Chlorella vulgaris Powder as an Eco-Friendly and Low-Cost Corrosion Inhibitor Against Carbon Steel Corrosion by HCl
Abstract
1. Introduction
2. Experimental
2.1. Preparation of C. vulgaris Powder
2.2. Metal Specimen Preparation
2.3. SEM Observation and Weight Loss
2.4. Electrochemical Corrosion Testing
2.5. Quantum Chemical Calculations
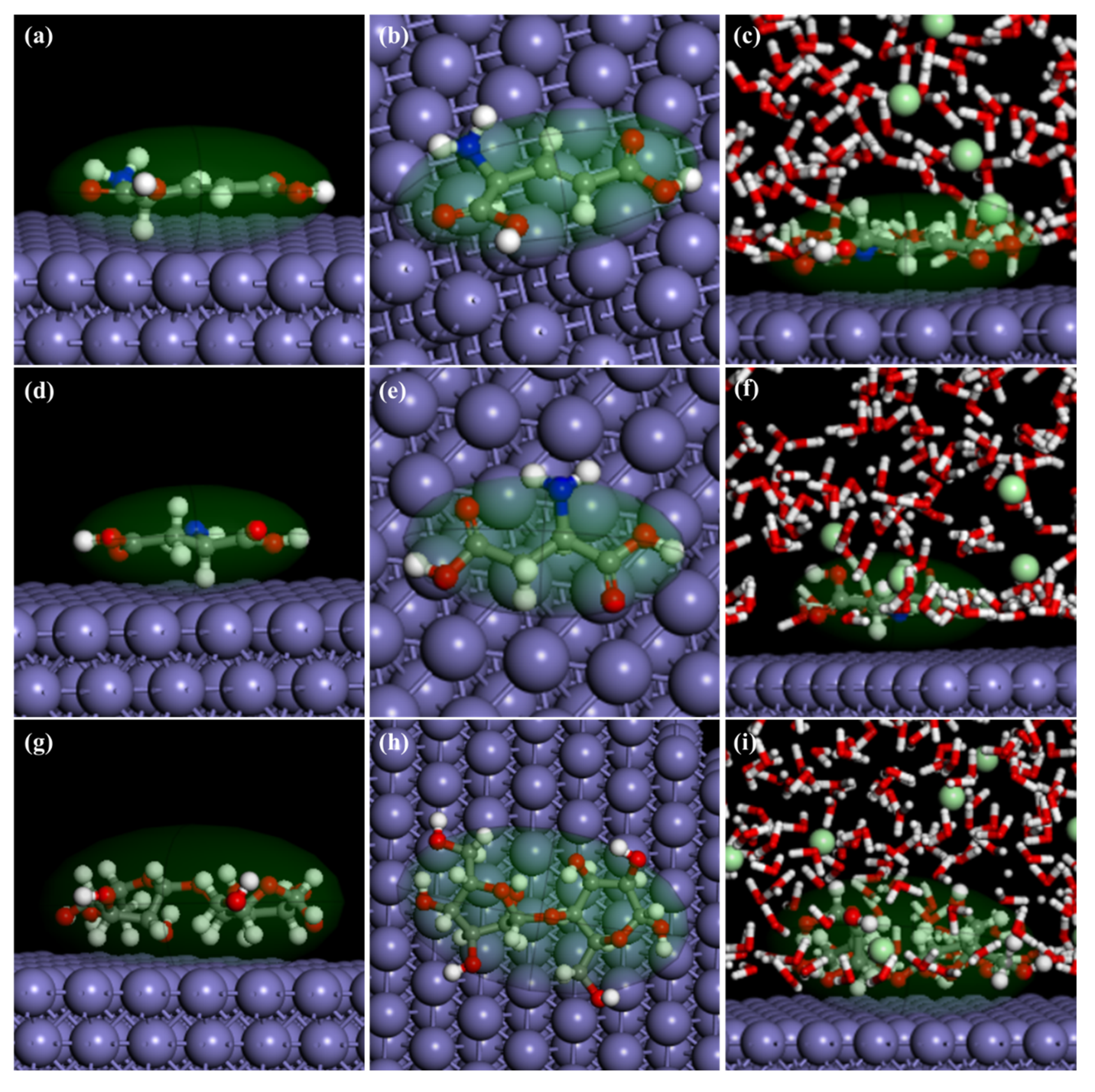
2.6. Molecular Dynamics Simulations
3. Results and Discussion
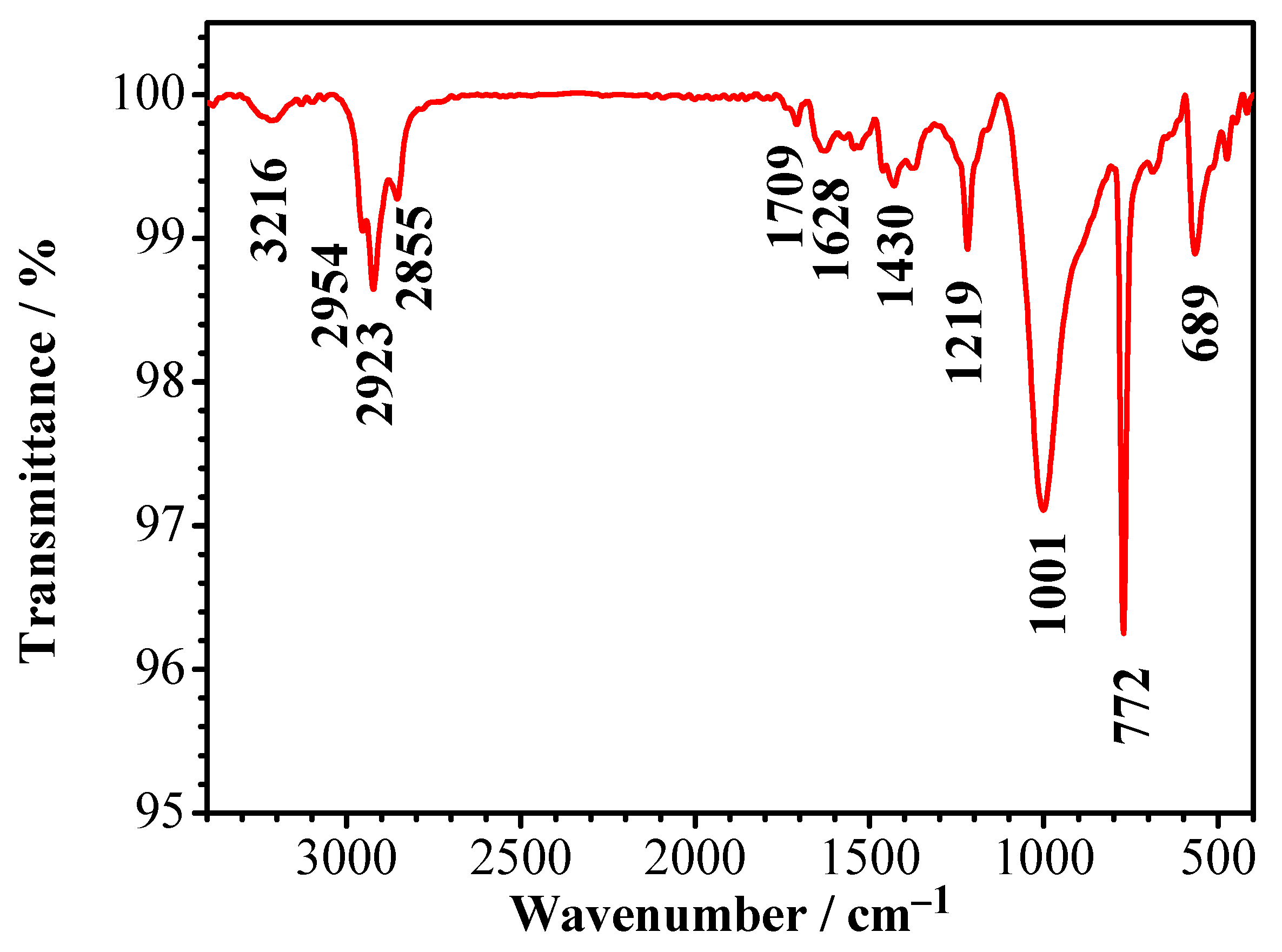
3.1. FT-IR Analysis of C. vulgaris Powder
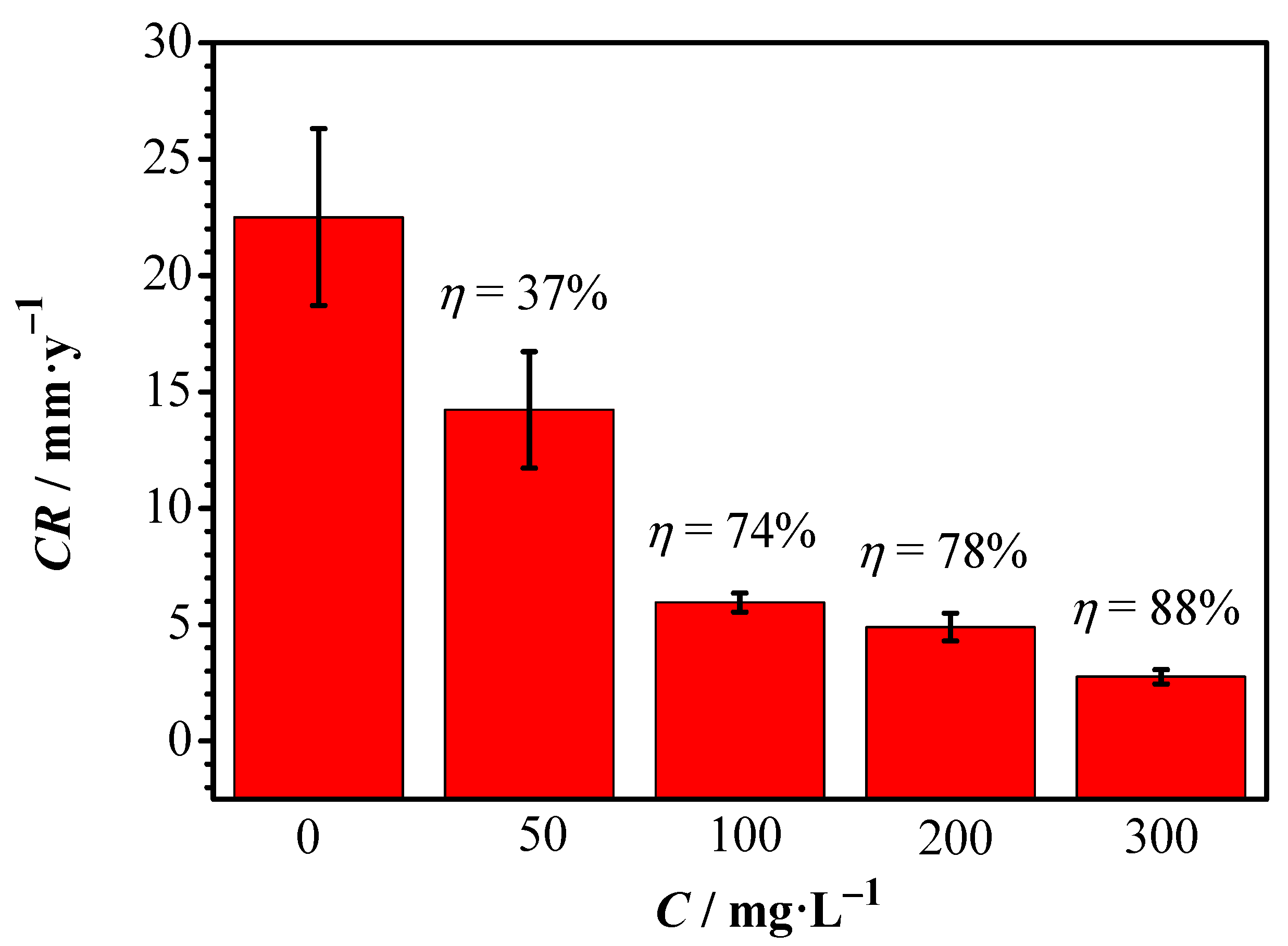
3.2. Weight Loss
3.3. EIS
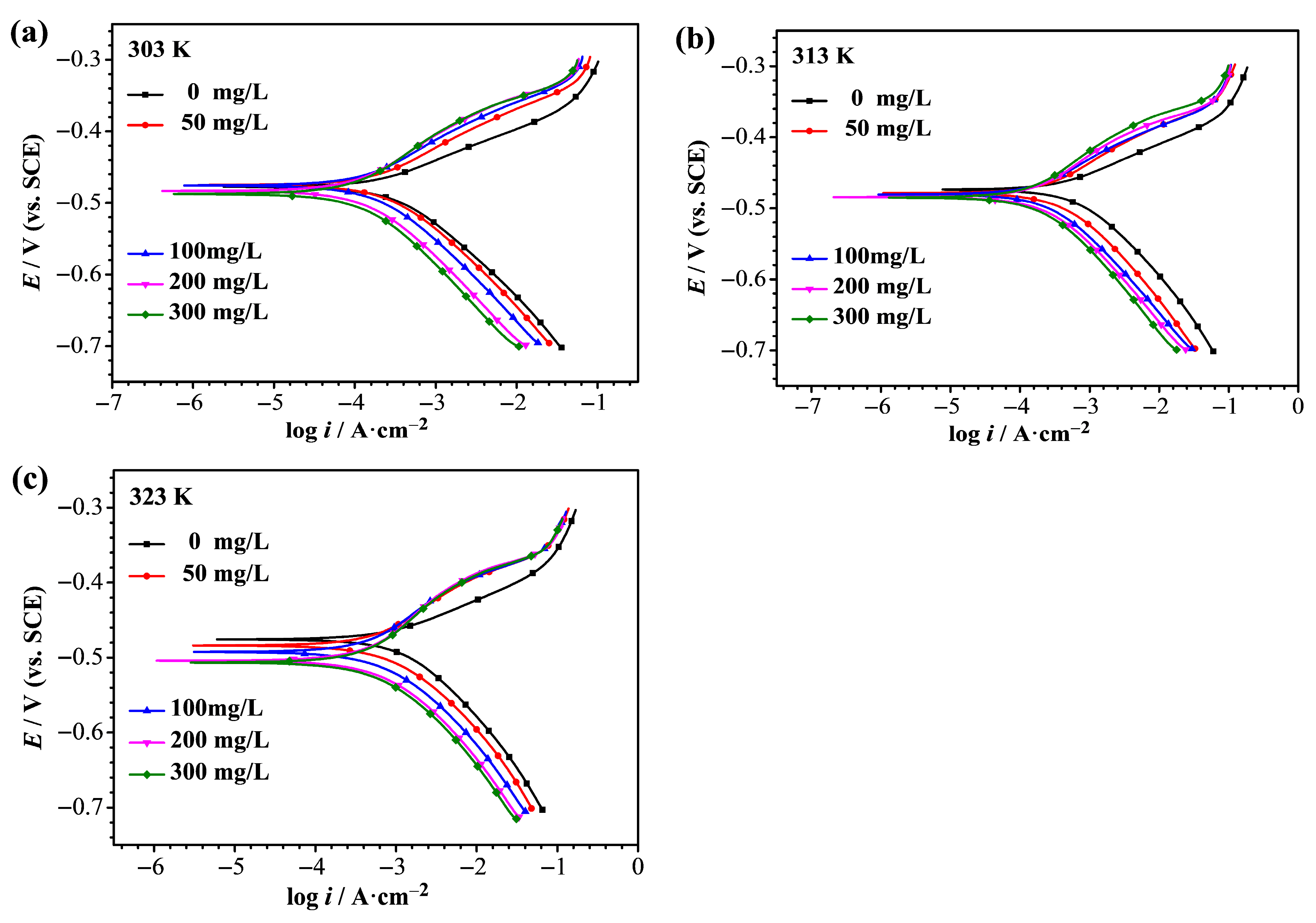
3.4. Potentiodynamic Polarization Analysis
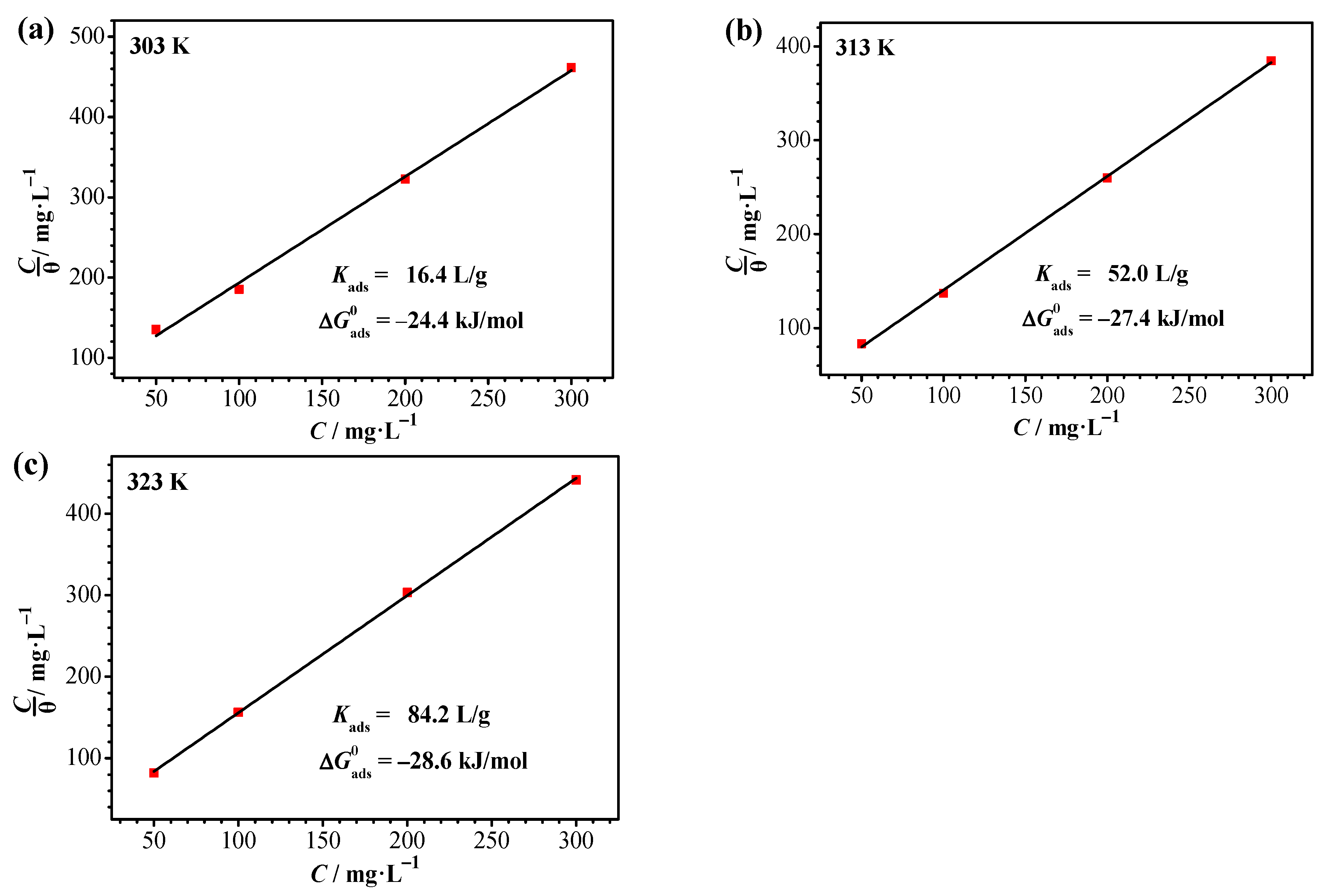
3.5. Inhibitor Adsorption Isotherm
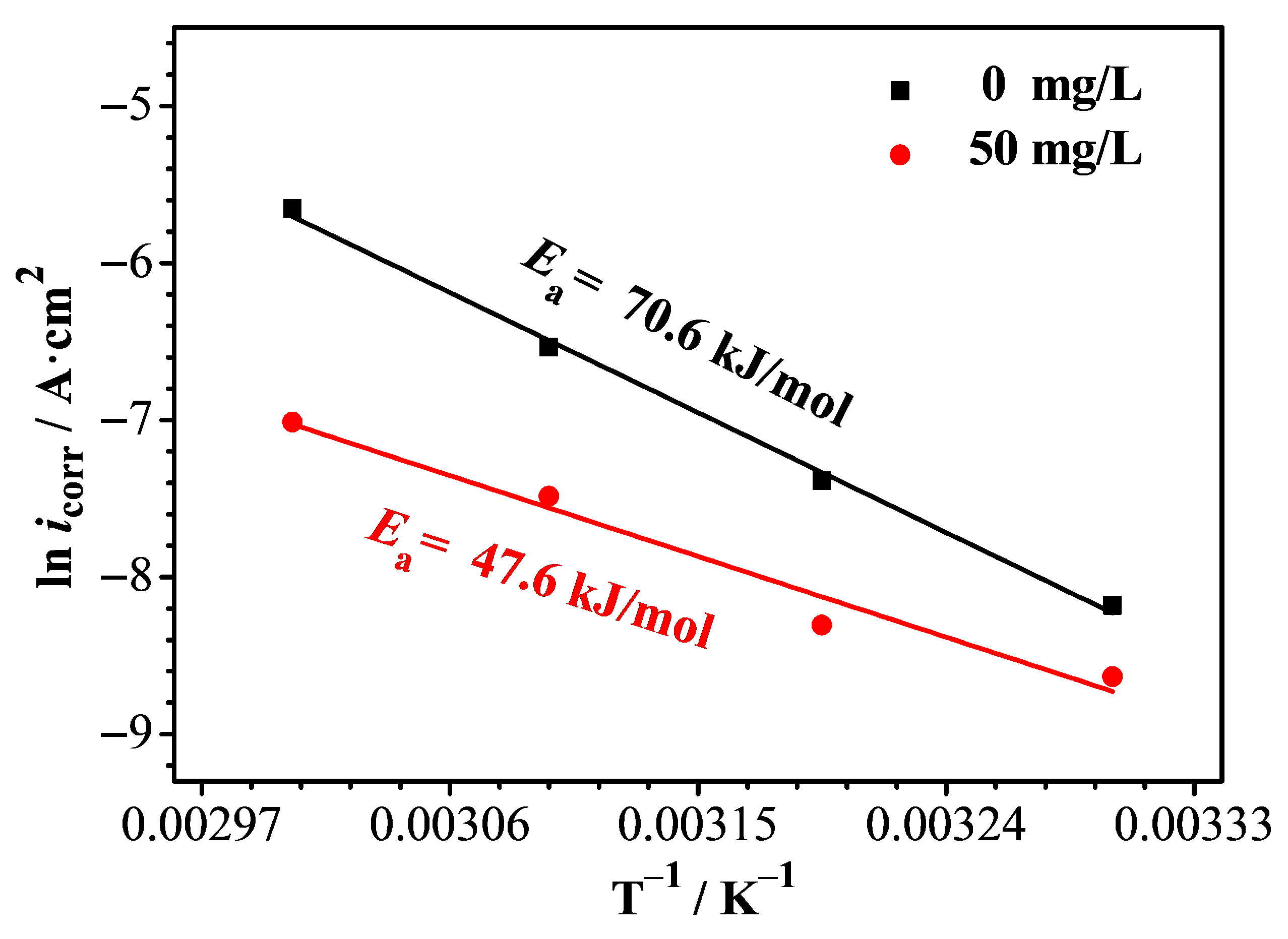
3.6. Activation Energy
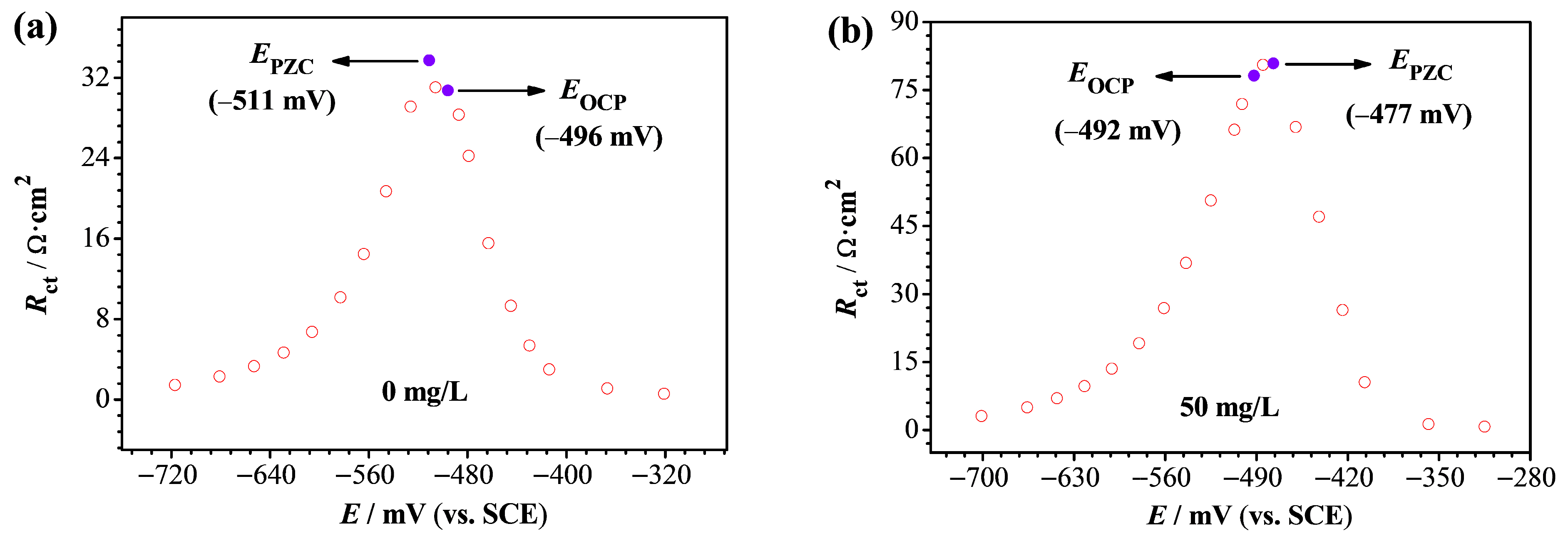
3.7. Potential of Zero Charge
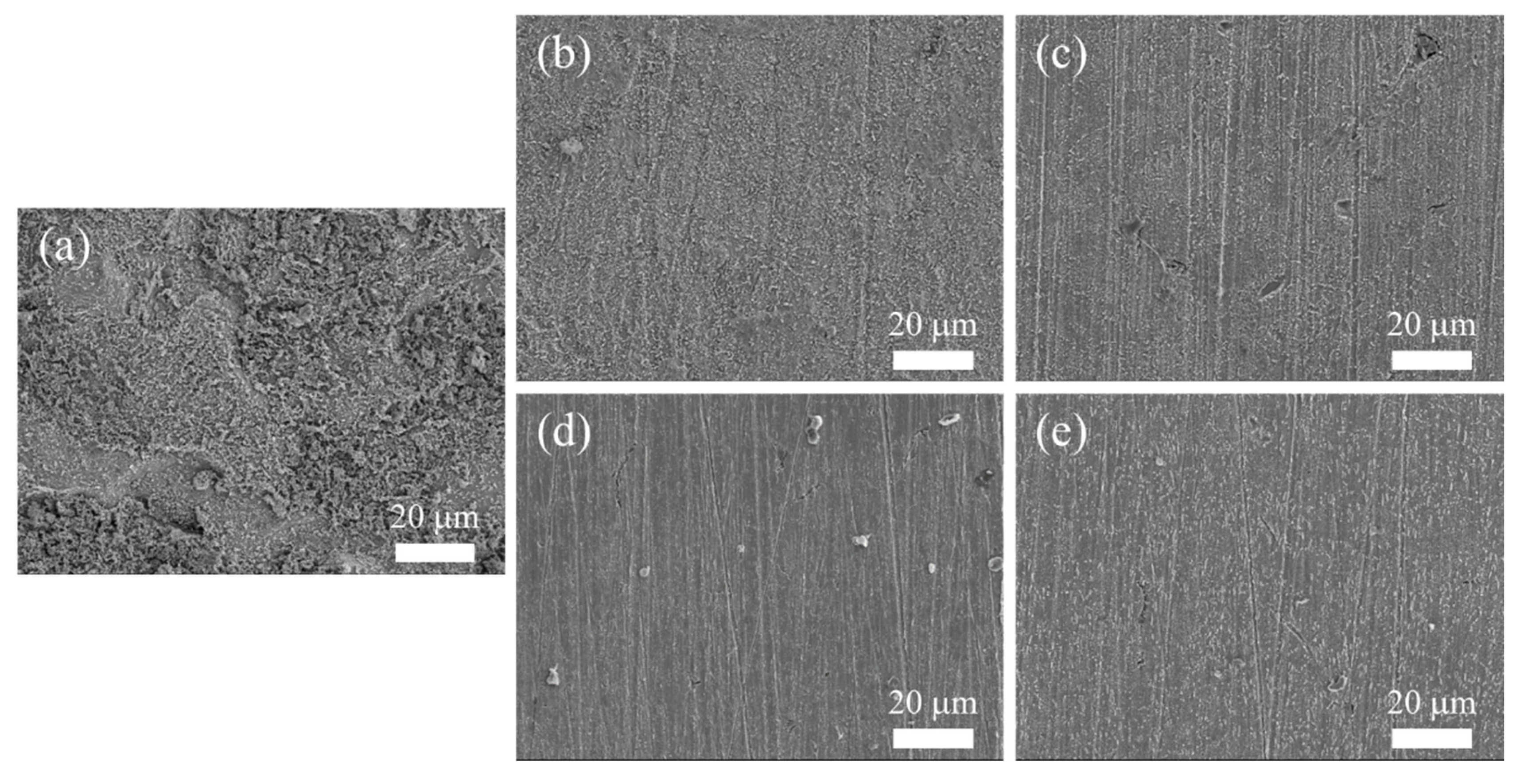
3.8. SEM Observation
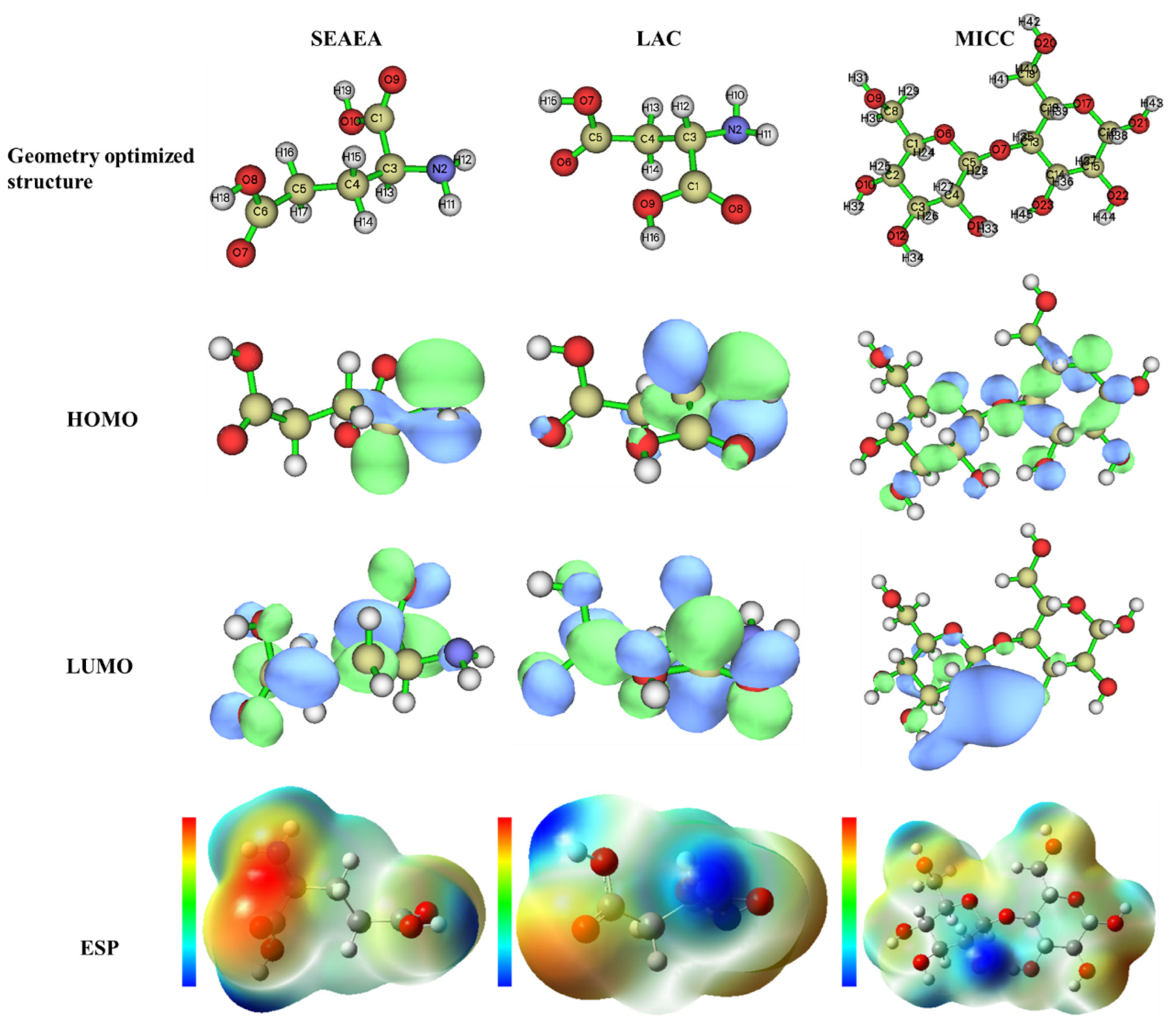
3.9. DFT
3.10. MD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, B.S.; Xu, N.; Zhang, Q.H.; Xuan, C.J.; Liu, H.F.; Zhang, G.A. Effect of benzyl substitution at different sites on the inhibition performance of pyrimidine derivatives for mild steel in highly acidic solution. J. Taiwan Inst. Chem. Eng. 2019, 95, 541–554. [Google Scholar] [CrossRef]
- Sayed, A.R.; El-lateef, H.M.A.; Mohamad, A.D.M. Polyhydrazide incorporated with thiadiazole moiety as novel and effective corrosion inhibitor for C-steel in pickling solutions of HCl and H2SO4. Macromol. Res. 2018, 26, 882–891. [Google Scholar] [CrossRef]
- Dohare, P.; Ansari, K.R.; Quraishi, M.A.; Obot, I.B. Pyranpyrazole derivatives as novel corrosion inhibitors for mild steel useful for industrial pickling process: Experimental and quantum chemical study. J. Ind. Eng. Chem. 2017, 52, 197–210. [Google Scholar] [CrossRef]
