Vacuum Distillation-Assisted Hydrometallurgical Route for Industrial Production of 99.999% Pure Gold from Au–Ag Alloys Feedstocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Methods
2.2. Experimental Principle and Equipment
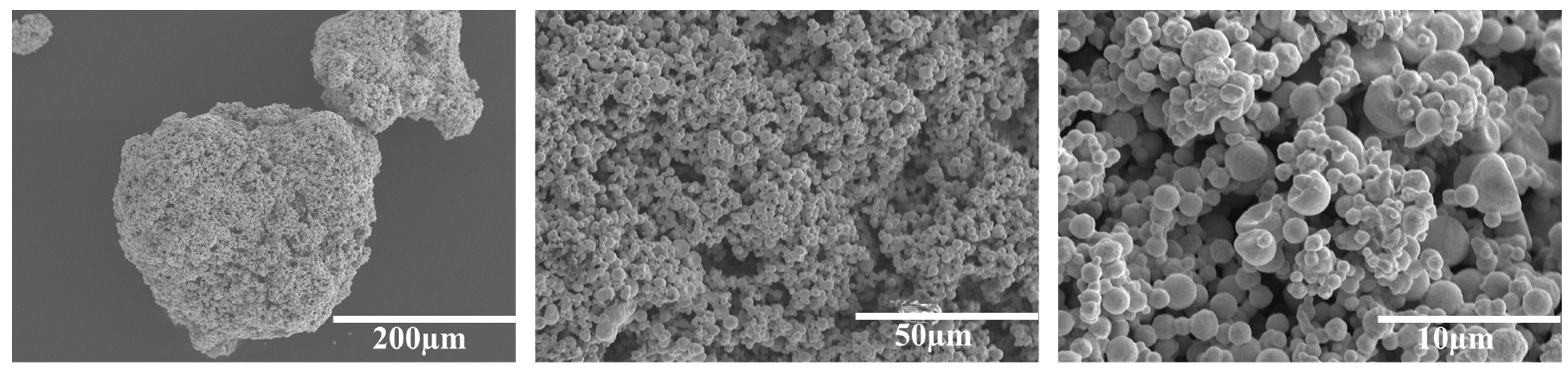
2.3. Characterization
3. Results and Discussion
3.1. Ag Removal During Vacuum Distillation
3.2. Optimization of Process Parameters for Aqua Regia Dissolution of Gold
3.2.1. Effect of Liquid-to-Solid Ratio on Au Leaching
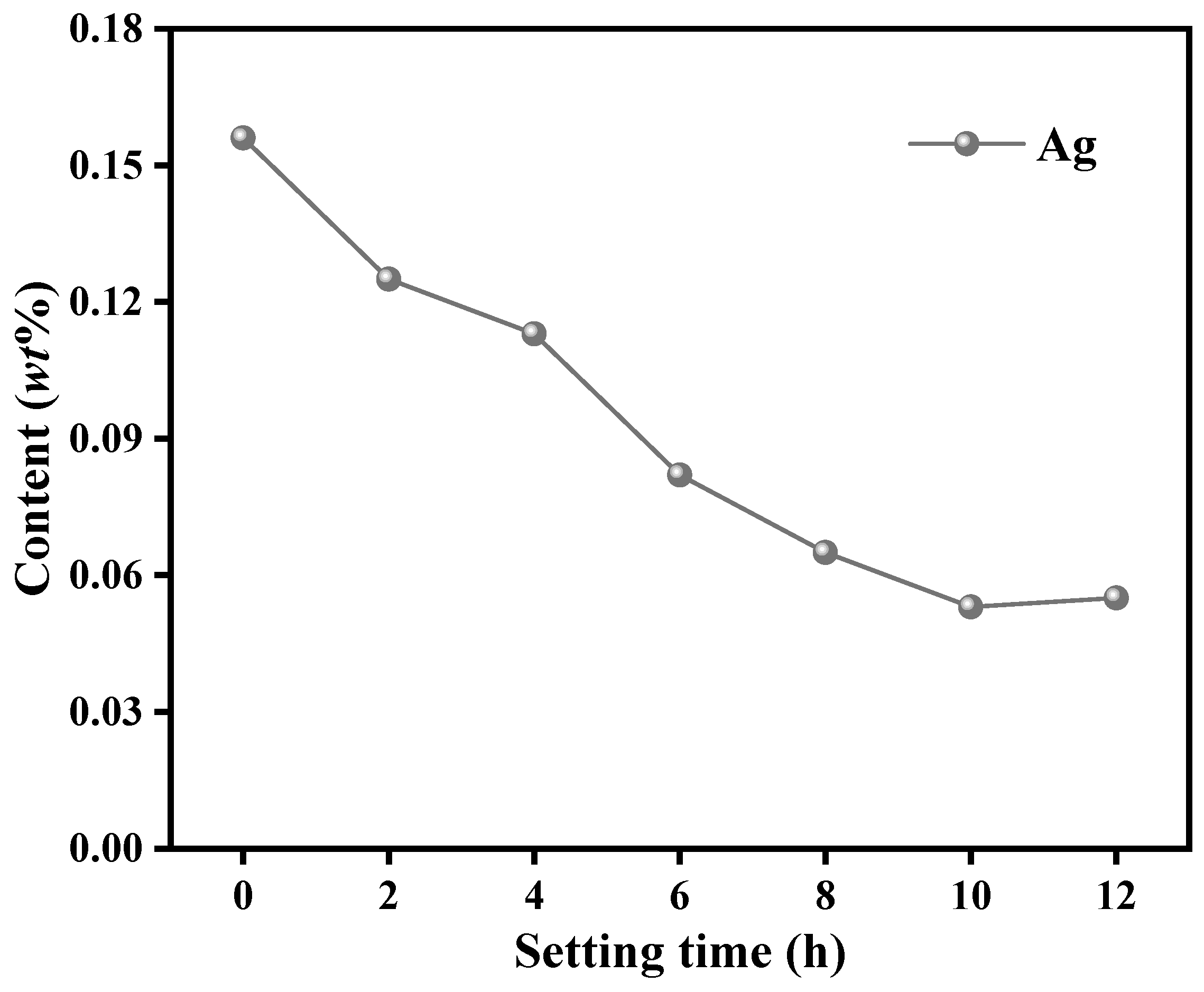
3.2.2. Effect of Liquid–Solid Ratio on Settling Time in Ag Removal
