Abstract
Chikungunya virus (CHIKV) is an arthritogenic alphavirus transmitted primarily via Aedes aegypti and Aedes albopictus mosquitoes. Since its identification, CHIKV remained confined to parts of Africa and Asia until the early 2000s, when it expanded to other continents, causing epidemics. Structurally, it is an enveloped virus with a positive-single-stranded RNA genome, which encodes four non-structural proteins (nsP1-nsP4), responsible for viral replication, and five structural proteins (C, E3, E2, 6K, and E1), which form the capsid and envelope. Of these proteins, glycoproteins E1 and E2 are essential for cell recognition and membrane fusion, determining infectivity and viral tropism. CHIKV replication occurs in the cytosol of different cell types, triggering an intense inflammatory and immune response, which manifests clinically as Chikungunya fever (CHIKF). Despite its epidemiological impact, current treatment is limited to symptomatic approaches, including the use of analgesics and anti-inflammatories, as no specific antiviral therapies are available. In response, promising advances are being made, including the development of vaccines, targeted antivirals, and immunotherapies. This article aims to review the main aspects of viral biology, epidemiology, and immunopathogenesis of CHIKV infection, in addition to discussing the main advances in the development of new therapeutic approaches for its control.
1. Introduction
Chikungunya virus (CHIKV) is an arthropod-borne virus (arbovirus) belonging to the Alphavirus genus and Togaviridae family, transmitted primarily by Aedes (Ae.) aegypti and Ae. albopictus mosquitoes. First identified in Tanzania in 1952, CHIKV has since emerged as a significant global public health concern, particularly in tropical and subtropical regions [1]. Clinically, CHIKV infection manifests with fever, hence it commonly being referred to as Chikungunya fever (CHIKF). However, there are other characteristics such as acute onset of high fever, rash, and debilitating polyarthralgia, which can persist for weeks or months [2]. In some cases, the disease progresses to a chronic phase, leading to long-term joint pain and disability, severely impacting patients’ quality of life [3]. Therefore, we will use the term CHIKV disease to reflect that fever is not the only symptom.
Over the past few decades, the incidence of CHIKV infections has increased dramatically, with major outbreaks reported across Africa, Asia, the Indian subcontinent, and the Americas [4]. The re-emergence and rapid geographic expansion of the virus highlight its growing importance in both epidemiological surveillance and clinical research [5]. From both scientific and clinical perspectives, CHIKV poses several significant challenges, such as the scarcity of effective treatments, unavailability of an approved vaccine (until recently), and the insufficient knowledge regarding its disease mechanisms and long-term effects [6].
This article aims to provide a comprehensive overview of CHIKV, focusing on its virological characteristics, epidemiology, immunopathogenesis, and the current state of research in the search for new therapeutic approaches. By highlighting the scientific and clinical relevance of CHIKV disease, the objective is to support further efforts in prevention, diagnosis, and treatment strategies.
2. General Aspects of Chikungunya
2.1. Epidemiology of Chikungunya Virus
The first reported epidemiological cases of fever, arthritis, and rashes resembling the CHIKF include cases in Zanzibar (Africa) in 1823, and an epidemic on the island of Saint Thomas (Caribbean) in 1827 and 1828 [7]. Some authors propose that the spread of the CHIKV beyond African territory may have begun in the mid-18th century when sailing ships carried humans and infected Ae. aegypti mosquitoes in sufficient numbers for the virus to circulate on board the ships, where the water stored for the crew was conducive to the reproduction and propagation of mosquitoes [8]. Since then, CHIKV has reached various territories around the world, resulting in outbreaks, endemics, and epidemics (Figure 1).
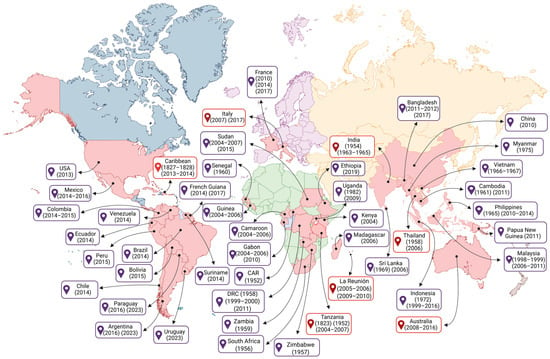
Figure 1.
Global epidemiology of the Chikungunya virus over time. Initially reported on the African continent, CHIKV, driven by trade and the traffic of travelers between different regions of the world, has spread to every continent except Antarctica. That figure presents a chronological sequence of CHIKV amplification around the world over the years, considering the year in which it was first reported or in which new outbreaks or epidemics occurred. The red text boxes highlight the countries where the first reported cases of the disease occurred on the continent. Each continent is represented by a color: blue (Americas), green (Africa), purple (Europe), orange (Asia), and brown (Oceania). Countries where CHIKV presence has been reported are shown in red. Please note that not all affected countries are shown. Created in BioRender. Oliveira, F. (2025) https://BioRender.com/15wrcvb (accessed on 12 October 2025).
However, it was not until 1952 that the CHIKV was isolated from the serum of a febrile patient during an outbreak on the Makonde plateau, located in south-eastern Tanzania in East Africa; and in 1953, it was isolated for the first time from Ae. aegypti mosquitoes, one of the main vectors of this virus [9]. Based on the disabling and debilitating symptoms presented by patients with severe arthralgia, the disease was given the name “Chikungunya” [10].
After the initial isolation of CHIKV, the first evidence of clinical infection was reported in an Indian patient in 1954 [11,12,13], as well as outbreaks in other African countries such as South Africa (1956), Zimbabwe (1957), the Democratic Republic of Congo (1958), Zambia (1959), Senegal (1960), Central African Republic (1982), and Uganda (1982) [14]. On the Asian continent, the first laboratory-confirmed outbreak occurred in Bangkok, Thailand (1958) [12]. In the following years, cases were recorded in other Asian countries, such as Cambodia (1961) [15], India (1963–1965, 1973) [16], the Philippines (1965), Vietnam (1966–1967), Sri Lanka (1969), Indonesia (1972), and Myanmar (1975) [12,17,18,19,20,21,22]. In the 2000s, new outbreaks of CHIKV were reported form several countries across the African continent, including the Kenya (2004), Cameroon (2006), Democratic Republic of Congo (1999–2000, 2011), Gabon (2006, 2007 and 2010), Madagascar (2006), Senegal (2009), Ethiopia (2019), and Sudan (2015) [23,24].
On the Asian continent, outbreaks of CHIKV disease were reported during 1998–1999 and 2001–2003 in Malaysia and Indonesia, respectively [25]. Following these outbreaks, CHIKV spread to the Indian Ocean islands, including Comoros, La Réunion, Mauritius, and Mayotte [26], and subsequently, infected air travelers from these epidemic regions reached Europe, Asia, and the Americas, contributing to the spread of CHIKV [8]. Between 2005 and 2006, more than 300,000 cases of CHIKV infection were reported on La Réunion Island [26], located in the Indian Ocean and belonging to France. Years later, between 2009 and 2010, the number of cases of CHIKV infection in La Réunion increased again [27]. During the outbreak in the Indian Ocean islands and Asia, cases of CHIKV were also reported in Europe and the Americas. Thus, since 2005, CHIKV transmission has reached global levels, with outbreaks in Asia, including Indonesia (1999–2016), Sri Lanka (2006), Malaysia (2006–2011), Thailand (1965, 2010–2014), Singapore (1965, 2006–2011), China (2010), Cambodia (2011), Bangladesh (2011–2012, 2017), and India (2005–2018) [28]. Moreover, in March 2025, La Réunion reported a new wave of outbreaks, with over 13,000 CHIKV cases in all the municipalities on the island. Due to the exponential number of cases and the increase in outbreaks, this led to the activation of level 4 of the OSERC “Arbovirus” system, corresponding to the circulation of an epidemic of medium intensity [29]. In addition, since the CHIKV outbreak in La Réunion in 2005, cases of infection have become increasingly frequent in Europe, with cases in Italy (2007, 2017), appearing to have originated from the introduction of the virus by a traveler returning from India, resulting in more than 200 cases of local transmission. Subsequently, there were new autochthonous cases in Lazio, in central Italy [24]. Later, another similar outbreak occurred in the southern region of France (2010, 2014, and 2017), which seems to have originated from the introduction of one of the CHIKV vectors, the Ae. albopictus mosquitoes [24,30].
In Oceania, the first reported cases of CHIKV infection were in 2011 related to an autochthonous transmission of CHIKV in New Caledonia, a French territory located in the Pacific Ocean, in the Melanesian region, characterizing the first time the virus was detected in the Pacific [31]. The following year, another outbreak was recorded in Papua New Guinea, also located in the Melanesian region of Oceania [32]. In 2013, outbreaks occurred in other Pacific regions, such as New Caledonia, Tonga, American Samoa, and the Independent State of Samoa. In 2015, there were also outbreaks in the Cook Islands [11]. In Australia, the first cases of CHIKV infections were reported in 2008, mainly from travelers infected in other countries [33]. Pyke et al. (2018) showed that CHIKV cases between 2010 and 2016 were imported into Australia by patients traveling from Southeast Asia, the Pacific, and the Americas [34].
CHIKV was reported as the cause of three epidemics in the Americas, with 671,268–1,089,982 cases reported per year between 2014 and 2016, and more than 97,000 cases per year from 2017 to 2023 [35]. The Asian lineage was introduced to the island of Saint Martin in the United States of America (USA) in October 2013 and from there spread through the Caribbean, Central America, northern South America, and North America, infecting almost 1,400,000 people [36,37]. In 2014, one of the largest CHIKV epidemics in the Americas occurred in the Latin Caribbean (Cuba, the Dominican Republic, Puerto Rico, Haiti, Guadeloupe, and Martinique), followed by the Non-Latin Caribbean region (Jamaica, the Bahamas, the US Virgin Islands, and Aruba). Later, the Central American and Andean regions were also affected [35]. In 2014, CHIKV arrived in the USA with local transmission occurring in Florida, Texas, Puerto Rico, and the USA Virgin Islands [38].
In South America, cases of CHIKV infection have been reported in all countries, this includes Argentina (2015), Bolivia (2015), Brazil (2014), Chile (2014), Colombia (2014–2015), Ecuador (2014), French Guiana (2014, 2017), Paraguay (2016, 2023), Peru (2015), Suriname (2023), Uruguay (2023), and Venezuela (2014) (PAHO/WHO). Among those countries, Brazil is considered the epicenter of the CHIKV epidemic in South America. Brazil is the largest and most populous country in Latin America, making it particularly susceptible to the CHIKV alphavirus. In addition, the country’s climate is suitable for the Ae. vector. In August 2014, local transmission of the Asian American sublineage of CHIKV was reported in the city of Oiapoque in the state of Amapá, and the new East Central South African (ECSA) sublineage (ECSA American) was detected in the city of Feira de Santana, in the state of Bahia [39]. The Asian American sublineage appears to have been introduced via French Guiana, which borders Brazil through the state of Amapá, and this sublineage appears to be restricted to two of the seven states that make up the Northern region (Amapá and Roraima) [39,40]. In subsequent years, the ECSA strain spread to other Brazilian northeastern states [40]. In 2016, the first detection of Ae. aegypti naturally infected with the ECSA genotype was reported, supporting the hypothesis that this species was acting as the main vector of CHIKV outbreaks [41]. In recent years, the ECSA American sublineage has become predominant in all Brazilian regions, as well as expanding into countries such as Haiti, Argentina, Uruguay, and Paraguay [42]. Furthermore, while the Asian American sublineage of CHIKV has not been reported since 2018 in the Americas, the ECSA American sublineage continues to cause outbreaks in Brazil, Uruguay, Paraguay, and Argentina [35]. By the year 2023, CHIKV cases in North America represent 0.3% of all reported cases in the Americas (12,172 out of 3,684,554), with most cases occurring in Mexico (12,034, equivalent to 92.7% of cases) [35]. In 2024, the American continent reported 1,008,430 cases, with the highest number occurring in North America, specifically in the USA (345,426 cases) (PAHO/WHO).
From January to May 2025, 220,000 cases and 80 deaths were reported in America, Africa, Asia, and the islands of the Indian Ocean, with a predominance of cases in South America [29]. Then, at the end of June and beginning of July 2025, 14 autochthonous cases were reported in France [43]. The continuing occurrence of CHIKV cases indicated that efforts to control and treat CHIKV disease was an ongoing major public health problem, especially for populations in tropical and subtropical countries, where the climate, fauna, and flora are conducive to vector survival and viral transmission.
2.2. Main Regional Genotypes of Chikungunya Virus over Time
The CHIKV has four distinct genotypes, recognized and classified according to the regions in which the virus has been detected or a genotypic adaptation has been recognized (Figure 2). The four main genotypes are the ECSA lineage, the West African lineage (also referred to as ECSA2), the Asian lineage, and the Indian Ocean Lineage (IOL) [34].
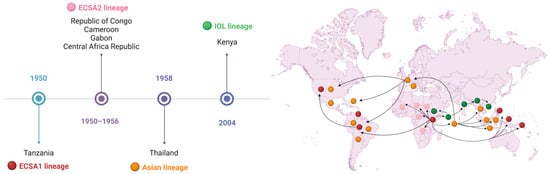
Figure 2.
The chronology of the emergence of the different Alphavirus chikungunya’s strains over time, following their geographical origin and subsequent spread. CHIKV has four main genotypes, resulting from adaptive mutations that have occurred over time. Chronologically, the first genotype described refers to the East South Central African lineage (ECSA1) (in red), followed by the West African lineage (ECSA2) (in pink), the Asian lineage (in yellow), and the Indian Ocean lineage (IOL) (in green), which spread to various countries around the world. Created in BioRender. Oliveira, F. (2025) https://BioRender.com/15wrcvb (accessed on 28 February 2025).
This geographical expansion of the different genotypes was facilitated by the intense circulation of infected people between countries. In particular, the ECSA lineage can be subdivided into ECSA1 (referring to the virus that infected the human population in Tanzania in 1950) and ECSA2 (which contains viral sequences obtained from the subsequent outbreaks that affected the Republic of Congo, Cameroon, Gabon, and the Central African Republic) [44].
Evolutionary studies have shown that the West African genotype evolved into a distinct variant when it spread to Asia, called the Asian genotype [45]. In 2004, during an outbreak in Lamu and Mombasa on the Kenyan coast, a new strain of ECSA was registered, which would later be classified as IOL, which circulated throughout the Indian Ocean region in 2005 and was introduced into India in 2006 [28], infecting around 2,000,000 people.
Tsetsarkin et al. (2007) carried out a more in-depth analysis of the microevolution of the CHIKV genome obtained during the outbreak on La Réunion Island in the Indian Ocean, identifying a mutation from alanine to valine at position 226 (E1-A226V) in the E1 glycoprotein (a viral envelope glycoprotein essential for virus–host cell fusion) [46]. This residue alteration conferred greater infectivity of the virus on the vector Ae. albopictus, amplifying the capacity for viral replication in the vector’s cells and dissemination to secondary organs, which, consequently, caused a slight increase in the transmission of the virus by Ae. albopictus, favoring it as the main vector in the region [46,47,48]. This mutation seems to have contributed to the viral spread in the region and the significant increase in case numbers. The CHIKV E1-226V variant has also been implicated in outbreaks occurring in Africa (Cameroon, Gabon, and Congo) [49,50,51], Europe (Italy) [52], and Southeast Asian regions (Malaysia, Singapore, Thailand, China, Cambodia, and Bhutan) [15,53,54,55,56].
Other adaptive mutations affecting the E2 gene (encoding the E2 glycoprotein, a surface protein crucial for virus infection and pathogenicity) have also been reported, resulting in increased transmission by the Ae. albopictus vector. These E2 protein adaptive mutations occurred on the Asian continent. The first to be described was E2-K252Q, which occurred in the state of Kerala (India) in 2007 and spread throughout Southeast Asia, where it was isolated from in 2008 [15,57,58]. In 2008, the adaptive mutations E2-V222I and E1-K211N were reported in Sri Lanka [59]. In 2009–2010, the adaptive mutation E2-L210Q was detected in India. All these mutations are related to the increased infection and transmission of CHIKV through Ae. albopictus mosquitoes [60].
Moreover, the different genotypes exhibit differences in their transmission cycles (Topic 2.3). While the Asian genotype seems to be transmitted mainly in an urban cycle with Ae. aegypti and Ae. albopictus mosquitoes, the African genotype seems to be more related to a sylvatic cycle, whose main vectors are the Ae. furcifer and Ae. africanus mosquitoes [61]. Furthermore, other adaptive mutations have been demonstrated that favor the transmission of the virus through the Ae. albopictus vector, such as T98A (E1) [62], L210Q (E2) [63], K252Q (E2) [63], I211T (E2) [64] and GD60D (E2) [64].
In particular, the genomes of the ECSA strain of CHIKV circulating in Brazil do not have the adaptive changes E1-A226 or E2-L210Q of Ae. albopictus, but have other mutations such as E1-K211T, E1-N335D, E1-A377V, E1-M407L, and E2-A103T, which seem to improve viral transmission in the Ae. aegypti vector, but not for Ae. albopictus [65]. These mutations significantly increased the infectivity of the virus (13×), its dissemination (15×), and transmission (62×) [65]. In South America, most of the CHIKV genomes available and used in research were obtained from samples collected between 2014 and 2015, the peak of the epidemic in the Americas, and, more recently, in samples obtained during recent epidemics in Brazil and Paraguay between the years 2021 and 2023. To date, 62.6% of all CHIKV genomes shared on NCBI GenBank are from Brazil, and 99.4% of them belong to the ECSA American sublineage [35].
Some factors have been identified as major influencers of the spread of CHIKV, such as the increase in air travel, allowing the flow of infected people between regions, the lack of prior exposure of human populations in the Indian Ocean basin and South Asia, the expansion of mosquito vectors such as Ae. albopictus into non-native regions (from native Asia to the islands of the Indian Ocean basin, Africa, and southern Europe), which has been facilitated by global trade, as well as the various adaptive mutations that the new strains of the virus have undergone over the years, conferring greater transmissibility and virulence [46,47,60,66].
Hence, monitoring these adaptive CHIKV mutations that occur during outbreaks and epidemics is essential. Since these genetic alterations can enhance viral transmission and survival. Thus, mapping and identifying these mutations could be crucial for controlling potential outbreaks and for developing new pharmacological therapies that include these alterations in viral structures.
2.3. Vectors of Chikungunya Virus
Ae. aegypti originated in sub-Saharan Africa and was first identified as an arbovirus vector in Cuba in 1900 [67]. This vector measures 4–7 cm and has a dark coloration (black or brown) with white or silver stripes arranged on the body and legs. In addition to having a high potential for pathogenic transmission to humans due to its purely anthropophilic habits and reproduction that target domestic (urban) and peridomestic environments [68,69,70,71]. This vector is more ecologically flexible compared to other vectors, Ae. albopictus, for example, because its geographic distribution is wider, especially in tropical and subtropical environments since this vector can be found in suburban and rural habitats. Furthermore, despite feeding mainly on humans, this vector can also infect a wide range of hosts, such as livestock, amphibians, reptiles, and birds, with different types of viruses [72,73].
First described in the Indian city of Calcutta, Ae. albopictus, also known as the “Asian tiger mosquito”, is native to Southeast Asia, the Western Pacific islands, and the Indian Ocean [74], and is frequently found in areas of extensive vegetation cover and more dispersed human populations; however, it has also been described in transitional environments with relatively low vegetation cover and generally coexisting with Ae. aegypti [75,76,77,78,79]. This mosquito has a black-and-white coloration, white bands on the legs, a median longitudinal band of silvery scales on the mesonotum, and a scaleless clypeus [80]. However, this species has spread throughout the tropics, mainly due to the development of human trade [81]. Despite being an opportunistic and zoophilic mosquito, when allowed to choose, this Aedes species shows a preference for feeding on human blood compared to the blood of other animals [82]. Furthermore, the results of some studies demonstrated that CHIKV was transmitted vertically from infected female Ae. albopictus to their offspring [83,84,85,86].
Ae. aegypti and Ae. albopictus use artificial container habitats for breeding and laying eggs so they can withstand dry conditions, ensuring survival even during unfavorable times, such as less rainy seasons, which facilitates the geographic expansion of these vectors [87,88,89,90]. While Ae. aegypti is restricted to warmer climates due to its inability to diapause, Ae. albopictus can be found in cooler and temperate regions because its eggs can enter diapause and overwinter—though the adults themselves can’t survive winter. [91,92]. The main breeding habitat of Ae. aegypti and Ae. albopictus is freshwater and their eggs are resistant to desiccation and high humidity, allowing them to hatch when conditions become favorable, giving rise to larvae [93]. The extensive geographic distribution of Ae. albopictus, jointly with mutations that improve the fitness and infectivity of CHIKV in this vector, may contribute to the expansion of the virus to temperate ecosystems in other regions, as observed by small outbreaks in France and Italy [52,94]. In addition, temperature changes are also an important influence of the vectorial capacity and transmission of CHIKV [92,95,96]. The increase in global temperature may also influence the migration of vectors restricted to warmer climates, such as Ae. aegypti, facilitating the spread of arboviruses, as seen in Europe, where the first autochthonous cases of flavivirus infections were reported [97,98,99].
Another factor that influences the transmission of CHIKV is the microbiota of the digestive tract of the mosquito vector. It has been described, for example, that the presence of the intracellular bacterium Wolbachia (Wb), which infects Ae. mosquitoes, interferes with the mosquito’s immune response, resulting in increased expression of cellular factors such as thioester-containing proteins (TEPs), C-type lectins, defensins, diptericin, glycosaminoglycan-binding protein B1 (GNBPB1), serine protease Z1A (PZ1A), cactus, and cecropin, which help neutralize CHIKV proliferation within the mosquito [100,101,102].
2.4. Transmission of Chikungunya Virus
CHIKV is transmitted by a sylvatic cycle (also known as enzootic) and an urban cycle (Figure 3). Transmission occurs mainly through the mosquito vectors Ae. albopictus and Ae. aegypti. However, CHIKV has also been detected in other species of the Ae. genus, such as Ae. fucifer, Ae. taylori, Ae. luteocephalus, Ae. africanus, Ae. neoafricanus and Ae. cordellieri [103]. The sylvatic cycle was demonstrated in the 1960s, in a study that isolated CHIKV from a pool of the forest mosquito Ae. africanus collected in the forest canopies of Uganda [104]. In this study, after infection of mice and rhesus monkeys with the virus, both species developed the disease, demonstrating that they are viable hosts for viral replication and propagation. Subsequently, another study using experimentally infected vervet monkeys (Chlorocebus pygerythrus) demonstrated that the animals produced CHIKV antibodies after infection, suggesting once again the involvement of non-human primates (NHP) in a sylvatic transmission cycle [105]. Thus, this cycle involves the transmission of the virus by forest-dwelling mosquito vectors to NHP (e.g., monkeys, baboons, and squirrels) [103]. Occasionally, humans may be accidentally infected when they move around or live near forests. In the urban cycle, the virus is transmitted by mosquito vectors to humans. CHIKV transmission depends on environmental factors, such as rainfall and altitude, ecological factors, including competent mosquito vectors, and social factors, including human mobility, socioeconomic status, and lifestyle [106,107,108,109].
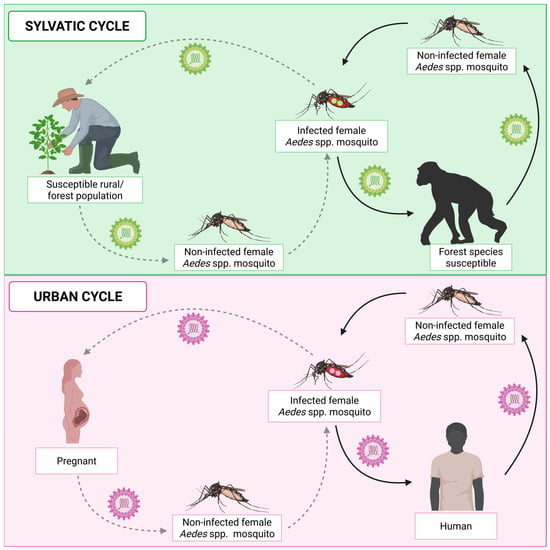
Figure 3.
Chikungunya virus transmission cycles. CHIKV transmission can occur through the sylvatic cycle or the urban cycle. The sylvatic cycle occurs in forest regions where infected female Aedes mosquitoes transmit the virus to non-human primates (NHPs). As a result, uninfected female Aedes mosquitoes, when feeding on infected NHPs, become infected and perpetuate the cycle. In addition, humans who live in or frequent forest regions can also participate in this cycle, as accidental hosts of the virus. The urban cycle occurs in regions densely populated by humans and involves the transmission of the virus mainly by Ae. aegypti and Ae. albopictus mosquitoes to humans. In the urban cycle, vertical transmission from mother to child can also occur. In this type of transmission, the infected mosquito transmits the virus to a pregnant woman. In turn, the virus can be transmitted to the fetus or baby during the breastfeeding period. Created in BioRender. Oliveira, F. (2025) https://BioRender.com/15wrcvb (accessed on 10 August 2025).
