In Salmonella Typhimurium and Bacillus subtilis, Nucleoid-Associated HU Proteins Are N-Terminally Acetylated
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Strain Construction
2.3. Plasmid Construction for Complementation and Overexpression
2.4. Purification of SeNatB and BsYfmK, SeHUα2, SeHUβ2, and SeHUαβ Proteins
2.4.1. SeNatB and BsYfmK
2.4.2. Native HU Heterodimer Purification
2.4.3. Plasmid-Derived SeHUα2, SeHUβ2, and Lysine Variants Purification
2.4.4. Purification of SeMsrA
2.4.5. Purification of SeMsrB
2.5. Thin-Layer Chromatography (TLC) Assay of Msr Activity
2.6. Mass Spectrometry Methods and Data Analysis
2.6.1. At the University of Georgia: Asp-N Digestion and LC-MS/MS Analysis
2.6.2. At the University of Wisconsin-Madison: Enzymatic “In Gel” Digestion
NanoLC-MS/MS
Data Analysis
2.7. In Vitro Acetylation Assays
2.8. In Vitro Deacetylation Assays
2.9. Galactosidase Assays
2.10. Electrophoretic Mobility Shift Assays (EMSAs)
3. Results
3.1. Nomenclature Clarification
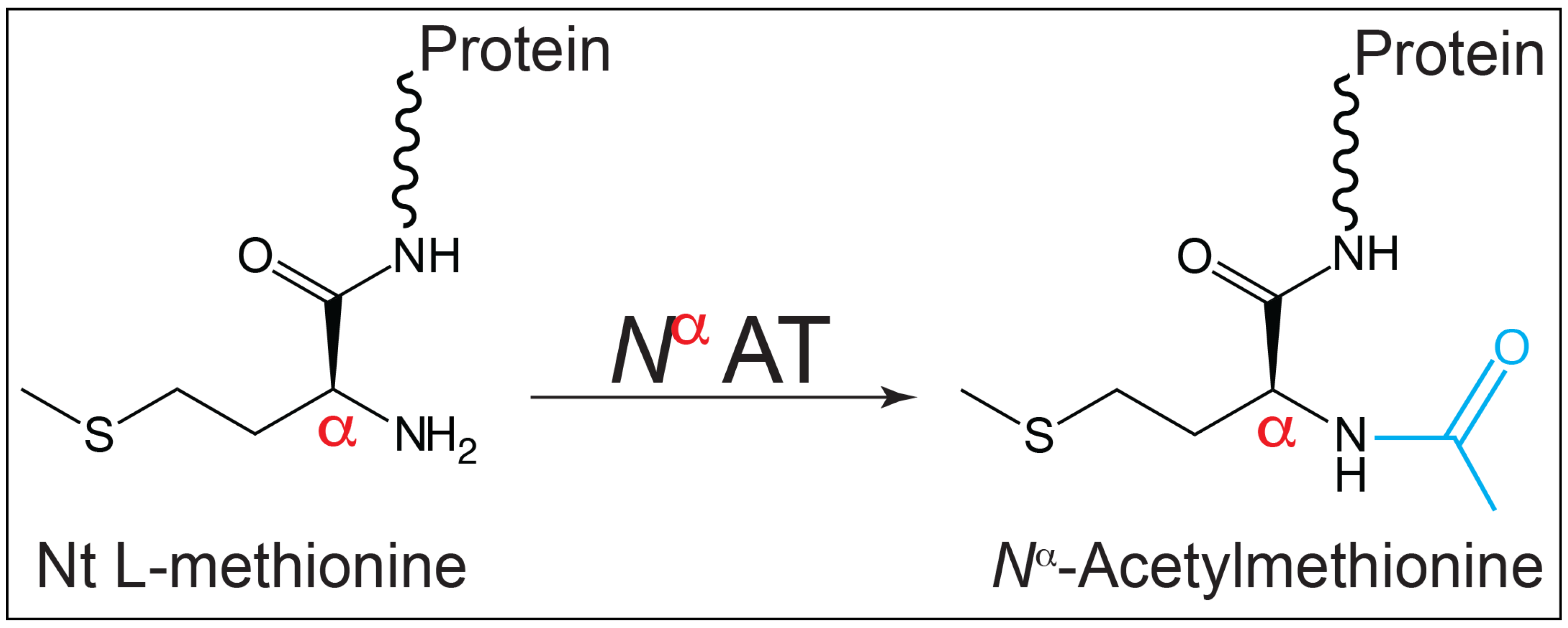
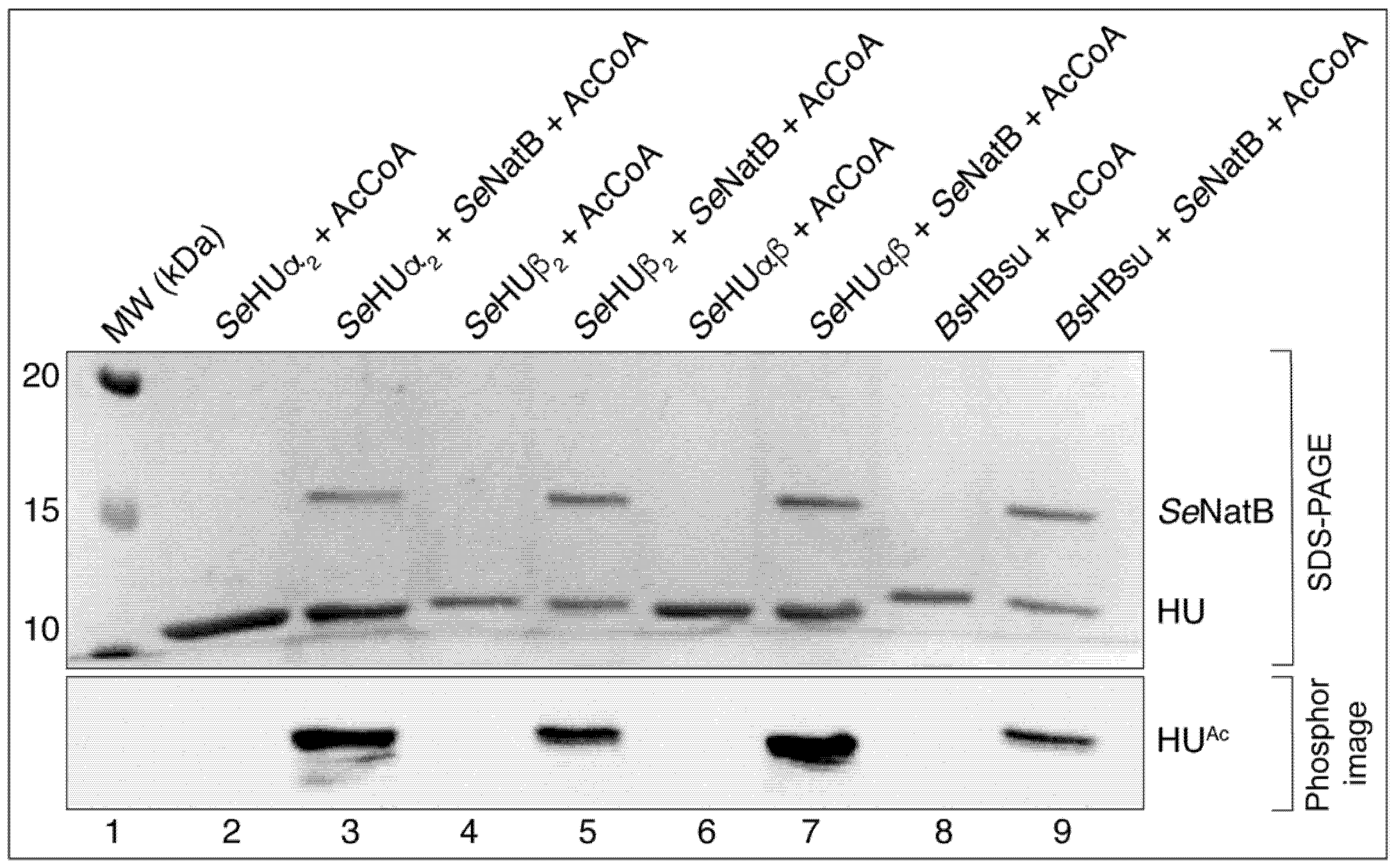
3.2. In S. Typhimurium, SeNatB Acetylated All Three Forms of HU
3.3. Several SeHU Variant Proteins Existed In Vivo
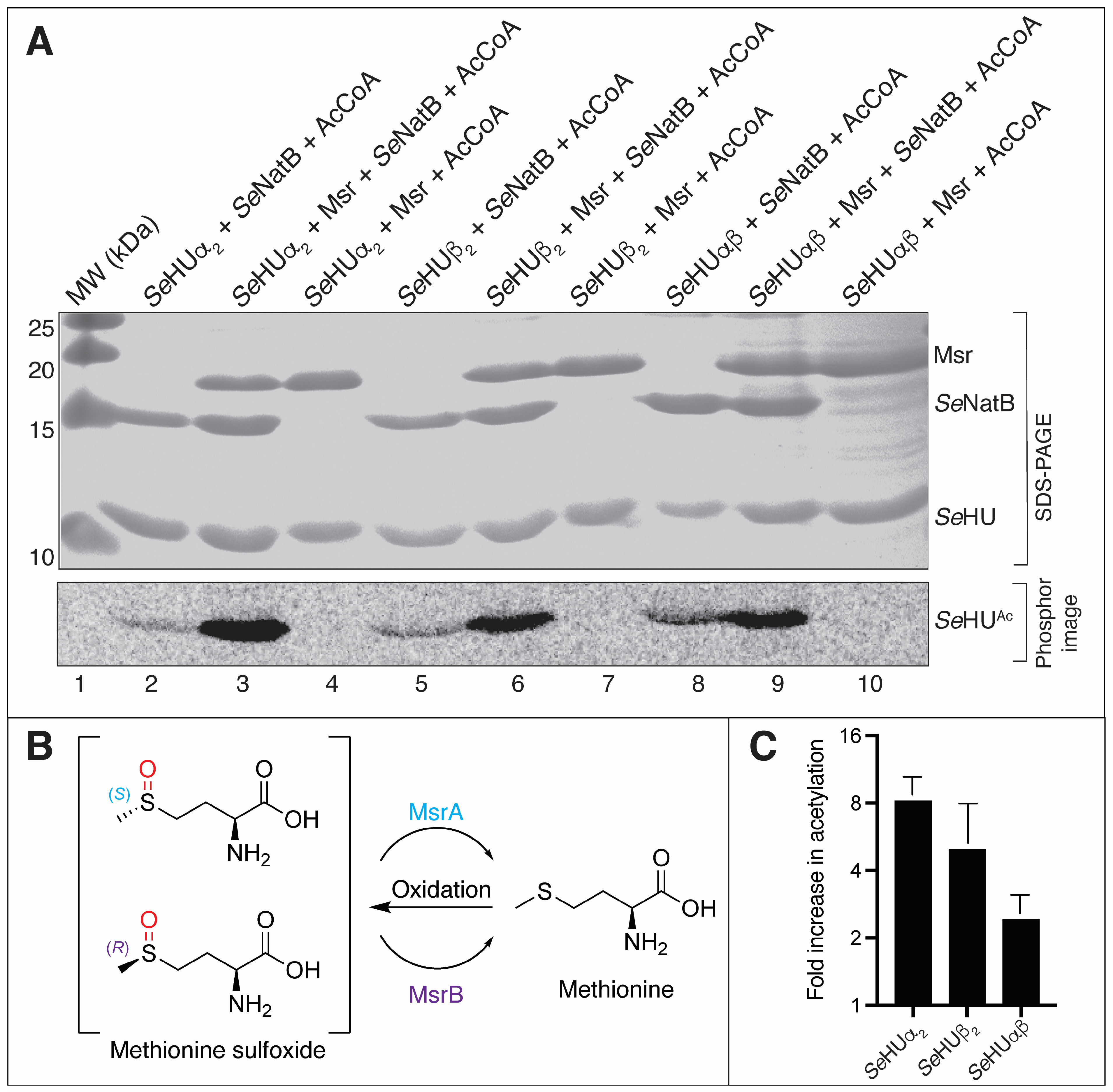
3.4. SeNatB Did Not Acetylate N-Terminal Methionine Sulfoxide Residues
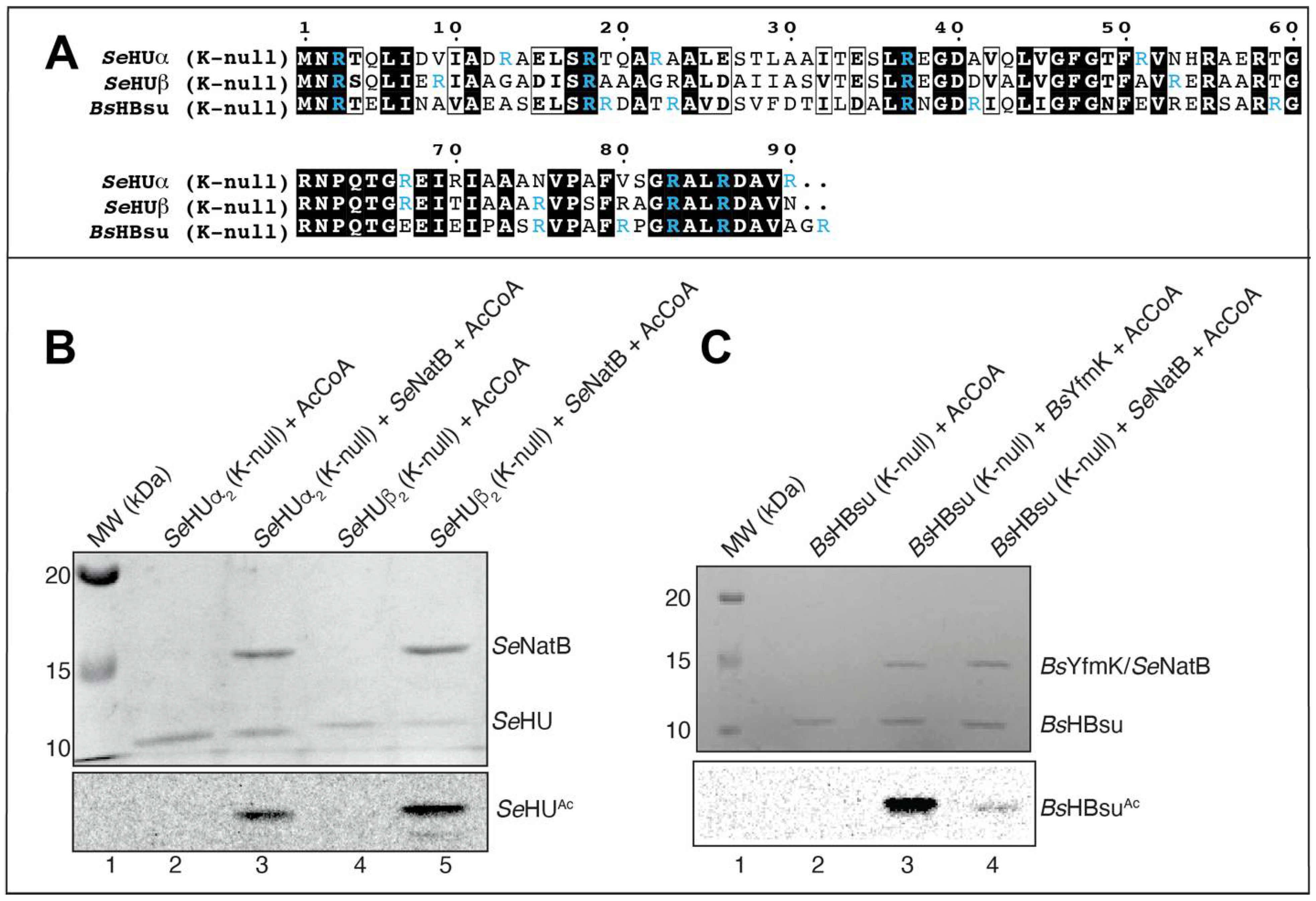
3.5. SeNatB and BsYfmK Acetylated Lysine-Null (K-Null) Variants of SeHU and HBsu Proteins In Vitro
3.6. N-Terminally Acetylated SeHU Proteins Could Not Be Deacetylated by the S. Typhimurium CobB Sirtuin Deacylase
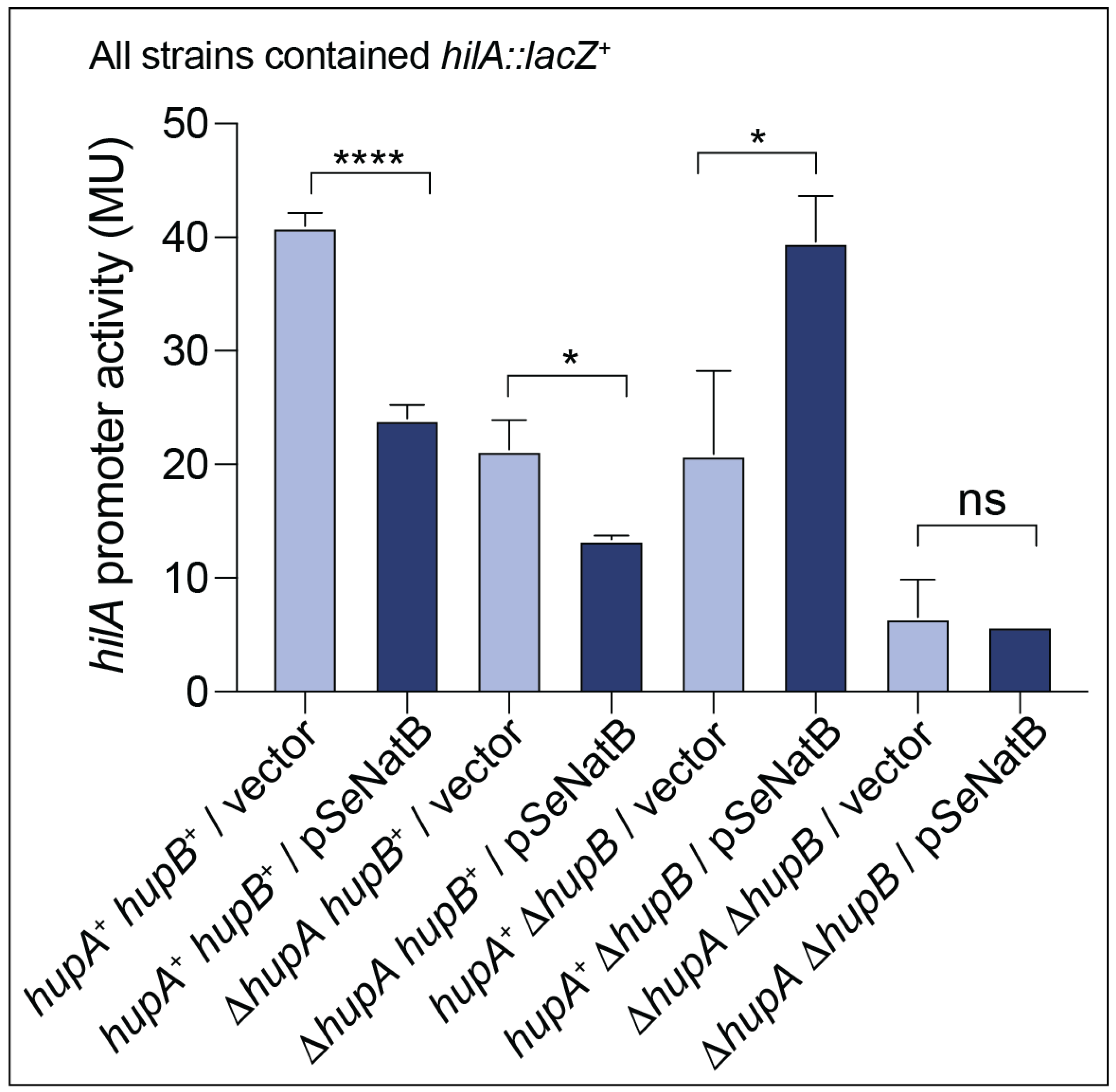
3.7. N-Terminal Acetylation of SeHU Proteins Altered SeHU-Dependent Gene Regulation
In Vivo Evidence
4. Discussion
4.1. Is the Nα Acetylation of HU Proteins a Response to Oxidative Damage?
4.2. Our Work Revealed Unknown Specificities of the B. subtilis YfmK Acyltransferase
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Dopfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J., II; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An overview of epidemiology, pathogenesis, and innovative approaches to mitigate the antimicrobial resistant infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Coburn, B.; Li, Y.; Owen, D.; Vallance, B.A.; Finlay, B.B. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 2005, 73, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Ramatla, T.; Khasapane, N.G.; Mlangeni, L.N.; Mokgokong, P.; Ramaili, T.; Ndou, R.; Nkhebenyane, J.S.; Lekota, K.; Thekisoe, O. Detection of Salmonella pathogenicity islands and antimicrobial-resistant genes in Salmonella enterica serovars Enteritidis and Typhimurium isolated from broiler chickens. Antibiotics 2024, 13, 458. [Google Scholar] [CrossRef]
- Hwang, C.S.; Shemorry, A.; Varshavsky, A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science 2010, 327, 973–977. [Google Scholar] [CrossRef]
- Shemorry, A.; Hwang, C.S.; Varshavsky, A. Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol. Cell 2013, 50, 540–551. [Google Scholar] [CrossRef]
- Xu, F.; Huang, Y.; Li, L.; Gannon, P.; Linster, E.; Huber, M.; Kapos, P.; Bienvenut, W.; Polevoda, B.; Meinnel, T.; et al. Two N-terminal acetyltransferases antagonistically regulate the stability of a nod-like receptor in Arabidopsis. Plant Cell 2015, 27, 1547–1562. [Google Scholar] [CrossRef]
- Sheikh, T.I.; de Paz, A.M.; Akhtar, S.; Ausio, J.; Vincent, J.B. MeCP2_E1 N-terminal modifications affect its degradation rate and are disrupted by the Ala2Val Rett mutation. Hum. Mol. Genet. 2017, 26, 4132–4141. [Google Scholar] [CrossRef]
- Behnia, R.; Panic, B.; Whyte, J.R.; Munro, S. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat. Cell Biol. 2004, 6, 405–413. [Google Scholar] [CrossRef]
- Forte, G.M.; Pool, M.R.; Stirling, C.J. N-terminal acetylation inhibits protein targeting to the endoplasmic reticulum. PLoS Biol. 2011, 9, e1001073. [Google Scholar] [CrossRef] [PubMed]
- Setty, S.R.; Strochlic, T.I.; Tong, A.H.; Boone, C.; Burd, C.G. Golgi targeting of ARF-like GTPase Arl3p requires its Nalpha-acetylation and the integral membrane protein Sys1p. Nat. Cell Biol. 2004, 6, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Trexler, A.J.; Rhoades, E. N-Terminal acetylation is critical for forming alpha-helical oligomer of alpha-synuclein. Protein Sci. 2012, 21, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef]
- Holmes, W.M.; Mannakee, B.K.; Gutenkunst, R.N.; Serio, T.R. Loss of amino-terminal acetylation suppresses a prion phenotype by modulating global protein folding. Nat. Commun. 2014, 5, 4383. [Google Scholar] [CrossRef]
