1. Introduction
More and more stringent regulations regarding ecological solutions are forcing us to move away from traditional concrete; in the production of concrete, a ton of CO
2 is produced from each ton of raw material produced [
1,
2]. The possible solutions are associated with the replacement of a part of traditional concrete with waste or industrial by-products, as well as the design of alternative materials such as geopolymers [
3,
4]. The second approach seems to be a more long-term approach to solving this problem. Geopolymers are the ecological alternative in construction to traditional cement, which is usually ordinary Portland cement [
5,
6]. One of the most important areas of construction is road infrastructure; a large part of the economy of various countries is based on it. Because of this, even partly replacing traditional materials with more ecological ones can bring about large-scale effects. Today’s roads have to not only fulfill strength requirements but must also withstand long-term temperature and load differences; some infrastructure cannot withstand these tests, and after one severe winter, asphalt cracks and holes may need to be repaired. This kind of construction also has to withstand frost and salt, which would prevent construction in many countries [
7,
8].
Previous investigations have shown the possibility of applying geopolymers as a material for road construction, including the application of industrial by-products such as fly ash or slag for material preparation [
9,
10]. Particular attention should be paid to the use of local waste for this purpose in order to limit the cost of transportation and the pollution connected to them. However, the implementation of such waste products seems very attractive; it has to be stressed that different waste streams have slightly different characteristics depending on local conditions, and because of that, they require additional research before application [
11,
12]. In some use cases, such kinds of waste, alongside computer methods, can play an important role in the optimization of geopolymers’ composition [
13].
Another important factor is obtaining proper compositions of used materials, which should include the prevention of cracking. One of the most popular methods of avoiding this phenomenon is the addition of fibers. Most previous research confirms the possibility of the application of different fibers for this purpose [
14,
15]. In this case, the best results are obtained by using carbon fibers. They not only prevent cracking but also do not influence compressive strength and enhance the flexural strength and fracture tongues of materials [
16,
17,
18]. When steel fibers are used, they are not harmful in the case of material surface degradation, for example, as a result of surface abrasion.
In recent years, micro- and nano-additives have also emerged as points of interest for scientists, as a potential admixture to compositions made especially for roads [
8]. Even a small amount of this additive can significantly affect the properties of the material. Currently, mineral nano-additives, such as nano-silica and nano-clays, are the most intensively researched solutions used in road construction, especially if we consider concrete applications [
8,
19,
20]. The other popular group is that of metal compounds [
8,
21]. Both metals and ceramics may be classified into one group of inorganic nano-additives. The addition of nanoparticles to this group could have different purposes. The most common are catalytic applications [
22]; enhancement of the physical and rheological properties, including the increased durability of asphalt mixtures [
21,
23]; improved rutting resistance [
24]; fatigue properties; and temperature resistance [
24,
25]. Despite a lot of benefits, the usage of nano-additives also brings some challenges. In the case of inorganic ones, the basic issue is their poor affinity to the organic materials used in road composition [
26,
27]. In the case of geopolymers (inorganic compounds), this problem does not appear.
Despite a relatively large amount of research in the field of geopolymers with nano-additives, geopolymer nanocomposites have not been widely investigated with the intention of applying them in the road industry. However, other investigations into geopolymers with nano-additives have shown their potential as a construction material, including some pavement technologies. Among others, one promising additive seems to be nano-silica, and research works have shown its potential to improve the mechanical properties of geopolymers. Previous works have shown that it enhances mechanical and rheological properties [
28,
29,
30], resistance to sulfate attack [
31], durability [
32], and thermal resistance [
33,
34]. In the case of metal and metal oxide nanoparticles, investigations have focused mainly on the addition of titanium dioxide and photocatalytic properties [
35]. Other types of compounds have been investigated in the area of antimicrobial properties and improving compressive strength [
33,
36].
The literature gap shows the necessity of designing more complex materials for road construction. The main motivation for the choice of this topic stemmed from questions regarding the feasibility, economic viability, and environmental impact of using geopolymers compared to traditional concrete. This article presents the process of research and experiments carried out to confirm the suitability of geopolymers for road infrastructure. The aim of the work was to determine the possibility of using geopolymer composites as materials for road infrastructure. The work included the synthesis of geopolymer materials and the examination of selected mechanical and physical properties.
2. Materials and Methods
2.1. Materials
For the geopolymer sample preparation, the following ingredients were used: fly ash from a power plant Skawina (CHP Skawina, Skawina, Poland); coal shale—a by-product from mining from the Silesia mine (Silesia mine, Czechowice-Dziedzice, Poland); short carbon fibers—3 mm (R&G GmbH, Waldenbuch, Germany); and nano-silica (Nanografi Nano Technology, Ankara, Turkey). The additives were selected to improve the material properties required for road application and give a synergic effect [
37]. Fly ash, carbon fiber, and nano-silica were used in an unaltered state. The coal shale, before its usage, was calcinated to improve its reactivity during geopolymer synthesis. At first, the coal shale was crushed, ground, and sifted through a special sieve to separate it from larger unground items. Then, the material was processed by calcination (a thermal activation) to remove carbon residues and achieve appropriate reactive microstructures. This process took place at a temperature of 800 °C for 24 h. In the next step, the material was characterized according to its chemical and mineralogical composition as well as its microstructure.
2.2. Raw Material Analysis
2.2.1. Fly Ash
In the first step, the elemental and oxide compositions were investigated to confirm the suitability of the material for the geopolymerization process, as shown in
Table 1 and
Table 2.
The elemental composition of fly ash showed the content of the typical elements such as silicon and aluminum. The analyzed fly ash also included an amount of iron (above 16%) and other elements, such as potassium, calcium, titanium, and sulfur. This composition is typical of fly ash from the energy industry [
38].
