Abstract
Due to climate change, electromobility and thus lithium-ion batteries are attracting increased interest. With a simultaneous increase in demand for raw materials like Li, Ni, Co, and Mn, their hydrometallurgical recycling is also gaining attention. The associated recoveries must be improved due to EU regulations. In a lab scale, metals are lost to the wrong filter cakes after leaching, cementation, and precipitations. Therefore, this work investigates the question of how many wash steps are suitable after each process step to optimize the recoveries and purity of filter cakes by comparing a reference process and a process with extended washing. The comparison showed that it is possible to recover up to 3.5% of Ni, Co, and Mn by extended washing at each step and in total nearly 100% of Li if wash water is recirculated. An investigation of the substeps of washing demonstrated that single wash steps are able to recover from 0.5% to 3.5% of Ni, Co, and Mn and from 1.6% to 8.7% of Li. The impact of extended washing on purity is shown by the analysis of filter cakes, where the purity of Fe and Al could be improved by 43.0% and for Ni, Co, and Mn by 48.0%. The paper closes with recommendations on how many wash steps are suitable after each process step.
1. Introduction
For several years, alternatives to combustion engines and thus electromobility have become the focus of science, industry, and society due to climate change [1,2]. Along with this, the significance of used lithium-ion batteries (LIBs) is strongly increasing as well [3,4]. In the period from 2010 to 2021, the growth rate amounted to 88%. Additionally, the global trend in electrical vehicles up to 2030 is expected to be exponential [1]. LIBs can be divided into different forms depending on their electrode material as follows: besides lithium cobalt(III) oxide (LCO), lithium nickel cobalt aluminum oxide (NCA), lithium iron phosphate (LFP), and lithium manganese oxide (LMO), lithium nickel manganese cobalt oxide (NMC) in particular is widely used [1,5,6,7,8]. Due to the increasing demand, the demand for the required valuable metals, such as lithium, nickel, cobalt, and manganese, is also rising [9,10,11,12]. With the aim of sustainable resource management and the goal of climate neutrality by 2050, EU regulation inevitably brings recycling into focus [13]. Recycling results in a reduction in waste streams and reduces imports and thus the CO2 footprint through sustainability [14,15]. In hydrometallurgical battery recycling, the valuable elements can be leached out of pretreated battery material (black mass) using different acids like H2SO4 or HCl and subsequent selective precipitations [8,16,17].
In a possible and simplified hydrometallurgical recycling process for LIBs according to Wang & Friedrich, the first step after acid leaching is Cu cementation, where Cu will precipitate with the addition of Fe [18,19,20]. After raising the pH value and oxidizing Fe from Fe2+ to Fe3+ by adding H2O2, Fe and Al will precipitate as hydroxide [21,22]. Following this, by further raising the pH value, Ni, Co, and Mn will precipitate in NMC precipitation [18,23,24]. Li will precipitate as Li2CO3 by crystallization using Na2CO3 in the last step [18,25]. This process can be enhanced by early-stage lithium recovery (ESLR), where Li will be leached out of black mass in a previous step by using deionised water before acid leaching [26,27].
The solids obtained in the different process steps are then separated from the suspension by filtration [28]. The resulting filter cakes cause problems, but at the same time they also have great potential. Due to their spongy structure, especially in the case of hydroxide precipitation, filter cakes retain a lot of solution and therefore unprecipitated ions [21,29,30]. These are missing in the subsequent precipitation steps, which reduces the total recoveries of the process. They also contaminate the filter cake by being retained unintentionally [21]. With the intention to counteract this problem, a filter cake can be washed with deionized water after filtration [28,29,30], but residues have been shown to remain in the filter cake. For this reason, this paper deals with the question of how many wash steps are effective and how much deionized water should be used to wash filter cakes.
Previous investigations regarding the washing of filter cakes are limited and date quite far back. Ruslim et al. examined the influence of important parameters, such as the wash and filtrate rates, on pre-dried filter cakes in an empirical manner. It was shown that a low humidity level at the time of detergent addition leads to poor wash performance due to uneven flow. Higher flow rates improve the moistening of the cake and promote the even distribution of the detergent, which is crucial for effective washing [31]. Huhtanen et al. investigated filtration, pressure, cake washing, and air drying, as well as the capacity of the filtration and purity of the filter cake, to create an empirical model for filter cake washing [32]. Additionally, important known variables and conditions, which affect the wash process, were pointed out [32,33]. Several basic facts of filtration were analyzed by Emmett et al. Main topics in this examination were the influence of the particle size distribution, flocculation, backwashing, solid concentration, and permeability of the filter cake [34]. Similar to this, Noerpel also examined the influence of particle size and pore size, with the addition of the mass flow of the wash liquid on the wash efficiency by washing filter cakes containing contaminated SiO2 particles [29]. M. T. Kuo chose a mathematical approach by deriving a differential equation for the filter cake wash cycle. The created model was validated with experimental results [35]. The investigation by Mocellin et al. is interesting with respect to the aspect of washing methods. By using Mn-containing slack, the filter cake was re-pulped after filtration [36]. Choundhury et al. dealt with the question of how much volume of wash liquid was needed and compared the created method with experimental values [37]. Kinnarinen et al. also considered the amount of wash liquid with the aim of reducing it to a minimum in cases involving washed bauxite [38]. In recent research, Sauer et al. investigated structures of filter cakes and the influence of the top layer in order to obtain information for process optimization [39], while Brückner et al. focused on different starting saturations of filter cakes [40].
Since none of the previous studies dealt with the number of wash steps, this study aims to clarify this question by determining how many wash steps result in an improvement compared to a process with only one wash step.
