Abstract
Kala-azar is a protracted disease caused by the protozoan Leishmania infantum (zoonotic) or L. donovani (anthroponotic), transmitted by sandflies. Patients present with fever, anemia, and hepatosplenomegaly, potentially progressing to hemorrhaging, secondary infections, and death. Its pathogenesis is linked to an exaggerated cytokine response. We studied 72 hospitalized patients, analyzing clinical data and outcomes in relation to L. infantum DNA loads in blood and bone marrow, and plasma concentrations of IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α, and TGF-β. Cytokine levels were found to be elevated. L. infantum kDNA loads in blood and bone marrow were strongly correlated and increased with disease duration. Higher parasite loads were observed in men, adults, and HIV-infected patients, and they were significantly associated with mortality. IL-6 was independently linked to sepsis. In multivariate analysis, IL-12 was the only cytokine inversely associated with blood parasite load. Parasite load, but not cytokine levels, correlated with disease severity, suggesting additional mechanisms drive progression. IL-12 appears to limit parasitemia, indicating a weak, persistent adaptive immune response that is ultimately overwhelmed by a progressive, inefficient, and inflammatory innate response.
1. Introduction
Kala-azar, or visceral leishmaniasis, is a lethal parasitic disease with protracted symptoms. Most patients report low-grade fever. Patients also report inappetence, diarrhea, and weight loss and appear malnourished and anemic, with an enlarged spleen and liver [1]. Jaundice, vomiting, and emaciation; signs of bleeding; and bacterial infections are frequent and may lead to death [2,3]. The disease is more common and more lethal at the extremes of age [4,5]. Immunosuppressed patients are also at higher risk [6,7].
Two species of protozoa are the cause of this disease. Leishmania donovani is the agent in East Africa and South Asia, and it is restricted to humans. Currently, L. donovani kala-azar has been brought under control in South Asia through a set of well-coordinated control measures [8]. L. infantum causes this disease among humans and other mammals in Central and Western Asia, the Mediterranean Basin, and the Americas. Unlike L. donovani kala-azar, the incidence of L. infantum kala-azar remains unaffected by control measures [9]. Transmission occurs mainly through being bitten by any of several species of sandflies. The flagellar promastigotes develop in the insects and culture media. After the infective bite, the promastigotes are phagocytized by neutrophils and monocytes. They lose their flagella and survive as amastigotes in macrophages distributed in the spleen, the liver Kupffer cells, bone marrow, and lymph nodes [10,11,12]. They circulate in the blood inside monocytes and neutrophils in very low concentrations [13,14].
There are no known Leishmania virulence factors that can directly harm mammal host cells or tissues and cause disease [3]. Instead, they lead to sickness through host responses as long as they surpass the host’s innate and acquired defenses and progressively multiply, generating the typical signs and symptoms and, eventually, death [15]. As with other infectious diseases, systemic inflammation is triggered by pro-inflammatory cytokines, while regulatory cytokines limit inflammation and presumably immunity [11,16]. This wave of cytokines is associated with hemorrhagic manifestations and bacterial infections [17].
How precisely the parasites and host intertwine such that they develop the lethal disease phenotype has been investigated but is still a matter of conjecture [18]. Evaluations of the role of the host showed that cytokines such as IL-6, IL-8, IFN-γ, and sCD14 might be involved in more life-threatening disease [17,19]. Similarly, the larger the L. infantum load, the more severe the disease [20,21]. Interestingly, the L. infantum genome explains around 80% of the mortality of human patients, suggesting a complex interaction between the parasite and the host response [22]. However, it is not known how the interplay of parasites and host molecules leads to death even after prompt diagnosis and treatment. Mortality has remained 10% over the years, and it is increasing in some parts of the world [23]. Therefore, this study was developed to shed light on the connections between L. infantum burden, a set of host molecules, life-threatening kala-azar, and immunity in order to better understand the pathogenesis of severe kala-azar.
2. Materials and Methods
2.1. Patients
All kala-azar patients were treated at the “Natan Portella” Tropical Disease Institute in Teresina, Brazil, and kala-azar cases confirmed through the presence of L. infantum amastigotes on bone marrow smears or the presence of promastigotes in culture were included in this study. This study was performed on a sample of 72 patients, sequentially admitted with available clinical information obtained by a single physician and with cryopreserved plasma, blood, and bone marrow samples obtained before treatment.
2.2. Medical Data
A detailed clinical history and physical examination—including information on hemorrhagic manifestations—were conducted upon admission for all hospitalized participants. The recorded bleeding variables included any history of hemorrhagic manifestations either upon admission or during a hospital stay—such as gingival or gastrointestinal bleeding, hematoma at the venipuncture site, petechiae, and bruising. These instances were categorized into two variables: ‘reported bleeding’, if not directly observed by the medical team, and ‘detected bleeding’, if clinically observed. The former was based on patient-reported symptoms, while the latter was identified by medical staff. A patient was considered to have a bacterial infection if signs or symptoms were present and if the infection was confirmed through X-rays, urinalysis, or culture results. Infections were categorized into two variables: ‘sepsis’ and ‘any bacterial infection’. Sepsis was defined by the presentation of hyperventilation, tissue hypoperfusion, decreased venous oxygen saturation, oliguria, or altered consciousness. Accordingly, the variable ‘sepsis’ referred to patients who met these criteria, while ‘any bacterial infection’ included all diagnosed bacterial infections, regardless of severity.
Since the number of deaths was too small for detecting statistically significant clinical associations with severe disease, the score system software Kala-Cal® was also used as a proxy for disease gravity. This system uses data such as edema, jaundice, dyspnea, HIV coinfection, vomiting, bacterial infections, and hemorrhages, and, indeed, its results showed significant associations with mortality. Data on this software have been published elsewhere [5], and this program can easily be accessed at https://www.sbmt.org.br/kalacal/ (accessed on 17 March 2024). In synthesis, disease severity was evaluated by six variables: “death”, “chance of death > 10% by using Kala-Cal®”, “reported bleeding”, “detected bleeding”, “sepsis”, and “any bacterial infection”.
2.3. DNA Isolation, Purity, and Standardization
Bone marrow and blood samples were collected in both heparinized and citrated tubes. Isolated parasites were stored in liquid nitrogen. DNA isolation was performed using the QIAmp DNA Blood Mini Kit (Hilden, Germany), according to the manufacturer’s instructions, with 200 μL of plasma or bone marrow. The purity and DNA concentration were evaluated using a spectrophotometer (NanoDrop ND-1000 spectrophotometer; Thermo Fisher Scientific, Waltham, MA, USA). DNA samples were normalized to a concentration of 5 ng/μL and concentrated or diluted with distilled water when necessary.
2.4. Quantitative PCR
Quantitative PCR was carried out by using the TaqMan probe to quantify L. infantum in plasma and bone marrow accurately. The target sequence for L. infantum detection consisted of FAM–TTT TGA ACG GGA TTT CTG-MGB-NFQ (GenBank AF169140). Specific primers based on kinetoplast DNA consisting of 5′–GGC GTT CTG CAA AAT CGG AAA A–3′ (forward) and 5′–CCG ATT TTT GGC ATT TTT GGT CGA T–3′ (reverse), (Applied Biosystems, Foster City, CA, USA) were used [21]. A standard curve was constructed using 10-fold serially diluted L. infantum DNA corresponding to 104 to 1 parasite per reaction.