3.3. Optimization of Solvent Extraction Process
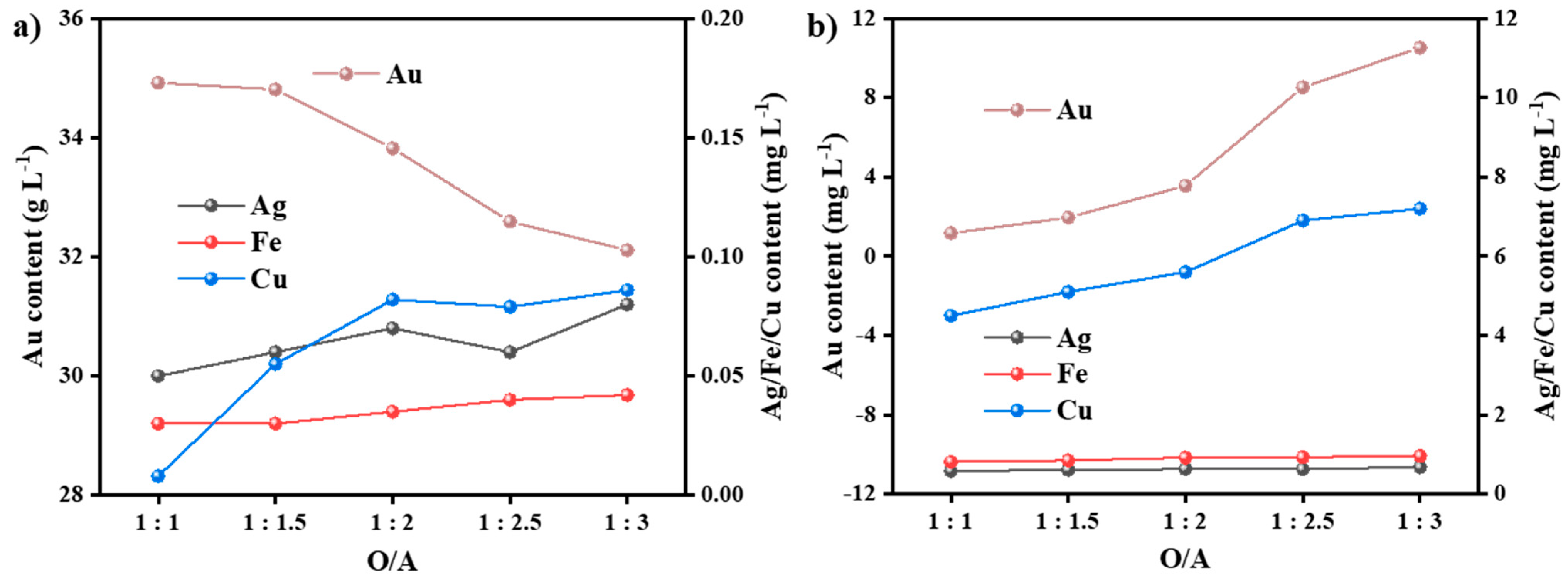
3.3.1. Effect of Phase Ratio (O/A)
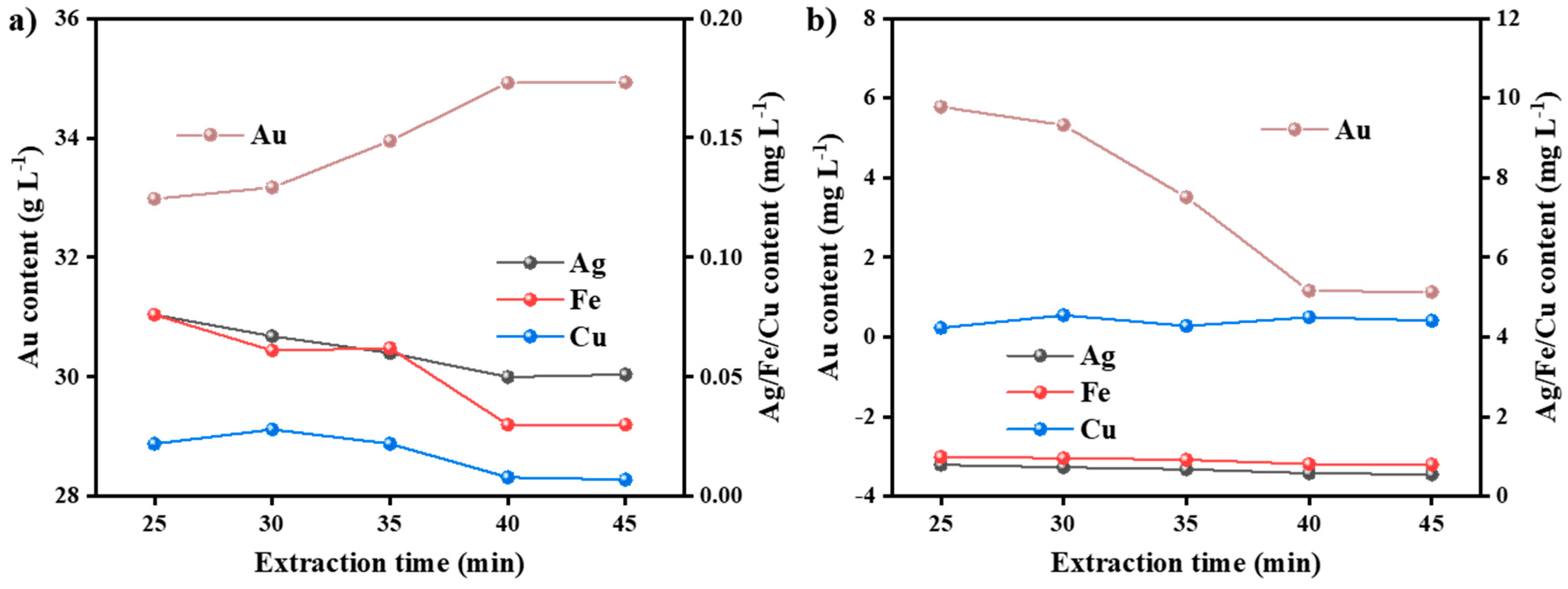
3.3.2. Effect of Extraction Time
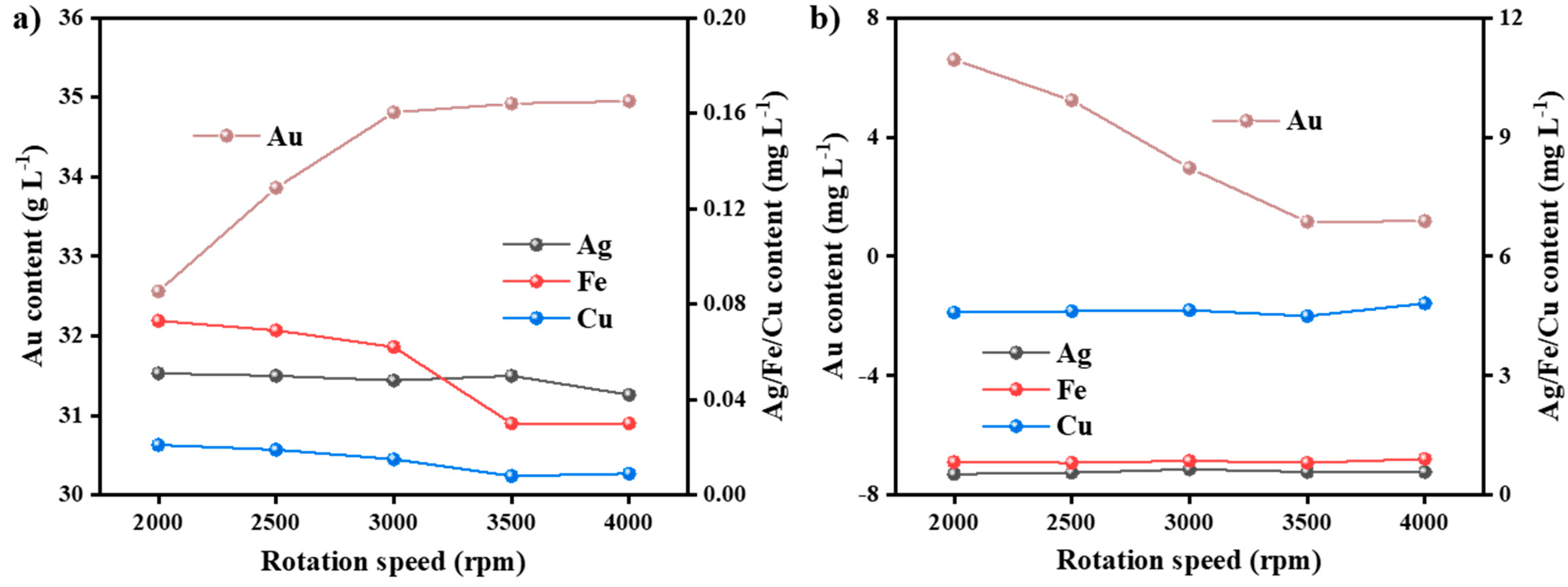
3.3.3. Impact of Rotational Speed
3.4. Optimization of Reverse Extraction and Reduction Process Parameters
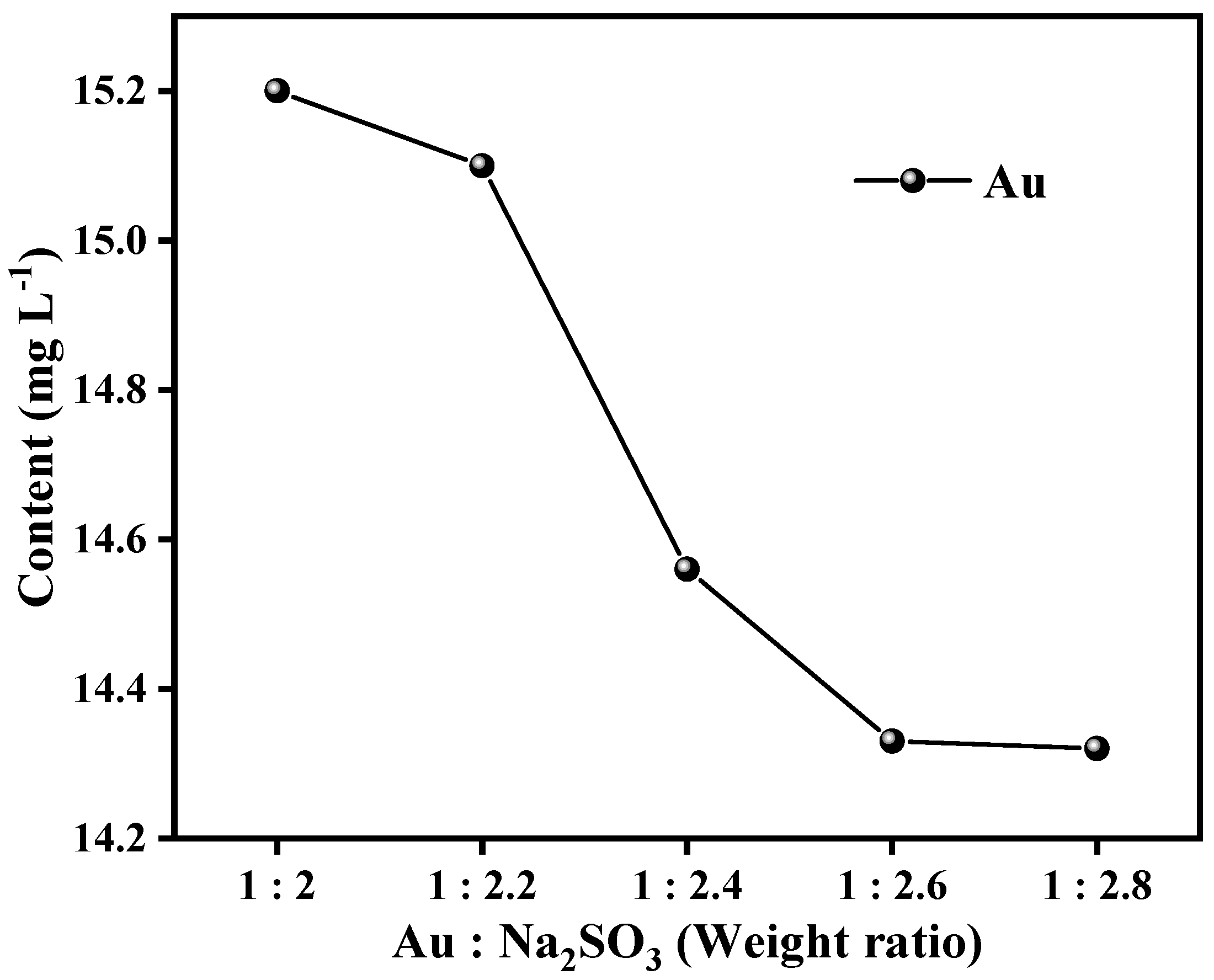
3.4.1. Effect of Na2SO3 Dosage
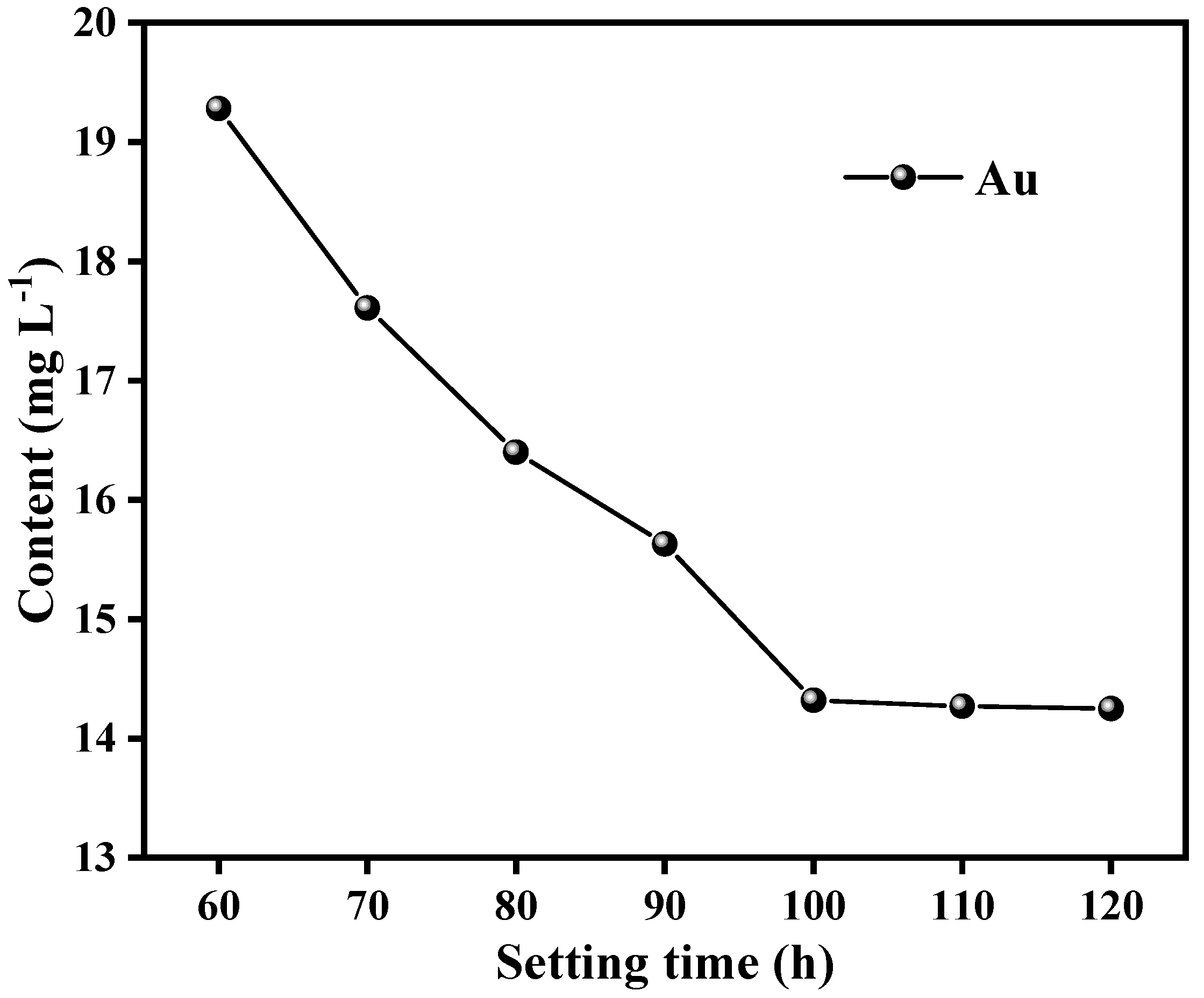
3.4.2. Effect of Stripping Extraction/Reduction Time
3.4.3. Analysis of Removal of Typical Impurity Elements
