Abstract
Vanadium nitride (VN) ceramic layers were deposited on 304L stainless steel specimens by direct current (DC) magnetron sputtering in an Ar/N2 gas mixture at substrate temperatures of 250 °C, 300 °C, and 350 °C. The obtained films were evaluated by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and atomic force microscopy (AFM). The results showed the existence of VN and V2N phases in the as-deposited coatings. It was found that the surface roughness parameter (Ra = 10 nm) decreased with increasing substrate temperatures up to 350 °C. The highest hardness (10.6 GPa) was achieved in the layer produced at 300 °C. The low values of plastic and elastic deformation, as well as a low friction coefficient (0.38), led to an enhancement in the coatings’ tribological properties. The film’s thickness increased with increasing temperature due to the presence of nucleation centers in the films. The highest thickness (557 nm) was achieved in the layer deposited at 350 °C. The electrochemical tests exhibited reliable protection against corrosion in strongly aggressive electrolytes. It has been proven that the temperature significantly affects the ceramic coatings’ structural, morphological, tribological, and corrosion properties.
1. Introduction
Nowadays, metallic materials are commonly used in industrial applications due to their desirable properties such as high strength, fatigue resistance, toughness, and inertness [1,2]. Many machine components, operating in abrasive environments, undergo degradation after a few years, which is attributed to severe environmental conditions where corrosion, high-temperature oxidation, and wear are concerned. Notably, 304L austenitic stainless steel is the most commonly used material for industrial applications due to its high heat stability and excellent corrosion resistance. Despite these good functional properties, this material exhibits poor wear resistance and low strength. These drawbacks can be overcome by applying a suitable technique for surface treatment. The materials’ functional properties, as well as the performance, reliability, safety, and lifetime of the cutting tools, can be improved by coating deposition [3].
Recently, VN coatings have gained attention due to their excellent mechanical properties, high melting point, good chemical resistance, good electrical conductivity, low friction coefficient, and good wear resistance. VN coatings are widely utilized for applications in various fields such as microelectronics, decorative, and protective coatings [4,5], supercapacitors, electrical systems for hybrid vehicles, and load leveling batteries during start up, acceleration, and braking, as well as memory storage systems and digital telecommunications systems [6,7,8].
VN films can be deposited by various techniques such as phase vapor deposition (PVD) and chemical vapor deposition (CVD) [9,10]. Huang et al. [11] aimed to find the optimum deposition conditions for VN thin coatings on Si substrates using unbalanced magnetron sputtering and investigated the effect of the most sensitive processing parameter on the structure and properties of as-deposited films. The results showed an excellent combination of properties, including high hardness, low electrical resistivity, and low residual stress in the VN layers. Aissani et al. [12] evaluated the influence of the thickness and nitrogen percentage on the structure, mechanical, and tribological properties of VN coatings deposited on Si and XC100 steel substrates using the reactive magnetron sputtering technique. Increasing the thickness and nitrogen concentration, the mechanical and tribological properties of VN coatings were improved. The electrochemical behavior of the reactive magnetron-sputtered VN coatings was investigated at different substrate bias voltages, revealing a greater corrosion rate and hardness at a higher bias voltage [13,14]. The unbalanced magnetron sputtering method was applied for the production of hard VN coatings to investigate the correlation between the preferred orientation and fracture toughness [15].
Ghimbeu et al. [16,17] produced VN films by the pulsed laser deposition (PLD) technique at room temperature and 500 °C to investigate the effect of temperature on the thickness of the obtained VN coatings. Cupric et al. [18] evaluated the influence of nitrogen pressure and substrate bias voltage on the structure and mechanical properties of vacuum-arc-deposited VN films. The result showed that higher nitrogen pressure and a bias voltage corresponded to high hardness and good adhesion to the substrate, as well as improved tribological behavior of the VN coatings.
Another method for the production of VN coatings involves using inductively coupled plasma (ICP)-assisted sputtering at various powers. Increasing ICP power, the coating microstructure became denser, and the hardness was enhanced [19]. Gao et al. [20] used the chemical vapor deposition in situ reactive deposition technique for VN film preparation on T12 steel substrates.
It has been reported that there is a correlation between the friction coefficient and the thickness of the coatings. Thicker VN films have the lowest friction coefficient of 0.4 [21,22,23,24]. The researchers in [25,26] investigated the influence of nitrogen partial pressure and negative bias voltage on the mechanical properties of VN coatings obtained on Si substrates by magnetron sputtering. The mechanical and tribological performance of VN films deposited by the arc-evaporation method was studied [27]. VN films were grown via reactive magnetron sputtering at various deposition angles, and it was found that a lower growth rate was obtained by raising the tilt angle [28].
The high-power impulse magnetron sputtering (HIPIMS) and direct current magnetron sputtering techniques were applied for VN film deposition on SiO2 substrates [29]. Fangfang et al. [30] enhanced the wear resistance of a magnetron-sputtered VN coating by Si addition.
It is obvious that VN films have been produced by different techniques on various substrates. One of the most common methods for VN layer deposition is direct current magnetron sputtering due to the use of non-toxic working gases and the high degree of smoothness, uniformity, and density of the deposited coatings. On the other hand, VN film deposition on 304L stainless steel substrates at various technological parameters has not been investigated in detail. For this purpose, we deposited VN films on 304L stainless steel substrates at temperatures of 250 °C, 300 °C, and 350 °C by the DC magnetron sputtering technique. Our study aims to investigate the effect of temperature on the structure, morphology, mechanical, and corrosion properties of VN coatings deposited on 304L stainless steel substrates.
2. Materials and Methods
2.1. VN Film Deposition and Applied Technology
VN coatings were deposited on 304L stainless steel substrates with the following composition: 0.03% C, 17.50–19.50% Cr, 2% Mn, 1% Si, 0.045% P, 0.015% S, 8–10.5% Ni, 0.1% N and Fe balance [31].
The experiments were carried out at temperatures of 250 °C, 300 °C, and 350 °C by direct current magnetron sputtering (DCMS). The substrates were mechanically polished and then loaded into the deposition chamber. The diameter of the sputtered target was 100 mm and the purity of V was 99.8%. Prior to the treatment, the samples were preliminarily polished to obliterate surface contamination after being loaded into the deposition chamber. Before the deposition process, the substrate surface was etched by Ar+ plasma for 10 min at a pulsed substrate bias of −1050 V to eliminate oxide layers from their surface, as well as the decrease in surface roughness which would have a negative effect on the obtained coatings’ properties. The vacuum chamber was evacuated to a base pressure of 8 × 10−2 mbar before the deposition process. After evacuation, the Ar and N2 mixture was introduced into the chamber by means of valves. The inert Ar and reactive N2 gas flows were regulated by vacuum meter indicators to determine the values of the working pressure. The technological regime for the VN film deposition includes three main processes:
- Cathode cleaning—this is a process of high-energy ion bombardment where 304L stainless steel substrates were etched by Ar positive ions for 10 min at a negative bias voltage of 1050 V to remove surface contamination involving the oxide layers from the substrate surface. This process was performed under the following technological conditions:
- Working pressure PAr = 8 Pa;
- Discharge voltage U = 900 V;
- Discharge current I = 0.1 A;
- Temperature T = 270 °C;
- Cleaning time t = 10 min.
- Deposition of intermediate pure V layer—this process was used to improve adhesion between the substrate and the coating. This process was performed under the following conditions:
- Working pressure PAr = 9 × 10−2 Pa;
- Discharge voltage U = 460 V;
- Discharge current I = 1 A;
- Temperature T = 250 °C;
- Deposition time t = 3 min.
- Deposition process of VN coatings—the production process took place in an Ar/N2 atmosphere and with a substrate bias voltage of −50 V.
- Nitrogen pressure PN2 = 2.4 × 10−2 Pa;
- Pressure ratio between reactive and inert gas PN2/PAr = 2.3;
- Voltage U = 575 V;
- Substrate temperatures—VN coatings were produced at temperatures of 250 °C, 300 °C, and 350 °C.
- Constant current I = 1 A;
- Deposition time t = 30 min.
2.2. XRD Analysis
The X-ray diffraction (XRD) method was assessed to evaluate the crystalline structure of the samples. The experiments were performed by the Empyrean system—Malvern Panalytical (Malvern, Worcestershire, United Kingdom)—equipped with a parabolic X-ray mirror, a parallel plate collimator, and a scintillation detector with Cu Kα radiation. The experiments were carried out at 2θ from 30 to 85° with a step size of 0.05° and a scan step time of 1.5 s. Phase identification was carried out using the ICDD (International Centre for Date Diffraction) database to determine the phases of the films.