- Ituen, E.B.; Solomon, M.M.; Umoren, S.A.; Akaranta, O. Corrosion inhibition by amitriptyline and amitriptyline based formulations for steels in simulated pickling and acidizing media. J. Pet. Sci. Eng. 2019, 174, 984–996. [Google Scholar] [CrossRef]
- Dhawan, H.; Sharma, D.K. Advances in the chemical leaching (inorgano-leaching), bio-leaching and desulphurisation of coals. Int. J. Coal Sci. Technol. 2019, 6, 169–183. [Google Scholar] [CrossRef]
- Fedoročková, A.; Hreus, M.; Raschman, P.; Sučik, G. Dissolution of magnesium from calcined serpentinite in hydrochloric acid. Miner. Eng. 2012, 32, 1–4. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: A review. J. Environ. Chem. Eng. 2017, 5, 246–273. [Google Scholar] [CrossRef]
- Mihajlović, M.B.P.; Radovanović, M.B.; Tasić, Ž.Z.; Antonijević, M.M. Imidazole based compounds as copper corrosion inhibitors in seawater. J. Mol. Liq. 2017, 225, 127–136. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Sorour, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 2019, 150, 183–193. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Qi, S.; Dong, D.; Cang, H.; Lu, G. The inhibition performance of long-chain alkyl-substituted benzimidazole derivatives for corrosion of mild steel in HCl. Corros. Sci. 2016, 102, 517–522. [Google Scholar] [CrossRef]
- Bedair, M.A. The effect of structure parameters on the corrosion inhibition effect of some heterocyclic nitrogen organic compounds. J. Mol. Liq. 2016, 219, 128–141. [Google Scholar] [CrossRef]
- Al-Fakih, A.M.; Abdallah, H.H.; Aziz, M. Experimental and theoretical studies of the inhibition performance of two furan derivatives on mild steel corrosion in acidic medium. Mater. Corros. 2019, 70, 135–148. [Google Scholar] [CrossRef]
