Abstract
Intermittent preventive treatment in pregnancy with sulfadoxine and pyrimethamine (IPTp-SP) is a key component in the malaria control strategy implemented in Africa. The aim of this study was to determine IPTp-SP adherence and coverage, and the impact on maternal infection and birth outcomes in the context of widespread SP resistance in the city of Douala, Cameroon. Clinical and demographic information were documented among 888 pregnant women attending 3 health facilities, from the antenatal care visit to delivery. Positive samples were genotyped for P. falciparum gene (dhfr, dhps, and k13) mutations. The overall IPTp-SP coverage (≥three doses) was 17.5%, and 5.1% received no dose. P. falciparum prevalence was 16%, with a predominance of submicroscopic infections (89.3%). Malaria infection was significantly associated with locality and history of malaria, and it was reduced among women using indoor residual spraying. Optimal doses of IPTp-SP were significantly associated with reduced infection among newborns and women (secundiparous and multiparous), but there was no impact of IPTp-SP on the newborn bodyweight. Pfdhfr-Pfdhps quintuple mutants were over-represented (IRNI-FGKAA, IRNI-AGKAA), and sextuple mutants (IRNI-AGKAS, IRNI-FGEAA, IRNI-AGKGS) were also reported. The Pfk13 gene mutations associated with artemisinin resistance were not detected. This study highlights the role of ANC in achieving optimal SP coverage in pregnant women, the mitigated impact of IPTp-SP on malaria outcomes, and the high prevalence of multiple SP-resistant P. falciparum parasites in the city of Douala that could compromise the efficacy of IPTp-SP.
Keywords:
malaria infection; IPTp-SP; pregnant women; effectiveness; dhfr; dhps; k13 mutations; Cameroon 1. Introduction
During pregnancy, especially in primiparous and secundiparous, women are at risk of several infectious diseases, including toxoplasmosis, leishmaniasis, and malaria [1,2]. Malaria in pregnancy (MiP) is an important public health concern in endemic areas, particularly in sub-Saharan Africa (sSA). In 2019, in the period of study, the World Health Organization (WHO) reported that 35% (~11.6 millions) of pregnancies in 33 sub-Saharan African countries with moderate-to-high risk transmission were exposed to malaria [3]. With the exception of Plasmodium knowlesi, all other species of human malaria parasites circulate in sSA, with P. falciparum being the predominant species [4]. Such infections can cause severe complications to the mother, her unborn baby, or child, and include maternal anemia, stillbirth, premature delivery, low birthweight, and growth retardation [5,6].
The management of MiP encompasses free distribution of long-lasting insecticide-treated nets, treatment of malaria cases, folic acid supplementation, and prevention of MiP through intermittent administration of sulfadoxine-pyrimethamine (SP), known as intermittent preventive treatment with SP during pregnancy (IPTp-SP) [3]. These strategies are implemented as part of antenatal care (ANC) services to prevent malaria and anemia in pregnant women living in moderate- to high-endemic areas. Since 2004, the WHO recommended IPTp-SP for preventing malaria episodes during pregnancy and for reducing the burden of MiP in sub-Saharan Africa, and to date, several malaria endemic countries have adopted IPTp-SP as a key preventive measure [3]. Many studies suggest a positive impact of IPTp-SP on maternal health and on morbidity and mortality in women and children [7,8,9]. However, considering the inadequacy of the two-dose regimen initially recommended to protect newborns from the deleterious effects of MiP during the third trimester, and that the beneficial effect of IPTp-SP on birthweight is dose-dependent [10,11], the WHO revised its policy in 2013. The revised WHO guidelines indicate that each pregnant woman should receive at least three doses of SP (IPTp-SP3+), also known as the optimal IPTp-SP dosage, with each dose administered at each ANC visit at one-month intervals, beginning in the second trimester and continuing until delivery [12].
In addition to the reported poor adherence and the low IPTp-SP uptake [13,14,15], many studies have highlighted that its effectiveness is reduced and compromised in areas where the prevalence of SP-resistant P. falciparum parasites is high in Africa [16], in the context where no other alternative drug with the same benefits as SP has yet been identified [17,18]. There is strong evidence that resistance to antimalarial drugs is associated with parasite genetic factors (single and crossed mutations in P. falciparum dihydrofolate reductase (Pfdhfr) and dihydropteroate synthetase (Pfdhps) genes). In this context, it is crucial to periodically monitor IPTp-SP coverage and effectiveness as well as the epidemiology of SP-resistant parasites among pregnant women in endemic areas, particularly in sSA which bears the bulk of the global MiP burden [3].
After the withdrawal of chloroquine and the adoption of the WHO policy in 2004, Cameroon have adopted and intensified the implementation of the revised WHO policy in 2013 with the aim to give at least three doses of SP between the 16th and the 36th weeks of gestation. Between 2014 and 2018, the proportion of pregnant women who received at least three doses of SP experienced a gradual progression, from 28% in 2014 to 38% in 2017, with regional disparities, and in the coastal region, this prevalence was estimated at 35% [19]. Cameroon is part of the WHO’s “High burden to High impact” strategy, which aims to reduce malaria by 2030 in the 11 highest-burden countries [20]. Research on the epidemiology of MiP, coverage, associated factors, and effectiveness of IPTp-SP is increasingly being conducted in Cameroon, particularly in the southwest, northwest, and central regions [21,22,23,24,25]. The present study was conducted in pregnant women living in the city of Douala, in the Littoral Region of Cameroon, where there is a paucity of data on the topic [26]. We determined the prevalence of malaria infection and submicroscopic parasitemia using quantitative polymerase chain reaction (qPCR), assessed the coverage and factors associated with IPTp-SP3+, and evaluated the effectiveness of IPTp-SP on selected maternal and birth outcomes. Additionally, P. falciparum malaria parasites were genotyped for mutations associated with SP and artemisinin (ART) resistance.
2. Materials and Methods
2.1. Study Sites
The study was conducted in the city of Douala (Littoral Region) (Figure 1). Douala is the economic capital of Cameroon, and it is divided into six districts (i.e., Douala I–VI districts). The population is greatly heterogeneous, with a predominance of three ethnic groups (Duala, Bassa, and Bamileke). The city is located in the tropical forest epidemiological area characterized by a diversity of ecosystems, heavy rainfall (1500–5000 mm3/year), and a humid climate. In this area, malaria transmission is perennial and holoendemic [27,28]. The main malarial species is P. falciparum, but other species including P. ovale and P. vivax have also been reported [29,30]. Three health facilities were included in the study, namely: (i) Deido District Hospital, located in the Douala I district, (ii) Bonassama District Hospital in the Douala IV district, and (iii) Nylon St Paul Maternity Clinic in the Douala III district. These three hospitals are represented as “Bonassama”, “Deido”, and “St Paul” in subsequent sections of this paper.
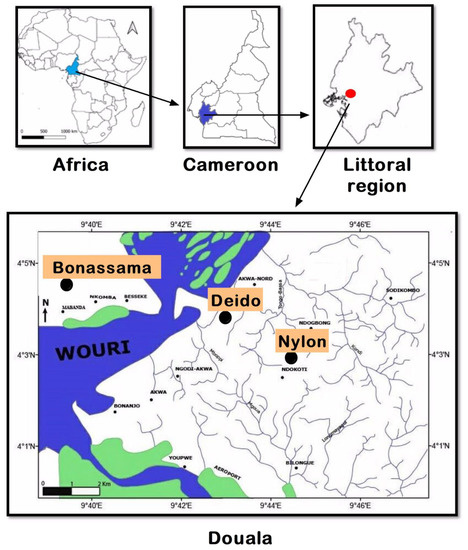
Figure 1.
Location of the study sites in the town of Douala, Littoral Region, Cameroon. Note: Three health facilities were included in three health districts, referred to as the Deido, Bonassama, and Nylon districts, located in Douala III district of the Littoral Region in Cameron, Central Africa Region.