Albumin was selected as the housekeeping gene to equalize the parasite count in the bone marrow. The number of parasites was expressed as a ratio with respect to the number of human nucleated cells. The primers were 5′-GCT GTC ATC TCT TGT GGG CTG T–3′(forward) and 5′–ACT CAT GGG AGC TGC TGG TTC–3′ (reverse). The probe was VIC-GG AGA GAT TTG TGT GGG CAT GAC A–TAMRA (GenBank NG009291) [24]. A standard curve was constructed using 10-fold serially diluted human cells DNA corresponding to 2 × 104 to 2 human nucleated cells per reaction.
Amplification and detection were performed using the StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Cycling parameters were 50 °C for 2 min, 95 °C for 10 min, and 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Standards, samples, and negative controls were analyzed in duplicates. The threshold cycle (Ct) value was plotted in accordance with the standard curve. The cut-off between reactions was 20%, and no deviation proportions were considered.
2.5. Plasma Cytokines
The plasma specimens were stored at −20 °C. Serum IL-1β, IL-6, IL-8, IL-10, TNF-α, and IL-12 levels were measured using a high-sensitivity multiplex inflammatory cytokine panel via cytometric bead array (CBA); we also measured the levels of the cytokines IL-17 and TGF-β, which were measured independently using CBA-flex set (BD Biosciences, San Jose, CA, USA) on the BDFAcs Array (BD Biosciences, San Jose, CA, USA), following the manufacturer’s instructions. Measurements of each sample were performed in duplicate, and the average of the two measurements was used. Standard curves were derived from the cytokine standards supplied with the kit and subjected to 10-fold dilution. The lower limits of detection for specific analyses ranged from 0.40 pg/mL for IL-8 to 0.04 pg/mL for IL-1β, 0.04 pg/mL for IL-6, 0.97 pg/mL for IL-10, 0.03 pg/mL for TNF-α, 0.06 pg/mL for IL-12, and 0.05 pg/mL for TGF-β, based on standard-curve dilution.
2.6. Statistical Analysis
Proportions and 95% confidence intervals were calculated for the clinical and demographic variables. The median and interquartile intervals, as well as the means, were calculated for kDNA loads. The Kolmogorov–Smirnov test was used to compare the plasma cytokines with the standard values taken from the literature [25]. The Wilcoxon rank-sum test was used to compare plasma and bone marrow kDNA loads according to demographic and clinical data. Similarly, this test was used to analyze the concentration of cytokines. Pearson’s correlation test was used to evaluate the correlation between death, “risk of death > 10% by Kala-Cal®”, “reported bleeding”, “detected bleeding”, “sepsis”, and “any bacterial infection”. The Spearman’s correlation test was used to test the correlation between the natural logarithm of plasma and bone marrow kDNA burdens and their correlation with the time of disease, as indicated by the time with fever. Finally, simple and multiple linear and quantile regression were applied to test whether the cytokines predicted the plasma and bone marrow kDNA loads. The statistical package Stata/IC 15.1 (College Station, TX, USA) was used to analyze the data.
3. Results
3.1. Study Population
Table 1 shows the characteristics of the study population. Forty-two of the patients were male (58.3%), and thirty were female (41.7%). The median age was 7.5, and the mean was 15.2 years. Seventeen participants (23.6%) were under two, 22 (30.6%) were under four, and 61 were children under 15. Six were older than 40 (8.3%). Among the 70 who were tested for HIV, 13 were positive (18.6%). Four (5.6%) patients died. Male sex was associated with HIV infection (p < 0.05).

Table 1.
Characteristics of the study population.
3.2. Clinical Findings
Table 1 also shows the clinical characteristics of the study population. The mean Kala-Cal® chance of death was 12.1%, and the median was 6.5. Twenty-five patients had a chance of death of at least 10% (34.7%). More than 40% had hemorrhages or infections. Fifteen patients had a “reported bleeding” manifestation (20.8%). Ten patients fulfilled the criteria for sepsis (14.1%). Four patients had some sort of bleeding during their hospital stay, e.g., “detected bleeding” (5.6%). A bacterial infection of any type was detected in 23 (31.9%) patients. Mortality was associated with “detected bleeding” (p < 0.05) and sepsis syndrome (p < 0.01), as well as with any bacterial infection (p < 0.10). The six markers of disease severity were significantly and positively correlated, except “death” plus “reported bleeding” and “probability of death > 10%” plus “sepsis” or “any bacterial infection”, which were positively but not statistically correlated. The Supplemental Table S1 provides information on the correlation matrix between these variables.
The chance of death calculated using Kala-Cal® was well correlated with the occurrence of death (r = 0.40, p < 0.001). As expected upon admission, the estimated chance of death among those who survived was 8.9%, much less than the chance for those who died later (65.3%).
3.3. Quantity of Parasite Load and Cytokines
Table 2 depicts the plasma and bone marrow L. infantum loads, plasma cytokine concentrations, and reference values. The median plasma kDNA concentration was 856.7 kDNA amastigote equivalents/mL (AEq/mL), and the mean was 3515.4 AEq/mL. The median bone marrow kDNA concentration was 55.7 AEq/106 DNA equivalents of human cells (HCEq), with an interquartile interval of 3.7–400.7 AEq/106HCEq. The standard deviation was 1.7 times greater than the mean plasma kDNA, while the metric for the same relationship was 3.2 times greater for bone marrow kDNA, demonstrating the higher variability of the bone marrow count. The standard values were assumed to be zero, although some asymptomatic patients may have harbored minimal plasma kDNA loads [26,27,28,29]. Regarding the cytokines, IL-6, IL-8, IL-10, and TGF-β had medians and means much higher than the values of a healthy population, while IL-1β and IL-12 exhibited a more modest increase. Despite the high plasma concentrations, cytokines were not detected in a relevant proportion of patients: 28% for IL-12, 19% for IL-1β, 9% for TNF-α, and 6% for IL-6.

Table 2.
Parasite load as measured by the concentration of kDNA and cytokines in the plasma of patients with kala-azar.
3.4. Plasma and Bone Marrow L. infantum Loads
The Spearman’s correlation test revealed a moderate but statistically significant correlation between the natural logarithms of the plasma and bone marrow parasite loads (r = 0.48, p < 0.001) (Figure 1). Bone marrow kDNA load without including human cell counts as the denominator in the parameter was poorly correlated with the parasite plasma load (r = 0.21, p > 0.05).
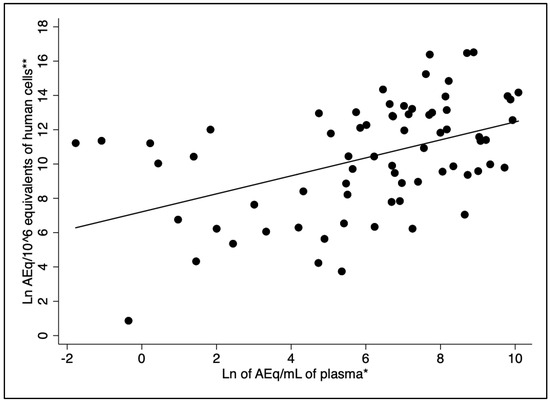
Figure 1.
Correlation between plasma and bone marrow parasite load (r = 0.48, p-value < 0.001). Legend: * Natural logarithm of kDNA amastigote-equivalents/mL of plasma (AEq/mL). ** Natural logarithm of HCEq/106 (amastigote-equivalents/DNA equivalents of human cells).