3.5. Comparative Evaluation of Proposed Process with Current Refining Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coetzee, L.L.; Theron, S.J.; Martin, G.J.; Merwe, J.-D.V.D.; Stanek, T.A. Modern Gold Deportments and Its Application to Industry. Miner. Eng. 2011, 24, 565–575. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Moradi Khaniabadi, P.; Jameel, M.S.; Oladzadabbasabadi, N.; Mohammed, S.A.; Abdullah, R.S.; Mehrdel, B. Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine. Int. J. Mol. Sci. 2022, 23, 7400. [Google Scholar] [CrossRef]
- Jha, M.K.; Gupta, D.; Lee, J.; Kumar, V.; Jeong, J. Solvent Extraction of Platinum Using Amine Based Extractants in Different Solutions: A Review. Hydrometallurgy 2014, 142, 60–69. [Google Scholar] [CrossRef]
- Yamada, M.; Rajiv Gandhi, M.; Kaneta, Y.; Kimura, N.; Katagiri, H. Thiodiphenol-Based n-Dialkylamino Extractants for Selective Platinum Group Metal Separation from Automotive Catalysts. Ind. Eng. Chem. Res. 2018, 57, 1361–1369. [Google Scholar] [CrossRef]
- GB/T 25933-2010; High Purity Gold. Standardization Administration of China: Beijing, China, 2010.
