in nucleotide sequences were detected. Only the M. granulomatis genotype B isolates clustered separately. Regarding amino acid sequences, all the M. granulomatis genotypes as well as the additional outgroup taxa clustered together.
Four genotypes of M. viride could be distinguished regarding RPB1. The Maximum Composite Likelihood (MCL) approach showed that isolates of M. viride genotypes A, D and the two isolates with previously unpublished genotype could be distinguished using this method. One isolate previously described as M. viride genotype D [NCBI Acc.-Nr. PV231549] clustered separately from the other isolates of M. viride genotype A regarding nucleotide sequences. Nevertheless, differentiation based on amino acid sequence was not possible. The M. viride genotype C isolates showed differences in nucleotide and amino acid sequences. Therefore, it clustered separately from the other isolates. Furthermore, there were significant differences in nucleotides that also translated into the amino acid sequences of the two M. viride genotype B isolates. This information could lead to a potential renaming of the isolates to M. viride genotype B1 [NCBI Acc.-Nr. PV231550] and M. viride genotype B2 [NCBI Acc.-Nr. PV231551], with M. viride genotype B1 having the lowest percentage of similarity with respect to the other isolates.
Notwithstanding, a clear distinction between M. viride and M. granulomatis genotypes could be made using this method.
4.4. Evaluation of the Phylogenetic Analysis Using NRPB2 (RPB2)
Regarding RPB2 nucleotides, the sequence similarity among the isolates of M. granulomatis and M. viride was >98.7%. M. granulomatis could be distinguished from M. viride with an identity of <98.7%. Regarding amino acids, M. granulomatis, M. viride, and the PARB-clade clustered together, as there were no significant differences in the CDS. In conclusion, sequencing of the RPB2 alone is not useful for analyzing the phylogenetic diversity of M. granulomatis and M. viride isolates.
4.5. Evaluation of the Phylogenetic Analysis Using EF1α
Similar results were obtained when analyzing the EF1α dataset. Even though the nucleotide sequences, including coding and non-coding regions, exhibited diversity among all the isolates, clear phylogenetic differentiation was not possible. The only isolates showing differences in amino acid sequences and therefore clustering apart from the others were the ones belonging to M. granulomatis genotype A. However, the amino acid sequence of EF1α showed more considerable variation among the PARB-clade isolates in comparison to M. granulomatis and M. viride.
5. Conclusions
Finally, after having sequenced and analyzed the data collected in this study, it can be postulated that an individual analysis of RPB1, RPB2, and EF1α is not sufficiently accurate for the specification of M. granulomatis and M. viride. However, a multilocus analysis of NRPB2 and EF1α represents a comprehensive and meaningful approach to differentiate between species complexes within M. granulomatis and M. viride as well as defining subspecies within these complexes. By using this method, the genotypes M. granulomatis A-D, previously postulated by Schmidt et al. [], could be reduced to M. granulomatis A-C. Furthermore, the genotypes M. viride A-D, previously postulated by Schmidt et al. [], could be subdivided into M. viride A, B1, B2, and C. In view of the genetic similarity exhibited by M. viride A and M. viride D, it is recommended that the designation M. viride genotype A1 be employed. Two new previously unclassified isolates could be referred to as M. viride genotype E based on the results of a phylogenetic analysis. The present findings serve to corroborate the hypothesis that an analysis of ITS and LSU can be usefully complemented by a multilocus analysis of NRPB2 and EF1a, providing a more profound insight into the phylogenetic divergence of Metarhizium spp.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14080745/s1, Figure S1: Phylogeny inferred from the amino acid analysis of 13 NRPB2 sequences (RPB1) of Metarhizium (M.) granulomatis—complex and 10 NRPB2 sequences (RPB1) of M. viride—complex; Figure S2: Phylogeny inferred from the amino acid analysis of 13 NRPB2 sequences (RPB2) sequences of Metarhizium (M.) granulomatis—complex and 10 NRPB2 sequences (RPB2) sequences of M. viride—complex; Figure S3: Phylogeny inferred from the amino acid analysis of 13 EF1α sequences of Metarhizium (M.) granulomatis—complex and 10 EF1α sequences of M. viride—complex; Figure S4: Microphotograph of the cloaca of a veiled chameleon (Chamaeleo calyptratus) with a granulomatous mycotic colorectitis; Table S1: Measurement of DNA quality of 23 frozen samples of Metarhizium (M.) granulomatis and M. viride by nanodrop spectrophotometry (NanoPhotometer® NP80, Implen GmbH, Munich, Germany).
