Identification of Fungi Causing Root Rot in Oregano Crops in Southern Peru: Morphological and Molecular Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Isolation and Purification of Fungi
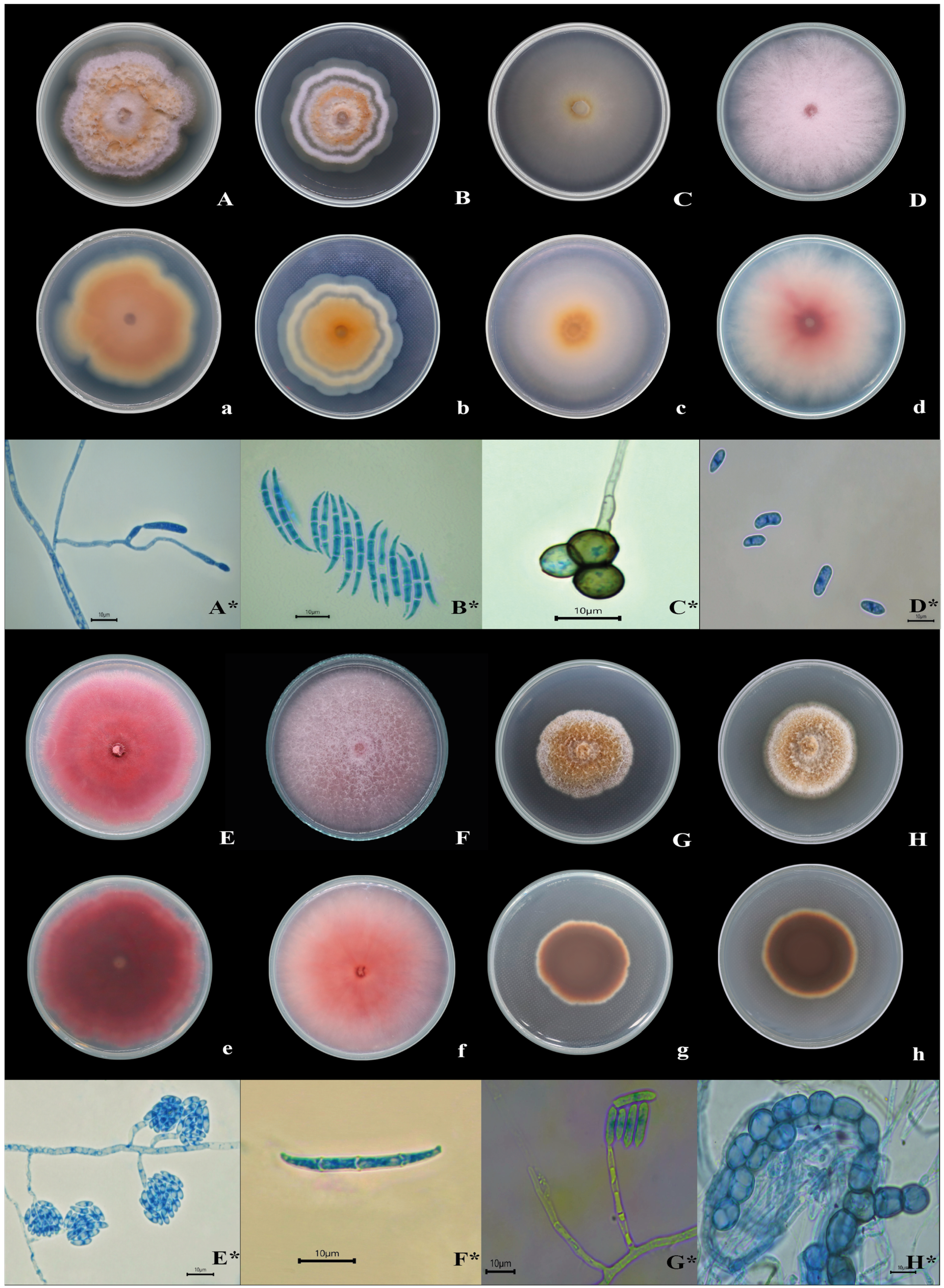
2.3. Macroscopic and Microscopic Morphological Characterization
2.4. DNA Extraction and Amplification
2.5. Phylogenetic Analysis
2.6. Pathogenicity Test
3. Results
3.1. Isolation and Characterization of Fungi Associated with Oregano Root Rot
3.2. Molecular Identification and Phylogenetic Analysis of the Fungi
3.3. Pathogenicity Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. Ecology, cultivation and utilization of the aromatic Greek oregano (Origanum vulgare L.): A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 545–552. [Google Scholar] [CrossRef]
- García-Pérez, E.; Castro-Álvarez, F.; Gutiérrez-Uribe, J.A.; García-Lara, S. Revision of the production, phytochemical composition, and nutraceutical properties of Mexican oregano. Rev. Mex. Cienc. Agríc. 2012, 3, 339–353. [Google Scholar]
- MINAGRI. Análisis De Mercado Del Orégano 2015–2019. Available online: https://repositorio.midagri.gob.pe/handle/20.500.13036/1105 (accessed on 1 January 2024).
