Abstract
The corrosion behaviour of new generation titanium alloys (β-type with low modulus) for medical implant applications is of paramount importance due to their possible detrimental effects in the human body such as release of toxic metal ions and corrosion products. In spite of remarkable advances in improving the mechanical properties and reducing the elastic modulus, limited studies have been done on the electrochemical corrosion behaviour of various types of low modulus titanium alloys including the effect of different beta-stabilizer alloying elements. This development should aim for a good balance between mechanical properties, design features, metallurgical aspects and, importantly, corrosion resistance. In this article, we review several significant factors that can influence the corrosion resistance of new-generation titanium alloys such as fabrication process, body electrolyte properties, mechanical treatments, alloying composition, surface passive layer, and constituent phases. The essential factors and their critical features are discussed. The impact of various amounts of α and β phases in the microstructure, their interactions, and their dissolution rates on the surface passive layer and bulk corrosion behaviour are reviewed and discussed in detail. In addition, the importance of different corrosion types for various medical implant applications is addressed in order to specify the significance of every corrosion phenomenon in medical implants.
1. Introduction
The need for biocompatible materials with a good combination of mechanical and electrochemical properties has been always of great significance for manufacturing medical implants, particularly load-bearing orthopaedic implants used for joint arthroplasty. Producing implants that can enhance the longevity in the body is imperative which, in turn, can reduce the possibility of implant failure; and hence, risky and expensive revision surgeries. Titanium and its alloys have long been used for biomedical implant applications as they offer a favourable set of properties including high specific strength, good biocompatibility and high corrosion resistance [1,2,3]. In terms of osteogenesis, titanium and its alloys have been reported to possess the greatest biocompatibility in comparison with the other biometals [4]. Nowadays, Ti-6Al-4V alloys are extensively used for the manufacture of load-bearing orthopaedic implants such as hip stems that are to live with the surrounding hard and soft tissues in the physiological media of the body [5,6].
Despite all the aforementioned advantages of Ti-6Al-4V alloy, research has identified some concerns around the toxicity of aluminium and vanadium present in this alloy. Neurological disorders, allergic reactions, Alzheimer’s disease and cytotoxicity are of some drawbacks associated with the release of Al and V into the body [7,8,9,10,11]. Also, from the mechanical behaviour perspective, the Young’s modulus of Ti-6Al-4V is approximately 110 GPa [12] that is significantly higher than the stiffness of the bone (typically 10–30 GPa) [5]. It is known that if there is a substantial discrepancy between the stiffness of the implant and the adjacent bone, significant stress shielding can occur under the mechanical loads of daily activities which can result in adverse effects such as bone loss, implant loosening, and peri-prosthetic fracture [11,13,14,15,16,17].
In order to address the issues associated with the most commonly used alloy of Ti-6Al-4V, there have been many efforts to develop a new class of titanium alloys (known as β-type titanium alloys) with reduced levels of stiffness. Any alteration in the chemical composition of Ti alloys may lead to stabilization of a certain phase and crystal structure: the high temperature Ti has a body-centered cubic (BCC) crystal structure, β-phase, while the low temperature phase (α) displays a hexagonal close-packed (HCP) structure (e.g., CP Ti) and the combination of the two phases (α + β) (e.g., Ti-6Al-4V) [18]. The β-type titanium alloys containing beta-stabilizing elements (e.g., Nb, Ta and Zr) exhibit many advantages such as lower Young’s moduli that are closer to that of the human bone (which can mitigate bone loss and implant loosening due to stress shielding), non-allergic and non-toxic elements such as Nb, Ta and Zr, excellent corrosion resistance due to the formation of more stable oxide layers and good biocompatibility [19,20]. Body fluid present in the immediate vicinity of implants contains organic and inorganic solutions of cations Mg2+, Ca2+, Na+, K+, and anions SO42−, Cl−, HCO3−, H2PO4−. Also, from the corrosion science perspective, due to the presence of such ions, the pH of body fluid may fluctuate from acidic to alkaline; and this can cause the open circuit potential (Eoc) to have different values in various parts of the implant surface. Hence, the adequate driving force of localized corrosion may increase which can speed up the degradation of the implant material [21].
Titanium alloys spontaneously form a passive oxide layer (mainly TiO2) that, to some extent, can protect these alloys against corrosion because of its thermodynamic stability, chemical inertia, and low solubility in the body fluids [22]. However, severe corrosion can occur when this passive oxide layer is mechanically disrupted. In such situations, aggressive Cl− ions may attack the implant material surface. For instance, fretting wear is known to occur at the interface of modular junctions (for example, head-neck taper junction of hip implants) due to relative micro-motions [23,24,25,26]. The fretting wear disrupts the passive oxide layer causing corrosion to occur in the alloy in vivo. Moreover, it is important to mention that metallic ions released due to the corrosion process can cause adverse reactions in the body resulting in implant revision surgeries due to the release of toxic ions in the body [23,27,28,29].
Now that new generation Ti alloys have been already developed with lower Young’s moduli (much closer to bone when compared to widely used Ti-6Al-4V) and that they have shown good biocompatibility, it is important to review their corrosion behaviour and electrochemical characteristics. There are various groups of new generation titanium alloys that may offer different levels of corrosion resistance in the body fluid depending on their chemistry (alloying elements and their amounts), and fabrication methods. Therefore, this review aims to provide useful information on the corrosion response of various Ti alloys that are increasingly being developed for the next generation medical implants.
2. In Vitro Electrochemical Corrosion Testing Procedures
The electrochemical corrosion testing procedures used for biometals (including low stiffness titanium alloys) involve several various parameters and testing methods depending on the objectives of the experimental work. Potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), open circuit potential (OCP) and other corrosion testing procedures can be applied in order to assess the corrosion behaviour. In addition, parameters such as time of exposure, corrosion medium or solution (electrolyte), pH and the electrolyte temperature can significantly affect the corrosion behaviour of titanium alloys due to the sensitivity of their passive oxide layer. Moreover, microstructural observations of the surface after anodization along with X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS) examinations can help scientists and researchers to explore corrosion mechanisms and the formation of passive layers with their chemical compositions.
Owing to their passive oxide layer, titanium-based alloys are resistant to some organic acids; for example, lactic acid and acetic acid. However, they can be corroded by some others such as citric acid [30,31]. Among halide anions, fluoride ions can more strongly cause dissolution of the passive oxide layer in titanium-based alloys [32]. It is known that fluoride ions are aggressive and they may cause pitting and crevice corrosion in dental implants [33]. Many commercial oral rinses include significant amounts of fluoride which can affect the electrochemical behaviour of orthodontic implants made of titanium alloys [23]. In a study by Li et al. [34], the effect of fluoride on the electrochemical parameters of Ti-24Nb-4Zr-8Sn (β-type Ti2448) alloy was examined in artificial saliva. Further increase of F− from (≤0.1 to 1%) degraded the corrosion resistance of the alloy. Also, all the corrosion resistance parameters extracted from the EIS measurements such as Rs (solution resistance), Rb (resistance of the inner barrier oxide layer) and Rp (resistance of the outer porous oxide layer) decreased and were in good agreement with Tafel diagram. Also, NaF concentration was found to influence the protective behaviour of the passive layer onto Ti-6Al-4V causing an increase in the corrosion current density in salvia solution [35]. Also, Huang [35] reported that the electrochemical properties of Ti-6Al-4V alter not only with the fluoride content, but also with the pH and the immersion time in Ringer’s solution. Robin and Carvalho [36] investigated the passivation of CP-Ti, Ti-13Nb-13Zr, Ti-5Nb-13Zr and Ti-20Nb-13Zr alloys for a pH range (2–7.5) in a Ringer’s solution with (1000 ppm) and without fluoride anions. The corrosion resistance parameters of the tested alloys reduced with a further increase of F− and decrease of pH. Also, according to the OCP and Bode diagrams, Ti-13Nb-13Zr showed a better corrosion resistance in comparison with the other tested alloys because of a spontaneous stable oxide layer (TiO2) formed on its surface. Although the chemical stability of Ti-Nb alloys is high (because of their excellent passive layer behaviour [37]), an increase in pH, in acidification of the Ringer’s solution from pH 7.5 to pH 0.5 resulted in negative corrosion potential shifts. The addition of NaCl further exacerbated the corrosion resistance [38,39,40,41,42].