2. Materials and Methods
The starting material for the experiments was NMC 111 LIB pouch cells. For thermal pretreatment, four discharged pouch cells were pyrolyzed in an electrically conductive pyrolysis furnace at IME RWTH Aachen at a furnace temperature of 650 °C for a duration of 150 min using a N2 protection gas atmosphere. After this, the Al frames of the batteries were removed, while the rest of the cells were shredded in a shredding mill (Pulverisette 25, Fritsch GmbH, Idar-Oberstein, Germany) with an adjusted gap width of 4 mm. The resulting material was sieved using a vibratory sieve shaker (AS 200 control, Retsch GmbH, Haan, Germany). The sieving time was 10 min, and the amplitude was set to 1.5. The smallest sieve had a mesh size of 250 µm to obtain material with a particle size smaller than 250 µm (hereinafter referred to as black mass) for the subsequent process. The composition are shown in Table 1.

Table 1.
Composition of the produced and used black mass with a particle size less than 250 µm.
With the intention to analyze the influence of the amount of wash water, a reference process was conducted with only one wash step after each process step (except after Li2CO3 precipitation). In each wash step, a filter cake was washed with a quantity of 200 mL deionized water. The filtration was carried out with filter paper with a retention area of 2–4 µm (MN 619, 240 mm, Macherey-Nagel GmbH & Co. KG, Düren, Germany) driven by a vacuum filtration at 0.3 bar with a Büchner funnel, suction flask, and vacuum pump (LVS 310 p, ILMVAC GmbH, Ilmenau, Germany). All filtrations were stopped when the drip interval (time from drop to drop) exceeded 60 s. Filter cakes were dried after filtration in a drying oven (UT 6060 Heraeus, Thermo Electron LED GmbH, Langenselbold, Germany) at 105 °C. The filtrates and filter cakes were analyzed by ICP-OES (Spectro ARCOS, Spectro Analytical Instruments GmbH, Kleve, Germany), CIC (Metrohm Ionenchromatographie IC 881 Compact IC Pro, Metrohm Deutschland GmbH & Co. KG, Filderstadt, Germany), and a gas analysis after combustion (Eltra CS 2000, ELTRA GmbH, Haan, Germany) for the elements C, Al, Cu, Ni, Co, Mn, Li, Fe, F, S, P, and Na. While C cannot be analyzed in liquid samples using these methods, O and H cannot be analyzed in solid and liquid samples. Based on previous XRD results, assumptions were made regarding the components to calculate the amount of O.
The analyzed reference process included six process steps, each followed by a filtration and one wash step (see Figure 1). In the first step, which was ESLR, 67.5 g of black mass was leached in 1350 mL H2O at room temperature (RT, 20 °C) for 120 min. Moving on to the filter cake of the subsequent filtration, the Li-poor black mass was leached with 750 mL of 2 M H2SO4 (96%, PanReac AppliChem GmbH, Darmstadt, Germany) at 80 °C for 120 min. Additionally, 37.5 mL of 50 g/L H2O2 (35%, Bernd Kraft GmbH, Den Haag, Netherlands) was added to the leaching process. The filtrate of the next filtration was used for Cu cementation. Therefore, 300 g/L NaOH (pellets, PanReac AppliChem GmbH, Darmstadt, Germany) was pipetted until a pH of 1.15 was reached. Then 5.65 g of Fe powder (99.5%, Merck KGaA, Darmstadt, Germany) was added to precipitate Cu over a reaction time of 30 min at 60 °C by cementation. The resulting filtrate was used in the fourth process step, named Fe-Al precipitation, where the pH was adjusted to 4.2 with a further 300 g/L of NaOH. After adding an additional 10 mL of 50 g/L H2O2, the reaction took place at 60 °C for 30 min. The received filtrate was used for NMC precipitation, where further 300 g/L NaOH was pipetted to reach a pH value of 10.4 at RT. The filtrate was used for Li2CO3 precipitation, which represented the last step of the process. Therefore, the solution was boiled down to 200 mL and 100 mL of 150 g/L Na2CO3 (≥99.5%, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was added using a burette. The reaction took place at 95 °C for a duration of 10 min. ESLR, NMC precipitation, and Li2CO3 precipitation were performed in a beaker on a laboratory stirrer (SLR, Xylem Analytics GmbH, Weilheim, Germany) with an agitator at 200 rpm. Since Li2CO3 precipitation was only partially effective due to excessive residual Li concentrations in the filtrate, it was calculated using the value of NMC filtration as if boiling down the solution. H2SO4 leaching, Cu cementation, and Fe-Al precipitation was carried out in a 1 L double-walled reactor that was heated with a thermostat (Eco Silver, LAUDA DR. R. WOBSER GMBH & CO. KG, Lauda-Königshofen, Germany). The fluids were homogenized using a mechanical stirrer (EUROSTAR 20 digital, IKA-Werke GmbH & Co. KG, Staufen, Germany). Temperature and pH value were set and recorded using an electrode (pH Sensor InPro4260i/SG, Mettler-Toledo GmbH, Gießen, Germany) in combination with a digital setup (developed and built at IME, RWTH Aachen, Germany).