2.3. XPS Analysis
X-ray photoelectron spectra (XPS) studies were performed in an electron spectrometer—VG ESCALAB II (Birches Industrial Estate, Sussex, UK)—using AlKα radiation with an energy of 1486.6 eV. The binding energies (BEs) were determined with an accuracy of ±0.1 eV. The changes in the composition and the chemical surroundings in the depth (5–10 nm) of the films were determined on the basis of the areas and binding energies of the C1s, O1s, N1s, and V2p photoelectron peaks (after Shirley-type subtraction of the background) and Scofield’s photoionization cross-sections. The analyses were performed by means of Collect 8.5-D-A software (version 8.5-D-A of the software).
2.4. AFM Investigations
The surface morphology, as well as the roughness of the VN layers and the initial material, were estimated by means of atomic force microscopy (AFM)—Oxford Instruments MFP-3D (Santa Barbara, CA, USA). The AFM equipment was used to characterize the surface topography of the obtained coatings. The studies were realized in a non-contact mode (AC-mode) of operation, and the scanned area for all samples was 40 × 40 μm2. During the measurements, Si-Tap300Al-G (standard type, budget sensors (Budget sensors, Sofia, Bulgaria) with a frequency of 300 kHz and an elasticity coefficient of k = 20 N/m were used. The data and the surface roughness parameter (roughness area, Ra) were analyzed and calculated by a special software—IgorPro 6.37 (available website: https://www.wavemetrics.com/software/igor-pro-637-installer, accessed on 12 August 2025).
2.5. Thickness
An optical microscope—3D Optical profiler, Zeta-20, Zeta Instruments (Milpitas, CA, USA)—was used to determine the thickness of the produced coatings. All experiments were performed at room temperature. The optical images of the layers were obtained using a magnification 50× objective lens. The Zeta system scans the samples over a user-specified vertical (or Z) range. The vertical (Z) resolution is <1 nm.
2.6. Mechanical Properties
The hardness and the elastic modulus were determined by a Nanomechanical Tester—Bruker (Billerica, MA, USA). The Young’s modulus and the hardness were calculated by the Oliver–Pharr method [32]. The software program contained 4 lines with 12 indentations each (a total of 48 indentations) and a spacing of 80 µm. Each indentation was made with a force of 10 mN.
2.7. Tribological Behavior
The tribological properties were assessed via dry slide examination by the ball-on-flat method with a ball from a hardened steel of UMT-2M tribotester—Bruker–CETR (Campbell, CA, USA). The experiments were carried out at a loading force of 2 N, for 5 and 10 min. All tests were performed at room temperature (~25 °C) and an air humidity of 30–40%. The experiments were performed at a constant velocity of 10 mm/s and a sliding distance of 1 cm.
2.8. Corrosion Studies
The used reagents in the corrosion studies were potassium hexacyanoferrates K3[Fe(CN)6] and K4[Fe(CN)6], salts for preparation of the buffer solutions (mono and dibasic potassium phosphates) and KCl, all of reagent grade (purity ≥ 98%), all of them used without further purification. Ultrapure water (0.055 μS cm−1, Adrona B30 Bio, Vilnius, Lithuania) and reagent-grade chemicals were used to prepare buffer solutions and the electrolytes for running electrochemical experiments.
The specimens under investigation were 2–3 mm thick circle-shaped lamellae with one side coated with VN and the rest being the initial material. Prior to the measurements, a crocodile clip was connected to each specimen. After that, the non-coated sides of each sample were insulated with a non-conductive polish and allowed to dry overnight under a fume cupboard. All electrochemical measurements were performed in a standard, non-compartmentalized electrochemical cell made of Pyrex glass with a working volume of 10–50 mL in a three-electrode configuration. The VN-coated surfaces were utilized as working electrodes, whilst a Ag/AgCl, 3 M KCl and a Pt wire were reference and counter electrodes, respectively, connected to a compact potentiostat–galvanostat, Autolab Vionic (Metrohm, Utrecht, The Netherlands), with an embedded frequency response analyzer (FRA-module) for carrying out electrochemical impedance spectroscopy, controlled by Intello software (4.3.5 version). The corrosion resistance of the specimens was examined as described previously [33]. Firstly, the possibility of the VN coatings protecting the substrate surface from corrosion was evaluated by the equilibrium potential (at zero current flowing through the electrochemical cell) in a 0.1 M KCl neutral aqueous solution. The protective function of the VN film against oxidative dissolution was determined by the impedance spectra (EIS) with frequencies ranged from 50 kHz to 1 Hz with 10 frequencies per decade in the same solution, where potassium hexacyanoferrates played the role of a redox probe. The current variation with the applied potential (known as the polarization characteristics of the coatings) was investigated by cyclic voltammetry at a scan rate of 50 mV/s in 0.1 M phosphate buffer with pH = 7.0, recorded over the potential region from −0.5 to 1.1 V vs. a Ag/AgCl, 3 M KCl reference system. The potentials were reported against this reference electrode.
3. Results and Discussion
3.1. XRD Analysis
Figure 1 shows the XRD results of the obtained VN films. The detected phases of the substrate correspond to γ-Fe and α-Fe, as determined using PDF #060696 and PDF #330397. Two other phases were detected, namely the VN and V2N ones using PDF #350768 and 331439, correspondingly. For all VN coatings, the diffraction peaks at 37.6° and 63.5° of the face-centered cubic (fcc) VN phase and reflections, corresponding to the (111) and (220) crystallographic planes, were observed. We also noticed the existence of other peaks for the V2N phase at 2θ = 41.7° and 65.8° which were associated with reflections in the (211) and (300) crystallographic planes. No amorphous-like halos were detected, which is normal since the studied samples were subjected to heating; however, the cooling speed was nowhere near high enough to cause the formation of an amorphous structure as opposed to a crystalline one. The presence of a VN phase was to be expected due to the applied surface coating, which had the same chemical composition. V2N is typically not found in nitrogen-rich environments [34]; however, a pre-applied V layer was used in order to increase the adhesion between the substrate and the VN layer. Due to this, it is possible that some N2 particles combined with the already applied V ones atop the surface of the 304L substrates and as a result the V2N phase was formed.
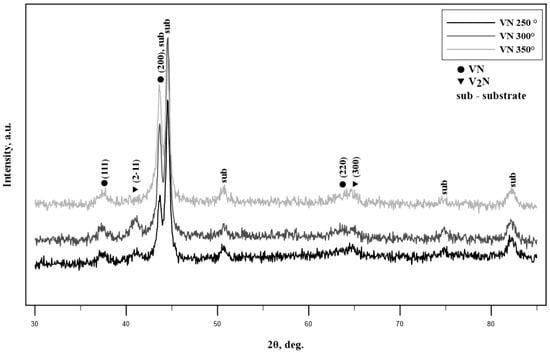
Figure 1.
XRD patterns of VN coatings at different temperatures.
No major change in the crystal structure was observed as a function of the applied temperature. Only the diffraction maxima intensity of the V2N phase reduced. Typically, in previous works it has been shown that obtaining a combination of VN, β-V2N, and the substrate increases the hardness and some functional properties of the specimens [35]. This usually happens in high-temperature environments. However, it is important to mention the application of a pure V interlayer atop the 304L SS substrates in order to improve the adhesion between the VN coating and the substrates. As a result of this, the parasitic V2N phase formed during the nitriding stage prior to the application of the VN coating. Applying the VN coatings at higher temperatures resulted in an increased energy of all particles included in the formed plasma flow (N2, VN, etc.). Due to the increased energy, the applied VN coatings are denser which reduces the diffraction maxima of the V2N phase. It is also possible that due to the higher temperature, the V2N phase acts as a diffusion barrier between the substrate and the VN coating. The latter attaches to the V2N phase and builds up off of it; however, this is just a hypothesis that was not proven during the present work. In any case, an obvious decrease in the V2N phase with an increase in temperature was noticed. This improves the purity of the formed VN films.
Due to the obvious interlapping of the VN phase with the substrate diffraction maxima, the texturing of the samples’ surfaces was unclear. However, it can be concluded based on the standalone diffraction maxima of the VN phase that isotropic properties of the samples are to be expected.