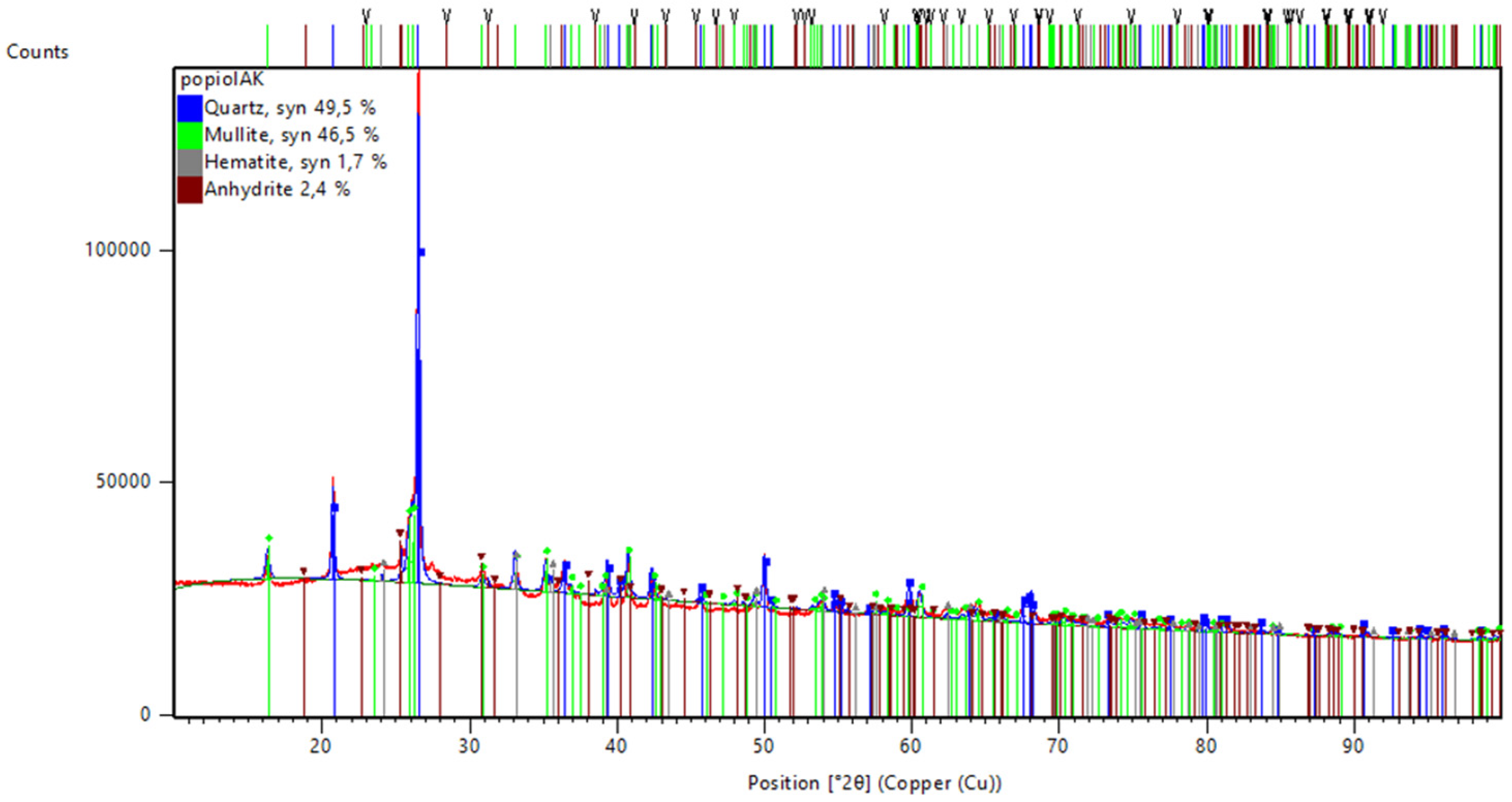
Figure 1 presents the results of the investigation of the mineral composition of the fly ash. The presented fly ash fulfilled the requirements for fly ash class F, following ASTM C618, including a CaO value of 4.576% (<10%), a total SiO
2 + Al
2O
3+ Fe
2O
3 value of 87.751% (>70%) and SO
3 of 1.395% (<5%) [
39]. This kind of fly ash is suitable for geopolymerization processes and should create a proper material structure [
11,
40].
The presented pattern shows that among the crystalline ingredients of fly ash, the main mineralogical components are quartz (49.5%) and mullite (46.5%). The quartz does not influence the reactivity of the material, but in the final composition, it allows the required strength to be obtained [
41]. Mullite is also hardly reactive; it reacts with alkaline solutions to a limited degree [
42]. However, mullite, similar to quartz, can ensure that the final products have enhanced strength and durability [
43,
44]. XRD analysis shows a small amount of hematite (1.7%) and anhydrite (2.4%).
SEM observations for the material are presented in
Figure 2.
Fly ash is characterized by the presence of many spherical particles. This kind of particle usually gives the fresh paste good workability and allows the amount of water used to be reduced [
45].
2.2.2. Coal Shale
According to the data presented in
Table 1, coal shale is mainly composed of silicon, aluminum, and iron. It also includes other elements, such as potassium, calcium, titanium, and sulfur.
