Yeast Diversity on Sandy Lake Beaches Used for Recreation in Olsztyn, Poland
Abstract
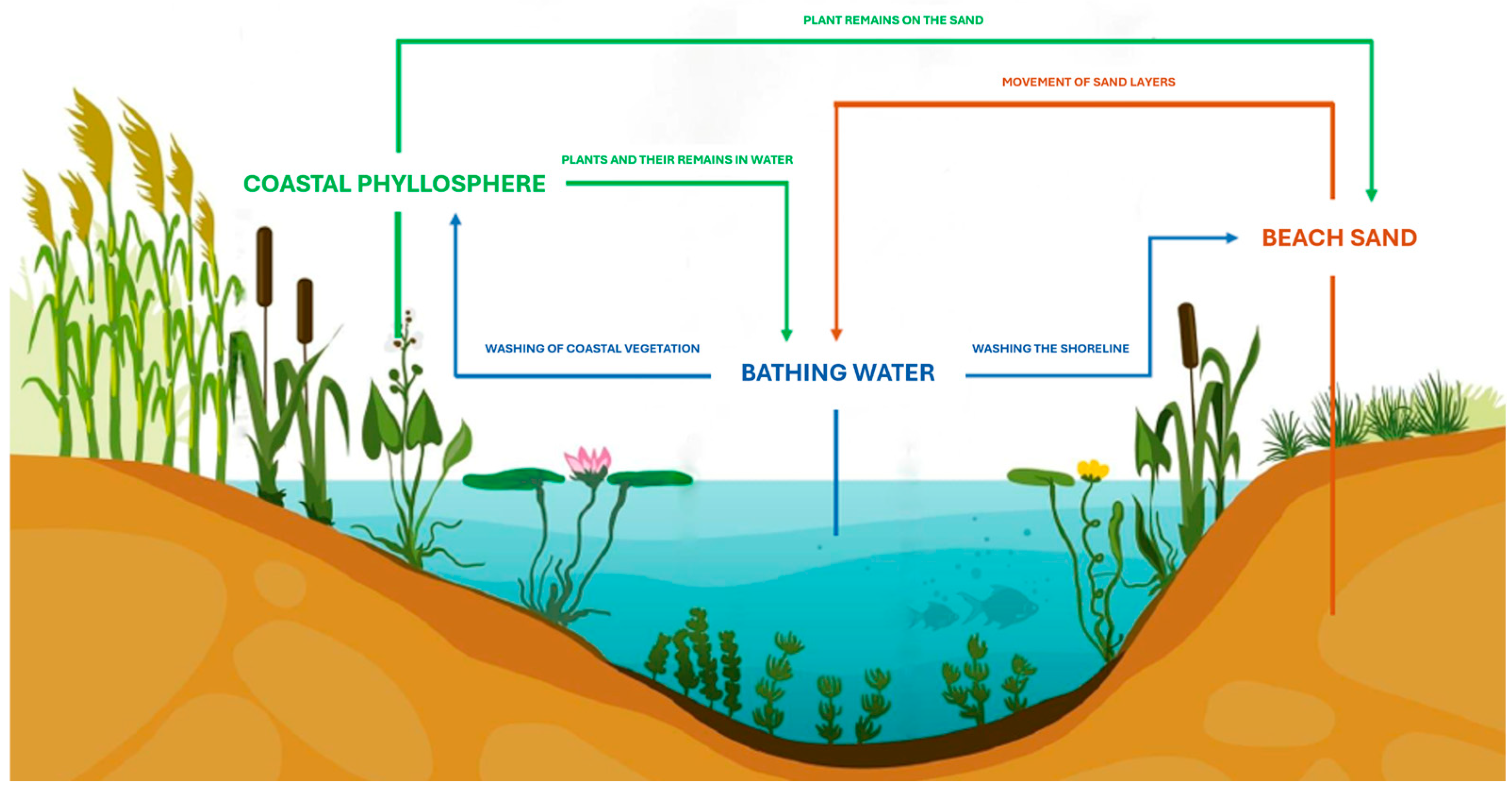
1. Introduction
2. Materials and Methods
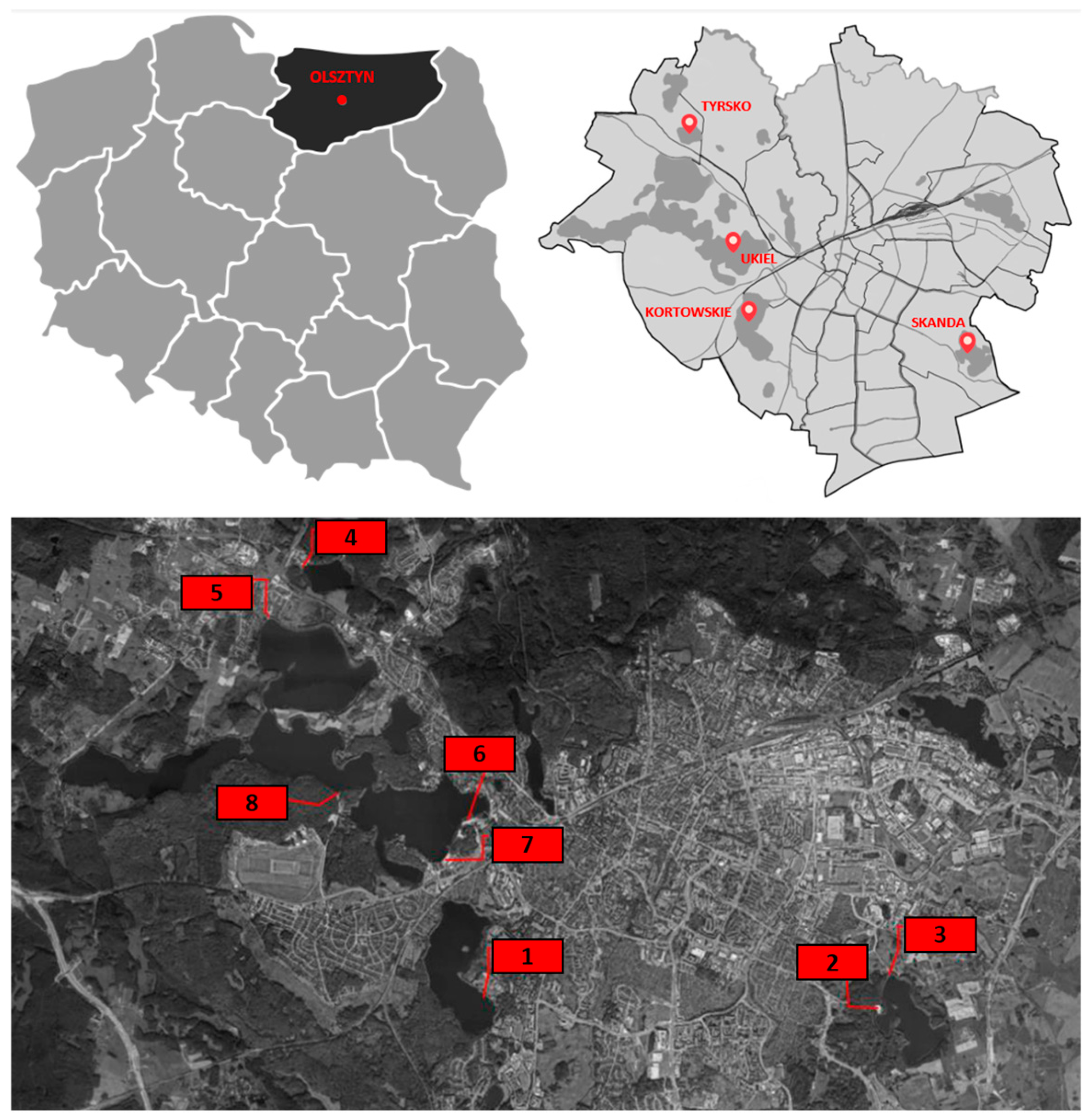
2.1. Research Area and Material
2.2. Sampling
2.3. Yeast Isolation and Cultivation
2.4. Yeast Count Assessment
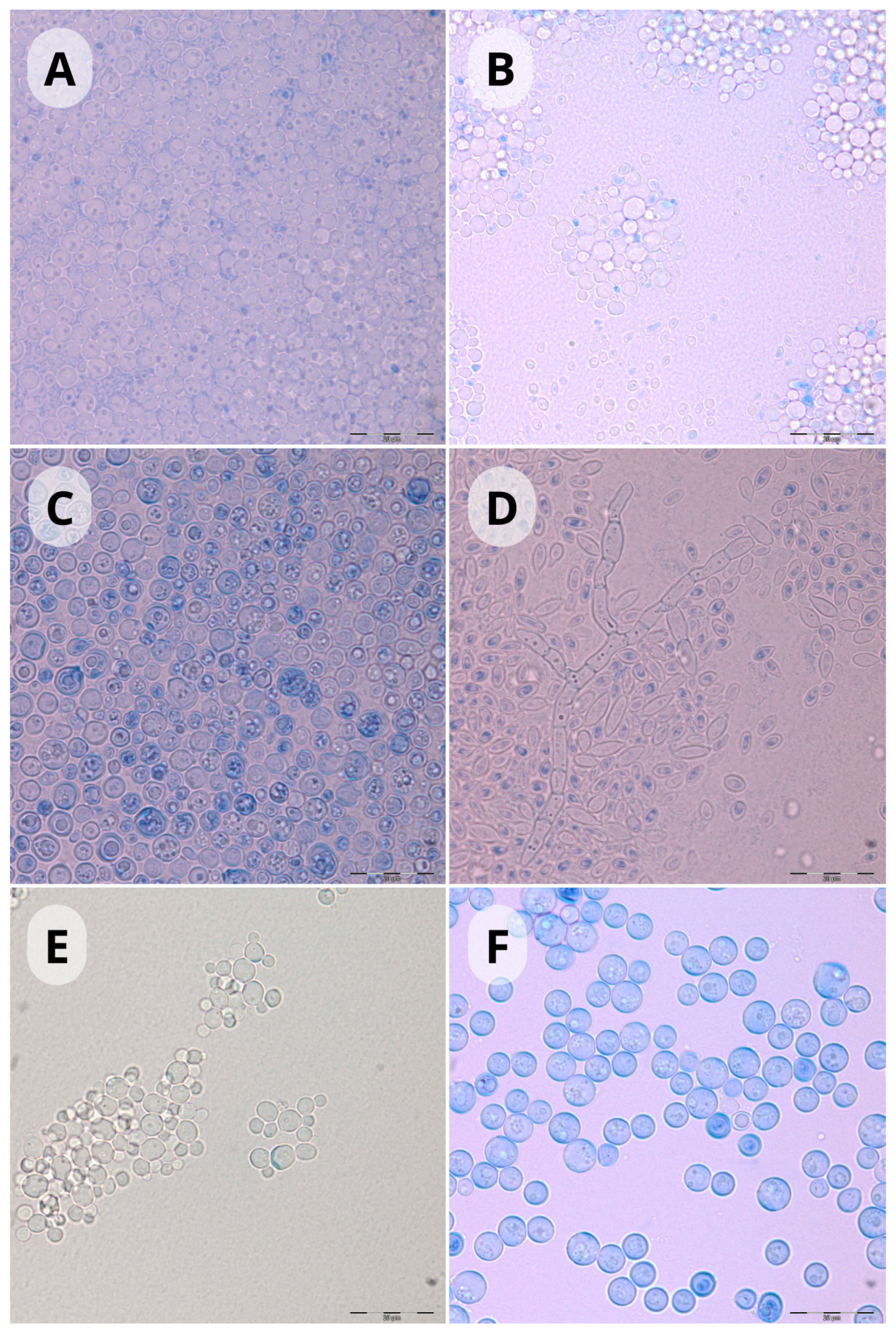
2.5. Yeast Identification
2.6. Statistical Analysis
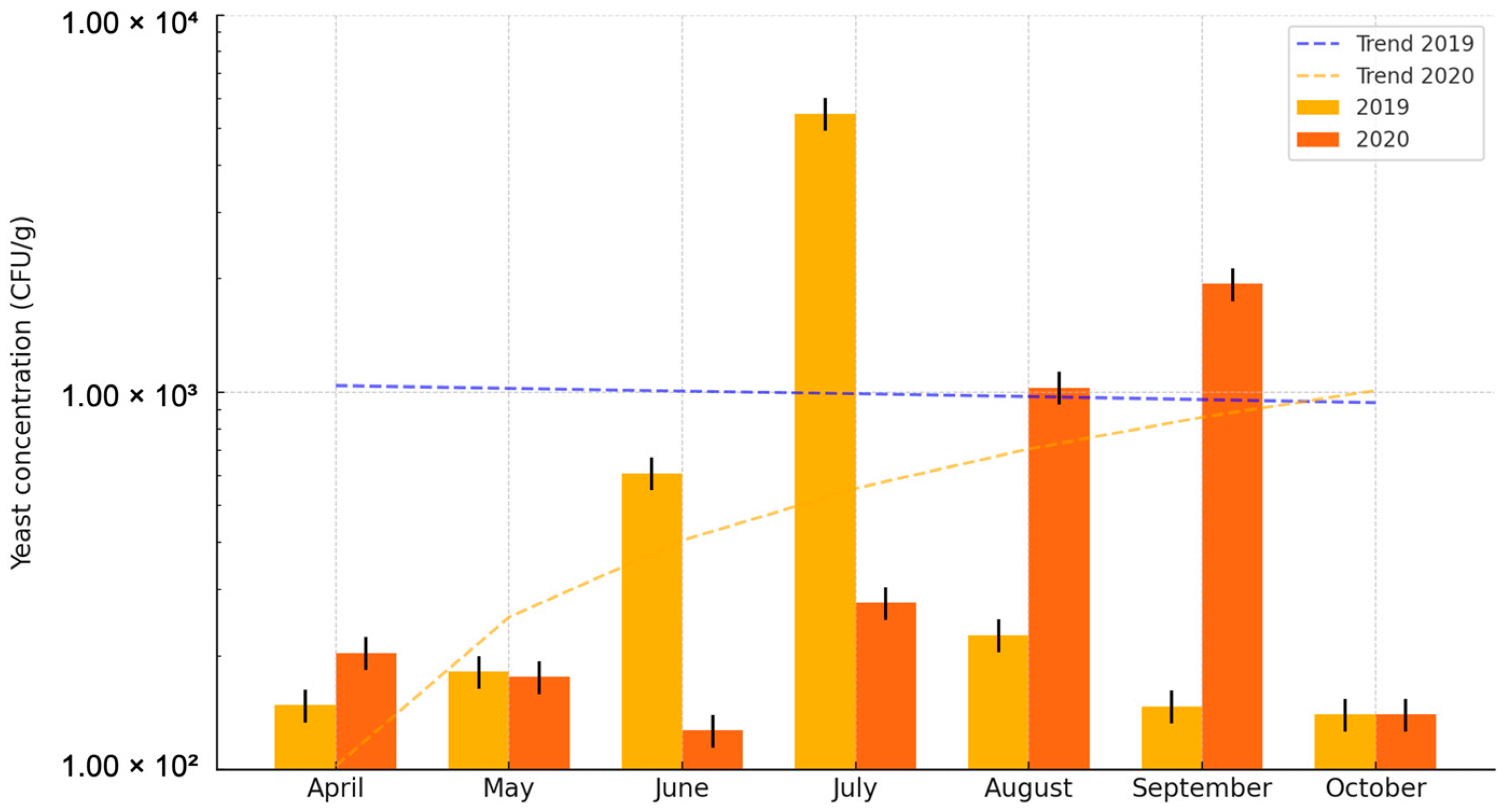
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A. Values of Meteorological Parameters During Sand Sampling
| SAMPLING PERIOD | TEMPERATURE | AVERAGE TEMPERATURE | AIR HUMIDITY | UV INDEX | ||
| MORNING | EVENING | MORNING | EVENING | |||
| APRIL/2019 | 6 °C | 14 °C | D 12 °C N 5 °C | 36% | 32% | 3/10 |
| MAY/2019 | 8 °C | 15 °C | D 15 °C N 8 °C | 73% | 29% | 4/10 |
| JUNE/2019 | 17 °C | 25 °C | D 25 °C N 15 °C | 64% | 45% | 7/10 |
| JULY/2019 | 20 °C | 21 °C | D 24 °C N 15 °C | 75% | 70% | 6/10 |
| AUGUST/2019 | 16 °C | 19 °C | D 22 °C N 14 °C | 85% | 51% | 5/10 |
| SEPTEMBER/2019 | 13 °C | 16 °C | D 18 °C N 11 °C | 75% | 51% | 4/10 |
| OCTOBER/2019 | 12 °C | 9 °C | D 13 °C N 7 °C | 91% | 91% | 2/10 |