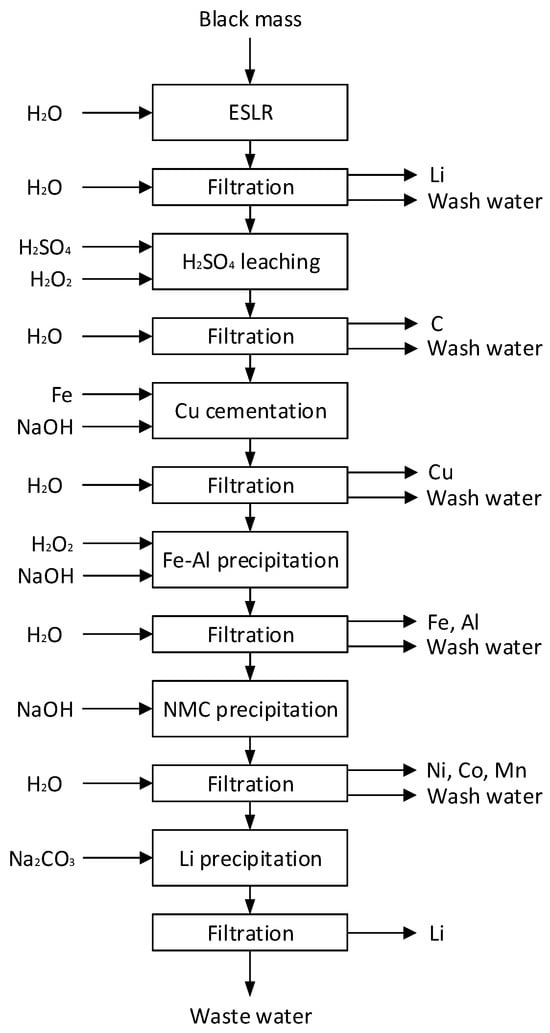
Figure 1.
Flowchart of the hydrometallurgical LIB recycling process, including filtration and wash steps (ESLR: early-stage lithium recovery); the filter cake from each process step is produced by the subsequent filtration; reference process: 1 time 200 mL H2O at each filtration; extended washing: 3 times 200 mL and 2 times 400 mL H2O at each filtration.
After completing the entire reference process above, the same process was carried out again with five wash steps after each process step, named extended washing. Wash step one through three were carried out as described above with 200 mL deionized water, washed from above until the drip interval exceeded 60 s. Due to the long filtration times, especially in hydroxide filtration, the filter cakes developed several cracks. As further washing would only have caused water to run through these cracks, wash steps 4 and 5 were changed. The filter cake was removed from the filter paper in each of these steps and dispersed in 200 mL deionized water for 5 min at 200 rpm. The suspension was filtered and washed with a further 200 mL deionized water, which means that 400 mL water in total was used in each of wash steps 4 and 5. After this, a sample of wash water was taken. In total, three reference experiments and five experiments with extended washing were performed.
The recovery R was calculated by relating the mass m of the respective elements (e) in the filter cakes () to the initial mass of the elements in black mass () as follows:
Only the element masses of the filter cakes where they precipitated were used to calculate the respective recovery. This was ESLR and Li precipitation for Li, H2SO4 leaching for C, Cu cementation for Cu, Fe-Al precipitation for Fe and Al, and NMC precipitation for Ni, Co, and Mn (compare to Figure 1). Since there was initially no Fe in the black mass, the added 5.65 g Fe was used as a reference for the calculation of Fe recoveries instead of 0. Assuming that the wash water is used as the starting liquid for sulfuric acid leaching in the process, the recovery by wash water was determined by the sum of the element masses in the wash water () of every step, divided again by the initial mass of the elements in black mass (see Equation (2)).
In the case of the hypothetical recirculation of the wash water into the process, the mass fraction of an element in the wash water must not be entirely attributed to the recovery. Since losses would still occur in the corresponding filter cake during recycling in subsequent process steps, these must be taken into account and deducted in the calculation. In the example of leaching, the mass of an element in wash water must be adjusted with the losses in Cu cementation, Fe-Al precipitation, and NMC precipitation taken into account by the mass fractions of an element in the respective filter cake to receive the realistic recirculated mass (see Equation (3)). The same applies to Equation (4) for Cu cementation and Equation (5) for Fe-Al precipitation. In case of NMC precipitation, there is no further adjustment necessary (see Equation (6)).
To determine the influence of single wash steps (), these recoveries relate to the mass of an element of the filtrate of the previous process instead of the initial amount in the black mass (see Equation (7)). This should ensure the evaluation of the wash steps regarding the indeed available elements without losses in previous process steps.
The calculation of the wash steps from leaching differs because they occur at the beginning of the process. Since there is no filtrate in use before, the calculation based on the initial mass is as follows:
3. Results and Discussion
This chapter compares the results of the reference process and extended washing regarding recoveries, then focuses on the efficiency of the different substeps of extended washing, and concludes with an examination of purities by comparing the compositions of filter cakes from the reference process and extended washing.
3.1. Comparison of Reference Process and Extended Washing
With the intention of demonstrating the influence of extended washing, the results from the reference process and extended washing are shown here side by side. In Figure 2 the comparison between the recoveries of Li, Ni, Co, and Mn from the reference process (a) and extended washing (b) can be seen. The reference process shows recoveries mainly for Ni (94.27%), Co (94.77%), and Mn (91.17%), with an additional approximately 4% in the single wash water step. In comparison, the main recoveries of Ni, Co, and Mn with extended washing are significantly lower (86.92%, 87.41%, and 80.43%) while more than 7.5% could be recovered by wash water. This directly points to a weakness in the process of extended washing. The high reduction in Ni, Co, and Mn recoveries is due to the increased number of process steps. In every single wash step, losses must be expected. On the other hand, the increased recoveries from wash water demonstrate the potential of this procedure. In the case of Li, the reduction in main recovery is approximately 3% smaller, while the increase in recovery from extended washing is nearly 6% higher. The biggest difference can be seen in recovery from ESLR (from 46.70% to 59.10%). However, it must be assumed that the additional wash water can be regarded as a higher liquid–solid ratio during ESLR because there was nearly twice as much water available (approximately 3 L instead of approximately 1.6 L) to leach Li out of the black mass, resulting in more less soluble LiF being dissolved from it. In summary, the total recovery of Ni, Co, and Mn has slightly decreased, while the total recovery of Li has risen sharply. The result of a total recovery of 101.27% can be attributed to the inhomogeneity of the initial black mass and measurement inaccuracy.