- Haque, J.; Ansari, K.R.; Srivastava, V.; Quraishi, M.A.; Obot, I.B. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: A combined experimental and theoretical approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on corrosion inhibitors: A short view. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Wang, J.; Hou, B.; Xiang, J.; Chen, X.; Gu, T.; Liu, H. The performance and mechanism of bifunctional biocide sodium pyrithione against sulfate reducing bacteria in X80 carbon steel corrosion. Corros. Sci. 2019, 150, 296–308. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 2012, 55, 407–415. [Google Scholar] [CrossRef]
- Umoren, S.A. Biomaterials for corrosion protection: Evaluation of mustard seed extract as eco-friendly corrosion inhibitor for X60 steel in acid media. J. Adhes. Sci. Technol. 2016, 30, 1858–1879. [Google Scholar] [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2018, 254, 377–382. [Google Scholar] [CrossRef]
- Jokar, M.; Farahani, T.S.; Ramezanzadeh, B. Electrochemical and surface characterizations of morus alba pendula leaves extract (MAPLE) as a green corrosion inhibitor for steel in 1 M HCl. J. Taiwan Inst. Chem. Eng. 2016, 63, 436–452. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Umoren, S.A.; Gasem, Z.M. Watermelon waste products as green corrosion inhibitors for mild steel in HCl solution. J. Environ. Chem. Eng. 2015, 3, 286–296. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.G.; Hosseini, M.; Safari, R.; Moshrefi, R.; Shiri, H.M. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, B.; Bao, H.; Xie, Y.; Mou, Y.; Li, P.; Chen, D.; Shi, Y.; Li, X.; Yang, W. Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 2019, 128, 49–55. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Huang, L.; Sun, K.; Ban, J.; Bi, J. Public perception of blue-algae bloom risk in Hongze Lake of China. Environ. Manag. 2010, 45, 1065–1075. [Google Scholar] [CrossRef]