| APRIL/2020 | 4 °C | 12 °C | D 13 °C N 5 °C | 69% | 29% | 3/10 |
| MAY/2020 | 6 °C | 6 °C | D 17 °C N 9 °C | 93% | 94% | 4/10 |
| JUNE/2020 | 12 °C | 18 °C | D 21 °C N 12 °C | 64% | 42% | 6/10 |
| JULY/2020 | 19 °C | 23 °C | D 22 °C N 14 °C | 85% | 59% | 6/10 |
| AUGUST/2020 | 19 °C | 24 °C | D 23 °C N 15 °C | 39% | 39% | 5/10 |
| SEPTEMBER/2020 | 13 °C | 16 °C | D 18 °C N 11 °C | 92% | 90% | 0/10 |
| OCTOBER/2020 | 11 °C | 8 °C | D 13 °C N 6 °C | 88% | 88% | 2/10 |
| D—average temperature of the month during the day, N—average temperature of the month at night. | ||||||
Appendix B. Estimated Number of Users of the “Ukiel” Recreational Complex and Supervised Beach on Lake Skanda During the Year—Data of the Sports and Recreation Center in Olsztyn
| LAKE UKIEL | ||||
| MONTH | 2019 | 2020 | ||
| SUM OF ENTRIES | SUM OF EXITS | SUM OF ENTRIES | SUM OF EXITS | |
| JANUARY | 32,751 | 29,601 | 14,557 | 13,889 |
| FEBRUARY | 64,786 | 84,381 | 4015 | 3385 |
| MARCH | 124,985 | 133,036 | 3458 | 3094 |
| APRIL | 106,946 | 126,388 | 1562 | 1918 |
| MAY | 142,397 | 132,035 | 8416 | 9846 |
| JUNE | 110,436 | 108,515 | 4343 | 5640 |