- Guo, Z.; Fan, X.; Liu, L.; Bian, Z.; Gu, C.; Zhang, Y.; Gu, N.; Yang, D.; Zhang, J. Achieving high-purity colloidal gold nanoprisms and their application as biosensing platforms. J. Colloid Interface Sci. 2010, 348, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Neto, I.F.; Soares, H.M. Simple and near-zero-waste processing for recycling gold at a high purity level from waste printed circuit boards. Waste Manag. 2021, 135, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Birloaga, I.; Vegliò, F. An Innovative Hybrid Hydrometallurgical Approach for Precious Metals Recovery from Secondary Resources. J. Environ. Manag. 2022, 307, 114567. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Wang, S.; Yang, Y.; Xin, X. Equilibrium, Thermodynamics and Kinetics Study on Au(III) Extraction by Gemini Surfactant with Different Spacer Length. Sep. Sci. Technol. 2019, 54, 985–995. [Google Scholar] [CrossRef]
- Kondo, K.; Sawada, M.; Matsumoto, M. Adsorption and Separation of Palladium and Platinum with Microcapsules Containing Tri-n-Octylamine Hydrochloride. J. Water Process Eng. 2014, 1, 115–120. [Google Scholar] [CrossRef]
- Panda, R.; Dinkar, O.S.; Jha, M.K.; Pathak, D.D. Recycling of Gold from Waste Electronic Components of Devices. Korean J. Chem. Eng. 2020, 37, 111–119. [Google Scholar] [CrossRef]
- Kyriakakis, G. Extraction of Gold from Platinum Group Metal (PGM) Ores. In Developments in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 897–917. ISBN 978-0-444-51730-2. [Google Scholar]
- Ji, H.; Guo, S.; Gao, L.; Yang, L.; Yan, H.; Zeng, H. Revolutionizing Titanium Production: A Comprehensive Review of Thermochemical and Molten Salt Electrolysis Processes. Int. J. Miner. Metall. Mater. 2025; accepted manuscript. [Google Scholar]
- Nose, K.; Okabe, T.H. Platinum Group Metals Production. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 751–770. ISBN 978-0-323-85373-6. [Google Scholar]
- Li, W.; Sun, N.; Stoner, B.; Jiang, X.; Lu, X.; Rogers, R.D. Rapid Dissolution of Lignocellulosic Biomass in Ionic Liquids Using Temperatures above the Glass Transition of Lignin. Green Chem. 2011, 13, 2038. [Google Scholar] [CrossRef]