CHIKV is transmitted primarily through the saliva of infected mosquitoes. When the female mosquito feeds on an infected person, the vector ingests the virus, which infects various tissues, such as the salivary glands. Subsequently, when the infected mosquito feeds on a naive (uninfected) individual, CHIKV is transmitted directly into the bloodstream or dermal tissue [4]. At this point, the components present in the saliva of the mosquito will be able to inhibit the interferon (IFN) signaling pathway, facilitating viral replication [110,111,112,113]. In the dermis, CHIKV can invade and replicate in dendritic cells (DCs) [114], macrophages [115], endothelial cells, epithelial cells [116], keratinocytes [117], melanocytes [118] and, above all, fibroblasts, which function as reservoirs for viral amplification [112,116,119,120,121]. CHIKV, like most alphaviruses, enters host cells through endocytosis mediated by the clathrin protein [122]. Moreover, the entry of CHIKV into the bloodstream seems to occur through viral binding, infection, or absorption by antigen-presenting cells, such as DCs and macrophages, which migrate to the lymphatic vessels and subsequently reach the bloodstream [123]. It is hypothesized that viral replication in the spleen, liver, endothelial cells, and monocytes contributes significantly to the high viremia typical of the acute phase [115,124,125,126]. From the blood, CHIKV can spread systemically and infect different tissues, such as those related to the symptoms of the disease: connective tissue, muscles and joints [115,119,121,127,128,129].
The first report of vertical transmission (mother to child) occurred during the La Réunion epidemic in 2005. On this occasion, the vertical transmission rate was close to 50% in mothers with high viremia during the intrapartum period [130]. Transmission from mother to fetus appears to occur through microtransfusions in the placental barrier or through the breakdown of the syncytiotrophoblast during uterine contractions [131,132]. In this context, the placenta seems to play a key role in this type of transmission, although it is not fully understood. CHIKV antigens have been detected in decidual cells, trophoblastic cells, endothelial cells, Hoffbauer cells and within the fetal capillaries of the placenta [133,134,135].
Chen et al. (2010) [136] evaluated CHIKV infection in pregnant Rhesus monkeys (Macaca mulatta, 7 and 15 years old and gestational days of 121–132 days) to evaluate the pathogenesis and the potential for transplacental transmission. The study demonstrated that viremia peaked 2–3 days after inoculation, with the development of intermittent fever (39.7 °C), increased joint temperature (1.19–9.4 °C) and circumference, appearance of erythematous skin rashes, severe leg swelling and high levels of inflammatory cytokines [interleukin (IL)-2, IL-6, IL-15, interleukin-1 receptor antagonist (IL1Ra), monocyte chemotactic protein-1 (MCP-1), and vascular endothelial growth factor (VEGF)]. In addition, through necropsy, viral ribonucleic acid (RNA) was observed in maternal lymphoid tissues associated with joints and the spinal cord. On the other hand, no viral RNA was detected in the germinal center in fetuses, which indicated the absence of transplacental transmission. This finding contrasts with studies conducted in humans, which demonstrate vertical transmission of CHIKV [119,137].
Moreover, it has been observed that postponing normal delivery or performing a cesarean section does not prevent CHIKV transmission from mother to fetus. Maternal infection results in obstetric complications such as miscarriage, pre-eclampsia, post-partum hemorrhage, premature birth, intrauterine death, oligohydramnios, and sepsis [138,139]. Infected neonates may present symptoms such as fever, refusal to breastfeed, skin rashes, skin hyperpigmentation, thrombocytopenia, and neurological complications, such as meningoencephalitis, cerebral edema, postnatal microcephaly and neurodevelopmental delay [140,141,142,143,144,145,146].
3. Virology and Structure of Chikungunya Virus
The term “togavirus” originated as informal jargon during early studies of arboviruses, particularly those isolated in the context of yellow fever research. The etymology is derived from the Latin toga, a Roman “mantle,” “cloak,” or “covering,” in reference to possessing a viral envelope [147]. Initially, the Togaviridae family included two genera: Alphavirus and Flavivirus. However, due to subsequent taxonomic revisions, flaviviruses were reclassified into their own family, Flaviviridae, based on distinct genomic and structural characteristics. As a result, the Togaviridae family now consists of a single genus, Alphavirus. All alphaviruses share a spherical virion architecture ~70 nm in diameter, and this genus includes CHIKV and other medically important viruses such as Sindbis virus (SINV), Semliki Forest virus, and Eastern equine encephalitis virus (EEEV) [147,148].
The CHIKV shares characteristics with Alphaviruses and consists of spherical particles approximately 70 nm in diameter and exhibits icosahedral symmetry with triangulation [147,149,150]. CHIKV genome consists of a single-strand positive-sense RNA molecule approximately 12 kb in length, featuring a 5′ capped structure and a 3′ polyadenylated (poly(A)) tail. The CHIKV genome is separated into two open reading frames (ORFs). The 5′ ORF encodes four non-structural proteins (nsP; nsP1–4) required for viral replication and the 3′ ORF encodes five structural proteins (Capsid, E1, E2, E3, and a 6K protein) (Figure 4) [149,151,152,153].
NsP are essential for virus replication, protein modification, and immune evasion. The nsPs are mainly produced as a single polyprotein with distinct roles in viral genome replication, also known as replicase complex [154,155]. NsP1 caps the 5′ end of the new viral RNA independently of the host–cell capping machinery. It is the only nsP reported to bind membranes, and its membrane affinity is enhanced by, but not dependent on, a palmitoylation site [156,157,158]. The nsP2 has RNA helicase and RNA triphosphatase (RTPase) activity in its N-terminal domain, and its C-terminus harbors a cysteine protease domain, which cleaves the polyprotein into individual nsPs [159,160]. NsP3 has adenosine diphosphate (ADP)-ribosyl hydrolase activity and interacts with several host–cell proteins [161,162,163]. NsP4 is the RNA-dependent RNA polymerase directly responsible for producing new viral RNA [164,165].
CHIKV structural proteins form the virion and are translated from subgenomic viral RNAs. Briefly, the 3′ ORF is transcribed into a subgenomic positive-stranded RNA, which encodes five structural proteins after subsequent cleavage and maturation steps. It is also expressed as a polyprotein processed by viral and cellular proteases [166,167,168].
Three main structural proteins are expressed: capsid protein (CP), E1, and E2 viral glycoproteins. Two supplementary small structural proteins, E3 and 6K, and its translational frameshift product, transferase (TF), are also synthesized. These act as stabilization and regulation functions involved in viral glycoprotein assembly and particle budding [167,169,170,171,172].
The structure of the CHIKV virion consists of a single-strand RNA encapsulated by 240 copies of the highly basic CP. This protein assembles into an icosahedral nucleocapsid exhibiting T = 4 symmetry, a defining characteristic of alphaviruses [167,173]. Beyond its structural role, the CP mediates the genomic encapsulation within the nucleocapsid and actively engages in the viral budding process through interactions with the cytoplasmic domain of E2 glycoprotein and finally the virion assembly [167,172,174,175,176,177].
This nucleocapsid is enveloped by a host-derived lipid bilayer that contains 80 trimeric glycoprotein spikes. Each spike consists of three E1-E2 heterodimers; the primary function of the E1 subunit is to mediate membrane fusion during viral entry, which facilitates the release of the viral genome into the host cell cytoplasm [178]. The E2 subunit is critical for receptor recognition and binding to the host cell surface [174,179]. Additionally, the E2 subunit plays a crucial role in viral entry by initiating clathrin-dependent endocytosis, which allows for the internalization of the virion into the host cell [180,181].
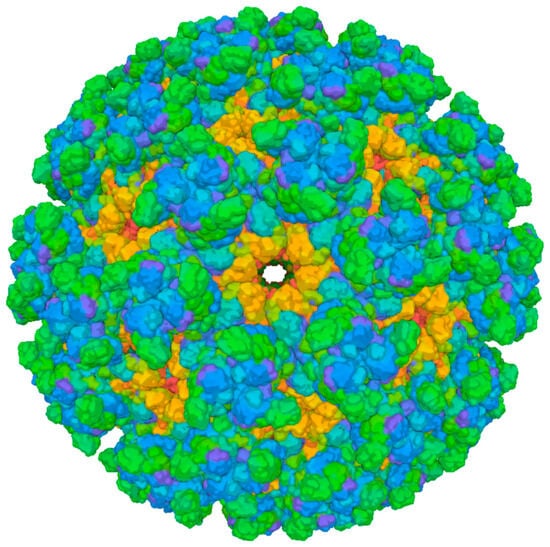
Figure 4.
Cryo-EM structure of Chikungunya virus strain Senegal 37997 VLP (PDB 6NK5). Surface representation of the virus-like particle derived from electron cryo-microscopy. The different colors represent different protein chains. Green represents the E2 protein complex, blue represents the E1 protein complex, and orange represents the Capsid protein complex. Image from the RCSB PDB (RCSB.org) of PDB ID 6NK5 [182]. Created in BioRender. Oliveira, F. (2025) https://BioRender.com/15wrcvb (accessed on 17 August 2025).
The E3 glycoprotein acts as a chaperone, properly folding the E2 glycoprotein and inhibiting premature conformational changes in the E2–E1 heterodimer through the acidic secretory pathway [167,182]. E3 and E2 are initially synthesized as the precursor p62 protein, which is cleaved by furin during maturation [183,184]. While E3 is typically cleaved in mature virions, it can remain bound to the E2–E1 heterodimer in some alphaviruses, such as SINV and CHIKV [173]. The dissociation of E3 also depends on factors like the culture medium’s pH and infected cells’ confluency [185,186].
The 6K protein, despite its small size, is essential for virion assembly and release [187]. It shares an N-terminus with the TF protein, but their C-termini differ due to ribosomal frameshifting [188]. Both 6K and TF are hypothesized to form ion channels and are found in low amounts in virion particles [189]. They contribute to viral budding [189] and pathogenesis, but their exact roles in glycoprotein processing, assembly, budding, and particle stability remain unclear [189,190]. Additionally, their hydrophobic nature has excluded them from recombinant protein preparations used for structural studies, further limiting understanding of their precise functions [167,191].
3.1. Membrane Fusion and Entry to Host Cells
Viral entry into host cells comprises a sequence of well-defined stages: attachment, receptor binding, endocytosis, and membrane fusion. The process initiates with attachment, wherein viral particles engage with the cellular surface through interactions with attachment factors, which results in the concentration of viral particles without necessarily inducing conformational changes in the viral envelope proteins. Subsequently, receptor binding occurs, characterized by specific interactions between viral proteins and host cell receptors that can provoke structural rearrangements essential for entry. The virus is then internalized via endocytosis, often facilitated by pathways dependent on clathrin or caveolin, culminating in the release of the viral genome into the cytoplasm and the initiation of infection [151,181,192,193].
3.1.1. Structural Components of Chikungunya Virus Involved in Entry
The entry of CHIKV into host cells is mediated by the structural proteins E1 and E2, which form heterodimers on the viral surface. These glycoproteins play essential roles in receptor binding and membrane fusion. Specifically, E2 is primarily responsible for the attachment to host cell receptors, while E1 facilitates the low-pH-dependent fusion process. During the viral assembly, the stability of the E1-E2 heterodimer is enhanced by the presence of the E3 glycoprotein, which prevents premature fusion [151,193].
E1 glycoprotein comprises a hydrophobic fusion loop (E1-FL) that inserts into the host membrane during the fusion process. Under low-pH conditions, E1 experiences conformational alterations, which result in the exposure of the fusion loop and facilitate the merger of membranes. Crucial residues such as E1-A/V226 and E1-V178 are instrumental in lipid sensing and fusion efficiency [170,181]. For instance, the E1-V178 residue is significantly conserved among most alphaviruses. Experimental mutation of this residue to alanine has been shown to result in a reduced dependence on cholesterol for CHIKV fusion [194].
E2 glycoprotein encompasses three structural domains, designated as A, B, and C. Domain A, centrally located, is surface-exposed and facilitates interactions with host receptors, such as matrix remodeling-associated protein 8 (Mxra8). Domain B, situated distally, encompasses the acid-sensitive region (ASR) and serves as a primary target for neutralizing antibodies. Domain C, which is embedded within the viral membrane, anchors E2 and exhibits reduced accessibility to immune detection [167,192].
The CP interacts with the cytoplasmic tail of E2, thereby facilitating the assembly and envelopment of the nucleocapsid. During the viral entry process, the capsid remains associated with the viral RNA, constituting the nucleocapsid core. After membrane fusion, the nucleocapsid is released into the cytoplasm, where the uncoating process occurs. The capsid disassembles, releasing the viral RNA, which is then available for replication [151,192].
3.1.2. Host Cell Receptors and Attachment Factors
The primary receptor for CHIKV is Mxra8, which interacts with domains A and B of the E2 protein. This interaction promotes the entry of the virus into fibroblasts, myocytes, and osteoblasts. Mxra8 functions as a conserved receptor across numerous alphaviruses, thereby establishing its relevance as a prospective target for therapeutic intervention [167,195]. Another receptor, Prohibitin-1 (PHB1), has been shown to interact specifically with E2 in certain cell types, including microglial cells. While PHB1 plays a role in facilitating viral attachment, it is not essential for viral entry, suggesting its function may be to enhance the concentration of virions at the cellular surface [193].
Glycosaminoglycans (GAGs) represent substantial, intricate carbohydrate macromolecules on the extracellular surfaces of diverse mammalian cell types. Notably, heparan sulfate plays a pivotal role as an attachment factor, facilitating the enhancement of viral attachment to the cellular surface [196]. These macromolecules can interact with various proteins and principally serve functions in cellular adhesion, proliferation, differentiation, and signal transduction [192]. Mutations in E2, including E2-R82, enhance GAG affinity, facilitating adaptation to cell culture while decreasing virulence in vivo [192,197,198].
Phosphatidylserine (PtdSer) receptors, such as T-cell immunoglobulin and mucin domain (TIM-1), the TAM family proteins (comprising Tyro3, Axl, and Mer), and CD300a, are capable of recognizing PtdSer present on the viral envelope [199,200]. These receptors enable entry by mimicking apoptosis, but they do not trigger the conformational changes necessary for fusion [181,201].
3.1.3. Mechanisms of Membrane Fusion
CHIKV enters host cells via clathrin-mediated endocytosis, facilitating the delivery of virions to early endosomes. The subsequent acidification of these endosomes, reaching a pH of approximately 6.0, triggers conformational alterations in the E1 protein, leading to the exposure of the hydrophobic fusion loop (E1-FL) [202]. This fusion loop is then inserted into the host cellular membrane, thereby initiating the process of membrane merger. Additionally, the presence of cholesterol and sphingomyelin within the host membrane significantly enhances the efficiency of this fusion process [151,193,203].
At low pH levels, E2 dissociates from E1, thereby facilitating the trimerization of E1 and the insertion of the fusion loop [202]. The ASR within E2, particularly the E2-H170 residue, contributes to the destabilization of the heterodimer. Furthermore, E3, which provides stability to E2 during the viral assembly process, is cleaved during viral maturation, consequently rendering the virus competent for fusion [167,192,204].
CHIKV demonstrates the ability to fuse with receptor-free liposomes, indicating that this fusion process is independent of any protein receptor. This fusion mechanism can be delineated into several distinct stages: the destabilization of the E2/E1 heterodimer, integrating the E1 protein into the target membrane, the trimerization of E1, and the subsequent formation of the fusion pore [205].
In summary, E1 trimers assemble into a ring-like structure on the host membrane, facilitating the proximity between viral and host membranes. This interaction leads to hemifusion, succeeded by the establishment and subsequent enlargement of a fusion pore. Consequently, the nucleocapsid is liberated into the cytoplasm, initiating the processes of uncoating and replication [170,206,207,208].
3.1.4. Endocytic Pathways Utilized by Chikungunya Virus
CHIKV primarily enters host cells through clathrin-mediated endocytosis (CME), a well-characterized and constitutive process in mammalian cells [209,210]. CME involves the formation of clathrin-coated pits at the plasma membrane, which encapsulate the virus. This process is mediated by a complex interplay of proteins, including adaptor protein-2 (AP-2), clathrin, dynamin, and epidermal growth factor receptor substrate 15 (Eps15) [209,210]. Dynamin, a large multidomain guanosine triphosphatase (GTPase), plays a critical role in the scission of clathrin-coated pits, leading to the formation of clathrin-coated vesicles that transport the virus into the cell [209,210]. Once internalized, the clathrin coat is rapidly removed, and the virus is delivered to early endosomes [193,211]. The acidic environment of the endosomes triggers conformational changes in the viral E1/E2 glycoproteins, facilitating the fusion of the viral envelope with the endosomal membrane and the subsequent release of the viral genome into the cytoplasm for replication [181].
Dynamin is a key mediator of CME and is essential for the pinching of endocytic vesicles from the plasma membrane. Its role in CHIKV entry has been well-documented, as inhibition of dynamin significantly reduces CHIKV infection [11,212,213]. Similarly, Eps15, a protein critical for the assembly of clathrin-coated pits, has been implicated in CHIKV entry. However, the involvement of Eps15 does not exclusively confirm CME, and studies have shown that CHIKV can enter cells via a clathrin-independent, Eps15-dependent pathway. They demonstrated this using knockdown of Eps15 and clathrin heavy chain, a major scaffold protein of the clathrin coat [214].
These findings indicated that the entry of the CHIKV is not exclusively limited to CME; rather, it may also engage other endocytic mechanisms that depend on the cellular context. CHIKV’s ability to utilize multiple entry pathways highlights the flexibility of its infection strategy and underscores the necessity for further research to thoroughly elucidate these underlying processes.
4. Immunological Aspects of Chikungunya Virus Infection
In this section, we will explore the inflammatory processes triggered by the host immune response following CHIKV entry. Focus will be given to the roles of cytokines, chemokines, and immune cells in modulating viral load—either promoting viral clearance or persistence—as well as the clinical symptoms resulting from these immunological mechanisms. The key aspects related to these responses are outlined below.
The acute phase is marked by high viral replication and dissemination from the primary sites of CHIKV infection, resulting in a peak in viremia and the manifestation of clinical symptoms characteristic of the acute phase [115]. The main target organs during infection include the liver, spleen, joints, and kidneys, allowing the infection of non-hematopoietic cells such as fibroblasts and endothelial cells. CHIKV replication is recognized by pattern recognition receptors (PRRs), such as NOD-like receptors (NLRs) and Toll-like receptors (TLRs), resulting in the downstream activation of nuclear factor kappa B (NF-kB) and the phosphorylation of interferon regulatory factor (IRF) 3, initiating the production and release of Type I Interferon (IFN-I) as well as other cytokines. Thus, at the site of skin infection, resident immune cells, such as lymphocytes, DCs, monocytes, and gamma delta T lymphocytes (γδ T cells), recognize the viral particles through PRRs and induce a rapid and robust production of IFN-I (Interferon alpha and beta [IFN-α/β]), along with other cytokines that will promote the additional recruitment of immune cells and the activation of adaptive immunity, essential for viral control and elimination. Consequently, with the release of chemoattractant molecules by resident cells, such as C-C motif chemokine ligand (CCL2)/MCP-1, there is an expressive migration of macrophages, neutrophils, natural killer (NK) cells and lymphocytes to the primary sites of infection (e.g., joints and muscles), leading to hypertrophy of synovial cells and adjacent synovial vessels [215], and subsequent joint pain in patients [115,216]. There is also the development of adaptive immunity, with a CD8+ T lymphocyte response in the early stages of infection and, later, the development of a CD4+ T cell response [217]. In addition, from the 3rd day after the onset of clinical symptoms, immunoglobulin (Ig) M antibodies for CHIKV can also be detected [218,219,220]. The chronic phase lasts longer than 3 weeks and can extend from months to years. It is characterized by signs and symptoms such as joint swelling, stiffness, arthralgia, and tendonitis/tenosynovitis. In this stage, there is a significant reduction in viral titers. However, viral particles can remain in macrophages and fibroblasts, since CHIKV can use these cells as replication reservoirs [121]. Pain is one of the most critical symptoms of the chronic phase of CHIKF. Some studies have shown that the E2 envelope protein induces the humoral immune response [221] and maturation of CD4+ T lymphocytes in patients [222], resulting in inflammation and swelling of the joints. In addition, the E2 protein is also involved in CHIKV-induced joint pain. Recently, Segato-Vendrameto et al. (2023) [223] demonstrated that the E2 protein activates dorsal root ganglion (DRG) neurons, resulting in calcium influx via Transient Receptor Potential Vanilloid 1 (TRPV1) and neuronal sensitization. As a result, the E2 protein leads to the development of mechanical and thermal hyperalgesia, which was reversed by the use of monoclonal antibodies directed against the E2 protein.
4.1. Innate Response
In CHIKV infection, the innate immune response contributes to antigen recognition, limitation of viral replication, and creating a microenvironment towards mounting an effective adaptive response. These functions are performed by immune cells such as dendritic cells, neutrophils, monocytes, macrophages, and NK cells. When activated, these cells begin to produce IFN-α, IFN-β, Interferon gamma (IFN-γ), tumor necrosis factor (TNF)-α, IL-6, IL-β, for example, which act to orchestrate the mechanisms of viral elimination. Next, we will explore important aspects of these cells in CHIKV infection.
DCs are resident antigen-presenting cells that capture, process, and present CHIKV antigens to T lymphocytes, initiating specific antiviral immune responses. Dermal DCs are the main activators of the IFN-I response to CHIKV and are primarily responsible for limiting viral replication and clinical progression [224]. The IFN-I signaling pathway is extremely important for controlling CHIKV infection, as evident using knockout (KO) animals and by genetic deletion of myeloid differentiation primary response gene 88 (MyD88) that resulted in increased viremia in mice [225,226,227]. In addition, when DCs are exposed to CHIKV, the activation of PRRs leads to the activation of signaling pathways, such as IFN-I and NF-kB, resulting in the activation of IκB-α and the release of TNF-α and IL-12p70 in CD11c+ CD86+ DCs, and increased production of IL-2 in CD4+ T cells [228]. The activation of these pathways and subsequent cytokine production contribute significantly to viral control and elimination from the host. In addition, Long et al. (2013) [229] demonstrated the functional importance of the dendritic cell immunoreceptor (DCIR) in arthritis triggered by CHIKV, suggesting CHIKV infection. In the study, the inhibition or absence of the receptor in DCs exposed to virus increased the expression of IL-10 and IL-6, and reduced IL-12, resulting in more severe disease (marked leukocyte infiltration, joint swelling and damage, and loss of body weight). In addition, receptor deficiency leads to delayed clearance of CHIKV [229].
Neutrophils are cells recruited by the onset of CHIKV infection. Neutrophil migration occurs through the production of chemokine (CXC motif) ligand (CXCL) 1 and CXCL2, and once at the site of infection, these cells can produce reactive oxygen species (ROS), cytokines (e.g., IFN-I), and neutrophil extracellular traps (NET), which contribute to the process of controlling acute CHIKV infection [230,231,232]. In these cells, IFN-I (IFN-α and IFN-β) signal through the same receptor, the IFN α/β receptor (IFNAR), but they have distinct functional mechanisms in the antiviral response against CHIKV. The KO mice demonstrated that IFN-α is essential for controlling viral replication and spread, and IFN-β acts primarily in modulating the inflammatory response [233]. Furthermore, the increase in neutrophils led to greater tissue damage at infected sites resulting in increased production of pro-inflammatory cytokines and chemokines (e.g., TNF-α, IL-6, CCL2, and CXCL1), edema, pain, and tissue damage, indicating that these cells not only aided in defense but also contributes to the worsening of immunopathology when present in excess [234]. Moreover, NETs induced in neutrophils in vitro were able to capture and neutralize CHIKV. In vivo (animal model), NET release depends on the activation of TLR7 and ROS generation. In patients with acute CHIKV infection, increased levels of the myeloperoxidase (MPO)-DNA complex (a NET marker) were detected, and there was a correlation between these levels and viral load in the blood. All these findings reinforce that NETs play an essential protective role in controlling the acute phase of CHIKV infection [232,235]. On the other hand, in musculoskeletal tissues during CHIKV infection, anti-inflammatory neutrophils (N2-subtype) infiltrates to modulate the inflammation. Consequently, it may compromise viral clearance and delay disease resolution [236]. Thus, these sets of cells are important for the initial control of the infection, but in the long term, neutrophil activity can contribute to the degree of the chronic phase of the disease [237,238].
Monocytes play an important role in restricting CHIKV infection, as their presence in synovial tissue is associated with elevated expression of IFN-α, which can inhibit viral replication [116,239,240]; and play an important role in the development of joint pathology [241]. Monocyte-derived macrophages migrate to the site of infection in response to the activation on chemoattractants, such as CCL2/MCP-1, induced by CHIKV [129,242]. Both resident and infiltrating macrophages produce IL-6, TNF-α, and granulocyte macrophage colony stimulating factor (GM-CSF), contributing to local inflammation [115,243]. Furthermore, these cells are susceptible to CHIKV infection and can act as viral reservoir and sites of replication with infiltration of NK cells and CD4+ T lymphocytes, which also contributes to the perpetuation of pain and polyarthralgia [221]; and induce antiviral control and the development of chronic arthritis due to IFN-α production [116,119,129,221,244]. The role of monocytes and macrophages is therefore dual. The inhibition by a monocyte chemotactic protein inhibitor (e.g., CCL2/MCP-1 and CCL8), reduce in osteoclastogenesis, preventing bone resorption [245]; and their depletion can result in increased neutrophil infiltration into joints, resulting in tissue damage and pain [245,246,247]. Furthermore, CHIKV can directly infect human osteoblasts, which increased expression of IL-6, activation of receptor activator of nuclear factor kappa-B ligand (RANKL), and inhibition of osteoprotegerin (OPG), which contributes to bone loss [248,249]. This dysregulation, associated with impaired osteoblast function, contributes to increased alkaline phosphatase levels, directly affecting bone mineralization [250]. Macrophages are essential sources of CCL2, regulating the migration of monocytes and NK cells to the site of infection [242,247]. However, a decreased monocytes/macrophages infiltration results in a compensatory neutrophil and eosinophil response, which can contribute to edema and tissue damage [247]. Therefore, targeting these cells requires a balanced approach to control both viral persistence and inflammation.