Surface microstructural characterization of β-type Ti alloys after electrochemical tests in various simulated body fluid solutions play an important role in verifying the corrosion test results. These observations can reveal corrosion aspects of the surface such as thickness of the passive layer, corrosion product phases and compounds, pitting and corrosion mechanism. Figure 1 shows scanning electron microscope (SEM) images of a Ti-24Nb-4Zr-8Sn alloy after immersion in 0.9% NaCl solution for various durations (24, 72, and 168 h) [36]. The results showed that the surface of the alloy after 24 h included a passive layer with no corrosion attack which was in agreement with the Bode diagram (Figure 2) so that the modulus of impedance at 0.01 Hz frequency increased after 24 h immersion and the phase angle at intermediate to low frequencies was closer to more negative angles in the Bode-phase diagram [43].

Figure 1.
Scanning electron microscope (SEM) images of Ti-24Nb-4Zr-8Sn alloy after immersion in 0.9% NaCl solution at 37 °C for: (a) 24 h, (b) 72 h, and (c) 168 h [43].
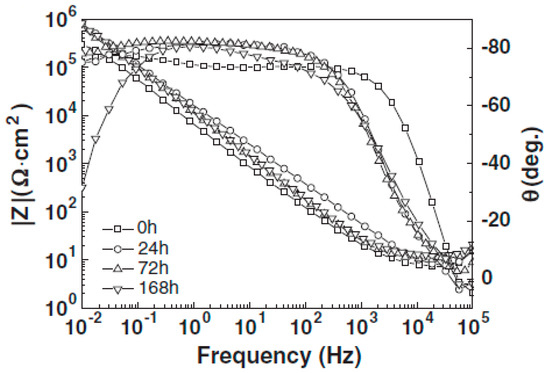
Figure 2.
Electrochemical impedance spectroscopy (EIS) spectra of Ti-24Nb-4Zr-8Sn alloy at different immersion hours in 0.9% NaCl solution [43].
SEM images of a biomedical β-type TiNbZrFe alloy after electrochemical testing in 0.9% NaCl and 0.2% NaF solutions showed that the deep corrosion pits in non-SMAT (surface mechanical attrition treated) samples were distributed densely on the surface which was verified with more fluctuations of the anodic branch of the Tafel diagram and the higher corrosion current density. However, the SMAT sample was covered completely with the passive oxide layer [44]. Atapour et al. [40] used secondary electron SEM micrographs of Ti-13Mo-7Zr-3Fe (as-received α + β) and (metastable β) samples after 50 h immersion in 5 M HCl at 37 °C. As can be seen in Figure 3a,c, the surface of the as-received α + β alloy after immersion shows that the β phase is preferentially dissolved, while in Figure 3b, as-received α + β includes no significant attack of the α phase and not even in grain boundaries which are more susceptible to corrosion due to having less density and more disorder on the atomic scale. Also, they concluded that once the attack initiated in the two-phase alloy (as-received α + β), the β phase seems to be more susceptible to dissolution than the α phase. In addition, the significant conclusion was that the localized galvanic series of α and β phases causes preferential corrosion of the β phase due to the presence of vanadium (V) as a beta-stabilizer element in Ti-6Al-4V alloy, and this is the main reason for higher corrosion rate of Ti-6Al-4V in comparison with Ti-13Mo-7Zr-3Fe (TMZF) (Figure 3c) [40].
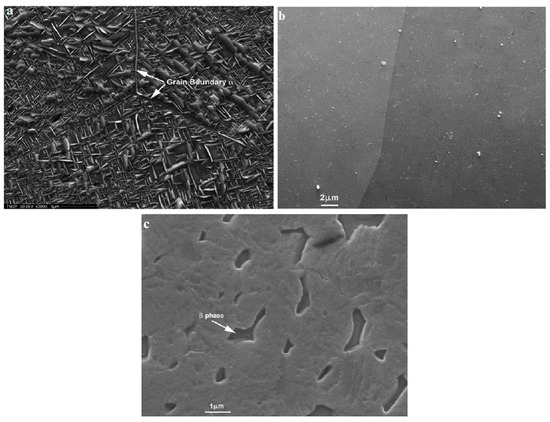
Figure 3.
Secondary electron SEM micrograph of Ti-13Mo-7Zr-3Fe: (a) as-received α + β, (b) metastable β, following 50 h immersion in 5 M HCl at 37 °C, and (c) secondary electron SEM micrograph of the surface corrosion aspects of Ti-6Al-4V ELI (extra-low interstitial) after 50 h immersion in 5 M HCl at 37 °C [40].
In a study by Lu et al. [45], the effect of α and β precipitations on the corrosion behaviour of β-type Ti-5Al-3Zr-4Mo-4Cr-4V alloy (Ti-1300) was investigated. Similar to the conclusion of [40], it was observed that both the equiaxed (the equiaxed microstructure of Ti-1300 alloy presents mainly relatively large equiaxed β-grains)/lamellar (the lamellar microstructure for Ti-1300 alloy is dominated by large quantities of precipitates as α phase and retained β phase) microstructures undergo a preferential corrosion degradation of α phase and the α + β interphase boundaries which create localized micro galvanic series between α and β phases (Figure 4a,b).
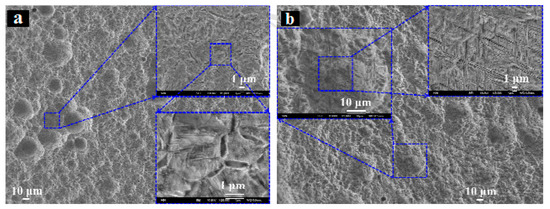
Figure 4.
SEM morphologies of Ti-1300 (Ti-5Al-3Zr-4Mo-4Cr-4V) surface in 5 M HCl solution: (a) lamellar, and (b) equiaxed microstructure [45].
It is important to note that many previous investigations on the corrosion behaviour of β-type titanium alloys (for biomedical applications) focused on the alloy composition only in one solution. As discussed previously, pH is an important factor which varies in various solutions. Hence, every solution may impose a different electrochemical result on an individual alloy and studying different simulated body fluid solutions would better exhibit the role of solution components on the biomedical corrosion procedure in these alloys.
Cheng-hao et al. [46] studied the corrosion characteristics of Ti-6Al-4V alloy in different simulated body fluids including Ringer’s solution (NaCl 8.5, KCl 0.2, CaCl2 0.2, NaHCO3 1.1 g/L), phosphate-buffered saline (PBS) solution (NaCl 8.0, KCl 0.2, Na2HPO4 1.15, KH2PO4 0.2 g/L) and Hank’s solution (NaCl 0.8, KCl 0.4, CaCl2 0.14, NaHCO3 0.35, C6H12O6 1.0, MgCl2. 6H2O 0.1, MgSO4·7H2O 0.06, Na2HPO4 0.06, KH2PO4 0.06 g/L) with different pH values. It was indicated that the order of corrosion rate (from highest to lowest) is in Ringer’s solution, PBS solution and Hank’s solution, respectively. It is reported that Na2HPO4 and KH2PO4 react with the metal surface and act as inhibitor by formation of a passive layer for the Hank’s solution. Also, a further reduction of pH decreases the corrosion resistance of the alloy in all the solutions (Table 1).

Table 1.
Corrosion current densities in different simulated body fluids for Ti-6Al-4V [46].
Also, different simulated body fluids were used by Hasena et al. [47] in order to evaluate the electrochemical behaviour of NiTi alloy. Figure 5 exhibits the OCP (open circuit potential) of all the tested solutions. Higher Cl− concentrations at 37 °C caused the most active potentials in HBSS (Hank’s balanced salt solution), Ringer’s solutions and the noblest (−291 mV) for HBSS after 3600 s exposure time. Also, it is claimed that in Hank’s solution with pH ranging from 1 to 9, the corrosion rate is highly affected by a further decrease in pH [48]. Many authors [49,50,51], explained that the Ringer’s solution seems to be more aggressive due to more concentrations of Cl− ions. It is recommended to refer to Tas’s work [52] for detailed reports on the effect of different ions on the corrosion rate of the tested alloys for each simulated body fluid solution.