2.2. Study Design, Study Population, and Sampling Strategy
The study was designed as a hospital-based prospective survey and conducted from December 2015 to December 2016 in three facilities in Douala, Littoral Region, Cameroon. After obtaining ethical and administrative authorizations, pregnant women were approached to explain the objectives of the study, and informed consent was obtained. Sociodemographic, clinical, and paraclinical data were collected using an ad hoc survey form. Blood samples were collected for anemia and malaria diagnosis. Each participant was administered an SP dose as per national guidelines and received education on malaria and IPTp-SP.
The target population consisted of pregnant women attending the health facilities for antenatal care (ANC) visits and delivery. Women willing to participate in the study and having signed an informed consent form were included. Women infected with human immunodeficiency virus/under cotrimoxazole therapy, with a documented history of allergy to sulfamides and having recently taken SP alone or in combination with folic acid, were excluded from the study.
The women were consecutively recruited in each of the health facilities to limit selection and information biases. The sample size was computed using Lorentz’ formula: N = p × (1 − p) × z2/d2, where N is the required minimum sample size for the study, p (33%, as previously reported in the Littoral Region [31]) is the proportion of pregnant women reported to have received at least one IPTp-SP dose, z is the statistic for the desired confidence interval (z = 1.96 for the 95% confidence interval), and d is the accepted margin of error (d = 0.05). Thus, the minimum sample size was estimated to be 340 pregnant women.
2.3. Data Collection
A structured questionnaire consisting of five sections was used to collect data from each pregnant woman and their baby. The first section of the questionnaire captured sociodemographic information (age, region of origin, residence, level of education, occupation, and management of health expenses). The second part was designed to document knowledge on IPTp-SP and evaluate the environmental risk of malaria infection. The participants were asked about the presence of water collections (e.g., lake, bog), the implementation of a malaria-related prevention plan at home (i.e., insecticide-treated net, coils, insecticide residual spraying), and continuous education towards malaria. The third and fourth sections documented gynecological and family history, and clinical and physical information on ongoing pregnancy, respectively. In this section, information such as the date of the last menstruations, probable date of delivery, number of pregnancies, number of children, number of abortions, and comorbidities (diabetes, hypertension) was collected. The fifth section aimed to report data on biological examinations along with data on babies.
2.4. Blood Collection, Hematological, and Parasitological Analyses
About 4 mL of peripheral blood (women attending ANC and at delivery) and 2–4 mL of placental blood on the newborn side (newborn infection among women reporting for delivery at the hospital) were collected into EDTA tubes. The collected blood was used to perform biological tests. For molecular analysis, 160 µL of peripheral or placental blood was spotted onto blotting paper (Whatman FTA® Elute) and stored at 4 °C until needed.
The hemoglobin (Hb) concentration and hematocrit (Hct) levels in the blood were determined using a portable hemoglobinometer (Hb Hemoglobin Test Strips, Mission® Plus Hb, San Diego, CA, USA). Anemia in pregnancy was defined as an Hb level below 11 g/dL (or Hct < 33%) [32]. Anemia status was further classified into three categories (light, moderate, and severe) according to the most recent WHO criteria [32].
Malaria infection was determined using a rapid diagnostic test (Pan SD Bioline) and Giemsa-stained blood films (thick and thin smears). The Pan SD Bioline RDT is an immunochromatographic test that qualitatively detects the presence of the histidine-rich protein 2, an antigen specifically produced by P. falciparum, and pan lactate dehydrogenase that is produced by all Plasmodium species. This RDT is recommended by the WHO for malaria diagnosis [33], and was performed in accordance with the manufacturer’s instructions. Results were categorized as valid (testing positive or negative, and a positive control line) and invalid (absence of the control line). Blood films and microscopic examination were performed as previously described [34]. Thin blood films were used to establish malaria species, while thick blood films were used to quantify malaria parasitemia. Parasites were counted against at least 200 leucocytes, and parasitemia was determined by assuming a leucocyte density of 8000/mm3 for each participant [35]. Readings of the blood films were performed by skilled microscopists at the Centre Pasteur Cameroon.
2.5. Molecular Analysis: Parasite Detection by PCR and Genotyping of the Pfdhfr, Pfdhps, and Pfk13 Genes by Sequencing
All molecular analyses were performed at the Noguchi Memorial Institute for Medical Research (NMIMR), Accra, Ghana, and included quantitative polymerase chain reaction (qPCR) of P. falciparum samples and genotyping of drug resistance-associated P. falciparum genes. The plasmodial DNA was extracted from the dried blood spots using the Chelex method, as previously described [36].
Two quantitative polymerase chain reaction (qPCR) protocols were used to track P. falciparum infections, targeting the var gene acidic terminal sequence (varATS) and the telomere-associated repetitive element 2 (TARE2), respectively.
Parasitemia was quantified by extrapolation of cycle thresholds (Ct) from a standard curve of 3D7 P. falciparum-infected erythrocytes culture after 40–45 cycles for TARE2qPCR and 45 cycles for ATSqPCR. A negative control with no DNA template was run in all reactions. A threshold parasite density of >2 parasites/µL was considered positive [37]. Positive (3D7) and negative (distilled water) controls were run for each amplification. Submicroscopic infections were defined as infections not detected by microscopy examination but detected using qPCR. The presence of P. falciparum DNA in the extracted DNA was examined in duplicate by qPCR.
All samples that were detected positive by TARE2 qPCR where genotyped for three genes, namely dihydrofolate reductase (Pfdhfr) and dihydropteroate synthase (Pfdhps) genes associated with SP resistance, and the propeller domain of the Kelch 13 gene (Pfk13) associated with artemisinin (ART) resistance, as previously described [36,38,39]. PCR products detectable as a clear band of the expected sizes were purified using Wizard SV Gel and the PCR Clean-Up System® (Promega, Madison, WI, USA) and sent for sequencing (GATC, Cologne, Germany) with the corresponding nested primers. The sequences generated were analyzed with the Chromas software [40], then aligned using the MEGA 5.2 software [41] and compared to the 3D7 P. falciparum reference genome. The SP resistance-associated mutations at positions 16, 51, 59, 108, and 164 in the Pfdhfr gene and positions 436, 437, and 540 in the Pfdhps gene were screened.
2.6. Statistical Analysis
Data were keyed into an Excel spreadsheet and then exported to StatView v5.03 for Windows (SAS Institute, Inc., Chicago, IL, USA) and GraphPad v7.03 for Windows (GraphPad PRISM, Inc., San Diego, CA, USA) for statistical analysis. Qualitative variables were presented as percentages, while quantitative variables were presented as mean ± standard deviation (SD). Pearson’s and Fisher’s exact tests were used to compare proportions. One-way analysis of variance and Student’s t-tests were used to compare mean values between ≥2 groups. Their non-parametric versions (i.e., Mann–Whitney and Kruskal–Wallis tests) were used as alternatives. Statistical significance was set at p-value < 0.05.
3. Results
3.1. Pregnant Women Included in the Study
A total of 1208 women were approached during the study, among them 888 were included based on different exclusion criteria (Figure 2).
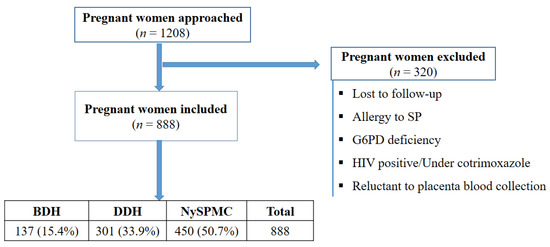
Figure 2.
Flow diagram of the study depicting the process of participant inclusion. Note: SP: Sulfadoxine-pyrimethamine, G6PD: glucose-6-phosphate dehydrogenase, HIV: human immunodeficiency virus, BDH: Bonassama District Hospital, DDH: Deido District Hospital, NySPMC: Nylon St Paul Maternity Clinic.