In CHIKV infection, the peak of NK cells occurs during the initial acute phase, around 3 days after the onset of symptoms, and correlates directly with viral load; and the persistence of this cell is associated with the progression into the chronic phase [251]. NK cell function is regulated by several classes of receptors expressed on their cells membranes, such as lectin C-type receptors [e.g., NKG2D (activating) and CD94/NKG2A (inhibiting)], killer cell immunoglobulin-like receptors (KIRs) [KIR2DS, KIR3DS, and their inhibitors KIR2DL and KIR3DL—activating and inhibitory variants that recognize human leukocyte antigen (HLA) and regulate the immune response], and natural cytotoxicity receptors (NCRs) (e.g., NKp30, NKp44, and NKp46, which detect cellular stress markers or viral antigens) [252]. The coordinated expression of activating and inhibitory receptors allows NK cells to respond in a balanced manner [252]. It has been observed that an imbalance of these receptors is associated with increased susceptibility of patients to CHIKV infection [253,254,255]. A high viral load in the acute and chronic phase can be associated with different NK cells (e.g., CD69+), and the release of cytokines like TNF-α and IFN-γ, and the persistence of these factors being correlated with persistent arthralgia [254,256,257]. The elevated expression of CD3− CD56+ NK cells, and expression of active NKG2C receptor and KIR2DL2/KIR2DL3 inhibitory receptors for HLA-C1 are related to higher viremia and the clearance of infected cells in the acute phase [241]. Also, the increase in the frequency of HLA-C2, which presents peptides to CD8+ T cells in combination with the expression of KIR2DL1 gene (receptor for HLA-C2), can worsen the infection [253,258]. Other studies have demonstrated that, after acute CHIKV infection, NK cells undergo a transient clonal expansion linked to increased viral load [251,254]. On the other hand, due to their cytotoxic activity, NK cell expansion may contribute to joint pathology [256]. Overall, NK cells contribute both to viral clearance and, potentially, to disease chronicity.
The innate immune response to CHIKV infection is detailed below and summarized in Table 1.

Table 1.
Different cellular immune responses triggered by Chikungunya virus infection.
4.2. Adaptative Immune Response
The adaptive immune response occurs via the activation of CD4+ and CD8+ T lymphocytes, which promote the elimination of infected cells and the secretion of cytokines (IFN-γ, IL-2, and TNF-α) that will amplify the antiviral functions of immune cells such as macrophages. In addition, the activation of B lymphocytes results in the production of neutralizing antibodies (Immunoglobulin (Ig)M and IgG) against CHIKV.
The role of T cells in the pathogenesis of alphaviruses infections is varied [274,275,276,277]. In fact, during the early phase of CHIKV infection, there is a predominance of CD8+ T cells, which are responsible for antiviral immunity, cytotoxic activities, and the destruction of infected cells to control virus replication, and those cells remain in the blood for 7–10 weeks post-infection [217,264,265]. Towards the end of the acute phase, the CD4+ T cell is responsible for modulating the activity of other immune cells—by producing IFN-γ, they stimulate cell-mediated and production of neutralizing antibodies [263]. Furthermore, an increase in the frequency of CD4+ T cells observed in patients with persistent CHIKV-associated arthralgia suggests a role in antiviral defense [278] and in inducing joint damage [221,222,262,279] by mechanism mediated by regulatory T cells (Tregs) and T cells, such as types I (Th1) and type 17 (Th17) helper T (Th) cell subsets [217,222,256,262,263,280].
During CHIKV infection, Th1 has promoted cell-mediated immunity, being able to activate macrophages to fight intracellular pathogens, producing cytokines like IFN-γ and TNF, and enhancing the cytotoxic activity of both NK cells and CD8+ T cells [266]. On the other hand, Th2 is involved mostly in humoral response, promoting B cell stimulation through IL-4, IL-5 and IL-13 signaling, and modulating Th1/Th17 inflammatory responses [256]. Th17 cell subsets can act in concert with Th1 or alone, increasing the production of the cytokines IL-17, IL-6, IL-21, and IL-22, and playing important roles in inflammation and tissue damage related with joint and muscle pain [263,270,281,282,283,284,285,286]. Treg cell activity decreases in parallel with the reduction in viral load, as their specific function protects against CHIKV-induced pathology in mice [263].
Cells infected with CHIKV are killed mainly by the action of cytotoxic immune cells, such as CD8+ T lymphocytes. Once activated, effector CD8+ T lymphocytes promote release of cytolytic granules through exocytosis or the granule-independent pathway, resulting in cytotoxicity, and produce important antiviral cytokines (e.g., IFN-γ) [284].
T cells play a complex role in CHIKV infection. CD8+ T cells dominate early, contributing to viral clearance through cytotoxicity and IFN-γ production, but may become exhausted during chronic infection, reducing their effectiveness. CD4+ T cells support immune coordination and antibody production but can also drive joint inflammation, especially through Th1 and Th17 responses. The expansion of these subsets, along with decreased Treg cell activity, is linked to tissue damage and chronic symptoms. Overall, while T cells are crucial for antiviral defense, their dysregulation may contribute to chronic CHIKV-associated pathology.
B lymphocytes and antibodies specific to CHIKV are essential for limiting viral replication and dissemination [129,273]. In addition, a study using serum samples from CHIKV-infected humans in Malaysia demonstrated that B cells produce neutralizing IgM antibodies targeting CHIKV surface glycoproteins E1 and E2 at day 6 post-infection, and the production of these antibodies is associated with lower levels of viremia [272]. Another study using human samples showed that CHIKV infection results in the production of IgG and the generation of memory B cells that can last up to 24 years after infection [271]. Furthermore, Hoarau et al. (2010) [221] demonstrated that E1/E2 and capsid proteins are the main factors that drive the humoral immune response in hospitalized patients with CHIKV, leading to increased production of IFN-γ and IL-12 and activation of cells such as NK cells and memory/effector B lymphocytes, these appear to have driven tissue damage, apoptosis, fibrosis and a polarized inflammatory response in CHIKV-induced arthritis, especially due to the persistence of viral RNA in synovial macrophages. CHIKV can elicit cellular and humoral immune responses through envelope and capsid proteins, contributing, for example, to the development of arthritis [221].
B cells and CHIKV-specific antibodies are critical for controlling viral replication and spread. In B-cell-deficient mouse models, infection leads to higher and persistent viremia, highlighting the importance of humoral immunity. Neutralizing IgM antibodies targeting CHIKV E1 and E2 glycoproteins appear early and are associated with reduced viremia, while long-term IgG and memory B cell responses can persist for decades. Despite this, chronic arthritis may occur independently of adaptive immunity, driven instead by viral persistence in tissues. Additionally, aging impairs B cell responses, contributing to prolonged infection and higher viral loads in elderly hosts.
The adaptive immune response to CHIKV infection is detailed below and summarized in Table 2.

Table 2.
Humoral immune and antibodies response activated to Chikungunya Virus infection.
5. Diagnosis and Clinical Management for Chikungunya Virus
The diagnosis of CHIKV infection is based on a combination of clinical, epidemiological, and laboratory data, and is essential to guide the initiation of treatment, which is currently limited to symptomatic relief and supportive therapies. Figure 5 summarizes the main diagnostic methods and treatments indicated for CHIKV.
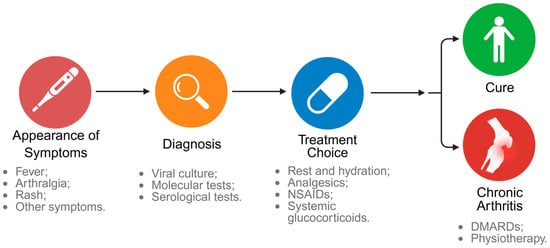
Figure 5.
Clinical evolution of Chikungunya infection. After the appearance of clinical signs such as fever, arthralgia and rash, the diagnosis is confirmed through viral culture, molecular or serological tests. Treatment is based on symptomatic relief with rest, hydration, analgesics, NSAIDs, and, in selected cases, systemic glucocorticoids. A clinical course can lead to cure or progression to chronic arthritis, which requires interventions such as the use of DMARDs and physical therapy. Created in BioRender. Oliveira, F. (2025) https://BioRender.com/15wrcvb (accessed on 14 July 2025).
Initially, clinical evaluation is based on the presence of classic symptoms such as sudden-onset fever (39 to 40 °C), severe and often debilitating arthralgia (reported in 85–90% of cases), rash (reported in 40–60% of cases), and other flu-like symptoms (e.g., headaches and gastrointestinal discomforts) [299]. Although the symptoms are nonspecific and similar to those of infections by other arboviruses, such as dengue virus (DENV) and zika virus (ZIKV), these signs may suggest a CHIKV infection [300]. The conjunctive presence of these characteristic symptoms is highly predictive of CHIKV infections in endemic regions [300]. However, specific laboratory diagnosis is necessary to confirm CHIKV infection, especially in regions where other arboviruses are endemic [5].
CHIKV infection can be diagnosed through detection of the virus itself, the viral genome or virus-specific antibodies, using viral culture, molecular, and serological tests [299,301]. Although viral isolation was considered the gold standard for viral detection for decades, these methods are now more commonly used in research rather than clinical diagnosis. This is due to the long time required to perform the test and the need for specialized equipment and trained professionals [299]. Among the molecular diagnostic methods, reverse transcription polymerase chain reaction (RT-PCR) is the most sensitive and is currently considered the gold standard for the detection of viral RNA [11,299,301]. However, RT-PCR is only effective during the acute phase of infection (within 5 to 7 days of symptom onset), where the viral load can reach levels high enough for detection [11,301]. Other molecular tests include isothermal methods and multiplex assays [299].
After the acute phase of CHIKV infection, serological tests that identify IgM and IgG antibodies are most commonly used, since IgM are detectable in the early stages of the disease and IgG are detectable for a long-time post-infection [299,301]. Among the serological tests, enzyme-linked immunosorbent assays (ELISA) are the most widely used due to their ability to detect antibodies produced during CHIKV disease several months after the initial infection [11,299]. However, although IgM and IgG antibodies to CHIKV are highly sensitive, they can cross-react with other alphaviruses [299,302], such as DENV, Mayaro, and o’nyong-nyong, leading to possible misdiagnosis [303,304] and requiring a more specific neutralization test to confirm the results.
Currently, there are no specific treatments for CHIKV infection; therefore, therapeutic strategies are based on supportive treatment, including pain management and anti-inflammatory medication. During the acute phase of infection, the recommended treatment is rest, adequate hydration, and analgesics, with acetaminophen being the first-line medication. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, can also be used, except in cases where dengue co-infection is suspected. The indicated analgesics and NSAIDs, as well as supportive therapies (e.g., hydration and rest), relieve fever and muscle and joint pain. However, when pain is not controlled with commonly used analgesics, more potent analgesics may be indicated [11,305]. Studies showed that broad-spectrum antivirals, such as ribavirin (RBV) and IFN, are effective against CHIKV, however more research on these drugs is needed for clinical implementation [5].
In cases of arthralgia or severe joint inflammatory responses, systemic glucocorticoids may be indicated. However, when symptoms progress to chronic arthritis, inflammatory arthritis treatment protocols are recommended, including disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), which are indicated to slow the progression of the disease. Together, physiotherapy is an important ally in the rehabilitation of patients with chronic post-CHIKV manifestations [305]. A retrospective study conducted in a Spanish center analyzed 119 patients with post-CHIKV complications, where the main clinical manifestation was persistent arthralgia (86% of patients). Management with targeted therapies resulted in clinical improvement in chronic cases, highlighting the importance of early diagnosis, specialized follow-up, and the adoption of interdisciplinary protocols to optimize treatment [306].
6. Emerging Therapies for Chikungunya Virus Infection
The growing disease burden and the risk of global outbreaks, coupled with the lack of specific treatments, have driven the development of targeted therapies. Emerging therapeutic strategies seek to interfere with viral replication, modulate the host immune response, or attenuate long-term inflammatory complications [307]. These approaches include antiviral agents, polyclonal and monoclonal antibodies, and therapeutic vaccines, many of which are still in preclinical phases or in early clinical trials [308]. This section and Figure 6 summarize the main therapeutic approaches under development against CHIKV.
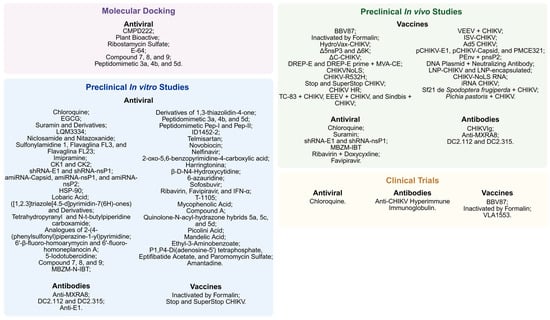
Figure 6.
Main therapeutic strategies under investigation against Chikungunya virus. Treatments are distributed according to their stage of development: Molecular Docking, in vitro and in vivo preclinical studies, and clinical trials. Approaches include antiviral compounds, antibodies, and experimental vaccines, highlighting the diversity of targets and mechanisms evaluated in combating CHIKV infection. Some treatments are identified by their acronym, and their full names can be found in topic 6. Created in BioRender. Oliveira, F. (2025) https://BioRender.com/15wrcvb (accessed on 18 August 2025).
6.1. Antiviral Agents
Over the past decade, the development of specific antiviral agents against CHIKV has been the subject of intense research. However, it is important to emphasize that to date there are no licensed antiviral agents that act directly against CHIKV, and most studies are in preclinical phases [307]. Table 3 summarizes the main antiviral agents in preclinical and clinical development against CHIKV. Below, we detail only the viral agents in preclinical development in vivo (animal models) and at the clinical stage.

Table 3.
Main antiviral agents under investigation against Chikungunya virus.
6.1.1. Entry and Fusion Inhibitors
The entry and fusion of CHIKV into host cells is a key event for the establishment of infection. Thus, inhibiting this initial step represents a promising strategy in the development of antivirals, since it prevents the multiplication of the virus and contributes to the reduction in viral load, inflammatory response, and clinical symptoms of the disease [364].
Among the antiviral agents, Chloroquine, a drug indicated for the treatment of malaria, systemic lupus erythematosus and rheumatoid arthritis [365], was the target of major investigations. However, in clinical phase studies, the treatment has not shown any benefit to the patient. Roques et al. (2018) demonstrated that in animal models, prophylactic administration of Chloroquine aggravated the infection by increasing viremia and slowing the immune response, while in humans, treatment modulated the levels of inflammatory markers with the potential to allow the adaptive immune response [310]. Other studies with similar effects are described in Table 3.
Suramin, a Food and Drug Administration (FDA)-approved compound for the treatment of trypanosomiasis, acts as a competitive inhibitor of GAGs and has been investigated for the treatment of CHIKV. Studies have shown that Suramin works by dose-dependent reduction in cytopathic effect, viremia, protein expression, and viral production when administered early [314]. Its mechanism of action involves direct interaction with envelope glycoproteins, blocking conformational changes necessary for viral binding and fusion [366]. In vivo studies, Suramin decreased viral loads, improved acute lesions, restored cartilage integrity, and reduced the number of chondrocytes in infected mice, confirming its role as a multifunctional inhibitor in early stages of CHIKV infection [316]. Other studies are presented in Table 3. However, despite promising results from different studies, clinical trials involving patients with hepatitis B [367] and acquired immunodeficiency syndrome (AIDS) [368] suggest that long-term treatment with this antiviral is directly related to the appearance of serious side effects.
6.1.2. RNA Interference
Interference RNAs (RNAi) are biological molecules that regulate gene expression. SiRNA promotes gene silencing by cleaving the corresponding messenger RNA (mRNA), preventing protein production. This mechanism has been explored as a potential antiviral therapy, being able to act both directly against the virus and in host cell genes [369]. Lam et al. (2012) developed three small loop interfering RNA (shRNA) sequences targeting the capsid, E1 and nsP1 genes of CHIKV [324]. The authors observed that shRNA-containing cells against E1 and nsP1 showed total suppression of virus production until the third day post-infection, while the shRNA directed to the capsid showed moderate inhibitory effects. The shRNA targeting the E1 protein blocked several geographic strains of CHIKV, without affecting the replication of other viruses (e.g., DENV and SINV) indicating high specificity. In addition, it has been shown to be effective in an in vivo model, fully protecting mice from CHIKV infection [324].
6.1.3. Non-Structural Protein Inhibitors
The nsP2 protein of CHIKV exerts multiple essential functions in viral replication, acting as protease, helicase, nucleoside-triphosphatase (NTPase), RNA triphosphatase (RNAse), and virulence factor by suppressing the host immune response via transcriptional shutdown [369]. In addition to its essential role in CHIKV replication, the early discovery of the 3D structure of nsP2 has made it the target of investigations for the development of anti-CHIKV drugs [364].
Mishra et al. (2016) investigated the antiviral activity of the compound 1-[(2-methylbenzimidazole-1-yl)methyl]-2-oxo-indolin-3-ylidene]amino]thiourea (MBZM-N-IBT) against CHIKV [340]. MBZM-N-IBT reduced plaque-forming unit (PFU) formation approximately 76% and viral RNA levels of nsP2 and E1 by approximately 65% and 24%, respectively. In addition, the viral protein expression of E2 and nsP2 were inhibited by 97%, indicating strong blockade of viral replication and translation. In summary, MBZM-N-IBT showed potent antiviral activity against CHIKV, with low toxicity and inhibition in the early and late phases of infection [340].
NsP4 is an essential polymerase for alphaviruses, responsible for the synthesis of viral RNA. Its C-terminal domain contains the typical structure of an RNA-dependent RNA polymerase (RdRp), with GDD motif at the active site. Because nsP4 is absent in humans and has conserved regions between several viruses (such as CHIKV, ZIKV, and DENV), it is a promising target for the development of broad-spectrum antivirals [369].
Nucleoside analogues (NAs) are synthetic molecules that mimic natural nucleosides and, upon intracellular activation by phosphorylation, can inhibit the synthesis of viral DNA or RNA, disrupting replication [370]. Ferreira et al. (2019) demonstrated that oral treatment with Sufosbuvir administered just before CHIKV infection significantly reduced paw edema in adult mice [352]. The treatment of newborn mice increased the survival of infected animals and prevented CHIKV-induced motor neuron impairment. After investigating the interaction between Sufosbuvir and the nsP4 enzyme, the authors identified that there were plausible hydrogen bonds and electrostatic interactions that underpin the potential mechanism presented by the treatment [352].
In addition, Franco et al. (2018) compared the activity of three broad-spectrum antiviral agents, including two NAs and IFN-α (Table 3), against CHIKV in different cell lines [353]. RBV, a guanosine analogue, inhibited viral RNA synthesis and depleted intracellular GTP by interference with inosine monophosphate dehydrogenase (IMPDH), but was of little promise when administered alone due to high toxicity at effective concentrations [353]. Other studies have investigated the mechanisms of action of RBV and proposed that it acts by directly inhibiting the nsP4 RdRp protein through interaction with the Cys483 residue [371] and that the combined administration of RBV with Doxycycline [354] or IFN-α [355] is effective against CHIKV infection without showing toxicity.
Franco et al. (2018) also demonstrated that Favipiravir (FAV or T-705), a purine analogue, acted as a substrate for CHIKV’s RdRp, promoting lethal mutagenesis and chain termination, resulting in inhibition of viral replication [353]. In addition, the compound showed good antiviral selectivity and low toxicity in human cells, suggesting its potential for clinical studies [353]. Together, Delang et al. (2014), investigated the action of FAV and identified that the K291R mutation in the highly conserved F1 motif of the RdRp of the +ssRNA viruses (nsP4 gene) is responsible for the resistance of CHIKV to the drug, pointing to this enzyme as a relevant molecular target [330]. Similar results were observed with the defluorinated analog of the FAV (T-1105) [330]. However, the effectiveness of treatment depends on the timing of administration: FAV is highly effective when used in the early stages of infection, preventing the spread of the virus to distant joints and tissues. Administration after this period has low efficacy against CHIKV infection [356].
6.1.4. Host-Directed Antivirals
Host factors can favor or inhibit viral replication and, therefore, are promising targets for the development of antivirals. However, as they also participate in essential functions of the body, their modulation can cause toxicity. Thus, ideally, therapies should specifically target virus–host interactions, without affecting vital cellular processes. A thorough and detailed review on host-directed antivirals is presented by Hucke and Bugert (2020) [369], Battisti et al. (2021) [364] and Haese et al. (2022) [372].
6.2. Antibodies
The use of antibodies represents a promising therapeutic strategy for the control of emerging and reemerging viral infections. Polyclonal antibodies (pAb) are responsible for recognizing multiple epitopes of the same antigen and are historically used in immune replacement therapies and in passive immunizations (e.g., against hepatitis A, rabies, and measles viruses). Monoclonal antibodies (mAbs), on the other hand, are responsible for recognizing a single epitope of the antigen and offer advantages in terms of safety, specificity, and pharmacological potential [373]. Table 4 summarizes the main studies in preclinical and clinical development with polyclonal and monoclonal antibodies against CHIKV. Below, we discuss in detail only the antibody therapies in preclinical development in vivo (animal models) and in the clinical phase.

Table 4.
Main studies under development with polyclonal and monoclonal antibodies against Chikungunya virus.
6.2.1. Polyclonal Antibodies
Couderc et al. (2009) investigated the use of human polyclonal immunoglobulins (CHIKVIg), extracted from individuals in the convalescent phase of CHIKV infection, in murine models [374]. Plaque reduction neutralization tests (PRNT) showed that the serum containing CHIKVIg has a high viral neutralization capacity. In adult mice deficient (−/−) for IFN-α/βr, administration before or shortly after CHIKV infection prevented the disease, which was confirmed by the absence of viremia and clinical signs. In addition, administration to CHIKV-infected newborn mice significantly reduced mortality, viral load, and symptoms. These results demonstrated that purification of antibodies against CHIKV from convalescent plasma is effective and can be considered as a strategy to prevent and treat CHIKV infections [374].
In addition to preclinical trials, phase I and II clinical trials have been conducted to evaluate the safety and tolerability of administering anti-CHIKV hyperimmune intravenous immunoglobulin (derived from plasma from convalescent individuals) to newborns via vertical transmission, as well as to select the safe and effective dose for the development of a new intervention. To date, there are no scientific publications that present the clinical results of this study [ClinicalTrials.gov: NCT02230263].
6.2.2. Monoclonal Antibodies
Through a Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas9-based genomic screening, the cell adhesion molecule Mxra8 was identified as a mediator of CHIKV and other alphaviruses, binding to the A and B domains of the E2 protein. CHIKV virions bind directly to Mxra8, allowing viral attachment and internalization by the cell. In vivo, blocking Mxra8 reduced viral load and clinical signs of infection [195]. In addition, it was identified that mAbs isolated from individuals exposed to CHIKV were protective because they prevented virions from binding to the Mxra8 receptor. Structural assays have revealed that antibodies bind to a conserved epitope on viral proteins, directly obstructing the interaction interface with Mxra8. These findings pave the way for the development of new antiviral strategies against CHIKV [377].
Two other mAbs isolated from individual’s plasma in the convalescent phase of CHIKV infection were characterized, namely DC2.112 and DC2.315. These antibodies bind to a highly conserved epitope of the E1 protein, allowing cross-neutralization of several alphaviruses. As a result, these antibodies showed weak neutralization, but significantly inhibited the viral release of infected cells, suggesting a new mechanism of viral control. They were also able to recruit myeloid cells to promote phagocytosis of infected cells. In vivo, both mAbs conferred robust protection against CHIKV infection, highlighting the E1 protein as a promising new target for the development of therapeutics and vaccines [375].
6.3. Vaccines
Several scientific efforts have been directed to the development of effective vaccines against CHIKV, aiming to prevent the infection and contain its spread in endemic areas. There are currently several vaccines against CHIKV in the preclinical and clinical phases, which include inactivated, chimeric, recombinant, DNA or RNA-based, VLP and live attenuated vaccines (LAV) vaccines [4]. Table 5 summarizes the main vaccines in preclinical and clinical development against CHIKV. Below, we focus on detailing only those vaccines currently in clinical development.

Table 5.
Main vaccines in development against Chikungunya virus.
The BBV87 vaccine was developed from the CHIK/03/06 strain (ECSA genotype, originating in India, 2006) cultured in Vero cells and inactivated by β-propiolactone (BPL). Phase I and II clinical trials demonstrated that healthy adults who received 2 or 3 doses of the vaccine had 100% seroconversion and a robust neutralizing antibody response that was maintained for at least 6 months. In addition, the vaccine was well tolerated, and adverse events were mild and self-limited [378]. Phase II and III clinical trials were conducted together to evaluate the safety and immunogenicity of two doses of the BBV87 vaccine in three endemic countries in Asia and Latin America, as well as to select the optimal dose for the confirmatory phase of the study. To date, there are no scientific publications that present the clinical results of this study [ClinicalTrials.gov: NCT 04566484].