- Santos, R.R.D.; Moreira, D.M.; Kunigami, C.N.; Aranda, D.A.G.; Teixeira, C.M.L.L. Comparison between several methods of total lipid extraction from Chlorella vulgaris biomass. Ultrason. Sonochem. 2015, 22, 95–99. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Whitney, C.G.; MacPherson, M.J.; Bhatti, S.; Banskota, A.H.; Stefanova, R.; McGinn, P.J. Biochemical characterization of microalgal biomass from freshwater species isolated in Alberta, Canada for animal feed applications. Algal Res. 2015, 11, 435–447. [Google Scholar] [CrossRef]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Nutrients and pharmaceuticals removal from wastewater by culture and harvesting of Chlorella sorokiniana. Bioresour. Technol. 2015, 185, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, D.; Dao, A.Q.; Zhang, G.; Lv, Y.; Liu, H. Study of corrosion behavior and mechanism of carbon steel in the presence of Chlorella vulgaris. Corros. Sci. 2015, 101, 84–93. [Google Scholar] [CrossRef]
- Liu, H.; Sharma, M.; Wang, J.; Cheng, Y.F.; Liu, H. Microbiologically influenced corrosion of 316L stainless steel in the presence of Chlorella vulgaris. Int. Biodeterior. Biodegrad. 2018, 129, 209–216. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Zhang, X.; Asif, M.; Zhang, T.; Hou, B.; Li, Y.; Xia, W.; Wang, H.; Liu, H. Corrosion Behavior of Aspergillus niger on 7075 Aluminum Alloy and the Inhibition Effect of Zinc Pyrithione Biocide. J. Electrochem. Soc. 2019, 166, G39. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, F.; Liu, H.; Zhang, T.; Li, Y.; Li, C.; Xia, W.; Wang, H.; Liu, H. Study of the corrosion behavior of Aspergillus niger on 7075-T6 aluminum alloy in a high salinity environment. Bioelectrochemistry 2019, 129, 10. [Google Scholar] [CrossRef]
- Zhang, G.A.; Hou, X.M.; Hou, B.S.; Liu, H.F. Benzimidazole Derivatives as Novel Inhibitors for the Corrosion of Mild Steel in Acidic Solution: Experimental and Theoretical Studies. J. Mol. Liq. 2019, 278, 413–427. [Google Scholar] [CrossRef]