3.5. Time of Disease, Plasma and Bone Marrow L. infantum Loads, Plasma Cytokines, and Severity
The plasma and bone marrow L. infantum kDNA loads increased with the duration of disease, as estimated by the duration of fever (Figure 2). The Spearman’s correlation coefficient for plasma kDNA over time was 0.33 (p < 0.005), and for bone marrow, it was 0.38 (p < 0.05). There was no correlation of any cytokine with time. According to the univariate linear regression analysis of the relationship between the natural logarithm of time with a fever and the natural logarithm of plasma kDNA load, the probability of rejecting the model was <0.005, and the p-value of the coefficient was 0.005. For predicting the natural logarithm of the bone marrow load, the probability of rejecting the model was <0.001, and the p-value of the coefficient was <0.001. There was no association between any cytokine and the duration of disease. A greater chance of death and “detected bleeding” were slightly correlated with the duration of a fever (ρ = 0.23, p > 0.05 and ρ = 0.24, p < 0.05, respectively).
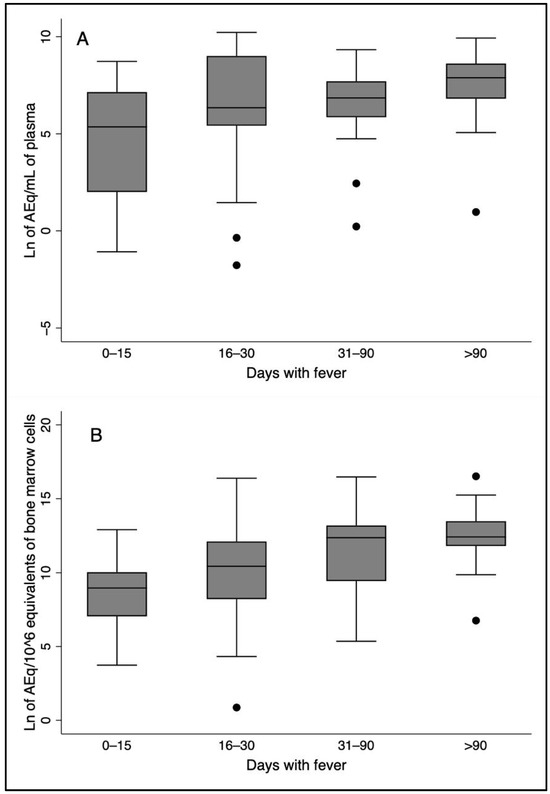
Figure 2.
Box plots showing parasite loads increasing with time of fever both in the plasma and in the bone marrow. The correlation coefficients with time represented in the plots were (A) parasite load in the plasma (ρ = 0.33, p-value < 0.005); (B) Parasite load in the bone marrow (ρ = 0.38, p-value < 0.001).
3.6. Plasma and Bone Marrow L. infantum Loads, Age, Sex, HIV Infection, and Kala-Azar Severity
Table 3 shows the relationships between L. infantum load and age, sex, HIV infection, and markers of kala-azar severity. Individuals 15 years of age or older had higher plasma median loads (p < 0.05), and patients 40 years old or older had the highest plasma loads (median = 9129.5 AEq/mL). The same trend was observed for bone marrow (p < 0.05). Plasma and bone marrow loads were higher in men (p < 0.05 and p > 0.05, respectively). HIV-infected patients had eight-times-higher plasma loads than non-infected patients (p < 0.01). On the other hand, in bone marrow, although the loads were higher, the difference was non-significant. In the multivariate quantile regression, HIV status did not stand out, and only sex and age were associated with plasma kDNA load.

Table 3.
Parasite load (concentration of Leishmania infantum amastigotes estimated using kDNA) in the plasma of patients with kala-azar.
The four patients who died had kDNA loads in their plasma that were a median of 14.2 times higher than those in the survivors but non-significantly associated with death, likely due to the small number of deceased patients. The bone marrow L. infantum loads were also higher in the deceased patients in comparison to those in the patients who survived, but not in a statistically significant manner. The concentration of kDNA in the plasma was 11.4 times higher in those with a chance of death estimated to be above 10% (p < 0.001). In the bone marrow, this concentration was 14.5 times higher in those with a chance of death greater than 10% (p < 0.005).
The associations for the two variables related to hemorrhagic phenomena, “reported bleeding” and “detected bleeding”, were not concordant regarding L. infantum loads in the blood and bone marrow. Plasma kDNA loads were ten times higher in the four patients with “detected bleeding” than in those with “reported bleeding”. The opposite was observed for bone marrow: the L. infantum load was not very high in those with “detected bleeding”, but it was 10 times higher in the patients with “reported bleeding”.
3.7. Plasma Cytokines, Age, Sex, HIV Infection, and Markers of Kala-Azar Severity
Table 4 shows the associations between age, sex, HIV infection, and the clinical manifestations of the severity of kala-azar with respect to the seven measured plasma cytokines. There was a paucity of statistically solid associations between plasma cytokines and the demographic and clinical data. IL-10 levels were almost two times higher in children (p-value < 0.05). IL-10 levels were also much higher in women than in men (p-value > 0.05). Similarly, IL-10 was the only cytokine associated with HIV infection, with its levels being significantly lower in those with HIV (p-value < 0.05). Possibly due to the small number of deaths in the study population (four), no cytokine had any statistically significant association with death. However, the median IL-6 level was noticeably, i.e., more than two times, higher in those who died. The proxy for death and disease severity used, i.e., the chance of death according to Kala-Cal®, showed no significant association with any cytokines. Only three patients with measured cytokines had “detected bleeding”. However, in these three patients, IL-6 levels were almost four times higher (p < 0.05). Only IL-8 was associated with “reported bleeding” (p-value < 0.05). Sepsis syndrome had some significant associations with cytokines. IL-12 levels were higher in those with the syndrome (p < 0.10), and the increases were nearly significant for IL-1β (p < 0.10), IL-6 (p < 0.10), and IL-10 (p-value > 0.05). After obtaining nearly all of the results, we found that the variable “any bacterial infection” was statistically associated with IL-1β (p-value < 0.05), IL-6 (p-value < 0.05), IL-8 (p-value < 0.05), IL-10 (p-value < 0.05), and TNF-a (p-value < 0.05). However, no statistically significant association would exist if the Bonferroni analysis for multiple comparisons were applied, that is, at a level of significance of the p-value < 0.001. In the multivariate linear regression, IL-6 was the only predictor of sepsis (p-value < 0.05), as confirmed in a multiple quantile regression. No cytokine was found to be able to predict the chance of death or any other clinical outcome at the p < 0.05 level.

Table 4.
Plasma concentration of cytokines according to demographic data, and HIV-infection, and markers of kala-azar severity.
3.8. Regression Analysis Between L. infantum Load and Plasma Cytokines
In the univariate linear regression analysis, IL-12 was found to negatively and significantly predict plasma kDNA levels (p < 0.01). Similarly, TNF-α also yielded negative and significant predictions (p < 0.05). However, when controlling for IL-12, the effect of TNF-α was no longer significant. In the multivariate linear regression, only IL-12 remained a significant predictor of plasma kDNA load (Supplemental Figure S1); no other cytokines were associated with this factor. Finally, no cytokines predicted kDNA levels in the bone marrow. The models’ adjusted R2 and pseudo R2, respectively, were low, indicating that the studied cytokines were poor predictors of L. infantum load.
4. Discussion
This study found that patients with kala-azar have a high inflammatory status and show signs of a substantial risk of death. On the other hand, the data also shows that children, women, and persons living with HIV have a more prominent regulatory immune response. Markedly, however, IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α, and TGF-β were not or only poorly associated with the risk of death. Moreover, while the levels of the studied cytokines did not increase with the duration of the disease, plasma and bone marrow L. infantum loads increased progressively. Additionally, plasma and bone marrow L. infantum loads were highly correlated, and parasite loads were higher among men, adults, and HIV-infected patients. Finally, higher plasma and bone marrow kDNA loads were associated with increased severity. Most plasma cytokines were not related to plasma or bone marrow L. infantum load, but higher plasma IL-12 levels were independently associated with lower L. infantum loads.