Harrison et al. (1971) described the production and evaluation of a formalin-inactivated vaccine for CHIKV [379]. To produce the vaccine, CHIKV was cultured in cell lines and inactivated by exposure to formaldehyde and subsequently purified and stabilized for human applications. Early-phase clinical trials have been conducted on adult volunteers, and the vaccine was well tolerated without significant adverse reactions and induced robust immune responses with high titers of neutralizing antibodies until the 14th day after the second dose. However, the persistence of antibodies was not evaluated, and the investigation was discontinued [379].
Based on animal model studies (Table 5), the attenuated vaccine VLA1553 (IXCHIQ®, manufactured by the company Valvena, Saint-Herblain, France) was developed and clinical phase trials began. The phase I clinical trial evaluated the safety and immunogenicity of three increasing doses of the vaccine, including revaccination after 6 or 12 months of the last dose administered. A single dose induced strong neutralizing antibody production in most participants and immune responses remained elevated for months after vaccination (100% seroconversion rate). In addition, the vaccine was well tolerated, with only mild to moderate adverse effects, including participants who received a second dose [383].
Subsequently, phase III clinical trials were initiated with the aim of evaluating the safety and immunogenicity of VLA1553 up to 180 days after vaccination. The results showed that only one dose of the vaccine was required to induce a rapid and intense seroconversion response and the antibody titer remained elevated for at least six months, indicating that there was long-lasting immunity. As in other trials, the vaccine was well tolerated in healthy adults, presenting light adverse effects such as pain at the injection site, headache, fever and mild myalgia [384]. Other studies have been initiated to evaluate the persistence of antibodies and the safety of the CHIKV vaccine for up to 2 years [385], and to evaluate the efficacy in endemic areas and in adolescents (between 12 and 17 years of age) [386]. Based on these studies and the results obtained, the VLA1553 vaccine has been approved by the FDA (Silver Spring, MD, USA; 2023), the European Medicines Agency (EMA; European Union; 2024) [404] and by the Brazilian Agency equivalent to FDA and EMA, the “Agência Nacional de Vigilância Sanitária” (ANVISA; Brasília, Brazil; 2025) [405], in addition to being recommended by the Centers for Disease Control (CDC) for adults traveling to regions with known outbreaks of CHIKV [406].
7. Future Perspectives and Conclusions
Despite the high infection rate of CHIKV and significant expansion into new geographic areas, there are still no specific treatments. It is necessary to develop mechanisms to distinguish CHIKV infection from other arboviruses, in addition to understanding the impact of arbovirus co-infections on clinical manifestations [407].
In recent decades, significant advances have been made in understanding the pathogenesis of CHIKV, but studies that prioritize the mechanisms involved in the pain response are needed, especially in severe cases [407]. Painkillers and anti-inflammatories are commonly prescribed for patients along with medical monitoring. These approaches may not be enough, as they only alleviate symptoms such as fever, arthritis, and myalgia and do not prevent persistent inflammation or progression to chronic pain [293]. This results in an impact on the quality of life of those patients [3]. Currently, treatments for CHIKV infection are mostly symptomatic, without a direct focus on modulating the underlying disease mechanism [407]. The lack of data on the mechanistic details involved in CHIKV infection highlights the need to seek specific therapeutic targets to control the disease.
Antiviral therapy has been shown to be effective at inhibiting CHIKV replication, but due to the lack of knowledge about the pathogenesis of CHIKV and the dynamics of viral mutation, the license for antiviral therapies remains unavailable [407]. Recently, the use of vaccines has been explored, but many affected regions have limited infrastructure, making government collaboration and public policies crucial to reducing costs and expanding their use in vulnerable populations. The high incidence rates and the need for new methods to control the vector highlight the importance of ensuring proper funding and resources for CHIKV-endemic countries, especially in low-income ones [407,408]. In addition, further research to expand development of licensed vaccines to pediatric, immunocompromised, and pregnant populations is needed [408].
Significant efforts have been made in recent years, but further research on the following topics is still necessary: the interruption of the transmission chain by controlling vector density and distribution, the diagnosis of CHIKV infection, treatments aimed at controlling symptoms based on the viral mechanism, and the redirection of current treatments towards more effective treatments [407].
Author Contributions
Conceptualization, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B., F.S.R.-O., C.Z., C.N.D.d.S., R.A., T.A.B., R.C. and W.A.V.; methodology, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B., F.S.R.-O., C.Z., C.N.D.d.S. and W.A.V.; software, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B. and F.S.R.-O.; validation, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B., F.S.R.-O., C.Z., C.N.D.d.S., R.A., T.A.B., R.C. and W.A.V.; formal analysis, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B., F.S.R.-O., C.Z., C.N.D.d.S., R.A., T.A.B., R.C. and W.A.V.; investigation, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B., F.S.R.-O., C.Z., C.N.D.d.S., R.A., T.A.B., R.C. and W.A.V.; data curation, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B., F.S.R.-O., C.Z., C.N.D.d.S., R.A., T.A.B., R.C. and W.A.V.; writing—original draft preparation, G.M.-C., J.A.C., K.M.Y., M.M.B. and B.H.S.B.; writing—review and editing, F.S.R.-O., C.Z., C.N.D.d.S., R.A., T.A.B., R.C. and W.A.V.; visualization, G.M.-C., J.A.C., K.M.Y., M.M.B., B.H.S.B., F.S.R.-O., C.Z., C.N.D.d.S., R.A., T.A.B., R.C. and W.A.V.; supervision, R.C. and W.A.V.; project administration, R.C. and W.A.V.; funding acquisition, R.C. and W.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (#203112/2020-2, #307852/2019-9, #309633/2021-4, #405027/2021-4, #427946/2018-2 and #405848/2025-0); PRONEX grant supported by SETI/Fundação Araucária and MCTI/CNPq, and Governo do Estado do Paraná (agreement #014/2017); Fundação Araucária (PBA/PROPPG 13/2021 agreements #276/2022-PBA and #250/2022-PBA; PBA/PROPPG 067/2024); Governo do Estado do Paraná, Conselho Paranaense de Ciência e Tecnologia, e Secretaria de Estado da Ciência, Tecnologia, e Ensino Superior (SETI) (dotação orçamentária #4560.19.571.06.6153; eprotocolo 1.234.745-0); Financiadora de Estudos e Projetos-FINEP; and CAPES (finance code #001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
During the preparation of this review, the authors used BioRender for the preparation of the illustrative figures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CHIKV | Chikungunya Virus |
| Ae | Aedes |
| CHIKF | Chikungunya Fever |
| USA | United States of America |
| ECSA | East Central South African |
| IOL | Indian Ocean Lineage |
| Wb | Wolbachia |
| TEP | Thioester-Containing Proteins |
| GNBPB1 | Glycosaminoglycan-Binding Protein B1 |
| PZ1A | Serine Protease Z1A |
| NHP | Non-Human Primate |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| IFN | Interferon |
| IFN-I | Type I Interferon |
| DC | Dendritic Cells |
| IL | Interleukin |
| IL1Ra | Interleukin-1 Receptor Antagonist |
| MCP-1 | Monocyte Chemotactic Protein-1 |
| VEGF | Vascular Endothelial Growth Factor |
| RNA | Ribonucleic Acid |
| SINV | Sindbis Virus |
| EEEV | Eastern Equine Encephalitis Virus |
| Poli(A) | Polyadenylated |
| ORF | Open Reading Frames |
| nsP | Non-Structural Proteins |
| RTPase | RNA triphosphatase |
| ADP | Adenosine Diphosphate |
| CP | Capsid Protein |
| TF | Transferase |
| Mxra8 | Matrix Remodeling-Associated Protein 8 |
| ASR | Acid-Sensitive Region |
| PHB1 | Prohibitin-1 |
| GAG | Glycosaminoglycans |
| PtdSer | Phosphatidylserine |
| TIM-1 | T-cell Immunoglobulin and Mucin Domain |
| CME | Clathrin-Mediated Endocytosis |
| AP-2 | Adaptor Protein-2 |
| Eps15 | Epidermal Growth Factor Receptor Substrate 15 |
| GTPase | Guanosine Triphosphatase |
| PRR | Pattern Recognition Receptors |
| NLR | NOD-Like Receptor |
| TLR | Toll-Like Receptor |
| NF-κB | Nuclear Factor Kappa B |
| IRF | Interferon Regulatory Factor |
| CCL | C-C Motif Chemokine Ligand |
| NK | Natural Killer |
| Ig | Immunoglobulin |
| TNF | Tumor Necrosis Factor |
| KO | Knockout |
| MyD88 | Myeloid Differentiation Primary Response Gene 88 |
| DCIR | Dendritic Cell Immunoreceptor |
| CXCL | Chemokine (CXC Motif) Ligand |
| TLR7 | Toll like receptor 7 |
| ROS | Reactive Oxygen Species |
| NET | Neutrophil Extracellular Traps |
| IFNAR | IFN Alpha/Beta Receptor |
| MPO | Myeloperoxidase |
| GM-CSF | Granulocyte Macrophage Colony Stimulating Factor |
| RANKL | Receptor Activator of Nuclear Factor Kappa-B Ligand |
| OPG | Osteoprotegerin |
| KIR | Killer Cell Immunoglobulin-Like Receptor |
| HLA | Human Leukocyte Antigen |
| NCR | Natural Cytotoxicity Receptor |
| Treg | Regulatory T |
| Th | Helper T Cells |
| IgM | Immunoglobulin M |
| IgG | Immunoglobulin G |
| DENV | Dengue Virus |
| ZIKV | Zika Virus |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| ELISA | Enzyme-Linked Immunosorbent Assays |
| NSAID | Nonsteroidal Anti-Inflammatory Drugs |
| RBV | Ribavirin |
| DMARD | Disease-Modifying Antirheumatic Drugs |
| MTX | Methotrexate |
| EGCG | Epigallocatechin-3-Gallate |
| FDA | Food and Drug Administration |
| AIDS | Acquired Immunodeficiency Syndrome |
| RNAi | Interference RNA |
| mRNA | Messenger Ribonucleic Acid |
| shRNA | Small Loop Interfering RNA |
| amiRNA | Artificial MicroRNA |
| MTase | Methyltransferase |
| GTase | Guanylyltransferase |
| GTP | Guanosine Triphosphate |
| SAR | Structure-Activity Studies |
| FHA | 6′-β-fluorohomoaryromycin |
| FHNA | 6′-fluoro-homoplanocin A |
| 5-IT | 5-iodotububercidin |
| NTPase | Nucleoside-Triphosphatase |
| RNAse | RNA Triphosphatase |
| MBZM-N-IBT | 1-[(2-methylbenzimidazole-1-yl)methyl]-2-oxo-indolin-3-ylidene]amino]thiourea |
| PFU | Plaque-Forming Unit |
| RdRp | RNA-dependent RNA polymerase |
| NA | Nucleoside Analogues |
| NHC | β-d-N4-hydroxycytidine |
| IMPDH | Inosine Monophosphate Dehydrogenase |
| FAV | Favipiravir |
| MPA | Mycophenolic Acid |
| PARP | Poly(ADP-Ribose) Polymerase |
| Bcl-2 | B-Cell Lymphoma 2 |
| PCA | Picolinic Acid |
| ITC | Isothermal Titration Calorimetry |
| SPR | Surface Plasmon Resonance |
| MDA | Mandelic Acid |
| EAB | Ethyl 3-Aminobenzoate (EAB) |
| AP4 | P1,P4-Di(adenosine-5′)tetraphosphate |
| EAC | Eptifibatide Acetate |
| PSU | Paromomycin Sulfate |
| pAb | Polyclonal Antibodies |
| mAb | Monoclonal Antibodies |
| PRNT | Plaque Reduction Neutralization Tests |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| LAV | Live Attenuated Vaccines |
| BPL | β-Propiolactone |
| EMA | European Medicines Agency |
| ANVISA | Agência Nacional de Vigilância Sanitária |
| CDC | Centers for Disease Control |
References
- Simo, F.B.N.; Burt, F.J.; Makoah, N.A. Chikungunya Virus Diagnosis: A Review of Current Antigen Detection Methods. Trop. Med. Infect. Dis. 2023, 8, 365. [Google Scholar] [CrossRef]
- Burt, F.J.; Rolph, M.S.; Rulli, N.E.; Mahalingam, S.; Heise, M.T. Chikungunya: A re-emerging virus. Lancet 2012, 379, 662–671. [Google Scholar] [CrossRef]
- da Cunha, R.V.; Trinta, K.S. Chikungunya virus: Clinical aspects and treatment. Mem. Inst. Oswaldo Cruz 2017, 112, 523–531. [Google Scholar] [CrossRef]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.d.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Khongwichit, S.; Chansaenroj, J.; Chirathaworn, C.; Poovorawan, Y. Chikungunya virus infection: Molecular biology, clinical characteristics, and epidemiology in Asian countries. J. Biomed. Sci. 2021, 28, 84. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.C.; Streblow, D.N.; Coffey, L.L. Chikungunya Virus Vaccines: A Review of IXCHIQ and PXVX0317 from Pre-Clinical Evaluation to Licensure. BioDrugs 2024, 38, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Reappearance of chikungunya, formerly called Dengue, in the Americas. Emerg. Infect. Dis. 2015, 21, 557–561. [Google Scholar] [CrossRef]
- Weaver, S.C.; Lecuit, M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef]
- Robinson, M.C. An epidemic of virus disease in southern province, tanganyika territory, in 1952–1953. I. clinical features. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 28–32. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Rahman, M.S.; Alom, J.; Hasan, M.S.; Johir, M.A.H.; Mondal, M.I.H.; Lee, D.Y.; Park, J.; Zhou, J.L.; Yoon, M.H. Microplastic particles in the aquatic environment: A systematic review. Sci. Total Environ. 2021, 775, 145793. [Google Scholar] [CrossRef]
- Freppel, W.; Silva, L.A.; Stapleford, K.A.; Herrero, L.J. Pathogenicity and virulence of chikungunya virus. Virulence 2024, 15, 2396484. [Google Scholar] [CrossRef]
- Hammon, W.M.D.; Rudnick, A.; Sather, G.E. Viruses associated with epidemic hemorrhage fevers of the Philippines and Thailand. Science 1960, 131, 1102–1103. [Google Scholar] [CrossRef]
- Wimalasiri-Yapa, B.M.C.R.; Stassen, L.; Huang, X.; Hafner, L.M.; Hu, W.; Devine, G.J.; Yakob, L.; Jansen, C.C.; Faddy, H.M.; Viennet, E.; et al. Chikungunya virus in Asia–Pacific: A systematic review. Emerg. Microbes Infect. 2019, 8, 70–79. [Google Scholar] [CrossRef]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its history in Africa and Asia and its spread to new regions in 2013–2014. J. Infect. Dis. 2016, 214, S436–S440. [Google Scholar] [CrossRef]
- Duong, V.; Andries, A.C.; Ngan, C.; Sok, T.; Richner, B.; Asgari-Jirhandeh, N.; Bjorge, S.; Huy, R.; Ly, S.; Laurent, D.; et al. Reemergence of chikungunya virus in Cambodia. Emerg. Infect. Dis. 2012, 18, 2066–2069. [Google Scholar] [CrossRef]
- Cecilia, D. Current status of dengue and chikungunya in India. WHO South-East Asia J. Public Health 2014, 3, 22. [Google Scholar] [CrossRef]
- Deller, J.J.; Russell, P.K. An analysis of fevers of unknown origin in American soldiers in Vietnam. Ann. Intern. Med. 1967, 66, 1129–1143. [Google Scholar] [CrossRef]
- Ming, C.K.; Thain, S.; Thaung, U.; Myint, K.S.; Swe, T.; Halstead, S.B.; Diwan, A.R. Clinical and laboratory studies on haemorrhagic fever in Burma, 1970-72. Bull. World Health Organ. 1974, 51, 227. [Google Scholar]
- Chikungunya Fever Among U.S. Peace Corps Volunteers—Republic of the Philippines. Morbidity and Mortality Weekly Report. 1986, 36, 794–795. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00000794.htm (accessed on 12 October 2025).
- Porter, K.R.; Tan, R.; Istary, Y.; Suharyono, W.; Sutaryo; Widjaja, S.; Ma’Roef, C.; Listiyaningsih, E.; Kosasih, H.; Hueston, L.; et al. A serological study of Chikungunya virus transmission in Yogyakarta, Indonesia: Evidence for the first outbreak since 1982. Southeast Asian J. Trop Med. Public Health 2004, 35, 408–415. [Google Scholar] [PubMed]
- Nimmannitya, S.; Halstead, S.B.; Cohen, S.N.; Margiotta, M.R. Dengue and chikungunya virus infection in man in Thailand, 1962-64. I. Observations on hospitalized patients with hemorrhagic fever. Am. J. Trop. Med. Hyg. 1969, 18, 954–971. [Google Scholar] [CrossRef]
- Yergolkar, P.N.; Tandale, B.V.; Arankalle, V.A.; Sathe, P.S.; Sudeep, A.B.; Gandhe, S.S.; Gokhle, M.D.; Jacob, G.P.; Hundekar, S.L.; Mishra, A.C. Chikungunya outbreaks caused by African genotype, India. Emerg. Infect. Dis. 2006, 12, 1580–1583. [Google Scholar] [CrossRef]
- Laras, K.; Sukri, N.C.; Larasati, R.P.; Bangs, M.J.; Kosim, R.; Djauzi, A.; Wandra, T.; Master, J.; Kosasih, H.; Hartati, S.; et al. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 128–141. [Google Scholar] [CrossRef]
- Bettis, A.A.; L’Azou Jackson, M.; Yoon, I.K.; Breugelmans, J.G.; Goios, A.; Gubler, D.J.; Powers, A.M. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Negl. Trop. Dis. 2022, 16, e0010069. [Google Scholar] [CrossRef]
- Erin Staples, J.; Breiman, R.F.; Powers, A.M. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Balleydier, E.; D’Ortenzio, E.; Bâville, M.; Filleul, L. Epidemiology of chikungunya infection on Reunion Island, Mayotte, and neighboring countries. Med. Mal. Infect. 2012, 42, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Borgherini, G.; Poubeau, P.; Staikowsky, F.; Lory, M.; Le Moullec, N.; Becquart, J.P.; Wengling, C.; Michault, A.; Paganin, F. Outbreak of chikungunya on Reunion Island: Early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis. 2007, 44, 1401–1407. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Shrivastava, S.; Cherian, S.; Gunjikar, R.S.; Walimbe, A.M.; Jadhav, S.M.; Sudeep, A.B.; Mishra, A.C. Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J. Gen. Virol. 2007, 88, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Chikungunya Virus Disease Worldwide Overview n.d. Available online: https://www.ecdc.europa.eu/en/chikungunya-monthly (accessed on 22 July 2025).
- Rezza, G. Chikungunya is back in Italy: 2007–2017. J. Travel Med. 2018, 25, tay004. [Google Scholar] [CrossRef]
- Dupont-Rouzeyrol, M.; Caro, V.; Guillaumot, L.; Vazeille, M.; D’Ortenzio, E.; Thiberge, J.M.; Baroux, N.; Gourinat, A.C.; Grandadam, M.; Failloux, A.B. Chikungunya virus and the mosquito vector aedes aegypti in new Caledonia (south pacific region). Vector-Borne Zoonotic Dis. 2012, 12, 1036–1041. [Google Scholar] [CrossRef]
- Horwood, P.F.; Reimer, L.J.; Dagina, R.; Susapu, M.; Bande, G.; Katusele, M.; Koimbu, G.; Jimmy, S.; Ropa, B.; Siba, P.M.; et al. Outbreak of Chikungunya Virus Infection, Vanimo, Papua New Guinea. Emerg. Infect. Dis. 2013, 19, 1535. [Google Scholar] [CrossRef] [PubMed]
- Viennet, E.; Knope, K.; Faddy, H.M.; Williams, C.R.; Harley, D. Assessing the threat of chikungunya virus emergence in Australia. Commun. Dis. Intell. Q Rep. 2013, 37, E136–E143. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; Moore, P.R.; McMahon, J. New insights into chikungunya virus emergence and spread from Southeast Asia. Emerg. Microbes Infect. 2018, 7, 1–3. [Google Scholar] [CrossRef]
- de Souza, W.M.; Ribeiro, G.S.; de Lima, S.T.S.; de Jesus, R.; Moreira, F.R.R.; Whittaker, C.; Sallum, M.A.M.; Carrington, C.V.F.; Sabino, E.C.; Kitron, U.; et al. Chikungunya: A decade of burden in the Americas. Lancet Reg. Health—Am. 2024, 30, 100673. [Google Scholar] [CrossRef] [PubMed]
- Cassadou, S.; Boucau, S.; Petit-Sinturel, M.; Huc, P.; Leparc-Goffart, I.; Ledrans, M. Emergence of chikungunya fever on the French side of Saint Martin island, October to December 2013. Eurosurveillance 2014, 19, 20752. [Google Scholar] [CrossRef]
- Leparc-Goffart, I.; Nougairede, A.; Cassadou, S.; Prat, C.; De Lamballerie, X. Chikungunya in the Americas. Lancet 2014, 383, 514. [Google Scholar] [CrossRef]
- Fischer, M.; Staples, J.E. Notes from the field: Chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. MMWR Morb. Mortal Wkly Rep. 2014, 63, 500–501. [Google Scholar]
- Nunes, M.R.T.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.G.; de Oliveira, L.F.; Azevedo Rdo, S.; da Silva, D.E.; da Silva, E.V.; da Silva, S.P.; et al. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef]
- Naveca, F.G.; Claro, I.; Giovanetti, M.; de Jesus, J.G.; Xavier, J.; Iani, F.C.M.; do Nascimento, V.A.; de Souza, V.C.; Silveira, P.P.; Lourenço, J.; et al. Genomic, epidemiological and digital surveillance of Chikungunya virus in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2018, 13, e0007065. [Google Scholar] [CrossRef]
- Costa-da-Silva, A.L.; Ioshino, R.S.; Petersen, V.; Lima, A.F.; Cunha Mdos, P.; Wiley, M.R.; Ladner, J.T.; Prieto, K.; Palacios, G.; Costa, D.D.; et al. First report of naturally infected Aedes aegypti with chikungunya virus genotype ECSA in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005630. [Google Scholar] [CrossRef]
- Burgueño, A.; Giovanetti, M.; Fonseca, V.; Morel, N.; Lima, M.; Castro, E.; Guimarães, N.R.; Iani, F.C.M.; Bormida, V.; Cortinas, M.N.; et al. Genomic and eco-epidemiological investigations in Uruguay reveal local Chikungunya virus transmission dynamics during its expansion across the Americas in 2023. MedRxiv 2023, 13, 2332672. [Google Scholar] [CrossRef]
- Seasonal Surveillance of Chikungunya Virus Disease in the EU/EEA 2025. Available online: https://chik-weekly.ecdc.europa.eu/ (accessed on 22 July 2025).