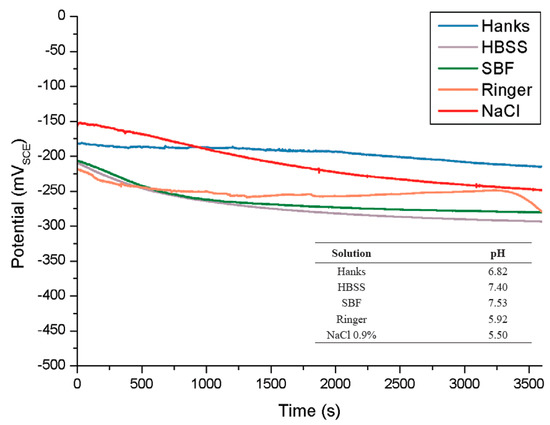
Figure 5.
Open circuit potentials (OCP) in different simulated body fluids at 37 °C and in 0.9% NaCl at 25 °C [47].
3. Fabrication Process
The corrosion and electrochemical behaviour of two distinct alloys with the same chemical composition but different manufacturing processes may be different. Various heat treatments and mechanical processes of Ti alloys including ageing and cold working are expected to influence the microstructure and alloy distribution with a direct consequence on their corrosion behaviour. Also, surface treatments such as anodizing and coatings can inventively alter the electrical current density passing through the bulk material [53,54,55]. Yun Bai et al. [56] showed that wrought Ti-6Al-4V has greater corrosion rate than electron beam melting (EBM)-produced Ti-6Al-4V. EBM-produced Ti-6Al-4V mainly consists of α and β phases, with a higher volume of β phase and much refined lamellar α/β phases and it was found that localized micro galvanic cells produced by α and β phases degrade the corrosion resistance of EBM-produced Ti-6Al-4V. In a study by Buciumeanu et al. [57], three different fabrication processes including casting, powder metallurgy (hot pressing, HP) and laser engineered net shaping (LENS) were used for Ti-6Al-4V specimens. The OCP measurements of HP, LENS and cast Ti-6Al-4V alloy were around −0.25 V, −0.03 V and −0.36 V, respectively. Hence, the LENS samples were found to have a better corrosion protection than the other two processes. This characteristic was attributed to the very high and localized cooling rates during the LENS process resulting in higher hardness and slight chemistry variation in the material.
Potentiodynamic polarization tests were performed for two deformed Ti-15Zr-12Nb alloys including: warm rolling (WR) at 660 °C for 1 h up to the 95% thickness reduction, and hot rolling (HR) from 880 °C to 660 °C up to the 70% thickness reduction using a multi-pass rolling process [58]. It is indicated that the HR sample with a coarse lamellar structure has higher corrosion current density (0.9976 mA/cm2) compared to the WR sample (0.8179 mA/cm2) with an ultra-fine microstructure and formation of more stable and passive surface layer. Also, in a study by Li et al. [59], corrosion characterization of two Ti-24Nb-2Zr (at. %) samples were investigated. Both samples were 75% deformed and then annealed at 800 °C for 1 h, one of them was also aged at 300 °C for 1 h. The electrochemical tests were performed to make a comparison against CP Ti alloy. The both deformed samples had higher passive current densities than CP Ti due to the precipitation of ω phase during the deformation and ageing processes which significantly destabilize the passive oxide film [56]. According to the results of [58,59], not any deformation procedure can improve the corrosion resistance of β type Ti alloys. There are a range of parameters such as deformation percent, annealing temperature and the texture of refined grains (e.g., uniform orientation) can affect the electrochemical behaviour of the alloy.
Five treated Ti-29Nb-13Ta-4.6Zr specimens were tested [60] namely: 87.5% cold-rolled titanium-niobium-tantalum-zirconium (TNTZCR), solutionized at 790 °C for 1 h (TNTZST), TNTZST aged at 400 °C for 72 h, multi-step-thermo-mechanical treatment (TNTZmulti), where the cold rolling (CR) and solution treatment (ST) were repeated 4 times, TNTZmulti aged at 400 °C for 72 h. and hot-swaged Ti-30Nb-10Ta-5Zr, which has 0.2% O and mirror surface produced by buff polishing. Figure 6 shows a higher level of corrosion resistance for the aged (TNTZmulti) and deformed (TNTZCR) samples when compared to the TNTZST and TNTZST aged at 400 °C. It is also shown that the critical current densities (Ic) and passive current densities (Ip) are at lowest for the TNTZmulti aged and TNTZmulti samples.
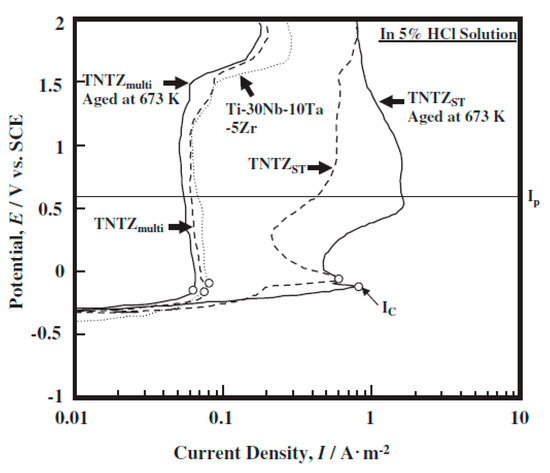
Figure 6.
Anodic polarization curves of TNTZST (Ti-29Nb-13Ta-4.6Zr), TNTZST aged at 673 K for 259.2 ks TNTZmulti, TNTZmulti aged at 673 K for 259.2 ks and hot swaged Ti-30Nb-10Ta-5Zr in 5% HCl solution at 310 K [60].
Two Ti-1.45Al-6.62Mo-4.53Fe-0.14O samples were solution treated at different temperatures of 750 °C (equiaxed β-grain structure with almost needle-like morphology of 10% primary α-phase) and 650 °C (equiaxed β-grain structure with almost needle-like morphology of 15% primary α-phase) for 30 min; and subsequently, both the samples were aged treated at 500 °C for 4 h. Through a comprehensive corrosion study, it was reported that the higher corrosion rate of 650 °C sample was due to the greater concentration of the α-phase which makes a local galvanic cell between the α and β phases [61]. It is important to note that thermal and mechanical treatments may alter the microstructure and phases in Ti alloys including β type Ti alloys. Therefore, the effect of these thermal and mechanical treatments on the corrosion behaviour of beta titanium alloys can be as important as the chemical composition. The corrosion current density reported in the literature [58] is (2 × 10−2 mA·cm−2) for the 650 °C sample which is about 34 times greater than that of 750 °C (5.9 × 10−4 mA·cm−2). It is thus indicated that a 100 °C increase in the solutionizing temperature of two equal alloys can greatly change the corrosion behaviour so that this might not happen even if the samples were made of two distinct precursors and initial composition. In another investigation [62], Ti-40Ta-22Hf-11.7Zr (TTHZ) beta Ti alloys were prepared in six samples, namely: as cast, ST (solution treated) and the other samples were then aged (STA) at 300 °C for 15 min, 1.5 h, 12 h and 24 h in argon, followed by air cooling (STA-15 min, STA-1.5 h, STA-12 h and STA-24 h), respectively. It was found that the ST sample has the best corrosion resistance consisting of a single beta phase, and with a further increase in ageing time, the α phase grows and the corrosion resistance decreases. Also, the STA-15 min sample had a small amount of α“ phase and β phase transformed into an α” phase without diffusion, so the corrosion resistance of the alloy tended to be the same as that in the solid solution due to the close thermodynamic properties of α″ to β phase which is the middle state of transition of α to β. Nishimura et al. [63] explained that the ageing heat treatment created α phase precipitations in the β phase of solutionized Ti-10Mn alloys which act as anodic particles [63]. Finally, the dissolution priority for α and β phases seems to be different in their various combinations. The single α phase is less corrosion resistant than β phase. However, according to [37], once the attack initiated in the two-phase (as-received α + β) alloy, the β phase appeared to be less resistant to continued dissolution than the α phase.
4. Chemical Composition
Admittedly, in order to achieve a superior biocompatible medical implant material, a comprehensive corrosion study is needed on the electrochemical reactions of the material with the synthetic physiological solutions [64,65]. Like other alloys, the electrochemical behaviour of beta titanium alloys depends, to a great extent, on many parameters such as electrolyte, microstructure and importantly chemical composition of the alloy (alloying elements and their amounts) [63,66,67]. Chemical composition is a major parameter which can influence the corrosion resistance of titanium alloys including new beta type titanium alloys. For instance, small additions of indium (In) to a beta-type Ti-Nb alloy (tested in Ringer’s solution) do not yield any negative effect on the corrosion behaviour and it was reported that a further addition of indium will enhance the corrosion resistance of the alloy [68]. Further addition of ruthenium (Ru) to Ti-20Nb-xRu (x = 0, 0.5, 1.0, 1.5 at. %) improves the corrosion resistance of this beta titanium alloy. The potentiodynamic polarization curves of Ru added samples in comparison to Ti-6Al-4V and CP Ti are presented in Figure 7. Approximately a 0.23 V difference in the corrosion potential of the Ru-free sample to nobler amounts and 1.6 V shift in comparison to the CP Ti and Ti-6Al-4V show a relatively better corrosion resistance [69].