| JULY | 174,234 | 160,329 | THE SUPERVISOR DID NOT KEEP A REGISTER DUE TO THE COVID-19 PANDEMIC | |
| AUGUST | 72,885 | 72,431 | ||
| SEPTEMBER | 53,825 | 57,711 | ||
| OCTOBER | 24,177 | 27,222 | ||
| NOVEMBER | 12,461 | 13,223 | ||
| DECEMBER | 10,828 | 11,499 | ||
| SUM | 930,711 | 956,371 | 36,351 | 37,772 |
| LAKE SKANDA | ||||
| MONTH | 2019 | 2020 | ||
| PEOPLE PER DAY | MONTHLY | PEOPLE PER DAY | MONTHLY | |
| JANUARY | THE SUPERVISORY DOES NOT KEEP A REGISTER (OFF-SEASON PERIOD) | |||
| FEBRUARY | ||||
| MARCH | ||||
| APRIL | ||||
| MAY | ||||
| JUNE | Approx. 150 (weekends 500) | Approx. 3600 | Approx. 150 (weekends 500) | Approx. 3300 |
| JULY | Approx. 9300 | Approx. 10,200 | ||
| AUGUST | Approx. 10,200 | Approx. 9000 | ||
| SEPTEMBER | Approx. 2100 | Approx. 1950 | ||
| OCTOBER | THE SUPERVISORY DOES NOT KEEP A REGISTER (OFF-SEASON PERIOD) | |||
| NOVEMBER | ||||
| DECEMBER | ||||
| SUM | - | Approx. 25,200 | - | Approx. 24,450 |
References
- World Health Organization (WHO). Guidelines for Recreational Water Quality; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240031302 (accessed on 15 May 2025).