- Singer, J.M.; Shaw, J.M. Mdm20 protein functions with Nat3 protein to acetylate Tpm1 protein and regulate tropomyosin-actin interactions in budding yeast. Proc. Natl. Acad. Sci. USA 2003, 100, 7644–7649. [Google Scholar] [CrossRef]
- Yang, D.; Fang, Q.; Wang, M.; Ren, R.; Wang, H.; He, M.; Sun, Y.; Yang, N.; Xu, R.M. Nalpha-acetylated Sir3 stabilizes the conformation of a nucleosome-binding loop in the BAH domain. Nat. Struct. Mol. Biol. 2013, 20, 1116–1118. [Google Scholar] [CrossRef]
- Monda, J.K.; Scott, D.C.; Miller, D.J.; Lydeard, J.; King, D.; Harper, J.W.; Bennett, E.J.; Schulman, B.A. Structural conservation of distinctive N-terminal acetylation-dependent interactions across a family of mammalian NEDD8 ligation enzymes. Structure 2013, 21, 42–53. [Google Scholar] [CrossRef]
- Scott, D.C.; Monda, J.K.; Bennett, E.J.; Harper, J.W.; Schulman, B.A. N-terminal acetylation acts as an avidity enhancer within an interconnected multiprotein complex. Science 2011, 334, 674–678. [Google Scholar] [CrossRef]
- Coulton, A.T.; East, D.A.; Galinska-Rakoczy, A.; Lehman, W.; Mulvihill, D.P. The recruitment of acetylated and unacetylated tropomyosin to distinct actin polymers permits the discrete regulation of specific myosins in fission yeast. J. Cell Sci. 2010, 123, 3235–3243. [Google Scholar] [CrossRef]
- Arnaudo, N.; Fernandez, I.S.; McLaughlin, S.H.; Peak-Chew, S.Y.; Rhodes, D.; Martino, F. The N-terminal acetylation of Sir3 stabilizes its binding to the nucleosome core particle. Nat. Struct. Mol. Biol. 2013, 20, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Kentache, T.; Jouenne, T.; De, E.; Hardouin, J. Proteomic characterization of Nalpha- and Nepsilon-acetylation in Acinetobacter baumannii. J. Proteom. 2016, 144, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Ouidir, T.; Jarnier, F.; Cosette, P.; Jouenne, T.; Hardouin, J. Characterization of N-terminal protein modifications in Pseudomonas aeruginosa PA14. J. Proteom. 2015, 114, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.R.; Escalante-Semerena, J.C. Modulation of the bacterial CobB sirtuin deacylase activity by N-terminal acetylation. Proc. Natl. Acad. Sci. USA 2020, 117, 15895–15901. [Google Scholar] [CrossRef]
