Abstract
Obligate intracellular parasites must efficiently invade host cells to complete their life cycle and facilitate transmission. For the malaria-causing parasite Plasmodium falciparum, the invasion of an erythrocyte is a critical process, and thereby a key target for intervention strategies. In this study, we investigate the role of the ApiAP2 family transcription factor PfAP2-06B (PF3D7_0613800) in the intraerythrocytic developmental cycle of P. falciparum and focus on its regulation of genes involved in erythrocyte invasion. Conditional knockdown of PfAP2-06B resulted in a defect in asexual growth and impaired erythrocyte invasion. Bulk RNA sequencing (RNA-seq) analysis revealed that PfAP2-06B modulates the expression of invasion-related genes during the schizont stage. Single-cell RNA sequencing indicated that PfAP2-06B influences invasion gene expression and contributes to stochastic variations in expression of cell-to-cell genes. These results underscore the critical function of PfAP2-06B in the process of erythrocyte invasion and suggest its potential as a target for novel malaria control strategies. Importance: Understanding gene regulation in Plasmodium falciparum is essential for uncovering mechanisms of parasite development and pathogenicity. The research underscores the pivotal role of PfAP2-06B in regulating critical aspects of Plasmodium intraerythrocytic development and host cell invasion, demonstrating that PfAP2-06B plays a key role in orchestrating stage-specific gene expression. These findings provide new insights into the transcriptional networks of P. falciparum and highlight PfAP2-06B as a potential target for therapeutic intervention. This work advances our understanding of malaria pathogenesis and developing effective interventions.
1. Introduction
The protozoan phylum Apicomplexa encompasses a diverse group of obligate intracellular parasites, many of which are of medical and veterinary importance [1]. Among these, Plasmodium falciparum is a prominent causative agent of human malaria, a disease that remains a major global health challenge, with 249 million malaria infections cases and 608,000 deaths in 2022 [2,3]. The life cycle of P. falciparum is complex, and involves both vertebrate and insect hosts; the intraerythrocytic developmental cycle (IDC) in the human host is central to disease pathogenesis [4].
In the human host, P. falciparum begins its asexual replication cycle with the invasion of erythrocytes by merozoites. This marks the start of the 48 h IDC, during which the parasite undergoes significant morphological and metabolic changes [5,6]. After erythrocyte invasion, the merozoite develops into a trophozoite, which metabolizes hemoglobin and acquires nutrients from serum. The intraerythrocytic parasite progresses through a series of developmental stages and, ultimately, to the schizont stage, during which the parasite replicates asexually via schizogony.
The division of the parasite nucleus and cytoplasm during schizogony results in 16 to 32 daughter merozoites, which are packaged within the schizont and are then released into the bloodstream following erythrocyte rupture [7]. Merozoites are equipped with a specialized set of proteins that are essential for a new round of erythrocyte invasion, including surface proteins such as merozoite surface proteins (MSPs), and micronemal and rhoptry organellar secreted proteins, which facilitate the recognition and binding of new erythrocytes [8,9,10]. The process of egress and invasion repeats cyclically, thus increasing the parasite burden in the bloodstream and resulting in the characteristic clinical symptoms of malaria, including fever, chills, and anemia.
Understanding the molecular and cellular mechanisms underlying the egress and invasion processes is crucial for developing new therapeutic strategies against P. falciparum and malaria. Despite significant advances in malaria treatment and prevention, the parasite’s complex life cycle and ability to rapidly evolve resistance to drugs pose ongoing challenges to controlling the disease [11]. Moreover, critical proteins that are exposed to the host immune system are typically expanded into antigenically variant gene families [12]. Therefore, elucidating the factors that regulate merozoite egress, invasion, and the subsequent immune responses is critical for identifying novel drug targets and vaccine candidates that may help in the fight against malaria [13,14].
In P. falciparum, epigenetic regulation through histone modifications and histone variants plays a central role in the control of gene expression [15]. The regulation of transcription in eukaryotes is influenced by specific transcription factors (TFs), which orchestrate developmental transcriptional programs [16,17]. Twenty-seven AP2 (ApiAP2) DNA-binding proteins unique to Apicomplexa have been characterized as central regulators of transcription, playing essential roles in developmental transitions, cellular differentiation, and environmental response mechanisms [15,18,19]. Among the ApiAP2 family proteins, the transcription factor AP2-I has been shown to be critical for erythrocyte invasion, suggesting its involvement in the regulation of genes associated with the invasion process [20]. The binding of AP2-I occurs prior to the recruitment of the bromodomain protein PfBDP1, which plays a critical role in the regulation of invasion-related genes. Another ApiAP2 TF, PF3D7_0613800, has been implicated in invasion, with its expression peaking during the late trophozoite to schizont stage. PF3D7_0613800 was identified as an interaction partner of PfBDP1 through mass spectrometry [21], and pull-down assays using a novel DNA motif identified by ATAC-seq [22] further support the interaction between PF3D7_0613800, AP2-I, and PfBDP1.
In this study, we employed CRISPR/Cas9 genomic editing technologies to investigate the function of PF3D7_0613800 throughout the Plasmodium IDC [23]. Using inducible gene knockdown, we confirm that PF3D7_0613800 encodes a Plasmodium-specific ApiAP2 transcription factor [24], which we designate as PfAP2-06B. Our results show that PfAP2-06B is essential for parasite growth, development, and invasion. Through growth curve assays, bulk RNA sequencing (RNA-seq), and single-cell RNA sequencing (scRNA-seq) analysis [25], we establish that PfAP2-06B regulates the expression of genes linked to invasion, with a specific expression pattern during the schizont phase of the intraerythrocytic life cycle.
2. Methods
2.1. Parasite Cultures
The Plasmodium falciparum 3D7 strain was grown in type O fresh human erythrocytes, following the described protocol, using RPMI 1640 medium (Gibco, Waltham, MA, USA) containing 0.5% Albumax I (Invitrogen, Waltham, MA, USA). Cultures were incubated at 37 °C under a gas mixture of 5% CO2, 5% O2, and 90% N2 at 37 °C [26]. Parasites were routinely synchronized at the ring stage using repeated 5% sorbitol treatments. For growth curve assays, a 5 h synchronization window was achieved by purifying schizonts with Percoll–sorbitol gradients (70% and 40% Percoll), followed by a 5% sorbitol treatment 5 h afterward [27].
2.2. Plasmid Construction
The pL6cs-AP2-06B-2xfkbp-gfp-2xfkb plasmid was constructed according to the previously described method [23,24,27]. The guide RNA was annealed using complementary oligonucleotides and ligated into the pL6cs plasmid between Xho I and Avr II sites. The C-terminal fragment of pfAP2-06B with 2×fkbp-gfp-2×fkbp was then inserted into Afl II and Asc I sites. The plasmid was verified by sequencing, transformed into Escherichia coli XL10 for amplification, and purified. Primers used for construction are listed in Supplementary Table S1.