Table 2 shows the oxide composition. The main component in this case is SiO
2. In the material, there is also a significant amount of Al
2O
3 and Fe
2O
3. All of these elements are supportive of a geopolymerization reaction.
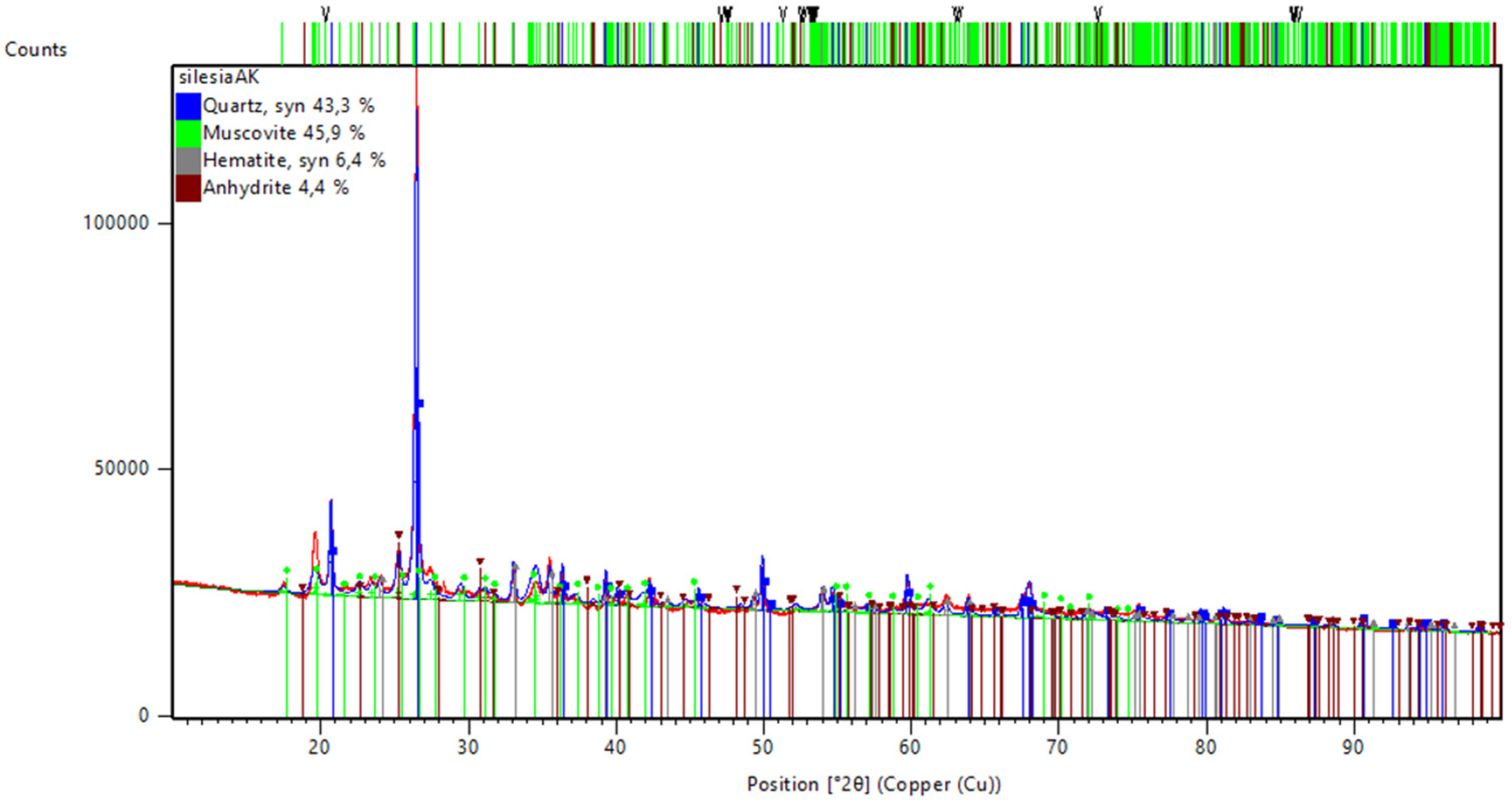
Figure 3 presents the results of the investigation of the mineral composition of coal shale “Silezia” after the calcination process.
The presented pattern shows the crystalline ingredients of the milled coal shale “Silezia”. The main component is quartz, with 43.3%, followed by muscovite, with 45.9%. The quartz, just as in the case of the fly ash, plays the role of filler in the material, allowing the required strength of the final composition to be obtained [
41]. Muscovite is only partly reactive and can be challenging when trying to obtain a proper geopolymer microstructure, in comparison with kaolinite, even after calcination [
46]. However, when this mineral is present, the geopolymerization process at a temperature above 70 °C seems to be important for the efficiency of the process, as well as ensuring a high pH value [
46,
47,
48]. XRD analysis shows a certain amount of hematite (6.4%) and anhydrite (4.4%). The presence of these minerals is higher than in fly ash, but still not very high. The lack of kaolinite in the analysis is caused by its change into an amorphous phase, becoming metakaolin after the calcination process. This mineral normally appears in coal shale [
40,
49].
SEM observations of the material are presented in
Figure 4.
The microstructure investigation in the case of coal shale shows sharp elements with irregular shapes, which are an effect of grinding the material.
2.2.3. Nano-Silica
According to the data presented in
Table 1 and
Table 2, nano-silica is mainly composed of SiO
2, which is also confirmed by the XRD analysis in
Figure 5.
Silicon oxide is an ingredient of nano-silica. The material has a highly amorphous character.
SEM observations of the material are presented in
Figure 6.
The nano-silica has particles of a regular shape; the small particles are very often agglomerated into larger clusters, which is more visible with higher magnification. To work effectively, nano-silica should be divided into smaller particles to be effectively integrated into the geopolymer matrix.