3.2. Sociodemographic and Gynecological Characteristics
Women aged 22–26 and 26–31 years accounted for 28% and 32.9% of the participants, respectively. The overall mean age of the participants was 26.90 ± 5.30 years. More than half of the women had completed secondary education, and a statistically significant difference was found between the level of education and health facilities (Table 1). The proportion of primiparous women was significantly higher in women attending the Deido Hospital (43.5%) than among those attending the Bonassama (33.8%) and St Paul (31.3%) Hospitals. The overall proportion of women having received IPTp-SP during their previous pregnancies was 70.2%.

Table 1.
Sociodemographic and gynecological characteristics of pregnant women.
3.3. Antenatal Care Visits and IPTp-SP Coverage
Analysis of the timing of the first ANC revealed that 29%, 36.2%, and 38.1% of women attended their first ANC at ≤16, 17–24, and 25+ weeks of pregnancy, respectively (Figure 3a). Women were 20.5 ± 4.9 weeks pregnant at their first ANC. No statistically significant association was found between gestational age and ANC visits (p = 0.62) (Figure 3a). At term, 5.1% (95% CI: 3.8–6.8%) of women had not yet received any dose of IPTp-SP. The IPTp coverage with one, two, three, or four doses of SP was 48.9% (95% CI: 45.5–52.1%), 28.5% (95% CI: 25.6–31.5%), 13.2% (95% CI: 11.1–15.6%), and 4.3% (95% CI: 3.2–5.9%), respectively. Thus, the overall proportion of women with adequate IPTp-SP coverage (at least three doses of SP, i.e., IPTp-SP3+) was 17.5% (95% CI: 15.1–20.2%).
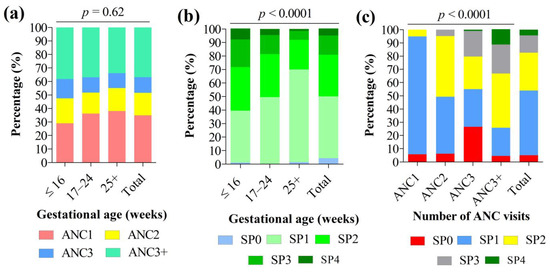
Figure 3.
Association between (a) gestational age and antenatal care visits, (b) gestational age and IPTp-SP coverage, and (c) antenatal care visits and IPTp-SP coverage. Note: IPTp-SP: Intermittent preventive treatment with sulfadoxine-pyrimethamine in pregnancy. Pearson’s chi square test was used to compare percentages. Statistically significant at p-value < 0.05.
The proportion of women attending their first ANC and receiving their first SP dose, and on time, was significantly higher in women with knowledge on IPTp-SP compared to their counterparts with no knowledge on IPTp-SP (27.4% vs. 18.7%, p = 0.04 for ANC, and 26.9% vs. 18.9%, p = 0.005 for SP dose). No statistically significant association was found between the timing of the first ANC, the first SP dose, and other participant characteristics (Supplementary File S1).
3.4. Factors Associated with Administration of at Least Three Doses of IPTp-SP (IPTp-SP3+) among Women at Delivery
Six determinants of optimal IPTp-SP coverage were identified based on multivariate logistic regression (participants’ age, level of education, number of ANC, timing of the first ANC, knowledge of IPTp-SP, and implementation of a prevention plan at home) (Table 2).

Table 2.
Determinants of uptake of ITPp-SP3+ among full-term women.
The uptake of the IPTp-SP optimal dose significantly increased with the level of education. Women having completed secondary and university studies had 21.61-fold (95% CI: 1.15–406.46, p = 0.007) and 6.30-fold (95% CI: 2.37–124.38, p = 0.002) higher uptake than their counterparts who had completed primary studies. An increase in age by one unit was associated with an increase in IPTp-SP3+ dose by 1.19 times (95% CI: 1.02–1.40, p = 0.03). Women having knowledge of IPTp-SP had three times more chance (OR = 2.91, 95% CI: 1.01–11.91, p = 0.04) to receive IPTp-SP3+ doses than those with no knowledge. Of note, the proportion of women with good knowledge of IPTp-SP significantly increased with the level of education, from 14.2% at the primary level to 26.2% at the university level (χ2 = 9.67, df = 3, p = 0.02). Interestingly, the chances of receiving optimal SP doses were reduced by 90% (OR = 0.10, 95% CI: 0.01–0.96, p = 0.04) in women attending their first ANC at ≥25 weeks of pregnancy compared to those attending at ≤16 weeks of pregnancy.
3.5. Prevalence of P. falciparum Malaria and Submicroscopic Infections in Peripheral Blood
It should be noted that LM, RDT, and PCR results were not available for all women included in the study, for various reasons. We therefore calculated the prevalence of P. falciparum malaria for the techniques using different sample sizes (Figure 4). The P. falciparum prevalence rates were 3.2% (25/789, 95% CI: 2.2–4.6%) for LM, 4.2% (34/785, 95% CI: 3.1–5.9%) for RDT, and 16.0% (81/505, 95% CI: 13.1–19.5%) for qPCR. By comparing LM and qPCR, we have noted that 67 of 75 infections (89.3%) detected by qPCR were not detected by LM. Thus, the overall proportion of submicroscopic infections using peripheral blood was 14.4% (67/465, 95% CI: 11.5–17.9%) (Figure 4).
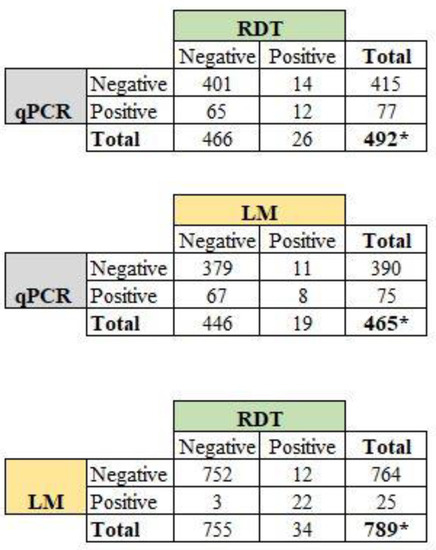
Figure 4.
Proportion of peripheral P. falciparum infections among the women by comparing diagnostic methods. Note: LM: light microscopy; RDT: rapid diagnostic test; qPCR: quantitative polymerase chain reaction. * Total sample size was below 888 as results for LM, RDT, and qPCR were not available for some women during the study.
Considering only samples with available results of malaria infection for all three methods (n = 465 women), the prevalence of peripheral P. falciparum infection was 4.1% (95% CI: 31–5.5%) for LM, 5.6% (95% CI: 3.9–7.0%) for RDT, and 16.1% (95% CI: 8.7–12.9%) for qPCR.
3.6. Factors Associated with qPCR-Based P. falciparum Malaria Infection
Based on univariate logistic regression analysis, health facility, no knowledge of IPTp-SP, and history of malaria were associated with malaria infection (Table 3). After adjusting in the multivariate analysis, three factors were statistically associated with malaria infection. The odds of P. falciparum infection were 3.03 (95% CI: 1.02–8.85, p = 0.03) and 4.37 (95% CI: 1.52–12.27, p = 0.003) times higher in women attending the Deido and St Paul health facilities, respectively, compared to women attending the Bonassama Hospital. Women with a history of malaria were twice as likely (95% CI: 1.08–3.73, p = 0.02) to be infected with P. falciparum compared to those with no history of infection. The risk of peripheral P. falciparum infection was reduced by 47% in those using indoor residual (IRS) spraying (aOR: 0.53, 95% CI: 0.30–0.95, p = 0.03).

Table 3.
Univariate and multivariate logistic analysis of factors associated with qPCR-based peripheral P. falciparum infection among pregnant women.