- Yüce, A.O.; Solmaz, R.; Kardaş, G. Investigation of inhibition effect of rhodanine-N-acetic acid on mild steel corrosion in HCl solution. Mater. Chem. Phys. 2012, 131, 615–620. [Google Scholar] [CrossRef]
- Li, X.L.; Chen, L.; Xie, B.; Lai, C.; He, J.Y.; Feng, J.S.; Yang, Y.G.; Ji, R.W.; Liu, M.N. Two semi flexible nonplanar double Schiff bases as corrosion inhibitors for mild steel in HCl solution: Experimental and theoretical investigations. J. Environ. Chem. Eng. 2023, 11, 110077. [Google Scholar] [CrossRef]
- Etzkorn, T.; Klotz, B.; Sørensen, S.; Patroescu, I.V.; Barnes, I.; Becker, K.H.; Platt, U. Gas-phase absorption cross sections of 24 monocyclic aromatic hydrocarbons in the UV and IR spectral ranges. Atmos. Environ. 1999, 33, 525–540. [Google Scholar] [CrossRef]
- Ashtarinezhad, A.; Shirazi, F.H.; Vatanpour, H.; Mohamazadehasl, B.; Panahyab, A.; Nakhjavani, M. FTIR-Microspectroscopy Detection of Metronidazole Teratogenic Effects on Mice Fetus. Iran. J. Pharm. Res. 2014, 13, 101–111. [Google Scholar]
- Alhazmi, A.H. FT-IR Spectroscopy for the Identification of Binding Sites and Measurements of the Binding Interactions of Important Metal Ions with Bovine Serum Albumin. Sci. Pharm. 2019, 87, 5. [Google Scholar] [CrossRef]
- Morais, C.L.M.; Shore, R.F.; Pereira, M.G.; Martin, F.L. Assessing Binary Mixture Effects from Genotoxic and Endocrine Disrupting Environmental Contaminants Using Infrared Spectroscopy. ACS Omega 2018, 3, 13399–13412. [Google Scholar] [CrossRef]
- Aja, M.; Jaya, M.; Nair, K.V.; Joe, I.H. FT-IR spectroscopy as a sentinel technology in earthworm toxicology. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014, 120, 534–541. [Google Scholar] [CrossRef]