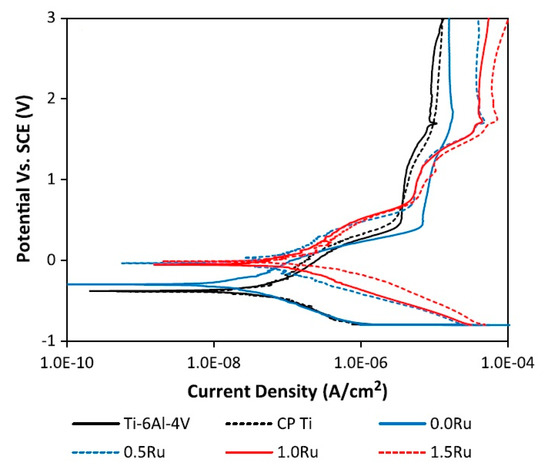
Figure 7.
Representative potentiodynamic polarization curves of titanium alloys in Hank’s balanced salt solution (HBSS) [69].
An electrochemical study was performed on the corrosion behaviour of two beta-type titanium alloys namely Ti-15Mo (TiMo) and Ti-29Nb-13Ta-4.6Zr (TNTZ) [70]. After one hour of immersion in Hank’s solution, the measured OCP for TiMo and TNTZ were −320 and −260 mV, respectively. This nobler potential of TNTZ beta titanium alloys related to the alloying elements increases the corrosion resistance. Using electrochemical impedance spectroscopy (EIS), the passivation behaviour of Ti-15Mo (TiMo) and Ti-29Nb-13Ta-4.6Zr (TNTZ) alloys was characterised. TNTZ showed two time constants at higher potentials, indicating the presence of two passive layers, namely an inner layer for corrosion resistance and an outer porous layer. Therefore, TNTZ seems to act better in orthopaedic applications [68]. Many authors have delved deeply in the electrochemical properties of low modulus Ti alloys including beta phase [71,72,73,74,75,76,77]. The Ti passive layer acts as a protective layer in various aqueous environments and its reaction in H2O is as follows:
Ti + H2O ↔ Ti(OH)4 + 2H2
Many alloying elements in titanium alloys lead to a better corrosion and electrochemical behaviour, and this greatly depends on the pH value of the environment. The oxide layer properties formed on the surface of Ti alloys highly depend on the alloying elements present in the passive layer. Factors such as diffusion rate of the alloying element ions in the passive oxide film and mutual solubility can specify the strength and stability of the surface film on Ti alloys [78,79,80,81,82]. Metikoš-Huković et al. [82] showed that the alloying of titanium with elements of higher valence electrons such as Nb (Ti-6Al-4V vs. Ti-6Al-6Nb) can cause the stabilization of the passive film and elimination of anion vacancies. The presence of Nb5+ cations increases the number of oxygen ions, which cancel out the anion vacancies and make the film less defective [83]. The disappearance of the anion vacancies lowers the diffusion rates across the passive layer.
Also, the formation of intermetallic precipitates may provide susceptible sites for localized corrosion and stabilization of an α/β phase structure which may result in a lower homogeneity in the passive layer; and consequently, lowering the breakdown potential of the passive layer [84,85].
Titanium-zirconium-molybdenum (TZM), as a functional alloy is not only used in biomedical applications but also in many other industrial applications. Hence, the electrolyte used for corrosion investigations for this alloy is mostly ordinary NaCl and NaOH solutions [86,87,88]. Zirconium has excellent solid solubility in titanium due to its near electrochemical and mechanical aspects to titanium. Therefore, alloying these two elements can lead to an improvement in the corrosion and mechanical behaviour of the alloy [89,90]. Any increase of molybdenum may lead to a decrease in β-transus temperature, and Mo has a higher β stabilizing impact on the TZM than Zr [91].
A common version of TZM alloys is lanthanum-doped titanium-zirconium-molybdenum (La-TZM) alloy with considerable properties such as high temperature oxidation, and better mechanical and physical functions. This wide range of features of La-TZM alloy have encouraged many scientists to investigate its corrosion behaviour too [92,93,94,95,96,97,98]. Deng et al. [98] showed that alloying lanthanum in TZM degrades the corrosion resistance. Since the corrosion potential difference of La and other alloying elements in La-TZM alloy is high, the micro galvanic cell series created by this potential difference in the alloy matrix can easily cause the initiation of pits on the surface leading to intergranular corrosion. Lanthanum plays the role of the second-phase particle which includes a nobler potential than the Mo matrix and spoils the integration of the oxide passive layer on the surface. Also, in Figure 8, it is shown that no significant passivation behaviour in the anodic branch of La-TZM alloy occurs while the passivation behaviour of the TZM is obviously illustrated by the reduction in the current density around −4 A/cm2. In another research [99], it is indicated that the corrosion resistance of TZM is better than La-TZM in alkaline and neutral environments including Cl− and OH−; while, on the contrary, the La-TZM shows a higher corrosion resistance than TZM in acidic media. Also, Hu et al. [99] indicated that the Cl− concentration is of great importance to evaluate the corrosion behaviour of TZM alloy. They showed that TZM alloys have a good corrosion resistance in 0.5 mol/L or 1.5 mol/L Cl− concentrations.
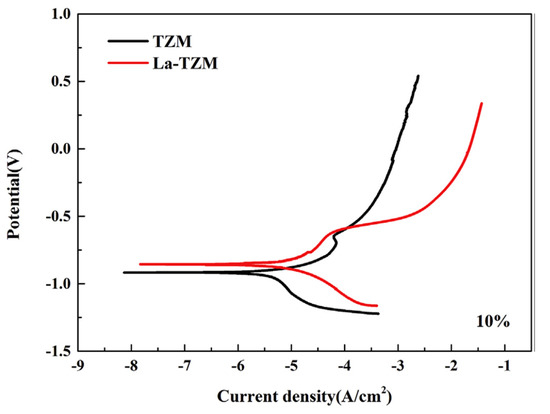
Figure 8.
Potentiodynamic polarization curves of TZM and La-TZM alloys [98].
Quaternary TNTZ (titanium–niobium–tantalum–zirconium) alloys were developed in the 1990′s [100]. These alloying elements improved the mechanical and corrosion behaviour of low modulus beta titanium alloys for biomedical applications. Nb is known as a β-stabilizer having a significant role in the spontaneous passivation of the alloy [101]. Also, from a microstructural point of view, a significant reduction of β-transus temperature seems to be present for the higher β-stabilizer content in some implants which include niobium in their composition [102,103]. Zr improves the passivity and lowers the active electrochemical reaction of the α phase of titanium [66] and Ta is considered as an element with an excellent capability in formation of passive oxide layer (Ta2O5) [104]. The most notable feature of TNTZ alloys, which improves its passive layer corrosion potential breakdown point in comparison with the other ternary and low modulus beta titanium alloys, is the strong bonding and adherence of their passive layers, near the corrosion potential of the alloying elements [105,106]. In a study reported by [107], it is shown that in Ti-20Nb-10Zr-5Ta alloy, Zr had the greatest influence on reducing the open circuit potential to more negative values and achieving a better protective passive layer behaviour. Milošev et al. [19] showed that addition of oxygen to TNT alloys may increase the chemical solubility of Ti due to its strong β-stabilizing effect and they tested the corrosion current density (0.045 μA/cm2). Also, they reported that the incorporation of different alloying oxides in the surface layer is the main reason of improvement in the corrosion behaviour of Ti-20Nb-10Zr-5Ta alloys.