- Krajewska-Kułak, E.; Łukaszuk, C.; Gniadek, A.; Macura, A.B.; van Damme-Ostapowicz, K.; Lewko, J.; Rolka, H.; Rozwadowska, E.; Guzowski, A. Zanieczyszczenie Powietrza w Pomieszczeniach Mieszkalnych, Ze Szczególnym Uwzglednieniem Roli Grzybów [Air Pollution in Aviation Air, with Detailed Consideration of the Role of Fungi]. Mikol. Lek. 2010, 17, 177–181. [Google Scholar]
- Ejdys, E.; Dynowska, M.; Biedunkiewicz, A.; Sucharzewska, E. An Overview of the Species of Fungi Occurring in School Rooms—A Four-Year Study. Pol. J. Environ. Stud. 2013, 22, 1691–1700. [Google Scholar]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1078–1089. [Google Scholar] [CrossRef]
- Wrzosek, M.; Ruszkiewicz-Michalska, M.; Sikora, K.; Damszel, M.; Sierota, Z. The Plasticity of Fungal Interactions. Mycol. Prog. 2017, 16, 101–108. [Google Scholar] [CrossRef]
- Kowalski, M.; Pastuszka, J.S. Effect of Ambient Air Temperature and Solar Radiation on Changes in Bacterial and Fungal Aerosols Concentrationin the Urban Environment. Ann. Agric. Environ. Med. 2018, 25, 259–261. [Google Scholar] [CrossRef]
- Yurkov, A.M. Yeasts of the Soil—Obscure but Precious. Yeast 2018, 35, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bałabański, T.; Biedunkiewicz, A. Threats Resulting from the Presence of Potentially Pathogenic Fungi in the Sport Changing Rooms—A Preliminary Study. Pol. J. Environ. Stud. 2023, 32, 3033–3042. [Google Scholar] [CrossRef]
- Gouka, L.; Raaijmakers, J.M.; Cordovez, V. Ecology and Functional Potential of Phyllosphere Yeasts. Trends Plant Sci. 2022, 27, 1109–1123. [Google Scholar] [CrossRef]
- Biedunkiewicz, A.; Sucharzewska, E.; Kulesza, K.; Nowacka, K.; Kubiak, D. Phyllosphere of Submerged Plants in Bathing Lakes as a Reservoir of Fungi—Potential Human Pathogens. Microb. Ecol. 2020, 79, 552–561. [Google Scholar] [CrossRef]
- Brandão, J.; Gangneux, J.P.; Arikan-Akdagli, S.; Barac, A.; Bostanaru, A.C.; Brito, S.; Bull, M.; Çerikçioğlu, N.; Chapman, B.; Efstratiou, M.A.; et al. Mycosands: Fungal Diversity and Abundance in Beach Sand and Recreational Waters—Relevance to Human Health. Sci. Total Environ. 2021, 781, 146598. [Google Scholar] [CrossRef]
- Brandão, J.; Albergaria, I.; Albuquerque, J.; José, S.; Grossinho, J.; Ferreira, F.C.; Raposo, A.; Rodrigues, R.; Silva, C.; Jordao, L.; et al. Untreated Sewage Contamination of Beach Sand from a Leaking Underground Sewage System. Sci. Total Environ. 2020, 740, 140237. [Google Scholar] [CrossRef]
- Biedunkiewicz, A.; Góralska, K. Microfungi Potentially Pathogenic for Humans Reported in Surface Waters Utilized for Recreation. Clean-Soil Air Water 2016, 44, 599–609. [Google Scholar] [CrossRef]
- Libkind, D.; Buzzini, P.; Turchetti, B. Yeasts in Continental and Seawater. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.-A., Yurkov, A.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–61. [Google Scholar]
- Fedorova, M.D.; Kurakov, A.V. Microbiota of Bottom Sediments in the Coastal Zone of Lake Baikal. Contemp. Probl. Ecol. 2023, 16, 492–508. [Google Scholar] [CrossRef]
- Nagahama, T. Yeast Biodiversity in Freshwater, Marine and Deep-Sea Environments. In Biodiversity and Ecophysiology of Yeasts; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 241–262. [Google Scholar]
- Monapathi, M.E.; Bezuidenhout, C.C.; James Rhode, O.H. Aquatic Yeasts: Diversity, Characteristics and Potential Health Implications. J. Water Health 2020, 18, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Biró, J.; Kucska, B.; Hancz, C. Factors Affecting Yeast Digestibility and Immunostimulation in Aquatic Animals. Animals 2024, 14, 2851. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Botha, A. The Importance and Ecology of Yeasts in Soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Biedunkiewicz, A.; Dynowska, M.; Ejdys, E.; Sucharzewska, E. Species Diversity of Yeast-like Fungi in Some Eutrophic Lakes in Olsztyn. Acta Mycol. 2013, 48, 61–71. [Google Scholar] [CrossRef]
- Nowacka, K.; Kulesza, K.; Glinka, P.; Sucharzewska, E.; Dynowska, M. The Phyllosphere as a Little-Known Reservoir of the Fusarium Genus, a Fungi of Importance to Medical Mycology. Ann. Parasitol. 2018, 64, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, H.; Wolfaardt, G.M.; Botha, A.; Van Rensburg, P.; Pretorius, I.S. Establishing a Risk-Assessment Process for Release of Genetically Modified Wine Yeast into the Environment. Can. J. Microbiol. 2009, 55, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Maciel, N.O.P.; Johann, S.; Brandão, L.R.; Kucharíková, S.; Morais, C.G.; Oliveira, A.P.; Freitas, G.J.C.; Borelli, B.M.; Pellizzari, F.M.; Santos, D.A.; et al. Occurrence, Antifungal Susceptibility, and Virulence Factors of Opportunistic Yeasts Isolated from Brazilian Beaches. Mem. Inst. Oswaldo Cruz 2019, 114, e180566. [Google Scholar] [CrossRef]
- Dynowska, M. The Variability of Fungi and Its Significance. In Mykologia Medyczna; Kurnatowska, A., Kurnatowski, P., Eds.; Edra Urban & Partner: Wrocław, Poland, 2018; pp. 17–25. [Google Scholar]
- Walker, J.K.M.; Ward, V.; Jones, M.D. Ectomycorrhizal Fungal Exoenzyme Activity Differs on Spruce Seedlings Planted in Forests versus Clearcuts. Trees-Struct. Funct. 2016, 30, 497–508. [Google Scholar] [CrossRef]
- Menu, E.; Filori, Q.; Dufour, J.C.; Ranque, S.; L’Ollivier, C. A Repertoire of the Less Common Clinical Yeasts. J. Fungi 2023, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Gregorczyk-Maga, I.; Kania, M.; Sulik-Tyszka, B.; Namysł, M.; Sepioło, A.; Romaniszyn, D.; Jachowicz-Matczak, E.; Wójkowska-Mach, J. Oral Myco- and Bacteriobiota and Yeast Infections in Mechanically Ventilated COVID-19 Patients. Microorganisms 2023, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Kurnatowska, A.; Kurnatowski, P. Mykologia Medyczna [Medical Mycology]; Edra Urban & Partner: Wrocław, Poland, 2018. [Google Scholar]