- Selhorst, P.; Makiala-Mandanda, S.; De Smet, B.; Mariën, J.; Anthony, C.; Binene-Mbuka, G.; de Weggheleire, A.; Ilombe, G.; Kinganda-Lusamaki, E.; Pukuta-Simbu, E.; et al. Molecular characterization of chikungunya virus during the 2019 outbreak in the Democratic Republic of the Congo. Emerg. Microbes Infect. 2020, 9, 1912–1918. [Google Scholar] [CrossRef]
- Powers, A.M.; Brault, A.C.; Tesh, R.B.; Weaver, S.C. Re-emergence of chikungunya and o’nyong-nyong viruses: Evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000, 81, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.S.; Fontenille, D.; Schuffenecker, I.; et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef]
- Weaver, S.C.; Forrester, N.L. Chikungunya: Evolutionary history and recent epidemic spread. Antivir. Res. 2015, 120, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Kassa, F.K.; Caron, M.; Nkoghé, D.; Leroy, E.M. A chikungunya outbreak associated with the vector aedes albopictus in remote villages of gabon. Vector-Borne Zoonotic Dis. 2012, 12, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Peyrefitte, C.N.; Bessaud, M.; Pastorino, B.A.M.; Gravier, P.; Plumet, S.; Merle, O.L.; Moltini, I.; Coppin, E.; Tock, F.; Daries, W.; et al. Circulation of Chikungunya virus in Gabon, 2006–2007. J. Med. Virol. 2008, 80, 430–433. [Google Scholar] [CrossRef]
- Peyrefitte, C.N.; Rousset, D.; Pastorino, B.A.M.; Pouillot, R.; Bessaud, M.; Tock, F.; Mansaray, H.; Merle, O.L.; Pascual, A.M.; Paupy, C.; et al. Chikungunya virus, Cameroon, 2006. Emerg. Infect. Dis. 2007, 13, 768–771. [Google Scholar] [CrossRef]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Wangchuk, S.; Chinnawirotpisan, P.; Dorji, T.; Tobgay, T.; Dorji, T.; Yoon, I.K.; Fernandez, S. Chikungunya fever outbreak, Bhutan, 2012. Emerg. Infect. Dis. 2013, 19, 1681–1684. [Google Scholar] [CrossRef]
- Wu, D.; Wu, J.; Zhang, Q.; Zhong, H.; Ke, C.; Deng, X.; Guan, D.; Li, H.; Zhang, Y.; Zhou, H.; et al. Chikungunya outbreak in Guangdong province, China, 2010. Emerg. Infect. Dis. 2012, 18, 493–495. [Google Scholar] [CrossRef]
- Ng, L.C.; Tan, L.K.; Tan, C.H.; Tan, S.S.Y.; Hapuarachchi, H.C.; Pok, K.Y.; Lai, Y.L.; Lam-Phua, S.G.; Bucht, G.; Lin, R.T.; et al. Entomologic and virologic investigation of chikungunya, Singapore. Emerg. Infect. Dis. 2009, 15, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Sam, I.C.; Chan, Y.F.; Chan, S.Y.; Loong, S.K.; Chin, H.K.; Hooi, P.S.; Ganeswrie, R.; Abubakar, S. Chikungunya virus of Asian and Central/East African genotypes in Malaysia. J. Clin. Virol. 2009, 46, 180–183. [Google Scholar] [CrossRef]
- Sreekumar, E.; Issac, A.; Nair, S.; Hariharan, R.; Janki, M.B.; Arathy, D.S.; Regu, R.; Mathew, T.; Anoop, M.; Niyas, K.P.; et al. Genetic characterization of 2006–2008 isolates of Chikungunya virus from Kerala, South India, by whole genome sequence analysis. Virus Genes 2010, 40, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Niyas, K.P.; Abraham, R.; Unnikrishnan, R.N.; Mathew, T.; Nair, S.; Manakkadan, A.; Issac, A.; Sreekumar, E. Molecular characterization of Chikungunya virus isolates from clinical samples and adult Aedes albopictus mosquitoes emerged from larvae from Kerala, South India. Virol. J. 2010, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, H.C.; Bandara, K.B.A.T.; Sumanadasa, S.D.M.; Hapugoda, M.D.; Lai, Y.L.; Lee, K.S.; Tan, L.K.; Lin, R.T.; Ng, L.F.; Bucht, G.; et al. Re-emergence of Chikungunya virus in South-east Asia: Virological evidence from Sri Lanka and Singapore. J. Gen. Virol. 2010, 91, 1067–1076. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Chen, R.; Yun, R.; Rossi, S.L.; Plante, K.S.; Guerbois, M.; Forrester, N.; Perng, G.C.; Sreekumar, E.; Leal, G.; et al. Multi-peaked adaptive landscape for chikungunya virus evolution predicts continued fitness optimization in Aedes albopictus mosquitoes. Nat. Commun. 2014, 5, 4084. [Google Scholar] [CrossRef]
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Chen, R.; Leal, G.; Forrester, N.; Higgs, S.; Huang, J.; Weaver, S.C. Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc. Natl. Acad. Sci. USA 2011, 108, 7872–7877. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Weaver, S.C. Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence. PLoS Pathog. 2011, 7, e1002412. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; McGee, C.E.; Volk, S.M.; Vanlandingham, D.L.; Weaver, S.C.; Higgs, S. Epistatic roles of E2 glycoprotein mutations in adaption of Chikungunya virus to Aedes albopictus and Ae. Aegypti mosquitoes. PLoS ONE 2009, 4, e6835. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; Costa, P.A.G.; Correa, I.A.; de Souza, M.R.M.; Calil, P.T.; da Silva, G.P.D.; Costa, S.M.; Fonseca, V.W.P.; da Costa, L.J. Chikungunya Virus: An Emergent Arbovirus to the South American Continent and a Continuous Threat to the World. Front. Microbiol. 2020, 11, 1297. [Google Scholar] [CrossRef] [PubMed]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.C.; Lavenir, R.; Pardigon, N.; Reynes, J.M.; Pettinelli, F.; et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006, 3, 1058–1070. [Google Scholar] [CrossRef]
- Reed, W.; Carroll, J. The Prevention of Yellow Fever. Public Health Pap. Rep. 1901, 27, 113. [Google Scholar]
- Shragai, T.; Tesla, B.; Murdock, C.; Harrington, L.C. Zika and chikungunya: Mosquito-borne viruses in a changing world. Ann. N. Y. Acad. Sci. 2017, 1399, 61–77. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Espinal, M.A.; Andrus, J.K.; Jauregui, B.; Waterman, S.H.; Morens, D.M.; Santos, J.I.; Horstick, O.; Francis, L.A.; Olson, D. Emerging and reemerging aedes-transmitted arbovirus infections in the region of the americas: Implications for health policy. Am. J. Public Health 2019, 109, 387–392. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends Parasitol. 2013, 29, 460–468. [Google Scholar] [CrossRef]
- Câmara, D.C.P.; Codeço, C.T.; Ayllón, T.; Nobre, A.A.; Azevedo, R.C.; Ferreira, D.F.; da Silva Pinel, C.; Rocha, G.P.; Honório, N.A. Entomological Surveillance of Aedes Mosquitoes: Comparison of Different Collection Methods in an Endemic Area in RIO de Janeiro, Brazil. Trop. Med. Infect. Dis. 2022, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Câmara, D.C.P.; Pinel Cda, S.; Rocha, G.P.; Codeço, C.T.; Honório, N.A. Diversity of mosquito (Diptera: Culicidae) vectors in a heterogeneous landscape endemic for arboviruses. Acta Trop. 2020, 212, 105715. [Google Scholar] [CrossRef] [PubMed]
- Honório, N.A.; Codeço, C.T.; Alves, F.C.; Magalhes, M.A.F.M.; Lourenço-De-Oliveira, R. Temporal distribution of aedes aegypti in different districts of Rio De Janeiro, Brazil, measured by two types of traps. J. Med. Entomol. 2009, 46, 1001–1014. [Google Scholar] [CrossRef]
- Braks, M.A.H.; Honório, N.A.; Lourenço-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent Habitat Segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honório, N.A.; Lima-Camara, T.N. The Asian tiger mosquito in Brazil: Observations on biology and ecological interactions since its first detection in 1986. Acta Trop. 2020, 205, 105386. [Google Scholar] [CrossRef] [PubMed]
- Tatem, A.J.; Hay, S.I.; Rogers, D.J. Global traffic and disease vector dispersal. Proc. Natl. Acad. Sci. USA 2006, 103, 6242–6247. [Google Scholar] [CrossRef]
- Van Den Hurk, A.F.; Hall-Mendelin, S.; Townsend, M.; Kurucz, N.; Edwards, J.; Ehlers, G.; Rodwell, C.; Moore, F.A.; McMahon, J.L.; Northill, J.A.; et al. Applications of a sugar-based surveillance system to track arboviruses in wild mosquito populations. Vector-Borne Zoonotic Dis. 2014, 14, 66–73. [Google Scholar] [CrossRef]
- Honório, N.A.; Wiggins, K.; Eastmond, B.; Câmara, D.C.P.; Alto, B.W. Experimental vertical transmission of chikungunya virus by brazilian and florida aedes albopictus populations. Viruses 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Chompoosri, J.; Thavara, U.; Tawatsin, A.; Boonserm, R.; Phumee, A.; Sangkitporn, S.; Siriyasatien, P. Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes. Parasit Vectors 2016, 9, 227. [Google Scholar] [CrossRef]
- Delatte, H.; Dehecq, J.S.; Thiria, J.; Domerg, C.; Paupy, C.; Fontenille, D. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector-Borne Zoonotic Dis. 2008, 8, 25–34. [Google Scholar] [CrossRef]
- Jain, J.; Kushwah, R.B.S.; Singh, S.S.; Sharma, A.; Adak, T.; Singh, O.P.; Bhatnagar, R.K.; Subbarao, S.K.; Sunil, S. Evidence for natural vertical transmission of chikungunya viruses in field populations of Aedes aegypti in Delhi and Haryana states in India—A preliminary report. Acta Trop. 2016, 162, 46–55. [Google Scholar] [CrossRef]
- Scott, T.W.; Takken, W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012, 28, 114–121. [Google Scholar] [CrossRef]
- Honório, N.A.; Nogueira, R.M.R.; Codeço, C.T.; Carvalho, M.S.; Cruz, O.G.; Magalhães, M.d.A.; de Araújo, J.M.; de Araújo, E.S.; Gomes, M.Q.; Pinheiro, L.S.; et al. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2009, 3, e545. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A.; Philip Lounibos, L. Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecol. Lett. 2005, 8, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Alto, B.W.; Juliano, S.A. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J. Med. Entomol. 2001, 38, 548–556. [Google Scholar] [CrossRef]
- Hawley, W.A.; Pumpuni, C.B.; Brady, R.H.; Craig, G.B. Overwintering Survival of Aedes albopictus (Diptera: Culicidae) Eggs in Indiana. J. Med. Entomol. 1989, 26, 122–129. [Google Scholar] [CrossRef]
- Reinhold, J.M.; Lazzari, C.R.; Lahondère, C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: A review. Insects 2018, 9, 158. [Google Scholar] [CrossRef]
- Pialoux, G.; Gaüzère, B.A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 2007, 7, 319–327. [Google Scholar] [CrossRef]
- Weaver, S.C.; Chen, R.; Diallo, M. Chikungunya virus: Role of vectors in emergence from enzootic cycles. Annu. Rev. Entomol. 2020, 65, 313–332. [Google Scholar] [CrossRef]
- Mercier, A.; Obadia, T.; Carraretto, D.; Velo, E.; Gabiane, G.; Bino, S.; Vazeille, M.; Gasperi, G.; Dauga, C.; Malacrida, A.R.; et al. Impact of temperature on dengue and chikungunya transmission by the mosquito Aedes albopictus. Sci. Rep. 2022, 12, 6973. [Google Scholar] [CrossRef]
- Zouache, K.; Fontaine, A.; Vega-Rua, A.; Mousson, L.; Thiberge, J.M.; Lourenco-De-Oliveira, R.; Caro, V.; Lambrechts, L.; Failloux, A.B. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141078. [Google Scholar] [CrossRef]
- Lazzarini, L.; Barzon, L.; Foglia, F.; Manfrin, V.; Pacenti, M.; Pavan, G.; Rassu, M.; Capelli, G.; Montarsi, F.; Martini, S.; et al. First autochthonous dengue outbreak in Italy, August 2020. Eurosurveillance 2020, 25, 2001606. [Google Scholar] [CrossRef]
- Cattaneo, P.; Salvador, E.; Manica, M.; Barzon, L.; Castilletti, C.; Di Gennaro, F.; Huits, R.; Merler, S.; Poletti, P.; Riccardo, F.; et al. Transmission of autochthonous Aedes-borne arboviruses and related public health challenges in Europe 2007–2023: A systematic review and secondary analysis. Lancet Reg. Health—Eur. 2025, 51, 101231. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The first local cases of Zika virus in Europe. Lancet 2019, 394, 1991–1992. [Google Scholar] [CrossRef] [PubMed]
- Buresova, V.; Hajdusek, O.; Franta, Z.; Loosova, G.; Grunclova, L.; Levashina, E.A.; Kopacek, P. Functional genomics of tick thioester-containing proteins reveal the ancient origin of the complement system. J. Innate Immun. 2011, 3, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Levashina, E.A.; Moita, L.F.; Blandin, S.; Vriend, G.; Lagueux, M.; Kafatos, F.C. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 2001, 104, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Saig, E.; Turley, A.P.; Ribeiro, J.M.C.; O’Neill, S.L.; McGraw, E.A. Human Probing Behavior of Aedes aegypti when Infected with a Life-Shortening Strain of Wolbachia. PLoS Negl. Trop. Dis. 2009, 3, e568. [Google Scholar] [CrossRef]
- Diallo, M.; Thonnon, J.; Traore-Lamizana, M.; Fontenille, D. Vectors of Chikungunya virus in Senegal: Current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999, 60, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Weinbren, M.P.; Haddow, A.J.; Williams, M.C. The occurrence of chikungunya virus in Uganda I. Isolation from mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1958, 52, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Paterson, H.E.; McIntosh, B.M. Further studies on the chikungunya outbreak in southern rhodesia in 1962. Ann. Trop. Med. Parasitol. 1964, 58, 52–55. [Google Scholar] [CrossRef]
- Moulay, D.; Pigné, Y. A metapopulation model for chikungunya including populations mobility on a large-scale network. J. Theor. Biol. 2013, 318, 129–139. [Google Scholar] [CrossRef]
- Rees, E.E.; Petukhova, T.; Mascarenhas, M.; Pelcat, Y.; Ogden, N.H. Environmental and social determinants of population vulnerability to Zika virus emergence at the local scale. Parasites Vectors 2018, 11, 290. [Google Scholar] [CrossRef]
- Shocket, M.S.; Anderson, C.B.; Caldwell, J.M.; Childs, M.L.; Couper, L.I.; Han, S.; Harris, M.J.; Howard, M.E.; Kai, M.P.; MacDonald, A.J.; et al. Environmental Drivers of Vector-Borne Diseases. In Population Biology of Vector-Borne Diseases; Oxford University Press: Oxford, UK, 2020; pp. 85–118. [Google Scholar] [CrossRef]
- Fischer, D.; Thomas, S.M.; Suk, J.E.; Sudre, B.; Hess, A.; Tjaden, N.B.; Beierkuhnlein, C.; Semenza, J.C. Climate change effects on chikungunya transmission in europe: Geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int. J. Health Geogr. 2013, 12, 51. [Google Scholar] [CrossRef]
- Puiprom, O.; Morales Vargas, R.E.; Potiwat, R.; Chaichana, P.; Ikuta, K.; Ramasoota, P.; Okabayashi, T. Characterization of chikungunya virus infection of a human keratinocyte cell line: Role of mosquito salivary gland protein in suppressing the host immune response. Infect. Genet. Evol. 2013, 17, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.-W.; Kini, R.M.; Ng, L.F.P. Mosquito Saliva Reshapes Alphavirus Infection and Immunopathogenesis. J. Virol. 2018, 92, e01004-17. [Google Scholar] [CrossRef]
- Wichit, S.; Diop, F.; Hamel, R.; Talignani, L.; Ferraris, P.; Cornelie, S.; Liegeois, F.; Thomas, F.; Yssel, H.; Missé, D. Aedes Aegypti saliva enhances chikungunya virus replication in human skin fibroblasts via inhibition of the type I interferon signaling pathway. Infect. Genet. Evol. 2017, 55, 68–70. [Google Scholar] [CrossRef]
- Agarwal, A.; Joshi, G.; Nagar, D.P.; Sharma, A.K.; Sukumaran, D.; Pant, S.C.; Parida, M.M.; Dash, P.K. Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression. Infect. Genet. Evol. 2016, 40, 126–135. [Google Scholar] [CrossRef]
- Brulefert, A.; Kraemer, M.; Cumin, M.; Selle, A.; Hoste, A.; Gad, H.H.; Rühl, J.; Madinier, J.B.; Chaloin, O.; Münz, C.; et al. Chikungunya Virus Envelope Protein E2 Provides a Vector for Targeted Antigen Delivery to Human Dermal CD14+ Dendritic Cells. J. Investig. Dermatol. 2021, 141, 2985–2989.e5. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Le Roux, K.; Prevost, M.C.; Fsihi, H.; et al. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007, 3, e89. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Wang, M.; Yang, F.; Wu, C.; Huang, D.; Xiong, L.; Wan, C.; Cheng, J.; Zhang, R. Differences in genome characters and cell tropisms between two chikungunya isolates of Asian lineage and Indian Ocean lineage. Virol. J. 2018, 15, 130. [Google Scholar] [CrossRef]
- Gasque, P.; Jaffar-Bandjee, M.C. The immunology and inflammatory responses of human melanocytes in infectious diseases. J. Infect. 2015, 71, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A mouse model for Chikungunya: Young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef] [PubMed]
- Ekchariyawat, P.; Hamel, R.; Bernard, E.; Wichit, S.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; Choumet, V.; Yssel, H.; Desprès, P.; et al. Inflammasome signaling pathways exert antiviral effect against Chikungunya virus in human dermal fibroblasts. Infect. Genet. Evol. 2015, 32, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Locke, M.C.; Cook, L.E.; Hiller, B.E.; Zhang, R.; Hedberg, M.L.; Monte, K.J.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Dermal and muscle fibroblasts and skeletal myofibers survive chikungunya virus infection and harbor persistent RNA. PLoS Pathog. 2019, 15, e1007993. [Google Scholar] [CrossRef]
- Kielian, M.; Chanel-Vos, C.; Liao, M. Alphavirus entry and membrane fusion. Viruses 2010, 2, 796–825. [Google Scholar] [CrossRef]
- Briant, L.; Desprès, P.; Choumet, V.; Missé, D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 2014, 464–465, 26–32. [Google Scholar] [CrossRef]
- Her, Z.; Malleret, B.; Chan, M.; Ong, E.K.S.; Wong, S.-C.; Kwek, D.J.C.; Tolou, H.; Lin, R.T.; Tambyah, P.A.; Rénia, L.; et al. Active Infection of Human Blood Monocytes by Chikungunya Virus Triggers an Innate Immune Response. J. Immunol. 2010, 184, 5903–5913. [Google Scholar] [CrossRef]
- Ruiz Silva, M.; Van Der Ende-Metselaar, H.; Mulder, H.L.; Smit, J.M.; Rodenhuis-Zybert, I.A. Mechanism and role of MCP-1 upregulation upon chikungunya virus infection in human peripheral blood mononuclear cells. Sci. Rep. 2016, 6, srep32288. [Google Scholar] [CrossRef]
- Broeckel, R.; Haese, N.; Messaoudi, I.; Streblow, D.N. Nonhuman primate models of chikungunya virus infection and disease (CHIKV NHP model). Pathogens 2015, 4, 662–681. [Google Scholar] [CrossRef]
- Lentscher, A.J.; McCarthy, M.K.; May, N.A.; Davenport, B.J.; Montgomery, S.A.; Raghunathan, K.; McAllister, N.; Silva, L.A.; Morrison, T.E.; Dermody, T.S. Chikungunya virus replication in skeletal muscle cells is required for disease development. J. Clin. Investig. 2020, 130, 1466–1478. [Google Scholar] [CrossRef]
- Morrison, T.E.; Oko, L.; Montgomery, S.A.; Whitmore, A.C.; Lotstein, A.R.; Gunn, B.M.; Elmore, S.A.; Heise, M.T. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: Evidence of arthritis, tenosynovitis, myositis, and persistence. Am. J. Pathol. 2011, 178, 32–40. [Google Scholar] [CrossRef]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya Virus Arthritis in Adult Wild-Type Mice. J. Virol. 2010, 84, 8021–8032. [Google Scholar] [CrossRef]
- Gérardin, P.; Barau, G.; Michault, A.; Bintner, M.; Randrianaivo, H.; Choker, G.; Lenglet, Y.; Touret, Y.; Bouveret, A.; Grivard, P.; et al. Multidisciplinary Prospective Study of Mother-to-Child Chikungunya Virus Infections on the Island of La Réunion. PLoS Med. 2008, 5, e60. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Carbonnier, M.; Pasquet, M.; Bouhmani, B.; Ghazouani, J.; Noormahomed, T.; Beullier, G.; Attali, T.; Samperiz, S.; Fourmaintraux, A.; et al. Mother-to-child transmission of chikungunya virus infection. Pediatr. Infect. Dis. J. 2007, 26, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Gérardin, P.; Couderc, T.; Bintner, M.; Tournebize, P.; Renouil, M.; Lémant, J.; Boisson, V.; Borgherini, G.; Staikowsky, F.; Schramm, F.; et al. Chikungunya virus–associated encephalitis. Neurology 2016, 86, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Salomão, N.; Brendolin, M.; Rabelo, K.; Wakimoto, M.; de Filippis, A.M.; Dos Santos, F.; Moreira, M.E.; Basílio-de-Oliveira, C.A.; Avvad-Portari, E.; Paes, M.; et al. Spontaneous Abortion and Chikungunya Infection: Pathological Findings. Viruses 2021, 13, 554. [Google Scholar] [CrossRef]
- Salomão, N.; Araújo, L.; Rabelo, K.; Avvad-Portari, E.; de Souza, L.; Fernandes, R.; Valle, N.; Ferreira, L.; Basílio-de-Oliveira, C.; Basílio-de-Oliveira, R.; et al. Placental Alterations in a Chikungunya-Virus-Infected Pregnant Woman: A Case Report. Microorganisms 2022, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Salomão, N.; Rabelo, K.; Avvad-Portari, E.; Basílio-de-Oliveira, C.; Basílio-de-Oliveira, R.; Ferreira, F.; Ferreira, L.; de Souza, T.M.; Nunes, P.; Lima, M.; et al. Histopathological and immunological characteristics of placentas infected with chikungunya virus. Front. Microbiol. 2022, 13, 1055536. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.I.; Clark, D.C.; Pesavento, P.; Lerche, N.W.; Luciw, P.A.; Reisen, W.K.; Brault, A.C. Comparative pathogenesis of epidemic and enzootic Chikungunya viruses in a pregnant Rhesus macaque model. Am. J. Trop. Med. Hyg. 2010, 83, 1249–1258. [Google Scholar] [CrossRef]
- Ferreira, F.C.P.A.D.M.; Filippis, A.M.B.; Moreira, M.E.L.; de Campos, S.B.; Fuller, T.; Lopes, F.C.R.; Brasil, P. Perinatal and Neonatal Chikungunya Virus Transmission: A Case Series. J. Pediatric Infect. Dis. Soc. 2024, 13, 576–584. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, N. Short-term pregnancy outcomes in patients chikungunya infection: An observational study. J. Family Med. Prim. Care 2019, 8, 985. [Google Scholar] [CrossRef]
- Escobar Vidarte, M.F.; Nieto Calvache, A.J.; Del Pilar Loaiza Osorio, S.; Barona Wiedmann, J.S.; Rosso Suarez, F. Pregnant Women Hospitalized with Chikungunya Virus Infection, Colombia, 2015. Emerg. Infect. Dis. 2017, 23, 1777–1783. [Google Scholar] [CrossRef]
- Bandeira, A.C.; Campos, G.S.; Sardi, S.I.; Rocha, V.F.D.; Rocha, G.C.M. Neonatal encephalitis due to Chikungunya vertical transmission: First report in Brazil. IDCases 2016, 5, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Gérardin, P.; Sampériz, S.; Ramful, D.; Boumahni, B.; Bintner, M.; Alessandri, J.L.; Carbonnier, M.; Tiran-Rajaoefera, I.; Beullier, G.; Boya, I.; et al. Neurocognitive Outcome of Children Exposed to Perinatal Mother-to-Child Chikungunya Virus Infection: The CHIMERE Cohort Study on Reunion Island. PLoS Negl. Trop. Dis. 2014, 8, e2996. [Google Scholar] [CrossRef]
- Ramos, R.; Viana, R.; Brainer-Lima, A.; Floreâncio, T.; Carvalho, M.D.; Van Der Linden, V.; Amorim, A.; Rocha, M.A.; Medeiros, F. Perinatal Chikungunya Virus-associated Encephalitis Leading to Postnatal-Onset Microcephaly and Optic Atrophy. Pediatr. Infect. Dis. J. 2018, 37, 94–95. [Google Scholar] [CrossRef]
- Meena, S.S.; Arya, S.; Meena, D.; Matlani, M.; Salhan, M. Neonatal Chikungunya: A case series. Trop. Dr. 2021, 51, 103–105. [Google Scholar] [CrossRef]
- Lenglet, Y.; Barau, G.; Robillard, P.Y.; Randrianaivo, H.; Michault, A.; Bouveret, A.; Gérardin, P.; Boumahni, B.; Touret, Y.; Kauffmann, E.; et al. Infection à Chikungunya chez la femme enceinte et risque de transmission materno-fœtale: Étude dans un contexte d’épidémie en 2005–2006 à l’île de la Réunion. J. Gynecol. Obstet. Biol. Reprod. 2006, 35, 578–583. [Google Scholar] [CrossRef]