Author Contributions
Conceptualization, J.W. and V.S.; methodology, J.W.; software, J.W. and V.S.; validation, V.S.; formal analysis, J.W.; investigation, J.W.; resources, V.S.; data curation, J.W.; writing—original draft preparation, J.W.; writing—review and editing, V.S. and J.W.; visualization, J.W.; supervision, V.S.; project administration, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Open Access Publishing Fund of Leipzig University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The NCBI Acc.-Nr. for the newly generated fragments of the RPB1 are PV231534, PV231535, PV231536, PV231537, PV231538, PV231539, PV231540, PV231541, PV231542, PV231543, PV231544, PV231545, PV231546, PV231547, PV231548, PV231549, PV231550, PV231551, PV231552, PV231553, PV231554, PV231555, PV231556. For RPB2, the NCBI Acc.-Nr. are PV231557, PV231558, PV231559, PV231560, PV231561, PV231562, PV231563, PV231564, PV231565, PV231566, PV231567, PV231568, PV231569, PV231570, PV231571, PV231572, PV231573, PV231574, PV231575, PV231576, PV231577, PV231578, PV231579. NCBI Acc.-Nr. for the EF1α are PV231580, PV231581, PV231582, PV231583, PV231584, PV231585, PV231586, PV231587, PV231588, PV231589, PV231590, PV231591, PV231592, PV231593, PV231594, PV231595, PV231596, PV231597, PV231598, PV231599, PV231600, PV231601, PV231602.
Acknowledgments
We are grateful to Zaida Rentería, Institute of Parasitology, University of Leipzig, Germany, for providing the equipment and material for PCR and the Core Unit DNA Technology, Faculty of Medicine, University of Leipzig, for sequencing.
Conflicts of Interest
The authors declare there are no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PCR | Polymerase chain reaction |
| SNP | Single-nucleotide polymorphism |
| CDS | Coding DNA sequence |
| NRPB2 | RNA polymerase II second largest subunit |
| RPB1 | First analyzed locus of the RNA polymerase II second largest subunit |
| RPB2 | Second analyzed locus RNA polymerase II second largest subunit |
| EF1α | Translation elongation factor 1 alpha |
References
- Sigler, L.; Gibas, C.F.C.; Kokotovic, B.; Bertelsen, M.F. Disseminated mycosis in veiled chameleons (Chamaeleo calyptratus) caused by Chamaeleomyces granulomatis, a new fungus related to Paecilomyces viridis. J. Clin. Microbiol. 2010, 48, 3182–3192. [Google Scholar] [CrossRef]
- Schmidt, V.; Klasen, L.; Schneider, J.; Hübel, J.; Cramer, K. Pulmonary fungal granulomas and fibrinous pneumonia caused by different hypocrealean fungi in reptiles. Vet. Microbiol. 2018, 225, 58–63. [Google Scholar] [CrossRef]
- Horgan, M.D.; Alexander, A.B.; Innis, C.; Stacy, B.A.; Gai, J.J.; Pesavento, P.A.; Highland, M.A.; Liguori, B.L.; Norton, T.M.; Wellehan, J.F.X.; et al. Pulmonary and coelomic mycoses due to Metarhizium and Beauveria species in reptiles. J. Zoo. Wildl. Med. 2022, 53, 605–612. [Google Scholar] [CrossRef]
- Warwick, C. Observations on disease-associated preferred body temperatures in reptiles. Appl. Anim. Behav. Sci. 1991, 28, 375–380. [Google Scholar] [CrossRef]
- Pough, F.H. Recommendations for the care of amphibians and reptiles in academic institutions. ILAR J. 1991, 33, S1–S21. [Google Scholar] [CrossRef]
- Schmidt, V.; Klasen, L.; Schneider, J.; Hübel, J.; Pees, M. Fungal dermatitis, glossitis and disseminated visceral mycosis caused by different Metarhizium granulomatis genotypes in veiled chameleons (Chamaeleo calyptratus) and first isolation in healthy lizards. Vet. Microbiol. 2017, 207, 74–82. [Google Scholar] [CrossRef]
- Schmidt, V.; Klasen, L.; Schneider, J.; Hübel, J.; Pees, M. Characterization of Metarhizium viride mycosis in veiled chameleons (Chamaeleo calyptratus), panther chameleons (Furcifer pardalis), and inland bearded dragons (Pogona vitticeps). J. Clin. Microbiol. 2017, 55, 832–843. [Google Scholar] [CrossRef]