2.3. Generation of Transgenic Parasite Lines
Transfections were conducted in uninfected erythrocytes using 100,000 ng of purified pL6cs-pfap2-06b-2xfkbp-gfp-2xfkbp plasmid, along with 100,000 ng of pUF1-Cas9-DSM1, followed by the addition of purified schizont stage 3D7 parasites. Parasites were cultured in the presence of 2.5 nM WR99210 and 1.5 μM DSM1 (Invitrogen), until live parasites were observed 3 weeks later in thin blood smears stained with Giemsa solution. The sequences at the designed integration sites were verified by PCR amplification of genomic DNA, followed by sequencing to confirm genetic editing. Transfections were then performed in uninfected erythrocytes using 100,000 ng of purified pLyn-FRB-mCherry plasmid and 2 μg/mL BSD, with the addition of schizont-stage PfAP2-06B-2xFKBP-GFP-2xFKBP parasites. Clonal lines of transgenic PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry (PfAP2-06B-KS) parasites were isolated by limiting dilution cloning. Primers used for verification are listed in Supplementary Table S2.
2.4. Growth Curve Assays
Parasites were tightly synchronized to a 5 h window and plated at 0.1% parasitemia with 2% hematocrit in a 6-well plate. PfAP2-06B-KS parasites were divided into two 2 mL dishes, one with 250 nM Rapa and the other as a control (EtOH of the same volume as Rapa). Parasitemia levels were monitored over the next three cycles by counting trophozoites in Giemsa-stained smears.
2.5. Immunofluorescence Assays
Immunofluorescence assays were performed to localize PfAP2-06B as described [28]. Schizont-stage parasites were fixed with 4% paraformaldehyde for 10 min, washed with PBS, and incubated with primary antibodies against GFP (1:500–1:1000) and mCherry (1:300). Secondary antibodies, AlexaFluor 488 goat anti-mouse IgG (1:500) and AlexaFluor 568 goat anti-rabbit IgG (1:500), were applied. For nuclear localization, samples were incubated with DAPI for 30 min. Preparations were visualized using a Nikon A1R microscope (Nikon, Tokyo, Japan) at 60× to 100× magnification, and images were acquired with NIS Elements software 4.2.0 and processed with Adobe Photoshop CS6 Extended v13.0.0.0.
2.6. Merozoite Counting Assay
To quantify the number of nuclei per schizont, highly synchronized parasites were cultured with or without knockdown (KD) drugs throughout their respective life cycles. The number of merozoites produced per schizont was assessed in mature segmenters. Schizont nuclei were Giemsa-stained, and the number of nuclei was counted in 26 mature schizonts.
2.7. Merozoite Release Assay
PfAP2-06B-KS parasites were sorbitol-synchronized as 0–3 h rings with 1% parasitemia, plated in replicate (three replicates for [+]Rapa and [−]Rapa controls; three replicates for [+]Rapa and [−]Rapa controls each with the addition of 200 nM ML-10 [29] at the trophozoite stage). Data are presented as mean +/− S.D. (or data range for N = 3 samples). At the 46, 48, and 50 h stages, calculate the schizont parasitemia using microscopy with Giemsa-stained blood smears (FANYIGX, Beijing, China).
2.8. Invasion Assays
100 μL erythrocytes were pretreated with 1 mg/mL trypsin at 37 °C for 1 h under gentle shaking, then washed twice with incomplete medium. Soybean trypsin inhibitor was subsequently added to the packed erythrocytes, which were incubated at room temperature for 10 min. Following three washes with medium, the erythrocytes were resuspended in an equal volume of medium and stored at 4 °C until use.
Invasion assay was performed to determine the invasion efficiency for proposed transgenic parasite lines. Briefly, mature schizonts were purified by centrifugation on 40%/70% Percoll gradient. Enriched schizont-infected erythrocytes were counted, and 8 × 105 schizonts were mixed with trypsin-treated erythrocytes at a 1:50 ratio in 200 μL complete medium, pH 7.2 (RPMI 1640, 24 mM HEPES, 360 μM hypoxanthine, 24 mM NaHCO3, 10 μg of gentamicin per mL, and 5% Albumax [GIBCO]), gassed with a 5% CO2, 5% O2, and 90% N2 mixture. Mixtures were added into 96-well plates with duplicate wells for each treatment. After incubation under standard conditions for 20 h, thin blood smears were prepared. Invasion efficiency was determined by microscopically counting ring-stage parasites, with at least 50 fields of the smear examined. Invasion efficiency was calculated by dividing the parasitemia after invasion by the pre-invasion baseline parasitemia.
2.9. Bulk RNA-Seq
PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry parasites were synchronized to a 5 h window with or without 250 nM Rapa treatment. At 38 to 42 h post-invasion (HPI), samples were collected during the schizont stage in TRIzol (two biological replicates). Total RNA was extracted following the manufacturer’s protocol (Zymo Research, Irvine, CA, USA). For strand-specific RNA-seq, poly(A) selection was performed using KAPA mRNA Capture Beads, followed by fragmentation to approximately 300–400 nucleotides. Library preparation was completed using the KAPA Stranded mRNA-Seq Kit (KK8421) (KAPA Biosystems, Wilmington, MA, USA), and sequencing was conducted on an Illumina HiSeq XTen system to generate 150 bp paired-end reads.
2.10. Bulk RNA-Seq Analysis
Low-quality and adaptor sequences were removed from the reads using Cutadapt (v1.16) with appropriate parameters. RNA sequencing reads were then aligned to the Plasmodium falciparum 3D7 genome (Pf 3D7 v45, obtained from PlasmoDB) using HISAT2 (v2.1.0) [30] in strand-specific mode (--rna-strandness RF). Mapped reads were assembled into transcripts based on the PlasmoDB GFF annotation files (Pf 3D7 v45) using featureCounts (v1.6.4) [31] with the following parameters: -M -p -B -C -s 2. Gene expression was quantified as fragments per kilobase of transcript sequence per million read pairs mapped (FPKM), using featureCounts output in R version 4.1.3. Differential gene expression was determined with a fold change threshold of >1.5 and a p-value < 0.05. Gene Ontology (GO) analysis of differentially expressed genes was performed using clusterProfiler, with a BH-adjusted p-value threshold of <0.01.
2.11. Single-Cell Suspension Preparation
Schizonts, with or without Rapa treatment, were collected by 40%/70% Percoll from culture and resuspended in 2–3 mL of 1x red blood cell lysis buffer (Invitrogen, 00-4333), then incubated at room temperature for 2–4 min. Cell viability was assessed using trypan blue exclusion, with a required viability of >85%. Single-cell suspensions were counted using a hemocytometer or Countess II Automated Cell Counter, and the concentration was adjusted to 400–1200 cells/μL.
2.12. GEM Generation and 3′Gene Expression Library Construction
Single-cell suspensions were prepared and loaded onto the 10× Chromium platform following the manufacturer’s instructions for the 10× Genomics Chromium Single-Cell 3′ Kit (V3) (10× Genomics, Pleasanton, CA, USA). cDNA amplification and library construction were carried out using the standard protocol. Sequencing of the libraries was performed on an Illumina NovaSeq 6000 system (paired-end, 150 bp multiplexed run) by Shanghai Personal Biotechnology (Shanghai, China).
