Abstract
The effects of the designed equal channel angular pressing (ECAP) procedures on microstructures, mechanical properties and corrosion resistances of newly developed nano-MgO/Mg–Zn–Ca composite materials have been investigated in this study. The die channel angles selected by the ECAP processes are 90°and 120°, and the corresponding composite materials are kept for 15 min in the ECAP mold at 300 °C before 1, 4, and 8 passes through route. It can be understood that the sizes of grains and second phases were significantly reduced because of continuous dynamic recrystallization (C-DRX) and mechanical shearing, and the ECAP process with the die angle of 90° shows more evidence of grain refinement owing to the higher shear stress. The obtained mechanical properties stipulated that both the yield and ultimate strength were improved after ECAP, which is related to the interaction of grain and texture evolution, while the elongation increases drastically from 14% (as-extruded state) to 34% (ECAP-ed state). Meanwhile, the improvement of corrosion resistance by microstructural evolution is more significant than adverse effects originating from the internal defects of the material itself as well as the defects originating from the number of passes. Ultimately, the conclusions were made based on the results regarding performance improvement by the optimized parameters designed and utilized in ECAP for this novel Mg composite material.
1. Introduction
In recent years, magnesium and its alloys have shown great potential in bone implantation as biodegradable metal biomaterials [1,2]. The main advantages of magnesium-based implants are the biodegradable and absorbable properties of magnesium, by which the degradation products of a magnesium alloy can be excreted or used in metabolic processes; magnesium is one of the most abundant cations in human cells and extracellular fluids, which is crucial for the formation of bones and teeth. Magnesium-based alloys have good biocompatibility, such as Mg–Zn–Ca matrix composites, with low development costs. The formation of stable intermetallic compounds between Zn, Ca, and Mg gives them high hardness and good creep resistance. However, the Mg–Zn–Ca alloy is difficult to manufacture as an implant that is easy to produce and hard at room temperature; it has processing problems and a fast corrosion rate. Therefore, it is proposed to add nano-MgO particles to the Mg–Zn–Ca alloy to improve its mechanical properties and corrosion resistance effectively [3,4]. The addition of MgO nanoparticles can effectively block the crack extension of the corrosion product layer and make the corrosion product layer denser [5]. Goh et al. [6] reported that adding MgO nanoparticles was an important factor in inhibiting the growth of grains, and the mechanical shearing process in the ECAP process made the MgO particles evenly distributed, then achieved the refinement of the grains. In the subsequent deformation process, the stable intermetallic compound Ca2Mg6Zn3 in Mg–Zn–Ca alloys limits grain size.
The hexagonal close-packed (hcp) structure of magnesium alloys results in very few slip systems at ordinary temperatures, resulting in processing performance being poor [7,8,9,10]. The severe plastic deformation (SPD) process imposes a large plastic strain on lightweight materials, improving the micro-plasticity of the materials and refining the microstructure. ECAP, one of the SPD methods, is a common method for refining grain and microstructure homogenization of hexagonal close-packed crystal structures like magnesium alloys [10,11]. During the ECAP deformation process of a magnesium alloy, the deformation energy storage begins to accumulate, but the high-energy state is unstable. High-temperature conditions can induce recrystallization behavior, and the deformation energy accumulated during the deformation process can be the driving force for recrystallization, thereby releasing the deformation energy through the recrystallization process to achieve a stable energy state [12,13]. Jin et al. [14] and Figueiredo et al. [15] proposed a grain refinement mechanism based on dynamic recrystallization and indicated that the new grains were formed by the initial grain boundaries during the deformation process. In addition to grain refinement, the size of MgO and other second phases decreases and are more evenly distributed in the matrix with the increase in the number of ECAP passes. During the deformation process, the recrystallized grains have a preferred orientation, and the non-basal slip system of the crystal is activated, which is beneficial to the improvement of its plasticity, deforming at a higher temperature. ECAP improves mechanical properties and the corrosion resistance of the specimens. The process of ECAP also leads to texture changes, and the texture softening can well support the experimental result of the formation of a geometric softer zone during compression deformation of the alloy [16,17].
Under the ECAP parameters of processing temperature (250 °C) and die channel angle (90°), L. B. Tong et al. [18] used routes of A, Bc and C to perform four passes of extrusion on a Mg–Zn–Ca alloy, and found that route of Bc is the most effective in grain refinement and formation of high-angle grain boundaries, which has great reference significance for optimizing the microstructure and mechanical properties of a Mg–Zn–Ca alloy. Zhang et al. [19] heated a specimen at 300 °C for 15 min in the mold, extruded the specimen into one, four and eight passes through the route BC, and found that the ECAP process has a significant effect on the microstructure homogenization of the composites, which is more obvious in the initial deformation stage. The work of Zhang et al. complemented the study of the microstructure and mechanical properties of Mg–Zn–Ca alloys under different numbers of passes in the route of Bc. Zhang et al. [20] conducted electrochemical tests and simulated body fluid immersion experiments on MgO/Mg–Zn–Ca composites; however, the authors discussed the effects of only two processing strategies on the corrosion resistance of this new composite material, which is not comprehensive for the study of the corrosion resistance of new composite materials. Although the previous work was rather valuable, it failed to systematically summarize the influence of different die channel angles and number of passes on the properties of MgO/Mg–Zn–Ca composites. In this work, the effects of two different mold angles (90° and 120°) and three passes (1,4, and 8) on the microstructure, mechanical properties and corrosion resistance of innovative magnesium alloys were systematically studied to perfect exploration on improving the properties of MgO/Mg–Zn–Ca composites.
2. Experimental Procedure
2.1. Melting and Fabrication of Composite Materials
The 0.6 wt.% MgO/Mg–3Zn–0.2Ca composite was prepared using high-purity magnesium (99.99%) ingots, Zn (99.99 wt.%) granules, a Mg–Ca master alloy (containing 25 wt.% Ca), and spherical MgO nanoparticles with an average diameter of 50–100 nm. Under the protection of an SF6/N2 gas mixture, pure magnesium ingots were melted at 720 °C, followed by the addition of calculated amounts of pure Zn and a Mg–Ca alloy into the molten magnesium, which was maintained at this temperature for 30 min. The MgO nanoparticles were then introduced at the bottom of a steel crucible preheated to 690 °C and subsequently incorporated into the molten magnesium. To ensure uniform dispersion of the MgO nanoparticles, a high-shear dynamic and static mixer was employed to disperse the nanoparticles within the Mg–3Zn–0.2Ca alloy melt. After successfully incorporating the MgO nanoparticles into the matrix melt, the composite melt underwent intensive shear mixing using a high-shear rotor–stator mixer at 4000 r/min for 5 min. The melt was then allowed to cool to 690 °C before being cast into a low-temperature steel mold at 680 °C. After solidification and cooling, the composite ingot was demolded. Following casting, all ingots underwent mechanical machining to remove surface oxidation scales.
2.2. Hot Extrusion
After the composite material was prepared, the ingots were first hot-extruded into square bars with a cross-section of 15 mm × 15 mm using a YJQ-300 extruder (Wuxi Linlian Machinery Manufacturing Co., Ltd., Jiangsu, China) (Figure 1c). Once the furnace temperature of the YJQ-300 extruder reached and stabilized at 300 °C, the extrusion was carried out at a steady speed of 2–3 mm/s. After extrusion, the square bars were cooled to room temperature and then cut into 15 mm × 15 mm × 100 mm sections to fit the ECAP die channel and facilitate the subsequent ECAP process.