3.7. Anemia and Its Association with Malaria Infection, Submicroscopic Infections, and IPTp-SP
The prevalence of maternal anemia was 36.3%, with most cases graded as mild (58.5%) (Figure 5a). Hemoglobin levels were slightly lower in P. falciparum-infected patients compared to their uninfected counterparts, but the difference was not statistically significant (p = 0.67) (Figure 5b). Similarly, hemoglobin levels were statistically similar between women with microscopic infections and those with submicroscopic infections (p = 0.08) (Figure 5c). No statistically significant association was found between the prevalence of anemia or submicroscopic infections and the participant characteristics (health facilities, age groups, education level, marital status, occupation, implementation of prevention plan, IPTp-SP doses, ITN use, gestational age at first ANC, gestational age at first IPTp-SP dose, parity) (Supplementary File S2).
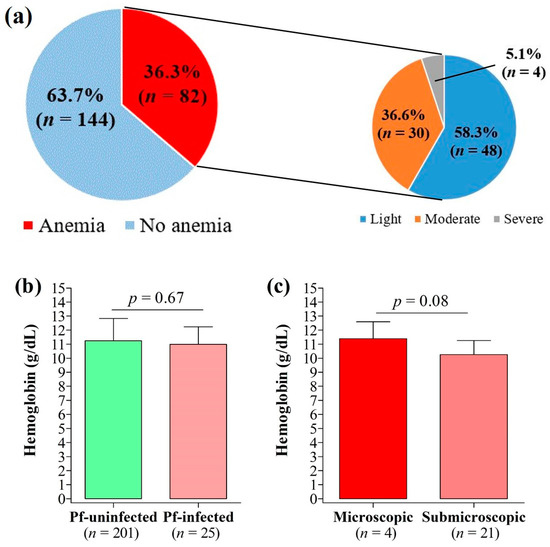
Figure 5.
Anemia and its relation to malaria infection. (a) Prevalence of anemia, (b) variation of hemoglobin count by peripheral malaria infection, and (c) variation of hemoglobin count by type of peripheral malaria infection.
3.8. Impact of SP Dose on Maternal and Birth Outcomes
In total, 182 babies were included in the study, 2 in Bonassama, 52 in Deido, and 118 in St Paul health facilities. The overall proportion of low birthweight (LBW) was 9.34% (17/182, 95% CI: 5.9–14.5%). The proportion of LBW in Deido and St Paul health facilities was 5.8% (3/52, 95% CI: 1.9–15.6%) and 11.9% (14/118, 95% CI: 7.2–18.9%), respectively.
With respect to parity, the prevalence of maternal P. falciparum infection was significantly reduced with SP doses in secundiparous and multiparous women (Table 4). In secundiparous, the prevalence of anemia ranged from 33.3% in women having received no SP dose to 7.7% in those having received more than three doses (p = 0.02). In multiparous women, the prevalence ranged from 25% to 0% (p = 0.01). The IPTp-SP dose had no significant effect on maternal anemia, LBW, or neonatal P. falciparum infection.

Table 4.
Effect of IPTp-SP on maternal and birth parameters.
3.9. Sulfadoxine-Pyrimethamine and Artemisinin Resistance Molecular Markers
Of the qPCR-positive samples, successful amplification of the Pfdhfr, Pfdhps, and Pfk13 genes was achieved for 37, 35, and 32 samples, respectively (Table 5). Mutations 51I (83.3%), 59R (97.3%), and 108N (97.3%) in the Pfdhfr gene, and 437G (94.3%) in the Pfdhps gene, were predominantly found. Quadruple mutants were found in 6 (17.1%) of 35 sequenced samples, represented by NRNI-FGKAA, IRNI-AAKAA, and IRNI-FAKAA. Quintuple mutants (IRNI-AGKAA and IRNI-FGKAA) were the most represented of the multiple mutations, with 71.4% of samples. Three samples harbored the sextuple mutant and one a septuple mutant. There were no ART resistance-associated mutations in the Pfk13 gene.

Table 5.
Frequency of Pfdhfr, Pfdhps, and Pfk13 genotypes among pregnant women.
4. Discussion
Malaria due to P. falciparum continues to impose enormous losses in humans, especially pregnant women and children. The implementation of strategies such as IPTp-SP has greatly reduced the malaria burden in pregnant women. In this study, we assessed the level of IPTp-SP adherence and coverage as well as its determinants and its impact on maternal and neonatal outcomes in the city of Douala, Cameroon.
Knowledge of IPTp-SP was also associated with IPTp-SP3+ uptake, consistent with a previous study in the city of Bamenda, Northwest Cameroon [25]. This is likely due to the level of education as we found that a high proportion of women with knowledge of IPTp-SP had completed university studies. Several studies reported the positive impact of educational level on the optimal adoption of IPTp-SP in different settings [42,43,44,45]. The higher the level of education of women, the more likely they are to understand health messages about IPTp-SP during ANC or through other communication channels. We have previously shown the key role of educational level on other aspects of malaria, including knowledge, attitudes, and practices towards malaria and its prevention [46,47].
The present study supports the role of the number of ANC visits in the optimal coverage of IPTp-SP among pregnant women, and similar findings have been reported elsewhere [21,43,45,48,49]. Again, our study highlighted the crucial role of the timing of the first ANC visit in optimally covering pregnant women with IPTp-SP, and similar findings were reported in Tanzania and Uganda [43,45]. The earlier a woman attends the ANC visit, the more likely she is to receive the optimal dose of SP, as well as other preventive measures, such as long-lasting insecticide-treated nets and malaria awareness. Less than 20% of the ANC attendees had received an optimal SP dose, and this low coverage rate was similarly reported in previous studies conducted in Uganda and Tanzania [43,45,49]. Women at delivery had low coverage with IPTp-SP, and this is particularly worrying as it questions the availability of IPTp-SP in health facilities. Other studies reported higher rates of optimal IPTp-SP coverage [15,25], and the discrepancies between these studies and ours may likely be due to differences in the study designs, as these studies focused on women who had given birth recently or within a few years before the study.
The risk of P. falciparum malaria infection was low in women implementing IRS at home. This finding underscores the importance of implementing additional preventive methods, such as IRS, LLINs, and IPTp-SP. It is evident that the combination of different methods significantly reduces the chances of contracting malaria parasites, as found by Fokam and colleagues, who observed improved hemoglobin levels in pregnant women using both bed nets and IPTp-SP [23]. Malaria risk also varied by health facility. Most of the women lived close to the facility they attended, and thus the findings may reflect geographical variations in malaria risk [15,42,50].
More than five percent of the P. falciparum infections were submicroscopic, and this is similar to rates reported elsewhere [51]. Again, submicroscopic infections accounted for 60.2% of all infections, which is consistent with studies in malaria settings where submicroscopic parasitemia were found to be predominant [52,53,54,55,56,57,58,59,60]. Detection of submicroscopic infections is particularly challenging for efficient control, as they often constitute undetected parasite reservoirs [57]. Molecular methods are the gold standard for tracking these infections, but they are expensive and difficult to apply in the field in endemic countries, especially in Africa where resources are generally limited.
The results regarding the impact of IPTp-SP on maternal and birth outcomes were contrasted in this study as this preventive strategy reduced the risk of maternal infection but was not associated with maternal anemia and neonatal parameters. The literature on the impact of IPTp-SP is similar to ours, with divergent results on the subject [15,26,58,59]. Differences in malaria settings, study design, host characteristics, parasite characteristics, and host–parasite interactions could likely explain these contrasting findings on the impact of IPTp-SP on malaria burden.