2.13. Single-Cell RNA-Seq Data Processing
Our study analyzed two Plasmodium falciparum single-cell RNA-seq datasets comprising 23,683 cells from the Rapa treatment group (mean 12,747 reads/cell) and 25,505 cells from the EtOH control group (mean 14,344 reads/cell), with 5536–5538 genes detected per sample. Initial processing using Cell Ranger (v8.0.0) generated raw expression matrices that were subsequently integrated through Seurat’s merge function (v4.1.0) with cell barcode prefixes distinguishing treatment groups. Doublet removal was performed using DoubletFinder prior to quality control filtering that excluded cells with <500 UMIs, <100 detected genes, >0.25% apicoplast-derived transcripts (PF3D7-API), or >2% mitochondrial gene expression (PF3D7-MIT). SCTransform normalization addressed sequencing depth variation while preserving biological heterogeneity, followed by selection of 1500 highly variable genes for dimensionality reduction. Principal component analysis with 12 PCs informed the construction of a shared nearest neighbor graph for Louvain clustering (resolution = 0.5). Uniform Manifold Approximation and Projection (UMAP) visualization in 2D space revealed distinct transcriptional states that were annotated into six developmental stages (early/late rings, trophozoites, and schizonts) based on established Plasmodium stage-specific markers through manual cluster merging. The final dataset retained high-quality single cells with clear cell cycle progression characteristics for downstream comparative analysis [32].
For temporal mapping of Plasmodium developmental stages, we integrated bulk RNA-seq time course data (24 timepoints spanning 4–52 h post-infection) with single-cell transcriptomes. To enhance cluster biological coherence, we performed harmonic integration using Harmony (30 harmony dimensions) with Leiden clustering at resolution = 2 in the corrected space. UMAP visualization utilized the first 30 harmony dimensions to preserve developmental continuum topology.
Temporal alignment employed dynamic time warping with extended Jaccard distance metric, comparing each cluster’s expression vector against standardized bulk RNA-seq profiles from synchronized parasites. Developmental timing assignments were validated through concordance checks between computationally predicted timepoints and manually annotated cell cycle stages.
3. Results
3.1. PfAP2-06B Is Essential for Plasmodium Falciparum Parasite Growth and Development
PfAP2-06B (PF3D7_0613800) is the largest transcription factor in the P. falciparum ApiAP2 family and contains three predicted DNA-binding domains [33]. The orthologue of PF3D7_0613800 in the rodent malaria parasite, Plasmodium berghei (PBANKA_0112100), was shown to be refractory to domain deletion, indicating that this predicted transcription factor might be crucial for parasite growth and development during the IDC [34]. To investigate the function of PF3D7_0613800, we endogenously tagged the C-terminal of PfAP2-06B with 2xFKBP-GFP-2xFKBP. A GFP tag between the two FKBPs allowed localization of the native target (Figure 1A). Following 20 days of culture and drug selection, we successfully obtained a 3D7/PfAP2-06B-2xFKBP-GFP-2xFKBP transgenic parasite line. Genomic DNA was extracted from the transgenic parasites, and two sets of PCR primers were utilized to confirm the accurate editing of the pfap2-06b locus (Figure 1B,C). Fluorescence microscopy was employed to investigate the expression levels and subcellular localization of the PfAP2-06B-2xFKBP-GFP-2xFKBP fusion protein. Similarly to other ApiAP2 transcription factors, PfAP2-06B localizes to the nucleus (Figure 1D).
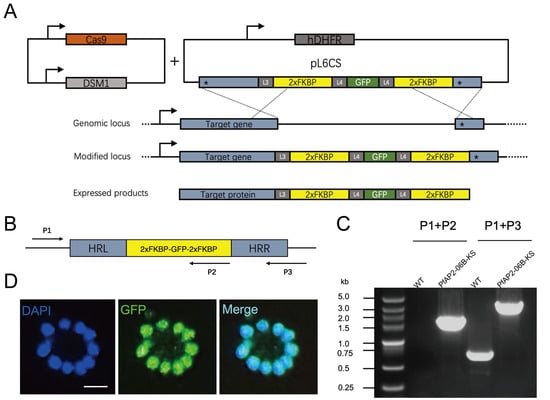
Figure 1.
Schematic of the plasmid and verification of PfAP2-06B-2xFKBP-GFP-2xFKBP transgenic parasite lines. (A) Generation of a PfAP2-06B transgenic parasite line tagged with 2xFKBP-GFP-2xFKBP at the C-terminus using the CRISPR/Cas9 gene editing system. ‘*’ denotes homologous sequences, arrow indicates the transcription direction. (B) Schematic diagrams for verification primers. Arrows indicate the direction of the primers. (C) The 2xFKBP-GFP-2xFKBP tagged PfAP2-06B transgenic line was verified by diagnostic PCR using P1, P2, and P3 primers. (D) Representative fluorescence microscopy images of PfAP2-06B-2xFKBP-GFP-2xFKBP late stage schizonts. Immunofluorescence assays using anti-GFP revealed perinuclear distribution of PfAP2-06B in the 3D7/PfAP2-06B-2xFKBP-GFP-2xFKBP line.
To create a knockdown parasite line, we introduced a plasma membrane mislocalizer, pLyn-FRB-mCherry, into the transgenic parasite line PfAP2-06B-2xFKBP-GFP-2xFKBP to create a line hereafter referred to as PfAP2-06B-KS (Figure 2A). In the absence of Rapa, the FRB and FKBP domains do not interact. However, upon the addition of Rapa, which binds to both FRB and FKBP, dimerization of these domains is induced. This interaction promotes the translocation of PfAP2-06B from the nucleus to the plasma membrane, ultimately leading to its degradation or reduction. As demonstrated by fluorescence microscopy, the introduction of Rapa to cultures of PfAP2-06B-KS parasites results in the translocation of PfAP2-06B from the nucleus to the plasma membrane. In contrast, in the absence of Rapa, PfAP2-06B remains predominantly localized in the nucleus (Figure 2B).
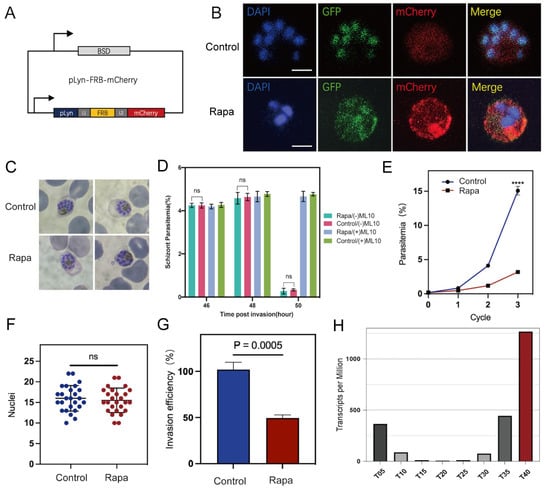
Figure 2.