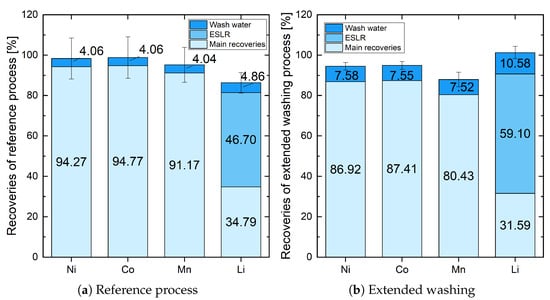
Figure 2.
Comparison of recoveries of Ni, Co, Mn, and Li in the reference process (1 time 200 mL H2O at each filtration) and extended washing (3 times 200 mL and 2 times 400 mL H2O at each filtration).
Figure 3 shows the comparison between the recoveries of Al, Fe, and C in the reference process and extended washing. It is apparent that the recovery of Al is very low in both cases (18.79% and 11.45%). This can be attributed to the insoluble phases in black mass, which were left in the system after H2SO4 leaching, and LiAlO2, which was removed by ESLR. Since only Al in Fe-Al precipitation is relevant for Al recovery, those two were not taken into account. The recovery of Al by wash water is negligible in both cases (less than 0.4%). Fe shows the same behavior as Ni, Co, and Mn. While the main recovery of the reference process was 91.81%, extended washing reduced it to 83.87% due to losses in the additional process steps. The wash steps were unsuccessful in terms of recovery (<0.5%). C had already demonstrated a good recovery in the reference process (95.44%), which slightly decreased due to losses in extended washing (92.80%). The recoveries by wash water could not be determined because the methods of analysis were not able to detect C in liquid solutions. Cu was not evaluated because previous analyses have shown that it precipitated almost completely during Cu cementation. However, the Cu cementation filter cake did not provide enough mass for analysis. In all other filter cakes, the Cu concentration was below the detection limit.
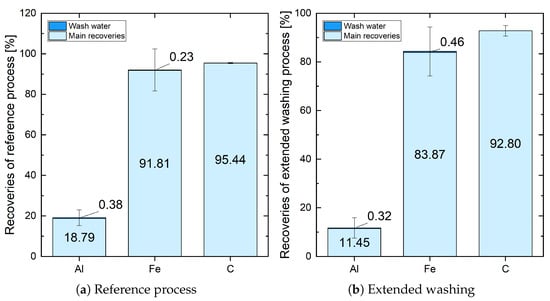
Figure 3.
Comparison of recoveries of Al, Fe, and C in the reference process (1 time 200 mL H2O at each filtration) and extended washing (3 times 200 mL and 2 times 400 mL H2O at each filtration).
3.2. Efficiency of Substeps of Extended Washing
This chapter will take a closer look at the recoveries at different wash steps to clarify how many are recommended. Figure 4 shows that the filtrate had already approx. 47% of Li recovered in ESLR. The first three wash steps added another approx. 2%. Wash steps 4 and 5 resulted in another approx. 4% in each case. Considering that twice the amount of wash water was used in those steps, it can be concluded that this is due to limited solubility of Li and its compounds in water. Nevertheless, the increase from around 47% to approx. 60% is very good. As the tendency of recovered Li persisted, it can be assumed that further wash steps lead to similar results. As already mentioned in Section 3.1, it can be compared to an increased liquid–solid ratio due to leaching with water in this process step.
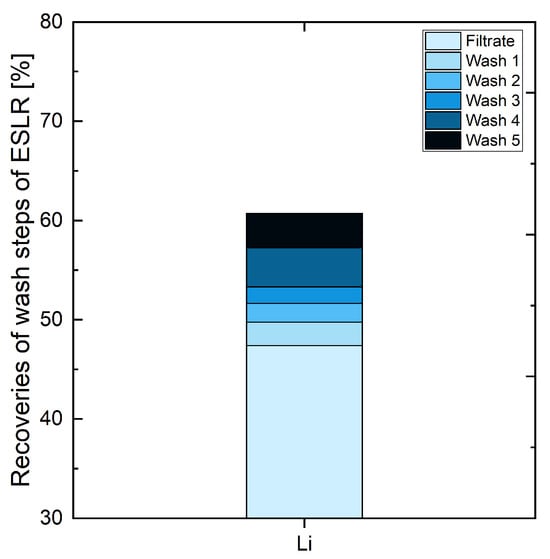
Figure 4.
Influence of the different wash steps in ESLR (early-stage lithium recovery).
By taking a look at the recoveries of the wash steps in leaching, it can be seen that washing the filter cake has nearly no effect (up to 0.4%, compare with Figure 5a). Due to the good permeability of the filter cake, it can be deduced that one wash step is sufficient regarding recoveries after leaching. Washing after Cu cementation shows a similar result. Figure 5b shows that the first wash step recovered nearly 0.5% of every element, but further wash steps did not result in more recovery. So, as with leaching, one wash step is sufficient after Cu cementation to recover as many of the target elements as possible.
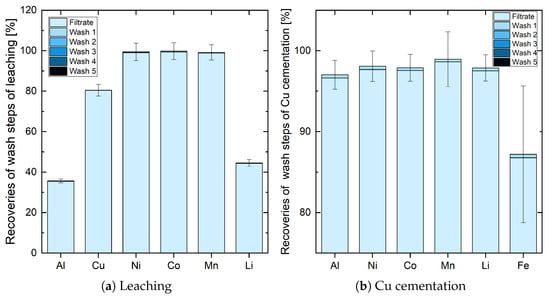
Figure 5.