IPTp-SP is still effective for the management of malaria in pregnant women in Cameroon [13,60]. Analyses of the Pfdhfr/Pfdhps genes identified the SP resistance-associated 59R and 108N mutations in Pfdhfr and 437G mutations in Pfdhps at rates close to 100%, and this is consistent with previous studies in Cameroon [61] and elsewhere [62]. The prevalence of the Pfdhfr 51I mutation (83.8%) found in this study was higher and not consistent with recent findings that showed that between 1980 and 2020, there was a significant decline in key mutations for Pfdhfr 51I (72.2–66.9%), Pfdhfr 59R (76.5–67.8%), and Pfdhfr 108N (90.8–67.6%), whereas the Pfdhps 437G mutation seems to be increasing over time (30.4–46.9%) [63]. In this study, we reported a higher level of the prevalence of 437G (94.3%). Both Pfdhps 540E and 581G mutations were found at rates < 5% among patients, lower than the prevalence where the WHO has recommended withdrawal of IPTp-SP, i.e., the prevalence of 581G > 10% and 540E > 95% [60]. Thus, our findings suggest the continuation of IPTp-SP use in MiP in Douala, but it should be interesting to conduct further studies with a higher number of Pfdhfr/Pfdhps sequences in the country to confirm these results. Nearly 89% of isolates carried the Pfdhfr IRN triple mutation, which is higher than that reported in one previous systematic review on mutation distribution in 2020 in Cameroon (~67.3%) and in Africa (~66% in 2020) [62,63]. In addition, quintuple mutants (I51R59N108-F436G437 and I51R59N108-A436G437) were predominantly found in isolates, and such mutants were previously reported in Cameroon and Ghana [61,64,65]. Interestingly, we noted that no wild-type at codon 436 was found in all isolates which were carrying either 436A or 436F. In African regions, most isolates carry S436 or 436A, and mutation 436F is generally found in low proportions [65].
We found Pfdhfr-Pfdhps sextuple mutants (I51R59N108-A436G437S613 and I51R59N108-F436G437E540) and septuple mutants (I51R59N108-A436G437G581S613) at prevalence rates below 11%. Such mutants, also known as fully resistant (I51R59N108-G437E540) and super resistant (I51R59N108-G437E540G581), confer a complete SP resistance and are strongly associated with treatment failures [66]. Only one isolate (2.9%) with five mutations associated with full resistance (i.e., I51R59N108-G437E540) was found in this study (this sample was otherwise with the F436 mutation), and this is in line with the previous low prevalence of this fully resistant mutation in Cameroon [61,63]. In Malawi and Myanmar, P. falciparum isolates with I51R59N108-G437E540 mutations are highly prevalent (>50%) [62]. Additionally, the I51R59N108-A436G437S613 sextuple mutant and the I51R59N108-A436G437G581S613 septuple mutant found at low rates in the present study have been reported in Cameroon, Ghana, Tanzania, and China [61,67,68,69]. In contrast, one study did not report quintuple, sextuple, or septuple mutants in febrile patients living in urban, semi-urban, or rural areas in Gabon [70]. Besides, these prevalence rates of quintuple and sextuple mutants could be underestimated considering the high fitness cost due to acquisition of such multiple mutation points, thereby reducing their density compared to susceptible strains in the absence of treatment. Thus, these multiple mutants likely could have been missed by detection methods in some patients due to the very-low-density parasites [70,71,72,73]. No mutation was identified in the sequenced Pfk13 propeller gene.
5. Conclusions
In conclusion, coverage with optimal SP was low in pregnant women. A set of determinants of optimal IPTp-SP coverage was identified, with ANC being one of the most crucial, and the timing of the first ANC visit and the number of ANC visits were found to be strongly associated. Infection with P. falciparum was relatively high, with a predominance of submicroscopic infections. IPTp-SP positively affected malaria infection in women, but no noticeable effects of this preventive measure were found on maternal anemia and birth outcomes, such as LBW and neonatal infection. This study underscores the role of ANC in achieving optimal SP coverage in pregnant women. The high circulation rate of multiple SP-resistant P. falciparum parasites found in this study could likely explain the limited effect of IPTp-SP on maternal and birth parameters, and this could compromise not only IPTp-SP efficacy but also that of seasonal malaria chemoprevention with SP+ amodiaquine, now widely implemented and recognized as highly effective in protecting young children in the Sahelian Belt, north of Cameroon. The findings underscore the need for continuous monitoring of IPTp effectiveness in high-burden countries and call for urgent consideration of the need to revise national guidelines for the management of MiP.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12060844/s1. Supplementary File S1: Timing of the first antenatal care visit, first dose of sulfadoxine-pyrimethamine, and the participants’ characteristics. Supplementary File S2: Prevalence of submicroscopic infections and anemia by patients’ characteristics.
Author Contributions
Conceptualization and designed the experiments, C.E.E.M.; software, C.E.E.M. and L.P.K.F.; validation, C.E.E.M., B.T., N.T.N. and A.S.E.; formal analysis, C.E.E.M., L.P.K.F. and A.A.; investigation, A.A. and B.T.; resources, A.R.E., I.C.P., P.E.E., S.B.E., G.T. and S.E.N.; data curation, L.P.K.F. and A.A.; writing—original draft preparation, C.E.E.M., L.P.K.F. and A.A.; writing—review and editing, all co-authors; English editing, L.A.; supervision, C.E.E.M. and A.S.E.; project administration, C.E.E.M.; funding acquisition, C.E.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Centre Pasteur Cameroon (Call for proposals for internal projects of the Centre Pasteur of Cameroon 2019, the CPC’s internal funding N°012/2015/CPC/DS) and genotyping by the French Institute for Sustainable Development (IRD).
Institutional Review Board Statement
The study was approved by the institutional review board of the University of Douala (No. CEI-UD/16/02/2015/T). Authorization was also issued by the Delegation of Public Health for the Littoral Region (No. 1313/AS/MINSANTE/DRSPL/BCASS). Objectives, benefits, and risks of the study were explained to participants in one of the two national languages (French or English).
Informed Consent Statement
Written consent was obtained from each pregnant woman prior to their inclusion in the study. They were informed about the confidential and voluntary aspect of the study and were free to withdraw at any time. Malaria-positive women were referred to the medical staff of each health facility for case management as per the national guidelines on malaria in pregnancy.
Data Availability Statement
The datasets analyzed for this study are included in the article. Additional data required are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to all the women who accepted to participate in the study. We also thank the medical and research staff of the different health facilities and Centre Pasteur Cameroon (Cameroon) and the Noguchi Memorial Institute for Medical Research (Ghana) for their technical assistance. The authors acknowledge the help of Stephane Koum (Department of Earth Sciences, The University of Douala, Cameroon) for generating maps of the study sites.
Conflicts of Interest
The authors declare having no competing interests with the publication of this paper.
Abbreviations
ANC: antenatal care, ART: artemisinin, G6PD: glucose-6-phosphate dehydrogenase, Hb: hemoglobin, Hct: hematocrit, HIV: human immunodeficiency virus, IPTp: intermittent preventive treatment in pregnancy, IRS: indoor residual spraying, ITN: insecticide-treated net, LBW: low birthweight, LM: light microscopy, MiP: malaria in pregnancy, NMIMR: Noguchi Memorial Institute for Medical Research, qPCR: quantitative polymerase chain reaction, RDT: rapid diagnostic test, SD: standard deviation, SP: sulfadoxine-pyrimethamine, sSA: sub-Saharan Africa, TARE2: telomere-associated repetitive element 2, varATS: var gene acidic terminal sequence, WHO: World Health Organization.
References
- Ahmed, M.; Sood, A.; Gupta, J. Toxoplasmosis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 44–50. [Google Scholar] [CrossRef]
- Curcio, A.M.; Shekhawat, P.; Reynolds, A.S.; Thakur, K.T. Neurologic infections during pregnancy. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 172, pp. 79–103. [Google Scholar]
- WHO (World Health Organization). World Malaria Report; WHO: Geneva, Switzerland, 2020; Volume WHO/HTM/GM, 299p, ISBN 978-92-4-001579-1. Available online: https://www.who.int/docs/default-source/malaria/world-malaria-reports/9789240015791-eng.pdf (accessed on 5 August 2022).