PfAP2-06B is essential for parasite growth and invasion. (A) Schematic of the pLyn-FRB-mCherry plasmid. (B) Representative fluorescence microscopy images of PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry late stage schizonts. Immunofluorescence assays (IFA) using anti-GFP revealed perinuclear distribution of PfAP2-06B in the 3D7/PfAP2-06B-2xFKBP-GFP-2xFKBP line using anti-mCherry revealed pLyn-FRB-mCherry. The first panel was treated as the Control (RAPA−), and the second panel was treated with RAPA (RAPA+). (C) Giemsa-stained blood smears after three life cycles of synchronized parasites treated with Rapa and ethanol as control. (D) Quantification of schizont at the 46, 48, and 50 h stages using microscopy with Giemsa-stained blood smears, to assess the schizonts rupture normally. No difference in Schizont Parasitemia (%) was observed between control (RAPA−) and treated (RAPA+). (E) Growth curves of PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry in the presence or absence of Rapa over three growth cycles (three biological replicates, average parasitemia ± s.d.). Comparisons between control (RAPA−) and treated (RAPA+) schizonts were performed with Student’s t-test. **** indicates p-value < 0.0001. (F) Nuclei in control (RAPA−) and treated (RAPA+) schizonts. p value of 0.556, unpaired t test; error bar is mean with ± standard deviation (s.d.). n.s., not significant. (G) Invasion efficiency of one life cycle’s control (RAPA−) and treated (RAPA+) 2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry parasites (three biological replicates, average parasitaemia ± s.d.). (H) Expression profiles of PfAP2-06B in the P. falciparum 3D7 strain throughout the intraerythrocytic developmental cycle according to RNA-seq transcriptomic analysis [22].
To evaluate the role of PfAP2-06B in parasite growth and development, the inducible knockdown line was tightly synchronized within a 5 h window and cultured in the presence or absence of Rapa [35]. Parasite progression was monitored across three consecutive blood-stage cycles using Giemsa-stained thin blood smears: at which time in the presence of Rapa the PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry parasites still maintained normal morphology as assessed by microscopy (Figure 2C). The quantification of schizonts at the 46, 48, and 50 h stages was performed using microscopy with Giemsa-stained blood smears to assess the normal rupture of schizonts (Figure 2D). To serve as a control, 200 nM of ML-10 was added to the trophozoites immediately prior to merozoite egress, effectively arresting P. falciparum growth [29]. The Rapa pool demonstrated normal schizont rupture, similar to the control, indicating no significant difference in rupture behavior between the two groups. However, compared to the control group, the knockdown of PfAP2-06B resulted in a greater than 70% reduction in parasite-infected erythrocytes (unpaired t-test, p-value < 0.0001; Figure 2E), indicating that PfAP2-06B is essential for the blood-stage growth and development of P. falciparum, but does not affect parasite morphology.
3.2. PfAP2-06B Modulates Parasite Invasion of Erythrocytes
To determine the underlying cause of the decreased number of infected erythrocytes following PfAP2-06B knockdown, we performed a merozoite counting assay after treatment with Rapa. Similarly to the controls, the experimental parasites developed into morphologically normal segmented schizonts, and there was no statistically significant difference in the number of nuclei in late schizonts of PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry parasites treated by Rapa (Figure 2F).
To evaluate the efficiency of erythrocyte invasion, we collected strictly synchronized late-stage schizonts, and introduced them into an equal volume of erythrocytes [36], both with and without Rapa treatment for one life cycle. The invasion efficiency was observed to decrease by approximately 40% following PfAP2-06B knockdown, which suggests that PfAP2-06B plays a role in parasite invasion of erythrocytes (Unpaired t-test, p-value = 0.0028, Figure 2G).
3.3. PfAP2-06B Impacts Egress and Invasion Gene Transcription at the Schizont Stage
Time-series expression profiling of the IDC in the P. falciparum 3D7 strain revealed that the PfAP2-06B mRNA transcript level reached its peak during the schizont stages (Figure 2H) [22]. To investigate the role of PfAP2-06B during this stage, transcriptome analyses were performed on the PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry parasites. Parasites were tightly synchronized within a 5 h window and cultured with or without Rapa. After reinvasion, parasites were harvested for bulk RNA-seq at 40 to 45 HPI. Analyses revealed significant effects of PfAP2-06B on gene transcription at this timepoint, with 404 genes downregulated by more than twofold. Given the pronounced decrease in erythrocyte invasion efficiency, we focused on 84 genes which are associated with egress and invasion (Figure 3A) [8], among which 60 genes were downregulated by more than two-fold (Figure 3B). The knockdown of AP2-06B disrupted the broader transcriptional network governing invasion and pathogenesis. Gene ontology (GO) analysis revealed that AP2-06B regulates a set of genes involved in invasion (Figure 4A), which is consistent with the results of the invasion efficiency assay, as well as genes implicated in cell adhesion, motility, and membrane trafficking. The disruption of these interconnected pathways likely contributes to the observed invasion defect and the altered developmental progression of the parasite.
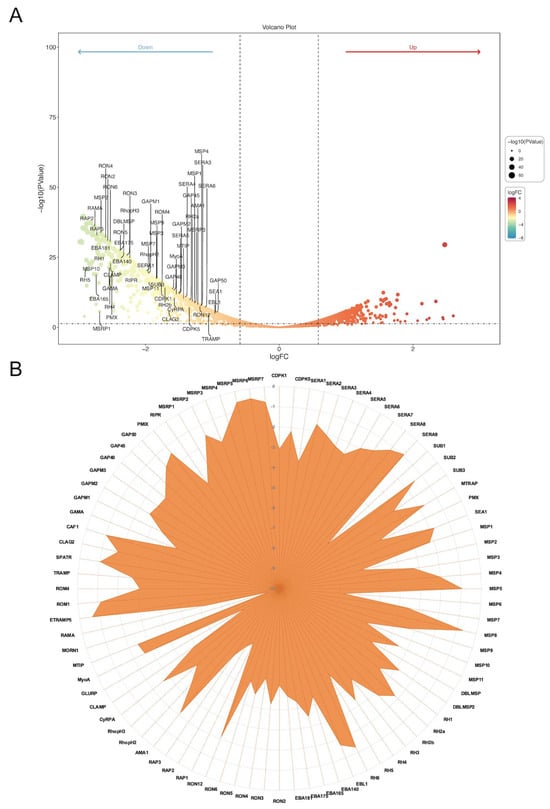
Figure 3.
PfAP2-06B impacts egress and invasion-related genes transcription. (A) Transcriptome changes in the PfAP2-06B knockdown schizonts (fold change of >2 and false discovery rate of <0.05). (B) Expression of 84 invasion-related gene transcripts upon AP2-06B knockdown, the vertical axis indicates the degree of decrease.
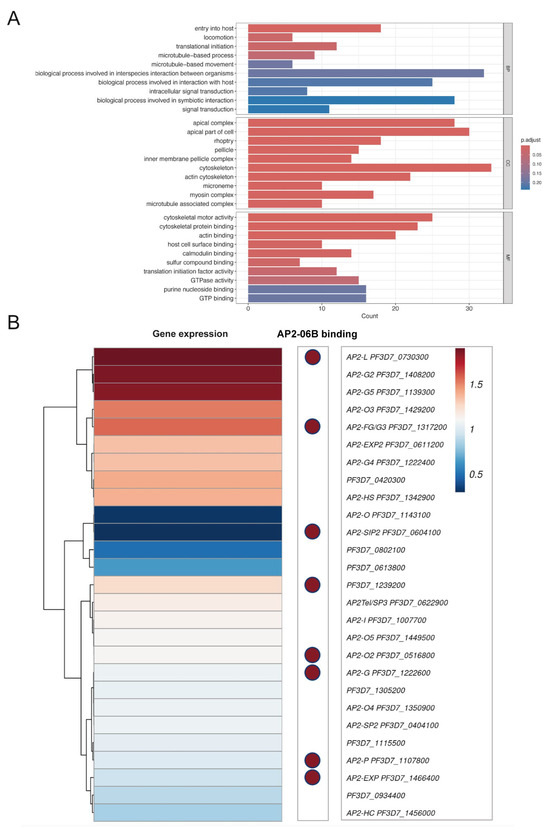
Figure 4.