The other biomedical low modulus beta type titanium alloys of great interest are the ternary TNZ (Titanium-Niobium-Zirconium) alloys. Robin et al. [36] showed that the corrosion resistance of Ti-13Nb-13Zr alloy is a little higher than Ti-5Nb-13Zr and Ti-20Nb-13Zr alloys in the Ringer’s solution excluding fluorides, and discussed that this is due to the lower Nb content of α-phase in Ti-13Nb-13Zr. In a TNZ system, Nb and Zr encourage the suppression of O2 evolution and cease the cathodic electron transfer reaction and can lower the active anodic dissolution reactions in the system [108]. Huang et al. [109] compared two alloys of Ti-13Nb-13Zr and Ti-15Mo, and concluded that Ti-13Nb-13Zr has a superior anticorrosion performance because of the Nb alloying element which annihilates the anion vacancies in the crystal lattice of the TiO2 passive layer, which is due to the formation of covalent bonds among Nb, Zr and Ti (4d3, 4d2, 3d2) respectively [66]. Also, Macdonald [110] presented the theory of the point defect model in surface layer whose presence can cause heterogeneity of passive layer and degradation of corrosion resistance [110]. Hence, Nb5+ particles can situate in the crystal lattice of TiO2 and fill the anion vacancies which results in lower defects and higher corrosion resistance [82].
An important ternary and quaternary group of beta titanium alloys including Sn alloying element has emerged recently. A study on Ti-32Nb-2Sn and Ti-32Nb-4Sn concluded that the higher Sn content improves the corrosion behaviour due to the higher passive layer quality [110]. Also, it must be considered that the Sn content up to 4 wt% to 6 wt% may cause cytotoxic effects in the human body and, therefore, further investigations should be undertaken [111]. Bai et al. [2] reported that Sn included beta titanium alloys show a wide range of passivation on anodic branch of Tafel diagram and it is a superior feature of these alloys.
5. General Significations of Corrosion
The complicated electrolytic behaviour of body fluid including a combination of attacking corrosion components creates an aggressive media for biomedical implants. Hence, controlling and improving the corrosion resistance of medical implants seems to be a significant concern in the manufacturing process of implants [112]. In spite of electrochemical parameters, mechanical deformation affects the corrosion rate as seen in [113] where a further amount of cold rolling lowered the corrosion rate. Thermodynamics and kinetics are the main two features of the electrochemical corrosion phenomenon. Thermodynamics specifies the oxidation-reduction reactions of the implant material adjacent to the body fluid media and the kinetics evaluates the probability of occurrence of reactions [114]. Common types of corrosion that can occur in beta type titanium alloys (when used in implants) are fretting, galvanic, pitting/crevice and uniform corrosion [115]. In an implant such as hip joint implants of which all the surface is in contact with the body tissues and fluids, the uniform corrosion is of great concern and pH variations can play a significant role in the corrosion degradation. Also, surface treatments and the thermodynamic stability and high adherence of the passive layer may control this type of corrosion [116]. In addition, fretting and wear corrosion must be considered in modular implants where metallic parts have direct contact (e.g., taper junction of hip implants). With regard to the advancement and development of low modulus beta titanium alloys, it is expected that successful implant materials withstand long-term uniform corrosion with corrosion rate of less than 1 µm/year [117]. For dental implants, pitting and galvanic corrosion should be more investigated since food debris is always deposited and can make a localized galvanic series following pitting corrosion [118]. Moreover, it is important to perform microstructural evaluations along with ordinary corrosion testing procedures when investigating pitting and galvanic corrosion in dental implant materials. Surface images of dental implants and any other implants susceptible to pitting corrosion can provide useful data about the morphology, distribution, depth and number of defects. Hence, further experiments would be more reliable and authentic.
6. Conclusions
In this review, low modulus β-type titanium alloys were investigated from a corrosion behaviour point of view. The important parameters that can affect the electrochemical and corrosion behaviour of these alloys were discussed. Investigations on the development of a suitable microstructure with optimal mechanical properties have been performed to design and fabricate low modulus β-type Ti-based alloys for medical implant applications. However, less attention has been paid to the electrochemical and corrosion behaviour of these new generation titanium alloys. Toxicity of biomedical implants depends, to a great extent, on the ion release rate of the alloy into the body which passes through the surface passive layer. Hence, the microstructure and composition of the passive layer and its adhesion and stability should be carefully evaluated in these alloys. Also, surface treatments and anodizing can greatly improve the quality of passive layers but the chemical composition of the surface coatings should be non-toxic. Surface microstructural observations of beta type Ti alloys after electrochemical tests in various simulated body fluid solutions play an important role in verifying corrosion test results. These observations can also reveal more data as to other corrosion aspects of the surface such as thickness of the passive layer, corroded phases and particles, pitting attacked areas and corrosion mechanism of the bulk. It is important to note that the physical and mechanical changes in beta-type Ti alloys may alter the microstructure and phases. Therefore, the effect of these physical (heat) and mechanical treatments on the corrosion behaviour of beta titanium alloys may be as important as the chemical composition. Also, the amount of α and β phases in the microstructure and their interaction and dissolution can affect the corrosion resistance. It is important to mention that various physical and mechanical treatments of beta titanium alloys directly affect the interaction and distribution of α and β phases which these phases not only alter the corrosion behaviour in the bulk material but also play an important role on the surface passive layer of implants.
Dissolution priority for the α and β phases seems to be different in their various combinations. The single α phase is less corrosion resistant than β phase. However, once the attack has initiated in the two-phase (α + β), the β phase appears to be less resistant to continued dissolution when compared to the α phase. It is suggested that more investigations should be performed on the corrosion resistance of α and β phases (separately and as mixed). Suitable amounts of α and β phases should be created in the microstructure of beta-titanium alloys including various alloying elements in order to have a high corrosion resistance and a stable surface passive layer. Afterwards, other physical, mechanical and chemical treatments such as surface treatment, heat treatments (e.g., ageing, solutionizing, and hardening) and various alloy fabrication processes should be taken into account.
Author Contributions
Conceptualization, R.H.O.; literature review, P.A.; writing—original draft preparation, P.A.; writing—review and editing, R.O. and R.G.; supervision, R.O. and R.G.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kunčická, L.; Kocich, R.; Lowe, T.C. Advances in metals and alloys for joint replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar]