- Oberto, J.; Nabti, S.; Jooste, V.; Mignot, H.; Rouviere-Yaniv, J. The HU regulon is composed of genes responding to anaerobiosis, acid stress, high osmolarity and SOS induction. PLoS ONE 2009, 4, e4367. [Google Scholar] [CrossRef]
- Claret, L.; Rouviere-Yaniv, J. Variation in HU composition during growth of Escherichia coli: The heterodimer is required for long term survival. J. Mol. Biol. 1997, 273, 93–104. [Google Scholar] [CrossRef]
- Bonnefoy, E.; Almeida, A.; Rouviere-Yaniv, J. Lon-dependent regulation of the DNA binding protein HU in Escherichia coli. Proc. Natl. Acad. Sci. USA 1989, 86, 7691–7695. [Google Scholar] [CrossRef]
- Carabetta, V.J.; Greco, T.M.; Cristea, I.M.; Dubnau, D. YfmK is an N(epsilon)-lysine acetyltransferase that directly acetylates the histone-like protein HBsu in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2019, 116, 3752–3757. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Bertani, G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 2004, 186, 595–600. [Google Scholar] [CrossRef] [PubMed]
- McKinney, J.; Guerrier-Takada, C.; Galan, J.; Altman, S. Tightly regulated gene expression system in Salmonella enterica serovar Typhimurium. J. Bacteriol. 2002, 184, 6056–6059. [Google Scholar] [CrossRef] [PubMed]
- Ellermeier, J.R.; Slauch, J.M. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J. Bacteriol. 2008, 190, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.W.; Botstein, D.; Roth, J.R. A Manual for Genetic Engineering: Advanced Bacterial Genetics; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1980. [Google Scholar]
- Chan, R.K.; Botstein, D.; Watanabe, T.; Ogata, Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology 1972, 50, 883–898. [Google Scholar] [CrossRef]
- VanDrisse, C.M.; Escalante-Semerena, J.C. New high-cloning-efficiency vectors for complementation studies and recombinant protein overproduction in Escherichia coli and Salmonella enterica. Plasmid 2016, 86, 1–6. [Google Scholar] [CrossRef][Green Version]
- Rocco, C.J.; Dennison, K.L.; Klenchin, V.A.; Rayment, I.; Escalante-Semerena, J.C. Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid 2008, 59, 231–237. [Google Scholar] [CrossRef]