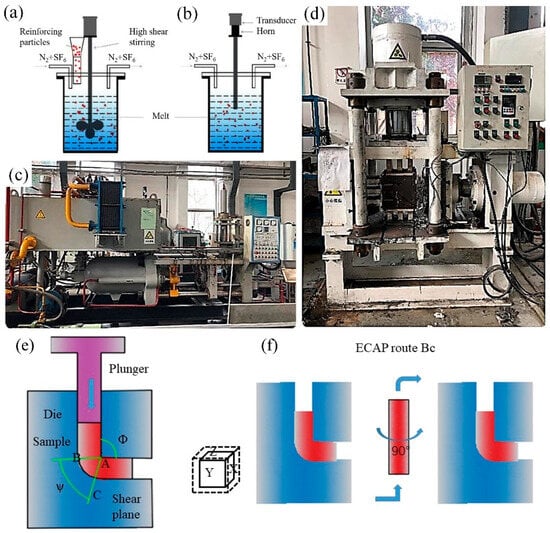
Figure 1.
Experimental equipment: (a) High-shear stirring, (b) Ultrasonic treatment, (c) YJQ-300 hot extruding machine, (d) ECAP machine, (e) ECAP machining principle and (f) ECAP route Bc.
2.3. ECAP Deformation of the Composite Material
A graphite-based alcohol lubricant was evenly applied to the surface of the pre-prepared sample and the die channel. The ECAP machine was heated to the specified temperature (300 °C), which was maintained for 30 min. The sample was placed into the die channel, and after both the sample and the die channel were maintained at that temperature for another 30 min, the ECAP processing was carried out according to the specified parameters. The extrusion path followed the Bc path, meaning that the sample was rotated 90° in the same direction before each subsequent pass.
2.4. ECAP Procedure
The die channel angles of the ECAP extruder were 90° and 120°. The selected route was (Figure 1f) as the passing channel for extruding the 1st, 4th and 8th passes after being kept in the ECAP mold at 300 °C for 15 min. The route was rotated 90° counterclockwise after each pass. Ignoring the friction between the sample and the channel, the accumulated equivalent strain values () of each of these processes were calculated by Equation (1), proposed by Iwahashi et al. [21].
where N represents the number of passes, φ the die channel angle, and ψ the corner angle. Through this formula, it can be found that when the angle is 90°, the total deformation strain is more significant than when it is 120°.
2.5. Microstructure Characterization
After ECAP, the cross-section microstructure of specimens in the extrusion direction (ED) was investigated. The specimens were mechanically polished to 5000# using SiC abrasive papers, and the etchant was composed of 2.75 g picric acid, 45 mL ethanol, 2.5 mL acetic acid, and 5 mL distilled water. A scanning electron microscope (SEM, Quanta FEG 250, FEI Co., Ltd., Oregon, OR, USA), and its energy-dispersive spectrometer (EDS) were relied on for microstructural observations. An X-ray diffractometer (Rigaku Ultima IV, Rigaku Corporation Co., Ltd., Tokyo, Japan) was used to scan in the range of 20°–80° at a speed of 8°·min−1, adopting a Cu target, a 9KW rotating anode target, and a working current of 40 kv, 150 mA. Electron back-scattered diffraction (EBSD) was used to detect textures in materials for further analyzing the extruded and ECAP-ed samples. Mechanical grinding and polishing methods were processed on the obtained specimens for the EBSD testing, and further ion polishing was performed using an ion etching machine (Leica RES101, Leica Microsystems Co., Ltd., Wetzlar, Germany). Owing to the difference in grain size, the original extruded and ECAP-ed specimens were measured with different magnifications for EBSD, and the Channel 5 software was relied on for data analysis.
2.6. Mechanical Test
Compression samples with dimensions of Φ4 mm × 8 mm were cut from the central region of the composite material using wire electrical discharge machining (Taizhou Hengtian Mechanical Manufacture Co., Ltd., Taizhou, Jiangsu, China) along the extrusion direction (ED). The mechanical test was conducted according to the GB/T 7314–2005 standard [22]. The two ends and the outer surface of the samples were polished to remove the oxide layer before testing. The samples were then positioned precisely on the compression platform of an OOL10 universal testing machine. No lubricant was applied between the sample and the compression tools. The test was terminated when the sample exhibited evident damage. Compression tests were conducted at a strain rate of 0.5 × 10−3 s−1 at room temperature, and the corresponding data were recorded to generate compression stress–strain curves.
2.7. Electrochemical Measurements
The size of the specimens for electrochemical testing was 5mm × 5mm × 3mm. To make the specimens reach the equilibrium potential, the specimens were placed in a constant-temperature simulated body fluid (SBF) at 37 °C for 30 min, then subjected to a traditional three-electrode system consisting of a platinum electrode, a saturated calomel electrode (SCE) and the specimen. The exposed surface of the specimen was 0.25 mm2, ground using silicon papers up to 3000#. Tests of potentiodynamic polarization were conducted at 37 °C on an electrochemical workstation (Zennium, Kronach, Germany) by exerting a potential from the self-corrosion of −500 mV to +500 mV at a scan rate of 1 mV/s.
3. Results and Discussion
3.1. Microstructure Analysis
The SEM of the as-extruded and ECAP-ed 0.6 wt.% MgO/Mg–3Zn–0.2Ca composite is shown in Figure 2, and the specific grain size is shown in Table 1. The average grain size and standard deviation were obtained through EBSD analysis. Figure 2a shows that the grain-size distribution of the as-extruded specimen is not homogenized, presenting the distribution of large grains around small grains. In the ECAP-ed specimens, the distribution of the microstructure was more uniform, and the grains evolved into equiaxial crystals. The significant changes in microstructure can be explained by the following aspects. The processing temperature is kept at 300 °C to reach the recrystallization temperature of Mg alloy during the ECAP process and the deformed grains recover and recrystallize, resulting in grain refinement. Nevertheless, the specimens are also subjected to strain along with the recrystallization process, such that the mechanical shearing effect breaks coarse grains into refined ones. Mechanical actions, such as shear stress concentration during extrusion, induce lattice twisting at grain boundaries, leading to the formation of high-angle grain boundaries and grain refinement. Dislocation accumulation and cross-slip contribute to a banded structure. As stress increases, sub-grain boundaries form and migrate, further promoting the development of high-angle grain boundaries and finer grains. Magnesium alloys have a low stacking fault energy (SFE), making it difficult for dislocations on slip planes to rearrange through climb and cross-slip. As a result, during hot deformation, the dynamic recovery process in magnesium alloys is relatively slow, causing local dislocation density to increase with strain, which consequently promotes the initiation of dynamic recrystallization [23,24].