PfAP2-06B knockdown transcriptome analysis. (A) Gene ontology (GO) analysis pathways and functional families enriched in PfAP2-06B-2xFKBP-GFP-2xFKBP::pLyn-FRB-mCherry heterochromatic target genes at the schizont stage (BH adjusted enrichment p-values of <0.01), BP (Biological Process), CC (Cellular Component) and MF (Molecular Function). (B) PfAP2 gene expression following PfAP2-06B knockdown and PfAP2-06B binding distribution at AP2 gene locus.
ChIP-seq analysis of PfAP2-06B indicated that the protein bound to the promoters of several egress and invasion genes that were downregulated in the PfAP2-06B knockdown strain (Supplementary Figure S1) [27], suggesting that PfAP2-06B directly regulates these genes. However, it did not bind to the promoters of most of the identified genes, indicating that the effects of PfAP2-06B knockdown on transcription are likely both direct and indirect. Altogether, the data suggest that PfAP2-06B is crucial for the expression of genes associated with egress and invasion.
3.4. PfAP2-06B Impacts the Transcription of Apiap2s and Binds to the Promoters of Eight Other Apiap2 Genes
The AP2 family of Plasmodium transcription factors are an important system for regulating stage-specific gene expression. The cooperation of AP2 transcription factors is a critical aspect of gene regulation in P. falciparum and drives the coordination of developmental stages and ensures the proper expression of genes required for parasite survival and transmission. The Plasmodium genome encodes a diverse family of AP2-like transcription factors that act in a network, with different members playing specific roles at different stages of the parasite life cycle. These factors often cooperate in a highly orchestrated manner to ensure timely gene expression during asexual replication, sexual differentiation, and host cell invasion.
To investigate the relationship between AP2-06B and other AP2 transcription factors, we analyze the expression of various AP2 factors in the AP2-06B knockdown strain, as well as the binding of AP2-06B through ChIP-seq analysis. In the PfAP2-06B knockdown strain, the transcription of Pfapiap2 genes exhibited notable changes; specifically, ap2-l, ap2-g2, and ap2-g5 were significantly upregulated, while ap2-o and ap2-sip2 were markedly downregulated (Figure 4B). PfAP2-06B was found to bind to the promoters of eight PfAP2 genes during the schizont stage (Supplementary Figure S1) [27], indicating that these genes are directly regulated by PfAP2-06B, including ap2-g, which plays a key role in gametocytogenesis [37]. Overall, these findings suggest that PfAP2-06B is involved in the regulation of different AP2 factors cooperation.
3.5. Differential Expression of Invasion Genes in Schizonts Reveals a Regulatory Role of PfAP2-06B in Modulating Developmental Stage-Specific Variability
Genetically identical cells are known to exhibit phenotypic variability even under the same environmental conditions. Single-cell transcriptomics is a rapidly advancing research tool, and provides a comprehensive and in-depth understanding of biological systems at the single-cell level. Recent studies have identified significant transcriptional variation during the schizont stage, characterized by a distinct set of highly variable invasion-related gene transcripts [38], which contrasts with data obtained from bulk RNA sequencing.
Single cell methodologies, such as 10x Genomics single-cell RNA sequencing (scRNA-seq), are well suited to compare developmental stages and, thereby, to refine differential transcriptional patterns [39], previously analyzed by “bulk” approaches. We employed 10x Genomics scRNA-seq to investigate the differential expression of invasion genes and to explore the impact of PfAP2-06B knockdown on these processes [40,41]. We focused on late-stage schizonts, to examine the transcriptional variability present in invasion genes arising from developmental differences between individual cells. Single parasites were segregated into 14 to 52 HPI groups (Figure 5A) based on pseudotemporal ordering [32], both for the PfAP2-06B knockdown strain and the control, using specific stage marker genes (Figure 5B). The expression of various invasion gene families exhibited distinct temporal patterns across different HPI groups, with peak expressions of the msp and ron gene families were observed between 40 and 44 HPI (Figure 6A) [42], while the eba and rh gene families showed peak expressions primarily at 46 HPI. In contrast, the sera and msrp gene families peaked between 34 and 36 HPI. These observations suggest variable expression levels of critical invasion-related gene transcripts in mature schizonts at different developmental stages.
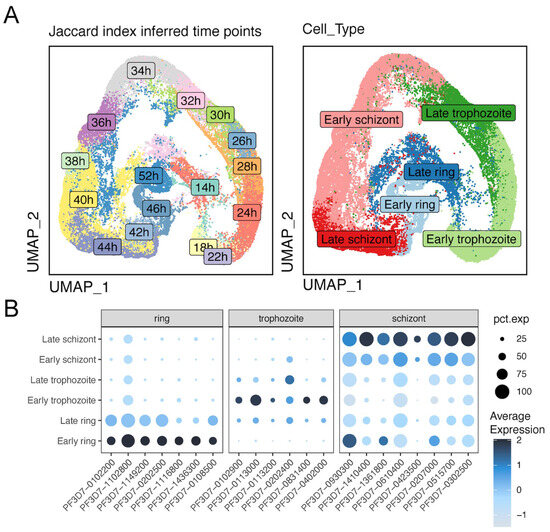
Figure 5.
Patterns of gene expression across different life cycle stages. (A) Left UMAP plot showing the distribution of subpopulations at different timepoints post-invasion (hpi) with an interval of 2 hr. Right UMAP plot showing the P. falciparum asexual development stage distribution. (B) Marker genes of ring, trophozoite, and schizont stages. pct.exp represents the percentage of expression.
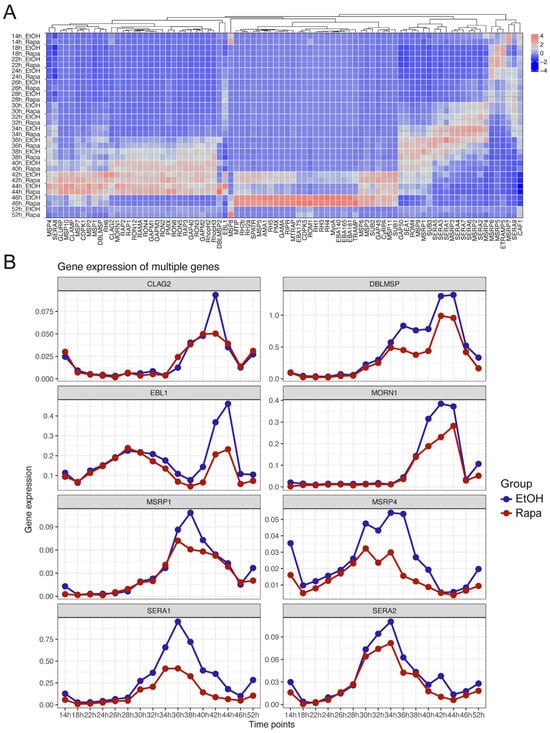
Figure 6.