2.2.4. Carbon Fibers
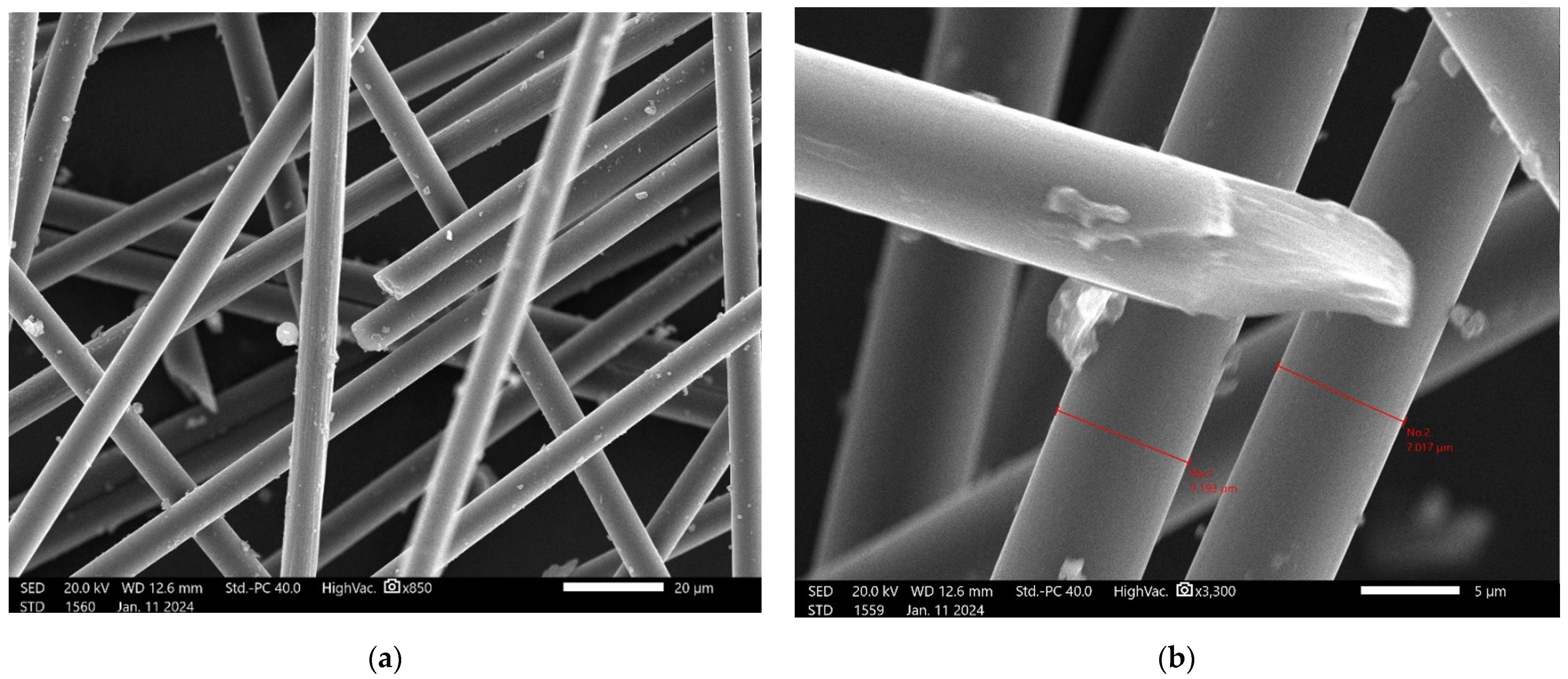
For the carbon fibers, SEM observations are presented in
Figure 7.
SEM investigation confirmed the regular character of the fibers. The measurements show that they all have a diameter of about 7 µm and smooth surfaces.
2.3. Samples Preparation
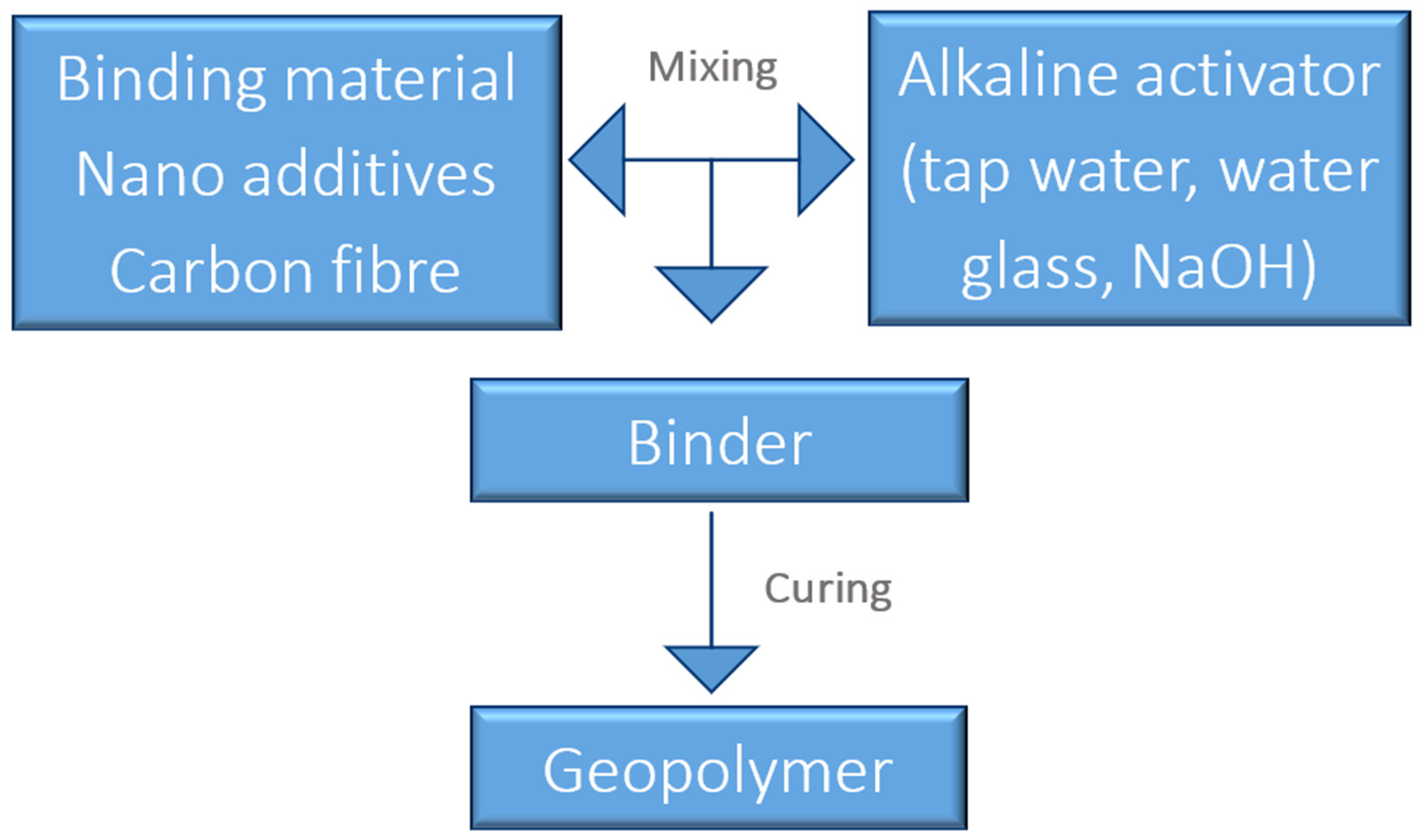
The day before the samples’ preparation, a 10 molar solution of a mixture of hydrated sodium hydroxide flakes with aqueous sodium silicate type R-145 (molar module 2.5, density 1.45 g/cm
3) in a ratio of 1:2.5 was synthesized. It was stored in laboratory conditions to equalize the concentrations. Next, the samples were made. To prepare the samples, the dry ingredients of the mass were combined (
Table 3), and then an alkaline solution was added to them.
The geopolymer composite was made based on FA with the addition of coal gangue, carbon fibers, and nano-silica. Each component was planned for the improvement of material properties. The role of coal gangue was the reinforcement of mechanical properties and lowering costs by using industrial by-products. CFs should enhance flexural strength and work against cracking in changeable preparations. Nano-silica should also improve physical and mechanical properties, including resistance against friction and limited water absorption. The reference sample was created based on FA to compare if the above additions brought about the expected effects.
The mass prepared in this way was mixed in a special laboratory mixer for 10 min. After this time, the geopolymer mass was transferred to appropriate forms, and the molds were placed on a vibrating table to remove air bubbles. The scheme of the samples’ preparation is presented in
Figure 8.
Then, the samples were placed in the oven for 24 h at 75 °C. The time was due to the laboratory’s schedule, and the setting time was between 8 and 12 h. The next day, they were unmolded and left in laboratory conditions for development for 28 days.
2.4. Methods
The elemental and oxide composition were investigated by X-ray fluorescence (XRF). The research was performed using the EDX-7200 from the company SHIMADZU (SHIMADZU EUROPA GmbH, Duisburg, Germany) and the software PCEDX Navi (Version: EDX-7000P). The materials were powdered, put into plastic, and covered by polypropylene foil. They were measured in an air atmosphere.
The mineralogical composition was measured by X-ray diffraction (XRD). The measurements were made using AERIS from the company Malvern PANalytical (PANalytical, Almelo, The Netherlands). The test was performed using a copper lamp. The detailed parameters of the machine’s settings were as follows: starting and ending angle—9.999–100 °2 Ɵ; measurement step—0.0027166 °2 Ɵ; time for measurement—340.425 s; total testing time—13 h 2 min 32 s; nickel filter; a 13 mm mask; knife in the low position; and a 1 mm gap. The measurements were made using powdered samples.