3.2. XPS Analysis
The surface composition and chemical state of VN films deposited at temperatures of 250 °C, 300 °C, and 350 °C were assessed by XPS measurement. The analysis showed that the chemical elements C, O, N, and V were registered on the surface and their quantitative composition is presented in Table 1.

Table 1.
XPS results of quantitative composition on the surface.
The surface analysis of the samples revealed distinct peaks corresponding to V2p and N1s. A layer of adventitious carbon was consistently observed on the sample surfaces, which can be removed by Ar ion sputtering. However, the sputtering was not performed, as Ar ion etching can damage the surface layer and potentially compromise the integrity of the VN coatings. The sample surfaces were not cleaned with ethanol or acetone to avoid introducing surface contaminants. The samples were prepared for analysis using protective measures to prevent surface contamination.
The presence of a high concentration of oxygen on the VN film surface is probably due to contamination or residual oxygen in the deposition vacuum chamber [22]. The C1s spectra and the V2p3/2 spectra are shown in Figure 2. It can be seen that the carbon spectra are decomposed into three peaks, which are attributed to the C-C bonds (adventitious carbon), C-N, and C = O. The V2p spectra contains two lines—the first one V2p3/2 is fixed at 513.7 eV and the second one V2p1/2 at 521.1 eV. The V2p1/2 peak overlapped with the satellite of the oxygen spectrum and, for this reason, only the V2p3/2 peak is decomposed. The deconvolution displayed two oxidation states of vanadium: V3+ in V-N (assigning to V2N phases), V3+ in V2O3, and V5+ in V2O5. These results are in the accordance with the data obtained in XRD analysis. The concentrations and binding energies of the different types of vanadium are presented in Table 2. The data exhibited a decrease in V-N phase concentration in the VN coatings with increased substrate temperature. This reduction is probably due to the low quantity of vanadium and nitrogen atoms that were deposited on the substrate surface and the lower rate during the deposition process [36]. The results in our study are in good agreement with those described by the authors of Reference [37]. The XPS spectra in the V2p, O1s, and N1s levels of the V layers obtained at different substrate temperatures are exhibited in Figure 3. The oxygen spectra have two strong features, corresponding to oxygen bound to vanadium at 530.4 eV and a carbonyl group at 532.2 eV. In the N1s spectra, the binding energies of the peaks are positioned at 396.8 eV and 400.1 eV and are assigned to V-N and C-N bonds, respectively [38,39]. The peak intensity, corresponding to the V-N phase, increased at a temperature of 350 °C as a result of the high energy of interaction between vanadium and nitrogen atoms [40].
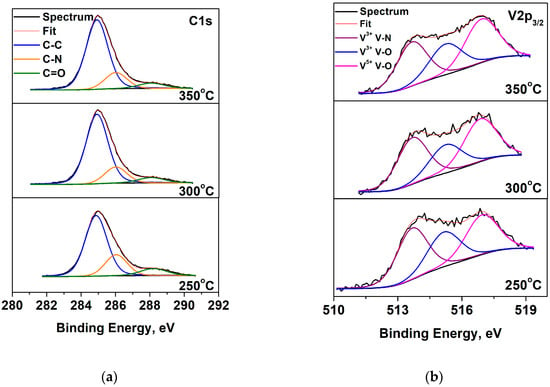
Figure 2.
Deconvolution of photoelectron spectra: (a) C1s; (b) V2p3/2 of VN layers at various temperatures.

Table 2.
The percentages of V from various species.
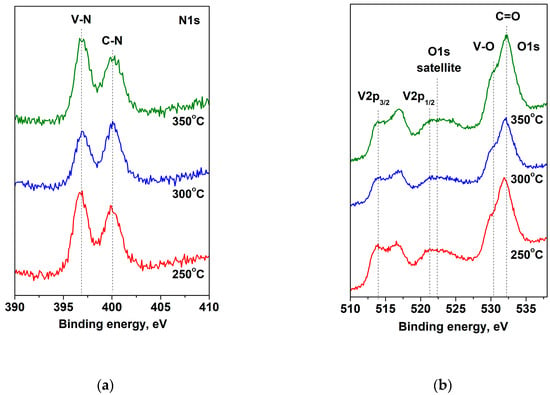
Figure 3.
Photoelectron spectra: (a) N1s; (b) O1s and V2p of VN layers, deposited at substrate different temperatures.
The tribological performance of vanadium is significantly influenced by its oxidation state. Various oxidation states of vanadium can form during tribological processes. At higher temperatures, vanadium and VN layers can oxidize, forming vanadium pentoxide (V2O5). This oxide film can act as a solid lubricant and has the ability to reduce friction by using a tribofilm (a protective film formed on the surfaces during sliding) and improve tribological properties at elevated temperatures [41,42].
The XPS observations showed the existence of V-N, C-N, V-O, and C=O bonds on the surface of the VN films. It can be noted that the temperature treatment has an impact on the quantity of V on the surface. This is reflected in the variation in concentration of the individual vanadium phases. A temperature-dependent trend was observed: a relatively high concentration at 250 °C, followed by a decrease at 300 °C, and a subsequent increase at 350 °C. However, the concentration at 350 °C does not exceed the level observed at 250 °C. The highest amount of vanadium is observed with the treatment of 250 °C, as confirmed by the table with the different species of vanadium.
3.3. AFM Analysis
The surface roughness of the VN films obtained at different temperatures on 304L stainless steel substrates was investigated by atomic force microscopy. 3D AFM surface images of VN coatings are given in Figure 4. The surface morphology of the bare material was also exhibited to estimate the effect of temperature on the surface properties of the initial material. The alteration of the substrate temperature during the magnetron sputtering process influences the surface roughness of the as-deposited VN films. It was found that the nano-roughness of the bare substrate is 216 nm. Applying a temperature of 250 °C to the initial material, the surface of the VN film became smoother and the roughness decreased significantly to 16 nm. Increasing the substrate temperature up to 300 °C led to a slight increase in the surface roughness up to 18 nm and a subsequent decrease to 10 nm at a substrate temperature of 350 °C. For all specimens, a wave-like topography was observed. The lower roughness is probably due to achieving the optimum ratio of the technological parameters such as the discharge voltage, discharge current, reactive and inert gas ratio, and appropriate substrate temperatures. These technological conditions are an important factor for films’ growth, phase composition, and structure. In addition, the lower roughness with increasing temperature is attributed to good homogeneity in the film’s composition and the enhanced density of the coating.
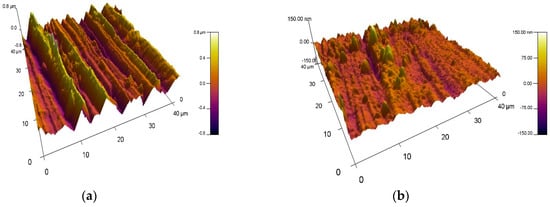
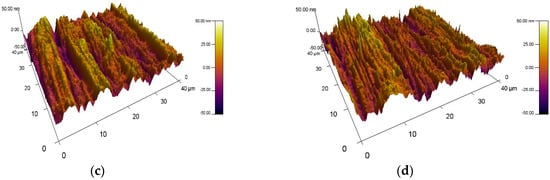
Figure 4.
Three-dimensional AFM images: (a) the base material and VN coatings deposited at (b) 250 °C; (c) 300 °C; and (d) 350 °C.
It is possible that the smoother surface contributes to improvement in the corrosion resistance of the films when they are exposed to chloride-containing solutions [43]. The lowest surface roughness was achieved for the VN coating produced at a temperature of 350 °C, as a result of the sufficiently increased ion flux and bombarding energy. On the other hand, the lower surface roughness is probably due to surface diffusion and thermal expansion processes [44]. When the temperature increases, the kinetic energy of V atoms on the surface is higher and they move more easily [45]. In this way, the surface diffusion was improved, irregularities were smoothed out, and the roughness decreased.
3.4. Thickness
The deposition temperature is one of the most important factors affecting the thickness and microstructure of the as-deposited VN coatings. The VN film thickness as a function of the substrate temperature is displayed in Figure 5, and optical 2D images are given in Figure 6.