Influence of the different wash steps in leaching and Cu cementation.
A big difference to the previous steps can be seen in the results of the wash steps after Fe-Al precipitation (see Figure 6a). The first wash steps received a good average recovery of about 1.5%. Due to the sponge-like structure of the hydroxide filter cakes, there are many unprecipitated ions of elements in the liquid, which are washed out by wash water. The second wash step already shows a reduced recovery of 0.9% on average, while the third has less than 0.7% left. The continuous reduction indicates a trend, suggesting that the recovery would have fallen even further. Because of long filtration times due to a typical thick hydroxide filter cake, cracks were formed, in which wash water passed through the cake without much effect. Supporting this theory, dispersing the filter cake led to an increase of more than 3.4% of all target elements. The dissolution of wash step 5 resulted in only approx. 0.6% for Ni, Co, and Mn. Regarding Li, this is shown more clearly. All available Li seems to be washed out in wash step 4 because the amount of Li in the liquid of wash step 5 was below the detection limit. Since the fourth wash step recovered most of it and the fifth recovered only a small amount, we arrive at the conclusion that five wash steps are sufficient after Fe-Al precipitation. After NMC precipitation, only the recovery of Li is of interest. Figure 6b shows the influence of the different wash steps of this. Since the filtrate had less than 80% of Li ions of the filtrate of Fe-Al precipitation left, approx. 20% must be available in the filter cake. Washing the first time recovered approx. 3%, the second time less than 1.8%, and the third time approx. 1.6%. This shows a behavior similar to washing after Fe-Al precipitation. Dispersing the filter cake in wash step 4 received here a high result as well, where approx. 8.7% of Li could be recovered. The fifth wash step was with less than 2.7%, which is significantly less, but it is debatable here whether further wash steps would have achieved more. As a result, nearly 18% of 20% could be recovered and it can be concluded that a minimum of five wash steps are advisable.
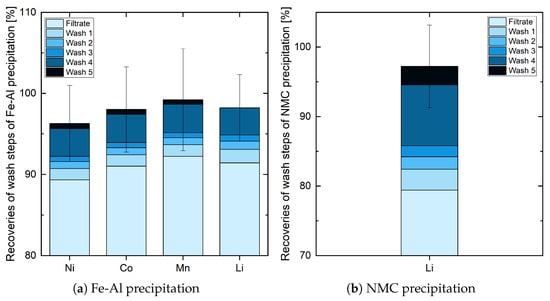
Figure 6.
Influence of the different wash steps in Fe-Al precipitation and NMC precipitation.
3.3. Impact of Extended Washing on the Purity of Filter Cakes
Besides the recovery, the purity is another important factor that can be used to evaluate the results. For this purpose, the compositions of the filter cakes are analyzed here. Additionally, the detailed influence of extended washing is examined by comparing with the cakes of the reference process.
Figure 7 compares the compositions of ESLR filter cakes. While the reference process in (a) shows 12.0% C, 1.0% Al, 18.1% Li, and 12.2% F, extended washing resulted in an increase of Li (20.4%), F (14.5%), and Al (1.3%), while C was reduced (11.2%) (see (b)). The unknown part was reduced from 56.1% to 51.8%. With the assumption that C was completely present as Li2CO3, Al as LiAlO2, and the remaining Li as LiOH, the amount of O can be calculated to be 49.1% in the reference process and 50.5% after extended washing. If F was available as LiF, more than 90% of filter cakes consisted of Li compounds in both cases. Due to the compositions of the filter cakes and the previous assumptions of chemical compounds, it can be concluded that the increased amount of wash water mainly dissolve additional LiF and LiAlO2. It can be inferred that washing the filter cakes has only a small impact on the purity. Even if the quantity of Li increases (see Section 3.1 and Section 3.2), the quality remains similar. This can be explained by the fact that at this point in the process no impurities by H2SO4 or NaOH entered the system.
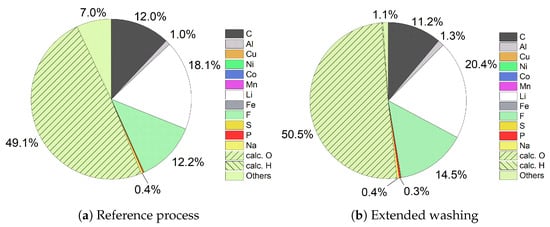
Figure 7.
Comparison of filter cake compositions of ESLR.
In case of leaching, the compositions of the filter cakes from the reference process and extended washing are nearly identical. The values of C (76.8%, 77.0%), Al (2.4%, 2.2%), and unknown components (20.6%, 20.5%) are very close to each other (see Figure 8). Due to the low retention capacity of the Li-poor black mass, one wash step is enough to clean the filter cake and achieve the results close to extended washing. The unknown part of the filter cakes can be attributed to O and H of hydrocarbons, which are partially still available because pyrolysis took place at a temperature of 650 °C.
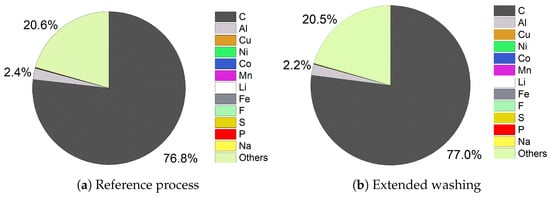
Figure 8.
Comparison of filter cake compositions of leaching.