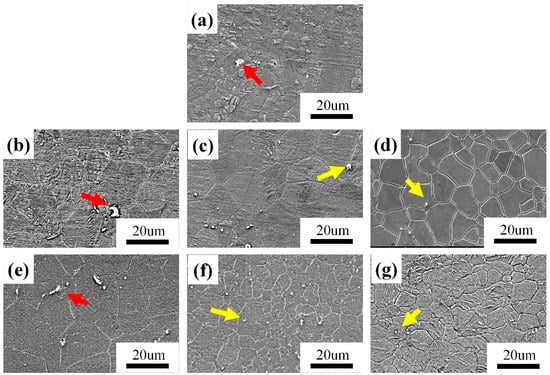
Figure 2.
SEM of 0.6wt.% MgO/Mg–3Zn–0.2Ca composite material. (a) As-extruded, (b) 1-pass ECAP at 120°, (c) 4-pass ECAP at 120°, (d) 8-pass ECAP at 120°, (e) 1-pass ECAP at 90°, (f) 4-pass ECAP at 90° and (g) 8-pass ECAP at 90°.

Table 1.
The average grain size of as-extruded and ECAP-ed specimens (um).
There are a large number of coarse grains in the extruded specimen, while the coarse grains are basically eliminated in the ECAP specimens. The specimens in the same pass of ECAP using different die channel angles exhibited different grain sizes. The specimens after ECAP when φ1 = 90° exhibited finer grains than the specimens after ECAP when φ2 = 120°, indicating that a larger amount of deformation occurred in the ECAP specimens at 90°; the higher strain led to more deformation energy being stored during ECAP, which promotes the process of recrystallization. Agwa et al. [25], using finite element (FE) simulation analysis, reported that enormous equivalent plastic strains occurred in the right-angle channel rather than in the obtuse-angle channel, which was consistent with the current experimental results. The grain size cannot invariably be decreased with an increased number of passes; when the number of passes reaches four, the grain size is in a stable state, and the dynamic recrystallization process achieves a balance. A larger strain in the ECAP-ed specimens results in a higher nucleation rate and finer grains. During the processing, a highly localized lattice misorientation around the particles can be caused in the deformation zone of the nano-Mgo particles, which can be used as an effective nucleation site for the newly recrystallized grains, achieving particle-stimulated nucleation (PSN) and MgO particles; a heterogeneous nucleating agent has a fixed effect on dislocations and grain boundaries during dynamic recrystallization, so adding MgO nanoparticles is beneficial to the grain refinement.
The flocculent second phase (as indicated by the red arrow) appears in the grain boundary, and the spherical second phase (as indicated by the yellow arrow) appears inside the grains, as shown in Figure 2. Table 2 shows the EDS result of the four-pass ECAP-ed specimen marked in Figure 3c. Through energy spectrum analysis and the XRD phase analysis results, it can be seen that the atomic ratio Mg:Ca:Zn is close to 6:2:3; therefore, it can be considered that the second phase at the grain boundary (A and B) are Ca2Mg6Zn3 molecules. Similarly, the flocculent second phases (C and D) are marked as MgZn compounds. The white particles at position E are identified as MgO, and it can be seen that the atomic percentage of Mg and O elements in Table 2 is not 1, because the size of the MgO particles is so small that the Mg matrix will be scanned in point scanning, resulting in a higher content of the Mg element.

Table 2.
EDS result of the 4-pass ECAP-ed specimen marked in Figure 3.
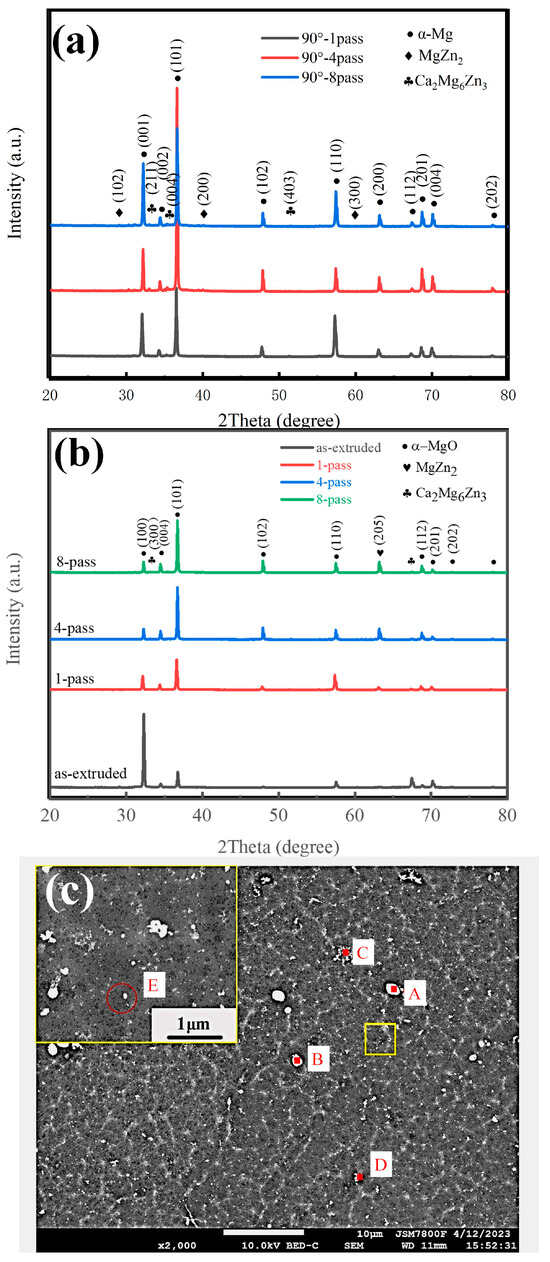
Figure 3.
XRD (a,b) of as-extruded composite 1-, 4-, 8-pass ECAP at 120° and 1-, 4-, 8-pass ECAP at 90°, and (c) SEM of 4-pass ECAP at 120°.
The banded and spherical second phases are larger and haphazardly distributed in the as-extruded specimen, which is related to the precipitation behavior during the casting process. After one-pass ECAP-ing, the distribution had a significant improvement; the refining effect of ECAP on the second phase was noticeable, and the refining effect was continuously improving, accompanying the increase in number of passes. The dimensions of the second phase, like the grain size, came into balance after four passes; therefore, it could be inferred that the sample after four-pass ECAP treatments could obtain better performance, and the increase in number of ECAP passes had little effect on its improvement. MgO particles exhibited pinning effects on dislocations and grain boundary movement during dynamic recrystallization, which also confirmed that MgO nanoparticles were an effective heterogeneous nucleating agent.
The as-extruded and ECAP-ed specimen XRD patterns are presented in Figure 3a,b. The as-extruded and ECAP-ed specimens show the same phase peaks: α-Mg matrix, MgZn2, and Ca2Mg6Zn3, indicating that the ECAP process can change the distribution of second phases but does not initiate a phase transition during ECAP [26]. MgO is not detected in the XRD results due to the low content, i.e., only 0.6 wt.%, which is below the measurable range of the instrument. The orientation of the second phase is also changed, such that the (004) orientation peak intensity of Ca2Mg6Zn3 increases, but the (643) orientation peak intensity decreases as the number of ECAP passes increases, as shown in Figure 3a. The intensity of (100) and (201) cylindrical orientation peaks of α-Mg decreases, while the intensity of (101), (102), and (103) peaks of basal and conical orientation increases, which will have a certain impact on the mechanical properties of the material, since greater basal slip and pyramidal slip can effectively improve the plasticity of the material. At φ1 = 90°, the (001) and (101) peak intensities of α-Mg in the composites are significantly improved, and new orientations of MgZn2 and Ca2Mg6Zn3 phases appear. This is because changing the channel angle generates higher strain, which facilitates the dynamic precipitation of the second phase.