- Bai, Y.; Li, S.J.; Prima, F.; Hao, Y.L.; Yang, R. Electrochemical corrosion behavior of Ti-24Nb-4Zr-8Sn alloy in a simulated physiological environment. Appl. Surf. Sci. 2012, 258, 4035–4040. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti-Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Yamamuro, T. Patterns of osteogenesis in relation to various biomaterials. J. Jpn. Soc. Biomater. 1989, 7, 19–23. [Google Scholar]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Hua, N.; Chen, W.; Zhang, L.; Li, G.; Liao, Z.; Lin, Y. Mechanical properties and bio-tribological behaviors of novel beta-Zr-type Zr-Al-Fe-Nb alloys for biomedical applications. Mater. Sci. Eng. C 2017, 76, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Niinomi, M.; Nakai, M.; Obara, S.; Fujii, H. Improved fatigue properties with maintaining low young’s modulus achieved in biomedical beta-type titanium alloy by oxygen addition. Mater. Sci. Eng. A 2017, 704, 10–17. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Fukui, H.; Ogawa, M.; Toda, H. Improvement in fatigue characteristics of newly developed beta type titanium alloy for biomedical applications by thermo-mechanical treatments. Mater. Sci. Eng. C 2005, 25, 248–254. [Google Scholar] [CrossRef]
- Du, Z.; Xiao, S.; Liu, J.; Lv, S.; Xu, L.; Kong, F.; Chen, Y. Hot deformation behavior of Ti-3.5Al-5Mo-6V-3Cr-2Sn-0.5Fe Alloy in α + β field. Metals 2015, 5, 216–227. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.H.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, R.; Fallahnezhad, K.; Kuppusami, S. An investigation on the wear resistance and fatigue behaviour of Ti-6Al-4V notched members coated with hydroxyapatite coatings. Materials 2016, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Raducanu, D.; Vasilescu, E.; Cojocaru, V.D.; Cinca, I.; Drob, P.; Vasilescu, C.; Drob, S.I. Mechanical and corrosion resistance of a new nanostructured Ti-Zr-Ta-Nb alloy. J. Mech. Behav. Biomed. Mater. 2011, 4, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer, H.; Niinomi, M.; Nakai, M.; Hieda, J.; Todaka, Y.; Akahori, T.; Miyazaki, T. Heterogeneous structure and mechanical hardness of biomedical β-type Ti-29Nb-13Ta-4.6Zr subjected to high-pressure torsion. J. Mech. Behav. Biomed. Mater. 2012, 10, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of low rigidity β-type titanium alloy for biomedical applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar]
- Takematsu, E.; Cho, K.; Hieda, J.; Nakai, M.; Katsumata, K.; Okada, K.; Niinomi, M.; Matsushita, N. Adhesive strength of bioactive oxide layers fabricated on TNTZ alloy by three different alkali-solution treatments. J. Mech. Behav. Biomed. Mater. 2016, 61, 174–181. [Google Scholar] [CrossRef]
- Bahl, S.; Das, S.; Suwas, S.; Chatterjee, K. Engineering the next-generation tin containing β titanium alloys with high strength and low modulus for orthopedic applications. J. Mech. Behav. Biomed. Mater. 2018, 78, 124–133. [Google Scholar] [CrossRef]
- Milošev, I.; Žerjav, G.; Moreno, J.M.C.; Popa, M. Electrochemical properties, chemical composition and thickness of passive film formed on novel Ti-20Nb-10Zr-5Ta alloy. Electrochim. Acta 2013, 99, 176–189. [Google Scholar] [CrossRef]
- Brett, C.; Muresan, I. The influence of artificial body fluids on metallic corrosion. In Key Engineering Materials; Trans Tech Publications: Zurich, Switzerland, 2002; Volume 230, pp. 459–462. [Google Scholar]
- Fallahnezhad, K.; Oskouei, R.H.; Badnava, H.; Taylor, M. An adaptive finite element simulation of fretting wear damage at the head-neck taper junction of total hip replacement: The role of taper angle mismatch. J. Mech. Behav. Biomed. Mater. 2017, 75, 58–67. [Google Scholar] [CrossRef]
- Oskouei, R.H.; Barati, M.R.; Farhoudi, H.; Taylor, M.; Solomon, L.B. A new finding on the in-vivo crevice corrosion damage in a CoCrMo hip implant. Mater. Sci. Eng. C 2017, 79, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Jiang, H.B.; Cha, J.Y.; Kim, K.M.; Hwang, C.J. The effect of fluoride-containing oral rinses on the corrosion resistance of titanium alloy (Ti-6Al-4V). Korean J. Orthod. 2017, 47, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Fallahnezhad, K.; Oskouei, R.H.; Taylor, M. Development of a fretting corrosion model for metallic interfaces using adaptive finite element analysis. Finite Elem. Anal. Des. 2018, 148, 38–47. [Google Scholar] [CrossRef]
- Fallahnezhad, K.; Farhoudi, H.; Oskouei, R.H.; Taylor, M. A finite element study on the mechanical response of the head-neck interface of hip implants under realistic forces and moments of daily activities: Part 2. J. Mech. Behav. Biomed. Mater. 2018, 77, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Fallahnezhad, K.; Farhoudi, H.; Oskouei, R.H.; Taylor, M. A finite element study on the mechanical response of the head-neck interface of hip implants under realistic forces and moments of daily activities: Part 1. J. Mech. Behav. Biomed. Mater. 2017, 75, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Hussenbocus, S.; Kosuge, D.; Solomon, L.B.; Howie, D.W.; Oskouei, R.H. Head-neck taper corrosion in hip arthroplasty. Biomed Res. Int. 2015, 2015, 758123. [Google Scholar] [CrossRef] [PubMed]
- Milimonfared, R.; Oskouei, R.; Taylor, M.; Solomon, L. The distribution and severity of corrosion damage at eight distinct zones of metallic femoral stem implants. Metals 2018, 8, 13–24. [Google Scholar] [CrossRef]
- Milimonfared, R.; Oskouei, R.H.; Taylor, M.; Solomon, L.B. An intelligent system for image-based rating of corrosion severity at stem taper of retrieved hip replacement implants. Med. Eng. Phys. 2018, 61, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Streitzel, R.; Hösch, A.; Kalbfleisch, H.; Buch, D. In vitro corrosion of titanium. Biomaterials 1998, 19, 1495–1499. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Azambuja, D.S. Electrochemical behavior of Ti and Ti6Al4V in aqueous solutions of citric acid containing halides. Mater. Res. 2006, 9, 387–392. [Google Scholar] [CrossRef]
- Schutz, R.W.; Thomas, D.E. Corrosion of titanium and titanium alloys. Metals Handb. Corros. 1987, 13, 669–706. [Google Scholar]
- Reclaru, L.; Meyer, J.M. Effects of fluorides on titanium and other dental alloys in dentistry. Biomaterials 1998, 19, 85–92. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Fan, Z.; Li, S.; Hao, Y.; Yang, R.; Gao, Y. Effect of fluoride on the corrosion behavior of nanostructured Ti-24Nb-4Zr-8Sn alloy in acidulated artificial saliva. J. Mater. Sci. Technol. 2018, 34, 1660–1670. [Google Scholar] [CrossRef]
- Huang, H.H. Effect of fluoride and albumin concentration on the corrosion behavior of Ti-6Al-4V alloy. Biomaterials 2003, 24, 275–282. [Google Scholar] [CrossRef]
- Robin, A.; Carvalho, O.A.S. Influence of pH and fluoride species on the corrosion behavior of ti-xnb-13zr alloys in ringer’s solution. Adv. Mater. Sci. Eng. 2013, 2013, 434975. [Google Scholar] [CrossRef]
- Reyes, K.M.; Kuromoto, N.K.; Claro, A.P.R.A.; Marino, C.E.B. Electrochemical stability of binary TiNb for biomedical applications. Mater. Res. Express 2017, 4, 075402. [Google Scholar] [CrossRef]
- Prashanth, K.; Zhuravleva, K.; Okulov, I.; Calin, M.; Eckert, J.; Gebert, A. Mechanical and corrosion behavior of new generation Ti-45Nb porous alloys implant devices. Technologies 2016, 4, 33. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Atapour, M.; Pilchak, A.L.; Frankel, G.S.; Williams, J.C. Corrosion behavior of β titanium alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 885–891. [Google Scholar] [CrossRef]
- Samuel, S.; Nag, S.; Nasrazadani, S.; Ukirde, V.; Bouanani, M.E.; Mohandas, A.; Nguyen, K.; Banerjee, R. Corrosion resistance and in vitro response of laser-deposited Ti-Nb-Zr-Ta alloys for orthopedic implant applications. J. Biomed. Mater. Res. Part A 2010, 94, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.C.; Vasilescu, C.; Drob, S.I.; Popa, M.; Drob, P.; Vasilescu, E. Electrodeposition, characterization, and corrosion stability of nanostructured anodic oxides on new Ti-15Zr-5Nb alloy surface. J. Nanomater. 2013, 2013, 3. [Google Scholar]