- Robillard, P.Y.; Boumahni, B.; Gérardin, P.; Michault, A.; Fourmaintraux, A.; Schuffenecker, I.; Carbonnier, M.; Djémili, S.; Choker, G.; Roge-Wolter, M.; et al. Transmission verticale materno-fœtale du virus chikungunya: Dix cas observés sur l’île de la Réunion chez 84 femmes enceintes. Presse Med. 2006, 35, 785–788. [Google Scholar] [CrossRef]
- Van Enter, B.J.D.; Huibers, M.H.W.; Van Rooij, L.; Steingrover, R.; Van Hensbroek, M.B.; Voigt, R.R.; Hol, J. Perinatal Outcomes in Vertically Infected Neonates During a Chikungunya Outbreak on the Island of Curaçao. Am. J. Trop. Med. Hyg. 2018, 99, 1415–1418. [Google Scholar] [CrossRef]
- Chazal, N.; Briant, L. Chikungunya Virus Entry and Replication. In Chikungunya Virus: Advances in Biology, Pathogenesis, and Treatment; Springer International Publishing: Cham, Switzerland, 2016; pp. 127–148. [Google Scholar] [CrossRef]
- Silva, G.S.; Richards, G.A.; Baker, T.; Amin, P.R. Encephalitis and myelitis in tropical countries: Report from the Task Force on Tropical Diseases by the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care 2017, 42, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Simizu, B.; Yamamoto, K.; Hashimoto, K.; Ogata, T. Structural proteins of Chikungunya virus. J. Virol. 1984, 51, 254–258. [Google Scholar] [CrossRef]
- Chmielewski, D.; Schmid, M.F.; Simmons, G.; Jin, J.; Chiu, W. Chikungunya virus assembly and budding visualized in situ using cryogenic electron tomography. Nat. Microbiol. 2022, 7, 1270–1279. [Google Scholar] [CrossRef]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. [Google Scholar] [CrossRef]
- Kril, V.; Aiqui-Reboul-Paviet, O.; Briant, L.; Amara, A. New Insights into Chikungunya Virus Infection and Pathogenesis. Annu. Rev. Virol. 2021, 8, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Vanderplasschen, A.; Law, M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002, 83, 2915–2931. [Google Scholar] [CrossRef]
- Ahola, T.; McInerney, G.; Merits, A. Alphavirus RNA replication in vertebrate cells. Adv. Virus Res. 2021, 111, 111–156. [Google Scholar] [CrossRef] [PubMed]
- Rupp, J.C.; Sokoloski, K.J.; Gebhart, N.N.; Hardy, R.W. Alphavirus RNA synthesis and non-structural protein functions. J. General. Virol. 2015, 96, 2483–2500. [Google Scholar] [CrossRef]
- Jones, R.; Bragagnolo, G.; Arranz, R.; Reguera, J. Capping pores of alphavirus nsP1 gate membranous viral replication factories. Nature 2021, 589, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Lampio, A.; Kilpeläinen, I.; Pesonen, S.; Karhi, K.; Auvinen, P.; Somerharju, P.; Kääriäinen, L. Membrane binding mechanism of an RNA virus-capping enzyme. J. Biol. Chem. 2000, 275, 37853–37859. [Google Scholar] [CrossRef] [PubMed]
- Ahola, T.; Kujala, P.; Tuittila, M.; Blom, T.; Laakkonen, P.; Hinkkanen, A.; Auvinen, P. Effects of Palmitoylation of Replicase Protein nsP1 on Alphavirus Infection. J. Virol. 2000, 74, 6725–6733. [Google Scholar] [CrossRef][Green Version]
- Karpe, Y.A.; Aher, P.P.; Lole, K.S. NTPase and 5′-RNA triphosphatase activities of chikungunya virus nsP2 protein. PLoS ONE 2011, 6, e22336. [Google Scholar] [CrossRef]
- Hardy, W.R.; Strauss, J.H. Processing the nonstructural polyproteins of sindbis virus: Nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and in trans. J. Virol. 1989, 63, 4653. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Hauer, D.; McPherson, R.L.; Utt, A.; Kirby, I.T.; Cohen, M.S.; Merits, A.; Leung, A.K.L.; Griffin, D.E. ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc. Natl. Acad. Sci. USA 2018, 115, E10457–E10466. [Google Scholar] [CrossRef]
- Kim, T.; Abraham, R.; Pieterse, L.; Yeh, J.X.; Griffin, D.E. Cell-Type-Dependent Role for nsP3 Macrodomain ADP-Ribose Binding and Hydrolase Activity during Chikungunya Virus Infection. Viruses 2022, 14, 2744. [Google Scholar] [CrossRef]
- Götte, B.; Liu, L.; McInerney, G.M. The enigmatic alphavirus non-structural protein 3 (nsP3) revealing its secrets at last. Viruses 2018, 10, 105. [Google Scholar] [CrossRef]
- Lello, L.S.; Bartholomeeusen, K.; Wang, S.; Coppens, S.; Fragkoudis, R.; Alphey, L.; Ariën, K.K.; Merits, A.; Utt, A. nsP4 Is a Major Determinant of Alphavirus Replicase Activity and Template Selectivity. J. Virol. 2021, 95, e0035521. [Google Scholar] [CrossRef]
- Laurent, T.; Kumar, P.; Liese, S.; Zare, F.; Jonasson, M.; Carlson, A.; Carlson, L.A. Architecture of the chikungunya virus replication organelle. Elife 2022, 11, e83042. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Snyder, J.E.; Kuhn, R. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef]
- Voss, J.E.; Vaney, M.C.; Duquerroy, S.; Vonrhein, C.; Girard-Blanc, C.; Crublet, E.; Thompson, A.; Bricogne, G.; Rey, F.A. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 2010, 468, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.W.; Gardner, J.; Geertsema, C.; Le, T.T.; Goh, L.; Vlak, J.M.; Suhrbier, A.; Pijlman, G.P. Effective Chikungunya Virus-like Particle Vaccine Produced in Insect Cells. PLoS Negl. Trop. Dis. 2013, 7, e2124. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.L.; Klose, T.; Urakami, A.; Hasan, S.S.; Akahata, W.; Rossmann, M.G. Structural studies of Chikungunya virus maturation. Proc. Natl. Acad. Sci. USA 2017, 114, 13703–13707. [Google Scholar] [CrossRef]
- Mangala Prasad, V.; Blijleven, J.S.; Smit, J.M.; Lee, K.K. Visualization of conformational changes and membrane remodeling leading to genome delivery by viral class-II fusion machinery. Nat. Commun. 2022, 13, 4772. [Google Scholar] [CrossRef]
- Tang, J.; Jose, J.; Chipman, P.; Zhang, W.; Kuhn, R.J.; Baker, T.S. Molecular links between the E2 envelope glycoprotein and nucleocapsid core in Sindbis virus. J. Mol. Biol. 2011, 414, 442–459. [Google Scholar] [CrossRef]
- Melancon, P.; Garoff, H. Processing of the Semliki Forest virus structural polyprotein: Role of the capsid protease. J. Virol. 1987, 61, 1301–1309. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Zhu, D.; Sun, Z.; Ma, J.; Wang, J.; Kong, L.; Wang, S.; Liu, Z.; Wei, L.; et al. Implication for alphavirus host-cell entry and assembly indicated by a 3.5Å resolution cryo-EM structure. Nat. Commun. 2018, 9, 5326. [Google Scholar] [CrossRef]
- Solignat, M.; Gay, B.; Higgs, S.; Briant, L.; Devaux, C. Replication cycle of chikungunya: A re-emerging arbovirus. Virology 2009, 393, 183–197. [Google Scholar] [CrossRef]
- Sun, S.; Xiang, Y.; Akahata, W.; Holdaway, H.; Pal, P.; Zhang, X.; Diamond, M.S.; Nabel, G.J.; Rossmann, M.G. Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization. Elife 2013, 2, e00435. [Google Scholar] [CrossRef]
- Selvarajah, S.; Sexton, N.R.; Kahle, K.M.; Fong, R.H.; Mattia, K.A.; Gardner, J.; Lu, K.; Liss, N.M.; Salvador, B.; Tucker, D.F.; et al. A Neutralizing Monoclonal Antibody Targeting the Acid-Sensitive Region in Chikungunya Virus E2 Protects from Disease. PLoS Negl. Trop. Dis. 2013, 7, e2423. [Google Scholar] [CrossRef]
- Khan, A.H.; Morita, K.; del Carmen Parquet, M.; Hasebe, F.; Mathenge, E.G.M.; Igarashi, A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J. Gen. Virol. 2002, 83, 3075–3084. [Google Scholar] [CrossRef]
- Li, L.; Jose, J.; Xiang, Y.; Kuhn, R.J.; Rossmann, M.G. Structural changes of envelope proteins during alphavirus fusion. Nature 2010, 468, 705–708. [Google Scholar] [CrossRef]
- Zhang, W.; Mukhopadhyay, S.; Pletnev, S.V.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Placement of the Structural Proteins in Sindbis Virus. J. Virol. 2002, 76, 11645–11658. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Helenius, A. Virus entry at a glance. J. Cell Sci. 2013, 126, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Solignat, M.; Gay, B.; Chazal, N.; Higgs, S.; Devaux, C.; Briant, L. Endocytosis of chikungunya virus into mammalian cells: Role of clathrin and early endosomal compartments. PLoS ONE 2010, 5, e11479. [Google Scholar] [CrossRef] [PubMed]
- Basore, K.; Kim, A.S.; Nelson, C.A.; Zhang, R.; Smith, B.K.; Uranga, C.; Vang, L.; Cheng, M.; Gross, M.L.; Smith, J.; et al. Cryo-EM Structure of Chikungunya Virus in Complex with the Mxra8 Receptor. Cell 2019, 177, 1725–1737.e16. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, I.; Simons, K. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc. Natl. Acad. Sci. USA 1988, 85, 8052–8056. [Google Scholar] [CrossRef]
- Zhang, X.; Fugère, M.; Day, R.; Kielian, M. Furin Processing and Proteolytic Activation of Semliki Forest Virus. J. Virol. 2003, 77, 2981–2989. [Google Scholar] [CrossRef]
- Sjöberg, M.; Lindqvist, B.; Garoff, H. Activation of the Alphavirus Spike Protein Is Suppressed by Bound E3. J. Virol. 2011, 85, 5644–5650. [Google Scholar] [CrossRef]
- Uchime, O.; Fields, W.; Kielian, M. The Role of E3 in pH Protection during Alphavirus Assembly and Exit. J. Virol. 2013, 87, 10255–10262. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Deng, C.L.; Li, J.Q.; Li, N.; Zhang, Q.Y.; Ye, H.Q.; Yuan, Z.M.; Zhang, B. Infectious Chikungunya Virus (CHIKV) with a Complete Capsid Deletion: A New Approach for a CHIKV Vaccine. J. Virol. 2019, 93, e00504-19. [Google Scholar] [CrossRef]
- Firth, A.E.; Chung, B.Y.W.; Fleeton, M.N.; Atkins, J.F. Discovery of frameshifting in Alphavirus 6K resolves a 20-year enigma. Virol. J. 2008, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.E.; Kulcsar, K.A.; Schultz, K.L.W.; Riley, C.P.; Neary, J.T.; Marr, S.; Jose, J.; Griffin, D.E.; Kuhn, R.J. Functional Characterization of the Alphavirus TF Protein. J. Virol. 2013, 87, 8511–8523. [Google Scholar] [CrossRef]
- Ramsey, J.; Mukhopadhyay, S. Disentangling the frames, the state of research on the alphavirus 6K and TF proteins. Viruses 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Loewy, A.; Smyth, J.; von Bonsdorff, C.H.; Liljeström, P.; Schlesinger, M.J. The 6-kilodalton membrane protein of Semliki Forest virus is involved in the budding process. J. Virol. 1995, 69, 469. [Google Scholar] [CrossRef] [PubMed]
- van Duijl-Richter, M.K.S.; Hoornweg, T.E.; Rodenhuis-Zybert, I.A.; Smit, J.M. Early events in chikungunya virus infection—From virus cell binding to membrane fusion. Viruses 2015, 7, 3647–3674. [Google Scholar] [CrossRef]
- Schnierle, B.S. Cellular attachment and entry factors for chikungunya virus. Viruses 2019, 11, 1078. [Google Scholar] [CrossRef]
- Kuo, S.C.; Chen, Y.J.; Wang, Y.M.; Tsui, P.Y.; Kuo, M.D.; Wu, T.Y.; Lo, S.J. Cell-based analysis of Chikungunya virus E1 protein in membrane fusion. J. Biomed. Sci. 2012, 19, 44. [Google Scholar] [CrossRef]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef]
- Heil, M.L.; Albee, A.; Strauss, J.H.; Kuhn, R.J. An Amino Acid Substitution in the Coding Region of the E2 Glycoprotein Adapts Ross River Virus To Utilize Heparan Sulfate as an Attachment Moiety. J. Virol. 2001, 75, 6303–6309. [Google Scholar] [CrossRef]
- Ashbrook, A.W.; Burrack, K.S.; Silva, L.A.; Montgomery, S.A.; Heise, M.T.; Morrison, T.E.; Dermody, T.S. Residue 82 of the Chikungunya Virus E2 Attachment Protein Modulates Viral Dissemination and Arthritis in Mice. J. Virol. 2014, 88, 12180–12192. [Google Scholar] [CrossRef]
- Thannickal, S.A.; Battini, L.; Spector, S.N.; Noval, M.G.; Álvarez, D.E.; Stapleford, K.A. Changes in the chikungunya virus E1 glycoprotein domain II and hinge influence E2 conformation, infectivity, and virus-receptor interactions. J. Virol. 2024, 98, e0067924. [Google Scholar] [CrossRef]
- Reyes Ballista, J.M.; Hoover, A.J.; Noble, J.T.; Acciani, M.D.; Miazgowicz, K.L.; Harrison, S.A.; Tabscott, G.A.L.; Duncan, A.; Barnes, D.N.; Jimenez, A.R.; et al. Chikungunya Virus Release is Reduced by TIM-1 Receptors Through Binding of Envelope Phosphatidylserine. BioRxiv 2024, 98, e0077524. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Z.; Chu, J.J.H. The interplay of viral and host factors in chikungunya virus infection: Targets for antiviral strategies. Viruses 2018, 10, 294. [Google Scholar] [CrossRef]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, J.M.; Boere, W.A.; Garoff, H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J. Virol. 1989, 63, 4991. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Gudigamolla, N.K.; Chowdary, T.K. Acidic pH-Induced Conformational Changes in Chikungunya Virus Fusion Protein E1: A Spring-Twisted Region in the Domain I-III Linker Acts as a Hinge Point for Swiveling Motion of Domains. J. Virol. 2020, 94, e01561-20. [Google Scholar] [CrossRef]
- Fields, W.; Kielian, M. A Key Interaction between the Alphavirus Envelope Proteins Responsible for Initial Dimer Dissociation during Fusion. J. Virol. 2013, 87, 3774–3781. [Google Scholar] [CrossRef]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar] [CrossRef]
- Blijleven, J.S.; Bouma, E.M.; van Duijl-Richter, M.K.S.; Smit, J.M.; van Oijen, A.M. Cooperative Chikungunya Virus Membrane Fusion and Its Substoichiometric Inhibition by CHK-152 Antibody. Viruses 2022, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- Van Duijl-Richter, M.K.S.; Blijleven, J.S.; van Oijen, A.M.; Smit, J.M. Chikungunya virus fusion properties elucidated by single-particle and bulk approaches. J. Gen. Virol. 2015, 96, 2122–2132. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Vaney, M.C.; Roussel, A.; Vigouroux, A.; Reilly, B.; Lepault, J.; Kielian, M.; Rey, F.A. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 2004, 427, 320–325. [Google Scholar] [CrossRef]
- Feng, F.; Bouma, E.M.; Hu, G.; Zhu, Y.; Yu, Y.; Smit, J.M.; Diamond, M.S.; Zhang, R. Colocalization of Chikungunya Virus with Its Receptor MXRA8 during Cell Attachment, Internalization, and Membrane Fusion. J. Virol. 2023, 97, e0155722. [Google Scholar] [CrossRef]
- Kirchhausen, T.; Owen, D.; Harrison, S.C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 2014, 6, a016725. [Google Scholar] [CrossRef] [PubMed]
- Gesprasert, G.; Wichukchinda, N.; Mori, M.; Shiino, T.; Auwanit, W.; Sriwanthana, B.; Pathipvanich, P.; Sawanpanyalert, P.; Miura, T.; Auewarakul, P.; et al. HLA-Associated Immune Pressure on Gag Protein in CRF01_AE-Infected Individuals and Its Association with Plasma Viral Load. PLoS ONE 2010, 5, e11179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoornweg, T.E.; van Duijl-Richter, M.K.S.; Ayala Nuñez, N.V.; Albulescu, I.C.; van Hemert, M.J.; Smit, J.M. Dynamics of Chikungunya Virus Cell Entry Unraveled by Single-Virus Tracking in Living Cells. J. Virol. 2016, 90, 4745–4756. [Google Scholar] [CrossRef]
- Perrais, D. Cellular and structural insight into dynamin function during endocytic vesicle formation: A tale of 50 years of investigation. Biosci. Rep. 2022, 42, BSR20211227. [Google Scholar] [CrossRef]
- Sigismund, S.; Woelk, T.; Puri, C.; Maspero, E.; Tacchetti, C.; Transidico, P.; Di Fiore, P.P.; Polo, S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 2005, 102, 2760–2765. [Google Scholar] [CrossRef]
- Zare, F.; Bokarewa, M.; Nenonen, N.; Bergström, T.; Alexopoulou, L.; Flavell, R.A.; Tarkowski, A. Arthritogenic Properties of Double-Stranded (Viral) RNA. J. Immunol. 2004, 172, 5656–5663. [Google Scholar] [CrossRef]
- Dupuis-Maguiraga, L.; Noret, M.; Brun, S.; Le Grand, R.; Gras, G.; Roques, P. Chikungunya disease: Infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Negl. Trop. Dis. 2012, 6, e1446. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, N.; Becquart, P.; Nkoghe, D.; Padilla, C.; Ndjoyi-Mbiguino, A.; Leroy, E.M. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J. Infect. Dis. 2011, 204, 115–123. [Google Scholar] [CrossRef]
- Suhrbier, A.; Jaffar-Bandjee, M.C.; Gasque, P. Arthritogenic alphaviruses-an overview. Nat. Rev. Rheumatol. 2012, 8, 420–429. [Google Scholar] [CrossRef]
- Panning, M.; Grywna, K.; Van Esbroeck, M.; Emmerich, P.; Drosten, C. Chikungunya Fever in Travelers Returning to Europe from the Indian Ocean Region, 2006—Volume 14, Number 3—March 2008—Emerging Infectious Diseases journal—CDC. Emerg. Infect. Dis. 2008, 14, 416–422. [Google Scholar] [CrossRef]
- Jain, J.; Nayak, K.; Tanwar, N.; Gaind, R.; Gupta, B.; Shastri, J.S.; Bhatnagar, R.K.; Kaja, M.K.; Chandele, A.; Sunil, S. Clinical, Serological, and Virological Analysis of 572 Chikungunya Patients From 2010 to 2013 in India. Clin. Infect. Dis. 2017, 65, 133–140. [Google Scholar] [CrossRef]
- Hoarau, J.-J.; Jaffar Bandjee, M.-C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Teo, T.H.; Chan, Y.H.; Lee, W.W.L.; Lum, F.M.; Amrun, S.N.; Her, Z.; Rajarethinam, R.; Merits, A.; Rötzschke, O.; Rénia, L.; et al. Fingolimod treatment abrogates chikungunya virus-induced arthralgia. Sci. Transl. Med. 2017, 9, eaal1333. [Google Scholar] [CrossRef]
- Segato-Vendrameto, C.Z.; Zanluca, C.; Zucoloto, A.Z.; Zaninelli, T.H.; Bertozzi, M.M.; Saraiva-Santos, T.; Ferraz, C.R.; Staurengo-Ferrari, L.; Badaro-Garcia, S.; Manchope, M.F.; et al. Chikungunya Virus and Its Envelope Protein E2 Induce Hyperalgesia in Mice: Inhibition by Anti-E2 Monoclonal Antibodies and by Targeting TRPV1. Cells 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Bryden, S.R.; Pingen, M.; Lefteri, D.A.; Miltenburg, J.; Delang, L.; Jacobs, S.; Abdelnabi, R.; Neyts, J.; Pondeville, E.; Major, J.; et al. Pan-viral protection against arboviruses by activating skin macrophages at the inoculation site. Sci. Transl. Med. 2020, 12, eaax2421. [Google Scholar] [CrossRef]
- Rudd, P.A.; Wilson, J.; Gardner, J.; Larcher, T.; Babarit, C.; Le, T.T.; Anraku, I.; Kumagai, Y.; Loo, Y.M.; Gale, M., Jr.; et al. Interferon Response Factors 3 and 7 Protect against Chikungunya Virus Hemorrhagic Fever and Shock. J. Virol. 2012, 86, 9888–9898. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; Burke, C.W.; Higgs, S.T.; Klimstra, W.B.; Ryman, K.D. Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology 2012, 425, 103–112. [Google Scholar] [CrossRef]
- Schilte, C.; Couderc, T.; Chretien, F.; Sourisseau, M.; Gangneux, N.; Guivel-Benhassine, F.; Kraxner, A.; Tschopp, J.; Higgs, S.; Michault, A.; et al. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J. Exp. Med. 2010, 207, 429–442. [Google Scholar] [CrossRef]
- Nghia, V.X.; van Giang, N.; Canh, N.X.; Ha, N.H.; Duong, N.T.; Hoang, N.H.; Xuan, N.T. Stimulation of dendritic cell functional maturation by capsid protein from chikungunya virus. Iran. J. Basic. Med. Sci. 2020, 23, 1268–1274. [Google Scholar] [CrossRef]
- Long, K.M.; Whitmore, A.C.; Ferris, M.T.; Sempowski, G.D.; McGee, C.; Trollinger, B.; Gunn, B.; Heise, M.T. Dendritic Cell Immunoreceptor Regulates Chikungunya Virus Pathogenesis in Mice. J. Virol. 2013, 87, 5697–5706. [Google Scholar] [CrossRef]
- Palha, N.; Guivel-Benhassine, F.; Briolat, V.; Lutfalla, G.; Sourisseau, M.; Ellett, F.; Wang, C.H.; Lieschke, G.J.; Herbomel, P.; Schwartz, O.; et al. Real-Time Whole-Body Visualization of Chikungunya Virus Infection and Host Interferon Response in Zebrafish. PLoS Pathog. 2013, 9, e1003619. [Google Scholar] [CrossRef]
- Muralidharan, A.; Reid, S.P. Complex Roles of Neutrophils during Arboviral Infections. Cells 2021, 10, 1324. [Google Scholar] [CrossRef]
- Hiroki, C.H.; Toller-Kawahisa, J.E.; Fumagalli, M.J.; Colon, D.F.; Figueiredo, L.T.M.; Fonseca, B.A.L.D.; Franca, R.F.O.; Cunha, F.Q. Neutrophil Extracellular Traps Effectively Control Acute Chikungunya Virus Infection. Front. Immunol. 2020, 10, 479140. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.E.; Locke, M.C.; Young, A.R.; Monte, K.; Hedberg, M.L.; Shimak, R.M.; Sheehan, K.C.F.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Distinct Roles of Interferon Alpha and Beta in Controlling Chikungunya Virus Replication and Modulating Neutrophil-Mediated Inflammation. J. Virol. 2019, 94, e00841-19. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, S.; de Melo Costa, V.R.; Santos, F.M.; de Sousa, C.D.F.; Moreira, T.P.; Gonçalves, M.R.; Félix, F.B.; Queiroz-Junior, C.M.; Campolina-Silva, G.H.; Nogueira, M.L.; et al. Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection. Cells 2022, 11, 2717. [Google Scholar] [CrossRef]
- Chen, J.; He, R.; Luo, J.; Yan, S.; Zhu, W.; Liu, S. Neutrophil Extracellular Traps in Viral Infections. Pathogens 2025, 14, 1018. [Google Scholar] [CrossRef]
- Stoermer, K.A.; Burrack, A.; Oko, L.; Montgomery, S.A.; Borst, L.B.; Gill, R.G.; Morrison, T.E. Genetic Ablation of Arginase 1 in Macrophages and Neutrophils Enhances Clearance of an Arthritogenic Alphavirus. J. Immunol. 2012, 189, 4047–4059. [Google Scholar] [CrossRef]
- Chang, A.Y.; Encinales, L.; Porras, A.; Pacheco, N.; Reid, S.P.; Martins, K.A.O.; Pacheco, S.; Bravo, E.; Navarno, M.; Mendoza, A.R.; et al. Frequency of Chronic Joint Pain Following Chikungunya Virus Infection: A Colombian Cohort Study. Arthritis Rheumatol. 2018, 70, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Gérardin, P.; Taylor, A.; Mostafavi, H.; Malvy, D.; Mahalingam, S. Chikungunya Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Disease Management. Arthritis Rheumatol. 2018, 70, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, H.; Abeyratne, E.; Zaid, A.; Taylor, A. Arthritogenic Alphavirus-Induced Immunopathology and Targeting Host Inflammation as A Therapeutic Strategy for Alphaviral Disease. Viruses 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Haist, K.C.; Burrack, K.S.; Davenport, B.J.; Morrison, T.E. Inflammatory monocytes mediate control of acute alphavirus infection in mice. PLoS Pathog. 2017, 13, e1006748. [Google Scholar] [CrossRef]
- Tanabe, I.S.B.; Tanabe, E.L.L.; Santos, E.C.; Martins, W.V.; Araújo, I.M.T.C.; Cavalcante, M.C.A.; Lima, A.R.V.; Câmara, N.O.S.; Anderson, L.; Yunusov, D.; et al. Cellular and Molecular Immune Response to Chikungunya Virus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 345. [Google Scholar] [CrossRef]
- Rulli, N.E.; Rolph, M.S.; Srikiatkhachorn, A.; Anantapreecha, S.; Guglielmotti, A.; Mahalingam, S. Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J. Infect. Dis. 2011, 204, 1026–1030. [Google Scholar] [CrossRef]
- Kumar, S.; Jaffar-Bandjee, M.C.; Giry, C.; Connen De Kerillis, L.; Merits, A.; Gasque, P.; Hoarau, J.J. Mouse macrophage innate immune response to chikungunya virus infection. Virol. J. 2012, 9, 313. [Google Scholar] [CrossRef]
- Ziegler, S.A.; Lu, L.; Rosa, A.P.A.T.; Xiao, S.; Tesh, R.B. An Animal Model for Studying the Pathogenesis of Chikungunya Virus Infection 2008. Available online: https://pubmed.ncbi.nlm.nih.gov/18606777/ (accessed on 22 July 2025).