Figure 4 shows the fraction content of grain type of the specimens. The as-extruded specimen exhibits a typical deformed structure; the fraction of recrystallized grain is just 7.4%, and most of the grains are substructure and deformed grains; the scale of substructure and deformed grains is close to 1:1, while in the ECAP-ed specimen, after eight passes at 120°, the recrystallized grains increase to 60.3%, and the fraction of deformed grains is 5.0%, which indicates an improvement in recrystallization. However, the grains have completed recrystallization in the ECAP-ed specimen after eight passes at 90°, almost no deformed grains are left, and the recrystallized grain is up to 71.5%. The main difference in eight-pass ECAP-ed specimens at different angles lies in the proportion of recrystallized grains and subgrains. In addition, the proportion of recrystallized grains in samples subjected to ECAP at 90° is higher because the ECAP-ed specimen at 90° obtains greater heterogeneity of the distribution of the stored energy in neighboring crystallites, which acts as a driving force for recrystallization during the recrystallization process, promoting its recrystallization behavior.
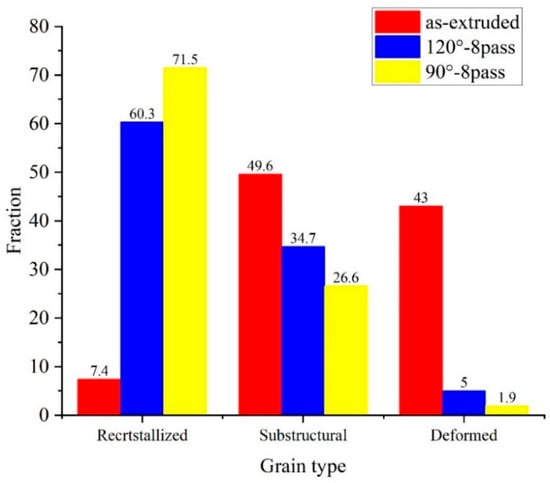
Figure 4.
Grain type of different ECAP-ed specimens.
Figure 5 presents the pole figures of the as-extruded and eight-pass ECAP-ed 0.6 wt.% MgO composite. The fabric texture {0001} <1010> in the as-extruded specimen indicates that the {0001} basal plane and <10–10> direction of most grains are parallel to the extrusion direction (ED), as shown in Figure 5a. The strong basal plane orientation of the coarse grains explains that the pole density of the extruded state (12.49) multiple of random distribution (MRD) is higher than that of the ECAP-ed specimens, indicating that the basal texture decreases after ECAP. The prismatic surface slip is the major deformation mechanism due to the equivalent axisymmetric compressive stress of extrusion and the axial tensile stress, while the {0001} basis plane is parallel to the c-axis direction, and {10–12} tensile twinning occurs when the plane is compressed or stretched along the c-axis direction. The research shows the {0001} basal texture is decomposed into {10–10} and {2-1–10} textures with the increase in the extent of compressive deformation, resulting in texture softening, which can decrease the yield strength [27]. Figure 5c presents the pole diagram of the composite after eight-pass ECAP. After the specimen is processed by eight-pass ECAP at 90°, it is subjected to strong shear force from passing through the included angle, and the basal plane slip system is activated to form a {11–20} <0001> texture; that is, the (0001) basal plane has a vertical oblique texture in the theoretical shear plane. The strong point in the pole figure is rotated about 70° counterclockwise around the ED direction, deviating from the orientation position of the basal plane and significantly weakening the basal plane texture. It is known that the rigid rotation of the lattice is 45° during the ECAP deformation at φ1 = 90°; however, the rotation of the lattice after eight passes is significantly larger than this angle, indicating the rotation of the lattice is not limited to the rotation of the crystal. In the early stage of deformation, strong shear deformation occurred during the ECAP process, and the coarse grains were broken to form cell blocks which rotated to match the strain between adjacent cell blocks to form a new texture. The grains had been refined, and the texture evolution in the grains was mainly formed by the rotation of the whole grains in the later stage of deformation, thereby gradually forming the ECAP shear texture.
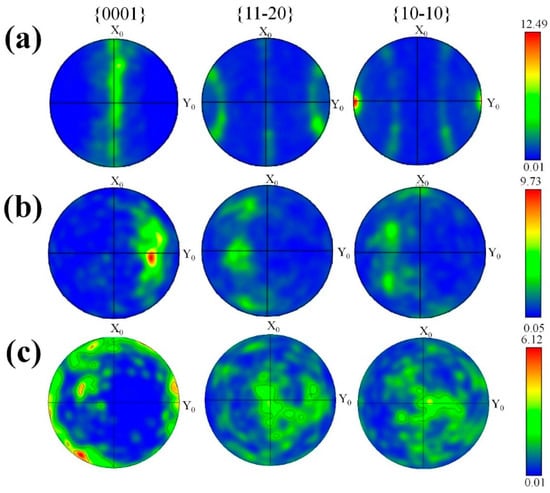
Figure 5.
Pole figures of composite. (a) As-extruded, (b) 8-pass ECAP at 120°, and (c) 8-pass ECAP at 90°.
Figure 6 presents the Kernel average misorientation (KAM) and local misorientation of the as-extruded and ECAP-ed specimens. The KAM method is used for Geometrically Necessary Dislocation (GND) of composite materials under different processes, using Equation (2) to calculate the GND density in different regions; the relation between KAM and GND is shown in Equation (2) [28].
where presents the GND density, presents the weighted average 4 of the local misorientation, μ presents the unit length of 75 nm, b presents the Burgers vector and B = 2.6 × 1015 m−2. The in as-extruded ECAP-ed specimens of eight passes at 120°and 90° are 0.071, 0.097, and 0.034, respectively, and the GND in as-extruded eight-pass ECAP-ed specimens at 120° and 90° is 2.522 × 1014 m−2, 1.846 × 1014 m−2, and 8.840 × 1013 m−2 [20].
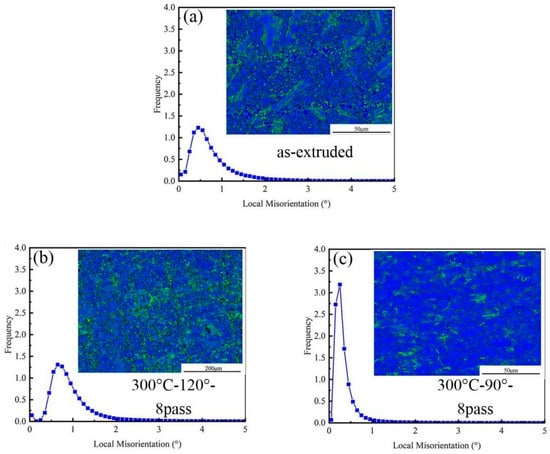
Figure 6.
Kernel average misorientation and local misorientation of specimens. (a) As-extruded, (b) 8-pass ECAP at 120°, and (c) 8-pass ECAP at 90°.