The filter cake from the Fe-Al precipitation in the reference process (Figure 9a) shows only one-fifth of the target elements Fe (18.9%) and Al (1.8%). With the assumption that both were available as hydroxides, it would account for up to two-fifths in total. While cathode materials like Ni (1.3%), Co (1.3%), and Mn (1.2%) were still left in small amounts after one wash step, the highest impurities came from Na (11.8%) with the use of NaOH and S (11.5%) and with the use of H2SO4. This can be explained by the sponge-like structure of the voluminous filter cake, which contains, even after the first wash step, many of dissolved ions. Provided that Na and S were present as Na2SO4 and Ni, Co, and Mn as hydroxide compounds, 21.5% of 46.6% of the unknown components can be attributed to O and 1.4% to H by calculation. The influence of extended washing can be seen in Figure 9b. The first thing to notice is the highly increased amount of Fe, with 40.5% of it having more than doubled. Al also increased from 1.8% to 2.5%, which results in a total amount of 84.8% target compositions if they are available as hydroxides. The amount of impurities dropped significantly by extended washing, resulting in low values for Ni (0.6%), Co (0.4%), and Mn (0.4%), as well as for S (2.9%), and especially Na, which was reduced to 0.8%. With the same assumptions regarding compounds like before, the amount of O can be calculated to be 40.1% and the amount of H to be 2.5%. It can be concluded that the dissolved ions, which were contained in the filter cake, were washed out by extended washing, leading to both an increased quantity and a sharply increased quality in the filter cake.
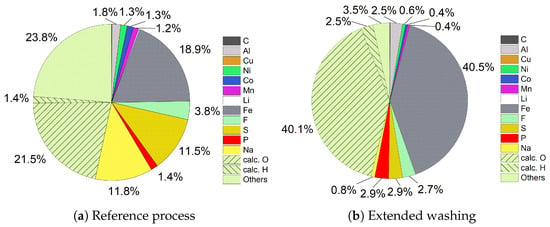
Figure 9.
Comparison of filter cake compositions of Fe-Al precipitation.
The filter cake compositions of NMC precipitation exhibit a similar result to Fe-Al precipitation. Figure 10a shows that Ni (8.9%), Co (8.9%), and Mn (8.1%) accounted for only a quarter (26.0%) of the filter cake. Together with Figure 10b, it can be seen that the amount of Na (13.7%) and S (12.7%) dropped down to 1.8% and 5.1% by extended washing. Removing these impurities led to a big increase in the proportion of the targeted cathode metals. After five wash steps, Ni (16.7%), Co (16.7%), and Mn (14.5%) accounted in sum for almost half (47.8%) of the filter cake. With the expectation that they were available as hydroxides, the share amounts to 76.2%. The calculated amount of O fell from 41.2% to 34.6% if Na and S were presented as Na2SO4 while the amount of H increased from 1.0% to 1.9%. As the NMC filter cake is like the Fe-Al filter cake based on hydroxides, the reasoning is identical. Due to the large amount of dissolved or unprecipitated ions in the voluminous filter cake, more than one wash step is necessary to achieve a good purity. In particular, the remaining amount of 5.1% of S suggests that more wash steps than five can lead to a further enhancement of the purity and thus improved the quality of the filter cake.
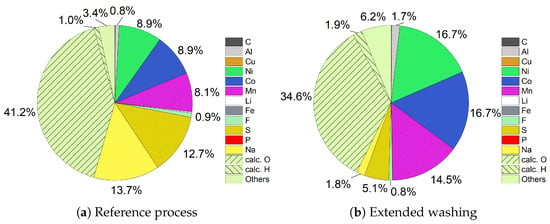
Figure 10.
Comparison of filter cake compositions of NMC precipitation.
4. Conclusions
The aim of this paper was the evaluation of a new process of washing filter cakes in hydrometallurgical LIB recycling to clarify the question of how many wash steps are suitable for the recovery and purity of filter cakes. For this purpose, a reference process with one wash step and a process with extended washing (five wash steps) was examined. At first, recoveries were compared. The positive aspect was the fact that extended washing increased recovery by wash water from approx. 4% to approx. 7.5% for NMC. Especially regarding Li, combining ESLR, wash water, and NMC filtrate, the recovery could be increased in total up to 100% by extended washing. On the other hand, extended washing has the disadvantage of process losses for all elements except Li due to excessive process steps.
The detailed investigation of the substeps of extended washing showed different results for the different process steps. In the case of ESLR, further wash steps are comparable with a higher liquid–solid ratio. Five wash steps resulted in 13% more Li. Regarding H2SO4 leaching and Cu cementation, the examination showed that one wash step is sufficient because of the low retention capacity and permeability of the filter cake. In contrast to this, Fe-Al precipitation and NMC precipitation benefited from the increased number of wash steps due to the sponge-like and voluminous filter cake, which is typically generated by hydroxides. The first three wash steps after Fe-Al precipitation yielded an average of approx. 2.9% for Ni, Co, and Mn, respectively. In the case of Li, more than 3.4% was even achieved. Especially when dispersing the filter cakes in step 4, this resulted in high recoveries. This step alone yielded approx. 3.4% recovered Ni, Co, Mn, and Li, respectively. Wash step 5 showed reduced effectiveness. In total, five wash steps were considered appropriate. Washing after NMC precipitation showed even greater effectiveness. Nearly 6% of Li could be recovered by the first three wash steps. Dispersing the filter cake in step 4 achieved more than 8.7%, while wash step 5 still reached 2.7%. In conclusion, a minimum of five wash steps were deemed suitable here.
Regarding purity, the compositions of the filter cakes were analyzed. In the case of ESLR, with an increase of Li from 18.1% in the reference process to 20.4% after extended washing, only a small improvement was achieved. The comparison of the filter cakes after H2SO4 leaching showed that more than one wash step resulted in no further improvement; thus, the filter cake retained the same quality. Fe-Al precipitation, on the other hand, showed an improvement from 18.9% Fe after the reference process to 40.5% after extended washing, which is a significant result. Additionally, impurities in the form of Na and S were removed and Ni, Co, and Mn were recovered by wash water, which also justifies five washing steps. Extended washing after NMC precipitation also achieved a great improvement. While filter cakes showed only less than 26% of NMC after the reference process, this accounted for nearly 48% when removing impurities by extended washing, which is why five wash steps are recommended here as well.