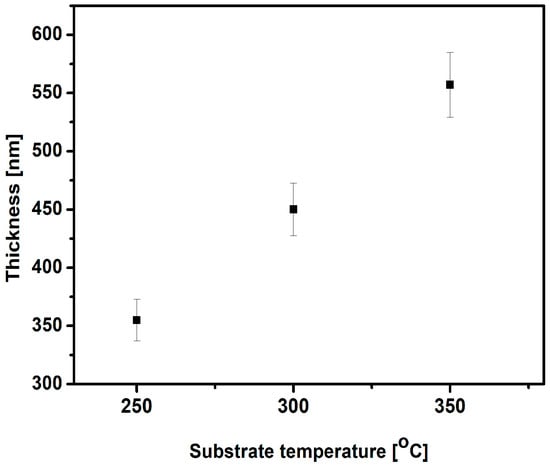
Figure 5.
Variation in the thickness with the substrate temperature.
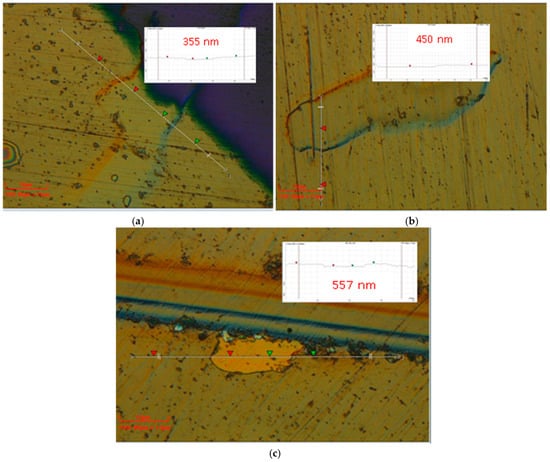
Figure 6.
Two-dimensional optical images of the VN coatings, deposited at (a) 250 °C; (b) 300 °C; (c) 350 °C.
The VN film thickness was determined by 3D Optical profiler. It measures the film thickness based on the surface height differences. When measuring the friction coefficient, chips occurred on the coatings where the thickness was measured. The markers showed the VN film thickness between two points. This is a surface with a profile of the thickness. The results exhibited a linear increase in the coating thickness with the increase in temperature. The VN coating deposited at 250 °C showed a thickness of 355 nm. Raising the temperature up to 350 °C, the thickness of the VN sample was enhanced (557 nm). The higher temperature during deposition led to thickening of the layer due to the higher kinetic energy of the vanadium atoms deposited on the surface and the improvement in adhesion between the bare material and the film. This phenomenon could have resulted in the presence of nucleation sites, which probably enhanced the thickness of the as-deposited film [46]. The nucleation process is an initial stage in the phase transition for formation of a new phase or structure in the coating. The phase transitions require enough energy to overcome the energetic barrier of new phase formation. Also, the nucleation sites provide the surface by lowing the energetic barrier and make it easier for production of the new phase. The highest thickness (557 nm) of the VN film is probably attributed to the denser structure and low compressive residual stresses, proving a lack of defects in the film during its growth [47]. Increasing the thickness led to a greater quantity of V2O5 in the film and good adhesion between the substrate and the coating. The thicker film provided low surface roughness, a coating with a high density, a reduction in the friction coefficient, and an enhancement in the tribological properties.
3.5. Mechanical Properties
The hardness and the elastic modulus of the VN films produced at substrate temperatures of 250 °C, 300 °C and 350 °C by the DC magnetron sputtering method are given in Figure 7, and load–indentation depth curves are displayed in Figure 8. A decrease in the hardness and the elastic modulus can be observed with increasing temperature due to a change in the films’ stresses. The highest hardness (10.6 GPa) was achieved in the film deposited at 300 °C, and the lowest value of hardness (9.5 GPa) was obtained at a temperature of 350 °C. The enhancement in the coating’s hardness is related to the highest quantity of V-N bonds in the layer. According to the authors of [48], the high hardness is a result of the good stoichiometry between vanadium and nitrogen ions. On the other hand, increasing the substrate temperature led to a decrease in the surface roughness and hardness due to VN film unevenness. In addition, the reduction in the coating’s hardness resulted in the presence of a V2O5 phase which was confirmed by the XPS measurements in our study.
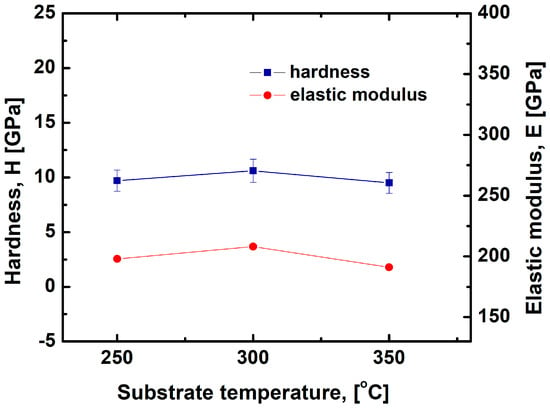
Figure 7.
Variation in hardness and elastic modulus of the VN films with the substrate temperature.
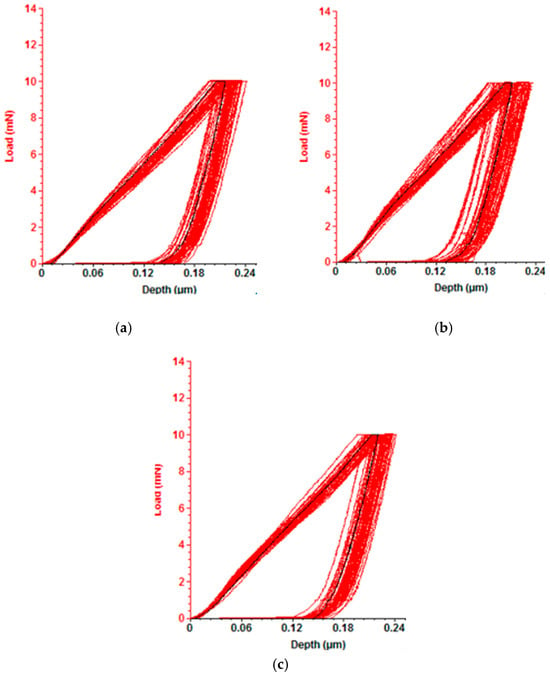
Figure 8.
Load–indentation depth curve: curves for VN coatings obtained with nanoindentation using a constant applied force of 10 mN at (a) 250 °C; (b) 300 °C; (c) 350 °C.
The elastic modulus characterizes the stiffness of the material to resist deformation under tension or compression. The highest Young’s modulus (208 GPa) was observed in the film deposited at a temperature of 300 °C, and the lowest modulus of elasticity (191 GPa) was achieved in the coating at a temperature of 350 °C. These values are significantly lower compared to the results reported by Huang et al., where the elastic modulus for VN films reaches 370 GPa [49]. According to the researchers in [50], this distinction is probably attributed to high compressive stresses and the smooth surface of the films. Zhang et al. [51] reported lower values of the elastic modulus due to a more porous structure in the coatings. The high values of elastic modulus corresponded to increased stiffness of the material and a small deformation [52]. According to the authors of [53], the mechanical behavior of the VN coatings depends on their structure. Films with a good density and homogeneity remarkably enhanced their hardness and elastic modulus [54,55]. In our study, the VN layer deposited at 300 °C was the densest and most homogeneous.
The hardness and elastic modulus ratios (H/E or H3/E2) have an important significance for determination of the materials’ functional properties. The H/E ratio evaluates the elastic deformation which is related to the ability of the film to resist loads. The H3/E2 parameter characterizes the plasticity of the film which undergoes irreversible deformation without any change in stress or load [56]. Table 3 gives information on the mechanical characteristics of the VN coatings produced at different temperatures.

Table 3.
Mechanical characteristics of the deposited VN films at different temperatures.
Temperature is an essential factor for the plasticity of metal materials. Upon increasing the temperature, plastic deformation is enhanced and the material becomes more malleable. This leads to the high strength of the material and its modern applications in industry. In our study, the obtained results are very close to each other but the highest values of the H/E (0.051) and H3/E2 (0.027) ratios are obtained for the VN coating at a temperature of 300 °C. On the other hand, the enhancement in elasticity and plasticity is probably related to the improved density of the film and the existence of the more stable V-N phase [57]. Lower values of H/E (0.048) and H3/E2 (0.023) are observed for the VN layer produced at 250 °C and it is an indicator of insufficient resistance to plastic deformation and susceptibility to wear. In addition, the high values of elastic deformation (H/E ratio) contributed to improvement in VN coatings’ toughness and tribological properties. The enhancement in the hardness and the elastic modulus can be associated with the lower concentration of oxygen (12.2%) on the surface of the film, as can be seen from the XPS results.