To study the microstructure, a JSM-IT200 InTouchScope™ scanning electron microscope (JEOL, Tokyo, Japan) was used. The samples were placed in a pot using carbon tape and covered by a gold layer to obtain conductivity. The observations were made at different magnifications; additionally, an automatic virtual ruler was involved in checking the grains’ dimensions.
Density was measured by using the geometric method by employing a caterpillar and laboratory weight. The test involved three samples of each type.
The compressive strength test was performed using MATEST (Matest, Treviolo, Italy). The test was provided according to the standard PN-EN 12390-3:2019 for the testing of concrete—Part 3: the Compressive strength of test specimens [
50]. Samples with dimensions of 50 mm × 50 mm × 50 mm were used for this test. The test involved three samples of each type.
The abrasion resistance was investigated using a Böhme disk (Matest, Treviolo, Italy). The main element of this device is an iron horizontal disc with a diameter of 750 mm and a 200 mm test track used to position specimens. The maximum speed of rotation was 30 rpm. The initial force was 294 ± 3 N on the specimen. The test consisted of abrading the sample surface using corundum powder (20 g of artificial corundum for each cycle). A single cycle consisted of 22 revolutions of the Böhme dial. Abrasion wear was measured after 16 cycles on each sample, and after each cycle, the sample was rotated 90° to ensure uniform abrasion in all directions. The samples used for the test were cubes of 50 mm × 50 mm × 50 mm. The sample height and weight were determined before and after the test. The test involved three samples of each type.
This process of investigating freeze–thaw resistance intended to examine the percentage of each sample’s weight loss over cycles of freezing and thawing (as a simulation of changeable temperature during the year in countries such as Poland and Kazakhstan) in a climatic chamber (WeissTechnik GMbH, Reiskirchen-Lindenstruth, Germany). Before starting the study, the samples were weighed; their weight was recorded, and then they were transferred to a chamber where they were exposed to cycles of temperature changes from −40 °C to 80 °C (
Table 1). Samples were tested in such cycles for 24 h. Each cycle lasted 90 min, which translates into 16 cycles per day, as shown in
Table 4.