- Armendariz, V.; Herrera, I.; Peralta-Videa, J.R.; Jose-Yacamanet, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanopart. Res. 2004, 6, 377–382. [Google Scholar] [CrossRef]
- Gamez, G.; Gardea-Torresdey, J.L.; Tiemann, K.J.; Parsons, J.; Dokken, K.; Yacaman, M.J. Recovery of gold (III) from multi-elemental solutions by alfalfa biomass. Adv. Environ. Res. 2003, 7, 563–571. [Google Scholar] [CrossRef]
- Reith, F.; Zammit, C.M.; Rogers, S.L.; McPhail, D.C.; Brugger, J. Potential utilisation of micro-organisms in gold processing: A review. Miner. Process. Extr. Metall. 2012, 121, 251–260. [Google Scholar] [CrossRef]
- Sharma, R.; Suhendra, N.F.; Jung, S.H.; Lee, H.I. Gold recovery at ultra-high purity from electronic waste using selective polymeric film. Chem. Eng. J. 2023, 451, 138506. [Google Scholar] [CrossRef]
- Xiong, Q.; Yuan, Z.; Zhang, Y.; Chen, Z.; Wei, K.; Ma, W. A new approach for separating copper, tin, and lead from photo-voltaic ribbon wastes. Sol. Energy 2025, 299, 113810. [Google Scholar] [CrossRef]
- Yi, X.; Qi, Y.; Li, F.; Shu, J.; Sun, Z.; Sun, S.; Chen, M.; Pu, S. Effect of electrolyte reuse on metal recovery from waste CPU slots by slurry electrolysis. Waste Manag. 2019, 95, 370–376. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.; Qiu, H.; He, L.; Zha, G.; Jiang, W.; Xu, B.; Yang, B.; Cao, X. Sustainable strategy for removing Ag impurities from crude gold via a vacuum distillation process to produce a 4N gold product. J. Clean. Prod. 2025, 500, 145248. [Google Scholar] [CrossRef]
- Yi, J.; Zha, G.; Huang, D.; Kong, X.; Yang, B.; Liu, D.; Xu, B. Effective Separation and Recovery of Valuable Metals from High Value-Added Lead Anode Slime by Sustainable Vacuum Distillation. J. Clean. Prod. 2021, 319, 128731. [Google Scholar] [CrossRef]
- YS/T 3027-2017; Chemical Analysis Methods for Crude Gold. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2017.
- Corti, C.W. Recovery and Refining of Gold Jewellery Scraps and Wastes. In The Santa Fe Symposium on Jewelry Manufacturing Technology; Met-Chem Research: Albuquerque, NM, USA, 2002; pp. 1–20. [Google Scholar]