- Preston, L.J.; Izawa, M.R.M.; Banerjee, N.R. Infrared spectroscopic characterization of organic matter associated with microbial bioalteration textures in basaltic glass. Astrobiology 2011, 11, 585–599. [Google Scholar] [CrossRef]
- Voll, D.; Beran, A. Dehydration process and structural development of cordierite ceramic precursors derived from FTIR spectroscopic investigations. Phys. Chem. Miner. 2002, 29, 545–551. [Google Scholar] [CrossRef]
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 2003, 45, 33–58. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Chauhan, D.S.; Lgaz, H.; Chung, I.-M. Polar group substituted imidazolium zwitterions as eco-friendly corrosion inhibitors for mild steel in acid solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Basik, M.; Mobin, M. Chondroitin sulfate as potent green corrosion inhibitor for mild steel in 1 M HCl. J. Mol. Struct. 2020, 1214, 128231. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Messali, M.; Larouj, M.; Lgaz, H.; Rezki, N.; Al-Blewi, F.F.; Aouad, M.R.; Chaouiki, A.; Salghi, R.; Chung, I.-M. A new schiff base derivative as an effective corrosion inhibitor for mild steel in acidic media: Experimental and computer simulations studies. J. Mol. Struct. 2018, 1168, 39–48. [Google Scholar] [CrossRef]
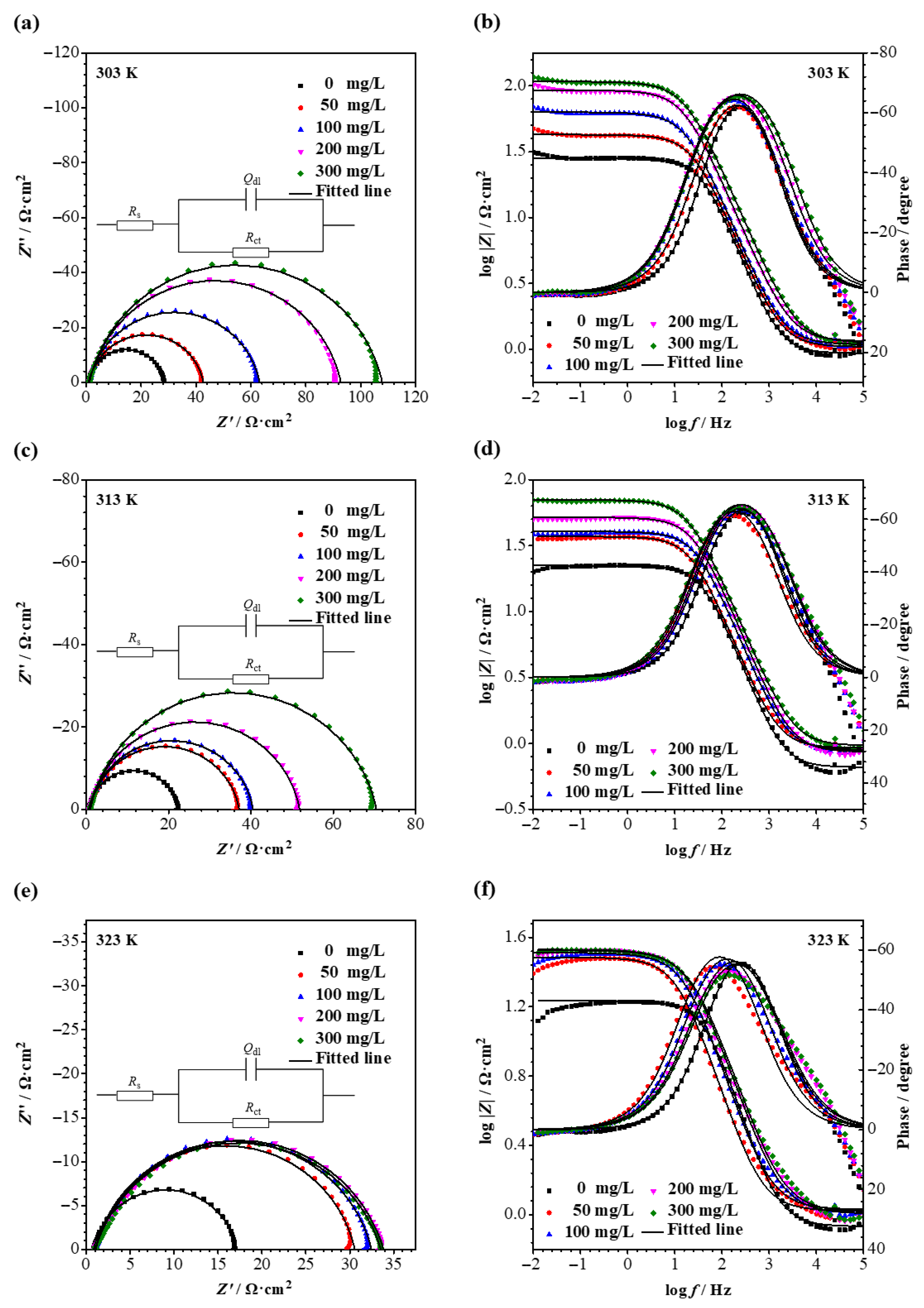
| T/K | C/mg L−1 | Rs/Ω cm2 | Qdl/Ω−1 sn cm−2 | n | Rct/Ω cm2 |
|---|---|---|---|---|---|
| 303 | 0 | 0.93 | 2.41 × 10−4 | 0.91 | 27.3 |
| 50 | 1.04 | 2.71 × 10−4 | 0.88 | 41.6 | |
| 100 | 1.09 | 2.56 × 10−4 | 0.88 | 62.1 | |
| 200 | 1.15 | 1.94 × 10−4 | 0.87 | 91.6 | |
| 300 | 1.12 | 1.64 × 10−4 | 0.86 | 108 | |
| 313 | 0 | 0.66 | 2.91 × 10−4 | 0.92 | 21.3 |
| 50 | 0.92 | 3.22 × 10−4 | 0.89 | 36.2 | |
| 100 | 0.89 | 2.37 × 10−4 | 0.89 | 39.6 | |
| 200 | 0.86 | 2.11 × 10−4 | 0.89 | 51.0 | |
| 300 | 0.96 | 2.04 × 10−4 | 0.87 | 69.5 | |
| 323 | 0 | 0.85 | 3.91 × 10−4 | 0.89 | 16.3 |