This study’s weakest point is its cross-sectional design, which did not allow the identification of causal relationships, except with death. Other key cytokines and molecules, such as IFN-γ, were not studied. Additionally, the levels of bone marrow cytokines were not measured, and spleen size was not regularly registered, hiding this organ’s importance in regard to the observed values. However, the findings are of interest for helping us to understand the pathogenesis of life-threatening kala-azar and highlighting the progressive immunological failure experienced by patients with kala-azar.
This study population reflects the well-known male and child predominance with respect to kala-azar caused by L. infantum [30]. The degree of HIV coinfection was higher than usual for this region and country [31,32]. Although mortality was lower in this sample, more than one-third of the patients had a chance of death estimated to be over 10% and hemorrhages and bacterial infections. These findings demonstrate how severe the disease can be and stress the importance of understanding the pathogenetic mechanisms of these complications. When compared with levels in blood donors [25], the plasma cytokine concentrations in the patients were very high, confirming the role of inflammation. Indeed, previous studies have already shown very high values of the cytokines IFN-γ, IL-8, TGF-β, L-10, and IL-6 in the plasma of patients from East Africa, Brazil, and India [33,34,35,36].
Children had higher concentrations of most cytokines than older patients, but only the levels of IL-10 reached statistical significance. This finding might support a previous observation and suggests children have a more immunotolerant state, independently of kala-azar infection [37]. Regarding sex, IL-10 levels were higher in females. Although another study on healthy adult individuals did not observe this difference, the present findings suggest that a sex-dependent IL-10 response in patients with kala-azar may actually exist [38].
Another previous study showed that HIV-infected patients undergoing antiretroviral therapy (ART) had lower IL-10 levels than both patients not on ART and long-term non-progressors [39,40]. In the present study, IL-10 levels were found to be lower in patients coinfected with HIV than in those non-coinfected, suggesting that HIV infection attenuates the regulatory innate response to kala-azar.
Based on observational data, it has been proposed that plasma IL-6 and IL-8, IFN-γ, IL-27, and soluble CD14 are the major mediators of the pathogenicity of kala-azar [3,17,19]. Due to its overlapping activity with respect to kala-azar, such as hemorrhages, anemia, hypoalbuminemia, and hyperglobulinemia, IL-6 was proposed to be the best explanatory cytokine for the complications of kala-azar [41]. However, in the present study, neither the chance of death upon hospital admission nor the occurrence of death revealed sufficiently strong associations between plasma cytokines and signs of severity. Indeed, when the p-values were corrected using the Bonferroni method for multiple comparisons, no association between clinical presentation and any of the seven studied cytokines was found. Additionally, in the multivariate linear regression, no cytokine was found to predict the chance of death. This set of data on the role of cytokines detected in the blood suggests that the core of the pathogenic phenomena that lead to severe kala-azar or death may not rely primarily on the direct effect of pro- or anti-inflammatory cytokines, as previously proposed [17,19].
In this study, we observed that the cytokine response does not change with time after the disease starts, and this finding may influence our comprehension of the framework for the pathogenesis of kala-azar. Although cytokine levels did not change, L. infantum loads and disease severity increased with time. This phenomenon does not seem to be determined by progressive spleen enlargement since parasite loads in the bone marrow also increased similarly.
The median plasma and bone marrow amounts of L. infantum kDNA in this sample were in the range of the concentrations previously found in Teresina and elsewhere using the same protocol [20,21,42]. As expected, plasma and bone marrow kDNA concentrations were highly correlated, indicating that bone marrow is balanced with systemic parasitism. Men and adults in general had higher plasma and bone marrow loads, likely due to the modulatory effects of testosterone and dihydrotestosterone on men, who constitute the majority of adult patients with kala-azar [43]. Interestingly, HIV infection was not independently associated with a higher parasite load. One possible explanation for this is that the patients with HIV had a relapsing course of kala-azar and, therefore, were undergoing secondary prophylaxis with liposomal amphotericin B for L. infantum to prevent kala-azar relapses.
The disease severity associated with L. infantum load is a relevant finding and deserves further discussion. Unfortunately, this study design does not allow for the assessment of the direction of causality, i.e., if L. infantum load worsens the disease via a linkage with a specific, unknown factor or if a broad, ongoing multifactorial lymphoid disruption leads to a non-specific, progressive, and generalized failure of immunity and then a higher parasite load.
Cytokines’ direct contributions to the manifestations of severe disease have already been studied [17,19], but here, plasma cytokines were also compared with plasma and bone marrow L. infantum kDNA loads, clinical presentation, and risk of death. IL-12 and TNF-α were found to be associated with plasma L. infantum load, but IL-12 was the only one found to be an independent predictor of plasma kDNA load, and, importantly, this interaction decreased L. infantum loads in a dose-dependent manner. Indeed, this finding is consistent with the canonical role of innate-immunity-derived IL-12, which promotes antigen-specific Th1 responses via T-cell activation, proliferation, and differentiation through the secretion of IFN-γ [12,44,45,46,47,48,49]. Unfortunately, this finding has not been forecasted, and IFN-γ was not analyzed in the present study. However, two previous articles analyzed the association between plasma cytokines and the blood load of L. donovani kala-azar in India and Africa [24,50]. They found a positive correlation with IL-10, TGF-β, and IL-17 but not with IFN-γ, TNF-α, IL-6, IL-4, IL-2, IL-12, and IL-22, suggesting that immunoregulatory cytokines are the primary controllers of blood parasite loads in L. donovani-derived kala-azar. However, although Teles et al. [51], in Brazil, and van Dijk et al. [36], in Uganda, also found a positive correlation with IL-10, they identified a negative correlation with IFN-γ. The difference between the data from India and the findings in Brazil and Uganda suggests that L. infantum and Indian L. donovani differ in terms of the host control of kala-azar: while there seems to be a more prominent role of a sustained, acquired, type Th1 response for the disease caused by L. infantum and Ugandan parasites, in the disease caused by Indian L. donovani, innate regulatory cytokines “deal the cards” and have an absolute, permissive effect on parasite load.
IL-12/IL-10 interaction is the line of balance in kala-azar: at the infection site, intracellular amastigote molecules drive the activity of the infected macrophages to promote a predominant IL-12/ IFN-γ or IL-10/IL-27 synthesis [10,15,52]. In the majority of infections, a Th1 response prevails, stimulating T-cells to synthesize IFN-γ, which triggers macrophages to produce free radicals that kill the amastigotes, and thus the infection is controlled [11,12,53,54]. In a small proportion of immunocompetent humans, IL-10/IL-27 prevails and disrupts macrophage IL-12 signaling to CD4+ and CD8+, blocking the secretion of IFN-γ and preventing macrophage activation for intracellular defense [48,54,55,56,57,58]. All these events may happen entirely at the innate-immunity level. However, with time, acquired immunity develops. If there is a stronger Th1-type response with T-cells secreting IFN-γ, memory T-lymphocytes generate Leishmania-specific clones, and the host becomes immune, as indicated by the high proportion of persons with cellular immunity to Leishmania who never develop symptoms and live in endemic areas [26,59]. However, if a regulatory profile is maintained, parasite load increases, disease and complications appear, and the host eventually dies. This study shows that despite the dominance of IL-10’s effects on L. donovani kala-azar, acquired immunity persistently influences parasitism in L. infantum-derived and Ugandan kala-azar.