- Dirección Regional de Agricultura Tacna. Serie Histórica Del Cultivo De Orégano En Tacna, Según Variables, Período 2018–2023. Available online: https://es.scribd.com/document/561155240/Produccion-y-Exportacion-de-Oregano-en-la-Region-de-Tacna (accessed on 1 February 2024).
- Salas-Portugal, F.; Alagón-de la Sota, P.C. Producción y exportación de orégano de la Región de Tacna. 2016. Available online: https://www.studocu.com/pe/document/universidad-nacional-de-san-cristobal-de-huamanga/metodos-numericos-aplicado/produccion-exportacion-oregano/30023893 (accessed on 1 May 2024).
- Tintaya, F.C.; Cutire, O.F. Economic results of oregano (Origanum vulgare L.) production in inter-Andean basins of the Tacna region, Perú. Idesia 2022, 40, 17–26. [Google Scholar] [CrossRef]
- Zimowska, B. Fungi threatening the cultivation of oregano (Origanum vulgare L.) in South-Eastern Poland. Acta Sci. Pol. Hortorum Cultus 2015, 14, 65–78. Available online: www.acta.media.pl (accessed on 1 May 2023).
- Yossen, V.; Conles, M. Eficacia de fungicidas in vitro para el control de Fusarium oxysporum y Fusarium proliferatum, agentes causales de marchitamiento en el cultivo de orégano en la Argentina. Rev. Ind. Agrícola Tucumán 2014, 91, 19–25. [Google Scholar]
- Chugnas Villena, I. Etiología Y Patogénesis De Fungosis Del Orégano (Origanum vulgare L.) En La Provincia De Cajamarca. Tesis de Licenciatura, Universidad Nacional de Cajamarca, Cajamarca, Peru, 2018. [Google Scholar]
- Díaz, T.S.; González, L.C. Efecto biocontrolador de Trichoderma harzianum rifai sobre Fusarium spp. en Leucaena leucocephala (Lam.) de Wit.(leucaena), Cedrela odorata L.(cedro) y Phitecellobium saman (Jacq.) Merr. Rev. Cient. Agroeco. 2013, 1, 165–172. [Google Scholar]
- Gargouri-Jbir, T.; Zitnick-Anderson, K.; Pasche, J.S.; Kalil, A. Characterization of Fusarium oxysporum f. sp. pisi associated with root rot of field pea in North Dakota and the effects of temperature on aggressiveness. Plant Dis. 2024, 108, 365–374. [Google Scholar] [CrossRef]
- Schwartz, H.F.; Mohan, S.K. Compendium of Onion and Garlic Diseases; APS Press: San Diego, CA, USA, 1995; pp. 1–54. [Google Scholar]
- Stankovic, S.; Levic, J.; Petrovic, T.; Logrieco, A.; Moretti, A. Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur. J. Plant Pathol. 2007, 118, 165–172. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, F.; Zhang, J.; Shang, W.; Hu, X. American ginseng root rot caused by Fusarium redolens in China. Plant Dis. 2021, 105, 2734. [Google Scholar] [CrossRef]
- Carlucci, A.; Lops, F.; Mostert, L.; Halleen, F.; Raimondo, M.L. Occurrence fungi causing black foot on young grapevines and nursery rootstock plants in Italy. Phytopathol. Mediterr. 2017, 56, 10–39. [Google Scholar] [CrossRef]
- Reis, P.; Cabral, A.; Nascimento, T.; Oliveira, H.; Rego, C. Diversity of Ilyonectria species in a young vineyard affected by black foot disease. Phytopathol. Mediterr. 2013, 52, 335–346. [Google Scholar]