- Bai, Y.; Hao, Y.L.; Li, S.J.; Hao, Y.Q.; Yang, R.; Prima, F. Corrosion behavior of biomedical Ti-24Nb-4Zr-8Sn alloy in different simulated body solutions. Mater. Sci. Eng. C 2013, 33, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cui, W.F.; Song, X.; Liu, G.; Zhou, L. Effects of surface nanocrystallization on corrosion resistance of β-type titanium alloy. Trans. Nonferr. Metals Soc. China 2014, 24, 2529–2535. [Google Scholar] [CrossRef]
- Lu, J.; Ge, P.; Li, Q.; Zhang, W.; Huo, W.; Hu, J.; Zhang, Y.; Zhao, Y. Effect of microstructure characteristic on mechanical properties and corrosion behavior of new high strength Ti-1300 beta titanium alloy. J. Alloys Compd. 2017, 727, 1126–1135. [Google Scholar] [CrossRef]
- Liang, C.; Mou, Z. Effect of different simulated body fluids on anti-corrosion bimetallic materials. Trans. Nonferr. Met. Soc. China 2001, 11, 579–582. [Google Scholar]
- Hansen, A.W.; Führ, L.T.; Antonini, L.M.; Villarinho, D.J.; Marino, C.E.B.; Malfatti, C.D.F. The electrochemical behavior of the NiTi alloy in different simulated body fluids. Mater. Res. 2015, 18, 184–190. [Google Scholar] [CrossRef]
- Trépanier, C.; Pelton, A.R. Effect of temperature and pH on the corrosion resistance of nitinol. In medical device materials. In Proceedings of the Materials and Processes for Medical Devices Conference, Baden-Baden, Germany, 3–7 October 2004. [Google Scholar]
- Shahrabi, T.; Sanjabi, S.; Saebnoori, E.; Barber, Z.H. Extremely high pitting resistance of NiTi shape memory alloy thin film in simulated body fluids. Mater. Lett. 2008, 62, 2791–2794. [Google Scholar] [CrossRef]
- Liang, C.-H.; Mou, Z.-Q. Effects of different simulated fluids on anticorrosion biometallic materials. Trans. Nonferr. Met. Soc. China 2001, 11, 579–582. [Google Scholar]
- Li, X.; Wang, J.; Han, E.H.; Ke, W. Influence of fluoride and chloride on corrosion behavior of NiTi orthodontic wires. Acta Biomater. 2007, 3, 807–815. [Google Scholar] [CrossRef]
- Tas, A.C. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef]
- Afzali, P.; Yousefpour, M.; Borhani, E. Evaluation of the effect of ageing heat treatment on corrosion resistance of Al-Ag alloy using electrochemical methods. J. Mater. Res. 2016, 31, 2457–2464. [Google Scholar] [CrossRef]
- Lopes, C.S.D.; Donato, M.T.; Ramgi, P. Comparative corrosion behaviour of titanium alloys (TI-15MO and TI-6AL-4V) for dental implants applications: A review. Corros. Prot. Mater. 2016, 35, 2. [Google Scholar]
- Geetha, M.; Mudali, U.K.; Gogia, A.K.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti-13Nb-13Zr alloy. Corros. Sci. 2004, 46, 877–892. [Google Scholar] [CrossRef]
- Bai, Y.; Gai, X.; Li, S.; Zhang, L.C.; Liu, Y.; Hao, Y.; Zhang, X.; Yang, R.; Gao, Y. Improved corrosion behaviour of electron beam melted Ti-6Al–4V alloy in phosphate buffered saline. Corros. Sci. 2017, 123, 289–296. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Shamsaei, N.; Thompson, S.M.; Silva, F.S.; Henriques, B. Tribocorrosion behavior of additive manufactured Ti-6Al-4V biomedical alloy. Tribol. Int. 2018, 119, 381–388. [Google Scholar] [CrossRef]
- Mohammed, M.T. Development of a new metastable beta titanium alloy for biomedical applications. Karbala Int. J. Mod. Sci. 2017, 3, 224–230. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Ma, G.; Liu, X.; Pan, D. Influence of ω phase precipitation on mechanical performance and corrosion resistance of Ti–Nb–Zr alloy. Mater. Des. 2016, 111, 421–428. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Fukui, H.; Suzuki, A. Fatigue, fretting fatigue and corrosion characteristics of biocompatible beta type titanium alloy conducted with various thereto-mechanical treatments. Mater. Trans. 2004, 45, 1540–1548. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Moustafa, M.M.; Al-Grafi, M.W.; El-Bagoury, N.; Amin, M.A. Effect of solution heat treatment on microstructure and wear and corrosion behavior of a two phase β-metastable titanium alloy. Int. J. Electrochem. Sci. 2016, 11, 3206–3226. [Google Scholar] [CrossRef]
- Lin, J.; Ozan, S.; Munir, K.; Wang, K.; Tong, X.; Li, Y.; Li, G.; Wen, C. Effects of solution treatment and aging on the microstructure, mechanical properties, and corrosion resistance of a β type Ti-Ta-Hf-Zr alloy. RSC Adv. 2017, 7, 12309–12317. [Google Scholar] [CrossRef]
- Nishimura, T. Effect of microstructure on the electrochemical behavior of Ti-10 Mass% Mn alloys in high chloride solution. J. Mater. Eng. Perform. 2016, 25, 443–450. [Google Scholar] [CrossRef]
- Mary, J.S.; Rajendran, S. Corrosion behaviour of metals in artificial body fluid an over view. Zaštita Materijala 2012, 53, 181–189. [Google Scholar]
- Nagalakshmi, R.; Rajendran, S.; Sathiyabama, J.; Pandiarajan, M.; Christy, J.L. Corrosion behaviour of biomaterials in synthetic biological solutions—An overview. Eur. Chem. Bull. 2013, 2, 171–179. [Google Scholar]
- Yu, S.Y.; Scully, J.R. Corrosion and passivity of Ti-13% Nb-13% Zr in comparison to other biomedical implant alloys. Corrosion 1997, 53, 965–976. [Google Scholar] [CrossRef]
- Reyes, K.M.; Kuromoto, N.K.; Claro, A.P.R.A.; Marino, C.E.B. Potentiodynamic polarization study of type 316L and 316LVM stainless steels for surgical implants in simulated body fluids. J. Chem. Pharm. Res. 2012, 4, 203–208. [Google Scholar]
- Gebert, A.; Oswald, S.; Helth, A.; Voss, A.; Gostin, P.F.; Rohnke, M.; Janek, J.; Calin, M.; Eckert, J. Effect of indium (In) on corrosion and passivity of a beta-type Ti-Nb alloy in Ringer’s solution. Appl. Surf. Sci. 2015, 335, 213–222. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Ping, D.H.; Yamabe-Mitarai, Y.; Wen, C. Impact of ruthenium on microstructure and corrosion behavior of β-type Ti-Nb-Ru alloys for biomedical applications. Mater. Des. 2014, 59, 303–309. [Google Scholar] [CrossRef]
- Jugowiec, D.; Łukaszczyk, A.; Cieniek, Ł.; Kowalski, K.; Rumian, Ł.; Pietryga, K.; Kot, M.; Pamuła, E.; Moskalewicz, T. Influence of the electrophoretic deposition route on the microstructure and properties of nano-hydroxyapatite/chitosan coatings on the Ti-13Nb-13Zr alloy. Surf. Coat. Technol. 2017, 324, 64–79. [Google Scholar] [CrossRef]
- Karthega, M.; Raman, V.; Rajendran, N. Influence of potential on the electrochemical behaviour of β titanium alloys in Hank’s solution. Acta Biomater. 2007, 3, 1019–1023. [Google Scholar] [CrossRef]
- Bolat, G.; Mareci, D.; Chelariu, R.; Izquierdo, J.; González, S.; Souto, R.M. Investigation of the electrochemical behaviour of TiMo alloys in simulated physiological solutions. Electrochim. Acta 2013, 113, 470–480. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Sutiman, D.; Gordin, D.M.; Gloriant, T. Evaluating electrochemical behaviour of recrystallized titanium alloys in Ringer’s solution. Mater. Corros. 2011, 62, 1117–1123. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Gordin, D.M.; Romas, M.; Sutiman, D.; Gloriant, T. Effect of Mo content on electrochemical behaviour of TiMo alloys for dental applications. Mater. Corros. 2010, 61, 829–837. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Bolat, G.; Cailean, A.; Grancea, V.; Sutiman, D. Electrochemical behaviour of Ti alloys containing Mo and Ta as β-stabilizer elements for dental application. Trans. Nonferr. Met. Soc. China 2013, 23, 3829–3836. [Google Scholar] [CrossRef]
- Lee, Y.R.; Han, M.K.; Kim, M.K.; Moon, W.J.; Song, H.J.; Park, Y.J. Effect of gold addition on the microstructure, mechanical properties and corrosion behavior of Ti alloys. Gold Bull. 2014, 47, 153–160. [Google Scholar] [CrossRef]
- Cremasco, A.; Messias, A.D.; Esposito, A.R.; Duek, E.A.D.R.; Caram, R. Effects of alloying elements on the cytotoxic response of titanium alloys. Mater. Sci. Eng. C 2011, 31, 833–839. [Google Scholar] [CrossRef]