- Tabor, S. Expression using the T7 RNA polymerase/promoter system. In Current Protocols in Molecular Biology; Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K., Eds.; Wiley Interscience: New York, NY, USA, 1990; Volume 2, pp. 16.2.1–16.2.11. [Google Scholar]
- Galloway, N.R.; Toutkoushian, H.; Nune, M.; Bose, N.; Momany, C. Rapid cloning for protein crystallography using Type IIS restriction enzymes. Cryst. Growth Des. 2013, 13, 2833–2839. [Google Scholar] [CrossRef]
- Pellegrini, O.; Oberto, J.; Pinson, V.; Wery, M.; Rouviere-Yaniv, J. Overproduction and improved strategies to purify the threenative forms of nuclease-free HU protein. Biochimie 2000, 82, 693–704. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Miller, F.D.; Hershberger, C.L. A quantitative beta-galactosidase alpha-complementation assay for fusion proteins containing human insulin B-chain peptides. Gene 1984, 29, 247–250. [Google Scholar] [CrossRef]
- Friedrich, U.A.; Zedan, M.; Hessling, B.; Fenzl, K.; Gillet, L.; Barry, J.; Knop, M.; Kramer, G.; Bukau, B. N(alpha)-terminal acetylation of proteins by NatA and NatB serves distinct physiological roles in Saccharomyces cerevisiae. Cell Rep. 2021, 34, 108711. [Google Scholar] [CrossRef] [PubMed]
- Polevoda, B.; Sherman, F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J. Mol. Biol. 2003, 325, 595–622. [Google Scholar] [CrossRef]
- Ingrosso, D.; Fowler, A.V.; Bleibaum, J.; Clarke, S. Specificity of endoproteinase Asp-N (Pseudomonas fragi): Cleavage at glutamyl residues in two proteins. Biochem. Biophys. Res. Commun. 1989, 162, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Tetaz, T.; Morrison, J.R.; Andreou, J.; Fidge, N.H. Relaxed specificity of endoproteinase Asp-N: This enzyme cleaves at peptide bonds N-terminal to glutamate as well as aspartate and cysteic acid residues. Biochem. Int. 1990, 22, 561–566. [Google Scholar] [PubMed]
- Levine, R.L.; Moskovitz, J.; Stadtman, E.R. Oxidation of methionine in proteins: Roles in antioxidant defense and cellular regulation. IUBMB Life 2000, 50, 301–307. [Google Scholar] [CrossRef]
- Sagher, D.; Brunell, D.; Hejtmancik, J.F.; Kantorow, M.; Brot, N.; Weissbach, H. Thionein can serve as a reducing agent for the methionine sulfoxide reductases. Proc. Natl. Acad. Sci. USA 2006, 103, 8656–8661. [Google Scholar] [CrossRef]