In Figure 6, green represents the local misorientation, which is caused by lattice distortion resulting from large deformations. In Figure 6a, the grain structure shows a higher content of green lines, which is due to the large strain experienced by the alloy during the hot extrusion process, leading to dislocation accumulation. The GND density of the eight-pass ECAP specimen at 120° in Figure 6b is high, indicating that after ECAP processing, the matrix generates a large number of dislocations. Under unidirectional compressive stress, dislocations form dislocation pile-ups during their movement, leading to higher stress concentrations around the matrix and precipitate particles. The activation of new dislocations increases the stress on the second-phase particles, ultimately causing microcracks due to the fracture of the second-phase particles and matrix. In Figure 6c, the grain structure is predominantly covered in blue, with less green, indicating that reducing the die angle results in a decrease in the GND density of the composite material. This is because, during ECAP deformation, recrystallization occurs within the 0.6 wt.% MgO/Mg–3Zn–0.2Ca composite material. The recrystallization process leads to a reduction in dislocation density, which can absorb a large number of dislocations and hinder strain hardening during deformation. This is the main reason for the observed strain softening and improved formability. As the die angle decreases, the recrystallized grain size reduces, and most of the dislocations generated during alloy deformation accumulate at the grain boundaries. Due to the presence of non-equilibrium grain boundaries, dislocations accumulated at the grain boundaries are more easily absorbed, triggering dynamic recovery. As a result, the GND density decreases, providing the magnesium alloy with higher stress and strain-hardening rates.
3.2. Mechanical Property
Since the Mg–Zn–Ca alloy under study is primarily intended for biomedical composites, it is likely to experience mainly compressive effects in the human body. Therefore, compression testing can reflect its mechanical behavior in the application environment. The compressive stress–strain curve of the as-extruded and ECAP-ed composite is shown in Figure 7, and the specific values are shown in Table 3. The ability to resist plastic deformation of ECAP-ed specimens was weaker than the as-extruded specimen; the one-pass ECAP-ed specimens at 90° and 120° had the lower ultimate compressive strength (UCS), while gradually rising with the increases in number of passes; the eight-pass ECAP-ed specimens achieved the approximate UCS with the as-extruded specimen. Recrystallization was an important factor in the decrease in UCS in the initial ECAP process. The specimen was subjected to significant deformation during the extrusion process, which caused the work-hardening effect; the formation of textures was the best proof, and the max pole density was 11.29 MRD in the as-extruded specimen. Higher temperatures promoted their recrystallization process, which reduced the orientation trend of the grains, leading to the evolution of their texture and reducing the work hardening when the ECAP process was conducted. It is worth noting that, although the ECAP-ed specimens obtain a similar UCS with the as-extruded specimen, this does not mean that both have the same performance, which mainly depends on their internal structural differences. In the as-extruded specimen, coarse grains are generally considered the source of stress concentration during working, which can cause cracks to initiate at these locations, finally leading to failure. In the ECAP-ed specimens, the stress concentration effect may not be obvious, so when the work hardening is lower than for the as-extruded specimen, the UCS still reaches a similar level because of the refinement and uniform distribution of grains; the performance is considered to be superior to the as-extruded specimen, so the ECAP process is expected to improve its performance without affecting its overall strength. The UCS of the one-pass and four-pass ECAP-ed specimens are at the same level, while the UCS increases after the eight-pass ECAP. Therefore, it can be inferred that dynamic recrystallization is in a balanced state when the number of passes is below four. However, as the number of passes increase, the recrystallization effect is suppressed because the grains are already very small, while the effect of work hardening is enhanced when the deformation rate remains unchanged. In polycrystals with small grain sizes, the non-uniform deformation of adjacent crystallites is not constrained, resulting in variations in stored energy. This variation maintains the driving force for recrystallization, which plays a key role in influencing the work-hardening effect.
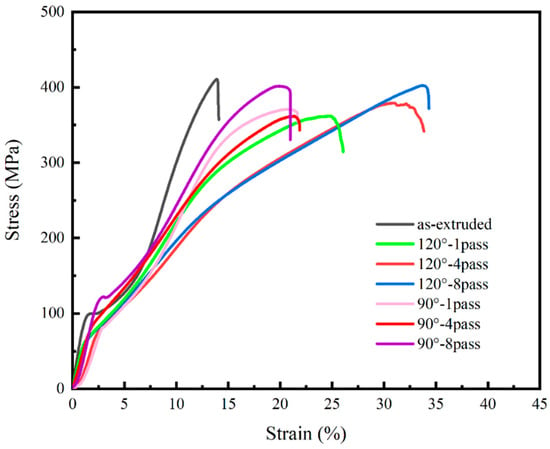
Figure 7.
Compressive stress–strain curve of as-extruded composite and 1-, 4-, 8-pass ECAP at 90° and 120°.

Table 3.
Compressive properties of the specimens.
There is an interesting phenomenon in Figure 7, which is that the yield strength of the alloy processed by high number of ECAP passes has no obvious change, although the grain size is refined. This phenomenon can be explained by the following aspects. After ECAP processing, there are still large grains in the material structure, which is not the complete grain refinement in the strict sense, and the phenomenon of uneven grain size appears. The preferred orientation of texture affects the mechanical properties of magnesium alloys [29]. Studies have shown that the elongation of the extruded magnesium alloy is greatly improved after equal channel angular extrusion, but the strength has not changed significantly, which is consistent with the findings of [17]. The compressive yield strength (CYS) has different performances at 90° and 120° after eight-pass ECAP; in the ECAP-ed specimens at 90°, the CYS increases with the increase in number of passes because of the effect of grain refinement, verified by the Hall–Petch effect. But it has the opposite trend in the ECAP-ed specimen at 120°; overall, it is in a relatively balanced state with a small downward trend, which is related to the texture softening effect; in the ECAP-ed specimen at 120°, the specimen is prone to form a basal fiber texture during the deformation processes, the {0001} basal plane is parallel to the c-axis direction, and {10–12} tensile twinning can occur when compressing or stretching along the c-axis direction. The {0001} basal texture is decomposed into {1010} and {2-1–10} textures with the increase in the degree of compressive deformation, resulting in texture softening, then a reduction in the CYS.
In the ECAP-ed specimens at 90° and 120°, the compressive strain of fracture (CSF) shows a great improvement, reaching 1.5 times and 2.4 times that of the as-extruded specimen, respectively. The improvement of CSF can be attributed to the homogenization of microstructure, grains, and second phases; in the as-extruded specimen, there exist many coarse grains which will act as areas of stress concentration. Meanwhile, the larger-sized phases can also be harmful to its performance; cracks can initiate in areas of stress concentration and spread along coarse grains, leading to a decrease in plasticity. The ECAP processing can refine the grains and phases and decrease the areas which can cause stress concentration. Meanwhile, the inhibiting effect of grain boundaries on crack propagation should not be underestimated. Cracks normally spread along the coarse grains and large phases; the ECAP-ed specimen has more grain boundaries and finer second phases, so the plasticity can be greatly improved by suppressing the propagation of cracks. The texture softening during ECAP can improve the anisotropy of the specimens and CSF. The value of CSF of the eight-pass ECAP-ed specimens at 120° is the highest; the specimens have the proximate grain size with it at 90° but exhibit better plasticity.