Transcriptional expression of invasion-related genes at the single-cell level following PfAP2-06B Knockdown. (A) Expression of 84 invasion-related genes at various timepoints with and without Rapa treatment (B) Time-dependent significant downregulation of invasion-related genes expression following PfAP2-06B knockdown.
Further comparison of invasion gene expression between the control and PfAP2-06B knockdown strains revealed that several genes, including ebl1, sera1, and msrp4, exhibited a marked decline in expression (Figure 6B) [43]. Additionally, genes such as clag2, dblmsp, morn1, msrp1, and sera2 showed a downward trend in expression. These findings underscore the regulatory role of PfAP2-06B in modulating invasion gene expression and highlight its influence on the stochastic variations observed in cell-to-cell gene expression.
4. Discussion
Our study reveals that PfAP2-06B, a key member of the largest transcription factor family in Plasmodium falciparum, plays a critical but uncharacterized role in the IDC of the malaria parasite [18]. Our data indicate that PfAP2-06B is crucial for parasite survival during the asexual blood stages, particularly at the schizont stage, and is essential for successful invasion of infected erythrocytes [44]. The inability to generate a PfAP2-06B knockdown strain, combined with the results from large-scale mutagenesis screens, strongly suggests that this transcription factor is crucial for maintaining parasite development and viability during these stages [45].
To investigate the function of PF3D7_0613800, we attempted to generate a ribozyme knockdown system for the gene [46], but it proved inefficient. While ribozymes are an emerging tool for manipulating gene expression in P. falciparum, their effectiveness is limited in knocking down larger proteins. Large proteins often play critical roles in complex biological processes, such as host cell invasion, immune evasion, and metabolism [47]. Knocking down such proteins requires highly efficient and specific gene-silencing techniques. Although ribozymes offer some advantages, they may not provide sufficient silencing to induce a phenotypic effect, particularly if the protein is part of a redundant or compensatory pathway. Given that PF3D7_0613800 is a 485 kDa protein [33], we subsequently selected a ‘knock sideways’ system to translocate the protein from the nucleus to the membrane.
At the molecular level, PfAP2-06B functions primarily as a stage-specific transcriptional regulator, with its peak expression occurring during the late trophozoite to schizont transition [22]. This stage-specific expression is consistent with the observed regulatory effects of PfAP2-06B on key genes involved in parasite egress and erythrocyte invasion. Using bulk RNA-seq analysis, we identified a substantial downregulation of approximately 60 genes related to invasion and egress in PfAP2-06B-KS parasites. The functional enrichment of these genes underscores the importance of maintaining the stochastic expression of invasion-related genes, which is likely crucial for successful parasite invasion across variable host cell surface receptors [20]. This is particularly significant given the dynamic nature of host–pathogen interactions during the blood stages of Plasmodium. The interaction of PfAP2-06B with other well-characterized transcriptional regulators, such as PfBDP1 and PfAP2-I, as revealed by mass spectrometry, suggests that PfAP2-06B likely operates within a regulatory network that governs invasion and egress events [21]. These interactions further support the hypothesis that the role of PfAP2-06B during erythrocyte invasion is tightly coordinated with other transcriptional factors involved in the parasite life cycle.
The association of PfAP2-06B with drug resistance mechanisms adds another layer of complexity to its role. Notably, mutations in PF3D7_0613800, the gene encoding PfAP2-06B, have been implicated in resistance to quinine and other antimalarial compounds, highlighting its potential involvement in the regulation of drug resistance [48]. This observation aligns with findings from chemo genetic screens, which identified mutations in PF3D7_0613800 as contributing to resistance against several Medicines for Malaria Venture (MMV) compounds [49,50,51]. In our previous work, we investigated the effects of the AP2-06B mutation and its knockdown on resistance to artemisinin and quinoline-based drugs [52]. Additionally, the regulation of PfAP2-06B expression by lysophosphatidylcholine (lysoPC) and PfAP2-G further complicates its role [53], suggesting that PfAP2-06B is responsive to lipid- and protein-mediated signaling pathways that could influence both parasite development and drug resistance. The essential function of PfAP2-06B during the schizont stage and its involvement in key processes such as parasite invasion, combined with its potential role in antimalarial drug resistance, positions it as an attractive target for therapeutic intervention.
In conclusion, this study highlights the crucial role of PfAP2-06B in regulating key aspects of Plasmodium development and host cell invasion. It also suggests that targeting this transcription factor could offer new insights into both therapeutic and prophylactic strategies against malaria. Further research into the detailed mechanisms by which PfAP2-06B regulates gene expression and interacts with other key proteins will be essential for advancing our understanding of malaria pathogenesis and developing effective interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14111076/s1, Figure S1: PfAP2-06B occupancy at representative apiap2 genes and invasion genes; Table S1: Primers used for construction and coding sequence; Table S2: Primers used for verification.
Author Contributions
Conceptualization, Q.S. and K.W.; Methodology, Q.S. and K.W.; Software, Y.G.; Validation, Y.G. and J.P.; Formal analysis, Y.G. and J.P.; Investigation, Q.S. and K.W.; Data curation, Y.G.; Writing – original draft, Q.S. and K.W.; Writing – review & editing, Q.S., K.W., Y.L., J.T., Q.Z. and J.C.; Supervision, J.T., Q.Z., J.C. and L.S.; Project administration, Y.L., J.T., Q.Z., J.C. and L.S.; Funding acquisition, Y.L., J.T., Q.Z., J.C. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant numbers 82372275, 82320108014, 81971967, 82172302, 82230077, W2411080), Shanghai Municipal Health Commission of Research Project (Grant no. 202240012), as well as the Shanghai Blue Cross Brain Hospital Co., Ltd. and the Shanghai Tongji University Education Development Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The Bulk RNA-seq data presented in the study are deposited in the SRA repository, accession number PRJNA1202365. The single-cell RNA-seq data generated in this study have been deposited in the SRA repository under accession number PRJNA1203001. The original contributions are included in the article and Supplementary Material. For further inquiries, please contact the corresponding authors.
Acknowledgments
We thank Guangxu Cao (Department of Obstetrics and Gynecology, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China) for scRNA-seq data analysis.