- Tucker, A.C.; Escalante-Semerena, J.C. Biologically active isoforms of CobB sirtuin deacetylase in Salmonella enterica and Erwinia amylovora. J. Bacteriol. 2010, 192, 6200–6208. [Google Scholar] [CrossRef]
- Schechter, L.M.; Jain, S.; Akbar, S.; Lee, C.A. The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infect. Immun. 2003, 71, 5432–5435. [Google Scholar] [CrossRef]
- Ouidir, T.; Jouenne, T.; Hardouin, J. Post-translational modifications in Pseudomonas aeruginosa revolutionized by proteomic analysis. Biochimie 2016, 125, 66–74. [Google Scholar] [CrossRef]
- Bonissone, S.; Gupta, N.; Romine, M.; Bradshaw, R.A.; Pevzner, P.A. N-terminal protein processing: A comparative proteogenomic analysis. Mol. Cell. Proteom. 2013, 12, 14–28. [Google Scholar] [CrossRef]
- Ouidir, T.; Kentache, T.; Hardouin, J. Protein lysine acetylation in bacteria: Current state of the art. Proteomics 2016, 16, 301–309. [Google Scholar] [CrossRef]
- Macek, B.; Forchhammer, K.; Hardouin, J.; Weber-Ban, E.; Grangeasse, C.; Mijakovic, I. Protein post-translational modifications in bacteria. Nat. Rev. Microbiol. 2019, 17, 651–664. [Google Scholar] [CrossRef] [PubMed]

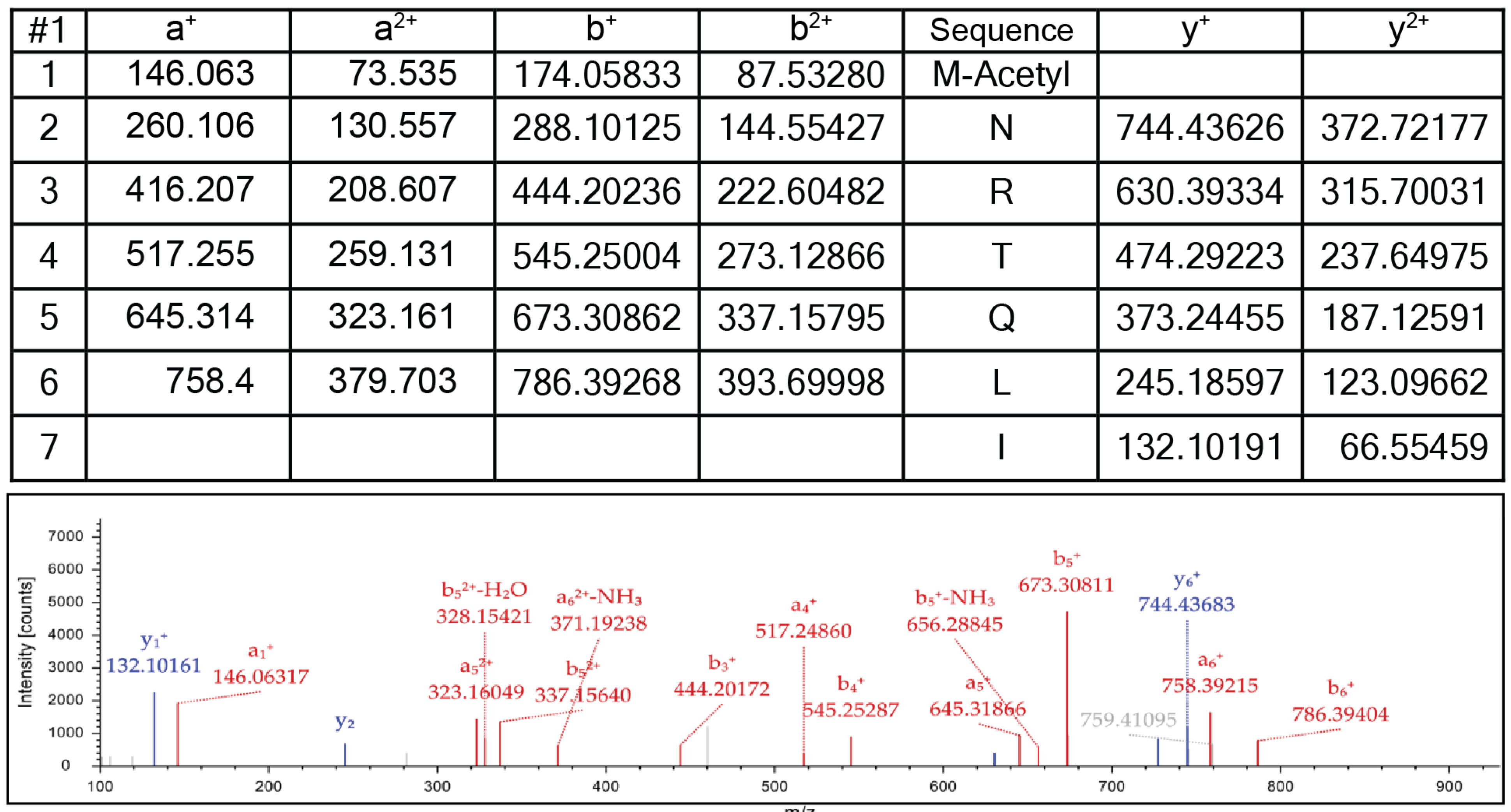
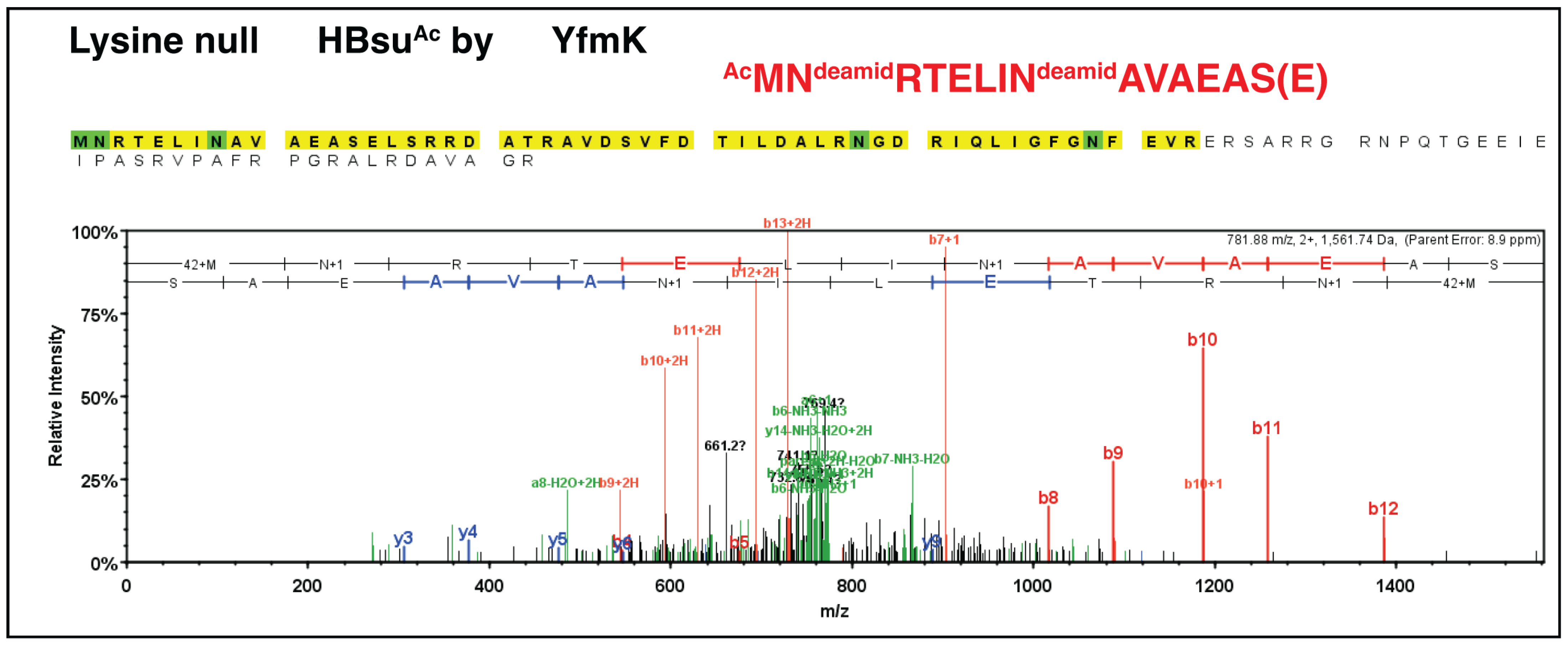
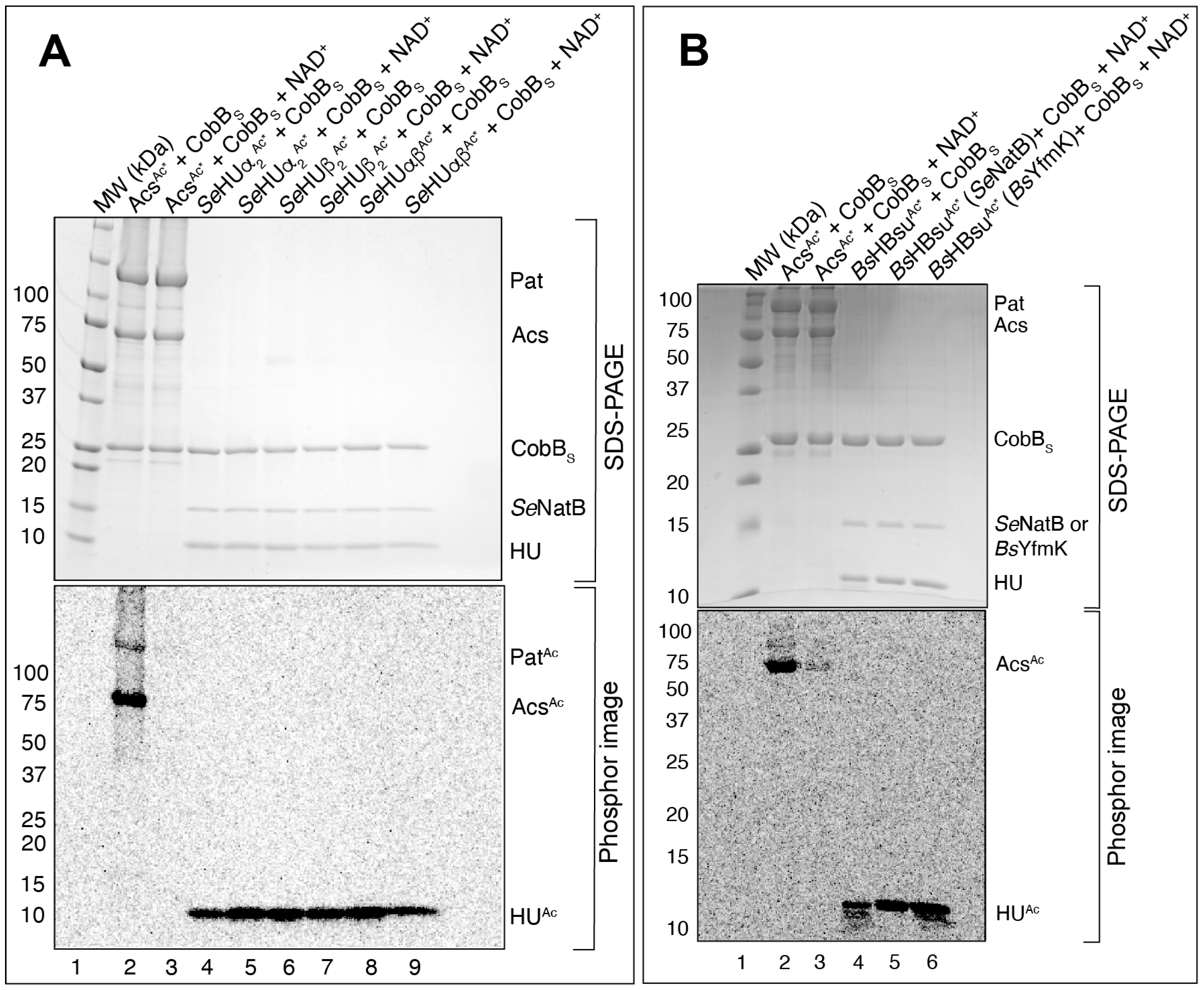
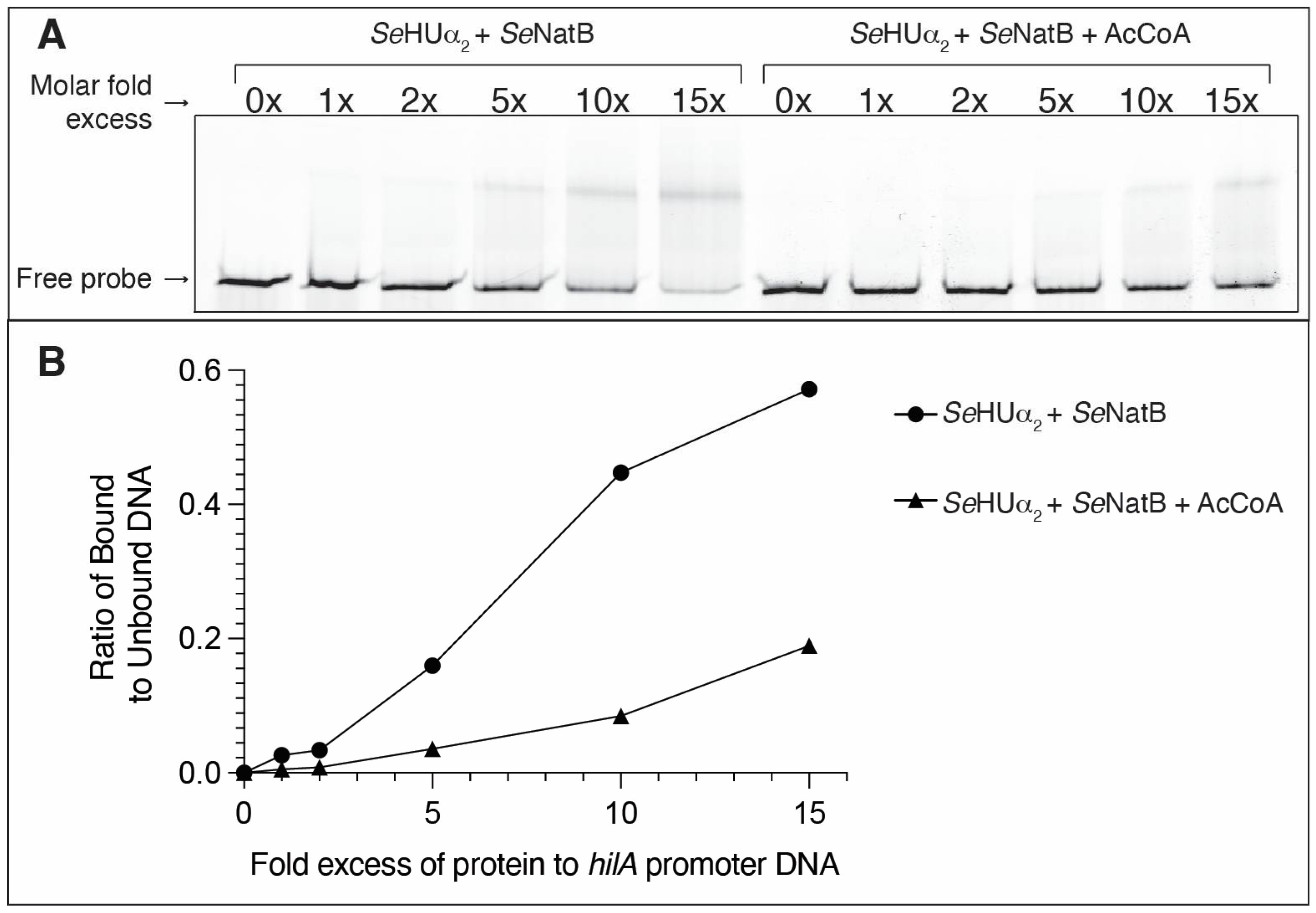

| Strain | Relevant Genotype | Reference/Source |
|---|---|---|
| S. enterica strains | ||
| JE6583 2 | metE205 ΔaraB9 | Kenneth Sanderson |
| JE6692 | metE205 ΔaraB9/pKD46 | |
| JE9637 3 | aadA::araCPBADT7-1 | [32] |
| JE13152 | aadA::araCPBADT7-1 ΔhupA102 ΔhupB104 lon-71 zaj-1034::Tn10 pat::MudI1734 | |
| JE7658 4 | S. enterica serovar Typhimurium 14028s (wild-type genome) | Joshua Fierer |
| Derivatives of JE7658 | ||
| JE18227 | attλ::pDX1::hilA′-lac+ | [33] |
| JE25581 | attλ::pDX1::hilA′-lac+ hupA101::kan+/pCV1 | |
| JE25582 | attλ::pDX1::hilA′-lac+ hupA101::kan+/pNatB10 | |
| JE25583 | attλ::pDX1::hilA′-lac+ hupB103::cat+/pCV1 | |
| JE25584 | attλ::pDX1::hilA′-lac+ hupB103::cat+/pNatB10 | |
| JE25588 | attλ::pDX1::hilA′-lac+ hupB103::cat+ hupA101::kan+/pCV1 | |
| JE25589 | attλ::pDX1::hilA′-lac+ hupB103::cat+ hupA101::kan+/pNatB10 | |
| E. coli Strains | ||