3.6. Friction Coefficient
Elastic and plastic deformations are associated with the films’ toughness and give information on the tribological performance of the films. The friction coefficients (COFs) of the VN coatings obtained at temperatures of 250 °C, 300 °C, and 350 °C are evaluated by ball-on-flat tests sliding an opposite tempered ball (with Cr coating) at room temperature and different times. The results are shown in Table 4.

Table 4.
Friction coefficients at different time of the VN films, deposited at 304L SS substrates temperatures.
From Table 4, it can be seen that when raising the friction time from 5 to 10 min, COF increased for all coatings. A similar trend is achieved with increasing temperature. Raising the VN coatings’ friction coefficient with an increase in friction time would be explained by the friction mechanism at sliding, owing to material transport from the ball on the coating [58]. The lowest COF (0.35) was measured for the VN film obtained at 250 °C for a friction time of 5 min, and the highest value was estimated for the VN film deposited at 300 °C for 10 min. The lower friction coefficient is probably due to an increase in the film density and the existence of V2O3 and V2O5 which is consistent with the XPS results. Similar statements were reported by the authors of [12]. According to them, the formation of vanadium oxides is the probable cause of the lower friction coefficient. No significant change in the friction coefficients is observed (0.45) for the layers at 300 and 350 °C and 5 min of testing.
With a longer time, the ball slides on the coating as much material is transferred and in this way the friction coefficient is enhanced [59]. Other authors have reported similar values of the friction coefficient (0.45) for the aforementioned films but at a higher deposition temperature [60]. Liu et al. [61] investigated the tribo-corrosion behavior of VN films and evaluated a friction coefficient under 0.2 at a temperature of 400 °C. The high values of plastic deformation lead to improved hardness of the VN film and good protection against wear [62]. García-Leon et al. [63] obtained a low friction coefficient (0.33) at low loads less than 10 N and high speed and low load.
There is a relationship between the surface roughness and friction coefficient for the investigated films. It was displayed that the friction coefficient (0.5226) increased with a decrease in the surface roughness (10 nm) for the VN sample obtained at 350 °C. Aissani et al. [12] showed a friction coefficient of 0.61 which can be explained by the formation of the unstable phase V2N. The enhancement in the friction coefficient with increasing temperature could result from the films’ wear debris [64,65]. On the other hand, the rougher a surface is, the higher the friction coefficient compared to surfaces without asperities. The friction is directly related to the wear. When the surface is uneven, the wear is faster. This is attributed to the lower effective contact area between the mating surfaces and the films’ tribological properties worsen.
3.7. Corrosion Resistance
The protective role that the coating of VN annealed at three different temperatures plays over the substrate was estimated on the basis of the following electrochemical techniques: determination of the equilibrium potential at open circuit (OCP measurements); impedance studies (EIS); and polarization studies implemented by means of cyclic voltammetry (CV). The corrosion protection of the samples, ensured by the VN films, was characterized by electrochemical studies carried out under equilibrium conditions with or without small perturbations (measurements of the open circuit potentials, OCP, and impedance studies). An open circuit technique (at zero current flowing through the cell, until an equilibrium value is reached) was used to determine the corrosion potentials of the as-deposited VN films. Table 5 shows the measured OCP values, reported vs. a Ag|AgCl, 3 M KCl reference electrode, determined in 0.1 M KCl, pH = 7.0.

Table 5.
Open circuit potentials of the VN-coated stainless steel (annealed at temperatures of 250, 300, and 350 °C) determined in 0.1 M KCl, neutral aqueous medium.
For comparison, 304L stainless steel initial material exhibited a corrosion potential of −0.652 mV [66] which is proof of an enhancement in the VN-coated specimen’s corrosion resistance, as can be seen from Table 5. Taking into account that the reference electrode is 200 mV more positive than the standard hydrogen electrode (SHE), the resulting positive values of the OCPs found for VN-coated samples produced at temperatures of 250, 300 and 350 °C undoubtedly indicated that these specimens would not corrode even in chloride-ion-containing solutions, as chlorides are evaluated to be one of the most aggressive corrosive agents. The second round of measuring the OCPs of the specimens was carried out under equivalent experimental conditions after polarization studies (performed in neutral phosphate buffer in the absence of chloride ions). The measured OCP values were found to be identical for the three studied samples, being even more positive than the initially measured OCPs. This finding implied that under the polarization conditions, surface passivation occurred, i.e., surface structures providing even greater corrosion protection were formed.
The above considered statements for the corrosion behavior of the investigated films are in good agreement with the EIS studies, as shown in Figure 9.
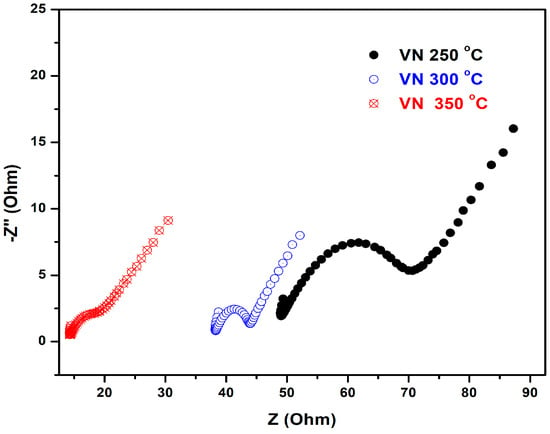
Figure 9.
Electrochemical impedance spectra (Nyquist plots) of VN-coated stainless steel samples, treated at the following temperatures: 250, 300, and 350 °C.
Electrochemical impedance spectroscopy (EIS) is a well-known qualitative method for evaluation of the corrosion behavior at the interface of the coating and electrolyte solution containing a redox probe. Also, the data obtained by this technique are reliable and can predict the long-term performance of the protective coatings. As can be seen from Figure 10, the impedance spectra of the VN films, deposited at temperatures of 250 and 300 °C, exhibited the appearance of a semi-circle, proving the formation of a homogeneous protective layer over their metallic surface. The EIS measurements showed the inability of redox particles to reach the conductive surface to reduce or oxidize over it, and the semi-circle’s diameter increased with the density of the protective VN layer. On the other hand, as the temperature of the treated sample raised, the diameters of the impedance spectra semi-circles for the samples under study decreased. The VN films produced at 250 and 300 °C demonstrated the best protection against corrosion, as can be observed from the semi-circle diameter of the Nyquist plot. The specimen obtained at 350 °C provided a much weaker protective ability to the metal substrate due to the much smaller resistance of the charge transfer as the semi-circle diameter reduced drastically.
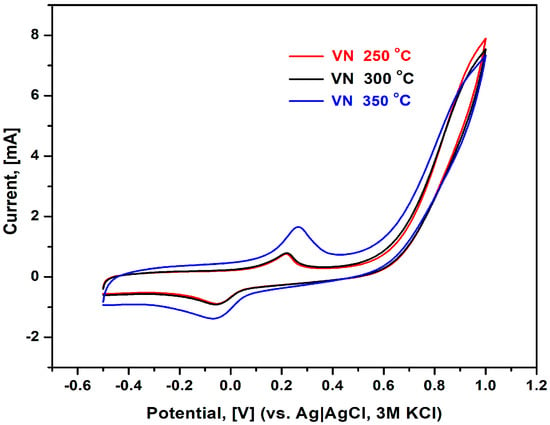
Figure 10.
Cyclic voltammograms of VN-coated stainless steel in 0.1 M phosphate buffer, pH = 7.0; scan rate 50 mV/s, room temperature.
The potentiodynamic technique, which was used to detect the presence of oxidation and reduction processes at the electrode–electrolyte interface, is cyclic voltammetry (CV). CV is a direct current electrochemical technique that examines the relationship between different current values at a potential varying linearly with time from an initial to an end value and then back to the beginning one. The polarization behavior of all VN samples was followed in the absence of strong corrosion agents, e.g., chloride ions, as can be deduced from Figure 10.