Therefore, the IL-12 response in L. infantum-derived kala-azar reported here and the IFN-γ response reported elsewhere suggest that an overwhelmed but enduring acquired immunity persists for a while during the course of this disease. Another reason for this hypothesis is that the secretion of IL-12 is maintained for a longer period after the disease is cured in comparison to what is observed for the cytokines secreted after the stimulation of the innate response, such as the rapid decrease—at one to two weeks—in the levels of the cytokines mentioned above, as previously reported [36,60]. Therefore, IL-12 seems to be part of acquired immunity, not innate immunity, since it is long-lasting due to the memory T-cells developed after earlier antigenic priming of T-cells, while IL-1, IL-6, IL-8, IL-10, and TNF-α, albeit at higher concentrations, last only during the antigenic stimulus, as characteristically occurs with respect to innate immunity. Nevertheless, although IL-10 has been described to be associated with T-regulatory cytokines as part of acquired immunity, it typically falls to very low levels after kala-azar is cured [34,35,36,61].
Similarly, the early decrease in IFN-γ levels observed in the study by Lima et al. [60] and van Dijk et al. [36] suggests that most plasma IFN-γ originates from cells that are part of the innate immune system, such as neutrophils, eosinophils, NK cells, or even T-cells, in an antigen-independent process [34,62,63], not from TCR antigen-specific CD4+ or CD8+ T-cells. Therefore, this study suggests that in kala-azar, innate and acquired immunity coexist with the disease. With treatment, the hidden cellular immunity is established, but relapses indicate that the parasite persists even after immunity develops and patients become asymptomatic, e.g., non-sterile immunes. This equilibrium may have advantages for both: long-lasting immunity at the cost of some chance of disease remission and transmission.
While L. infantum-derived kala-azar still shows signs of some effective defense, L. donovani-derived kala-azar does not. The two parasites lead to remarkable phenotypic differences, despite being relatively genetically similar. L. infantum-based kala-azar seems to derive from the older L. donovani kala-azar [64]. While L. donovani kala-azar is mostly transmitted among humans, L. infantum kala-azar is zoonotic, with a breadth of mammal hosts [1]. L. donovani kala-azar develops in older individuals, while L. infantum kala-azar affects younger, immunocompetent persons, primarily children, indicating a higher force of infection [30]. L. donovani kala-azar leads to post-kala-azar dermal leishmaniasis, which is rare in infections with L. infantum [65]. There are other clinical and epidemiological differences between the two species, but comparative, well-controlled, head-to-head studies on these two species and places still need to be performed, covering topics ranging from genomic analysis to innate and acquired immunity and pathogenesis.
Another open question from this study is why bone marrow L. infantum load did not show an association with IL-12 or TNF-a similar to that shown by plasma load. One explanation is that bone marrow control of Leishmania parasitism has in situ peculiarities not captured by the plasma cytokines analyzed. An insightful study investigated the association between bone marrow cytokines and local L. infantum burden. The findings revealed that IFN-γ was associated with a reduction in bone marrow parasite loads, whereas interleukin-10 (IL-10) correlated with an increase in parasite burden. Interestingly, a strong and statistically significant positive correlation between IFN-γ and IL-10 levels was also observed. However, IL-12 or TNF-a were not found to be associated with parasite burden [51]. These findings illustrate the strongly opposing effects of concomitant and protective versus permissive cytokines, each one downregulating (or upregulating) the other, in the bone marrow. It was not clear if the local findings in bone marrow can be generalized for blood.
Since cytokines are pleiotropic and redundant, engaging in synergistic actions, it is difficult to understand their individual roles in diseases [57]. Here, the risks of death and complications were not firmly associated with specific cytokines, except the association of IL-6 with sepsis in the multivariate regression analysis. However, since sepsis is the main cause of cytokine storms [57,66], it is not valid to infer that the increase in IL-6 levels was due to L. infantum instead of lipopolysaccharide (LPS) from opportunistic bacteria. Remarkably, mortality and the chance of death were not associated with any plasma cytokines. Although IFN-γ was not analyzed in this study, it does not seem to be a candidate for explaining kala-azar severity since its side effects are mild in a human host and do not match the symptoms of complicated kala-azar [67].
Therefore, the important question is how a microorganism without virulence factors that do not directly harm human cells or tissues could lead to death [15,18]. A progressive rise in parasite load indicates a failing acquired immunity, suggesting that a higher parasite load is a consequence and not a cause of immune failure. Two distinct mechanisms without the direct and specific action of cytokines may explain the global defense failure. One is T-cell exhaustion, and the other is spleen disorganization. T-cell exhaustion is a dysfunction of T-cells, mostly CD8+ cells, occurring naturally and during chronic infections and cancer [68]. The other alternative is the disorganization of lymph nodes and spleen architecture, a phenomenon that disrupts the white pulp structure [69]. Both mechanisms are exacerbated in kala-azar infections and may hypothetically result in an increasing amastigote load, progressive acquired immune failure, and, thereafter, an increased probability of complications and death [70,71,72]. These two mechanisms of immune deterioration occur simultaneously in kala-azar, but a clear molecular link between them has not been identified yet [73]. Hence, these processes seem to be accelerated in kala-azar by an elusive systemic factor, likely linked to the prolonged and intense inflammatory status; lymphotoxin-β and IL-27 are plausible mediators of this phenomenon [74,75].
In summary, L. infantum load was associated with life-threatening kala-azar, but the corresponding mechanism is unknown. In contrast, circulating cytokines were poorly associated with phenotypes of severe disease. As expected, IL-12 has an enduring, strong, negative effect on L. infantum proliferation during kala-azar infection. Parasite load worsened with time, but cytokine load did not, suggesting a cytokine-independent immunological failure process that results in progressively severe disease and death. However, there are still no key host factors leading to complications and death by kala-azar. Consequently, it may only be conjectured that the long-term immunological consequences of sustained infection and inflammation, such as persistent immune activation, may lead to immune exhaustion and overall immunological disorganization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14070615/s1, Figure S1: Above: output of the multivariate linear regression analysis for prediction of of plasma parasite kDNA load by plasma cytokines. Bellow: output of the multivariate quantile regression analysis for prediction of plasma parasite kDNA load by plasma cytokines. Table S1: Pearson’s correlation coefficient matrix between variables used to estimate the severity of kala-azar.
Author Contributions
Conceptualization, I.d.S.S. and C.H.N.C.; Methodology, I.d.S.S., D.A.Z., J.C.d.S., D.R.A. and J.C.d.S.A.; Formal Analysis, I.d.S.S. and C.H.N.C.; Investigation, D.L.C., I.d.S.S., D.A.Z., J.C.d.S., D.R.A. and J.C.d.S.A.; Resources, I.d.S.S. and C.H.N.C.; Data Curation, I.d.S.S. and C.H.N.C.; Writing—Original Draft Preparation, I.d.S.S. and C.H.N.C.; Writing—Review and Editing, I.d.S.S., C.H.N.C. and D.L.C.; Visualization, G.R.F.; Supervision, C.H.N.C.; Project Administration, C.H.N.C.; Funding Acquisition, C.H.N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Pesquisas—CNPq number 475102/2011-8.
Institutional Review Board Statement
The project was approved by the Research Ethics Committee of the Federal University of Piauí (CAE: 44037015.3.0000.5314), by the precepts of the Resolution of the National Health Council-CNS 466/12, which deals with the Guidelines and Norms for Research Involving Human Subjects.
Informed Consent Statement
All patients included in the study, or their guardians, signed an informed consent form.
Data Availability Statement
Data is available through permission given directly by the authors IS and CC.