| E. coli C41 (λDE3) | pka12::kan+ ompT hsdS (rBmB) gal (λDE3) | Laboratory collection |
| E. coli C43 (λDE3) | F-ompT gal hsdSB (rB-mB-) dcm lon C41 (λDE3) | Laboratory collection |
| Plasmid | Genotype | Description | Source |
|---|---|---|---|
| pCV1 | araC+bla+ | ParaBAD expression vector | [36] |
| pNatB10 | natB+bla+ | S. enterica natB+ cloned into pCV1 | [24] |
| pTEV6 | bla+ | N-terminal rTEV-cleavable MBP-His fusion overexpression vector | [37] |
| pYfmk1 | yfmK+bla+ | B. subtilis yfmK+ cloned into pTEV6 | |
| pNatB6 | natB+bla+ | S. enterica natB+ cloned into pTEV6 | [24] |
| pTEV18 | bla+ | N-terminal rTEV-cleavable His fusion overexpression vector | [36] |
| pMsrA2 | msrA+ bla+ | (Stm4408) msrA cloned into pTEV18 | |
| pMsrB1 | msrB+bla+ | stm1291 (yeaA+ also called msrB) cloned into pTEV18 | |
| pT7-7 | bla+ | Overexpression vector with T7 promoter | [38] |
| pSAPKO-WT | kan+ | Derivative of pET28b with BspQI cut sites and T7 promoter for overexpression of tagless vectors | [39] |
| pHBSU1 | hbs+kan+ | hbs+ cloned into pSAPKO-WT | |
| pHUPB3 | hupB+bla+ | Encodes HupAWT in pT7-7 | |
| pHUPA5 | hupA+bla+ | hupB+ cloned into pT7-7 (Contains extra start ATG) | |
| pHUPA6 | hupA+bla+ | Encodes HupAK3R,K13R,K18R,K22R,K37R,K51R,K67R,K83R,K86R,K90R in pT7-7 | |
| pHUPA7 | hupA+bla+ | Encodes HupBWT with deleted extra ATG in pT7-7; mutagenized pHUPA3 | |
| pHUPB14 | hupA+bla+ | Encodes HupBK3R,K9R,K18R,K37R,K53R,K67R,K75R,K83R,K86R in pT7-7 | |
| pHUPB15 | hupB+bla+ | Encodes HupBWT with deleted extra ATG in pT7-7; mutagenized pHUPB5 | |
| pKD46 | exo+bet+gam+bla+ | Expression of lambda Red recombinase system | [24] |
| Primer Name | Primer Sequence 5′ → 3′ |
|---|---|
| pYiaC10_F_BspQI | nngctcttcnttcatgattcgcaaatcccagagtgaagac |
| pYiaC10_R_BspQI | nngctcttcnttattacggcgtttgatccgcctgccaac |
| msrB_F_BspQI | nngctcttcnttcatgagcacgtttaaagtgag |
| msrB_R_BspQI | nngctcttcnttatcagcctttcagttgat |
| Yfmk_F_BspQI | nngctcttcnttcatggcttcaatagacagg |
| YfmK_R_BspQI | nngctcttcnttatcagttgcgaagaatcag |
| HBsu_F_sapkowT | nngctcttcnatgatgaacaaaacagaact |
| HBsu_R_sapkowT | nngctcttcnttaagttgccggaaaataa |
| Del_M1_HupA1 | ctttaagaaggagatatacatatgaacaagactcaactgattgatgta |
| Del_M1_HupA2 | tacatcaatcagttgagtcttgttcatatgtatatctccttcttaaag |