As compressive deformation increases, the {0001} basal texture decomposes into {10–10} and {2-1–10} textures, leading to texture softening and reduced yield strength. The weakening effect of the extrusion-induced texture surpasses the strengthening effect of grain refinement, while grain refinement activates more slip systems, enhancing plastic deformation. During early ECAP processing, UCS decreases due to dislocations and defects introduced in the composite. As ECAP continues, dislocation accumulation reaches a critical level, hindering movement and making deformation more difficult. With more ECAP passes, the second-phase size decreases, reducing crack initiation and improving strength. A smaller channel angle decreases plasticity due to increased strain and work hardening. The high yield strength results from grain refinement, second-phase and texture strengthening. According to the Hall–Petch relationship, smaller grain size increases yield strength, while the Orowan mechanism suggests second-phase dispersion contributes to strength. In magnesium alloys, basal slip dominates at room temperature [30]. A strong basal texture inhibits basal slip activation, raising yield strength. Additionally, MgO and other second-phase particles at grain boundaries exert a pinning effect, effectively hindering dislocation motion and grain boundary sliding, thereby enhancing the composite’s strength.
Figure 8 presents the Schmid factors of as-extruded and eight-pass ECAP-ed specimens. Different colors in the figure represent different deformation capabilities of the grains, and the difficulty of opening the slip in different colors in the figure represent different deformation capability of the grains. In the as-extruded specimen, the distribution of the Schmid factor has a large deviation, indicating a large difference in grain orientation, which explains the reason for the low plasticity of the as-extruded specimen. The texture softening effect and the recrystallization process make the distribution of the Schmid factor of the ECAP specimens finer, and that of the eight-pass ECAP-ed specimen at 120° has a smooth trend; the higher area is greater than 0.3, which is preferred to a soft orientation which undergoes greater deformation and stores more energy [18].
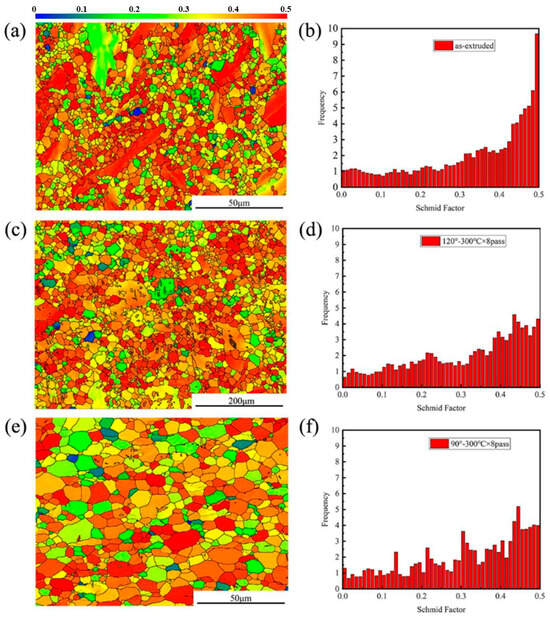
Figure 8.
Schmid factor of different specimens. (a,b) As-extruded, (c,d) 8-pass ECAP at 120° and (e,f) 8-pass ECAP at 90°.
3.3. Corrosion Resistance
The dynamic potential polarization curves of composites, corrosion potential (), and corrosion current density () are shown in Figure 9 and Figure 10, respectively; is the cathode Tafel slope, and is the anode Tafel slope in Figure 10. The corrosion resistance of the prepared innovative magnesium alloy was reflected by dynamic potential polarization curves and impedance spectroscopy to explore the effect of microstructure on corrosion resistance. Table 4 shows the result of corrosion potential (Ecorr), corrosion current (Icorr), cathode Tafel slope (bc), and anode Tafel slope (ba); the Tafel slope represents the blocking of the electrode process. Impedance spectroscopy, as shown in Figure 11, reflects the effectiveness of the passivation film formed on the surface. The radius of the capacitive loop is affiliated with the surface film resistance (R1); the small capacitive reactance loop represents the charge transfer resistance (R2), while CPE1 and CPE2 are the constant phase elements of the charge transfer on the surface film, as shown in Figure 11c.
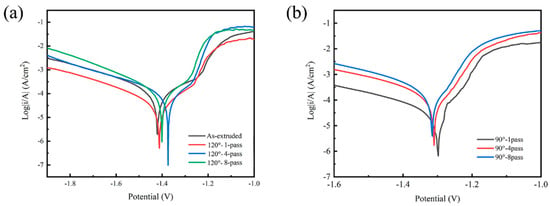
Figure 9.
Dynamic potential polarization curves of composites. (a) As-extruded and 1-, 4-, 8-pass ECAP at 120°, and (b) 1-, 4-, 8-pass ECAP at 90°.
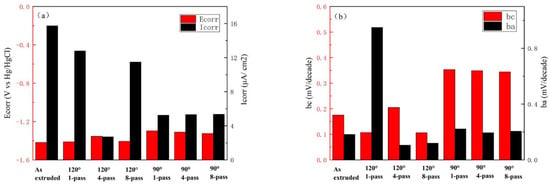
Figure 10.
Electrochemical parameters for different specimens in SBF solution. (a) Corrosion potential and corrosion current density of extruded and ECAP-ed specimens, and (b) cathode Tafel slope and anode Tafel slope of extruded and ECAP-ed specimens.

Table 4.
Electrochemical parameters for different specimens in SBF solution.
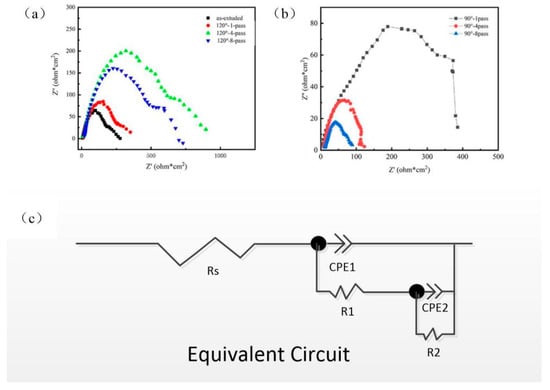
Figure 11.
Impedance spectroscopy of composites. (a) As-extruded and 1,4,8-pass ECAP at 120°; and (b) 1,4,8-pass ECAP at 90°; (c) Equivalent circuit model of specimens under corrosion in SBF Solution.
The corrosion potential of the as-extruded specimens is the lowest, but the corrosion current density is the highest, indicating that the as-extruded specimens have the largest corrosion rate. The factors that affect corrosion performance are related to the size of the second phase, which is usually considered the main reason for causing potential differences and accelerating the corrosion process. Larger second phases will exacerbate the pitting process, and the distribution state of the second phase will also affect the corrosion performance; the specimens which have an interrupted distribution of the second phase have better corrosion resistance. The electrochemical corrosion between the second phase and the substrate will be separated independently; because the presence of grain boundaries prevents the diffusion of corrosion and allows it to exist in a small area without affecting its overall corrosion performance, the continuous distribution of the second phase leads to intensified intergranular corrosion, and the corrosion effect will intensify. Moreover, the strengthening effect of grain boundaries will suppress corrosion performance, as the size of corrosive ions decreases. The more grain boundaries there are, the stronger the ability to inhibit the propagation of these corrosive ions; therefore, as the number of grain boundaries increases, the corrosion resistance improves. The ECAP-ed specimens of 90° display the lower and higher , and the ECAP-ed sample of 90°-1-pass has the best corrosion resistance. With a greater strain created by adjusting the mold angle, the reduced significantly, attributing to a decline in the dimension of the second phase and a multiplication grain boundary density during ECAP. From the dynamic potential polarization curves, the corrosion resistance of ECAP-ed specimens was improved by increasing strain to refine grain size; when specimens are of the same grain size, it is necessary to analyze the inner structures.