- Jablokov, V.R.; Nutt, M.J.; Richelsoph, M.E.; Freese, H.L. The application of Ti-15Mo beta titanium alloy in high strength structural orthopaedic applications. J. ASTM Int. 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical stability and corrosion resistance of Ti-Mo alloys for biomedical applications. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef] [PubMed]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Guillemot, F. Recent advances in the design of titanium alloys for orthopedic applications. Expert Rev. Med. Dev. 2005, 2, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Metikoš-Huković, M.; Kwokal, A.; Piljac, J. The influence of niobium and vanadium on passivity of titanium-based implants in physiological solution. Biomaterials 2003, 24, 3765–3775. [Google Scholar] [CrossRef]
- Kubaschewski, O.; Hopkins, B.E. Oxidation of Metals; Kubaschewski, V.O., Hopkins, B.E., Eds.; Butterworths Scientific Publications: London, UK, 1998. [Google Scholar]
- Watanabe, T.; Shindo, T.; Naito, H. Effect of iron content on the breakdown potential for pitting of titanium in NaCl solutions. In Sixth World Conference on Titanium IV; Cedex: Paris, France, 1988; pp. 1735–1740. [Google Scholar]
- Shoesmith, D.W.; Ikeda, B.M. The resistance of titanium to pitting, microbially induced corrosion and corrosion under unsaturated conditions. Atomic Energy Canada 1997, 30, 96–557. [Google Scholar]
- Banerjee, D.; Williams, J.C. Perspectives on titanium science and technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, Z.; Liu, W.; Zhang, D.; Zhao, H.; Wang, L.; Li, X. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Ho, W.F.; Cheng, C.H.; Pan, C.H.; Wu, S.C.; Hsu, H.C. Structure, mechanical properties and grindability of dental Ti-10Zr-X alloys. Mater. Sci. Eng. C 2009, 29, 36–43. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wu, S.-C.; Sung, Y.-C.; Ho, W.-F. The structure and mechanical properties of as-cast Zr–Ti alloys. J. Alloys Compd. 2009, 488, 279–283. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Araújo, R.O.; Lourenço, M.L.; Kuroda, P.A.B.; Buzalaf, M.A.R.; Grandini, C.R. Effect of the substitutional elements on the microstructure of the Ti-15Mo-Zr and Ti-15Zr-Mo systems alloys. J. Mater. Res. Technol. 2015, 4, 180–185. [Google Scholar] [CrossRef]
- Yang, F.; Wang, K.S.; Hu, P.; He, H.C.; Kang, X.Q.; Wang, H.; Liu, R.Z.; Volinsky, A.A. La doping effect on TZM alloy oxidation behavior. J. Alloys Compd. 2014, 593, 196–201. [Google Scholar] [CrossRef]
- Hu, P.; Hu, B.L.; Wang, K.S.; Song, R.; Yang, F.; Yu, Z.T.; Tan, J.F.; Cao, W.C.; Liu, D.X.; An, G.; et al. Strengthening and elongation mechanism of Lanthanum-doped Titanium-Zirconium-Molybdenum alloy. Mater. Sci. Eng. A 2016, 678, 315–319. [Google Scholar] [CrossRef]
- Hu, P.; Yang, F.; Deng, J.; Chang, T.; Hu, B.; Tan, J.; Wang, K.; Cao, W.; Feng, P.; Yu, H. High temperature mechanical properties of TZM alloys under different lanthanum doping treatments. J. Alloys Compd. 2017, 711, 64–70. [Google Scholar] [CrossRef]
- Hu, P.; Zhou, Y.; Chang, T.; Yu, Z.; Wang, K.; Yang, F.; Hu, B.; Cao, W.; Yu, H. Investigation on compression behavior of TZM and La2O3doped TZM Alloys at high temperature. Mater. Sci. Eng. A 2017, 687, 276–280. [Google Scholar] [CrossRef]
- Wang, K.S.; Tan, J.F.; Hu, P.; Yu, Z.T.; Yang, F.; Hu, B.L.; Song, R.; He, H.C.; Volinsky, A.A. La2O3 effects on TZM alloy recovery, recrystallization and mechanical properties. Mater. Sci. Eng. A 2015, 636, 415–420. [Google Scholar] [CrossRef]
- Hu, B.L.; Wang, K.S.; Hu, P.; Zhou, Y.H.; Yang, F.; Li, Q.W.; Yu, H.L. Effect of lanthanum in arc erosion of Titanium-Zirconium-Molybdenum alloy. J. Alloys Compd. 2017, 696, 522–528. [Google Scholar] [CrossRef]
- Deng, J.; Wang, K.S.; Hu, P.; Zhou, Y.H.; Chang, T.; Hu, B.L.; Feng, P.F.; Song, R.; An, G. Electrochemical behavior and microstructural characterization of lanthanum-doped titanium-zirconium-molybdenum alloy. J. Alloys Compd. 2018, 763, 687–694. [Google Scholar] [CrossRef]
- Hu, P.; Song, R.; Li, X.J.; Deng, J.; Chen, Z.Y.; Li, Q.W.; Yu, H.L. Influence of concentrations of chloride ions on electrochemical corrosion behavior of titanium-zirconium-molybdenum alloy. J. Alloys Compd. 2017, 708, 367–372. [Google Scholar] [CrossRef]
- Davidson, J.; Kovacs, P. Biocompatible Low Modulus Titanium Alloy for Medical Implants. U.S. Patent 5,169,597, 8 December 1992. [Google Scholar]
- O’Brien, B.; Stinson, J.; Carroll, W. Development of a new niobium-based alloy for vascular stent applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 303–312. [Google Scholar] [CrossRef]
- Arciniegas, M.; Manero, J.M.; Peña, J.; Gil, F.J.; Planell, J.A. Study of new multifunctional shape memory and low elastic modulus Ni-free Ti alloys. Metall. Mater. Trans. A 2008, 39, 742–751. [Google Scholar] [CrossRef]
- Arciniegas, M.; Peña, J.; Manero, J.M.; Paniagua, J.C.; Gil, F.J. Quantum parameters for guiding the design of Ti alloys with shape memory and/or low elastic modulus. Philos. Mag. 2008, 88, 2529–2548. [Google Scholar] [CrossRef]
- Okazaki, Y. A new Ti-15Zr-4Nb-4Ta alloy for medical applications. Curr. Opin. Solid State Mater. Sci. 2001, 5, 45–53. [Google Scholar] [CrossRef]
- Saji, V.S.; Jeong, Y.H.; Choe, H.C. A comparative study on corrosion behavior of Ti-35Nb-5Ta-7Zr, Ti-6Al-4V and CP-Ti in 0.9 wt% NaCl. Corros. Sci. Technol. 2009, 8, 139–142. [Google Scholar]
- Rajabi, F.; Hanzaki, A.Z.; Abedi, H.R.; Farghadany, E. Corrosion behavior of thermo-mechanically processed biomedical Ti-29Nb-13Ta-4.6Zr. J. Alloys Compd. 2017, 725, 23–31. [Google Scholar] [CrossRef]
- Popa, M.; Vasilescu, E.; Drob, P.; Raducanu, D.; Moreno, J.M.C.; Ivanescu, S.; Vasilescu, C.; Drob, S.I. Microstructure, mechanical, and anticorrosive properties of a new Ti-20Nb-10Zr-5Ta alloy based on nontoxic and nonallergenic elements. Met. Mater. Int. 2012, 18, 639–645. [Google Scholar] [CrossRef]
- Hussein, M.A.; Kumar, A.M.; Yilbas, B.S.; Al-Aqeeli, N. Laser nitriding of the newly developed Ti-20Nb-13Zr at.% biomaterial alloy to enhance its mechanical and corrosion properties in simulated body fluid. J. Mater. Eng. Perform. 2017, 26, 5553–5562. [Google Scholar] [CrossRef]
- Huang, L.; Chen, T.H.; Chiang, C.C.; Lin, S.Y. Electrochemical corrosion and mechanical properties of two biomedical titanium alloys. Int. J. Electrochem. Sci. 2018, 13, 2779–2790. [Google Scholar] [CrossRef]
- Macdonald, D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434. [Google Scholar] [CrossRef]
- Moraes, P.E.; Contieri, R.J.; Lopes, E.S.; Robin, A.; Caram, R. Effects of Sn addition on the microstructure, mechanical properties and corrosion behavior of Ti–Nb–Sn alloys. Mater. Charact. 2014, 96, 273–281. [Google Scholar] [CrossRef]
- Hallab, N.J.; Anderson, S.; Stafford, T.; Glant, T.; Jacobs, J.J. Lymphocyte responses in patients with total hip arthroplasty. J. Orthop. Res. 2005, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Peña, J.; Gil, F.J.; Manero, J.M. Low modulus Ti–Nb–Hf alloy for biomedical applications. Mater. Sci. Eng. C 2014, 42, 691–695. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Gilbert, J.L.; Urbani, R.M. Corrosion of metal orthopaedic implants. J. Bone Jt. Surg. 1989, 80, 268–282. [Google Scholar] [CrossRef]
- Gittens, A.; Olivares-Navarrete, R.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Corrosion in titanium dental implants/prostheses—A review. Trends Biomater. Artif. Organs 2011, 25, 34–46. [Google Scholar]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Patents Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Sargeant, A.; Goswami, T. Hip implants: Paper V. Physiological effects. Mater. Des. 2006, 27, 287–307. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).