It can be seen that all studied samples were characterized by the appearance of a pair of two well-defined peaks: the first one was observed on the forward scan at 200–250 mV, while the peak on the backward scan raised at –100 mV, thus implying an irreversible redox process. The sample behavior at applied potentials exceeding 550 mV was described by a sharp current increase, and the shape of cyclic voltammograms is typical for faradaic processes, e.g., sample dissolution. No signs of such were observed, however, after removing the samples from the electrolytic solution where the corrosion tests were performed. It is plausible that a new surface compound is formed upon testing the samples in the neutral phosphate buffer, as suggested by the positive shift in the measured OCP (Table 5, see OCP2). Analogously to EIS studies, both specimens deposited at 250 and 300 °C manifested smaller faradaic currents compared to the sample treated at a temperature of 350 °C.
In order to investigate the consequences of the polarization tests on the examined samples, a second round of impedance studies was performed under equivalent experimental conditions (Figure 11). The resulting EIS spectra suggested that both the shape and the charge transfer resistance changed drastically: unlike the Nyquist plots of the samples obtained with the first impedance tests that varied from sample to sample, the second ones appeared very similar, with scarcely expressed semi-circles, followed by a linear tail tilted at 45°. The last finding indicates strong diffusion control over the redox probe reaching the metal surface. The other important finding was the vastly reduced charge transfer resistance as compared with the first impedance tests: for the VN film at 250 °C, its decay is the most substantial (~20 times), while the other two samples indicated a reduction in the charge transfer resistance of eight and five times for VN coatings at 300 °C and 350 °C, respectively.
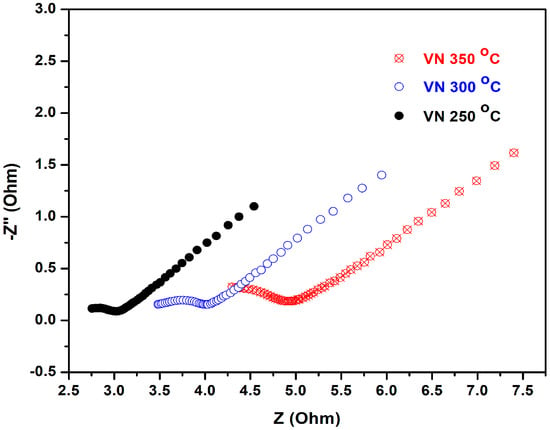
Figure 11.
Impedance studies of VN: electrochemical impedance spectra (Nyquist plots) of VN-coated stainless steel samples treated at different temperatures. The EIS tests followed the polarization measurements.
The surface roughness and adhesion of the coatings have an important role for their protection against corrosion processes. The good adhesion between the coating and the substrate material decreased the roughness and improved the corrosion resistance of the films [67]. As regards the bare substrate, namely 304L stainless steel, the significant value of the roughness (216 nm) was responsible for a decrease in the potential in the strong aggressive solutions.
Summarizing, the performed studies on the corrosion resistance of VN-coated stainless steel indicated that the layer of VN deposited over the sample’s surface definitely protects the steel from corrosion even in the presence of strong corrosion agents (e.g., chloride ions).
The obtained results in our research exhibited a good possibility for the deposition of nanostructured VN coatings with low roughness and a small friction coefficient. Increasing the temperature led to an improvement in the mechanical properties of the as-deposited VN films and adhesion between the substrate and the film, as well as a reduction in the surface roughness. The electrochemical tests revealed a remarkable positive shift in the corrosion potential for all films in comparison with the initial material whose potential was negative. The best mechanical, tribological, and corrosion properties were shown for VN films deposited at 250 °C and 300 °C, which is in good agreement with the XPS results obtained in our research.
4. Conclusions
In our research, VN coatings films were deposited on 304L stainless steel substrates at temperatures of 250, 300, and 350 °C by a DC magnetron sputtering process to evaluate their effect on the structural, mechanical, and tribological properties, as well as the corrosion behavior of the obtained coatings. The XRD results showed an obvious decrease in the V2N phase with the increase in temperature that led to an improvement in the purity of the formed VN films. XPS analysis confirmed the growth of VN. The surface roughness decreased (10 nm) with an increase in the temperature up to 350 °C as a result of good homogeneity in the film composition and improved density of the coating. The VN films’ thickness increased (557 nm) with the temperature due to the presence of nucleation centers in the films. The lowest friction coefficient (0.3546) was achieved in the VN film deposited at 250 °C for 5 min as a result of the existence of vanadium oxides, V2O3 and V2O5, which can improve the tribological properties of the coating. The smoother surface and good adhesion between the film and the initial material contributed to the improvement in the physical–mechanical properties of the film–substrate system. The electrochemical measurements showed that the sample with the highest protection against corrosion processes was VN coating, produced at a temperature of 250 °C. It can be concluded that the applied technological conditions are suitable for successful deposition of VN coatings, as well as for the enhancement of mechanical, tribological, and corrosion properties and their use in modern industrial applications.
Author Contributions
Conceptualization, S.R.; methodology, S.R., D.D., N.I., N.D. and V.S.; formal analysis, S.R., D.D., N.I., N.D., V.S.,V.K., M.S. and G.A.; investigation, S.R., D.D., N.I., N.D., V.S., V.K., M.S. and G.A.; writing—original draft preparation, S.R.; writing—review and editing, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
Nina Dimcheva gratefully acknowledges access to the research infrastructure of the Centre for Competence “Personalized Innovative Medicine, PERIMED-2 (BG Programme “Research, Innovation, and Digitalization for Smart Transformation” 2021-2027, co-financed by the EU, grant BG16RFPR002-1.014-0007).
Data Availability Statement
The original data presented in the study are openly available.
Acknowledgments
Research equipment of the Distributed Research Infrastructure INFRAMAT, part of the Bulgarian National Roadmap for Research Infrastructures, supported by the Bulgarian Ministry of Education and Science, was used in this investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Y.; Lee, J.-W.; Duh, J.-G. Mechanical strengthening in self-lubricating Can/VN multilayer coatings for improved high-temperature tribological characteristics. Surf. Coat. Technol. 2016, 303, 12–17. [Google Scholar] [CrossRef]
- Hovsepian, P.; Luo, Q.; Robinson, G.; Pittman, M.; Howard, M.; Doerwald, D.; Tietema, R.; Sim, W.; Deeming, A.; Zens, T. TiAlN/VN superlattice structured PVD coatings: A new alternative in machining of aluminum alloys for aerospace and automotive components. Surf. Coat. Technol. 2006, 201, 265. [Google Scholar] [CrossRef]
- Chabanon, A.; Michau, A.; Schlegel, M.; Gündüz, D.; Puga, B.; Miserque, F.; Schuster, F.; Maskrot, H.; Pareige, C.; Cadel, E.; et al. Surface Modification of 304L Stainless Steel and Interface Engineering by HiPIMS Pre-Treatment. Coatings 2022, 12, 727. [Google Scholar] [CrossRef]
- Caicedo, J.; Zambrano, G.; Aerators, W.; Escobar-Alarcon, L.; Camps, E. Mechanical and electrochemical characterization of vanadium nitride (VN) thin films. App. Surf. Sci. 2011, 258, 312–320. [Google Scholar] [CrossRef]