Conflicts of Interest
The authors declare that this study received funding from Shanghai Blue Cross Brain Hospital Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Paul, A.S.; Saha, S.; Engelberg, K.; Jiang, R.H.; Coleman, B.I.; Kosber, A.L.; Chen, C.-T.; Ganter, M.; Espy, N.; Gilberger, T.W.; et al. Parasite Calcineurin Regulates Host Cell Recognition and Attachment by Apicomplexans. Cell Host Microbe 2015, 18, 49–60. [Google Scholar] [CrossRef]
- Malaria Genomic Epidemiology Network. A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature 2015, 526, 253–257. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World Malaria Report 2023; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.-H.; Duraisingh, M.T. The Molecular Basis of Erythrocyte Invasion by Malaria Parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef]
- Singh, S.; Chitnis, C.E. Molecular Signaling Involved in Entry and Exit of Malaria Parasites from Host Erythrocytes. Cold Spring Harb. Perspect. Med. 2017, 7, a026815. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Auth, T.; Gov, N.S.; Satchwell, T.J.; Hanssen, E.; Zuccala, E.S.; Riglar, D.T.; Toye, A.M.; Betz, T.; Baum, J.; et al. Membrane-Wrapping Contributions to Malaria Parasite Invasion of the Human Erythrocyte. Biophys. J. 2014, 107, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Reilly, H.B.; Wang, H.; Steuter, J.A.; Marx, A.M.; Ferdig, M.T. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int. J. Parasitol. 2007, 37, 1599–1607. [Google Scholar] [CrossRef]
- Boyle, M.J.; Langer, C.; Chan, J.-A.; Hodder, A.N.; Coppel, R.L.; Anders, R.F.; Beeson, J.G. Sequential Processing of Merozoite Surface Proteins during and after Erythrocyte Invasion by Plasmodium falciparum. Infect. Immun. 2014, 82, 924–936. [Google Scholar] [CrossRef]
- Das, S.; Hertrich, N.; Perrin, A.J.; Withers-Martinez, C.; Collins, C.R.; Jones, M.L.; Watermeyer, J.M.; Fobes, E.T.; Martin, S.R.; Saibil, H.R.; et al. Processing of Plasmodium falciparum Merozoite Surface Protein MSP1 Activates a Spectrin-Binding Function Enabling Parasite Egress from RBCs. Cell Host Microbe 2015, 18, 433–444. [Google Scholar] [CrossRef]
- Tham, W.-H.; Lim, N.T.Y.; E Weiss, G.; Lopaticki, S.; E Ansell, B.R.; Bird, M.; Lucet, I.; Dorin-Semblat, D.; Doerig, C.; Gilson, P.R.; et al. Plasmodium falciparum Adhesins Play an Essential Role in Signalling and Activation of Invasion into Human Erythrocytes. PLoS Pathog. 2015, 11, e1005343. [Google Scholar] [CrossRef]
- Fidock, D.A.; Nomura, T.; Talley, A.K.; Cooper, R.A.; Dzekunov, S.M.; Ferdig, M.T.; Ursos, L.M.; Sidhu, A.B.; Naudé, B.; Deitsch, K.W.; et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 2000, 6, 861–871. [Google Scholar] [CrossRef]
- Dzikowski, R.; Templeton, T.J.; Deitsch, K. Variant antigen gene expression in malaria. Cell. Microbiol. 2006, 8, 1371–1381. [Google Scholar] [CrossRef]
- Pino, P.; Caldelari, R.; Mukherjee, B.; Vahokoski, J.; Klages, N.; Maco, B.; Collins, C.R.; Blackman, M.J.; Kursula, I.; Heussler, V.; et al. A multistage antimalarial targets the plasmepsins IX and X essential for invasion and egress. Science 2017, 358, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.-A.; Fowkes, F.J.I.; Beeson, J.G. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell. Mol. Life Sci. 2014, 71, 3633–3657. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Babu, M.M.; Iyer, L.M.; Aravind, L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 2005, 33, 3994–4006. [Google Scholar] [CrossRef] [PubMed]
- Pieszko, M.; Weir, W.; Goodhead, I.; Kinnaird, J.; Shiels, B. ApiAP2 Factors as Candidate Regulators of Stochastic Commitment to Merozoite Production in Theileria annulata. PLoS Neglected Trop. Dis. 2015, 9, e0003933. [Google Scholar] [CrossRef]
- Kaneko, I.; Iwanaga, S.; Kato, T.; Kobayashi, I.; Yuda, M. Genome-Wide Identification of the Target Genes of AP2-O, a Plasmodium AP2-Family Transcription Factor. PLoS Pathog. 2015, 11, e1004905. [Google Scholar] [CrossRef]
- Campbell, T.L.; De Silva, E.K.; Olszewski, K.L.; Elemento, O.; Llinás, M. Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite. PLoS Pathog. 2010, 6, e1001165. [Google Scholar] [CrossRef]
- De Silva, E.K.; Gehrke, A.R.; Olszewski, K.; León, I.; Chahal, J.S.; Bulyk, M.L.; Llinás, M. Specific DNA-binding by Apicomplexan AP2 transcription factors. Proc. Natl. Acad. Sci. USA 2008, 105, 8393–8398. [Google Scholar] [CrossRef]
- Santos, J.M.; Josling, G.; Ross, P.; Joshi, P.; Orchard, L.; Campbell, T.; Schieler, A.; Cristea, I.M.; Llinás, M. Red Blood Cell Invasion by the Malaria Parasite Is Coordinated by the PfAP2-I Transcription Factor. Cell Host Microbe 2017, 21, 731–741.e710. [Google Scholar] [CrossRef]
- Josling, G.A.; Petter, M.; Oehring, S.C.; Gupta, A.P.; Dietz, O.; Wilson, D.W.; Schubert, T.; Längst, G.; Gilson, P.R.; Crabb, B.S.; et al. A Plasmodium falciparum Bromodomain Protein Regulates Invasion Gene Expression. Cell Host Microbe 2015, 17, 741–751. [Google Scholar] [CrossRef]
- Toenhake, C.G.; Fraschka, S.A.-K.; Vijayabaskar, M.S.; Westhead, D.R.; van Heeringen, S.J.; Bártfai, R. Chromatin Accessibility-Based Characterization of the Gene Regulatory Network Underlying Plasmodium falciparum Blood-Stage Development. Cell Host Microbe 2018, 23, 557–569.E9. [Google Scholar] [CrossRef]
- Ghorbal, M.; Gorman, M.; MacPherson, C.R.; Martins, R.M.; Scherf, A.; Lopez-Rubio, J.J. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat. Biotechnol. 2014, 32, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, J.; Flemming, S.; Reichard, N.; Soares, A.B.; Mesén-Ramírez, P.; Jonscher, E.; Bergmann, B.; Spielmann, T. A genetic system to study Plasmodium falciparum protein function. Nat. Methods 2017, 14, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Poran, A.; Nötzel, C.; Aly, O.; Mencia-Trinchant, N.; Harris, C.T.; Guzman, M.L.; Hassane, D.C.; Elemento, O.; Kafsack, B.F.C. Single-cell RNA sequencing reveals a signature of sexual commitment in malaria parasites. Nature 2017, 551, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, S.; Wei, G.; Tang, J.; Zhao, Y.; Wang, F.; He, X.; Guo, G.; Shang, X.; Yu, X.; et al. Rrp6 Regulates Heterochromatic Gene Silencing via ncRNA RUF6 Decay in Malaria Parasites. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Shang, X.; Wang, C.; Fan, Y.; Guo, G.; Wang, F.; Zhao, Y.; Sheng, F.; Tang, J.; He, X.; Yu, X.; et al. Genome-wide landscape of ApiAP2 transcription factors reveals a heterochromatin-associated regulatory network during Plasmodium falciparum blood-stage development. Nucleic Acids Res. 2022, 50, 3413–3431. [Google Scholar] [CrossRef]