Acknowledgments
The authors acknowledge the Instituto de Doenças Tropicais Natan Portella for the full support of the study, including patient evaluation, laboratory data use, and the infra-structure of the Laboratory of Leishmaniasis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.M.; Carvalho, E.M.; Rocha, H. Bacterial Infections in Patients with Visceral Leishmaniasis. J. Infect. Dis. 1990, 162, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.H.N.; Werneck, G.L.; Costa, D.L.; Holanda, T.A.; Aguiar, G.B.; Carvalho, A.S.; Cavalcanti, J.C.; Santos, L.S. Is severe visceral leishmaniasis a systemic inflammatory response syndrome? A case control study. Rev. Soc. Bras. Med. Trop. 2010, 43, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, M.J.A.d.Q.; Cavalcanti, N.V.; Alves, J.G.B.; Fernandes Filho, M.J.C.; Correia, J.B. Risk Factors for Death in Children with Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2010, 4, e877. [Google Scholar] [CrossRef]
- Costa, D.L.; Rocha, R.L.; Chaves, E.d.B.F.; Batista, V.G.d.V.; Costa, H.L.; Costa, C.H.N. Predicting death from kala-azar: Construction, development, and validation of a score set and accompanying software. Rev. Soc. Bras. Med. Trop. 2016, 49, 728–740. [Google Scholar] [CrossRef]
- Akuffo, H.; Costa, C.; van Griensven, J.; Burza, S.; Moreno, J.; Herrero, M. New insights into leishmaniasis in the immunosuppressed. PLoS Negl. Trop. Dis. 2018, 12, e0006375. [Google Scholar] [CrossRef]
- Kurizky, P.S.; Marianelli, F.F.; Cesetti, M.V.; Damiani, G.; Sampaio, R.N.R.; Gonçalves, L.M.T.; Sousa, C.A.F.d.; Martins, S.S.; Vernal, S.; Mota, L.M.H.d.; et al. A comprehensive systematic review of leishmaniasis in patients undergoing drug-induced immunosuppression for the treatment of dermatological, rheumatological and gastroenterological diseases. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e28. [Google Scholar] [CrossRef]
- Rahim, S.; Karim, M.M. The Elimination Status of Visceral Leishmaniasis in Southeast Asia Region. Acta Parasitol. 2024, 69, 1704–1716. [Google Scholar] [CrossRef]
- da Rocha, I.C.M.; dos Santos, L.H.M.; Coura-Vital, W.; da Cunha, G.M.R.; Magalhães, F.d.C.; da Silva, T.A.M.; Morais, M.H.F.; Oliveira, E.; Reis, I.A.; Carneiro, M. Effectiveness of the Brazilian Visceral Leishmaniasis Surveillance and Control Programme in reducing the prevalence and incidence of Leishmania infantum infection. Parasit. Vectors 2018, 11, 586. [Google Scholar] [CrossRef]
- McCall, L.I.; Zhang, W.W.; Matlashewski, G. Determinants for the Development of Visceral Leishmaniasis Disease. PLoS Pathog. 2013, 9, e1003053. [Google Scholar] [CrossRef]
- Volpedo, G.; Pacheco-Fernandez, T.; Bhattacharya, P.; Oljuskin, T.; Dey, R.; Gannavaram, S.; Satoskar, A.R.; Nakhasi, H.L. Determinants of Innate Immunity in Visceral Leishmaniasis and Their Implication in Vaccine Development. Front. Immunol. 2021, 12, 748325. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Islam, N.A.K.; Barinberg, D.; Soulat, D.; Schleicher, U.; Rai, B. The immunomicrotope of Leishmania control and persistence. Trends Parasitol. 2024, 40, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Zacarias, D.A.; Silva, V.C.; Rolão, N.; Costa, D.L.; Costa, C.H. Comparison of optical microscopy and quantitative polymerase chain reaction for estimating parasitaemia in patients with kala-azar and modelling infectiousness to the vector Lutzomyia longipalpis. Mem. Inst. Oswaldo Cruz 2016, 111, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.R.; Santos-Oliveira, J.R.; Silva-Freitas, M.L.; Honda, M.; Costa, D.L.; Da-Cruz, A.M.; Costa, C.H.N. Biomarkers of disease severity in patients with visceral leishmaniasis co-infected with HIV. Cytokine 2022, 149, 155747. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S. Major Molecular Factors Related to Leishmania Pathogenicity. Front. Immunol. 2022, 13, 847797. [Google Scholar] [CrossRef]
- Samant, M.; Sahu, U.; Pandey, S.C.; Khare, P. Role of Cytokines in Experimental and Human Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2021, 11, 624009. [Google Scholar] [CrossRef]
- Costa, D.L.; Rocha, R.L.; Carvalho, R.M.A.; Lima-Neto, A.S.; Harhay, M.O.; Costa, C.H.N.; Barral-Neto, M.; Barral, A.P. Serum cytokines associated with severity and complications of kala-azar. Pathog. Glob. Health 2013, 107, 78–87. [Google Scholar] [CrossRef]
- Costa, C.H.N.; Chang, K.P.; Costa, D.L.; Cunha, F.V.M. From Infection to Death: An Overview of the Pathogenesis of Visceral Leishmaniasis. Pathogens 2023, 12, 969. [Google Scholar] [CrossRef]
- dos Santos, P.L.; de Oliveira, F.A.; Santos, M.L.B.; Cunha, L.C.S.; Lino, M.T.B.; de Oliveira, M.F.S.; Bomfim, M.O.M.; Silva, A.M.; de Moura, T.R.; de Jesus, A.R.; et al. The Severity of Visceral Leishmaniasis Correlates with Elevated Levels of Serum IL-6, IL-27 and sCD14. PLoS Negl. Trop. Dis. 2016, 10, e0004375. [Google Scholar] [CrossRef]
- Silva, J.M.; Zacarias, D.A.; de Figueirêdo, L.C.; Soares, M.R.A.; Ishikawa, E.A.Y.; Costa, D.L.; Costa, C.H.N. Bone Marrow Parasite Burden among Patients with New World Kala-Azar is Associated with Disease Severity. Am. Soc. Trop. Med. Hyg. 2014, 90, 621–626. [Google Scholar] [CrossRef]
- Zacarias, D.A.; Rolão, N.; de Pinho, F.A.; Sene, I.; Silva, J.C.; Pereira, T.C.; Costa, D.L.; Costa, C.H.N. Causes and consequences of higher Leishmania infantum burden in patients with kala-azar: A study of 625 patients. Trop. Med. Int. Health 2017, 22, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Grace, C.A.; Sousa Carvalho, K.S.; Sousa Lima, M.I.; Costa Silva, V.; Reis-Cunha, J.L.; Brune, M.J.; Forrester, S.; Pedrozo e Silva de Azevedo, C.d.M.; Costa, D.L.; Speed, D.; et al. Parasite Genotype Is a Major Predictor of Mortality from Visceral Leishmaniasis. MBio 2022, 13, e0206822. [Google Scholar] [CrossRef] [PubMed]
- Cota, G.; Erber, A.C.; Schernhammer, E.; Simões, T.C. Inequalities of visceral leishmaniasis case-fatality in Brazil: A multilevel modeling considering space, time, individual and contextual factors. PLoS Negl. Trop. Dis. 2021, 15, e0009567. [Google Scholar] [CrossRef] [PubMed]
- Kildey, K.; Rooks, K.; Weier, S.; Flower, R.L.; Dean, M.M. Effect of age, gender and mannose-binding lectin (MBL) status on the inflammatory profile in peripheral blood plasma of Australian blood donors. Hum. Immunol. 2014, 75, 973–979. [Google Scholar] [CrossRef]