- Berlanas, C.; López-Manzanares, B.; Gramaje, D. Estimation of viable propagules of black-foot disease pathogens in grapevine cultivated soils and their relation to production systems and soil properties. Plant Soil. 2017, 417, 467–479. [Google Scholar] [CrossRef]
- Cabral, A.; Groenewald, J.Z.; Rego, C.; Oliveira, H.; Crous, P.W. Cylindrocarpon root rot: Multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol. Prog. 2012, 11, 655–688. [Google Scholar] [CrossRef]
- Erper, I.; Ozer, G.; Alkan, M.; Zholdoshbekova, S.; Turkkan, M. First report of Dactylonectria torresensis causing black root rot of strawberries in Kyrgyzstan. J. Plant Pathol. 2021, 103, 379–380. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Pei, W.; Zheng, G. First report of root rot caused by Dactylonectria torresensis on Bletilla striata (Baiji) in Yunnan, China. Plant Dis. 2021, 105, 698. [Google Scholar] [CrossRef]
- Manici, L.M.; Kelderer, M.; Franke-Whittle, I.H.; Rühmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Top, A.; Insam, H.; Naef, A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Sánchez, J.; Iturralde, P.; Koch, A.; Tello, C.; Martínez, D.; Proaño, N.; Martínez, A.; Viera, W.; Ayala, L.; Flores, F. Dactylonectria and Ilyonectria species causing black foot disease of Andean Blackberry (Rubus glaucus Benth) in Ecuador. Diversity 2019, 11, 218. [Google Scholar] [CrossRef]
- Pečenka, J.; Eichmeier, A.; Peňázová, E.; Baránek, M.; León, M.; Armengol, J. First report of Dactylonectria torresensis causing black-foot disease on grapevines in the Czech Republic. Plant Dis. 2018, 102, 2038. [Google Scholar] [CrossRef]
- Crous, P.W. Taxonomy and Pathology of Cylindrocladium (Calonectria) and Allied Genera; APS Press: St. Paul, MN, USA, 2002. [Google Scholar]
- Chaverri, P.; Salgado, C.; Hirooka, Y.; Rossman, A.Y.; Samuels, G. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Stud. Mycol. 2011, 68, 57–78. [Google Scholar] [CrossRef]
- Linares, M.Y. Fusarium spp.: Un Modelo Para El Análisis De Patógenos Multihospedero. Master’s Thesis, Pontificia Universidad Javeriana, Bogota, Colombia, 2010. [Google Scholar]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda-Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Zerillo, M.M.; Adhikari, B.N.; Hamilton, J.P.; Buell, C.R.; Lévesque, C.A.; Tisserat, N. Carbohydrate-active enzymes in Pythium and their role in plant cell wall and storage polysaccharide degradation. PLoS ONE 2013, 8, E72572. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; McLellan, H.; Boevink, P.C.; Birch, P.R. All roads lead to susceptibility: The many modes of action of fungal and oomycete intracellular effectors. Plant Commun. 2020, 1, 100050. [Google Scholar] [CrossRef]
- Bakker, M.G.; Brown, D.W.; Kelly, A.C.; Kim, H.S.; Kurtzman, C.P.; Mccormick, S.P.; O’Donnell, K.L.; Proctor, R.H.; Vaughan, M.M.; Ward, T.J. Fusarium mycotoxins: A trans-disciplinary overview. Can. J. Plant Pathol. 2018, 40, 161–171. [Google Scholar] [CrossRef]