The most important points are summarized as follows:
- One wash step is sufficient after H2SO4 leaching and Cu cementation.
- Extended washing with five wash steps is recommended after Fe-Al precipitation and NMC precipitation for improved recovery.
- Single wash steps of extended washing are able to recover 0.5% to 3.5% of Ni, Co, and Mn and 1.6% to 8.7% of Li.
- Extended washing improved the purity of Fe-Al compounds in the Fe-Al filter cake from 41.3% to 84.8% and the purity of NMC compounds in the NMC filter cake from 41.3% to 76.2%
- Nearly 100% of Li can be recovered by using extended washing, if wash water is recirculated.
The losses of target metals by an increased number of process steps can be reduced by an adjustment of the wash process of dispersing filter cakes, leading to an optimized process. In addition, it should be investigated whether a change in the order of the wash steps and thus dispersing filter cakes earlier will lead to a different result. Furthermore, the recirculation of wash water into the process should be analyzed, as increasing the amount of used water would make scaling up uneconomical.
Author Contributions
Conceptualization, D.D. and F.D.; methodology, D.D. and M.A.; software, D.D.; validation, D.D. and M.A.; formal analysis, D.D. and M.A.; investigation, D.D. and M.A.; resources, D.D. and F.D.; data curation, D.D. and M.A.; writing—original draft preparation, D.D.; writing—review and editing, D.D., M.A., F.D., and B.F.; visualization, D.D.; supervision, F.D. and B.F.; project administration, D.D. and F.D.; funding acquisition, D.D., F.D., and B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry for Education and Research (BMBF) and the Projektträger Jülich (PTJ) under Project SIMTEGRAL (Integrated multi-scale system simulation and sustainability assessment of primary and circular raw material supply chains for lithium-ion batteries), grant number 03XP0331C, which was part of the competence cluster for battery recycling greenBatt.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author.
Acknowledgments
The authors acknowledge all project partners and colleagues for their support and feedback.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kresse, C.; Bastian, D.; Bookhagen, B.; Frenzel, M. Lithium-Ionen-Batterierecycling in Deutschland und Europa. 2022. Available online: https://savearchive.zbw.eu/bitstream/11159/12253/1/180609875X_0.pdf (accessed on 13 November 2025).
- Jármai, K.; Cservenák, Á. Vehicle and Automotive Engineering 4; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Alanazi, F. Electric Vehicles: Benefits, Challenges, and Potential Solutions for Widespread Adaptation. Appl. Sci. 2023, 13, 6016. [Google Scholar] [CrossRef]
- König, A.; Nicoletti, L.; Schröder, D.; Wolff, S.; Waclaw, A.; Lienkamp, M. An Overview of Parameter and Cost for Battery Electric Vehicles. World Electr. Veh. J. 2021, 12, 21. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Hydrometallurgical recycling technologies for NMC Li-ion battery cathodes: Current industrial practice and new R&D trends. RSC Sustain. 2023, 1, 1932–1951. [Google Scholar] [CrossRef]
- Wang, Y.; An, N.; Wen, L.; Wang, L.; Jiang, X.; Hou, F.; Yin, Y.; Liang, J. Recent progress on the recycling technology of Li-ion batteries. J. Energy Chem. 2021, 55, 391–419. [Google Scholar] [CrossRef]
- Chen, X.; Cao, L.; Kang, D.; Li, J.; Li, S.; Wu, X. Hydrometallurgical Processes for Valuable Metals Recycling from Spent Lithium-Ion Batteries. In Recycling of Spent Lithium-Ion Batteries; An, L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 93–139. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Maisel, F.; Neef, C.; Marscheider-Weidemann, F.; Nissen, N.F. A forecast on future raw material demand and recycling potential of lithium-ion batteries in electric vehicles. Resour. Conserv. Recycl. 2023, 192, 106920. [Google Scholar] [CrossRef]
- Hu, S.; He, S.; Jiang, X.; Wu, M.; Wang, P.; Li, L. Forecast and Suggestions on The Demand of Lithium, Cobalt, Nickel and Manganese Resources in China’s New Energy Automobile Industry. IOP Conf. Ser. Earth Environ. Sci. 2021, 769, 042018. [Google Scholar] [CrossRef]
- Tang, C.; Tukker, A.; Mogollón, J.M. The demand and recycling potential for lithium, cobalt, and nickel in the European electric-mobility transition. Environ. Res. Commun. 2025, 7, 061007. [Google Scholar] [CrossRef]
- Europäische Union. VERORDNUNG (EU) 2023/1542 DES EUROPÄISCHEN PARLAMENTS UND DES RATES vom 12. Juli 2023 über Batterien und Altbatterien, zur Änderung der Richtlinie 2008/98/EG und der Verordnung (EU) 2019/1020 und zur Aufhebung der Richtlinie 2006/66/EG. 12 July 2023. Available online: https://eur-lex.europa.eu/legal-content/DE/TXT/?uri=CELEX%3A32023R1542 (accessed on 13 November 2025).