- Porcino, G.N.; Carvalho, K.S.S.; Braz, D.C.; Costa Silva, V.; Costa, C.H.N.; de Miranda Santos, I.K.F. Evaluation of methods for detection of asymptomatic individuals infected with Leishmania infantum in the state of Piauí, Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007493. [Google Scholar] [CrossRef]
- Das, V.N.R.; Bimal, S.; Siddiqui, N.A.; Kumar, A.; Pandey, K.; Sinha, S.K.; Topno, R.K.; Mahentesh, V.; Singh, A.K.; Lal, C.S.; et al. Conversion of asymptomatic infection to symptomatic visceral leishmaniasis: A study of possible immunological markers. PLoS Negl. Trop. Dis. 2020, 14, e0008272. [Google Scholar] [CrossRef]
- Chakravarty, J.; Hasker, E.; Kansal, S.; Singh, O.P.; Malaviya, P.; Singh, A.K.; Chourasia, A.; Singh, T.; Sudarshan, M.; Singh, A.P.; et al. Determinants for progression from asymptomatic infection to symptomatic visceral leishmaniasis: A cohort study. PLoS Negl. Trop. Dis. 2019, 13, e0007216. [Google Scholar] [CrossRef]
- Virginia Batista Vieira, A.; Farias, P.C.S.; Silva Nunes Bezerra, G.; Xavier, A.T.; Sebastião Da Costa Lima Júnior, M.; Silva, E.D.D.; Barbosa Júnior, W.L.; Medeiros, Z.M. Evaluation of molecular techniques to visceral leishmaniasis detection in asymptomatic patients: A systematic review. Expert Rev. Mol. Diagn. 2021, 21, 493–504. [Google Scholar] [CrossRef]
- Harhay, M.O.; Olliaro, P.L.; Vaillant, M.; Chappuis, F.; Lima, M.A.; Ritmeijer, K.; Costa, C.H.; Costa, D.L.; Rijal, S.; Sundar, S.; et al. Who Is a Typical Patient with Visceral Leishmaniasis? Characterizing the Demographic and Nutritional Profile of Patients in Brazil, East Africa, and South Asia. Am. Soc. Trop. Med. Hyg. 2011, 84, 543–550. [Google Scholar] [CrossRef]
- Leite de Sousa-Gomes, M.; Romero, G.A.S.; Werneck, G.L. Visceral leishmaniasis and HIV/AIDS in Brazil: Are we aware enough? PLoS Negl. Trop. Dis. 2017, 11, e0005772. [Google Scholar] [CrossRef]
- Machado, C.A.L.; Sevá, A.d.P.; Silva, A.A.F.A.e.; Horta, M.C. Epidemiological profile and lethality of visceral leishmaniasis/human immunodeficiency virus co-infection in an endemic area in Northeast Brazil. Rev. Soc. Bras. Med. Trop. 2021, 54, e0795. [Google Scholar] [CrossRef] [PubMed]
- Hailu, A.; van der Poll, T.; Berhe, N.; Kager, P.A. Elevated plasma levels of interferon (IFN)-gamma, IFN-gamma inducing cytokines, and IFN-gamma inducible CXC chemokines in visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2004, 71, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Peruhype-Magalhães, V.; Martins-Filho, O.A.; Prata, A.; Silva, L.D.A.; Rabello, A.; Teixeira-Carvalho, A.; Figueiredo, R.M.; Guimarães-Carvalho, S.F.; Ferrari, T.C.A.; Van Weyenbergh, J.; et al. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-γ and interleukin-10 and low frequency of tumour necrosis factor-α+ monocytes are hallmarks of active human visceral Leishmaniasis due to Leishmania chagasi infection. Clin. Exp. Immunol. 2006, 146, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.A.; Saluja, S.; Salotra, P. Elevated levels of interferon-γ, interleukin-10, and interleukin-6 during active disease in Indian kala azar. Clin. Immunol. 2006, 119, 339–345. [Google Scholar] [CrossRef]
- van Dijk, N.J.; Carter, J.; Kiptanui, D.; Mens, P.F.; Schallig, H.D.F.H. A case–control study on risk factors for visceral leishmaniasis in West Pokot County, Kenya. Trop. Med. Int. Health 2024, 29, 904–912. [Google Scholar] [CrossRef]
- Decker, M.L.; Grobusch, M.P.; Ritz, N. Influence of Age and Other Factors on Cytokine Expression Profiles in Healthy Children—A Systematic Review. Front. Pediatr. 2017, 5, 255. [Google Scholar] [CrossRef]
- Ringleb, M.; Javelle, F.; Haunhorst, S.; Bloch, W.; Fennen, L.; Baumgart, S.; Drube, S.; Reuken, P.A.; Pletz, M.W.; Wagner, H.; et al. Beyond muscles: Investigating immunoregulatory myokines in acute resistance exercise—A systematic review and meta-analysis. FASEB J. 2024, 38, e23596. [Google Scholar] [CrossRef]
- Tasca, K.I.; Correa, C.R.; Caleffi, J.T.; Mendes, M.B.; Gatto, M.; Manfio, V.M.; de Camargo, C.C.; Tavares, F.C.; Biasin, M.; de Souza, L.d.R. Asymptomatic HIV People Present Different Profiles of sCD14, sRAGE, DNA Damage, and Vitamins, according to the Use of cART and CD4+ T Cell Restoration. J. Immunol. Res. 2018, 2018, 7531718. [Google Scholar] [CrossRef]
- Guedes, D.L.; Silva, E.D.d.; Castro, M.C.A.B.; Júnior, W.L.B.; Ibarra-Meneses, A.V.; Tsoumanis, A.; Adriaensen, W.; van Griensven, J.; Pereira, V.R.A.; Medeiros, Z.M.d. Comparison of serum cytokine levels in symptomatic and asymptomatic HIV-Leishmania coinfected individuals from a Brazilian visceral leishmaniasis endemic area. PLoS Negl. Trop. Dis. 2022, 16, e0010542. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Mary, C.; Faraut, F.; Lascombe, L.; Dumon, H. Quantification of Leishmania infantum DNA by a Real-Time PCR Assay with High Sensitivity. J. Clin. Microbiol. 2004, 42, 5249–5255. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Albuquerque, L.P.; da Silva, A.M.; de Araújo Batista, F.M.; de Souza Sene, I.; Costa, D.L.; Costa, C.H.N. Influence of sex hormones on the immune response to leishmaniasis. Parasite Immunol. 2021, 43, e12874. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W.; Rubin, B.Y.; Rothermel, C.D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J. Clin. Investig. 1983, 72, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Reiner, N.E.; Ng, W.; Wilson, C.B.; McMaster, W.R.; Burchett, S.K. Modulation of in vitro monocyte cytokine responses to Leishmania donovani. Interferon-gamma prevents parasite-induced inhibition of interleukin 1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-alpha and in. J. Clin. Investig. 1990, 85, 1914–1924. [Google Scholar] [CrossRef]
- Heinzel, F.P.; Schoenhaut, D.S.; Rerko, R.M.; Rosser, L.E.; Gately, M.K. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 1993, 177, 1505–1509. [Google Scholar] [CrossRef]
- Murray, H.W.; Hariprashad, J. Interleukin 12 is effective treatment for an established systemic intracellular infection: Experimental visceral leishmaniasis. J. Exp. Med. 1995, 181, 387–391. [Google Scholar] [CrossRef]
- Ghalib, H.W.; Piuvezam, M.R.; Skeiky, Y.A.; Siddig, M.; Hashim, F.A.; El-Hassan, A.M.; Russo, D.M.; Reed, S.G. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J. Clin. Investig. 1993, 92, 324–329. [Google Scholar] [CrossRef]