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef]
- Ngai, M.; Weckman, A.M.; Erice, C.; McDonald, C.R.; Cahill, L.S.; Sled, J.G.; Kain, K.C. Malaria in pregnancy and adverse birth outcomes: New mechanisms and therapeutic opportunities. Trends Parasitol. 2020, 36, 127–137. [Google Scholar] [CrossRef]
- Saito, M.; Briand, V.; Min, A.M.; McGready, R. Deleterious effects of malaria in pregnancy on the developing fetus: A review on prevention and treatment with antimalarial drugs. Lancet Child Adolesc. Health 2020, 4, 761–774. [Google Scholar] [CrossRef]
- Kayentao, K.; Garner, P.; van Eijk, A.M.; Naidoo, I.; Roper, C.; Mulokozi, A.; MacArthur, J.R.; Luntamo, M.; Ashorn, P.; Doumbou, O.K.; et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: Systematic review and meta-analysis. JAMA 2013, 309, 594–604. [Google Scholar] [CrossRef]
- ter Kuile, F.O.; van Eijk, A.M.; Filler, S.J. Resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy. JAMA 2007, 297, 2603–2616. [Google Scholar] [CrossRef]
- Mpogoro, F.J.; Matovelo, D.; Dosani, A.; Ngallaba, S.; Mugono, M.; Mazigo, H.D. Uptake of intermittent preventive treatment with sulphadoxine-pyrimethamine for malaria during pregnancy and pregnancy outcomes: A cross-sectional study in Geita district, North-Western Tanzania. Malar. J. 2014, 13, 455. [Google Scholar] [CrossRef]
- Kayentao, K.; Kodio, M.; Newman, R.D.; Maiga, H.; Doumtabe, D.; Ongoiba, A.; Coulibaly, D.; Salam Keita, A.; Maiga, B.; Mungai, M.; et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J. Infect. Dis. 2005, 191, 109–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maiga, O.M.; Kayentao, K.; Traore, B.T.; Djimde, A.; Traore, B.; Diallo, M.; Ongoiba, A.; Doumtabé, D.; Doumbo, A.; Traoré, M.S. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine for the prevention of malaria during pregnancy in Mali: A randomized controlled trial. Clin. Infect. Dis. 2011, 53, 215–223. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP); World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.afro.who.int/sites/default/files/2017-06/iptp-sp-updated-policy-brief-24jan2014.pdf (accessed on 5 August 2022).
- Yoah, A.T.; Fru-Cho, J.; Kah, E.; Njukang, E.; Wirsiy, F.S.; Duamor, C.T.; Mboudou, E.T. Impact of Adherence to a Full Course of Intermittent Preventive Treatment of Malaria in Pregnancy on Pregnancy Outcome in Muyuka Health District: A Cross-Sectional Study. Int. Arch. Public Health Community Med. 2018, 2, 008. [Google Scholar]
- Essiben, F.; Foumane, P.; De Nguefack, M.A.T.; Eko, F.E.; Njotang, P.N.; Enow, R.M.; Mboudou, E.T. Factors associated with the failure of Intermittent Preventive Treatment for malaria among pregnant women in Yaounde. Pan Afr. Med. J. 2016, 23, 152. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4894739/pdf/PAMJ-23-152.pdf (accessed on 15 August 2022). (In French). [PubMed]
- Anchang-Kimbi, J.K.; Kalaji, L.N.; Mbacham, H.F.; Wepnje, G.B.; Apinjoh, T.O.; Ngole Sumbele, I.U.; Dione-Odom, J.; Tita, A.T.N.; Achidi, E.A. Coverage and effectiveness of intermittent preventive treatment in pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) on adverse pregnancy outcomes in the Mount Cameroon area, South West Cameroon. Malar. J. 2020, 19, 100. [Google Scholar] [CrossRef]
- van Eijk, A.M.; Larsen, D.A.; Kayentao, K.; Koshy, G.; Slaughter, D.E.C.; Roper, C.; Okell, L.C.; Desai, M.; Gutman, J.; Khairallah, C.; et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Massougbodji, A.; Tubert-Bitter, P.; Briand, V.; Journot, V.; Cot, M.; Escolano, S. Mefloquine versus sulfadoxine–pyrimethamine for intermittent preventive treatment in pregnancy: A joint analysis on efficacy and tolerability. Am. J. Trop. Med. Hyg. 2015, 93, 300–304. [Google Scholar] [CrossRef][Green Version]
- Roh, M.E.; ter Kuile, F.O.; Rerolle, F.; Glymour, M.M.; Shiboski, S.; Gosling, R.; Gutman, J.; Kakuru, A.; Desai, M.; Kajubi, R.; et al. Overall, anti-malarial, and non-malarial effect of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine on birthweight: A mediation analysis. Lancet Glob. Health 2020, 8, e942–e953. [Google Scholar] [CrossRef]
- Ministry of Public Health, National Observatory of Public Health, World Health Organization. Report of the Monitoring of the 100 Key Health Indicators in Cameroon in 2017. Available online: http://cdnss.minsante.cm/sites/default/files/Fr_Rapport-de-Suivi-des-100-Indicateurs-Cl%C3%A9s-de-Sant%C3%A9-Au-Cameroun-en-2017%20%28Derniere%20version%29.pdf (accessed on 15 August 2022). (In French).
- WHO (World Health Organization). High Burden to High Impact: A Targeted Malaria Response; WHO: Geneva, Switzerland, 2018. [Google Scholar] [CrossRef]
- Anchang-Kimbi, J.K.; Achidi, E.A.; Apinjoh, T.O.; Mugri, R.N.; Chi, H.F.; Tata, R.B.; Nkegoum, B.; Mendimi, J.M.; Sverremark-Ekström, E.; Troye-Blomberg, M. Antenatal care visit attendance, intermittent preventive treatment during pregnancy (IPTp) and malaria parasitaemia at delivery. Malar. J. 2014, 13, 162. [Google Scholar] [CrossRef]
- Babakhanyan, A.; Tutterrow, Y.L.; Bobbili, N.; Salanti, A.; Wey, A.; Fogako, J.; Leke, R.J.; Leke, R.G.F.; Taylor, D.W. Influence of intermittent preventive treatment on antibodies to VAR2CSA in pregnant Cameroonian women. Am. J. Trop. Med. Hyg. 2016, 94, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Fokam, E.B.; Ngimuh, L.; Anchang-Kimbi, J.K.; Wanji, S. Assessment of the usage and effectiveness of intermittent preventive treatment and insecticide-treated nets on the indicators of malaria among pregnant women attending antenatal care in the Buea Health District, Cameroon. Malar. J. 2016, 15, 172. [Google Scholar] [CrossRef]
- Leonard, N.; Eric, F.B.; Judith, A.K.K.; Samuel, W. Factors associated to the use of insecticide treated nets and intermittent preventive treatment for malaria control during pregnancy in Cameroon. Arch. Public. Health 2016, 74, 5. [Google Scholar] [CrossRef]
- Diengou, N.H.; Cumber, S.N.; Nkfusai, C.N.; Mbinyui, M.S.; Viyoff, V.Z.; Bede, F.; Akwah, L.; Tsoko-Gwegweni, J.M.; Anchang-Kimbi, J. Factors associated with the uptake of intermittent preventive treatment of malaria in pregnancy in the Bamenda health districts, Cameroon. Pan Afr. Med. J. 2020, 35, 42. [Google Scholar] [CrossRef]
- Tonga, C.; Kimbi, H.K.; Anchang-Kimbi, J.K.; Nyabeyeu, H.N.; Bissemou, Z.B.; Lehman, L.G. Malaria risk factors in women on intermittent preventive treatment at delivery and their effects on pregnancy outcome in Sanaga-Maritime, Cameroon. PLoS ONE 2013, 8, e65876. [Google Scholar] [CrossRef]
- Snow, R.W.; Noor, A.M. Malaria Risk Mapping in Africa: The Historical Context to the Information for Malaria (INFORM) Project; Working Paper in Support of the INFORM Project Funded by the Department for International Development and the Wellcome Trust; Kenya Medical Research Institute (KEMRI): Nairobi, Kenya, 2015. [Google Scholar]
- Antonio-Nkondjio, C.; Ndo, C.; Njiokou, F.; Bigoga, J.D.; Awono-Ambene, P.; Etang, J.; Same Ekobo, A.; Wondji, C.S. Review of malaria situation in Cameroon: Technical viewpoint on challenges and prospects for disease elimination. Parasite Vectors 2019, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Kojom Foko, L.; Kouemo Motse, F.D.; Kamgain Mawabo, L.; Pande, V.; Singh, V. First evidence of local circulation of Plasmodium ovale curtisi and reliability of a malaria rapid diagnostic test among febrile outpatients in Douala, Cameroon. Infect. Genet. Evol. 2021, 91, 104797. [Google Scholar] [CrossRef]
- Ngassa Mbenda, H.G.; Gouado, I.; Das, A. An additional observation of Plasmodium vivax malaria infection in Duffy-negative individuals from Cameroon. J. Infect. Dev. Ctries. 2016, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Public Health. 2012 Activity Report of the National Malaria Control Program. Yaoundé: 2012. Available online: https://pnlp.cm/wp-content/uploads/2020/05/Rapport-annuel-PNLP-2012-du-24-07-2013.pdf (accessed on 5 August 2022). (In French).