- Mahyapour, H.; Mohammadnejad, S. Optimization of the Operating Parameters in Gold Electro-Refining. Miner. Eng. 2022, 186, 107738. [Google Scholar] [CrossRef]
- Feather, A.; Sole, K.C.; Bryson, L.J. Gold Refining by Solvent Extraction—The Minataur Process. J. S. Afr. Inst. Min. Metall. 1997, 97, 169–173. [Google Scholar]
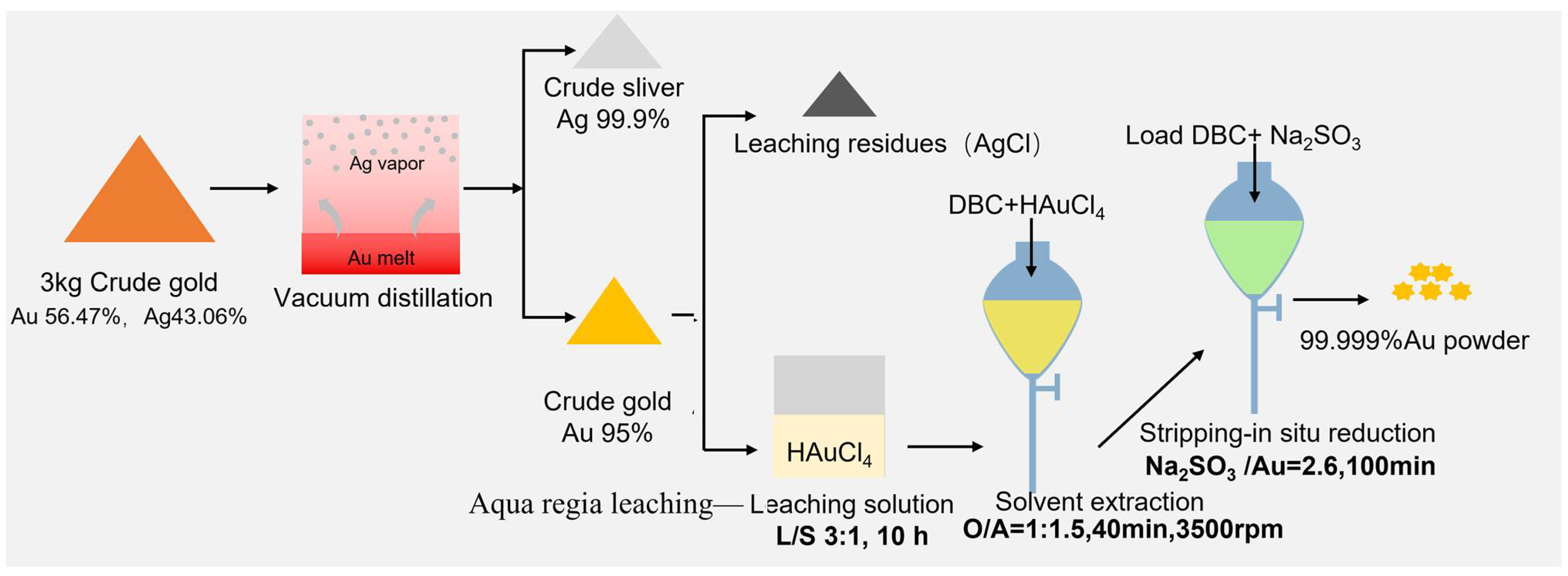
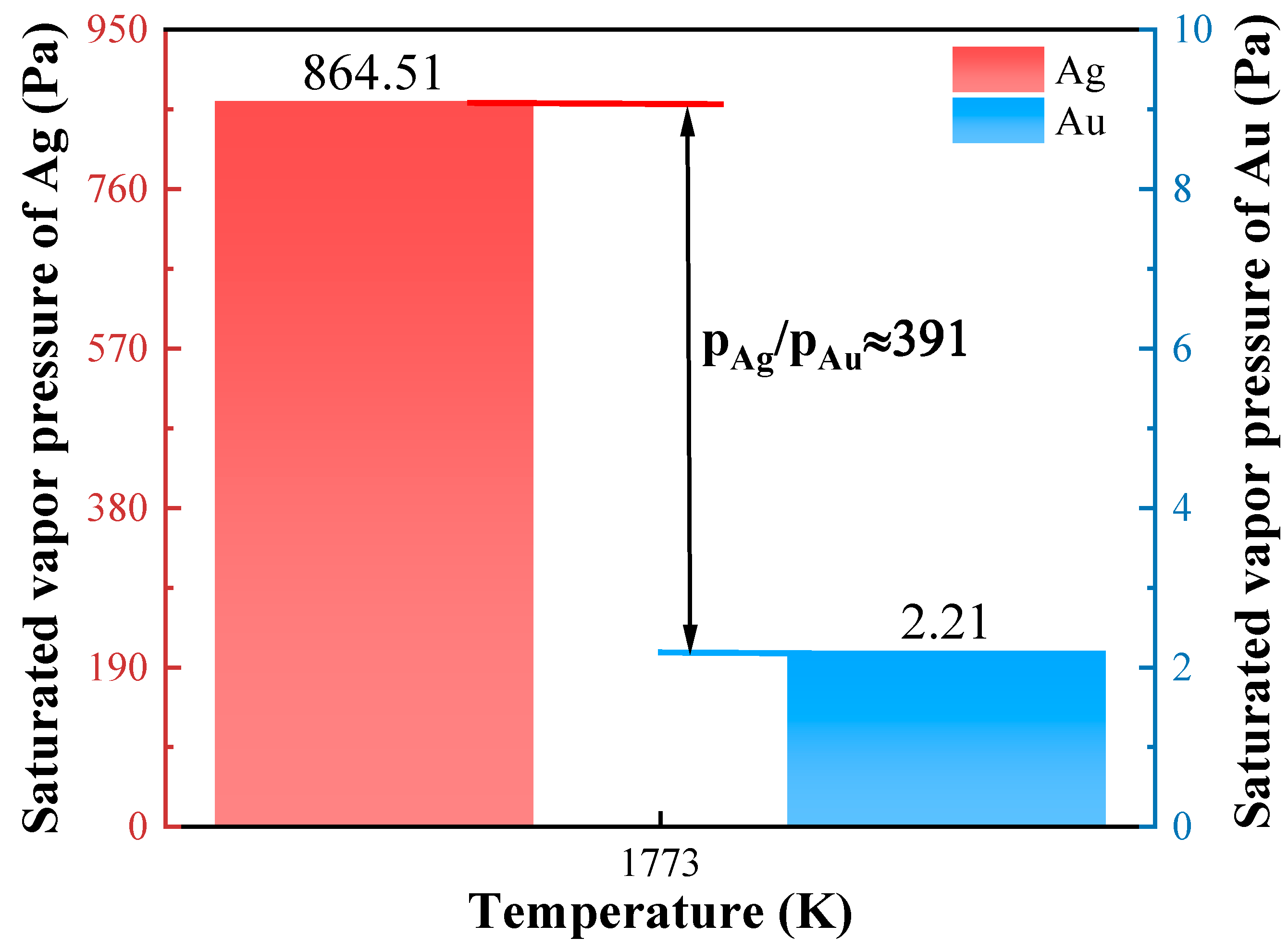
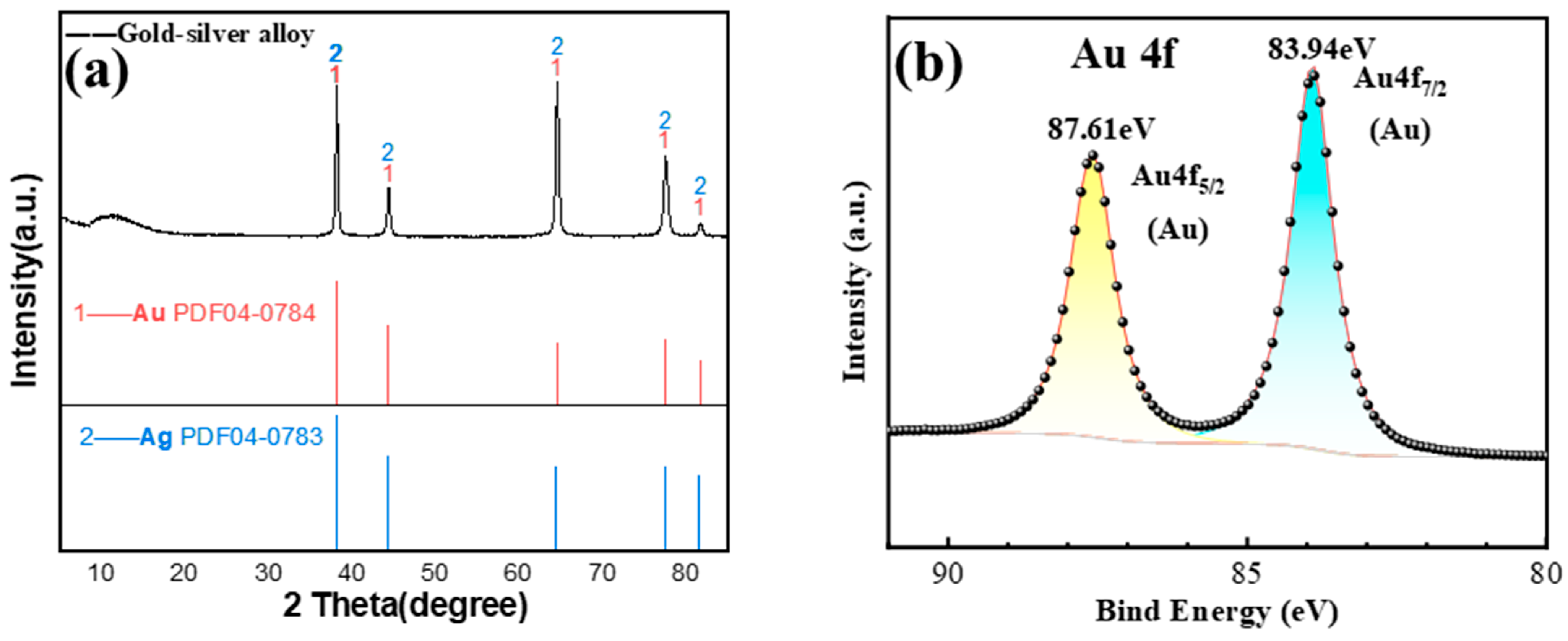
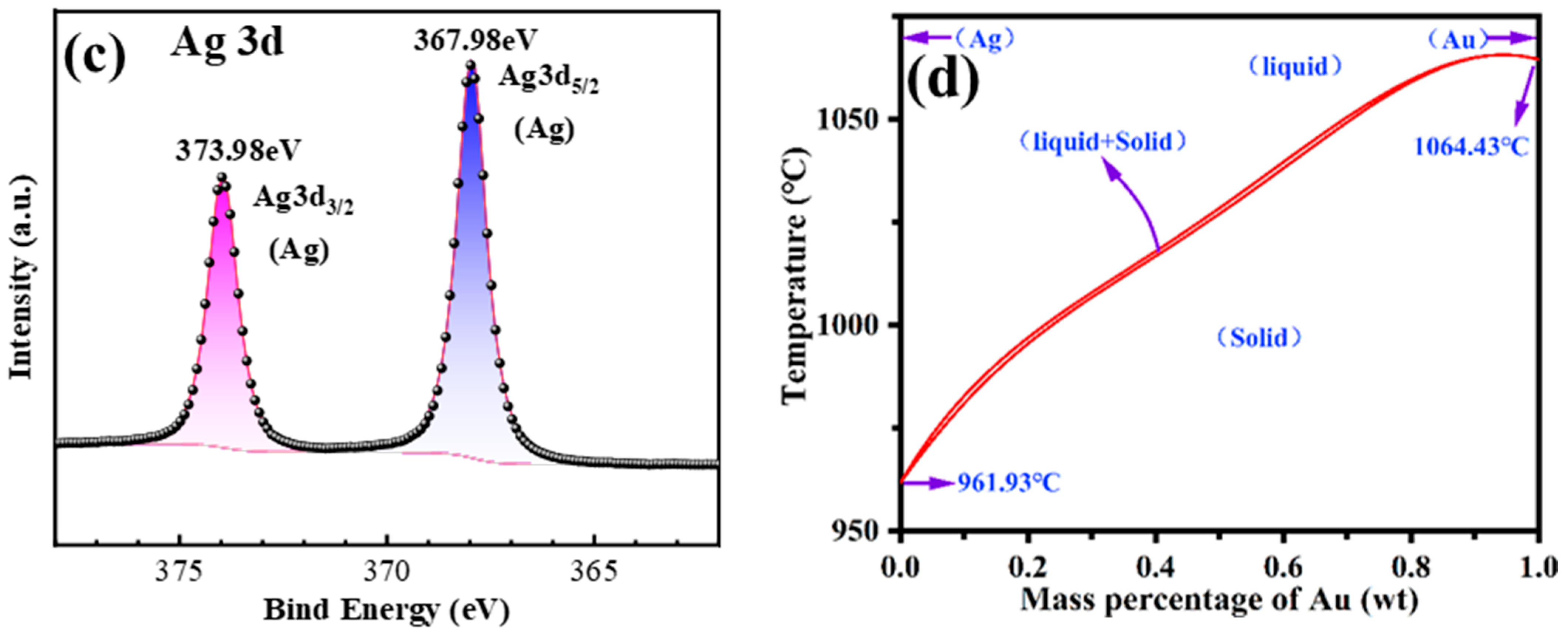
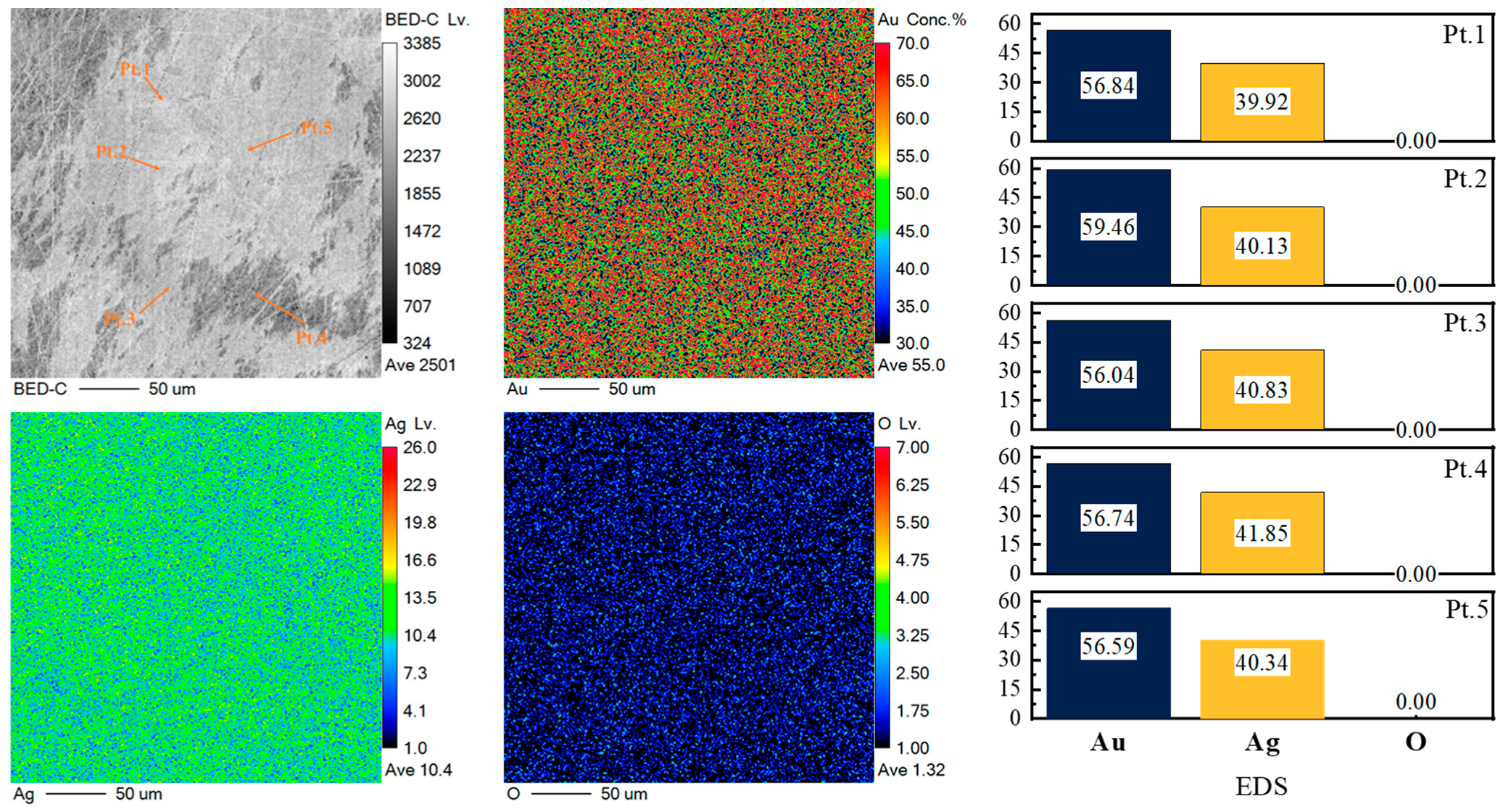
| Sample | Mass (g) | Content (%) | Recovery (%) | ||
|---|---|---|---|---|---|
| Au | Ag | Au | Ag | ||
| Feedstock | 2974.32 | 56.49 | 43.06 | / | / |
| Volatile | 1195.43 | 0.02 | 99.47 | 0.011 | 92.844 |
| Residue | 1772.78 | 94.76 | 5.17 | 99.989 | 7.156 |
| Exp. No | Liquid-to-Solid Ratio (%) | Mass of Gold in Filter Residue (g) | Leaching Rate of Gold (%) |
|---|---|---|---|
| 1 | 6:1 | 0.390 | 99.81 |
| 2 | 4:1 | 1.792 | 99.40 |
| 3 | 3:1 | 3.24 | 99.19 |
| 4 | 2:1 | 16.398 | 97.26 |
| Treatment Process | Au | Ag | Cu | Fe | ||||
|---|---|---|---|---|---|---|---|---|
| Product Content (wt%) | Removal Rate (%) | Product Content (wt%) | Removal Rate (%) | Product Content (wt%) | Removal Rate (%) | Product Content (wt%) | Removal Rate (%) | |
| Vacuum distillation | 94.76 | 0.01 | 99.47% | 92.84 | / | / | / | / |
| Aqua regia dissolution | 13.94 | 0.19 | 0.053 | 98.78 | / | 0.00 | / | 0.00 |
| Solvent extraction | 2.63 | 0.14 | 2.52 | 0.00 | 2.30 | 0.00 | 2.19 | 0.00 |
| Stripping—in situ reduction | 99.999 | 0.001 | 0.00014 | 99.99 | 0.00005 | 99.99 | 0.00014 | 99.99 |
| Total recovery rate (%) | 99.67 | 0.0004 | 0.0001 | 0.00006 | ||||
| Category | Content of Impurity Elements (ppm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ag | Fe | Cu | Pb | Ti | Sn | Si | Mg | |
| Gold powder product | 1.4 | 1.4 | 0.5 | <0.5 | <0.2 | <0.2 | <2 | 0.3 |
| AU99999 standard | <2 | <2 | <1 | <1 | <2 | <1 | <2 | <1 |
| As | Bi | Cr | Ni | Mn | Pd | Zn | Sb | |
| Gold powder product | <0.5 | <0.2 | <0.2 | 0.19 | <0.2 | <0.2 | <0.2 | <0.4 |