| 50 | 1.05 | 7.94 × 10−4 | 0.86 | 29.5 | |
| 100 | 1.07 | 5.99 × 10−4 | 0.85 | 31.4 | |
| 200 | 1.01 | 5.21 × 10−4 | 0.83 | 32.8 | |
| 300 | 1.03 | 5.04 × 10−4 | 0.82 | 32.5 |
| T/K | C/mg L−1 | βa/V dec−1 | βc/V dec−1 | Ecorr/V (vs. SCE) | icorr/A cm−2 | ηi/% |
|---|---|---|---|---|---|---|
| 303 | 0 | 0.057 | −0.096 | −0.478 | 2.81 × 10−4 | − |
| 50 | 0.061 | −0.090 | −0.475 | 1.78 × 10−4 | 37 | |
| 100 | 0.064 | −0.091 | −0.476 | 1.28 × 10−4 | 54 | |
| 200 | 0.073 | −0.096 | −0.483 | 1.06 × 10−4 | 62 | |
| 300 | 0.077 | −0.100 | −0.487 | 9.88 × 10−5 | 65 | |
| 313 | 0 | 0.057 | −0.100 | −0.474 | 6.20 × 10−4 | − |
| 50 | 0.058 | −0.088 | −0.479 | 2.47 × 10−4 | 60 | |
| 100 | 0.055 | −0.086 | −0.480 | 1.65 × 10−4 | 73 | |
| 200 | 0.061 | −0.086 | −0.484 | 1.42 × 10−4 | 77 | |
| 300 | 0.067 | −0.092 | −0.485 | 1.39 × 10−4 | 78 | |
| 323 | 0 | 0.068 | −0.126 | −0.476 | 1.45 × 10−3 | − |
| 50 | 0.069 | −0.091 | −0.484 | 5.61 × 10−4 | 61 | |
| 100 | 0.076 | −0.091 | −0.493 | 5.28 × 10−4 | 64 | |
| 200 | 0.098 | −0.099 | −0.504 | 4.93 × 10−4 | 66 | |
| 300 | 0.095 | −0.100 | −0.507 | 4.63 × 10−4 | 68 |
| Molecule | ||||||||
|---|---|---|---|---|---|---|---|---|
| SEAEA | −0.3811 | −6.9683 | 6.5872 | 6.9683 | 0.3811 | 3.6747 | 3.2936 | 0.1738 |
| LAC | −0.3164 | −6.9351 | 6.6187 | 6.9351 | 0.3164 | 3.62575 | 3.3093 | 0.1804 |
| MICC | 0.8135 | −7.0580 | 7.8715 | 7.058 | −0.8135 | 3.12225 | 3.9357 | 0.2156 |
| SEAEA | LAC | MICC | MICC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 0.020 | 0.252 | C1 | 0.021 | 0.268 | C1 | 0.008 | 0.003 | C13 | 0.061 | 0.003 |
| N2 | 0.370 | 0.035 | N2 | 0.385 | 0.031 | C2 | 0.001 | 0.005 | C14 | 0.064 | 0.010 |
| C3 | 0.051 | 0.034 | C3 | 0.049 | 0.040 | C3 | 0.005 | 0.015 | C15 | 0.017 | 0.005 |
| C4 | 0.035 | 0.022 | C4 | 0.031 | 0.018 | C4 | 0.008 | 0.044 | C16 | 0.011 | 0.002 |