- Ayada, H.; Dhioui, B.; Mazouz, H.; El Harrak, A.; Jaiti, F.; Ouhmidou, B.; Diouri, M.; Moumni, M. In silico comparative genomic analysis unravels a new candidate protein arsenal specifically associated with Fusarium oxysporum f. sp. albedinis pathogenesis. Sci. Rep. 2022, 12, 19098. [Google Scholar] [CrossRef]
- Manici, L.M.; Caboni, E.; Caputo, F.; Frattarelli, A.; Lucioli, S. Phytotoxins from Dactylonectria torresensis involved in replant disease of fruit trees. Rhizosphere 2021, 17, 100300. [Google Scholar] [CrossRef]
- Wang, Z.; Nilsson, R.H.; James, T.Y.; Dai, Y.; Townsend, J.P. Future perspectives and challenges of fungal systematics in the age of big data. In Biology of Microfungi. Fungal Biology; Li, D.W., Ed.; Springer: Cham, Switzerland, 2016; pp. 25–46. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar] [CrossRef]
- Antil, S.; Abraham, J.S.; Sripoorna, S.; Maurya, S.; Dagar, J.; Makhija, S.; Bhagat, P.; Gupta, R.; Sood, U.; Lal, R.; et al. DNA barcoding, an effective tool for species identification: A review. Mol. Biol. Rep. 2023, 50, 761–775. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- O’Donnell, K.; Whitaker, B.K.; Laraba, I.; Proctor, R.H.; Brown, D.W.; Broders, K.; Kim, H.-S.; McCormick, S.P.; Busman, M.; Aoki, T.; et al. DNA sequence-based identification of Fusarium: A work in progress. Plant Dis. 2022, 106, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Geiser, D.M.; Jiménez-Gasco, M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Sulca Quispe, L. Eficacia de Trichoderma harzianum y Bacillus subtilis en el Control de la Pudrición Radicular (Cylindrocarpon destructans) del Orégano (Origanum vulgare L.), bajo Condiciones de Invernadero. Ph.D. Thesis, Universidad Nacional Jorge Basadre Grohmann, Tacna, Peru, 2023. [Google Scholar]
- French, E.R.; Hebert, T.T. Metodos De Investigacion Fitopatologica; Catie, B.O.I., Ed.; Instituto Interamericano de Ciencias Agrícolas: San José, Costa Rica, 1982; Volume 43, p. 165. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. Media—Recipes and Preparation. In The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006; pp. 5–14. [Google Scholar]
- Torbati, M.; Arzanlou, M.; Sandoval-Denis, M.; Crous, P.W. Multigene phylogeny reveals new fungicolous species in the Fusarium tricinctum species complex and novel hosts in the genus Fusarium from Iran. Mycol. Prog. 2019, 18, 119–133. [Google Scholar] [CrossRef]
- Lombard, L.; Van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Crous, P.W.; Groenewald, J.Z.; Risède, J.M.; Simoneau, P.; Hywel-Jones, N.L. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004, 50, 415–430. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Maddison, D.W.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Versión 3.61. 2018. Available online: http://mesquiteproject.org (accessed on 3 May 2024).
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree; Institute of Evoltionary Biology, University of Edinburgh: Edinburgh, Scotland, 2018; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 27 May 2024).