- Dynowska, M.; Sucharzewska, E.; Biedunkiewicz, A. Enzymatic Activity of Fungi of the Genus Candida Isolated from the Skin and the Digestive Tract in People and from Municipal Sewage. Acta Mycol. 2001, 36, 293–302. [Google Scholar] [CrossRef]
- Dynowska, M.; Biedunkiewicz, A. Mikrogrzyby Wód o Potencjalnych Właściwościach Bioindykacyjnych [Water Microfungi with Potential Bioindicative Properties]. In Biologiczne Metody Oceny Stanu Środowiska. Tom 2. Ekosystemy Wodne [Biological Methods of Assessing the State of the Environment. Volume 2. Water Ecosystems]; Ciecierska, H., Dynowska, M., Eds.; Mantis: Olsztyn, Poland, 2013; pp. 284–310. [Google Scholar]
- Wójcik, A.; Kurnatowski, P.; Błaszkowska, J. Potentially Pathogenic Yeasts from Soil of Children’s Recreational Areas in the City of Łódź (Poland). Int. J. Occup. Med. Environ. Health 2013, 26, 477–487. [Google Scholar] [CrossRef]
- Wójcik, A.; Błaszkowska, J.; Kurnatowski, P.; Góralska, K. Sandpits as a Reservoir of Potentially Pathogenic Fungi for Children. Ann. Agric. Environ. Med. 2016, 23, 542–548. [Google Scholar] [CrossRef]
- Echevarría, L.; Iqbal, M.N. Identification of Fungi and Yeasts from the Sands of the Pyramids of Giza, in Cairo, Egypt. PSM Biol. Res. 2021, 6, 13–18. [Google Scholar]
- Kulesza, K.; Biedunkiewicz, A.; Nowacka, K.; Dynowska, M.; Urbaniak, M.; Stępień, Ł. Dishwashers as an Extreme Environment of Potentially Pathogenic Yeast Species. Pathogens 2021, 10, 446. [Google Scholar] [CrossRef]
- Dynowska, M.; Ejdys, E. Mikologia Laboratoryjna–Przygotowanie Materiału Badawczego i Diagnostyka [Laboratory Mycology–Preparation of Research Material and Diagnostics]; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2011. [Google Scholar]
- Ergül, C.; Çalişkan, E. Endospore Formed Bacteria and Staining Techniques. In Science, Ecology and Engineering Research in the Globalizing World; Christov, I., Strauss, E., Gad, A.-A., Curebal, I., Eds.; World. St. Kliment Ohridski University Press: Sofia, Bulgaria, 2018; pp. 362–374. [Google Scholar]
- De Hoog, G.; Guarro, J.; Gené, J.; Figueras, M.J. Atlas of Clinical Fungi, 2nd ed.; Centraalbureau voor Schimmelcultures/Universitat Rovira and Virgili: Reus, Spain, 2000. [Google Scholar]
- De Hoog, S.; Guarro, J.; Gené, J.; Ahmed, S.; Al-Hatmi, A.; Figueras, M.; Vitale, R. Atlas of Clinical Fungi: The Ultimate Benchtool for Diagnostics: Part α: Introductions, Lower Fungi, Basidiomycetes, Yeasts, Filamentous Ascomycetes A-B, 4th ed.; Westerdijk Institute/Universitat Rovira i Virgili: Reus, Spain, 2019. [Google Scholar]
- De Hoog, S.; Guarro, J.; Gené, J.; Ahmed, S.; Al-Hatmi, A.; Figueras, M.; Vitale, R. Atlas of Clinical Fungi: The Ultimate Benchtool for Diagnostics: Part β: Filamentous Ascomycetes C-Z, 4th ed.; Westerdijk Institute/Universitat Rovira i Virgili: Reus, Spain, 2019. [Google Scholar]
- Abdullabekova, D.A.; Magomedova, E.S.; Magomedov, G.G.; Kachalkin, A.V. Yeasts as an Element of Ampelocenosis Soil Biodiversity in an Arid Climate. Arid Ecosyst. 2021, 11, 299–303. [Google Scholar] [CrossRef]
- Novak Babič, M.; Gunde-Cimerman, N.; Breskvar, M.; Džeroski, S.; Brandão, J. Occurrence, Diversity and Anti-Fungal Resistance of Fungi in Sand of an Urban Beach in Slovenia—Environmental Monitoring with Possible Health Risk Implications. J. Fungi 2022, 8, 860. [Google Scholar] [CrossRef]
- Frenkel, M.; Serhan, H.; Blum, S.E.; Fleker, M.; Sionov, E.; Amit, S.; Gazit, Z.; Gefen-Halevi, S.; Segal, E. What Is Hiding in the Israeli Mediterranean Seawater and Beach Sand. J. Fungi 2022, 8, 950. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia Coli: Ecology and Public Health Implications—A Review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Brandão, J.; Weiskerger, C.; Valério, E.; Pitkänen, T.; Meriläinen, P.; Avolio, L.; Heaney, C.D.; Sadowsky, M.J. Climate Change Impacts on Microbiota in Beach Sand and Water: Looking Ahead. Int. J. Environ. Res. Public Health 2022, 19, 1444. [Google Scholar] [CrossRef]
- Kossowski, J. Relationship of Daily Amplitudes of Soil Temperature in the Near-Surface Layer with Air Temperature Amplitudes and Other Meteorological Elements [Związek Amplitud Dobowych Temperatury Gleby w Warstwie Przypowierzchniowej z Amplitudami Temperatury Powietrz. Acta Agrophysica 2005, 5, 657–667. [Google Scholar]
- Cuartero, J.; Querejeta, J.I.; Prieto, I.; Frey, B.; Alguacil, M.M. Warming and Rainfall Reduction Alter Soil Microbial Diversity and Co-Occurrence Networks and Enhance Pathogenic Fungi in Dryland Soils. Sci. Total Environ. 2024, 949, 175006. [Google Scholar] [CrossRef]
- Shah, A.H.; Abdelzaher, A.M.; Phillips, M.; Hernandez, R.; Solo-Gabriele, H.M.; Kish, J.; Scorzetti, G.; Fell, J.W.; Diaz, M.R.; Scott, T.M.; et al. Indicator Microbes Correlate with Pathogenic Bacteria, Yeasts and Helminthes in Sand at a Subtropical Recreational Beach Site. J. Appl. Microbiol. 2011, 110, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, J.F.; Grace, D.; Strand, T. The Consequences of Human Actions on Risks for Infectious Diseases: A Review. Infect. Ecol. Epidemiol. 2015, 5, 30048. [Google Scholar] [CrossRef]
- Kauppinen, A.; Al-Hello, H.; Zacheus, O.; Kilponen, J.; Maunula, L.; Huusko, S.; Lappalainen, M.; Miettinen, I.; Blomqvist, S.; Rimhanen-Finne, R. Increase in Outbreaks of Gastroenteritis Linked to Bathing Water in Finland in Summer 2014. Eurosurveillance 2017, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skowera, B.; Wojkowski, J. Relation of Soil Temperature with Air Temperature at the Jurassic River Valley. Ecol. Eng. 2017, 18, 18–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grishkan, I.; Kidron, G.J. Vertical Divergence of Cultured Microfungal Communities Through the Depth in Different Soil Formations at Nahal Nizzana, Western Negev Desert, Israel. Geomicrobiol. J. 2016, 33, 564–577. [Google Scholar] [CrossRef]