- van Hoof, G.; Robertz, B.; Verrecht, B. Towards Sustainable Battery Recycling: A Carbon Footprint Comparison between Pyrometallurgical and Hydrometallurgical Battery Recycling Flowsheets. Metals 2023, 13, 1915. [Google Scholar] [CrossRef]
- Fahimi, A.; Ducoli, S.; Federici, S.; Ye, G.; Mousa, E.; Frontera, P.; Bontempi, E. Evaluation of the sustainability of technologies to recycle spent lithium-ion batteries, based on embodied energy and carbon footprint. J. Clean. Prod. 2022, 338, 130493. [Google Scholar] [CrossRef]
- An, L. (Ed.) Recycling of Spent Lithium-Ion Batteries; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 10, S3632–S3639. [Google Scholar] [CrossRef]
- Wang, H.; Friedrich, B. Development of a Highly Efficient Hydrometallurgical Recycling Process for Automotive Li–Ion Batteries. J. Sustain. Metall. 2015, 1, 168–178. [Google Scholar] [CrossRef]
- Agrawal, R.; Kapoor, M.L. Theoretical considerations on the cementation of copper with iron. J. S. Afr. Inst. Min. Metall. 1982, 82, 106–111. [Google Scholar]
- Gupta, C.K.; Mukherjee, T.K. Hydrometallurgy in Extraction Processes; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Chernyaev, A.; Zhang, J.; Seisko, S.; Louhi-Kultanen, M.; Lundström, M. Fe3+ and Al3+ removal by phosphate and hydroxide precipitation from synthetic NMC Li-ion battery leach solution. Sci. Rep. 2023, 13, 21445. [Google Scholar] [CrossRef]
- Cai, G.; Fung, K.Y.; Ng, K.M.; Wibowo, C. Process Development for the Recycle of Spent Lithium Ion Batteries by Chemical Precipitation. Ind. Eng. Chem. Res. 2014, 53, 18245–18259. [Google Scholar] [CrossRef]
- Tawonezvi, T.; Zide, D.; Nomnqa, M.; Madondo, M.; Petrik, L.; Bladergroen, B.J. Recovery of NixMnyCoz(OH)2 and Li2CO3 from spent Li-ionB cathode leachates using non-Na precipitant-based chemical precipitation for sustainable recycling. Chem. Eng. J. Adv. 2024, 17, 100582. [Google Scholar] [CrossRef]
- Monhemius, A.J. Precipitation diagrams for metal hydroxides, sulphides, arsenates and phosphates. Trans. Inst. Min. Metall. 1977, 86, C202–C206. [Google Scholar]
- Anwar Ul Haq, R. Thermodynamics and Precipitation Kinetics of Lithium Carbonate (Li2CO3). Master’s Thesis, School of Chemical Enginering, Aalto University, Espoo, Finland, 2019. [Google Scholar]
- Schwich, L.; Schubert, T.; Friedrich, B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals 2021, 11, 177. [Google Scholar] [CrossRef]
- Yan, Z.; Sattar, A.; Li, Z. Priority Lithium recovery from spent Li-ion batteries via carbothermal reduction with water leaching. Resour. Conserv. Recycl. 2023, 192, 106937. [Google Scholar] [CrossRef]
- Anlauf, H. (Ed.) Wet Cake Filtration: Fundamentals, Equipment, and Strategies; Wiley-VCH: Hoboken, NJ, USA, 2019. [Google Scholar]
- Noerpel, S.; Siauw, V.; Nirschl, H. Filter Cake Washing of Mesoporous Particles. Chem. Eng. Technol. 2012, 35, 661–667. [Google Scholar] [CrossRef]
- Seupel, S.; Peuker, U.A. Displacement washing of filter cakes from porous particles. Sep. Purif. Technol. 2021, 274, 118141. [Google Scholar] [CrossRef]
- Ruslim, F.; Nirschl, H.; Stahl, W.; Carvin, P. Optimization of the wash liquor flow rate to improve washing of pre-deliquored filter cakes. Chem. Eng. Sci. 2007, 62, 3951–3961. [Google Scholar] [CrossRef]
- Huhtanen, M.; Salmimies, R.; Kinnarinen, T.; Häkkinen, A.; Ekberg, B.; Kallas, J. Empirical Modelling of Cake Washing in a Pressure Filter. Sep. Sci. Technol. 2012, 47, 1102–1112. [Google Scholar] [CrossRef]
- Svarovsky, L. (Ed.) Solid-Liquid Seperation, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Emmett, R.C.; Dahlstrom, D.A. Liquid-solid separation factors in hydrometallurgical leach circuit design. Can. J. Chem. Eng. 1959, 37, 3–8. [Google Scholar] [CrossRef]
- Kuo, M.T. Filter cake washing performance. AIChE J. 1960, 6, 566–568. [Google Scholar] [CrossRef]
- Mocellin, J. COM 2015: Hosting AMCAA: 54th Annual Conference of Metallurgists Hosting America’s Conference on Aluminium Alloys: August 23–26, Fairmont Royal York Hotel, Toronto, ON, Canada: Proceedings; Canadian Institute of Mining Metallurgy and Petroleum: Westmount, QC, Canada, 2015. [Google Scholar]
- Choudhury, A.P.R.; Dahlstrom, D.A. Prediction of cake–washing results with continuous filtration equipment. AIChE J. 1957, 3, 433–438. [Google Scholar] [CrossRef]
- Kinnarinen, T.; Lubieniecki, B.; Holliday, L.; Helsto, J.J.; Häkkinen, A. Recovery of sodium from bauxite residue by pressure filtration and cake washing. Int. J. Miner. Process. 2015, 141, 20–26. [Google Scholar] [CrossRef]
- Sauer, F.; Löwer, E.; Henn, H.; Peuker, U.; Hoffner, B. Displacement Washing of Filter Cakes With a Fine Particle Top Layer. Chem. Eng. Technol. 2025, 48, e202400394. [Google Scholar] [CrossRef]
- Brückner, A.; Sprott, T.; Peuker, U.A.; Hoffner, B. Influence of pre-dewatering on the success of cake washing. Sep. Sci. Technol. 2023, 58, 175–187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).