The entire study took 36 days. This value was estimated taking into consideration the predicted lifetime of the material for the road (10 years) and the number of days with a transition to 0 °C in Poland (57) [
51].
4. Discussion and Future Studies
The preliminary results show the high relevance of the properties of the geopolymer composition for road applications. Information about the obtained results and a comparison with basic requirements are presented in
Table 10.
In the case of scaling up this technology for practical application, further investigation is required. The most important area of research should include the following aspects.
Environmental safety, including leaching tests for ready compositions; despite the raw materials not showing hazardous components, environmental safety should be additionally confirmed before further applications.
Additional research for material durability, also in corrosive environments and on the polluted surface, as well as reactions with carbon dioxide; the surface has to be resistant to the most popular pollutants on the road, i.e., those associated with acid rain and other elements that potentially can appear on the road, including oils.
Long-term cracking resistance—freeze–thaw studies should be continued for longer periods, e.g., for 25 years. Additionally, there should be experiments with extreme cold or high humidity.
The above tests should be carried out before the first stage of scalability and real-world applications on a medium scale. Next steps may involve improving compositions with additional components, such as nano-titanium dioxide, which can be applied to obtain other desired properties, including photocatalytic ones. Also, before application on a wider scale, technology has to be improved, as well as the process of production. There are also some limitations connected to wider applications of geopolymers, including a lack of proper standards for these materials, which can make practical application on a wider scale (beyond experimental installations) difficult.
5. Conclusions
A new material dedicated to road application has been synthesized and investigated. The most important results are as follows.
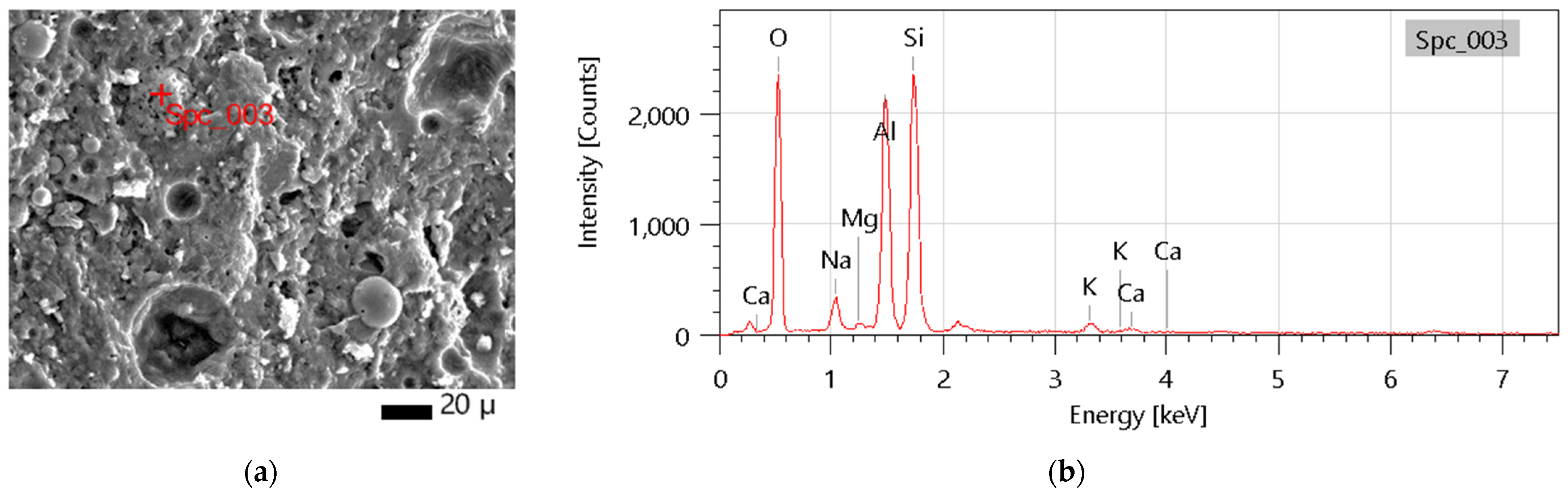
The chemical composition (elemental and oxide) of the final composition is in line with investigations performed for raw materials and typical compositions of geopolymers. The main ingredients are SiO2 and Al2O3, which build the main structure of the material.