- Bautista, J.; Campelo, M.; Luna, D.; Luque, J.; Marinas, J. Gas-phase selective oxidation of toluene on TiO2–sepiolite supported vanadium oxides: Influence of vanadium loading on conversion and product selectivities. Catal. Today 2007, 128, 183–190. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, Y.; Zhao, L.; Ran, F. Recent advances of fabricating vanadium nitride nanocompositions for high-performance anode materials of supercapacitors. J. Energy Storage 2024, 75, 109564. [Google Scholar] [CrossRef]
- Jrondi, A.; Buvat, G.; Pena, F.; Marinova, M.; Huvé, M.; Brousse, T.; Roussel, P.; Lethien, C. Major improvement in the cycling ability of pseudocapacitive vanadium nitride films for micro-supercapacitor. Adv. Energy Mater. 2023, 13, 2203462. [Google Scholar] [CrossRef]
- Adalati, R.; Sharma, M.; Sharma, S.; Kumar, A.; Malik, G.; Boukherroub, R.; Chandra, R. Metal nitrides as efficient electrode material for supercapacitors: A review. J. Energy Storage 2022, 56, 105912. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Mohimi, E.; Zhang, Z.; Mallek, J.; Liu, S.; Trinh, B.; Shetty, P.; Girolami, G.; Abelson, J. Low temperature chemical vapor deposition of superconducting vanadium nitride thin films. J. Vac. Sci. Technol. A 2019, 37, 031509. [Google Scholar] [CrossRef]
- Huang, J.; Yu, G. Optimization of deposition processing of VN thin films using design of experiment and single-variable (nitrogen flow rate) methods. Mater. Chem. Phys. 2019, 224, 246–256. [Google Scholar] [CrossRef]
- Aissani, L.; Alhussein, A.; Nouveau, C.; Ghelani, L.; Zaabat, M. Influence of film thickness and Ar/N2 plasma gas on the structure and performance of sputtered vanadium nitride coatings. Surf. Coat. Technol. 2019, 378, 124948. [Google Scholar] [CrossRef]
- Lebreton, A.; Lethien, C.; Coleman, J.; Brousse, T.; Barbé, J. Tuning Deposition Conditions for VN Thin Films Electrodes for Microsupercapacitors: Influence of the Substrate Bias Voltage. J. Electrochem. Soc. 2025, 172, 040523. [Google Scholar] [CrossRef]
- Liao, M.-J.; Gotoh, Y.; Tsuji, H.; Ishikawa, J. Crystallographic structure and composition of vanadium nitride films deposited by direct sputtering of a compound target. J. Vac. Sci. Technol. A 2004, 22, 146–150. [Google Scholar] [CrossRef]
- Huang, J.-H.; Wei, L.-J.; Ting, I.-S. Evaluation of fracture toughness of VN hard coatings: Effect of preferred orientation. Mater. Chem. Phys. 2022, 275, 125253. [Google Scholar] [CrossRef]
- Ghimbeu, M.; Sima, F.; Ostaci, R.; Socol, G.; Mihailescu, I.; Vix-Guterl, C. Crystalline vanadium nitride ultra-thin films obtained at room temperature by pulsed laser deposition. Surf. Coat. Technol. 2012, 211, 158–162. [Google Scholar] [CrossRef]
- Xiang, W.; Drogoff, B.; Chaker, M. An innovative method to achieve large-scale high-quality VO2 thin films: Oxidation of vanadium nitride material deposited by sputtering. Appl. Surf. Scie 2023, 633, 157607. [Google Scholar] [CrossRef]
- Cupric, A.; Gilewicz, A.; Tolmachova, G.; Klimenko, I.; Kolodiy, I.; Vasilenko, R.; Warcholinski, B. Effect of Nitrogen Pressure and Substrate Bias Voltage on Structure and Mechanical Properties of Vacuum Arc Deposited VN Coatings. Metallur. Mater. Trans. A 2023, 54, 4438–4455. [Google Scholar]
- Chun, S.-Y. Properties of VNCoatings Deposited by ICPAssisted Sputtering: Effect of ICPPower. J. Korean Ceramic Soc. 2017, 54, 38–42. [Google Scholar] [CrossRef]
- Gao, M.; Xu, X.; Li, H. Investigation on preparation of vanadium nitride hard coating by in-situ method technique. Mater. Lett. 2020, 274, 128045. [Google Scholar] [CrossRef]
- Jia-Hong, H.; Cheng-Han, L.; Ge-Ping, Y. Texture evolution of vanadium nitride thin films. Thin Solid Film. 2019, 688, 137415. [Google Scholar] [CrossRef]
- Aissani, L.; Mamoun, F.; Chadli, A.; Samad, M.; Cheriet, A.; Salhi, F.; Nouveau, C.; Wei, S.; Orbison, A.; Alhussein, A. Investigating the effect of nitrogen on the structural and tribo-mechanical behavior of vanadium nitride thin films deposited using R.F. magnetron sputtering. J. Mater. Scie 2021, 56, 17319–17336. [Google Scholar] [CrossRef]
- Zhaobing, C.; Jibin, P.; Liping, W.; Qunji, X. Synthesis of a new orthorhombic form of diamond in varying- C VN films: Microstructure, mechanical and tribological properties. Appl. Surf. Sci. 2019, 481, 767–776. [Google Scholar] [CrossRef]
- Hongjian, G.; Bo, L.; Jianyi, W.; Wenyuan, C.; Zhenyu, Z.; Wenzhen, W.; Junhong, J. Microstructures, mechanical and tribological properties of VN films deposited by PLD technique. RSC Adv. 2016, 6, 33403–33408. [Google Scholar] [CrossRef]
- Yuexiu, Q.; Zhang, S.; Li, B.; Wei, J.-L.; Dongliang, Z. Influence of nitrogen partial pressure and substrate bias on the mechanical properties of VN coatings. Procedia Eng. 2012, 36, 217–225. [Google Scholar] [CrossRef]
- Gueddaoui, H.; Schmerber, G.; Abes, M.; Guemmaz, M.; Parlebas, J.C. Effects of experimental parameters on the physical properties of non-stoichiometric sputtered vanadium nitrides films. Catal. Today 2006, 113, 270–274. [Google Scholar] [CrossRef]
- Fallqvist, M.; Olsson, M. The influence of surface defects on the mechanical and tribological properties of VN-based arc-evaporated coatings. Wear 2013, 297, 1111–1119. [Google Scholar] [CrossRef]
- Krause, B.; Kaufholz, M.; Kotapati, S.; Schneider, R.; Müller, E.; Gerthsen, D.; Wochner, P.; Baumbach, T. Angle-resolved X-ray reflectivity measurements during off-normal sputter deposition of VN. Surf. Coat. Technol. 2015, 277, 52–57. [Google Scholar] [CrossRef]
- Hajihoseini, H.; Gudmundsson, J. Vanadium and vanadium nitride thin films grown by high power impulse magnetron sputtering. J. Phys. D Appl. Phys. 2017, 50, 505302. [Google Scholar] [CrossRef]
- Fangfang, G.; Zhu, P.; Fanping, M. Enhancing the wear resistance of magnetron sputtered VN coating by Si addition. Wear 2016, 354–355, 32–40. [Google Scholar] [CrossRef]
- AlHazaa, A.; Haneklaus, N. Diffusion Bonding and Transient Liquid Phase (TLP)Bonding of Type 304 and 316 Austenitic Stainless Steel—A Review of Similar and Dissimilar Material Joints. Metals 2020, 10, 613. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Rabadzhiyska, S.; Dechev, D.; Ivanov, N.; Ivanova, T.; Strijkova, V.; Katrova, V.; Rupetsov, V.; Dimcheva, N.; Valkov, S. Wear and Corrosion Resistance of ZrN Coatings Deposited on Ti6Al4V Alloy for Biomedical Applications. Coatings 2024, 14, 1434. [Google Scholar] [CrossRef]
- Galesic, I.; Bolbesen, B. Formation of vanadium nitride by rapid thermal processing. Thin Solid Film. 1999, 349, 14–18. [Google Scholar] [CrossRef]
- Galesic, I.; Angelkort, C.; Lewalter, H.; Berendes, A.; Kolbesen, B. Formation of Tansition Metal Nitrides by Rapid Thermal Processing (RTP). Phys. Stat. Sol. 2000, 177, 15–26. [Google Scholar] [CrossRef]
- Jinghua, L.; Fengfan, L.; Weiwei, L.; Xin, L. Effect of calcination temperature on the microstructure of vanadium nitride/nitrogen doped graphene nanocomposites as anode materials in electrochemical capacitors. Inorg. Chem. Front. 2019, 6, 164–171. [Google Scholar]
- Osonkie, V.; Chukwunenye, P.; Cundari, T.; Kelbera, J. Plasma modification of vanadium oxynitride surfaces: Characterization by in situ XPS experiments and DFT calculations. J. Chem. Phys. 2020, 153, 144709. [Google Scholar] [CrossRef]