Figure 11 presents the impedance spectroscopy of these specimens. The capacitive behavior is large, owing to the charge transfer process of Mg → Mg2+ at the double layer formed by the surface film, while the capacitance loop is small because of the relaxation of the corrosion product film formed on the alloy surface; the resistance of this oxide film is low due to its porous and partially protective properties [31]. The composite materials have capacitive arcs in the high-frequency region and the low-frequency region, indicating that local corrosion occurs in the electrochemical corrosion process. The AC impedance spectrum shows that the capacitor arc processed by ECAP tends to continue to spread, indicating that the constant temperature capacitor arc tends to continue to spread, indicating that the constant temperature ECAP processing has not broken and does not destroy the uniform and slow corrosion of the substrate. Interface bonding between MgO and the matrix has a significant impact on corrosion initiation, while grain homogenization slows down the potential difference between large and small grains, which can explain the phenomenon of improving corrosion resistance and reducing the corrosion rate. Larger-size agglomerates in the extruded state and good interfacial bonding will not cause damage to the matrix at the initiation of corrosion. Dynamic recovery and recrystallization processes homogenize the structure and reduce the susceptibility of corrosion before ECAP, and the cathode current density of the composites after ECAP is the lowest, indicating that the ECAP process improves the cathode reaction resistance. Generally speaking, the bulk deposits act as a cathode during the corrosion process, causing more severe corrosion.
The MgO particles in the ECAP samples are finer and more uniform due to the mechanical shearing, which creates the uniform structure of the second phase without lumps, which improves the corrosion resistance, making the corrosion surface protective film denser and complete, accelerates the passivation speed of the magnesium alloy, reduces the secondary corrosion, and thus reduces the corrosion rate. During the corrosion process of the 0.6 wt.% MgO/Mg–3Zn–0.2Ca composite, the grain boundaries can serve as the cathodic protection matrix and generate Mg(OH)2, which can form a protective film that improves the corrosion resistance. But the Cl- existing in humans can damage the Mg(OH)2 protective film, causing localized pitting corrosion, and grain refinement will bring more flaws. The as-extruded specimen has many refined grains, whose microstructure is the most inhomogeneous, and the volume fraction of the second phase is the largest; therefore, the corrosion resistance of the as-extruded specimen is the worst [32].
4. Conclusions
The microstructure, mechanical properties, and corrosion resistance of a 0.6wt.% MgO/Mg3Zn0.2Ca composite treated with ECAP (1-, 4-, 8-pass) with die channel angles of 90° and 120° were studied and compared with that of the extruded state. The main results are as follows:
1. The microstructure homogenization of the composite material is affected by the ECAP process significantly; the function of mechanical shear and recovery recrystallization during ECAP can not only refine the grains but also homogenize the second phases. Due to the higher total deformation strain of the ECAP-ed specimen at the die channel angle of 90°, the grain size and second phases are more uniform than those at 120°, and the proportion of recrystallized grains is higher, which causes the dislocation density to be lower than that at 120°. After the ECAP, the {10–10} cylindrical texture strength of the ECAP-ed specimen decreases, yet the {0001} basal texture and {11–20} pyramidal texture are enhanced.
2. The results show that the {0001} basal texture is decomposed into {10–10} and {2-1–10} textures with the increase in compression deformation degree, resulting in texture softening, thus reducing the yield strength. Grain refinement can activate more slip systems, thereby improving the plastic deformation ability of the material. In addition, the size of the second phase decreases, which reduces the possibility of cracking at the second phase and increases the strength with the increase in number of passes.
3. The electrochemical measurement results show that the corrosion resistance of the ECAP-ed sample is stronger due to grain refinement and homogenization; however, the corrosion resistance decreases due to the defects caused by ECAP with the increase in number of passes. The experimental results indicate that the ECAP-ed sample of 90°-1-pass has the best corrosion resistance.
Author Contributions
Conceptualization, X.Z., J.H., J.T. and L.Z.; methodology, L.Z.; software, J.T.; validation, P.Z., Y.W., and Z.J.; formal analysis, Y.W. and Z.J.; Investigation, P.Z. and Z.J.; resources, X.Z. and J.T.; data curation, J.H. and L.Z.; writing—original draft preparation, X.Z. and J.T.; writing—review and editing, J.H. and L.Z.; visualization, Y.W. and Z.J.; supervision, Y.W. and Z.J.; project administration, J.H. and L.Z.; funding acquisition, J.H., J.T. and Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Special Fund for Frontier Technology Research/Advanced Materials of Suzhou. Fund No.: SYG202353.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, D.; Zhang, D.; Yuan, Q.; Liu, L.; Li, H.; Xiong, L.; Guo, X.; Yan, Y.; Yu, K.; Dai, Y.; et al. In Vitro and in Vivo Assessment of the Effect of Biodegradable Magnesium Alloys on Osteogenesis. Acta Biomater. 2022, 141, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, D.; Nazarov, A.; Shishkunova, M.; Asfandiyarov, R.; Raab, A.; Sementeeva, Y. Bulk Ultrasonic Treatment of Magnesium and Mg-Zn-Zr Alloy Subjected to ECAP. J. Alloys Compd. 2025, 1014, 178632. [Google Scholar] [CrossRef]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on Magnesium and Magnesium-Based Alloys as Biomaterials for Bone Immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar]
- Wang, X.J.; Nie, K.B.; Sa, X.J.; Hu, X.S.; Wu, K.; Zheng, M.Y. Microstructure and Mechanical Properties of SiCp/MgZnCa Composites Fabricated by Stir Casting. Mater. Sci. Eng. A 2012, 534, 60–67. [Google Scholar] [CrossRef]
- Tang, C.; Lyu, S.; Zhao, Z.; Chen, M. Effects of MgO Nano Particles on the Mechanical Properties and Corrosion Behavior of Mg–Zn–Ca Alloy. Mater. Chem. Phys. 2023, 297, 127380. [Google Scholar] [CrossRef]