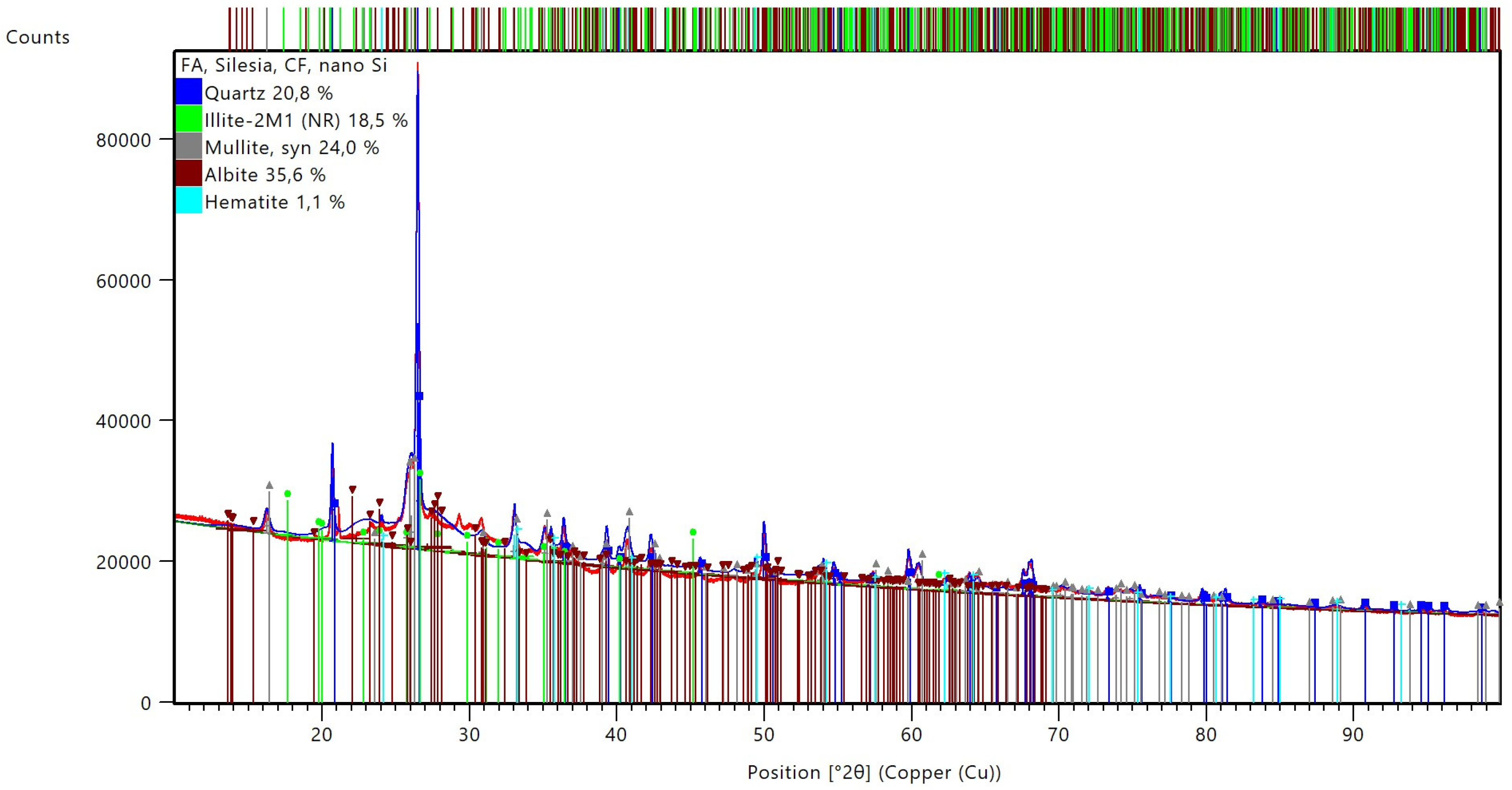
The mineralogical composition is a geopolymer material based on quartz, mullite, and hematite that is not transformed during the geopolymerization process, alongside other components, such as illite and albite, that are the results of the manufacturing process.
The additives allow for a reduction in the weight of the composition from 1.78 to 1.53 g/cm3.
The compressive strength of the investigated paste is almost 35 MPa for the reference sample and almost 38 MPa for the reference sample. The addition of fine aggregate or coarse aggregate can increase the value of this composition.
The additives improve the abrasion resistance of geopolymers. Compared to the reference samples, a lower mass loss was noted.
The additives enhance the freeze–thaw resistance of the geopolymers.
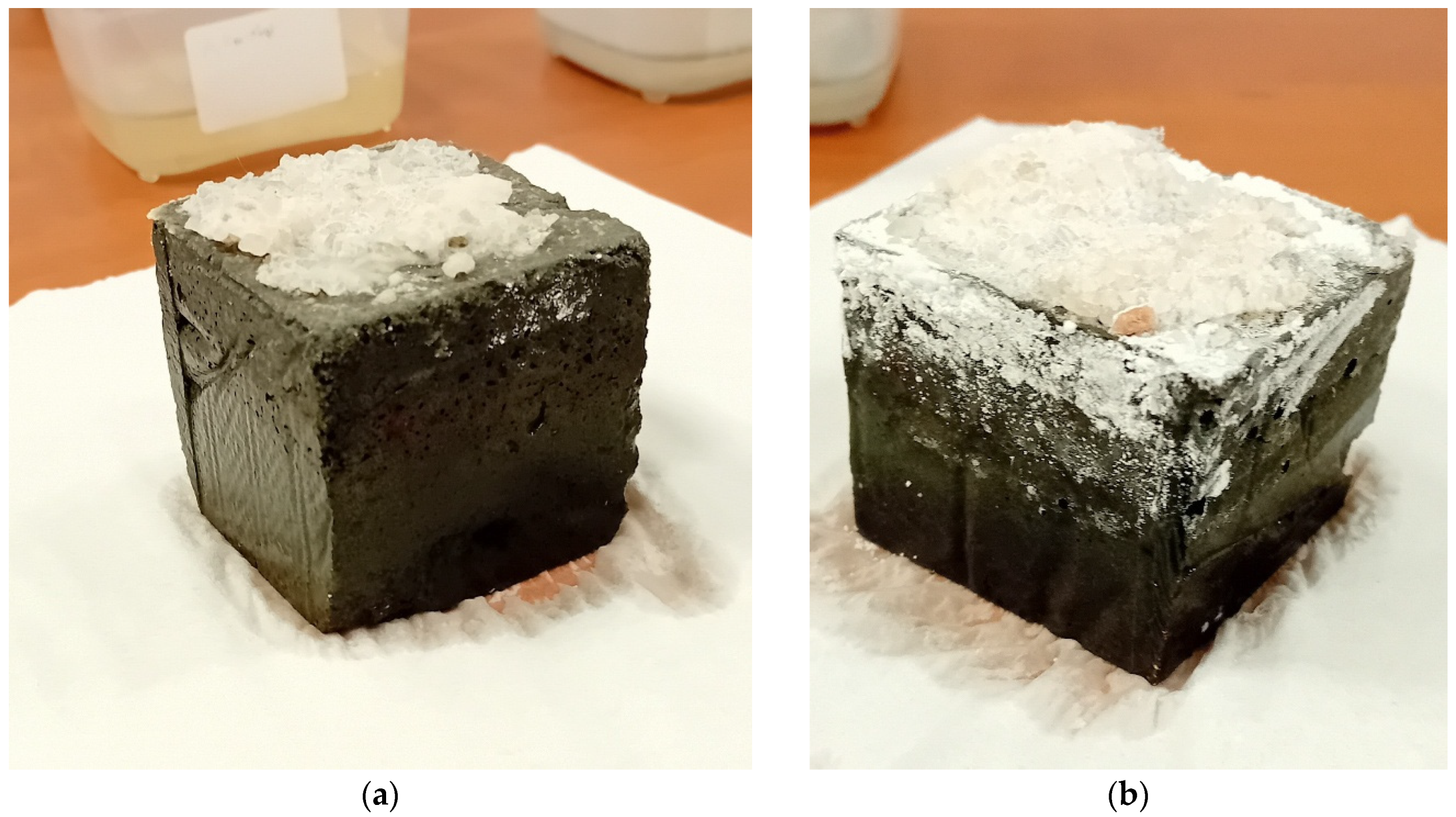
Visual observation of samples with a salt layer showed that salt does not significantly influence the structure of the material.