- Shang, X.; Wang, C.; Shen, L.; Sheng, F.; He, X.; Wang, F.; Fan, Y.; He, X.; Jiang, M. PfAP2-EXP2, an Essential Transcription Factor for the Intraerythrocytic Development of Plasmodium falciparum. Front. Cell Dev. Biol. 2022, 9, 782293. [Google Scholar] [CrossRef]
- Platon, L.; Baker, D.A.; Ménard, D. Modified Plasmodium falciparum Ring-Stage Survival Assay with ML10 Kinase Inhibitor. Antimicrob. Agents Chemother. 2023, 67, e0001723. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Kucharski, M.; Tripathi, J.; Nayak, S.; Zhu, L.; Wirjanata, G.; van der Pluijm, R.W.; Dhorda, M.; Dondorp, A.; Bozdech, Z. A comprehensive RNA handling and transcriptomics guide for high-throughput processing of Plasmodium blood-stage samples. Malar. J. 2020, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Jeninga, M.D.; Quinn, J.E.; Petter, M. ApiAP2 Transcription Factors in Apicomplexan Parasites. Pathogenetics 2019, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Modrzynska, K.; Pfander, C.; Chappell, L.; Yu, L.; Suarez, C.; Dundas, K.; Gomes, A.R.; Goulding, D.; Rayner, J.C.; Choudhary, J.; et al. A Knockout Screen of ApiAP2 Genes Reveals Networks of Interacting Transcriptional Regulators Controlling the Plasmodium Life Cycle. Cell Host Microbe 2017, 21, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, A.K.; Green, J.L.; Satyam, R.; Salunke, R.P.; Lenz, T.; Shuaib, M.; Isaioglou, I.; Abel, S.; Gupta, M.; Esau, L.; et al. DNA-binding protein PfAP2-P regulates parasite pathogenesis during malaria parasite blood stages. Nat. Microbiol. 2023, 8, 2154–2169. [Google Scholar] [CrossRef]
- Gaur, D.; Storry, J.R.; Reid, M.E.; Barnwell, J.W.; Miller, L.H. Plasmodium falciparum is able to invade erythrocytes through a trypsin-resistant pathway independent of glycophorin B. Infect. Immun. 2003, 71, 6742–6746. [Google Scholar] [CrossRef]
- Kafsack, B.F.; Rovira-Graells, N.; Clark, T.G.; Bancells, C.; Crowley, V.M.; Campino, S.G.; Williams, A.E.; Drought, L.G.; Kwiatkowski, D.P.; Baker, D.A.; et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 2014, 507, 248–252. [Google Scholar] [CrossRef]
- Subudhi, A.K.; O’dOnnell, A.J.; Ramaprasad, A.; Abkallo, H.M.; Kaushik, A.; Ansari, H.R.; Abdel-Haleem, A.M.; Ben Rached, F.; Kaneko, O.; Culleton, R.; et al. Malaria parasites regulate intra-erythrocytic development duration via serpentine receptor 10 to coordinate with host rhythms. Nat. Commun. 2020, 11, 2763. [Google Scholar] [CrossRef]
- Howick, V.; Andrew, J.R.; Virginia, M.H.; Virginia, M.H.; Tallulah, A.; Andrew, R.; Tallulah, S.A.; Haynes, H.; Haynes, H.; Adam, J.R.; et al. The Malaria Cell Atlas: A comprehensive reference of single parasite transcriptomes across the complete Plasmodium life cycle. bioRxiv 2019. [Google Scholar] [CrossRef]
- Germain, P.-L.; Lun, A.; Macnair, W.; Robinson, M.D. Doublet identification in single-cell sequencing data using scDblFinder. F1000Research 2021, 10, 979. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M., 3rd; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 84, 3573–3587.e29. [Google Scholar] [CrossRef]
- Tripathi, J.; Zhu, L.; Nayak, S.; Stoklasa, M.; Bozdech, Z. Stochastic Expression of Invasion Genes in Plasmodium falciparum Schizonts. Nat. Commun. 2021, 13, 3004. [Google Scholar] [CrossRef]
- Thomas, J.A.; Tan, M.S.Y.; Bisson, C.; Borg, A.; Umrekar, T.R.; Hackett, F.; Hale, V.L.; Vizcay-Barrena, G.; Fleck, R.A.; Snijders, A.P.; et al. A protease cascade regulates release of the human malaria parasite Plasmodium falciparum from host red blood cells. Nat. Microbiol. 2018, 3, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 2018, 360, eaap7847. [Google Scholar] [CrossRef] [PubMed]
- Bushell, E.; Gomes, A.R.; Sanderson, T.; Anar, B.; Girling, G.; Herd, C.; Metcalf, T.; Modrzynska, K.; Schwach, F.; Martin, R.E.; et al. Functional Profiling of a Plasmodium Genome Reveals an Abundance of Essential Genes. Cell 2017, 170, 260–272.e8. [Google Scholar] [CrossRef] [PubMed]
- Prommana, P.; Uthaipibull, C.; Wongsombat, C.; Kamchonwongpaisan, S.; Yuthavong, Y.; Knuepfer, E.; A Holder, A.; Shaw, P.J. Inducible knockdown of Plasmodium gene expression using the glmS ribozyme. PLoS ONE 2013, 8, e73783. [Google Scholar] [CrossRef]
- Das, D.; Krishnan, S.R.; Roy, A.; Bulusu, G. A network-based approach reveals novel invasion and Maurer’s clefts-related proteins in Plasmodium falciparum. Mol. Omics 2019, 15, 431–441. [Google Scholar] [CrossRef]
- Ehrlich, H.Y.; Bei, A.K.; Weinberger, D.M.; Warren, J.L.; Parikh, S. Mapping partner drug resistance to guide antimalarial combination therapy policies in sub-Saharan Africa. Proc. Natl. Acad. Sci. USA 2021, 118, e2100685118. [Google Scholar] [CrossRef]
- Wendler, J.P.; Okombo, J.; Amato, R.; Miotto, O.; Kiara, S.M.; Mwai, L.; Pole, L.; O’Brien, J.; Manske, M.; Alcock, D.; et al. A Genome Wide Association Study of Plasmodium falciparum Susceptibility to 22 Antimalarial Drugs in Kenya. PLoS ONE 2014, 9, e96486. [Google Scholar] [CrossRef]
- Adjalley, S.H.; Scanfeld, D.; Kozlowski, E.; Llinás, M.; Fidock, D.A. Genome-wide transcriptome profiling reveals functional networks involving the Plasmodium falciparum drug resistance transporters PfCRT and PfMDR1. BMC Genom. 2015, 16, 1090. [Google Scholar] [CrossRef]
- Ecker, A.; Lehane, A.M.; Fidock, D.A. Molecular markers of Plasmodium resistance to antimalarials. In Treatment and Prevention of Malaria: Antimalarial Drug Chemistry, Action and Use; Milestones in Drug Therapy; Springer: Basel, Switzerland, 2011. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, C.; Yang, W.; Ma, X.; Tang, J.; Zhang, J.; Zhu, G.; Wang, Y.; Liu, Y.; He, X. Plasmodium falciparum transcription factor AP2-06B is mutated at high frequency in Southeast Asia but does not associate with drug resistance. Front. Cell. Infect. Microbiol. 2024, 14, 1521152. [Google Scholar] [CrossRef]
- Brancucci, N.M.B.; Gerdt, J.P.; Wang, C.; De Niz, M.; Philip, N.; Adapa, S.R.; Zhang, M.; Hitz, E.; Niederwieser, I.; Boltryk, S.D.; et al. Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell 2017, 171, 1532–1544.e1515. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).