- Gaetán, S.A.; Madia, M.S.; Pérez, A. Recent outbreak of Fusarium crown and root rot caused by Fusarium solani on marjoram in Argentina. Australas. Plant Dis. Notes. 2007, 2, 15–16. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Crous, P.W. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Pers. Mol. Phylogeny Evol. Fungi 2018, 41, 109–129. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Nouri, M.T.; Trouillas, F.P. Taxonomy and multi-locus phylogeny of Cylindrocarpon-like species associated with diseased roots of grapevine and other fruit and nut crops in California. Fungal Syst. Evol. 2019, 4, 59–75. [Google Scholar] [CrossRef]
- Rana, A.; Sahgal, M.; Johri, B.N. Fusarium oxysporum: Genomics, diversity and plant–host interaction. In Developments in Fungal Biology and Applied Mycology; Springer: Singapore, 2017; pp. 159–199. [Google Scholar] [CrossRef]
- Guan, Y.M.; Lu, B.H.; Wang, Y.; Gao, J.; Wu, L.J. First report of root rot caused by Fusarium redolens on ginseng (Panax ginseng) in Jilin province of China. Plant Dis. 2014, 98, 844. [Google Scholar] [CrossRef]
- Bozoğlu, T.; Özer, G.; Imren, M.; Paulitz, T.C.; Dababat, A.A. First report of crown rot caused by Fusarium redolens on wheat in Kazakhstan. Plant Dis. 2021, 105, 3302. [Google Scholar] [CrossRef]
- Hamini-Kadar, N.; Edel-Hermann, V.; Gautheron, N.; Steinberg, C. First report of Fusarium commune and Fusarium redolens causing crown and root rot on tomato in Algeria. New Dis. Rep. 2010, 22, 3. [Google Scholar] [CrossRef]
- Galindo-Solís, J.M.; Fernández, F.J. Endophytic fungal terpenoids: Natural role and bioactivities. Microorganisms 2022, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Nigro, F.; Antelmi, I.; Sion, V.; Parente, P.; Pacifico, A. First report of Dactylonectria torresensis causing foot and root rot of olive trees. Plant Dis. 2019, 103, 768. [Google Scholar] [CrossRef]
- Erper, I.; Agustí-Brisach, C.; Tunali, B.; Armengol, J. Characterization of root rot disease of kiwifruit in the Black Sea region of Turkey. Eur. J. Plant Pathol. 2013, 136, 291–300. [Google Scholar] [CrossRef]
- Berlanas, C.; Ojeda, S.; López-Manzanares, B.; Andrés-Sodupe, M.; Bujanda, R.; Martínez-Diz, M.P.; Diaz-Losada, E.; Gramaje, D. Occurrence and diversity of black-foot disease fungi in symptomless grapevine nursery stock in Spain. Plant Dis. 2020, 104, 94–104. [Google Scholar] [CrossRef]
- Langenhoven, S.D.; Halleen, F.; Spies, C.F.; Stempien, E.; Mostert, L. Detection and quantification of black foot and crown and root rot pathogens in grapevine nursery soils in the Western Cape of South Africa. Phytopathol. Mediterr. 2018, 57, 519–537. [Google Scholar] [CrossRef]
- Farh, M.E.A.; Kim, Y.J.; Kim, Y.J.; Yang, D.C. Cylindrocarpon destructans/Ilyonectria radicicola-species complex: Causative agent of ginseng root-rot disease and rusty symptoms. J. Ginseng Res. 2018, 42, 9–15. [Google Scholar] [CrossRef]
- Johnson, J.M.; Oelmüller, R. Mutualism or parasitism: Life in an unstable continuum. What can we learn from the mutualistic interaction between Piriformospora indica and Arabidopsis thaliana? Rev. Endocytobiosis Cell Res. 2009, 19, 81–111. [Google Scholar]
- Manici, L.M.; Kelderer, M.; Caputo, F.; Saccà, M.L.; Nicoletti, F.; Topp, A.R.; Mazzola, M. Involvement of Dactylonectria and Ilyonectria spp. in tree decline affecting multi-generation apple orchards. Plant Soil 2018, 425, 217–230. [Google Scholar] [CrossRef]
- Tacna Regional Directorate of Agriculture. Agricultural Statistical Yearbook; Tacna Regional Directorate of Agriculture: Tacna, Peru, 2023. Available online: https://cms.agritacna.gob.pe/uploads/statistics/agricola/2024/b76fd885-e791-419b-941c-51a141345c1d.pdf (accessed on 2 July 2025).
- Tacna Regional Directorate of Agriculture. Agricultural Statistical Yearbook; Tacna Regional Directorate of Agriculture: Tacna, Peru, 2022. Available online: https://cms.agritacna.gob.pe/uploads/statistics/agricola/2022/e4fab05f-6c3e-4bc6-b30d-d41c93e9b6c8.pdf (accessed on 2 July 2025).