| DelM1_HupB1 | tttaactttaagaaggagatatacatatgaataaatctcaactgatcgaaaaaattgc |
| DelM1_HupB2 | gcaattttttcgatcagttgagatttattcatatgtatatctccttcttaaagttaaa |
| SeHupB_K3R_P1T77 | tttcgatcagttgagatctattcaccatatgtatatctccttcttaaagt |
| SeHupB_K3R_P2T77 | actttaagaaggagatatacatatggtgaatagatctcaactgatcgaaa |
| SeHupB_K9R_P1 | cagcccctgcagcaattctttcgatcagttgagat |
| SeHupB_K9R_P2 | atctcaactgatcgaaagaattgctgcaggggctg |
| QC1_K18R_HupB | caggggctgatatctctagggctgcggctg |
| QC2_K18R_HupB | cagccgcagccctagagatatcagcccctgc |
| QC1_K37R_HupB | tcatccccttctctcagagattcggtaacagaagca |
| QC2_K37R_HupB | tgcttctgttaccgaatctctgagagaaggggatga |
| QC1_K75R_HupB | ctcggcactctggcagcggcgatggt |
| QC2_K75R_HupB | accatcgccgctgccagagtgccgag |
| QC1_ K83R_86R_HupB | taccgcgtctctcagcgctctacctgcacggaaactcg- |
| QC2_ K83R_86R_HupB | cgagtttccgtgcaggtagagcgctgagagacgcggta |
| hilA_R1_FAM | taaaatgtggcatgataatagt |
| hilA_F1 | ctattgcaatgaggcca |
| yfmk_F_KpnI-pTEV6 | nnnggtaccatggcttcaatagacagg |
| yfmk_R_NotI-pTEV6 | nnngcggccgctcagttgcgaagaatcag |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parks, A.R.; Will, J.L.; Mathew, L.G.; Massier, S.; Hardouin, J.; Escalante-Semerena, J.C. In Salmonella Typhimurium and Bacillus subtilis, Nucleoid-Associated HU Proteins Are N-Terminally Acetylated. Pathogens 2025, 14, 616. https://doi.org/10.3390/pathogens14070616
Parks AR, Will JL, Mathew LG, Massier S, Hardouin J, Escalante-Semerena JC. In Salmonella Typhimurium and Bacillus subtilis, Nucleoid-Associated HU Proteins Are N-Terminally Acetylated. Pathogens. 2025; 14(7):616. https://doi.org/10.3390/pathogens14070616
Chicago/Turabian StyleParks, Anastacia R., Jessica L. Will, Liju G. Mathew, Sébastien Massier, Julie Hardouin, and Jorge C. Escalante-Semerena. 2025. "In Salmonella Typhimurium and Bacillus subtilis, Nucleoid-Associated HU Proteins Are N-Terminally Acetylated" Pathogens 14, no. 7: 616. https://doi.org/10.3390/pathogens14070616
APA StyleParks, A. R., Will, J. L., Mathew, L. G., Massier, S., Hardouin, J., & Escalante-Semerena, J. C. (2025). In Salmonella Typhimurium and Bacillus subtilis, Nucleoid-Associated HU Proteins Are N-Terminally Acetylated. Pathogens, 14(7), 616. https://doi.org/10.3390/pathogens14070616