- Goh, C.S.; Gupta, M.; Wei, J.; Lee, L.C. Characterization of High Performance Mg/MgO Nanocomposites. J. Compos. Mater. 2007, 41, 2325–2335. [Google Scholar] [CrossRef]
- Han, W.Z.; Zhang, Z.F.; Wu, S.D.; Li, S.X. Investigation on the Geometrical Aspect of Deformation during Equal-Channel Angular Pressing by in-Situ Physical Modeling Experiments. Mater. Sci. Eng. A 2008, 476, 224–229. [Google Scholar] [CrossRef]
- Luis-Pérez, C.J.; Luri-Irigoyen, R.; Gastón-Ochoa, D. Finite Element Modelling of an Al-Mn Alloy by Equal Channel Angular Extrusion (ECAE). J. Mater. Process. Technol. 2004, 153–154, 846–852. [Google Scholar] [CrossRef]
- Horky, J.; Bryła, K.; Krystian, M.; Mozdzen, G.; Mingler, B.; Sajti, L. Improving Mechanical Properties of Lean Mg–Zn–Ca Alloy for Absorbable Implants via Double Equal Channel Angular Pressing (D-ECAP). Mater. Sci. Eng. A 2021, 826, 142002. [Google Scholar] [CrossRef]
- Li, J.; He, T.; Du, X.Y.; Vereschaka, A.; Zhang, J.J. Regulating Hardness Homogeneity and Corrosion Resistance of Al-Zn-Mg-Cu Alloy via ECAP Combined with Inter-Pass Aging. Mater. Charact. 2024, 218, 114489. [Google Scholar] [CrossRef]
- Gazizov, M.R.; Mironov, S.Y.; Holmestad, R.; Gazizova, M.Y.; Kaibyshev, R.O. Effect of ECAP and Aging on Microstructure of an Al-Cu-Mg-Si Alloy. Mater. Charact. 2024, 218, 114500. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Wang, C.; Sun, J.; Bai, J.; Xue, F.; Jiang, J.; Ma, A. Potential of Multi-Pass ECAP on Improving the Mechanical Properties of a High-Calcium-Content Mg-Al-Ca-Mn Alloy. J. Magnes. Alloys 2019, 7, 617–627. [Google Scholar] [CrossRef]
- Cabibbo, M.; Santecchia, E.; Mengucci, P.; Bellezze, T.; Viceré, A. The Role of Cryogenic Dipping Prior to ECAP in the Microstructure, Secondary-Phase Precipitation, Mechanical Properties and Corrosion Resistance of AA6012 (Al-Mg-Si-Pb). Mater. Sci. Eng. A 2018, 716, 107–119. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, D.; Wu, X.; Yin, K.; Yan, K. Drag Effects of Solute and Second Phase Distributions on the Grain Growth Kinetics of Pre-Extruded Mg-6Zn Alloy. J. Mater. Sci. Technol. 2016, 32, 1260–1266. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Langdon, T.G. Analysis of the Creep Behavior of Fine-Grained AZ31 Magnesium Alloy. Mater. Sci. Eng. A 2020, 787, 139489. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, A.; Xu, B.; Jiang, J.; Sun, J. Corrosion Behavior of AZ91 Mg Alloy with a Heterogeneous Structure Produced by ECAP. Corros. Sci. 2021, 187, 109517. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Beyerlein, I.J.; Zhilyaev, A.P.; Langdon, T.G. Evolution of Texture in a Magnesium Alloy Processed by ECAP through Dies with Different Angles. Mater. Sci. Eng. A 2010, 527, 1709–1718. [Google Scholar] [CrossRef]
- Tong, L.B.; Zheng, M.Y.; Hu, X.S.; Wu, K.; Xu, S.W.; Kamado, S.; Kojima, Y. Influence of ECAP Routes on Microstructure and Mechanical Properties of Mg-Zn-Ca Alloy. Mater. Sci. Eng. A 2010, 527, 4250–4256. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Xin, Y.; Cai, Y.; Han, J. Effect of Equal Channel Angular Pressing on Microstructure and Mechanical Performance of Innovative Nano MgO-Added Mg-Zn-Ca Composite as a Biomaterial. Mater. Lett. 2021, 304, 130604. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Song, J.; Liu, Z.; Chen, M.; Han, J.; Jiang, Z. Effect of ECAP on Corrosion Behavior of Innovative Nano MgO/Mg-Zn-Ca Composite as a Biomedical Material in Simulated Body Environment. Mater. Today Commun. 2023, 35, 106345. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Horita, Z.; Nemoto, M.; Langdon, T.G. An Investigation of Microstructural Evolution during Equal-Channel Angular Pressing. Acta Mater. 1997, 45, 4733–4741. [Google Scholar] [CrossRef]
- GB/T 7314–2005; Metallic materials-Compresion testing at ambient temperature. National Standardization Administration: Beijing, China, 2005.
- Khani, S.; Aboutalebi, M.R.; Salehi, M.T.; Samim, H.R.; Palkowski, H. Microstructural Development during Equal Channel Angular Pressing of As-Cast AZ91 Alloy. Mater. Sci. Eng. A 2016, 678, 44–56. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y. Dynamic Recrystallization Mechanism, Texture Evolution Development and Mechanical Characteristics of a Mg–8.7Gd–4.18Y–0.42Zr Magnesium Alloy by ECAP. Prog. Nat. Sci. Mater. Int. 2024, 34, 376–388. [Google Scholar] [CrossRef]
- Agwa, M.A.; Ali, M.N.; Al-Shorbagy, A.E. Optimum Processing Parameters for Equal Channel Angular Pressing. Mech. Mater. 2016, 100, 1–11. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, F.; Ye, P.; Su, Y.; Long, M. A Bimodal Grain Structured Al-Mg-Sc-Zr Alloy with Excellent Strength and Ductility by the Combination of Hot Rolling and Thermal-ECAP. Mater. Today Commun. 2025, 43, 111632. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Ma, M.; Li, X.; Shi, G.; Yuan, J.; Chen, D.; Zhang, K. Study on the Effect of the ECAP Deformation on the Organization and Properties of the Extruded Mg-Sn-Al Alloys. Mater. Lett. 2024, 357, 135775. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Ponge, D.; Demir, E.; Raabe, D. Orientation Gradients and Geometrically Necessary Dislocations in Ultrafine Grained Dual-Phase Steels Studied by 2D and 3D EBSD. Mater. Sci. Eng. A 2010, 527, 2738–2746. [Google Scholar] [CrossRef]
- Paudel, Y.R.; Indeck, J.; Hazeli, K.; Priddy, M.W.; Inal, K.; Rhee, H.; Barrett, C.D.; Whittington, W.R.; Limmer, K.R.; El Kadiri, H. Characterization and Modeling of {101¯2} Twin Banding in Magnesium. Acta Mater. 2020, 183, 438–451. [Google Scholar] [CrossRef]
- Wang, Y.; Choo, H. Influence of Texture on Hall-Petch Relationships in an Mg Alloy. Acta Mater. 2014, 81, 83–97. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, Q.X.; Chen, G.Q.; Cao, X.; Zhang, S.; Pan, J.L.; Zhang, G.; Shi, Q.Y. Enhanced Corrosion Resistance of AZ91 Magnesium Alloy through Refinement and Homogenization of Surface Microstructure by Friction Stir Processing. Corros. Sci. 2018, 138, 284–296. [Google Scholar] [CrossRef]
- Cubides, Y.; Ivan Karayan, A.; Vaughan, M.W.; Karaman, I.; Castaneda, H. Enhanced Mechanical Properties and Corrosion Resistance of a Fine-Grained Mg-9Al-1Zn Alloy: The Role of Bimodal Grain Structure and β-Mg17Al12 Precipitates. Materialia 2020, 13, 100840. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).