- Bacellar, O.; Brodskyn, C.; Guerreiro, J.; Barral-Netto, M.; Costa, C.H.; Coffman, R.L.; Johnson, W.D.; Carvalho, E.M. Interleukin-12 Restores Interferon- Production and Cytotoxic Responses in Visceral Leishmaniasis. J. Infect. Dis. 1996, 173, 1515–1518. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, R.; Katara, G.K.; Singh, L.C.; Negi, N.S.; Ramesh, V.; Salotra, P. Quantification of Parasite Load in Clinical Samples of Leishmaniasis Patients: IL-10 Level Correlates with Parasite Load in Visceral Leishmaniasis. PLoS ONE 2010, 5, e10107. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Ghosh, S.; Ejazi, S.A.; Rahaman, M.; Pandey, K.; Ravi Das, V.N.; Das, P.; Goswami, R.P.; Saha, B.; Ali, N. Induction of IL-10 and TGFβ from CD4+CD25+FoxP3+ T Cells Correlates with Parasite Load in Indian Kala-azar Patients Infected with Leishmania donovani. PLoS Negl. Trop. Dis. 2016, 10, e0004422. [Google Scholar] [CrossRef]
- Teles, L.d.F.; Viana, A.G.; Cardoso, M.S.; Pinheiro, G.R.G.; Bento, G.A.; Lula, J.F.; Soares, T.d.C.M.; Fujiwara, R.T.; Carvalho, S.F.G.d. Evaluation of medullary cytokine expression and clinical and laboratory aspects in severe human visceral leishmaniasis. Parasite Immunol. 2021, 43, e12880. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.A.; Kumar, R.; Gautam, S.; Nylén, S.; Singh, O.P.; Sundar, S.; Sacks, D. IL-27 and IL-21 Are Associated with T Cell IL-10 Responses in Human Visceral Leishmaniasis. J. Immunol. 2011, 186, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Badaró, R.; Reed, S.G.; Jones, T.C.; Johnson, W.D. Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J. Clin. Investig. 1985, 76, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Bacellar, O.; Brownell, C.; Regis, T.; Coffman, R.L.; Reed, S.G. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J. Immunol. 1994, 152, 5949–5956. [Google Scholar] [CrossRef]
- Karp, C.L.; El-Safi, S.H.; Wynn, T.A.; Satti, M.M.; Kordofani, A.M.; Hashim, F.A.; Hag-Ali, M.; Neva, F.A.; Nutman, T.B.; Sacks, D.L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J. Clin. Investig. 1993, 91, 1644–1648. [Google Scholar] [CrossRef]
- Nylén, S.; Sacks, D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007, 28, 378–384. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Fabri, A.; Kandara, K.; Coudereau, R.; Gossez, M.; Abraham, P.; Monard, C.; Cour, M.; Rimmelé, T.; Argaud, L.; Monneret, G.; et al. Characterization of Circulating IL-10-Producing Cells in Septic Shock Patients: A Proof of Concept Study. Front. Immunol. 2021, 11, 615009. [Google Scholar] [CrossRef]
- D’Oliveira Júnior, A.; Costa, S.R.M.; Bispo Barbosa, A.; Orge Orge, M.d.L.G.; Carvalho, E.M. Asymptomatic Leishmania chagasi Infection in Relatives and Neighbors of Patients with Visceral Leishmaniasis. Mem. Inst. Oswaldo Cruz 1997, 92, 15–20. [Google Scholar] [CrossRef]
- Lima, S.; Braz, D.; Silva, V.; Farias, T.; Zacarias, D.A.; Silva, J.C.; Costa, C.H.N.; Costa, D.L. Biomarkers of the early response to treatment of visceral leishmaniasis: A prospective cohort study. Parasite Immunol. 2021, 43, e12797. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Munk, R.B.; Sugiyama, K.; Ghosh, P.; Sasaki, C.Y.; Rezanka, L.; Banerjee, K.; Takahashi, H.; Sen, R.; Longo, D.L. Antigen-Independent IFN-γ Production by Human Naïve CD4+ T Cells Activated by IL-12 Plus IL-18. PLoS ONE 2011, 6, e18553. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Cho, M.J.; Choi, J.M. Author Correction: Bystander CD4+ T cells: Crossroads between innate and adaptive immunity. Exp. Mol. Med. 2023, 55, 1275. [Google Scholar] [CrossRef] [PubMed]
- Reis-Cunha, J.L.; Grace, C.A.; Ahmed, S.; Harnqvist, S.E.; Lynch, C.M.; Boité, M.C.; Barcellos, G.; Lachaud, L.; Bastien, P.; Munt, H.; et al. The global dispersal of visceral leishmaniasis occurred within human history. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zijlstra, E.E. The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasit. Vectors 2016, 9, 464. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Miller, C.H.T.; Maher, S.G.; Young, H.A. Clinical Use of Interferon-γ. Ann. N.Y. Acad. Sci. 2009, 1182, 69–79. [Google Scholar] [CrossRef]
- Baessler, A.; Vignali, D.A.A. T Cell Exhaustion. Annu. Rev. Immunol. 2024, 42, 179–206. [Google Scholar] [CrossRef]
- Sonar, S.A.; Watanabe, M.; Nikolich, J.Ž. Disorganization of secondary lymphoid organs and dyscoordination of chemokine secretion as key contributors to immune aging. Semin. Immunol. 2023, 70, 101835. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Singh, N.; Singh, A.K.; Rai, M.; Sacks, D.; Sundar, S.; Nylén, S. CD8 T Cell Exhaustion in Human Visceral Leishmaniasis. J. Infect. Dis. 2014, 209, 290–299. [Google Scholar] [CrossRef]
- Silva-O’Hare, J.; de Oliveira, I.S.; Klevorn, T.; Almeida, V.A.; Oliveira, G.G.S.; Atta, A.M.; de Freitas, L.A.R.; Dos-Santos, W.L.C. Disruption of Splenic Lymphoid Tissue and Plasmacytosis in Canine Visceral Leishmaniasis: Changes in Homing and Survival of Plasma Cells. PLoS ONE 2016, 11, e0156733. [Google Scholar] [CrossRef] [PubMed]
- Hermida, M.d.-R.; de Melo, C.V.B.; Lima, I.d.S.; Oliveira, G.G.d.S.; Dos-Santos, W.L.C. Histological Disorganization of Spleen Compartments and Severe Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2018, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.L.; da Silva, A.V.A.; Pereira, L.d.O.R.; Figueiredo, F.B.; Mendes Junior, A.A.V.; Menezes, R.C.; Mendes-da-Cruz, D.A.; Boité, M.C.; Cupolillo, E.; Porrozzi, R.; et al. Pro-Cellular Exhaustion Markers are Associated with Splenic Microarchitecture Disorganization and Parasite Load in Dogs with Visceral Leishmaniasis. Sci. Rep. 2019, 9, 12962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, L.; Gao, J.; Yang, Y.; Hu, C.; Guo, B.; Zhu, B. T lymphocytes maintain structure and function of fibroblastic reticular cells via lymphotoxin (LT)-B. BMC Immunol. 2014, 15, 33. [Google Scholar] [CrossRef]
- Montes de Oca, M.; de Labastida Rivera, F.; Winterford, C.; Frame, T.C.M.; Ng, S.S.; Amante, F.H.; Edwards, C.L.; Bukali, L.; Wang, Y.; Uzonna, J.E.; et al. IL-27 signalling regulates glycolysis in Th1 cells to limit immunopathology during infection. PLOS Pathog. 2020, 16, e1008994. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).