- Klasen, L.; Pees, M.; Schmidt, V. Simultaneous detection of Metarhizium viride, Metarhizium granulomatis and Metarhizium anisopliae species complex in veiled chameleons (Chamaeleo calyptratus), panther chameleon (Furcifer pardalis) and a central bearded dragon (Pogona vitticeps). Berl. Münchener Tierärztliche Wochenschrift 2019, 132, 428–434. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Kepler, R.M.; Humber, R.A.; Bischoff, J.F.; Rehner, S.A. Clarification of generic and species boundaries for Metarhizium and related fungi through multigene phylogenetics. Mycologia 2014, 106, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Khonsanit, A.; Thanakitpipattana, D.; Tasanathai, K.; Noisripoom, W.; Lamlertthon, S.; Himaman, W.; Houbraken, J.; Samson, R.A.; Luangsa-ard, J. Revisiting Metarhizium and the description of new species from Thailand. Stud Mycol. 2020, 95, 171–251. [Google Scholar] [CrossRef]
- Malkus, A.; Linda Chang, P.F.; Zuzga, S.M.; Chung Kren Shao, J.; Cunfer, B.M.; Arseniuk, E.; Ueng, P.P. RNA polymerase II gene (RPB2) encoding the second largest protein subunit in Phaeosphaeria nodorum and P. avenaria. Mycol. Res. 2006, 110, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. Metarhizium frigidum sp. nov.: A cryptic species of M. anisopliae and a member of the M. flavoviride complex. Mycologia 2006, 98, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Mülhardt, C. Der Experimentator: Molekularbiologie/Genomics; Spektrum Akademischer Verlag: Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. Molecular evolutionary genetics analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: Cambridge, MA, USA, 1969; pp. 21–132. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Rehner, S.A.; Kepler, R.M. Species limits, phylogeography and reproductive mode in the Metarhizium anisopliae complex. J. Invertebr. Pathol. 2017, 148, 60–66. [Google Scholar] [CrossRef]
- Du, L.C. Plant Protection. South China Agricultural University: Guangzhou, China, 2021; unpublished work. [Google Scholar]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef]
- Saud, Z.; Kortsinoglou, A.M.; Kouvelis, V.N.; Butt, T.M. Telomere length de novo assembly of all 7 chromosomes and mitogenome sequencing of the model entomopathogenic fungus, Metarhizium brunneum, by means of a novel assembly pipeline. BMC Genom. 2021, 22, 87. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, G.; Zheng, P.; Shang, Y.; Su, Y.; Zhang, X.; Liu, X.; Zhan, S.; Leger, R.J.S.; Wang, C. Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proc. Natl. Acad. Sci. USA 2014, 111, 16796–16801. [Google Scholar] [CrossRef]
- Zhang, X. Molecular Identification of Metarhizium Strains Isolated from Pests and Soil in China. Anhui Provincial Key Laboratory for Microbial Pest Control, Anhui Agricultural University: Hefei, China, 2014; submitted. [Google Scholar]
- Nielsen, K.N.; Salgado, J.F.M.; Natsopoulou, M.E.; Kristensen, T.; Stajich, J.E.; De Fine Licht, H.H. Diploidy within a haploid genus of entomopathogenic fungi. Genome Biol. Evol. 2021, 13, evab158. [Google Scholar] [CrossRef] [PubMed]
- Inglis, G.D.; Duke, G.M.; Goettel, M.S.; Kabaluk, J.T.; Ortega-Polo, R. Biogeography and genotypic diversity of Metarhizium brunneum and Metarhizium robertsii in northwestern North American soils. Can. J. Microbiol. 2019, 65, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Khonsanit, A.; Luangsa-ard, J.J.; Thanakitpipattana, D.; Noisripoom, W.; Chaitika, T.; Kobmoo, N. Cryptic diversity of the genus Beauveria with a new species from Thailand. Mycol. Prog. 2020, 19, 291–315. [Google Scholar] [CrossRef]
- Yang, Y.T.; Chen, C.Y.; Yang, Y.Y. Phylogenetic Analysis of Beauveria bassiana. Agricultural Research and Extension Station, MOA, Plant Protection Laboratory: Taoyuan City, Taiwan, 2024; submitted. [Google Scholar]
- Dalla Nora, D. Fungos Entomopatogenicos para o Controle do Percevejo da Soja. Ciencia do Solo, Universidade Federal de Santa Maria: Santa Maria, Brazil, 2019; submitted. [Google Scholar]
- Stackebrandt, E.; Ebers, J. Taxonomic parameters revisited: Tarnished gold standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).