| C5 | 0.013 | 0.008 | C5 | 0.011 | 0.004 | C5 | 0.024 | 0.005 | O17 | 0.026 | 0.003 |
| C6 | 0.009 | 0.009 | O6 | 0.030 | 0.017 | O6 | 0.016 | 0.012 | C18 | 0.014 | 0.001 |
| O7 | 0.017 | 0.015 | O7 | 0.017 | 0.012 | O7 | 0.090 | 0.006 | C19 | 0.019 | 0.001 |
| O8 | 0.013 | 0.005 | O8 | 0.038 | 0.212 | C8 | 0.003 | 0.003 | O20 | 0.035 | 0.002 |
| O9 | 0.043 | 0.207 | O9 | 0.029 | 0.142 | O9 | 0.003 | 0.002 | O21 | 0.017 | 0.003 |
| O10 | 0.037 | 0.155 | O10 | 0.005 | 0.006 | O22 | 0.050 | 0.008 | |||
| O11 | 0.031 | 0.126 | O23 | 0.113 | 0.035 | ||||||
| O12 | 0.012 | 0.026 |
| Systems | (kJ Mol−1) | (kJ Mol−1) |
|---|---|---|
| Fe/SEAEA | 87.983781 | −87.983781 |
| Fe/LAC | 175.2569 | −175.2569 |
| Fe/MICC | 134.3232 | −134.3232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, Z.; Li, X.; Lai, J.; Cao, S.; Liu, G.; Wang, X.; Lyu, Y.; Wang, J.; Yang, J. Chlorella vulgaris Powder as an Eco-Friendly and Low-Cost Corrosion Inhibitor Against Carbon Steel Corrosion by HCl. Metals 2026, 16, 109. https://doi.org/10.3390/met16010109
Li Z, Li X, Lai J, Cao S, Liu G, Wang X, Lyu Y, Wang J, Yang J. Chlorella vulgaris Powder as an Eco-Friendly and Low-Cost Corrosion Inhibitor Against Carbon Steel Corrosion by HCl. Metals. 2026; 16(1):109. https://doi.org/10.3390/met16010109
Chicago/Turabian StyleLi, Zhong, Xiaolong Li, Jianfeng Lai, Shaohua Cao, Guoqiang Liu, Xiaowan Wang, Yan Lyu, Junlei Wang, and Jike Yang. 2026. "Chlorella vulgaris Powder as an Eco-Friendly and Low-Cost Corrosion Inhibitor Against Carbon Steel Corrosion by HCl" Metals 16, no. 1: 109. https://doi.org/10.3390/met16010109
APA StyleLi, Z., Li, X., Lai, J., Cao, S., Liu, G., Wang, X., Lyu, Y., Wang, J., & Yang, J. (2026). Chlorella vulgaris Powder as an Eco-Friendly and Low-Cost Corrosion Inhibitor Against Carbon Steel Corrosion by HCl. Metals, 16(1), 109. https://doi.org/10.3390/met16010109