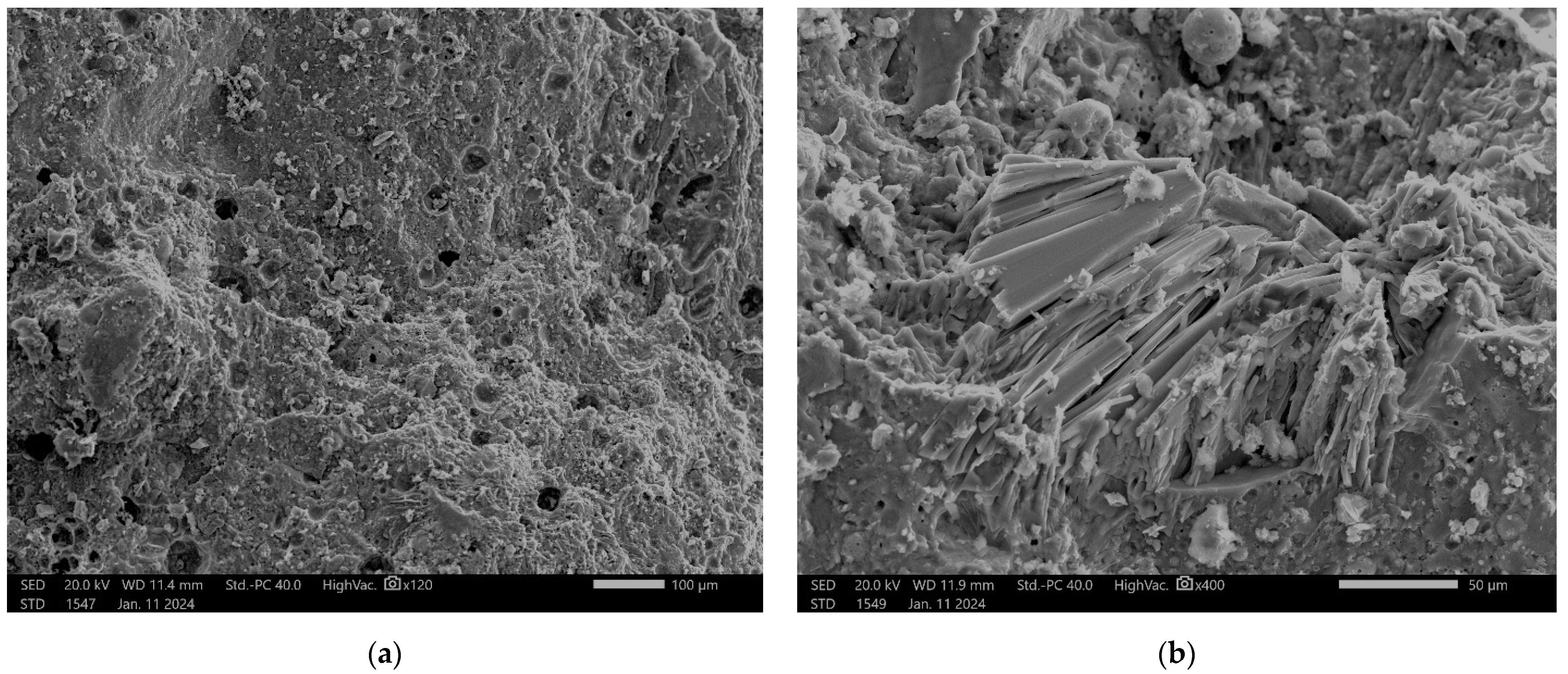
Microstructure investigation showed a structure typical of geopolymer materials. At a large magnification, in the structure, we observed some undissolved particles which came from fly ash. No incoherence or agglomeration of the additives was visible in the matrix.
The preliminary results show the high relevance of the properties of geopolymer compositions for road applications. However, for practical applications, further investigations are required to confirm the environmental safety of the material in leaching tests, alongside additional research on material durability. Moreover, material synthesis with the usage of additional components, such as nano-titanium dioxide, can be applied to obtain other desired properties, including photocatalytic ones.