- Mooiman, C.; Bouwknegt, J.; Dekker, W.J.C.; Wiersma, S.J.; Ortiz-Merino, R.A.; De Hulster, E.; Pronk, J.T. Critical Parameters and Procedures for Anaerobic Cultivation of Yeasts in Bioreactors and Anaerobic Chambers. FEMS Yeast Res. 2021, 21, foab035. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, H.; Lu, Y.; Aljohani, R.; Al-Amad, A.; Yoell, H.; Xu, J. Global Patterns in Culturable Soil Yeast Diversity. iScience 2021, 24, 103098. [Google Scholar] [CrossRef] [PubMed]
- Kaewkrajay, C.; Putchakarn, S.; Limtong, S. Cultivable Yeasts Associated with Marine Sponges in the Gulf of Thailand, South China Sea. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2021, 114, 253–274. [Google Scholar] [CrossRef]
- Góralska, K.; Biedunkiewicz, A. Human Mycobiota-Selected Ontocenoses of Students of Natural Science and Medicine. J. Bacteriol. Parasitol. 2016, 7, 3–8. [Google Scholar] [CrossRef]
- Salah, H.A.; Temerk, H.A.; Salah, N.A.; Alshehri, S.R.Z.; Al-Harbi, J.A.; Mawad, A.M.M.; Khaled, K.A.M.; Hesham, A.E.L.; Amein, K.A. Production and Optimization of Xylanase and α-Amylase from Non-Saccharomyces Yeasts (Pichia Membranifaciens). J. Pure Appl. Microbiol. 2021, 15, 452–461. [Google Scholar] [CrossRef]
- Mullen, A.A.; Lynch, C.D.; Hill, S.M.; Holohan, C.P.; Cróinín, T.Ó.; Wolfe, K.H.; Brien, C.E.O. Crossm Draft Genome Sequences of Two Natural Isolates of the Yeast. Genome Announc. 2018, 6, 1–2. [Google Scholar] [CrossRef]
- Desnos-Ollivier, M.; Ragon, M.; Robert, V.; Raoux, D.; Gantier, J.C.; Dromer, F. Debaryomyces Hansenii (Candida famata), a Rare Human Fungal Pathogen Often Misidentified as Pichia guilliermondii (Candida guilliermondii). J. Clin. Microbiol. 2008, 46, 3237–3242. [Google Scholar] [CrossRef]
- Drumonde-Neves, J.; Franco-Duarte, R.; Lima, T.; Schuller, D.; Pais, C. Yeast Biodiversity in Vineyard Environments Is Increased by Human Intervention. PLoS ONE 2016, 11, e0160579. [Google Scholar] [CrossRef]
- Lossow, K.; Gawrońska, H.; Mientki, C.; Łopata, M.; Wiśniewski, G. Olsztyn Lakes, Trophic Status, Threats [Jeziora Olsztyna, Stan Troficzny, Zagrożenia]; Studio Przygotowawcze Wydawnictw “Edycja” s.c.: Olsztyn, Poland, 2005. [Google Scholar]
- Do Espírito Santo, E.P.T.; Monteiro, R.C.; da Costa, A.R.F.; Marques-da-Silva, S.H. Molecular Identification, Genotyping, Phenotyping, and Antifungal Susceptibilities of Medically Important Trichosporon, Apiotrichum, and Cutaneotrichosporon Species. Mycopathologia 2020, 185, 307–317. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Wang, C.; Gu, Y.; Cao, S.; Huang, X.; Wen, Y.; Zhao, Q.; Wu, R.; Wen, X.; et al. Identification, Genotyping, and Pathogenicity of Trichosporon Spp. Isolated from Giant Pandas (Ailuropoda Melanoleuca). BMC Microbiol. 2019, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Breuer, U.; Harms, H. Debaryomyces hansenii—An Extremophilic Yeast with Biotechnological Potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, Epidemiology, Pathogenicity and Antifungal Resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Martin, H.; Kavanagh, K.; Velasco-Torrijos, T. Targeting Adhesion in Fungal Pathogen Candida albicans. Future Med. Chem. 2021, 13, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, R.; Sanchez, H.; Andreu, C.; Andes, D.; del Olmo, M. Formation and Characterization of Biofilms Formed by Salt-Tolerant Yeast Strains in Seawater-Based Growth Medium. Appl. Microbiol. Biotechnol. 2021, 105, 2411–2426. [Google Scholar] [CrossRef]
- Silva, L.; Ramirez, M.; Osorio, E. Yeast Diversity Associated to Sediments and Water from Two Colombian Artificial Lakes. Braz. J. Microbiol. 2014, 45, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Li, L.; Xiao, A.; Cao, Y.; Chen, Y.; Cai, H. Identification and Characterization of a New Naringinase-Producing Strain, Williopsis Californica Jmudeb007. World J. Microbiol. Biotechnol. 2011, 27, 2857–2862. [Google Scholar] [CrossRef]
- Bertout, S.; Gouveia, T.; Krasteva, D.; Pierru, J.; Pottier, C.; Bellet, V.; Arianiello, E.; Salipante, F.; Roger, F.; Drakulovski, P. Search for Cryptococcus neoformans/Gattii Complexes and Related Genera (Filobasidium, Holtermanniella, Naganishia, Papiliotrema, Solicoccozyma, Vishniacozyma) Spp. Biotope: Two Years Surveillance of Wild Avian Fauna in Southern France. J. Fungi 2022, 8, 227. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, H.; Qiu, L.; Fu, Y.; Zhu, X.; Zhang, H.; Wang, J.; Chen, D. Appetite Suppression and Interleukin 17 Receptor Signaling Activation of Colonic Mycobiota Dysbiosis Induced by High Temperature and High Humidity Conditions. Front. Cell. Infect. Microbiol. 2021, 11, 657807. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, H.; Qiu, Y.; Xu, Z.; Xie, Q.; Ding, W.; Liu, H.; Li, G. Dysbiosis of Gastric Mucosal Fungal Microbiota in the Gastric Cancer Microenvironment. J. Immunol. Res. 2022, 2022, 6011632. [Google Scholar] [CrossRef]
- Gupta, S.; Paul, K.; Kaur, S. Diverse Species in the Genus Cryptococcus: Pathogens and Their Non-Pathogenic Ancestors. IUBMB Life 2020, 72, 2303–2312. [Google Scholar] [CrossRef]
- Passer, A.R.; Coelho, M.A.; Billmyre, R.B.; Nowrousian, M.; Mittelbach, M.; Yurkov, A.M.; Averette, A.F.; Cuomo, C.A.; Sun, S.; Heitman, J. Genetic and Genomic Analyses Reveal Boundaries between Species Closely Related to Cryptococcus Pathogens. mBio 2019, 10, e00764-19. [Google Scholar] [CrossRef]
- Lief, M.H.; Caplivski, D.; Bottone, E.J.; Lerner, S.; Vidal, C.; Huprikar, S. Exophiala Jeanselmeiinfection in Solid Organ Transplant Recipients: Report of Two Cases and Review of the Literature. Transpl. Infect. Dis. 2011, 13, 73–79. [Google Scholar] [CrossRef]