- Chen, W.; Foo, S.-S.; Taylor, A.; Lulla, A.; Merits, A.; Hueston, L.; Forwood, M.R.; Walsh, N.C.; Sims, N.A.; Herrero, L.J.; et al. Bindarit, an Inhibitor of Monocyte Chemotactic Protein Synthesis, Protects against Bone Loss Induced by Chikungunya Virus Infection. J. Virol. 2015, 89, 581–593. [Google Scholar] [CrossRef]
- Burt, F.J.; Chen, W.; Miner, J.J.; Lenschow, D.J.; Merits, A.; Schnettler, E.; Kohl, A.; Rudd, P.A.; Taylor, A.; Herrero, L.J.; et al. Chikungunya virus: An update on the biology and pathogenesis of this emerging pathogen. Lancet Infect. Dis. 2017, 17, e107–e117. [Google Scholar] [CrossRef]
- Poo, Y.S.; Nakaya, H.; Gardner, J.; Larcher, T.; Schroder, W.A.; Le, T.T.; Major, L.D.; Suhrbier, A. CCR2 Deficiency Promotes Exacerbated Chronic Erosive Neutrophil-Dominated Chikungunya Virus Arthritis. J. Virol. 2014, 88, 6862–6872. [Google Scholar] [CrossRef]
- Noret, M.; Herrero, L.; Rulli, N.; Rolph, M.; Smith, P.N.; Li, R.W.; Roques, P.; Gras, G.; Mahalingam, S. Interleukin 6, RANKL, and osteoprotegerin expression by chikungunya virus-infected human osteoblasts. J. Infect. Dis. 2012, 206, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Her, Z.; Ong, E.K.S.; Chen, J.M.; Dimatatac, F.; Kwek, D.J.C.; Barkham, T.; Yang, H.; Rénia, L.; Leo, Y.S.; et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 2011, 203, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.M.; Keating, M.K.; Shieh, W.J.; Bhatnagar, J.; Bollweg, B.C.; Levine, R.; Blau, D.M.; Torres, J.V.; Rivera, A.; Perez-Padilla, J.; et al. Clinical Characteristics, Histopathology, and Tissue Immunolocalization of Chikungunya Virus Antigen in Fatal Cases. Clin. Infect. Dis. 2021, 73, E345–E354. [Google Scholar] [CrossRef]
- Petitdemange, C.; Becquart, P.; Wauquier, N.; Béziat, V.; Debré, P.; Leroy, E.M.; Vieillard, V. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011, 7, e1002268. [Google Scholar] [CrossRef] [PubMed]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef]
- Petitdemange, C.; Wauquier, N.; Jacquet, J.M.; Theodorou, I.; Leroy, E.; Vieillard, V. Association of HLA class-i and inhibitory KIR genotypes in gabonese patients infected by chikungunya or dengue type-2 Viruses. PLoS ONE 2014, 9, e108798. [Google Scholar] [CrossRef]
- Petitdemange, C.; Wauquier, N.; Devilliers, H.; Yssel, H.; Mombo, I.; Caron, M.; Nkoghé, D.; Debré, P.; Leroy, E.; Vieillard, V. Longitudinal Analysis of Natural Killer Cells in Dengue Virus-Infected Patients in Comparison to Chikungunya and Chikungunya/Dengue Virus-Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0004499. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E.; Franco-Paredes, C.; Ault, S.K.; Periago, M.R. The neglected tropical diseases of Latin America and the Caribbean: A review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl. Trop. Dis. 2008, 2, e300. [Google Scholar] [CrossRef]
- Teo, T.-H.; Her, Z.; Tan, J.J.L.; Lum, F.-M.; Lee, W.W.L.; Chan, Y.-H.; Ong, R.Y.; Kam, Y.W.; Leparc-Goffart, I.; Gallian, P.; et al. Caribbean and La Réunion Chikungunya Virus Isolates Differ in Their Capacity To Induce Proinflammatory Th1 and NK Cell Responses and Acute Joint Pathology. J. Virol. 2015, 89, 7955–7969. [Google Scholar] [CrossRef]
- Thanapati, S.; Ganu, M.; Giri, P.; Kulkarni, S.; Sharma, M.; Babar, P.; Ganu, A.; Tripathy, A.S. Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum. Immunol. 2017, 78, 370–374. [Google Scholar] [CrossRef]
- Khakoo, S.I.; Jamil, K.M. KIR/HLA interactions and pathogen immunity. BioMed Res. Int. 2011, 2011, 298348. [Google Scholar] [CrossRef]
- Webster, B.; Werneke, S.W.; Zafirova, B.; This, S.; Coléon, S.; Décembre, E.; Paidassi, H.; Bouvier, I.; Joubert, P.E.; Duffy, D.; et al. Plasmacytoid dendritic cells control dengue and Chikungunya virus infections via IRF7-regulated interferon responses. Elife 2018, 19, e34273. [Google Scholar]
- De Castro-Jorge, L.A.; De Carvalho, R.V.H.; Klein, T.M.; Hiroki, C.H.; Lopes, A.H.; Guimarães, R.M.; Fumagalli, M.J.; Floriano, V.G.; Agostinho, M.R.; Slhessarenko, R.D.; et al. The NLRP3 inflammasome is involved with the pathogenesis of Mayaro virus. PLoS Pathog. 2019, 15, e1007934. [Google Scholar] [CrossRef]
- Chêne, A.; Donati, D.; Guerreiro-Cacais, A.O.; Levitsky, V.; Chen, Q.; Falk, K.I.; Orem, J.; Kironde, F.; Wahlgren, M.; Bejarano, M.T. A Molecular Link between Malaria and Epstein–Barr Virus Reactivation. PLoS Pathog. 2007, 3, e80. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chang, J.; Côrtes, F.H.; Ha, C.; Villalpando, J.; Castillo, I.N.; Gálvez, R.I.; Grifoni, A.; Sette, A.; Romero-Vivas, C.M.; et al. Chikungunya virus-specific CD4+ T cells are associated with chronic chikungunya viral arthritic disease in humans. Cell Rep. Med. 2025, 6, 102134. [Google Scholar] [CrossRef] [PubMed]
- Teo, T.-H.; Lum, F.-M.; Claser, C.; Lulla, V.; Lulla, A.; Merits, A.; Rénia, L.; Ng, L.F. A Pathogenic Role for CD4+ T Cells during Chikungunya Virus Infection in Mice. J. Immunol. 2013, 190, 259–269. [Google Scholar] [CrossRef]
- Davenport, B.J.; Bullock, C.; McCarthy, M.K.; Hawman, D.W.; Murphy, K.M.; Kedl, R.M.; Diamond, M.S.; Morrison, T.E. Chikungunya Virus Evades Antiviral CD8+ T Cell Responses To Establish Persistent Infection in Joint-Associated Tissues. J. Virol. 2020, 94, e02036-19. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Yeang, H.X.A.; Fox, J.M.; Taffner, S.; Malkova, O.N.; Oh, S.T.; Kim, A.H.J.; Diamond, M.S.; Lenschow, D.J.; Yokoyama, W.M. Brief report: Chikungunya viral arthritis in the United States: A mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 1214–1220. [Google Scholar] [CrossRef]
- Brito RMde, M.; de Melo, M.F.; Fernandes, J.V.; Valverde, J.G.; Matta Guedes, P.M.; de Araújo, J.M.G.; Nascimento, M.S.L. Acute Chikungunya Virus Infection Triggers a Diverse Range of T Helper Lymphocyte Profiles. Viruses 2024, 16, 1387. [Google Scholar] [CrossRef]
- Yamada, H.; Nakashima, Y.; Okazaki, K.; Mawatari, T.; Fukushi, J.-I.; Oyamada, A.; Fujimura, K.; Iwamoto, Y.; Yoshikai, Y. Preferential Accumulation of Activated Th1 Cells Not Only in Rheumatoid Arthritis But Also in Osteoarthritis Joints. J. Rheumatol. 2011, 38, 1569–1575. [Google Scholar] [CrossRef]
- Venugopalan, A.; Ghorpade, R.P.; Chopra, A. Cytokines in acute chikungunya. PLoS ONE 2014, 9, e111305. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Gomes, I.C.; Leite, J.; Santana, A.; Macêdo, B.; Fonseca, R.; Keesen, T.; Carregaro, V.; Silva, J. Evaluation TH17 and Treg cells during chronic chikungunya infection. J. Immunol. 2019, 202, 140.20. [Google Scholar] [CrossRef]
- Kulkarni, S.P.; Ganu, M.; Jayawant, P.; Thanapati, S.; Ganu, A.; Tripathy, A.S. Regulatory T cells and IL-10 as modulators of chikungunya disease outcome: A preliminary study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2475–2481. [Google Scholar] [CrossRef]
- Powers, J.M.; Lyski, Z.L.; Weber, W.C.; Denton, M.; Streblow, M.M.; Mayo, A.T.; Haese, N.N.; Nix, C.D.; Rodríguez-Santiago, R.; Alvarado, L.I.; et al. Infection with chikungunya virus confers heterotypic cross-neutralizing antibodies and memory B-cells against other arthritogenic alphaviruses predominantly through the B domain of the E2 glycoprotein. PLoS Negl. Trop. Dis. 2023, 17, e0011154. [Google Scholar] [CrossRef]
- Chua, C.L.; Sam, I.C.; Chiam, C.W.; Chan, Y.F. The neutralizing role of IgM during early Chikungunya virus infection. PLoS ONE 2017, 12, e0171989. [Google Scholar] [CrossRef]
- Hawman, D.W.; Stoermer, K.A.; Montgomery, S.A.; Pal, P.; Oko, L.; Diamond, M.S.; Morrison, T.E. Chronic Joint Disease Caused by Persistent Chikungunya Virus Infection Is Controlled by the Adaptive Immune Response. J. Virol. 2013, 87, 13878–13888. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Griffin, D.E. Extensive immune-mediated hippocampal damage in mice surviving infection with neuroadapted Sindbis virus. Virology 2003, 311, 28–39. [Google Scholar] [CrossRef][Green Version]
- Griffin, D.E. Recovery from viral encephalomyelitis: Immune-mediated noncytolytic virus clearance from neurons. Immunol. Res. 2010, 47, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Brooke, C.B.; Deming, D.J.; Whitmore, A.C.; White, L.J.; Johnston, R.E. T Cells Facilitate Recovery from Venezuelan Equine Encephalitis Virus-Induced Encephalomyelitis in the Absence of Antibody. J. Virol. 2010, 84, 4556–4568. [Google Scholar] [CrossRef]
- Yun, N.E.; Peng, B.H.; Bertke, A.S.; Borisevich, V.; Smith, J.K.; Smith, J.N.; Poussard, A.L.; Salazar, M.; Judy, B.M.; Zacks, M.A.; et al. CD4+ T cells provide protection against acute lethal encephalitis caused by Venezuelan equine encephalitis virus. Vaccine 2009, 27, 4064–4073. [Google Scholar] [CrossRef]
- Beddingfield, B.J.; Sugimoto, C.; Wang, E.; Weaver, S.C.; Russell-Lodrigue, K.E.; Killeen, S.Z.; Kuroda, M.J.; Roy, C.J. Phenotypic and Kinetic Changes of Myeloid Lineage Cells in Innate Response to Chikungunya Infection in Cynomolgus Macaques. Viral Immunol. 2022, 35, 192–199. [Google Scholar] [CrossRef]
- Teo, T.; Howland, S.W.; Claser, C.; Gun, S.Y.; Poh, C.M.; Lee, W.W.; Lum, F.M.; Ng, L.F.; Rénia, L. Co-infection with Chikungunya virus alters trafficking of pathogenic CD8+ T cells into the brain and prevents Plasmodium-induced neuropathology. EMBO Mol. Med. 2018, 10, 121–138. [Google Scholar] [CrossRef]
- Barr, T.A.; Brown, S.; Mastroeni, P.; Gray, D. B Cell Intrinsic MyD88 Signals Drive IFN-γ Production from T Cells and Control Switching to IgG2c. J. Immunol. 2009, 183, 1005–1012. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, C.; Nandakumar, K.S. Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediat. Inflamm. 2020, 2020, 3830212. [Google Scholar] [CrossRef] [PubMed]
- Beringer, A.; Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 2019, 15, 491–501. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular pathogens: Host immunity and microbial persistence strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Fox, J.M.; Diamond, M.S. Immune-Mediated Protection and Pathogenesis of Chikungunya Virus. J. Immunol. 2016, 197, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Poo, Y.S.; Alves, J.C.; Almeida, R.P.; Mostafavi, H.; Tang, P.C.H.; Bucala, R.; Teixeira, M.M.; Taylor, A.; Zaid, A.; et al. Interleukin-17 Contributes to Chikungunya Virus-Induced Disease. MBio 2022, 13, e0028922. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Baldoni, N.R.; Cardoso, C.S.; Oliveira, C.D.L. Biomarkers of severity and chronification in chikungunya fever: A systematic review and meta-analysis. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e16. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.S.; Kam, Y.W.; Lee, B.; Hapuarachchi, H.C.; Wimal, A.; Ng, L.C.; Ng, L.F. A systematic meta-analysis of immune signatures in patients with acute chikungunya virus infection. J. Infect. Dis. 2015, 211, 1925–1935. [Google Scholar] [CrossRef]
- Ng, L.F.P.; Chow, A.; Sun, Y.J.; Kwek, D.J.C.; Lim, P.L.; Dimatatac, F.; Ng, L.C.; Ooi, E.E.; Choo, K.H.; Her, Z.; et al. IL-1β, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS ONE 2009, 4, e4261. [Google Scholar] [CrossRef]
- Lee, N.; Wong, C.K.; Lam, W.Y.; Wong, A.; Lim, W.; Lam, C.W.K.; Cockram, C.S.; Sung, J.J.; Chan, P.K.; Tang, J.W. Chikungunya fever, Hong Kong. Emerg. Infect. Dis. 2006, 12, 1790–1792. [Google Scholar] [CrossRef]
- Chaaitanya, I.K.; Muruganandam, N.; Sundaram, S.G.; Kawalekar, O.; Sugunan, A.P.; Manimunda, S.P.; Ghosal, S.R.; Muthumani, K.; Vijayachari, P. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011, 24, 265–271. [Google Scholar] [CrossRef]
- Pott, F.; Postmus, D.; Brown, R.J.P.; Wyler, E.; Neumann, E.; Landthaler, M.; Goffinet, C. Single-cell analysis of arthritogenic alphavirus-infected human synovial fibroblasts links low abundance of viral RNA to induction of innate immunity and arthralgia-associated gene expression. Emerg. Microbes Infect. 2021, 10, 2151–2168. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, S.; Parray, H.A.; Das, S. Chikungunya and arthritis: An overview. Travel Med. Infect. Dis. 2021, 44, 102168. [Google Scholar] [CrossRef]
- Gualberto Cavalcanti, N.; MeloVilar, K.; Branco Pinto Duarte, A.L.; Jesus Barreto de Melo Rêgo, M.; Cristiny Pereira, M.; da Rocha Pitta, I.; Diniz Lopes Marques, C.; Galdino da Rocha Pitta, M. IL-27 in patients with Chikungunya fever: A possible chronicity biomarker? Acta Trop. 2019, 196, 48–51. [Google Scholar] [CrossRef]
- Banerjee, N.; Mukhopadhyay, S. Oxidative damage markers and inflammatory cytokines are altered in patients suffering with post-chikungunya persisting polyarthralgia. Free Radic. Res. 2018, 52, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Kelvin, A.A.; Banner, D.; Silvi, G.; Moro, M.L.; Spataro, N.; Gaibani, P.; Cavrini, F.; Pierro, A.; Rossini, G.; Cameron, M.J.; et al. Inflammatory cytokine expression is associated with Chikungunya virus resolution and symptom severity. PLoS Negl. Trop. Dis. 2011, 5, e1279. [Google Scholar] [CrossRef]
- Reddy, V.; Mani, R.S.; Desai, A.; Ravi, V. Correlation of plasma viral loads and presence of Chikungunya IgM antibodies with cytokine/chemokine levels during acute Chikungunya virus infection. J. Med. Virol. 2014, 86, 1393–1401. [Google Scholar] [CrossRef]
- Priya, R.; Patro, I.K.; Parida, M.M. TLR3 mediated innate immune response in mice brain following infection with Chikungunya virus. Virus Res. 2014, 189, 194–205. [Google Scholar] [CrossRef]
- Natrajan, M.S.; Rojas, A.; Waggoner, J.J. Beyond Fever and Pain: Diagnostic Methods for Chikungunya Virus. J. Clin. Microbiol. 2019, 57, e00350-19. [Google Scholar] [CrossRef]
- Simon, F.; Javelle, E.; Cabie, A.; Bouquillard, E.; Troisgros, O.; Gentile, G.; Leparc-Goffart, I.; Hoen, B.; Gandjbakhch, F.; Rene-Corail, P.; et al. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med. Mal. Infect. 2015, 45, 243–263. [Google Scholar] [CrossRef]
- Burdino, E.; Calleri, G.; Caramello, P.; Ghisetti, V. Unmet Needs for a Rapid Diagnosis of Chikungunya Virus Infection. Emerg. Infect. Dis. 2016, 22, 1837. [Google Scholar] [CrossRef]
- Cavrini, F.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. Chikungunya: An emerging and spreading arthropod-borne viral disease. J. Infect. Dev. Ctries. 2009, 3, 744–752. [Google Scholar] [CrossRef]
- Hassing, R.J.; Leparc-Goffart, I.; Tolou, H.; van Doornum, G.; van Genderen, P.J. Cross-reactivity of antibodies to viruses belonging to the Semliki forest serocomplex. Eurosurveillance 2010, 15, 19588. [Google Scholar] [CrossRef]
- da Rocha Queiroz Lima, M.; de Lima, R.C.; de Azeredo, E.L.; Dos Santos, F.B. Analysis of a Routinely Used Commercial Anti-Chikungunya IgM ELISA Reveals Cross-Reactivities with Dengue in Brazil: A New Challenge for Differential Diagnosis? Diagnostics 2021, 11, 819. [Google Scholar] [CrossRef]
- Hua, C.; Combe, B. Chikungunya Virus-Associated Disease. Curr. Rheumatol. Rep. 2017, 19, 69. [Google Scholar] [CrossRef]
- de la Calle-Prieto, F.; Barriga, J.J.; Arsuaga, M.; de Miguel, R.; Díaz-Menéndez, M. Clinical profile and management of a Spanish single-center retrospective cohort of patients with post-chikungunya associated complications. Travel Med. Infect. Dis. 2024, 60, 102726. [Google Scholar] [CrossRef]
- Ghildiyal, R.; Gabrani, R. Antiviral therapeutics for chikungunya virus. Expert. Opin. Ther. Pat. 2020, 30, 467–480. [Google Scholar] [CrossRef]
- Mahalingam, S.; Tharmarajah, K.; Zaid, A. Chikungunya: Vaccines and therapeutics. F1000Research 2017, 6, 2114. [Google Scholar] [CrossRef]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Lakshmana Rao, P.V.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against chikungunya virus in vero cells. J. Med. Virol. 2010, 82, 817. [Google Scholar] [CrossRef] [PubMed]
- Roques, P.; Thiberville, S.D.; Dupuis-Maguiraga, L.; Lum, F.M.; Labadie, K.; Martinon, F.; Gras, G.; Lebon, P.; Ng, L.F.P.; de Lamballerie, X.; et al. Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection. Viruses 2018, 10, 268. [Google Scholar] [CrossRef]
- Chopra, A.; Saluja, M.; Venugopalan, A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 2014, 66, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Sliva, K.; Von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral Res. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Lu, J.W.; Hsieh, P.S.; Lin, C.C.; Hu, M.K.; Huang, S.M.; Wang, Y.M.; Liang, C.Y.; Gong, Z.; Ho, Y.J. Synergistic effects of combination treatment using EGCG and suramin against the chikungunya virus. Biochem. Biophys. Res. Commun. 2017, 491, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Wang, Y.M.; Lu, J.W.; Wu, T.Y.; Lin, L.I.; Kuo, S.C.; Lin, C.C. Suramin inhibits chikungunya virus entry and transmission. PLoS ONE 2015, 10, e0133511. [Google Scholar] [CrossRef]
- Albulescu, I.C.; Van Hoolwerff, M.; Wolters, L.A.; Bottaro, E.; Nastruzzi, C.; Yang, S.C.; Tsay, S.C.; Hwu, J.R.; Snijder, E.J.; van Hemert, M.J. Suramin inhibits chikungunya virus replication through multiple mechanisms. Antivir. Res. 2015, 121, 39–46. [Google Scholar] [CrossRef]
- Kuo, S.C.; Wang, Y.M.; Ho, Y.J.; Chang, T.Y.; Lai, Z.Z.; Tsui, P.Y.; Wu, T.Y.; Lin, C.C. Suramin treatment reduces chikungunya pathogenesis in mice. Antivir. Res. 2016, 134, 89–96. [Google Scholar] [CrossRef]
- Henß, L.; Beck, S.; Weidner, T.; Biedenkopf, N.; Sliva, K.; Weber, C.; Becker, S.; Schnierle, B.S. Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry. Virol. J. 2016, 13, 149. [Google Scholar] [CrossRef]
- Hwu, J.R.; Gupta, N.K.; Tsay, S.C.; Huang, W.C.; Albulescu, I.C.; Kovacikova, K.; van Hemert, M.J. Bis(benzofuran–thiazolidinone)s and bis(benzofuran–thiazinanone)s as inhibiting agents for chikungunya virus. Antivir. Res. 2017, 146, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.F.S.; Gomes, M.G.M.; de Aquino, T.M.; de Araújo-Júnior, J.X.; de Souza, S.J.M.; Cavalcante, J.P.M.; Santos, E.C.D.; Bassi, Ê.J.; Silva-Júnior, E.F.D. Computer-Aided Design, Synthesis, and Antiviral Evaluation of Novel Acrylamides as Potential Inhibitors of E3-E2-E1 Glycoproteins Complex from Chikungunya Virus. Pharmaceuticals 2020, 13, 141. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lu, J.W.; Lin, C.C.; Chin, Y.F.; Wu, T.Y.; Lin, L.I.; Lai, Z.Z.; Kuo, S.C.; Ho, Y.J. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antivir. Res. 2016, 135, 81. [Google Scholar] [CrossRef]
- Wintachai, P.; Thuaud, F.; Basmadjian, C.; Roytrakul, S.; Ubol, S.; Désaubry, L.; Smith, D.R. Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol. Immunol. 2015, 59, 129–141. [Google Scholar] [CrossRef]
- Wichit, S.; Hamel, R.; Bernard, E.; Talignani, L.; Diop, F.; Ferraris, P.; Liegeois, F.; Ekchariyawat, P.; Luplertlop, N.; Surasombatpattana, P.; et al. Imipramine Inhibits Chikungunya Virus Replication in Human Skin Fibroblasts through Interference with Intracellular Cholesterol Trafficking. Sci Rep. 2017, 7, 3145. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Tiwari, M.; Santhosh, S.R.; Parida, M.; Lakshmana Rao, P.V. RNA interference mediated inhibition of Chikungunya virus replication in mammalian cells. Biochem. Biophys. Res. Commun. 2008, 376, 718–722. [Google Scholar] [CrossRef]
- Lam, S.; Chen, K.C.; Ng, M.M.L.; Chu, J.J.H. Expression of Plasmid-Based shRNA against the E1 and nsP1 Genes Effectively Silenced Chikungunya Virus Replication. PLoS ONE 2012, 7, e46396. [Google Scholar] [CrossRef]
- Saha, A.; Bhagyawant, S.S.; Parida, M.; Dash, P.K. Vector-delivered artificial miRNA effectively inhibited replication of Chikungunya virus. Antivir. Res. 2016, 134, 42. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; Haystead, T.; Das, P.K.; Merits, A.; Ng, M.L.; Vasudevan, S.G. Chikungunya virus nsP3 & nsP4 interacts with HSP-90 to promote virus replication: HSP-90 inhibitors reduce CHIKV infection and inflammation in vivo. Antivir. Res. 2014, 103, 7–16. [Google Scholar] [CrossRef]
- Feibelman, K.M.; Fuller, B.P.; Li, L.; LaBarbera, D.V.; Geiss, B.J. Identification of small molecule inhibitors of the Chikungunya virus nsP1 RNA capping enzyme. Antivir. Res. 2018, 154, 124. [Google Scholar] [CrossRef]
- Delang, L.; Li, C.; Tas, A.; Quérat, G.; Albulescu, I.C.; De Burghgraeve, T.; Guerrero, N.A.; Gigante, A.; Piorkowski, G.; Decroly, E.; et al. The viral capping enzyme nsP1: A novel target for the inhibition of chikungunya virus infection. Sci. Rep. 2016, 6, 31819. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Gómez-SanJuan, A.; Delang, L.; Li, C.; Bueno, O.; Gamo, A.M.; Priego, E.M.; Camarasa, M.J.; Jochmans, D.; Leyssen, P.; et al. Antiviral activity of [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones against chikungunya virus targeting the viral capping nsP1. Antivir. Res. 2017, 144, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Delang, L.; Guerrero, N.S.; Tas, A.; Quérat, G.; Pastorino, B.; Froeyen, M.; Dallmeier, K.; Jochmans, D.; Herdewijn, P.; Bello, F.; et al. Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. J. Antimicrob. Chemother. 2014, 69, 2770–2784. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sanjuan, A.; Gamo, A.M.; Delang, L.; Pérez-Sánchez, A.; Amrun, S.N.; Abdelnabi, R.; Jacobs, S.; Priego, E.M.; Camarasa, M.J.; Jochmans, D.; et al. Inhibition of the Replication of Different Strains of Chikungunya Virus by 3-Aryl-[1,2,3]triazolo[4,5-d] pyrimidin-7(6 H)-ones. ACS Infect. Dis. 2018, 4, 605–619. [Google Scholar] [CrossRef]