| AU99999 standard | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Cd | Al | Ir | Pt | Rh | Te | Se | ||
| Gold powder product | <0.2 | <0.5 | <0.6 | <0.2 | <0.2 | <0.2 | <0.02 | |
| AU99999 standard | <1 | <1 | <1 | <1 | <1 | / | / | |
| Method | Main Principle | Raw Material Requirements | Gold Product Purity | Gold Yield | Ref. |
|---|---|---|---|---|---|
| Pyrometallurgy | High-temperature smelting and oxidation | High-grade alloys (>90% Au) | 99.0–99.5% | 95–98% | [25] |
| Chemical Refining | Aqua regia or chlorination dissolution + precipitation | Au > 85%, Ag < 8% | 99.5–99.99% | 65~80% | [22] |
| Electrolytic Refining | Dissolution at anode and Au deposition at cathode | 75.02% Au 15.27% Cu 7.37% Ag | 99.95–99.99% | 98–99% | [26] |
| Aqua regia leaching–solvent extraction | Aqua regia dissolution → Fe removal → acidification → selective extraction of Au with dibutyl carbitol (DBC) → acid scrubbing → ammonia stripping → reduction to gold powder → nitric acid purification | 60–75% Au 4% Ag | >5 N | ~97% | [27] |
| This work | vacuum distillation removes Ag → selective Au dissolution → organic extraction → in situ reduction | Au~56% Ag~43% | >5 N | >99.98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Zha, G. Vacuum Distillation-Assisted Hydrometallurgical Route for Industrial Production of 99.999% Pure Gold from Au–Ag Alloys Feedstocks. Metals 2025, 15, 1271. https://doi.org/10.3390/met15111271
Wu W, Zha G. Vacuum Distillation-Assisted Hydrometallurgical Route for Industrial Production of 99.999% Pure Gold from Au–Ag Alloys Feedstocks. Metals. 2025; 15(11):1271. https://doi.org/10.3390/met15111271
Chicago/Turabian StyleWu, Weihuang, and Guozheng Zha. 2025. "Vacuum Distillation-Assisted Hydrometallurgical Route for Industrial Production of 99.999% Pure Gold from Au–Ag Alloys Feedstocks" Metals 15, no. 11: 1271. https://doi.org/10.3390/met15111271
APA StyleWu, W., & Zha, G. (2025). Vacuum Distillation-Assisted Hydrometallurgical Route for Industrial Production of 99.999% Pure Gold from Au–Ag Alloys Feedstocks. Metals, 15(11), 1271. https://doi.org/10.3390/met15111271