| Degree | Percentage (%) | Description of the Damage |
|---|---|---|
| 0 | 0 | Healthy plant. |
| 1 | 1–20 | Less than 5 secondary roots out of 10 evaluated showed dark brown necrosis, and small lesions on the main root, with no impact on the aerial part. |
| 2 | 21–40 | Less than 5 basal leaves with yellowing, curling, and epinasty out of 10 evaluated, with slight defoliation. Necrosis of the main root and more than 8 out of 10 secondary roots with dark brown coloration, necrotic rootlets in brown. Crown with brown necrosis and slight vascular discoloration. |
| 3 | 41–60 | More than 5 basal branches out of 10 evaluated with yellowing, curling of several branches, wilting, epinasty, leaf necrosis, and slight defoliation. Black necrosis in the main root, crown, and more than 5 secondary roots out of 10 evaluated, with bark peeling of the secondary root, dark brown necrosis of rootlets, and vascular discoloration in the crown. |
| 4 | 61–80 | Foliar yellowing of the entire aerial part and defoliation of basal branches. Main root and several secondary roots with black necrosis. Crown with vascular discoloration and black necrosis. |
| 5 | 81–100 | Dead plant. |
| Degree | Percentage (%) | Description of the Damage |
|---|---|---|
| 0 | 0 | Healthy plant. |
| 1 | 1–20 | Small lesions on the main root, with no impact on the aerial part. |
| 2 | 21–40 | Less than 5 secondary roots out of 10 evaluated with brown necrosis and slight discoloration in the crown, with mild wilting. |
| 3 | 41–60 | More than 5 basal leaves out of 10 evaluated with yellowing, curling, mild wilting, epinasty, slight defoliation, and death of central branches. Necrosis of the main root, more than 5 secondary roots, and rootlets out of 10 evaluated with dark brown necrosis. Crown with dark brown necrosis and slight vascular discoloration. |
| 4 | 61–80 | Foliar yellowing of the entire aerial part, defoliation of basal branches, and several dead branches. Main root and several secondary roots with dark brown necrosis. Crown with necrosis and severe vascular discoloration. |
| 5 | 81–100 | Dead plant. |
| Species | Isolation Code | Host |
|---|---|---|
| Dactylonectria torresensis | FARU0310 | Oregano Root |
| Dactylonectria torresensis | FARU0311 | |
| Fusarium iranicum | LUAD0312 | |
| Fusarium iranicum | LUAD0313 | |
| Fusarium oxysporum | LUAD0314 | Oregano Wreath |
| Fusarium oxysporum | LUAD0315 | |
| Fusarium redolens | LUAD0316 | |
| Fusarium iranicum | LUAD0317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe-Mamani, R.A.; Sulca-Quispe, L.; Huanca-Mamani, W.; Garcia-Castillo, M.G.; Muñoz-Torres, P.; Sepúlveda-Chavera, G. Identification of Fungi Causing Root Rot in Oregano Crops in Southern Peru: Morphological and Molecular Analysis. Pathogens 2025, 14, 746. https://doi.org/10.3390/pathogens14080746
Quispe-Mamani RA, Sulca-Quispe L, Huanca-Mamani W, Garcia-Castillo MG, Muñoz-Torres P, Sepúlveda-Chavera G. Identification of Fungi Causing Root Rot in Oregano Crops in Southern Peru: Morphological and Molecular Analysis. Pathogens. 2025; 14(8):746. https://doi.org/10.3390/pathogens14080746
Chicago/Turabian StyleQuispe-Mamani, Rubí Adelin, Liduvina Sulca-Quispe, Wilson Huanca-Mamani, Mirna G. Garcia-Castillo, Patricio Muñoz-Torres, and German Sepúlveda-Chavera. 2025. "Identification of Fungi Causing Root Rot in Oregano Crops in Southern Peru: Morphological and Molecular Analysis" Pathogens 14, no. 8: 746. https://doi.org/10.3390/pathogens14080746
APA StyleQuispe-Mamani, R. A., Sulca-Quispe, L., Huanca-Mamani, W., Garcia-Castillo, M. G., Muñoz-Torres, P., & Sepúlveda-Chavera, G. (2025). Identification of Fungi Causing Root Rot in Oregano Crops in Southern Peru: Morphological and Molecular Analysis. Pathogens, 14(8), 746. https://doi.org/10.3390/pathogens14080746