- Moesslacher, J.; Battisti, V.; Delang, L.; Neyts, J.; Abdelnabi, R.; Pürstinger, G.; Urban, E.; Langer, T. Identification of 2-(4-(Phenylsulfonyl)piperazine-1-yl)pyrimidine Analogues as Novel Inhibitors of Chikungunya Virus. ACS Med. Chem. Lett. 2020, 11, 906–912. [Google Scholar] [CrossRef]
- Kovacikova, K.; Morren, B.M.; Tas, A.; Albulescu, I.C.; Van Rijswijk, R.; Jarhad, D.B.; Shin, Y.S.; Jang, M.H.; Kim, G.; Lee, H.W.; et al. 6′-β-Fluoro-Homoaristeromycin and 6′-Fluoro-Homoneplanocin A Are Potent Inhibitors of Chikungunya Virus Replication through Their Direct Effect on Viral Nonstructural Protein 1. Antimicrob. Agents Chemother. 2020, 64, e02532-19. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jarhad, D.B.; Jang, M.H.; Kovacikova, K.; Kim, G.; Yoon, J.; Kim, H.R.; Hyun, Y.E.; Tipnis, A.S.; Chang, T.S.; et al. Identification of 6′-β-fluoro-homoaristeromycin as a potent inhibitor of chikungunya virus replication. Eur. J. Med. Chem. 2019, 187, 111956. [Google Scholar] [CrossRef]
- Mudgal, R.; Mahajan, S.; Tomar, S. Inhibition of Chikungunya virus by an adenosine analog targeting the SAM-dependent nsP1 methyltransferase. FEBS Lett. 2019, 594, 678. [Google Scholar] [CrossRef]
- Meena, M.K.; Kumar, D.; Kumari, K.; Kaushik, N.K.; Kumar, R.V.; Bahadur, I.; Vodwal, L.; Singh, P. Promising inhibitors of nsp2 of CHIKV using molecular docking and temperature-dependent molecular dynamics simulations. J. Biomol. Struct. Dyn. 2022, 40, 5827–5835. [Google Scholar] [CrossRef]
- Khan, N.; Bhat, R.; Patel, A.K.; Ray, P. Discovery of small molecule inhibitors of chikungunya virus proteins (nsP2 and E1) using in silico approaches. J. Biomol. Struct. Dyn. 2021, 39, 1373–1385. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, D.; Giri, R. Targeting the nsp2 Cysteine Protease of Chikungunya Virus Using FDA Approved Library and Selected Cysteine Protease Inhibitors. Pathogens 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Puusepp, L.; Varghese, F.S.; Utt, A.; Ahola, T.; Kananovich, D.G.; Lopp, M.; Merits, A.; Karelson, M. Design and Validation of Novel Chikungunya Virus Protease Inhibitors. Antimicrob. Agents Chemother. 2016, 60, 7382. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Mamidi, P.; Kumar, S.; Basantray, I.; Saswat, T.; Das, I.; Nayak, T.K.; Chattopadhyay, S.; Subudhi, B.B.; et al. Inhibition of Chikungunya Virus Replication by 1-[(2-Methylbenzimidazol-1-yl) Methyl]-2-Oxo-Indolin-3-ylidene] Amino] Thiourea(MBZM-N-IBT). Sci. Rep. 2016, 6, 20122. [Google Scholar] [CrossRef]
- De, S.; Ghosh, S.; Keshry, S.S.; Mahish, C.; Mohapatra, C.; Guru, A.; Mamidi, P.; Datey, A.; Pani, S.S.; Vasudevan, D.; et al. MBZM-N-IBT, a Novel Small Molecule, Restricts Chikungunya Virus Infection by Targeting nsP2 Protease Activity In Vitro, In Vivo, and Ex Vivo. Antimicrob. Agents Chemother. 2022, 66, e00463-22. [Google Scholar] [CrossRef]
- Jadav, S.S.; Sinha, B.N.; Hilgenfeld, R.; Pastorino, B.; De Lamballerie, X.; Jayaprakash, V. Thiazolidone derivatives as inhibitors of chikungunya virus. Eur. J. Med. Chem. 2015, 89, 172–178. [Google Scholar] [CrossRef] [PubMed]
- El-Labbad, E.M.; Ismail, M.A.H.; Abou Ei Ella, D.A.; Ahmed, M.; Wang, F.; Barakat, K.H.; Abouzid, K.A. Discovery of novel peptidomimetics as irreversible CHIKV NsP2 protease inhibitors using quantum mechanical-based ligand descriptors. Chem. Biol. Drug Des. 2015, 86, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Mudgal, R.; Narwal, M.; Kaur, R.; Singh, V.A.; Malik, A.; Chaudhary, M.; Tomar, S. Chikungunya virus inhibition by peptidomimetic inhibitors targeting virus-specific cysteine protease. Biochimie 2018, 149, 51–61. [Google Scholar] [CrossRef]
- Lucas-Hourani, M.; Lupan, A.; Desprès, P.; Thoret, S.; Pamlard, O.; Dubois, J.; Guillou, C.; Tangy, F.; Vidalain, P.O.; Munier-Lehmann, H. A phenotypic assay to identify chikungunya virus inhibitors targeting the nonstructural protein nsP2. J. Biomol. Screen. 2013, 18, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.K.; Soni, A.; Singh Yadav, S.P.; Kumar, A.; Gaurav, N.; Raghavendhar, S.; Sharma, P.; Sunil, S.; Ashish; Jayaram, B.; et al. Evaluation of novobiocin and telmisartan for anti-CHIKV activity. Virology 2020, 548, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Bhakat, S.; Delang, L.; Kaptein, S.; Neyts, J.; Leyssen, P.; Jayaprakash, V. Reaching beyond HIV/HCV: Nelfinavir as a potential starting point for broad-spectrum protease inhibitors against dengue and chikungunya virus. RSC Adv. 2015, 5, 85938–85949. [Google Scholar] [CrossRef]
- Zhang, S.; Garzan, A.; Haese, N.; Bostwick, R.; Martinez-Gzegozewska, Y.; Rasmussen, L.; Streblow, D.N.; Haise, M.T.; Pathak, A.K.; Augelli-Szafran, C.E.; et al. Pyrimidone inhibitors targeting Chikungunya Virus nsP3 macrodomain by fragment-based drug design. PLoS ONE 2021, 16, e0245013. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thiruchelvan, M.; Lee, R.C.H.; Chen, H.; Chen, K.C.; Ng, M.L.; Chu, J.J. Inhibition of Chikungunya Virus Replication by Harringtonine, a Novel Antiviral That Suppresses Viral Protein Expression. Antimicrob. Agents Chemother. 2013, 57, 155. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami, M.; Tao, S.; Zandi, K.; Hsiao, H.M.; Jiang, Y.; Hammond, E.; Amblard, F.; Russell, O.O.; Merits, A.; Schinazi, R.F. Characterization of β-d-N4-Hydroxycytidine as a Novel Inhibitor of Chikungunya Virus. Antimicrob. Agents Chemother. 2017, 61, e02395-16. [Google Scholar] [CrossRef]
- Briolant, S.; Garin, D.; Scaramozzino, N.; Jouan, A.; Crance, J.M. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: Synergistic effect of interferon-α and ribavirin combination. Antivir. Res. 2004, 61, 111–117. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Reis, P.A.; de Freitas, C.S.; Sacramento, C.Q.; Hoelz, L.V.B.; Bastos, M.M.; Mattos, M.; Rocha, N.; Gomes de Azevedo Quintanilha, I.; da Silva Gouveia Pedrosa, C.; et al. Beyond Members of the Flaviviridae Family, Sofosbuvir Also Inhibits Chikungunya Virus Replication. Antimicrob. Agents Chemother. 2019, 63, e01389-18. [Google Scholar] [CrossRef]
- Franco, E.J.; Rodriquez, J.L.; Pomeroy, J.J.; Hanrahan, K.C.; Brown, A.N. The effectiveness of antiviral agents with broad-spectrum activity against chikungunya virus varies between host cell lines. Antivir. Chem. Chemother. 2018, 26, 2040206618807580. [Google Scholar] [CrossRef]
- Rothan, H.A.; Bahrani, H.; Mohamed, Z.; Teoh, T.C.; Shankar, E.M.; Rahman, N.A.; Yusof, R. A Combination of Doxycycline and Ribavirin Alleviated Chikungunya Infection. PLoS ONE 2015, 10, e0126360. [Google Scholar] [CrossRef]
- Gallegos, K.M.; Drusano, G.L.; D′argenio, D.Z.; Brown, A.N. Chikungunya Virus: In Vitro Response to Combination Therapy With Ribavirin and Interferon Alfa 2a. J. Infect. Dis. 2016, 214, 1192. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Jochmans, D.; Verbeken, E.; Neyts, J.; Delang, L. Antiviral treatment efficiently inhibits chikungunya virus infection in the joints of mice during the acute but not during the chronic phase of the infection. Antivir. Res. 2018, 149, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Dhanwani, R.; Patro, I.K.; Rao, P.V.L.; Parida, M.M. Cellular IMPDH enzyme activity is a potential target for the inhibition of Chikungunya virus replication and virus induced apoptosis in cultured mammalian cells. Antivir. Res. 2011, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Orba, Y.; Sasaki, M.; Kobayashi, S.; Carr, M.J.; Nobori, H.; Sato, A.; Hall, W.W.; Sawa, H. Discovery of a novel antiviral agent targeting the nonstructural protein 4 (nsP4) of chikungunya virus. Virology 2017, 505, 102–112. [Google Scholar] [CrossRef]
- Marra, R.K.F.; Kümmerle, A.E.; Guedes, G.P.; Barros Cde, S.; Gomes, R.S.P.; Cirne-Santos, C.C.; Paixão, I.C.N.P.; Neves, A.P. Quinolone-N-acylhydrazone hybrids as potent Zika and Chikungunya virus inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126881. [Google Scholar] [CrossRef]
- Sharma, R.; Fatma, B.; Saha, A.; Bajpai, S.; Sistla, S.; Dash, P.K.; Parida, M.; Kumar, P.; Tomar, S. Inhibition of chikungunya virus by picolinate that targets viral capsid protein. Virology 2016, 498, 265–276. [Google Scholar] [CrossRef]
- Sharma, R.; Kesari, P.; Kumar, P.; Tomar, S. Structure-function insights into chikungunya virus capsid protein: Small molecules targeting capsid hydrophobic pocket. Virology 2018, 515, 223–234. [Google Scholar] [CrossRef]
- Fatma, B.; Kumar, R.; Singh, V.A.; Nehul, S.; Sharma, R.; Kesari, P.; Kuhn, R.J.; Tomar, S. Alphavirus capsid protease inhibitors as potential antiviral agents for Chikungunya infection. Antivir. Res. 2020, 179, 104808. [Google Scholar] [CrossRef]
- Dey, D.; Siddiqui, S.I.; Mamidi, P.; Ghosh, S.; Kumar, C.S.; Chattopadhyay, S.; Ghosh, S.; Banerjee, M. The effect of amantadine on an ion channel protein from Chikungunya virus. PLoS Negl. Trop. Dis. 2019, 13, e0007548. [Google Scholar] [CrossRef]
- Battisti, V.; Urban, E.; Langer, T. Antivirals against the Chikungunya Virus. Viruses 2021, 13, 1307. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef]
- Albulescu, I.C.; White-Scholten, L.; Tas, A.; Hoornweg, T.E.; Ferla, S.; Kovacikova, K.; Smit, J.M.; Brancale, A.; Snijder, E.J.; van Hemert, M.J. Suramin Inhibits Chikungunya Virus Replication by Interacting with Virions and Blocking the Early Steps of Infection. Viruses 2020, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Loke, R.H.T.; Anderson, M.G.; Coleman, J.C.; Murray-Lyon, I.M.; Tsiquaye, K.N.; Zuckerman, A.J. Suramin treatment for chronic active hepatitis B—Toxic and ineffective. J. Med. Virol. 1987, 21, 97–99. [Google Scholar] [CrossRef]
- Kaplan, L.D.; Wolfe, P.R.; Volberding, P.A.; Feorino, P.; Abrams, D.I.; Levy, J.A.; Abrams, D.I.; Kiprov, D.; Wong, R.; Kaufman, L.; et al. Lack of response to suramin in patients with AIDS and AIDS-related complex. Am. J. Med. 1987, 82, 615–620. [Google Scholar] [CrossRef]
- Hucke, F.I.L.; Bugert, J.J. Current and Promising Antivirals Against Chikungunya Virus. Front. Public Health 2020, 8, 618624. [Google Scholar] [CrossRef]
- Eyer, L.; Nencka, R.; de Clercq, E.; Seley-Radtke, K.; Růžek, D. Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses. Antivir. Chem. Chemother. 2018, 26, 2040206618761299. [Google Scholar] [CrossRef]
- Rozen-Gagnon, K.; Stapleford, K.A.; Mongelli, V.; Blanc, H.; Failloux, A.B.; Saleh, M.C.; Vignuzzi, M. Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models. PLoS Pathog. 2014, 10, e1003877. [Google Scholar] [CrossRef] [PubMed]
- Haese, N.; Powers, J.; Streblow, D.N. Small Molecule Inhibitors Targeting Chikungunya Virus. Curr. Top. Microbiol. Immunol. 2022, 435, 107–139. [Google Scholar] [CrossRef]
- Dessain, S.K.; Adekar, S.P.; Berry, J.D. Exploring the Native Human Antibody Repertoire to Create Antiviral Therapeutics. Hum. Antib. Ther. Viral Dis. 2008, 317, 155. [Google Scholar] [CrossRef]
- Couderc, T.; Khandoudi, N.; Grandadam, M.; Visse, C.; Gangneux, N.; Bagot, S.; Prost, J.F.; Lecuit, M. Prophylaxis and therapy for chikungunya virus infection. J. Infect. Dis. 2009, 200, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Kafai, N.M.; Winkler, E.S.; Gilliland, T.C.; Cottle, E.L.; Earnest, J.T.; Jethva, P.N.; Kaplonek, P.; Shah, A.P.; Fong, R.H.; et al. Pan-protective anti-alphavirus human antibodies target a conserved E1 protein epitope. Cell 2021, 184, 4414–4429.e19. [Google Scholar] [CrossRef]
- Masrinoul, P.; Puiprom, O.; Tanaka, A.; Kuwahara, M.; Chaichana, P.; Ikuta, K.; Ramasoota, P.; Okabayashi, T. Monoclonal antibody targeting chikungunya virus envelope 1 protein inhibits virus release. Virology 2014, 464–465, 111–117. [Google Scholar] [CrossRef]
- Powell, L.A.; Miller, A.; Fox, J.M.; Kose, N.; Klose, T.; Kim, A.S.; Bombardi, R.; Tennekoon, R.N.; Dharshan de Silva, A.; Carnahan, R.H.; et al. Human mAbs Broadly Protect against Arthritogenic Alphaviruses by Recognizing Conserved Elements of the Mxra8 Receptor-Binding Site. Cell Host Microbe 2020, 28, 699–711.e7. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Sumathy, K.; Sahastrabuddhe, S.; Excler, J.L.; Kochhar, S.; Smith, E.R.; Gurwith, M.; Chen, R.T.; Benefit-Risk Assessment of VAccines by TechnolOgy Working Group (BRAVATO, ex-V3SWG). A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for an inactivated viral vaccine against Chikungunya virus. Vaccine 2022, 40, 5263. [Google Scholar] [CrossRef]
- Harrison, V.R.; Eckels, K.H.; Bartelloni, P.J.; Hampton, C. Production and evaluation of a formalin-killed Chikungunya vaccine. J. Immunol. 1971, 107, 643–647. [Google Scholar] [CrossRef]
- Tiwari, M.; Parida, M.; Santhosh, S.R.; Khan, M.; Dash, P.K.; Rao, P.V.L. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine 2009, 27, 2513–2522. [Google Scholar] [CrossRef]
- Slifka, D.K.; Raué, H.P.; Weber, W.C.; Andoh, T.F.; Kreklywich, C.N.; DeFilippis, V.R.; Streblow, D.N.; Slifka, M.K.; Amanna, I.J. Development of a next-generation chikungunya virus vaccine based on the HydroVax platform. PLoS Pathog. 2022, 18, e1010695. [Google Scholar] [CrossRef] [PubMed]
- Hallengärd, D.; Kakoulidou, M.; Lulla, A.; Kümmerer, B.M.; Johansson, D.X.; Mutso, M.; Lulla, V.; Fazakerley, J.K.; Roques, P.; Le Grand, R.; et al. Novel Attenuated Chikungunya Vaccine Candidates Elicit Protective Immunity in C57BL/6 mice. J. Virol. 2014, 88, 2858. [Google Scholar] [CrossRef] [PubMed]
- Wressnigg, N.; Hochreiter, R.; Zoihsl, O.; Fritzer, A.; Bézay, N.; Klingler, A.; Lingnau, K.; Schneider, M.; Lundberg, U.; Meinke, A.; et al. Single-shot live-attenuated chikungunya vaccine in healthy adults: A phase 1, randomised controlled trial. Lancet Infect. Dis. 2020, 20, 1193–1203. [Google Scholar] [CrossRef]
- Schneider, M.; Narciso-Abraham, M.; Hadl, S.; McMahon, R.; Toepfer, S.; Fuchs, U.; Hochreiter, R.; Bitzer, A.; Kosulin, K.; Larcher-Senn, J.; et al. Safety and immunogenicity of a single-shot live-attenuated chikungunya vaccine: A double-blind, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2023, 401, 2138. [Google Scholar] [CrossRef]
- McMahon, R.; Toepfer, S.; Sattler, N.; Schneider, M.; Narciso-Abraham, M.; Hadl, S.; Hochreiter, R.; Kosulin, K.; Mader, R.; Zoihsl, O.; et al. Antibody persistence and safety of a live-attenuated chikungunya virus vaccine up to 2 years after single-dose administration in adults in the USA: A single-arm, multicentre, phase 3b study. Lancet Infect. Dis. 2024, 24, 1383–1392. [Google Scholar] [CrossRef]
- Buerger, V.; Hadl, S.; Schneider, M.; Schaden, M.; Hochreiter, R.; Bitzer, A.; Kosulin, K.; Mader, R.; Zoihsl, O.; Pfeiffer, A.; et al. Safety and immunogenicity of a live-attenuated chikungunya virus vaccine in endemic areas of Brazil: Interim results of a double-blind, randomised, placebo-controlled phase 3 trial in adolescents. Lancet Infect. Dis. 2025, 25, 114–125. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, Z.-R.; Li, N.; Pei, X.-R.; Li, X.-D.; Deng, C.-L.; Ye, H.Q.; Zhang, B. High-Titer Self-Propagating Capsidless Chikungunya Virus Generated in Vero Cells as a Strategy for Alphavirus Vaccine Development. J. Virol. 2022, 96, e01480-21. [Google Scholar] [CrossRef] [PubMed]
- Roques, P.; Ljungberg, K.; Kümmerer, B.M.; Gosse, L.; Dereuddre-Bosquet, N.; Tchitchek, N.; Hallengärd, D.; García-Arriaza, J.; Meinke, A.; Esteban, M.; et al. Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight 2017, 2, e83527. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Liu, X.; Zaid, A.; Goh, L.Y.H.; Hobson-Peters, J.; Hall, R.A.; Merits, A.; Mahalingam, S. Mutation of the N-Terminal Region of Chikungunya Virus Capsid Protein: Implications for Vaccine Design. MBio 2017, 8, e01970-16. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Teo, T.; Utt, A.; Tan, J.J.; Amrun, S.N.; Bakar, F.A.; Yee, W.X.; Becht, E.; Lee, C.Y.; Lee, B.; et al. Mutating chikungunya virus non-structural protein produces potent live-attenuated vaccine candidate. EMBO Mol. Med. 2019, 11, e10092. [Google Scholar] [CrossRef]
- Carrau, L.; Rezelj, V.V.; Noval, M.G.; Levi, L.I.; Megrian, D.; Blanc, H.; Weger-Lucarelli, J.; Moratorio, G.; Stapleford, K.A.; Vignuzzi, M. Chikungunya Virus Vaccine Candidates with Decreased Mutational Robustness Are Attenuated In Vivo and Have Compromised Transmissibility. J. Virol. 2019, 93, e00775-19. [Google Scholar] [CrossRef]
- Piper, A.; Ribeiro, M.; Smith, K.M.; Briggs, C.M.; Huitt, E.; Nanda, K.; Spears, C.J.; Quiles, M.; Cullen, J.; Thomas, M.E.; et al. Chikungunya Virus Host Range E2 Transmembrane Deletion Mutants Induce Protective Immunity against Challenge in C57BL/6J Mice. J. Virol. 2013, 87, 6748. [Google Scholar] [CrossRef]
- Wang, E.; Volkova, E.; Adams, A.P.; Forrester, N.; Xiao, S.Y.; Frolov, I.; Weaver, S.C. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine 2008, 26, 5030. [Google Scholar] [CrossRef]
- Wang, E.; Kim, D.Y.; Weaver, S.C.; Frolov, I. Chimeric Chikungunya Viruses Are Nonpathogenic in Highly Sensitive Mouse Models but Efficiently Induce a Protective Immune Response. J. Virol. 2011, 85, 9249. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Auguste, A.J.; Kaelber, J.T.; Luo, H.; Rossi, S.L.; Fenton, K.; Leal, G.; Kim, D.Y.; Chiu, W.; Wang, T.; et al. A Chikungunya Fever Vaccine Utilizing an Insect-Specific Virus Platform. Nat. Med. 2016, 23, 192. [Google Scholar] [CrossRef]
- Wang, D.; Suhrbier, A.; Penn-Nicholson, A.; Woraratanadharm, J.; Gardner, J.; Luo, M.; Le, T.T.; Anraku, I.; Sakalian, M.; Einfeld, D.; et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine 2011, 29, 2803. [Google Scholar] [CrossRef]
- Mallilankaraman, K.; Shedlock, D.J.; Bao, H.; Kawalekar, O.U.; Fagone, P.; Ramanathan, A.A.; Ferraro, B.; Stabenow, J.; Vijayachari, P.; Sundaram, S.G.; et al. A DNA vaccine against Chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl. Trop. Dis. 2011, 5, e928. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Ramanathan, A.A.; Kawalakar, O.; Sundaram, S.G.; Tingey, C.; Bian, C.B.; Muruganandam, N.; Vijayachari, P.; Sardesai, N.Y.; Weiner, D.B.; et al. Nonstructural Protein 2 (nsP2) of Chikungunya Virus (CHIKV) Enhances Protective Immunity Mediated by a CHIKV Envelope Protein Expressing DNA Vaccine. Viral Immunol. 2013, 26, 75. [Google Scholar] [CrossRef]
- Muthumani, K.; Block, P.; Flingai, S.; Muruganantham, N.; Chaaithanya, I.K.; Tingey, C.; Wise, M.; Reuschel, E.L.; Chung, C.; Muthumani, A.; et al. Rapid and Long-Term Immunity Elicited by DNA-Encoded Antibody Prophylaxis and DNA Vaccination Against Chikungunya Virus. J. Infect. Dis. 2016, 214, 369. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.; Li, X.; Huang, W.; Fang, E.; Li, W.; Liu, X.; Liu, M.; Li, J.; Li, M.; et al. Construction and immunogenicity of an mRNA vaccine against chikungunya virus. Front. Immunol. 2023, 14, 1129118. [Google Scholar] [CrossRef]
- Abeyratne, E.; Tharmarajah, K.; Freitas, J.R.; Mostafavi, H.; Mahalingam, S.; Zaid, A.; Zaman, M.; Taylor, A. Liposomal Delivery of the RNA Genome of a Live-Attenuated Chikungunya Virus Vaccine Candidate Provides Local, but Not Systemic Protection After One Dose. Front. Immunol. 2020, 11, 304. [Google Scholar] [CrossRef]
- Szurgot, I.; Ljungberg, K.; Kümmerer, B.M.; Liljeström, P. Infectious RNA vaccine protects mice against chikungunya virus infection. Sci. Rep. 2020, 10, 21076. [Google Scholar] [CrossRef]
- Saraswat, S.; Athmaram, T.N.; Parida, M.; Agarwal, A.; Saha, A.; Dash, P.K. Expression and Characterization of Yeast Derived Chikungunya Virus Like Particles (CHIK-VLPs) and Its Evaluation as a Potential Vaccine Candidate. PLoS Negl. Trop. Dis. 2016, 10, e0004782. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.C.; Streblow, Z.J.; Kreklywich, C.N.; Denton, M.; Sulgey, G.; Streblow, M.M.; Marcano, D.; Flores, P.N.; Rodriguez-Santiago, R.M.; Alvarado, L.I.; et al. The Approved Live-Attenuated Chikungunya Virus Vaccine (IXCHIQ®) Elicits Cross-Neutralizing Antibody Breadth Extending to Multiple Arthritogenic Alphaviruses Similar to the Antibody Breadth Following Natural Infection. Vaccines 2024, 12, 893. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária, A. IXCHIQ (Vacina Chikungunya): Novo Registro 2025. Available online: https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/novos-medicamentos-e-indicacoes/ixchiq-vacina-chikungunya-novo-registro (accessed on 21 July 2025).
- Travel Health Notices; CDC. Chikungunya in Timor-Leste—Level 2—Practice Enhanced Precautions 2024. Available online: https://wwwnc.cdc.gov/travel/notices/level2/chikungunya-timor-leste (accessed on 21 July 2025).
- Henderson Sousa, F.; Ghaisani Komarudin, A.; Findlay-Greene, F.; Bowolaksono, A.; Sasmono, R.T.; Stevens, C.; Barlow, P.G. Evolution and immunopathology of chikungunya virus informs therapeutic development. DMM Dis. Models Mech. 2023, 16, dmm049804. [Google Scholar] [CrossRef] [PubMed]
- Cherian, N.; Bettis, A.; Deol, A.; Kumar, A.; Di Fabio, J.L.; Chaudhari, A.; Yimer, S.; Fahim, R.; Endy, T. Strategic considerations on developing a CHIKV vaccine and ensuring equitable access for countries in need. NPJ Vaccines 2023, 8, 123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).