- WHO (World Health Organization). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva. 2011. 6p. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?sequence=22&isAllowed=y (accessed on 15 August 2022).
- WHO. Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs: Round 8 (2016–2018); WHO: Geneva, Switzerland, 2018; Volume 8, Available online: https://www.who.int/publications/i/item/9789241514965 (accessed on 5 August 2022).
- Cheesbrough, M. District Laboratory Practice in Tropical Countries; Part 2: Se; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Trape, J.-F. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Plowe, C.V.; Djimde, A.; Bouare, M.; Doumbo, O.; Wellems, T.E. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: Polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 1995, 52, 565–568. Available online: https://www.ajtmh.org/view/journals/tpmd/52/6/article-p565.xml (accessed on 5 August 2022). [CrossRef] [PubMed]
- Diallo, A.; Ndam, N.T.; Moussiliou, A.; Dos Santos, S.; Ndonky, A.; Borderon, M.; Oliveau, S.; Lalou, R.; Le Hesran, J.Y. Asymptomatic carriage of plasmodium in urban Dakar: The risk of malaria should not be underestimated. PLoS ONE 2012, 7, e31100. [Google Scholar] [CrossRef]
- Arya, A.; Kojom Foko, L.P.; Chaudhry, S.; Sharma, A.; Singh, V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions–India and sub-Saharan Africa. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 43–56. [Google Scholar] [CrossRef]
- Pearce, R.J.; Drakeley, C.; Chandramohan, D.; Mosha, F.; Roper, C. Molecular determination of point mutation haplotypes in the dihydrofolate reductase and dihydropteroate synthase of Plasmodium falciparum in three districts of Northern Tanzania. Antimicrob. Agents Chemother. 2003, 47, 1347–1354. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC152520/pdf/0879.pdf (accessed on 5 August 2022). [CrossRef]
- Goodstadt, L.; Ponting, C.P. CHROMA: Consensus-based colouring of multiple alignments for publication. Bioinformatics 2001, 17, 845–846. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Akpa, C.O.; Akinyemi, J.O.; Umeokonkwo, C.D.; Bamgboye, E.A.; Dahiru, T.; Adebowale, A.S.; Oyeneye Ajayi, I. Uptake of intermittent preventive treatment for malaria in pregnancy among women in selected communities of Ebonyi State, Nigeria. BMC Pregnancy Childbirth 2019, 19, 457. [Google Scholar] [CrossRef] [PubMed]
- Okethwangu, D.; Opigo, J.; Atugonza, S.; Kizza, C.T.; Nabatanzi, M.; Biribawa, C.; Kyabayinze, D.; Ario, A.R. Factors associated with uptake of optimal doses of intermittent preventive treatment for malaria among pregnant women in Uganda: Analysis of data from the Uganda Demographic and Health Survey, 2016. Malar. J. 2019, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Pons-Duran, C.; Llach, M.; Sacoor, C.; Sanz, S.; Macete, E.; Arikpo, I.; Ramirez, M.; Meremikwu, M.; Ndombo Ndombe, D.; Mendes, S.; et al. Coverage of intermittent preventive treatment of malaria in pregnancy in four sub-Saharan countries: Findings from household surveys. Int. J. Epidemiol. 2021, 50, 550–559. [Google Scholar] [CrossRef]
- Mushi, V.; Mbotwa, C.H.; Zacharia, A.; Ambrose, T.; Moshi, F.V. Predictors for the uptake of optimal doses of sulfadoxine-pyrimethamine for intermittent preventive treatment of malaria during pregnancy in Tanzania: Further analysis of the data of the 2015–2016 Tanzania demographic and health survey and malaria indicators. Malar. J. 2021, 20, 75. [Google Scholar] [CrossRef]
- Kojom Foko, L.P.; Lehman, L.G. Knowledge and beliefs towards malaria and associated factors among residents of the town of Douala, Cameroon. Arch. Curr. Res. Int. 2018, 14, 1–17. [Google Scholar] [CrossRef]
- Mbohou Nchetnkou, C.; Kojom Foko, L.P.; Lehman, L.G. Knowledge, attitude, and practices towards malaria among employees from enterprises in the town of Douala, Cameroon. BioMed Res. Int. 2020, 2020, 8652084. [Google Scholar] [CrossRef]
- Azizi, S.C. Uptake of intermittent preventive treatment for malaria during pregnancy with Sulphadoxine-Pyrimethamine in Malawi after adoption of updated World Health Organization policy: An analysis of demographic and health survey 2015–2016. BMC Public Health 2020, 20, 335. [Google Scholar] [CrossRef]
- Wafula, S.T.; Mendoza, H.; Nalugya, A.; Musoke, D.; Waiswa, P. Determinants of uptake of malaria preventive interventions among pregnant women in eastern Uganda. Malar. J. 2021, 20, 5. [Google Scholar] [CrossRef]
- Lehman, L.G.; Kojom Foko, L.; Tonga, C.; Nyabeyeu, H.; Eboumbou, E.C.; Kouodjip Nono, L.; Kangam, L.; Ngapmen, A.L.; Assomo Ndemba, P.B.; Matip, I.; et al. Epidemiology of malaria using LED fluorescence microscopy among schoolchildren in Douala, Cameroon. Int. J. Trop. Dis. Health 2018, 29, 1–13. [Google Scholar] [CrossRef]
- Unger, H.W.; Rosanas-Urgell, A.; Robinson, L.J.; Ome-Kaius, M.; Jally, S.; Umbers, A.J.; Pomat, W.; Mueller, I.; Kattenberg, E.; Rogerson, S.J. Microscopic and submicroscopic Plasmodium falciparum infection, maternal anaemia and adverse pregnancy outcomes in Papua New Guinea: A cohort study. Malar. J. 2019, 18, 302. [Google Scholar] [CrossRef]
- Singh, N.; Bharti, P.K.; Singh, M.P.; Singh, R.; Yeboah-Antwi, K.; Desai, M.; Udhayakumar, V.; Muniyandi, M.; Hamer, D.H.; Wylie, B.J. What is the burden of submicroscopic malaria in pregnancy in central India? Pathog. Glob. Health 2015, 109, 30–38. [Google Scholar] [CrossRef]
- Cohee, L.M.; Kalilani-Phiri, L.; Boudova, S.; Joshi, S.; Mukadam, R.; Seydel, K.B.; Mawindo, P.; Thesing, P.; Kamiza, S.; Makwakwa, K.; et al. Submicroscopic malaria infection during pregnancy and the impact of intermittent preventive treatment. Malar. J. 2014, 13, 274. [Google Scholar] [CrossRef]