- Badali, H.; Najafzadeh, M.J.; Van Esbroeck, M.; van den Enden, E.; Tarazooie, B.; Meis, J.F.G.M.; de Hoog, G.S. The Clinical Spectrum of Exophiala jeanselmei, with a Case Report and in Vitro Antifungal Susceptibility of the Species. Med. Mycol. 2009, 48, 318–327. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Ngan, A.H.Y.; Tsang, C.C.C.; Ling, I.W.H.; Chan, J.F.W.; Leung, S.Y.; Yuen, K.Y.; Lau, S.K.P. Clinical Spectrum of Exophiala Infections and a Novel Exophiala Species, Exophiala hongkongensis. J. Clin. Microbiol. 2013, 51, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Biedunkiewicz, A.; Schulz, Ł. Fungi of the Genus Exophiala in Tap Water—Potential Etiological Factors of Phaeohyphomycoses. Mikol. Lek. 2012, 19, 23–26. [Google Scholar]
| No. | Species | MI | DV | BSL | RG | SUM | M | E | d10 | d50 | S | US | s19 | s20 | LK | LS | LT | LU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aureobasidium pullulans (de Bary & Löwenthal) G. Arnaud 1918 | + | A | 1 | 1 | 5 | 4 | 1 | 2 | 3 | 1 | 4 | 1 | 4 | 1 | 0 | 3 | 1 |
| 2 | Barnettozyma californica (Lodder) Kurtzman, Robnett & Bas.-Powers 2008 | + | A | 1 | 1 | 12 | 7 | 5 | 11 | 1 | 8 | 4 | 8 | 4 | 2 | 0 | 1 | 9 |
| 3 | ** Candida albicans (C.P. Robin) Berkhout 1923 | A | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 4 | Citeromyces matritensis (Santa María) Santa María 1957 | A | 1 | 1 | 8 | 5 | 3 | 8 | 0 | 6 | 2 | 2 | 6 | 2 | 5 | 0 | 1 | |
| 5 | Clavispora lusitaniae Rodr. Mir. 1979 (anamorfa: Candida lusitaniae) | A | 2 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 6 | Cryptococcus amylolentus (Van der Walt, D.B. Scott & Klift) Golubev 1981 | B | 1 | 1 | 4 | 1 | 3 | 3 | 1 | 0 | 4 | 0 | 4 | 3 | 0 | 1 | 0 | |
| 7 | Cryptococcus uniguttulatus (Wolfram & Zach) Phaff & Fell 1970 | B | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 8 | Cutaneotrichosporon jirovecii (Frágner) Xin Zhan Liu, F.Y. Bai, M. Groenew. & Boekhout 2015 | + | B | 2 | 2 | 16 | 8 | 8 | 12 | 4 | 4 | 12 | 15 | 1 | 4 | 6 | 1 | 5 |
| 9 | Cutaneotrichosporon moniliiforme (E. Guého & M.T. Sm.) Xin Zhan Liu, F.Y. Bai, M. Groenew. & Boekhout 2015 | + | B | 2 | 2 | 28 | 18 | 10 | 17 | 11 | 6 | 22 | 25 | 3 | 8 | 9 | 3 | 8 |
| 10 | Cyniclomyces guttulatus (C.P. Robin) Van der Walt & D.B. Scott 1971 | A | 1 | 1 | 2 | 2 | 0 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | |
| 11 | ** Debaryomyces hansenii (Zopf) Lodder & Kreger-van Rij 1984 | + | A | 1 | 1 | 24 | 7 | 17 | 10 | 14 | 13 | 11 | 5 | 19 | 4 | 7 | 2 | 11 |
| 12 | Dothiora sorbi (Wahlenb.) Fuckel 1870 | + | A | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 13 | Exophiala bergeri Haase & de Hoog 1999 | A | 2 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | |
| 14 | Exophiala castellanii Iwatsu, Nishim. & Miyaji 1984 | A | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | |
| 15 | Exophiala jeanselmei (Langeron) McGinnis & A.A. Padhye 1977 | A | 2 | 2 | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | |
| 16 | ** Geotrichum albidum (Lagerh.) H.Y. Zhu, X.Z. Liu & F.Y. Bai 2024 | A | 1 | 1 | 3 | 2 | 1 | 3 | 0 | 2 | 1 | 0 | 3 | 1 | 2 | 0 | 0 | |
| 17 | Geotrichum galactomycetum H.Y. Zhu, X.Z. Liu & F.Y. Bai 2024 | A | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | |
| 18 | Hanseniaspora osmophila (Niehaus) Phaff, M.W. Mill. & Shifrine 1956 | A | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 19 | Isabelozyma rhagii (Diddens & Lodder) Q.M. Wang, Yurkov, Boekhout & F.Y. Bai 2024 | A | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | |
| 20 | Komagataella pastoris (Guillierm.) Y. Yamada, M. Matsuda, K. Maeda & Mikata 1995 | A | 1 | 1 | 3 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 2 | |
| 21 | Kondoa malvinella (Fell & I.L. Hunter) Y. Yamada, Nakagawa & I. Banno 1989 | B | 1 | 1 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | |
| 22 | Kregervanrija fluxuum (Phaff & E.P. Knapp) Kurtzman 2006 (anamorfa: Candida vini) | A | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 23 | Leucosporidium scottii Fell, Statzell, I.L. Hunter & Phaff 1970 | B | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 24 | Lipomyces lipofer (Den Dooren) Lodder & Kreger-van Rij 1952 | + | A | 1 | 1 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| 25 | Lodderomyces elongisporus (Recca & Mrak) Van der Walt 1971 | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 26 | Metschnikowia pulcherrima Pitt & M.W. Mill. 1968 | A | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | |
| 27 | Moniliella spathulata (de Hoog) C.A. Rosa & Lachance 2009 | B | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | |
| 28 | Mycogloea nipponica Bandoni 1998 | B | 1 | 1 | 6 | 2 | 4 | 4 | 2 | 4 | 2 | 0 | 6 | 1 | 3 | 0 | 2 | |
| 29 | ** Nadsonia commutata Golubev 1973 | A | 1 | 1 | 3 | 3 | 0 | 1 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 3 | |
| 30 | Nadsonia fulvescens var. elongata (Konok.) Golubev & M.T. Sm. 1989 | A | 1 | 1 | 5 | 3 | 2 | 3 | 2 | 0 | 5 | 4 | 1 | 3 | 2 | 0 | 0 | |
| 31 | Naganishia albida (Saito) Xin Zhan Liu, F.Y. Bai, M. Groenew. & Boekhout 2015 | B | 1 | 1 | 3 | 1 | 2 | 3 | 0 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 2 | |
| 32 | ** Nakaseomyces glabratus (H.W. Anderson) Sugita & M. Takash 2022 | A | 2 | 2 | 12 | 6 | 6 | 8 | 4 | 7 | 5 | 3 | 9 | 0 | 5 | 1 | 6 | |
| 33 | Octosporomyces octosporus (Beij.) Kudryavtsev 1960 | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | |
| 34 | Ogataea angusta (Teun., H.H. Hall & Wick.) S.O. Suh & J.J. Zhou 2010 | A | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | |
| 35 | Ogataea minuta (Wick.) Y. Yamada, K. Maeda & Mikata 1994 | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 36 | Oosporidium margaritiferum Stautz 1931 | A | 1 | 1 | 6 | 5 | 1 | 3 | 3 | 1 | 5 | 0 | 6 | 2 | 3 | 0 | 1 | |
| 37 | Papiliotrema laurentii (Kuff.) Xin Zhan Liu, F.Y. Bai, M. Groenew. & Boekhout 2015 | + | B | 1 | 1 | 4 | 3 | 1 | 3 | 1 | 2 | 2 | 0 | 4 | 0 | 2 | 0 | 2 |
| 38 | Papiliotrema pseudoalba (Nakase & M. Suzuki) Xin Zhan Liu, F.Y. Bai, M. Groenew. & Boekhout 2015) | B | 1 | 1 | 3 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 3 | 0 | 0 | 0 | |