- Liu, H.-H.; Zhang, H.-L.; Xu, H.-B.; Lou, T.-P.; Sui, Z.-T.; Zhang, Y. Hierarchically nanostructured vanadium nitride microspheres assembled with porous nanosheets fabricated by a template-free route. Ceram. Int. 2018, 44, 1583–1588. [Google Scholar] [CrossRef]
- Ling, C.; Yajid, M.; Tamin, M.; Kamarudin, M.; Taib, M.; Nosbi, N.; Wan, A. Effect of substrate roughness and PVD deposition temperatures on hardness and wear performance of AlCrN-coated WC-Co. Surf. Coat. Technol. 2022, 436, 128304. [Google Scholar] [CrossRef]
- Sánchez, E.; Sanchéz, M.; Ipaz, L.; Aperador, W.; Caicedo, J.; Amaya, C.; Hernández, M.; Landaverde, F.; Beltran, E.; Muñoz-Saldaña, J.; et al. Mechanical, tribological, and electrochemical behavior of Cr1 xAlxN coatings deposited by r.f. reactive magnetron co-sputtering method. Appl. Surf. Sci. 2010, 256, 2380–2387. [Google Scholar] [CrossRef]
- Ponomarev, I.; Polcar, T.; Nicolini, P. Tribological properties of V2O5 studied via reactive molecular dynamics simulations. Tribol. Inter. 2021, 154, 106750. [Google Scholar] [CrossRef]
- Ge, C.; Zhang, B.; Xu, X.; Lyu, X.; Ma, X.; Li, T.; Lu, X.; Liu, Z. Tribofilm distribution and tribological analysis of piston ring-cylinder liner interfaces under realistic engine conditions. Tribol. Inter. 2025, 201, 110250. [Google Scholar] [CrossRef]
- Kong, Q.; Ji, L.; Li, H.; Liu, X.; Wang, Y.; Chen, J.; Zhou, H. Influence of substrate bias voltage on the microstructure and residual stress of CrN films deposited by medium frequency magnetron sputtering. Mater. Scien. Enginer. B 2011, 176, 850–854. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Shen, Y. Temperature effect on surface roughening of thin films. Surf. Sci. 2005, 595, 20–29. [Google Scholar] [CrossRef]
- Li, J.; Song, Z.; Liu, Z.; Xie, X.; Guan, P.; Zhu, Y. Exploring Surface-Driven Mechanisms for Low-Temperature Sintering ofNanoscale Copper. Appl. Sci. 2025, 15, 476. [Google Scholar] [CrossRef]
- Chang, H.; Huang, P.; Yeh, J.; Davison, A.; Tsau, C.; Yang, C. Influence of substrate bias, deposition temperature and post-deposition annealing on the structure and properties of multi-principal-component (AlCrMoSiTi)N coatings. Surf. Coat. Technol. 2008, 202, 3360–3366. [Google Scholar] [CrossRef]
- Camacho-Espinosa, E.; Rosendo, E.; Díaz, T.; Oliva, A.; Rejon, V.; Peña, J. Effects of temperature and deposition time on the RF- sputtered CdTe films preparation. Superf. Vacío 2014, 27, 15–19. [Google Scholar]
- Aissani, L.; Fellah, M.; Nouveau, C.; Samade, M.; Montagne, A.; Iost, A. Structural and mechanical properties of Cr–Zr–N coatings with different Zr content. Surf. Eng. 2017, 35, 1–9. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.; Pierson, J.; Petrzhik, M.; Alnot, M.; Levashov, E.; Shtansky, D. Effect of nitrogen partial pressure on the structure, physical and mechanical properties of CrB2 and Cr–B–N films. Thin Solid Film. 2009, 517, 2675–2680. [Google Scholar] [CrossRef]
- Aissani, L.; Nouveau, C.; Walock, M.; Djebaili, H.; Djelloul, A. Influence of vanadium on structure, mechanical and tribological properties of CrN coatings. Surf. Eng. 2015, 31, 779–788. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, F.; Yang, Y.; Yan, M.; Zhang, Y.; Guo, J.; Li, H. Effects of sputtering gas on microstructure and tribological properties of titanium nitride films. Appl. Surf. Sci. 2019, 488, 61–69. [Google Scholar] [CrossRef]
- Zhang, B.; Qigang, H.; Zhang, J.; Han, Z.; Niu, S.; Luquan, R. Advanced bio-inspired structural materials: Local properties determine overall performance. Mater. Today 2020, 41, 177–197. [Google Scholar] [CrossRef]
- Guo, H.; Lu, C.; Zhang, Z.; Liang, B.; Jia, J. Comparison of microstructures and properties of VN and VN/Ag nanocomposite films fabricated by pulsed laser deposition. Appl. Phys. A 2018, 124, 694. [Google Scholar] [CrossRef]
- Pfeiler-Deutschmann, M.; Mayrhofer, P.; Chladil, K.; Penoy, M.; Michotte, C.; Kathrein, M. Effect of wavelength modulation of arc evaporated Ti–Al–N/Ti–Al–V–N multilayer coatings on microstructure and mechanical/tribological properties. Thin Solid Film. 2015, 581, 20–24. [Google Scholar] [CrossRef]
- Pfeiler, M.; Kutschej, K.; Penoy, M.; Michotte, C.; Mitterer, C.; Kathrein, M. The effect of increasing V content on structure, mechanical and tribological properties of arc evaporated Ti–Al–V–N coatings. Int. J. Refract. Met. Hard Mater. 2009, 27, 502–506. [Google Scholar] [CrossRef]
- Naghashzadeh, A.; Shafyei, A.; Sourani, F. Nanoindentation and Tribological Behavior of TiN-TiCN-TiAlN Multilayer Coatings on AISI D3 Tool Steel. J. Mater. Eng. Perform. (JMEP) 2022, 31, 4335–4342. [Google Scholar] [CrossRef]
- Lv, Y.; Ji, L.; Liu, X.; Li, H.; Zhou, H. Influence of substrate bias voltage on structure and properties of the CrAlN films deposited by unbalanced magnetron sputtering. Appl. Phys. Lett. 2012, 258, 3864–3870. [Google Scholar] [CrossRef]
- Gassner, G.; Mayrhofer, P.; Kutschej, K.; Mitterer, C.; Kathreinc, M. A new low friction concept for high temperatures: Lubricious oxide formation on sputtered VN coatings. Tribol. Lett. 2004, 17, 751–756. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, S.; Li, B.; Wang, Y.; Lee, J.-W.; Li, F.; Zhao, D. Improvement of tribological performance of CrN coating via multilayering with VN. Surf. Coat. Technol. 2013, 231, 357–363. [Google Scholar] [CrossRef]
- Wiklund, U.; Casas, B.; Stavlid, N. Evaporated vanadium nitride as a friction material in dry sliding against stainless steel. Wear 2006, 261, 2–8. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Xie, X.; Qin, J.; Wang, Y. The tribo-corrosion behavior of monolayer VN and multilayer VN/C hard coatings under simulated seawater. Ceram. Int. 2021, 47, 25655–25663. [Google Scholar] [CrossRef]
- Cai, Z.; Pu, J.; Lu, X.; Jiang, X.; Wang, L.; Xue, Q. Improved tribological property of VN film with the design of pre-oxidized layer. Ceram. Int. 2019, 45, 6051–6057. [Google Scholar] [CrossRef]
- García-León, R.; Martínez-Trinidad, J.; Zepeda-Bautista, R.; Campos-Silva, I.; Guevara-Morales, A.; Martínez-Londoño, J.; Barbosa-Saldaña, J. Dry sliding wear test on borided AISI 316L stainless steel under ball-on-flat configuration: A statistical analysis. Tribol. Int. 2021, 157, 106885. [Google Scholar] [CrossRef]
- Muhammed, M.; Javidani, M.; Heidari, M.; Jahazi, M. Enhancing the Tribological Performance of Tool Steels for Wood-Processing Applications: A Comprehensive Review. Metals 2023, 13, 1460. [Google Scholar] [CrossRef]
- Ju, H.; Yu, D.; Xu, J.; Yu, L.; Geng, Y.; Gao, T.; Yi, G.; Bian, S. Microstructure, mechanical, and tribological properties of niobium vanadium carbon nitride films. J. Vac. Sci. Technol. A 2018, 36, 1–7. [Google Scholar] [CrossRef]
- Malik, G.; Kumar, A.; Adalati, R.; Sharma, S.; Bansal, A.; Chandra, R. Enhanced electrochemical corrosion resistance of SS(304L) alloy with nano-pyramids c-Tina layer for saline media application. J. Alloys Metallur. Syst. 2023, 3, 100028. [Google Scholar] [CrossRef]
- Toloei, A.; Stoilov, V.; Northwood, D. The Relationship Between Surface Roughness and Corrosion. In Proceedings of the ASME 2013 International Mechanical Engineering Congress & Exposition IMECE 65498, San Diego, CA, USA, 15–21 November 2013; American Society of Mechanical Engineers: New York, NY, USA, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).