- Elbadry, M.A.; Tagliamonte, M.S.; Raccurt, C.P.; Lemoine, J.F.; Existe, A.; Boncy, J.; Weppelmann, T.A.; Dame, J.B.; Okech, B.A. Submicroscopic malaria infections in pregnant women from six departments in Haiti. Trop. Med. Int. Health 2017, 22, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Ndam, N.T.; Tornyigah, B.; Dossou, A.Y.; Escriou, G.; Nielsen, M.A.; Salanti, A.; Issifou, S.; Massougbodji, A.; Chippaux, J.P.; Deloron, P. Persistent Plasmodium falciparum infection in women with an intent to become pregnant as a risk factor for pregnancy-associated malaria. Clin. Infect. Dis. 2018, 67, 1890–1896. [Google Scholar] [CrossRef]
- Sumbele, I.U.N.; Teh, R.N.; Nkeudem, G.A.; Mekachie, S.S.; Moyeh, M.N.; Shey, R.A.; Mounchili Shintouo, C.; Mbigha Ghogomu, S.; El-Saber Batiha, G.; Alkazmi, L.; et al. Asymptomatic and sub-microscopic Plasmodium falciparum infection in children in the Mount Cameroon area : A cross-sectional study on altitudinal influence, haematological parameters and risk factors. Malar. J. 2021, 20, 382. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.; Slater, H.; Nash, R.; Bousema, T.; Drakeley, C.; Ghani, A.C.; Ghani, A.V.; Okell, L.C. Global patterns of submicroscopic Plasmodium falciparum malaria infection: Insights from a systematic review and meta-analysis of population surveys. Lancet Microb. 2021, 2, e366–e374. [Google Scholar] [CrossRef] [PubMed]
- Anto, F.; Agongo, I.H.; Asoala, V.; Awini, E.; Oduro, A.R. Intermittent preventive treatment of malaria in pregnancy: Assessment of the sulfadoxine-pyrimethamine three-dose policy on birth outcomes in rural Northern Ghana. J. Trop. Med. 2019, 2019, 6712685. [Google Scholar] [CrossRef] [PubMed]
- Mikomangwa, W.P.; Minzi, O.; Mutagonda, R.; Baraka, V.; Mlugu, E.M.; Aklillu, E.; Kamuhabwa, A.A.R. Effect of sulfadoxine-pyrimethamine doses for prevention of malaria during pregnancy in hypoendemic area in Tanzania. Malar. J. 2020, 19, 160. [Google Scholar] [CrossRef]
- Amimo, F.; Lambert, B.; Magit, A.; Sacarlal, J.; Hashizume, M.; Shibuya, K. Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in Africa: A systematic analysis of national trends. BMJ Glob. Health 2020, 5, e003217. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, P.; Menard, S.; Iriart, X.; Nsango, S.E.; Tchioffo, M.T.; Abate, L.; Awono-Ambene, P.H.; Morlais, I.; Berry, A. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé Cameroon: Emergence of highly resistant pfdhfr/pfdhps alleles. J. Antimicrob. Chemother. 2015, 70, 2566–2571. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Chhibber-Goel, J.; Verma, I.; Gopinathan, S.; Parvez, S.; Sharma, A. Geographical spread and structural basis of sulfadoxine-pyrimethamine drug-resistant malaria parasites. Int. J. Parasitol. 2021, 51, 505–525. [Google Scholar] [CrossRef]
- Niba, P.T.N.; Nji, A.M.; Evehe, M.S.; Ali, I.M.; Netongo, P.M.; Ngwafor, R.; Moyeh, M.N.; Ngum Ngum, L.; Ndum, O.E.; Acho, F.A.; et al. Drug resistance markers within an evolving efficacy of anti-malarial drugs in Cameroon: A systematic review and meta-analysis (1998–2020). Malar. J. 2021, 20, 32. [Google Scholar] [CrossRef]
- Amenga-Etego, L.N.; Asoala, V.; Agongo, G.; Jacob, C.; Goncalves, S.; Awandare, G.A.; Rockett, K.A.; Kwiatkowski, D. Temporal evolution of sulfadoxine-pyrimethamine resistance genotypes and genetic diversity in response to a decade of increased interventions against Plasmodium falciparum in northern Ghana. Malar. J. 2021, 20, 152. [Google Scholar] [CrossRef]
- Mama, A.; Ahiabor, C.; Tornyigah, B.; Frempong, N.A.; Kusi, K.A.; Adu, B.; Courtin, D.; Houzé, S.; Deloron, P.; Ofori, M.F.; et al. Intermittent preventive treatment in pregnancy with sulfadoxine-pyrimethamine and parasite resistance: Cross-sectional surveys from antenatal care visit and delivery in rural Ghana. Malar. J. 2022, 21, 107. [Google Scholar] [CrossRef]
- Naidoo, I.; Roper, C. Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013, 29, 505–515. [Google Scholar] [CrossRef]
- Zhao, L.; Pi, L.; Qin, Y.; Lu, Y.; Zeng, W.; Xiang, Z.; Qin, P.; Chen, X.; Li, C.; Zhang, Y.; et al. Widespread resistance mutations to sulfadoxine-pyrimethamine in malaria parasites imported to China from Central and Western Africa. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bwire, G.M.; Mikomangwa, W.P.; Kilonzi, M. Occurrence of septuple and elevated Pfdhfr-Pfdhps quintuple mutations in a general population threatens the use of sulfadoxine-pyrimethamine for malaria prevention during pregnancy in eastern-coast of Tanzania. BMC Infect. Dis. 2020, 20, 530. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Cheng, W.; Yao, Y.; Tan, H.; Wu, K.; Li, J. Molecular surveillance of anti-malarial resistance Pfdhfr and Pfdhps polymorphisms in African and Southeast Asia Plasmodium falciparum imported parasites to Wuhan, China. Malar. J. 2020, 19, 434. [Google Scholar] [CrossRef] [PubMed]
- Voumbo-Matoumona, D.F.; Kouna, L.C.; Madamet, M.; Maghendji-Nzondo, S.; Pradines, B.; Lekana-Douki, J.B. Prevalence of Plasmodium falciparum antimalarial drug resistance genes in Southeastern Gabon from 2011 to 2014. Infect. Drug Resist. 2018, 11, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Sarah-Matio, E.M.; Guillochon, E.; Nsango, S.E.; Abate, L.; Ngou, C.M.; Bouopda, G.A.; Feufack-Donfack, L.B.; Bayibéki, A.N.; Tchioffo Tsapi, M.; Talman, A.; et al. Genetic Diversity of Plasmodium falciparum and Distribution of Antimalarial Drug Resistance Mutations in Symptomatic and Asymptomatic Infections. Antimicrob. Agents Chemother. 2022, 66, e0018822. [Google Scholar] [CrossRef] [PubMed]
- Bushman, M.; Morton, L.; Duah, N.; Quashie, N.; Abuaku, B.; Koram, K.A.; Dimbu, P.R.; Plucinski, M.; Gutman, J.; Lyaruu, P.; et al. Within-host competition and drug resistance in the human malaria parasite Plasmodium falciparum. Proc. Biol. Sci. 2016, 283, 20153038. [Google Scholar] [CrossRef] [PubMed]
- Eldh, M.; Hammar, U.; Arnot, D.; Beck, H.P.; Garcia, A.; Liljander, A.; Mercereau-Puijalon, O.; Migot-Nabias, F.; Mueller, I.; Ntoumi, F.; et al. Multiplicity of Asymptomatic Plasmodium falciparum Infections and Risk of Clinical Malaria: A Systematic Review and Pooled Analysis of Individual Participant Data. J. Infect. Dis. 2020, 221, 775–785. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).