| 39 | ** Pichia fermentans Lodder 1932 | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| 40 | Pichia membranifaciens (E.C. Hansen) E.C. Hansen 1904 | A | 1 | 1 | 6 | 4 | 2 | 5 | 1 | 2 | 4 | 4 | 2 | 0 | 5 | 0 | 1 | |
| 41 | Pichia pseudolambica (M.T. Sm. & Poot) H.Y. Zhu, X.Z. Liu & F.Y. Bai 2024 | + | A | 1 | 1 | 5 | 4 | 1 | 3 | 2 | 0 | 5 | 1 | 4 | 0 | 3 | 0 | 2 |
| 42 | Pichia terricola Van der Walt 1957 | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 43 | Rhodotorula diobovata (S.Y. Newell & I.L. Hunter) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout 2015 | B | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| 44 | Saccharomyces bayanus Sacc. 1895 | A | 1 | 1 | 3 | 1 | 2 | 0 | 3 | 2 | 1 | 0 | 3 | 1 | 2 | 0 | 0 | |
| 45 | Saccharomyces cerevisiae (Desm.) Meyen 1838 | A | 1 | 1 | 5 | 1 | 4 | 4 | 1 | 1 | 4 | 1 | 4 | 0 | 3 | 1 | 1 | |
| 46 | Saccharomyces mikatae G.I. Naumov, S.A. James, E.S. Naumova, E.J. Louis & I.N. Roberts 2000 | A | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | |
| 47 | ** Saccharomycodes ludwigii (E.C. Hansen) E.C. Hansen 1904 | A | 1 | 1 | 3 | 2 | 1 | 3 | 0 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | |
| 48 | Saitozyma podzolica (Babeva & Reshetova) Xin Zhan Liu, F.Y. Bai, M. Groenew. & Boekhout 2015 | + | B | 1 | 1 | 3 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 2 |
| 49 | Schizosaccharomyces pombe Lindner 1893 | A | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 50 | Schwanniomyces capriottii M. Suzuki & Kurtzman 2010 | + | A | 1 | 1 | 5 | 4 | 1 | 3 | 2 | 2 | 3 | 5 | 0 | 2 | 0 | 1 | 2 |
| 51 | Schwanniomyces occidentalis Klöcker 1909 | A | 1 | 1 | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | |
| 52 | ** Schwanniomyces polymorphus (Klöcker) M. Suzuki & Kurtzman 2010 | A | 1 | 1 | 8 | 4 | 4 | 5 | 3 | 2 | 6 | 3 | 5 | 0 | 4 | 2 | 2 | |
| 53 | Schwanniomyces vanrijiae (Van der Walt & Tscheuschner) M. Suzuki & Kurtzman 2010 | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | |
| 54 | Solicoccozyma aeria (Saito) Yurkov 2015 | + | B | 1 | 1 | 11 | 7 | 4 | 10 | 1 | 3 | 8 | 3 | 8 | 7 | 1 | 0 | 3 |
| 55 | Sporobolomyces xanthus (Nakase, G. Okada & Sugiy.) Boekhout 1991 | B | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 56 | Sydowia polyspora (Bref. & Tavel) E. Müll. 1953 | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 57 | Tausonia pullulans (Lindner) Xin Zhan Liu, F.Y. Bai, M. Groenew. & Boekhout 2015 | + | B | 1 | 1 | 6 | 2 | 4 | 5 | 1 | 1 | 5 | 6 | 0 | 2 | 2 | 0 | 2 |
| 58 | Thelebolus globosus Brumm. & de Hoog 2005 | + | A | 1 | 1 | 2 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 |
| 59 | Torulaspora globosa (Klöcker) Van der Walt & Johannsen 1975 | A | 1 | 1 | 4 | 2 | 2 | 4 | 0 | 1 | 3 | 2 | 2 | 0 | 3 | 0 | 1 | |
| 60 | Vanderwaltozyma polyspora (Van der Walt) Kurtzman 2003 | A | 1 | 1 | 5 | 3 | 2 | 3 | 2 | 0 | 5 | 3 | 2 | 1 | 3 | 1 | 0 | |
| 61 | Vanrija humicola (Dasz.) R.T. Moore 1980 | B | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | |
| 62 | Wickerhamomyces anomalus (E.C. Hansen) Kurtzman, Robnett & Bas.-Powers 2008 (anamorfa: Candida peliculosa) | A | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 63 | Bullera sp. | B | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | |
| 64 | * Cryptococcus sp. | + | B | 1/2 | 1/2 | 5 | 3 | 2 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 1 | 0 | 2 |
| 65 | Dipodascus sp. | A | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 66 | Kluyveromyces sp. | A | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Sum * | - | - | - | 264 | 147 | 117 | 175 | 89 | 100 | 164 | 115 | 149 | 63 | 87 | 27 | 87 | ||
| No. | SPECIES | MI | DV | BSL | RG | SUM | M | E | d10 | d50 | S | US | s19 | s20 | LK | LS | LT | LU |
| No. | Species | Research Season | Lake |
|---|---|---|---|
| 1 | Nakaseomyces glabratus (H.W. Anderson) Sugita & M. Takash 2022 Pichia fermentans Lodder 1932 | 2019 | Ukiel |
| 2 | Debaryomyces hansenii (Zopf) Lodder & Kreger-van Rij 1984 Nadsonia commutata Golubev 1973 | 2019 | Ukiel |
| 3 | Debaryomyces hansenii (Zopf) Lodder & Kreger-van Rij 1984 Nadsonia commutata Golubev 1973 | 2019 | Ukiel |
| 4 | Candida albicans (C.P. Robin) Berkhout 1923 Geotrichum albidum (Lagerh.) H.Y. Zhu, X.Z. Liu & F.Y. Bai 2024 | 2020 | Skanda |
| 5 | Saccharomycodes ludwigii (E.C. Hansen) E.C. Hansen 1904 Schwanniomyces polymorphus (Klöcker) M. Suzuki & Kurtzman 2010 | 2020 | Tyrsko |
| Parameter | Isolates Number | Average Strains Concentration |
|---|---|---|
| Temperature | Independent of the parameter (R = 0.2991; p = 0.1355) | * Depends on temperature (R = 0.4339; p = 0.0210) |
| Air humidity | Independent of the parameter (R = −0.0837; p = 0.6716) | Independent of the parameter (R = −0.0766; p = 0.6984) |
| UV index | Independent of the parameter (R = 0.1056; p = 0.5926) | Independent of the parameter (R = 0.3396; p = 0.0770) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bałabański, T.; Biedunkiewicz, A.; Jastrzębski, J.P. Yeast Diversity on Sandy Lake Beaches Used for Recreation in Olsztyn, Poland. Pathogens 2025, 14, 744. https://doi.org/10.3390/pathogens14080744
Bałabański T, Biedunkiewicz A, Jastrzębski JP. Yeast Diversity on Sandy Lake Beaches Used for Recreation in Olsztyn, Poland. Pathogens. 2025; 14(8):744. https://doi.org/10.3390/pathogens14080744
Chicago/Turabian StyleBałabański, Tomasz, Anna Biedunkiewicz, and Jan P. Jastrzębski. 2025. "Yeast Diversity on Sandy Lake Beaches Used for Recreation in Olsztyn, Poland" Pathogens 14, no. 8: 744. https://doi.org/10.3390/pathogens14080744
APA StyleBałabański, T., Biedunkiewicz, A., & Jastrzębski, J. P. (2025). Yeast Diversity on Sandy Lake Beaches Used for Recreation in Olsztyn, Poland. Pathogens, 14(8), 744. https://doi.org/10.3390/pathogens14080744










